ABSTRACT
Uterine fibroids (leiomyomas) are the most common benign tumors associated with excessive deposition of extracellular matrix (ECM)-associated proteins that increase fibroid tumorigenicity. Herein, we determined the expression levels of vitamin D receptor (VDR) protein in human uterine fibroids and compared these levels to those in adjacent normal myometrium. Using Western blot analysis, we found that more than 60% of uterine fibroids analyzed (25 of 40) expressed low levels of VDR. We also found that the biologically active 1,25-dihydroxyvitamin D3 (1,25[OH]2D3), which functions via binding to its nuclear VDR, induced VDR in a concentration-dependent manner and reduced ECM-associated fibrotic and proteoglycans expression in immortalized human uterine fibroid cell line (HuLM). At 1–10 nM concentrations, 1,25(OH)2D3 significantly induced (P < 0.05) nuclear VDR, which was further stimulated by higher concentrations of 1,25(OH)2D3 in HuLM cells. 1,25(OH)2D3 at 10 nM also significantly reduced (P < 0.05) the protein expression of ECM-associated collagen type 1, fibronectin, and plasminogen activator inhibitor-1 (PAI-1) in HuLM cells. We also found that 1,25(OH)2D3 reduced mRNA and protein expressions of proteoglycans such as fibromodulin, biglycan, and versican in HuLM cells. Moreover, the aberrant expression of structural smooth muscle actin fibers was reduced by 1,25(OH)2D3 treatment in a concentration-dependent manner in HuLM cells. Taken together, our results suggest that human uterine fibroids express reduced levels of VDR compared to the adjacent normal myometrium and that treatment with 1,25(OH)2D3 can potentially reduce the aberrant expression of major ECM-associated proteins in HuLM cells. Thus, 1,25(OH)2D3 might be an effective, safe, nonsurgical treatment option for human uterine fibroids.
Keywords: 1,25-dihydroxyvitamin D3 (1,25[OH]2D3); extracellular matrix (ECM); fibroids; vitamin D receptor (VDR)
1,25-dihydroxyvitamin D3 is an antifibrotic agent that suppresses major extracellular matrix-associated protein expression in human uterine fibroid cells and might be useful as an alternative therapeutic option for fibroid treatment.
INTRODUCTION
Uterine fibroids (leiomyoma) are the most common benign tumors associated with excessive symptoms, including vaginal bleeding, pelvic pain, recurrent miscarriage, and preterm labor [1, 2]. They are the most commonly cited reason for hysterectomy in the United States [3]. Although the initiating factors that lead to the development of uterine fibroids are not well understood, results of numerous studies support the idea that estrogen and progesterone play important roles in the growth of uterine fibroids [4, 5]. African-American women are at 3- to 4-fold greater risk of developing uterine fibroids than Caucasian women [6]. In addition, hypovitaminosis D, a condition in which the vitamin D levels are lower than the normal physiological levels, is approximately 10-fold more prevalent in African-American women (40%–45%) compared to Caucasian women (4%) [7]. However, the exact reasons for this higher occurrence of hypovitaminosis D in African-American women are not well understood. Moreover, the correlation between vitamin D3 and uterine fibroids is also not well documented.
Uterine fibroids are characterized by the deposition of excessive extracellular matrix (ECM)-associated proteins, and the process is known as fibrosis [8]. Studies have demonstrated genes that encode ECM proteins are expressed abnormally in uterine fibroids [9, 10]. The transforming growth factor-beta (TGF-β) family are multifunctional peptides that regulate diverse biological functions, including cell growth, differentiation, inflammation, apoptosis, and tissue remodeling, and these processes are important to tissue fibrosis [11–13]. TGF-β has been identified in a variety of normal and transformed mammalian cells and tissues [11]. These profibrotic cytokines are overexpressed in a wide range of fibrotic tissues, including uterine fibroids [13–15]. TGF-β has been shown to upregulate the synthesis of many ECM proteins associated in the process of fibrosis [16]. The ECM-associated genes such as collagen type 1 and fibronectin were overexpressed in uterine fibroids as compared to normal myometrium [17, 18]. TGF-β stimulates ECM production in uterine fibroids by inducing the expression of collagen type 1, fibronectin, laminin, and proteoglycans [11, 19–21]. These multifunctional peptides promote fibroproliferative and tumorigenic processes in different organ systems, including uterine fibroids [22].
Vitamin D3 is known as the primary form of dietary vitamin D. Vitamin D3 itself is biologically inactive and must be metabolized to its biologically active forms. After it is consumed in the diet or synthesized in the epidermis, vitamin D3 enters the circulation and is transported to the liver, where it is hydroxylated to form 25-hydroxyvitamin D3, the major circulating form of vitamin D3. The 25-hydroxyvitamin D3 is then enzymatically catalyzed in the kidney to form the biologically active 1,25-dihydroxyvitamin D3 (1,25[OH]2D3), which exerts the physiological effects within the body [23]. A previous study has demonstrated that 1,25(OH)2D3 can act as a strong growth inhibitor and induce apoptosis in human breast cancer cells [24]. 1,25(OH)2D3 can suppress proliferation of normal and malignant cells by inducing differentiation and apoptosis [25]. An analog of 1,25(OH)2D3 exhibits potential antitumor activity in vivo in a murine squamous cell carcinoma model [26]. Recently, we and others have shown a growth inhibitory function of 1,25(OH)2D3 on human uterine fibroid cells [27, 28]. We have also shown that 1,25(OH)2D3 reduces fibroid tumor growth in vivo in an Eker rat model [29]. Moreover, we have shown that 1,25(OH)2D3 can attenuate TGF-β-induced protein expression in human uterine fibroid cell line [30]. Our recent study also demonstrated the risk of lower levels of serum vitamin D3 (both 25-hydroxyvitamin D3 and 1,25[OH]2D3) in the development of human uterine fibroids [31]. In addition, several recent epidemiological studies demonstrated the risk of lower levels of serum vitamin D3 in the development of human uterine fibroids [32, 33]. To our knowledge, however, no study has yet established the lower levels of vitamin D receptor (VDR) in human uterine fibroids compared to the adjacent normal myometrium and the risk associated in the development of human uterine fibroids. In addition, the biological function of 1,25(OH)2D3 in the regulation of major ECM-associated proteins, which are involved in the process of fibrosis, is also not known. Thus, the present study was undertaken to evaluate the risk associated with reduced levels of VDR protein in human uterine fibroid tumors and to determine the biological function of 1,25(OH)2D3 in the regulation of ECM-associated proteins, which are key in the process of fibrosis.
MATERIALS AND METHODS
Cell Lines and Cultures
Both immortalized human uterine fibroid cell line (HuLM) and human normal uterine smooth muscle cell line (UTSM) were a generous gift from Dr. Darlene Dixon (National Institute of Environmental Health Sciences, Research Triangle Park, NC) [34]. These cell lines were cultured and maintained as described previously [27, 30].
Reagents and Antibodies
The bioactive 1,25[OH]2D3 was purchased from Sigma Biochemicals. Smooth muscle cell culture medium (SmBM) was purchased from Lonza. Antibodies were purchased as follows: anti-collagen type 1 (catalog no. 70R-CR007X) from Fitzgerald; anti-plasminogen activator inhibitor-1 (PAI-1; catalog no. sc-8979), anti-fibromodulin (catalog no. sc-33772), anti-biglycan (catalog no. sc-33788), anti-versican (catalog no. sc-25831), and anti-VDR (catalog no. sc-1008) from Santa Cruz Biotechnology; and anti-fibronectin (catalog no. F3648), anti-α-actin (catalog no. A5228), fluorescein isothiocyanate (FITC)-conjugated phalloidin (F-actin; catalog no. P5282), and monoclonal anti-β-actin (catalog no. A5441) from Sigma Biochemicals.
Protein Extraction from Tissue Samples
Uterine fibroid tumors and samples of the adjacent normal myometrium tissue were collected from 40 individuals who underwent hysterectomy at clinics in Texas and Tennessee to surgically remove their uterine fibroids. Tissue samples were collected from different ethnic groups, including African-American, Caucasian, and Hispanic patients who underwent hysterectomy (abdominal, vaginal, and laparoscopic). All subjects were from 35 to 53 years old, and their uterine fibroid sizes were from 2.5 to 14 cm. Tissue samples were also collected from the Meharry Clinic under an approved IRB protocol (Protocol 090630WJR246 11). All patients received no hormone supplementations, including vitamin D3, for at least 3 mo before the hysterectomy was performed. Extraction of proteins from tissue samples was performed as described previously [35, 36] with minor modifications. Briefly, a portion (3–5 mm3) of each uterine fibroid and the adjacent normal myometrium tissue samples was chopped into small pieces, homogenized in lysis buffer containing 50 mM Tris-HCL (pH 7.5), 150 mM NaCl, 10 mM ethylenediaminetetra-acetic acid, 0.5% (wt/vol) Nonidet P-40, 0.5 mM dithiothreitol, 0.1% (wt/vol) SDS, 0.5% (wt/vol) sodium deoxycholate, 5 mM sodium fluoride, 1.0 mM phenylmethylsulfonyl fluoride, and 3 μg/ml of each protease inhibitor such as leupeptin, pepstatin, and aprotinin. Tissue homogenates were sonicated to solubilize proteins and left on ice for approximately 30 min, vortexed intermittently, and then centrifuged at 14 000 rpm for 20 min at 4°C. Clean supernatants were collected into new Eppendorff tubes, and then the protein concentration of each sample was determined using Bradford Protein Assay reagent (Bio-Rad) according to the manufacturer's instructions. To determine the expression of VDR in uterine fibroids and the adjacent normal myometrium, equal amounts of each protein lysate were examined by Western blot analysis using anti-VDR and anti-β-actin (loading control) antibodies.
Western Blot Analysis
For analysis of protein expressions, HuLM and UTSM cells were serum starved overnight and then treated with increasing concentrations of 1,25(OH)2D3 (0, 1, 10, 100, and 1000 nM) for 48 h, as indicated in the figure legends. The 0 nM concentration of 1,25(OH)2D3 in each individual experiment served as the vehicle control. Cell lysates were prepared using the above-described lysis buffer, and Western blot analysis was performed as previously described [30, 37]. Equal amounts of solubilized proteins from either tissue or cultured cells (30–40 μg) were resolved in 8% and 10% SDS-PAGE. The proteins were transferred onto polyvinylidene fluoride membranes (Bio-Rad) and incubated with specific primary antibodies for 2 h at room temperature, followed by a 1-h incubation with appropriate horseradish peroxidase-conjugated secondary antibodies. For detection of proteoglycan proteins such as fibromodulin, biglycan, or versican in Western blots, we used Santa Cruz antibodies. The anti-fibromodulin antibody has previously been used to show the protein expression in uterine fibroids and myometrium [38]. This antibody has also been used to examine the expression of fibromodulin in fibroproliferative disorders such as hypertrophic scarring [39]. On the other hand, the anti-biglycan antibody has also recently been used to examine the expression of biglycan in hypertrophic scarring [39], and the anti-versican antibody has been used to examine the expression of versican protein in overused supraspinatus tendon [40] and atherosclerosis disorders [41]. Moreover, the anti-fibronectin antibody has previously been used to determine fibronectin protein in Western blots [29, 42]. The antigen-antibody complexes in Western blots were detected with an enhanced chemiluminescence detection system (Amersham Biosciences). Specific protein bands were visualized after exposure of autoradiography films and developing the films using an automatic x-ray developer. The intensity of each protein band was quantified using image-analysis software and normalized against β-actin, and then the normalized ratio was used to generate data graphs.
RNA Isolation and First-Stand cDNA Synthesis by Reverse Transcriptase
To examine the effects of 1,25(OH)2D3 on mRNA levels of proteoglycans such as fibromodulin, biglycan, and versican, HuLM cells were plated in 60-mm dishes, serum starved for 20 h, and then treated with increasing concentrations of 1,25(OH)2D3 (0, 1, 10, 100, and 1000 nM) for 48 h. Cells were collected from the plates, and total cellular RNA was isolated using RNeasy Protect kit (Qiagen) as described previously [43]. RNA concentration was determined using a NanoVue system (GE Healthcare).
First-strand cDNA was synthesized using the RT synthesis kit (GoScript Reverse Transcription System, Catalog no. A5000, Promega). First-strand cDNA was prepared by reverse transcriptase using 1 μg of total RNA in a 20-μl reaction volume containing oligo-dT primers, dNTPs, MgCl2, RNase inhibitor, and reverse transcriptase according to the manufacturer's instruction. The reaction mixture was incubated for 1 h at 42°C and stopped by incubation at 70°C for 10 min.
Quantitative Real-Time PCR
Quantitative real-time PCR was performed to determine the mRNA expression of fibromodulin, biglycan, and versican using total RNA from 1,25(OH)2D3-treated HuLM cells as described above. Primer sequences were obtained from the published literature [44, 45]. Primer sequences were as follows: fibromodulin, 5′-TTTTATCATCGTTCTGCCTTCATG-3′ (sense) and 5′-TGTTTGCGGGACCTTAGGAA-3′ (antisense); biglycan, 5′-TTGCCCCCAAACCTGTACTG-3′ (sense) and 5′-AAAACCGGTGTCTGGGACTCT-3′ (antisense); versican, 5′-TGGAATGATGTTCCCTGCAA-3′ (sense) and 5′-AAGGTCTTGGCATTTTCTACAACAG-3′ (antisense); and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 5′-ATGGGGAAGGTGAAGGTCG-3′ (sense) and 5′-TAAAAGCAGCCCTGGTGACC-3′ (antisense). Both sense and antisense primers for fibromodulin, biglycan, versican, and GAPDH were purchased from Qiagen. Amplification reactions were performed using a SYBR Green PCR Master Mix reagent kit (Qiagen/SA Bioscience). Briefly, 50 ng of each cDNA were added to the Master Mix containing appropriate primer sets (200 nM each) and SYBR Green in a 25-μl reaction volume. All samples were analyzed in triplicate measurements. Real-time PCR analysis was performed using a MyiQ5 machine (Bio-Rad). Amplification of all genes was performed under the following cycling conditions: denaturation at 95°C for 10 min and then 45 cycles of 95°C for 15 sec and 60°C for 1 min. Synthesis of a DNA product of the expected size was confirmed by melting-curve analysis. Relative quantitation of target gene expression was calculated by the comparative cycle threshold (Ct) method, which normalizes the copy number of the target gene to that of an endogenous reference gene. For each cDNA sample, the Ct value of target sequence was used. GAPDH was chosen as the reference housekeeping gene based on the majority of the previous studies. The Ct values of each gene were normalized to those of GAPDH, and the resultant ratios were used to express data.
Immunofluorescence Analysis
Both HuLM and UTSM cells were cultured on sterile glass cover slips, serum starved for 20 h, and treated with increasing concentrations of 1,25(OH)2D3 (0, 10, 100, and 1000 nM) for 48 h. The 0 nM concentration of 1,25(OH)2D3 in each individual experiment was considered as the vehicle control. Cells were fixed in a 3.7% formaldehyde solution at room temperature for 15 min. After washing in PBS, cells were permeabilized for 10 min using 0.2% Triton X-100/PBS, and then nonspecific binding was inhibited by blocking for 30 min in blocking/incubation solution containing 10% (wt/vol) fetal bovine serum/0.1% (wt/vol) Triton X-100/PBS. Incubation with either primary anti-fibronectin or anti-collagen type 1 or anti-VDR antibody (1:50 dilution each) and CY3- or FITC-conjugated secondary antibody (1:150 dilution) were performed for 1 h at room temperature. Cells were washed for 15 min (three washes of 5 min each) with the above-described incubation solution, air-dried, and mounted onto microscopic slides with one drop of VECTASHIELD solution (Vector Laboratories) containing 4′,6 diamidino-2-phenylindole. For staining with smooth muscle f-actin, cells were incubated with 2 μg/ml of FITC-conjugated phalloidin followed by washing with PBS for 15 min (three washes of 5 min each). Fluorescent images were taken using an Axiovert 100 M inverted microscope. Analyses of images were performed using image-analysis software (MCY-INST-10, MVIA, Inc.). Staining signals for structural smooth muscle f-actin (green) were visualized by fluorescent microscopy. Intensities of the signals were visually compared between the control and 1,25(OH)2D3-treated cells.
Laser Confocal Microscopy
Both HuLM and UTSM cells were cultured on sterile glass cover slips and treated with increasing concentrations of 1,25(OH)2D3 (0, 10, 100, and 1000 nM) for 48 h. Cells were fixed, permeabilized, and processed for immunofluorescence analysis using anti-collagen type 1 and anti-fibronectin antibodies and FITC-conjugated phalloidin as described above. Staining signals for collagen type 1 (red), fibronectin (red), and structural smooth muscle f-actin (green) were visualized using laser confocal fluorescent microscopy. A TE2000-U C1 confocal laser scanning microscope (Nikon) was used to collect fluorescence emissions with the 505- to 550-nm filter set. Confocal images were captured at 400× magnification under conditions similar to those described previously [30]. The EZ C1 confocal software (Nikon) was then used to reconstruct serial z-plane images into a three-dimensional (3D) rendering, which was then transferred to PowerPoint (Microsoft) as described previously [30].
Statistical Analysis
All statistical data analyses were performed using Student t-test. The Student t-test was used to assess the significance of differences in levels of VDR in human uterine fibroids and the adjacent normal myometrium (M) samples. The Student t-test was also used to assess the significance of differences both between untreated control versus 1,25(OH)2D3-treated data points and within the 1,25(OH)2D3-treated data points. Values were considered to be statistically significant at the 95% confidence level for P < 0.05. Data are presented as the mean ± SD.
RESULTS
Human Uterine Fibroids Expressed Lower Levels of VDR than Adjacent Normal Myometrium
We recently demonstrated an association of lower levels of serum vitamin D3 with increasing size of uterine fibroids [31]. Additionally, the levels of serum vitamin D3 were also lower in women with uterine fibroids as compared to the healthy counterpart. The biologically active vitamin D3, 1,25(OH)2D3, exerts its function in the cell system through interacting with the VDR [23]. The VDR is a nuclear receptor that functions as a transcription factor and plays a major role in the modulation of gene expression by interacting with the VDR-response element (VDRE) in the promoter region of target genes. We hypothesized that reduced levels of VDR might be an important risk factor for the pathogenesis of human uterine fibroids due to inadequate function of 1,25(OH)2D3. To test this hypothesis, we performed Western blot analysis for VDR expression using protein lysates that were prepared from human uterine fibroids and the adjacent normal myometrium tissues. We used rabbit polyclonal anti-VDR antibody from Santa Cruz Biotechnology that recognized approximately 56-kDa VDR protein. This anti-VDR antibody has previously been used successfully [29, 46]. We found that more than 60% of uterine fibroid tumors analyzed (25 of 40) showed reduced levels of VDR as compared to the adjacent normal myometrium (Supplemental Fig. S1; available online at www.biolreprod.org). The total Western blot data for VDR expression are shown in the Supplemental Data (Supplemental Fig. S1). To further evaluate whether the reduced levels of VDR in these 25 uterine fibroids were statistically significant, we determined the mean values of VDR levels in both uterine fibroids and the adjacent normal myometrium. These mean values of VDR were used to generate the graph shown in Figure 1. The reduced levels of VDR in those fibroid tumors were statistically very significant (P = 0.0002) compared to levels in the adjacent normal myometrium. These results suggest that reduced levels of VDR might be an important risk factor for the pathogenesis of human uterine fibroids.
FIG. 1.
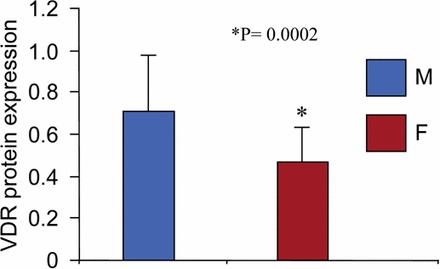
Human uterine fibroids expressed lower levels of VDR compared to the adjacent normal myometrium. Expression levels of VDR protein were analyzed in human fibroid tumors (n = 40) and the adjacent normal myometrium using Western blot analysis (see Supplemental Fig. S1). Twenty-five of the uterine fibroid (F) tumors showed reduced levels of VDR compared to the adjacent normal myometrium (M; see asterisks in Supplemental Fig. S1). The normalized values of VDR levels from these 25 uterine fibroids and the adjacent normal myometrium (see Supplemental Fig. S1) were used to calculate the mean, which was then used to generate the graph. Student t-test was used to calculate the P-value (P = 0.0002) with 95% confidence. Data are presented as the mean ± SD.
1,25(OH)2D3 Treatment Induced VDR Expression in Cultured HuLM Cells
The 1,25(OH)2D3 has been shown to exerts its biological function by interacting with and inducing/activating VDR [23]. 1,25(OH)2D3 has also been shown to inhibit proliferation and promote differentiation of human cancer cells through the activation of VDR, which is a transcription factor of the nuclear receptor superfamily [47]. To test whether 1,25(OH)2D3 can sensitize HuLM cells via the induction of VDR, we performed Western blot analysis using lysates from cultured HuLM cells treated with increasing concentrations of 1,25(OH)2D3 (0, 1, 10, 100, and 1000 nM) for 8, 24, and 48 h. Equal amounts of each cell lysates were analyzed by Western blots using anti-VDR antibody. We found that 1,25(OH)2D3 treatment induced VDR expression in a concentration-dependent manner in HuLM cells (Fig. 2A). At low concentrations (1–10 nM), 1,25(OH)2D3 significantly induced VDR expression at 48 h compared to untreated control (P < 0.05) (Fig. 2A). Because 1,25(OH)2D3 showed a concentration-dependent effect at the 48-h time point, we used only the 48-h time point for the following experiments with HuLM cells. To further test the subcellular localization of VDR protein, we performed immunofluorescence analysis after treating HuLM cells with increasing concentrations of 1,25(OH)2D3 for 48 h. At the 10 nM concentration, 1,25(OH)2D3 induced nuclear VDR staining, which was further stimulated by higher concentrations of 1,25(OH)2D3 in HuLM cell (Fig. 2B). We further examined the levels of VDR in lysates from UTSM cells treated with 1,25(OH)2D3 for 48 h. Similar to HuLM cells, equal amounts of each lysate from UTSM cells were analyzed for VDR expression. We found that 1,25(OH)2D3 induced VDR levels in a concentration-dependent manner in UTSM cells, whereas the induction was not as strong as that in HuLM cells (Fig. 2C, left panel). We also analyzed VDR expression in UTSM cells after the treatment with 1,25(OH)2D3 for the 8- and 24-h time points. However, 1,25(OH)2D3 was enabled to induce VDR levels consistently (data not shown). Moreover, we compared the endogenous levels of VDR in both cell types and observed that HuLM cells expressed comparatively lower levels of VDR than UTSM cells (Fig. 2C, right panel). To further test whether 1,25(OH)2D3 can also induce nuclear VDR in UTSM cells, we performed similar immunofluorescence analysis. We found that 1,25(OH)2D3 induced nuclear VDR staining in UTSM cells. These results suggest that 1,25(OH)2D3 can potentially sensitize HuLM cells while not markedly affecting normal UTSM cells. Thus, these results suggest that 1,25(OH)2D3 potentially induces VDR protein expression in HuLM cells.
FIG. 2.
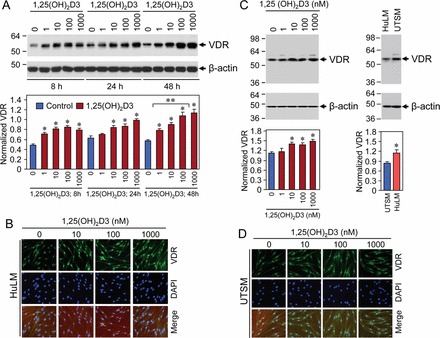
Treatment with 1,25(OH)2D3 induced VDR protein levels in HuLM cells. A and C) HuLM cells were treated with increasing concentrations of 1,25(OH)2D3 (0, 1, 10, 100, and 1000 nM) for 8, 24, and 48 h (A), and UTSM cells were similarly treated with increasing concentrations of 1,25(OH)2D3 as described for 48 h (C). Equal amounts of each cell lysate from both cell types were examined by Western blot analysis using anti-VDR antibody. Equal amounts of lysates from HuLM and UTSM cells were also compared for VDR expression. Western blot analysis with anti-β-actin antibody was used as a loading control. The intensity of each protein band was quantified and normalized to the corresponding β-actin, and then the signal ratio (VDR:β-actin) was used to generate the graphs. *P < 0.05 when compared to control, **P < 0.05 when compared between 1,25(OH)2D3-treated data points. Data are presented as the mean ± SD (n = 3 independent measurements). B and D) Immunofluorescence analysis was performed after culturing both HuLM and UTSM cells on sterile glass cover slips and treatment with increasing concentrations of 1,25(OH)2D3 (0, 10, 100, and 1000 nM) for 48 h. Treated cells were fixed, permeabilized, and incubated with anti-VDR antibody, followed by staining with FITC (green)-conjugated rabbit secondary antibody. Staining signals for VDR expression was monitored using a fluorescent microscopy. Nuclei of cells were staining with 4′,6 diamidino-2-phenylindole. Original magnification ×200 (B and D).
1,25(OH)2D3 Reduced Collagen Type 1 Protein Expression in Cultured HuLM Cells
Collagen type 1 is the most abundant collagen subtype within human, and it plays an important role in the process of fibrosis. We tested whether 1,25(OH)2D3 can affect collagen type 1 protein expression using HuLM cells treated with increasing concentrations of 1,25(OH)2D3 for 48 h. Cell lysates were examined by Western blot analysis using anti-collagen type 1 antibody, which has negligible (<1%) cross-reactivity with collagen types 2–6. We have previously used this anti-collagen type 1 antibody in Western blot analysis [29]. In the present study, we found that at the 10 nM concentration, 1,25(OH)2D3 significantly reduced collagen type 1 protein expression in HuLM cells compared with the untreated control (P < 0.05) (Fig. 3A). We observed that the anti-collagen type 1 antibody recognized approximately 140 kDa as a major protein band by reducing SDS-PAGE (Fig. 3, A and E). 1,25(OH)2D3 also reduced collagen type 1 protein levels in a concentration-dependent manner in HuLM cells. To further evaluate the pattern of collagen type 1 protein expression in uterine fibroid cells and compare it to that in normal myometrial cells, both HuLM and UTSM cells were treated with 1,25(OH)2D3 for 48 h and then analyzed by immunofluorescence. Collagen type 1 staining was positive in both HuLM and UTSM cells. However, more intense and disorganized collagen type 1 staining was observed in HuLM cells compared with UTSM cells (Figs. 3, B and C). UTSM cells expressed considerably higher amount of endogenous VDR (Fig. 2C, right). Treatment with 1,25(OH)2D3 reduced those disorganized staining of collagen type 1 in HuLM cells, whereas no obvious changes of collagen type 1 occurred in UTSM cells (Fig. 3E). Confocal microscopy revealed a similar staining pattern of collagen type 1 in UTSM cells, whereas disorganized collagen type 1 was present in HuLM cells (Fig. 3D). Furthermore, 1,25(OH)2D3 reduced the presence of disorganized collagen type 1 in HuLM cells (Fig. 3D). To further examine the effect of 1,25(OH)2D3 on collagen expression in UTSM cells, we also performed Western blot analysis using lysates from UTSM cells treated with 1,25(OH)2D3. We found that 1,25(OH)2D3 did not markedly reduce collagen type 1 expression in UTSM cells (Fig. 3E). These results suggest a potential role of 1,25(OH)2D3 in the reduction of excessive disorganized production of collagen type 1 in HuLM cells.
FIG. 3.
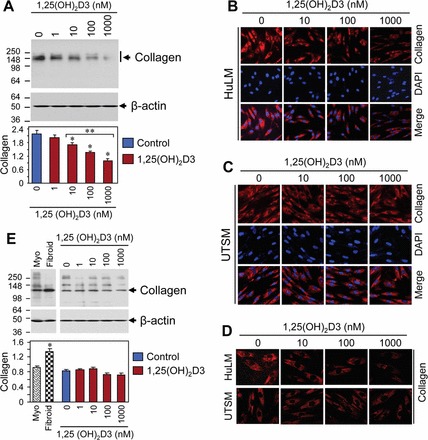
Effect of 1,25(OH)2D3 on protein expression of collage type 1 (collagen) in HuLM and UTSM cells. A) HuLM cells were serum starved and treated with increasing concentrations of 1,25(OH)2D3 (0, 1, 10, 100, and 1000 nM) for 48 h. Equal amounts of each cell lysate were examined by Western blot analysis using anti-collagen type 1 antibody. Western blot analysis with anti-β-actin antibody was used as a loading control. The intensity of each protein band was quantified and normalized to the corresponding β-actin, and then the signal ratio (collagen:β-actin) was used to generate the graph. *P < 0.05 when compared to control, **P < 0.05 when compared between 1,25(OH)2D3-treated data points. Data are presented as the mean ± SD (n = 3 independent measurements). B and C) Immunofluorescence analysis was performed using HuLM (B) and UTSM (C) cells cultured on sterile glass cover slips. Cells were serum starved and treated with increasing concentrations of 1,25(OH)2D3 for 48 h. Treated cells were fixed, permeabilized, and then incubated with anti-collagen type 1 antibody, followed by staining with CY3 (red)-conjugated specific secondary antibody. Staining signals for collagen type 1 (red) were monitored using a fluorescent microscopy. Nuclei of cells were staining with 4′,6 diamidino-2-phenylindole. D) Immunofluorescence analysis was performed as described above, and pictures were captured using laser confocal microscopy. E) UTSM cells were treated with 1,25(OH)2D3 as described above, and then cell lysates were examined by Western blot analysis for collagen type 1 protein expression. In addition, protein lysates from human uterine fibroid tumor (fibroid) and the adjacent myometrium (Myo) were also analyzed for collagen type 1 protein expression. Data were analyzed as described above. Original magnification ×200 (B–D).
1,25(OH)2D3 Reduced Fibronectin Protein Expression in Cultured HuLM Cells
Fibronectin is a key component in ECM, which plays an important role in the process of fibrosis. To test whether 1,25(OH)2D3 treatment affects the levels of fibronectin protein, we performed Western blot analysis using lysates from HuLM cells treated with increasing concentrations of 1,25(OH)2D3. We found that at the 10 nM concentration, 1,25(OH)2D3 significantly reduced fibronectin expression compared to control (P < 0.05) (Fig. 4A). 1,25(OH)2D3 also reduced fibronectin levels in a concentration-dependent manner in HuLM cells (Fig. 4A). To examine the effect of 1,25(OH)2D3 on UTSM cells, we also performed similar Western blot analysis using lysates from UTSM cells treated with 1,25(OH)2D3. We found that 1,25(OH)2D3 reduced fibronectin expression in UTSM cells as compared to the untreated control. However, fibronectin expression in UTSM cells was not consistently reduced with 1,25(OH)2D3 doses (Fig. 4E). To further evaluate the pattern of fibronectin expression in uterine fibroid cells and compare it to that in normal myometrial cells, both HuLM and UTSM cells were treated with 1,25(OH)2D3 for 48 h and then analyzed by immunofluorescence. Fibronectin staining was observed in both HuLM and UTSM cells, whereas more intense and disorganized staining signals were observed in HuLM cells compared to UTSM cells (Fig. 4, B and C). We found that treatment with 1,25(OH)2D3 reduced those disorganized staining of fibronectin in HuLM cells, whereas no obvious changes occurred in UTSM cells (Fig. 4, B and C). Additionally, confocal microscopy demonstrated parallel and organized fibronectin staining in UTSM cells, whereas more disorganized signals were observed in HuLM cells (Fig. 4D). Furthermore, treatment with 1,25(OH)2D3 reduced excessive fibronectin staining in HuLM cells. These results suggest that 1,25(OH)2D3 can effectively reduce the excessive production of fibronectin in HuLM cells.
FIG. 4.
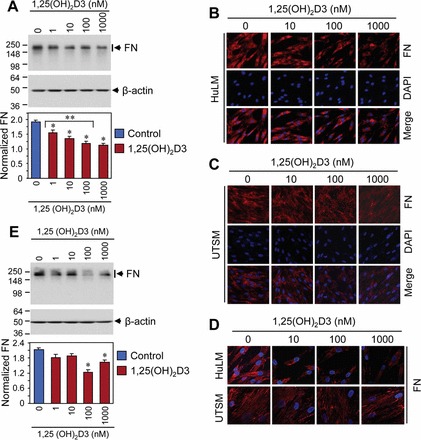
Effect of 1,25(OH)2D3 on protein expression of fibronectin (FN) in HuLM and UTSM cells. A and E) Protein lysates were prepared from 1,25(OH)2D3-treated HuLM (A) and UTSM (E) cells as indicated. Equal amounts of each cell lysate were examined by Western blot analysis using rabbit polyclonal anti-fibronectin antibody. Western blot with anti-β-actin antibody was used as a loading control. The intensity of each protein band was quantified and normalized to the corresponding β-actin, and then the signal ratio (FN:β-actin) was used to generate the graphs. *P < 0.05 when compared to control, **P < 0.05 when compared between 1,25(OH)2D3-treated data points. Data are presented as mean ± SD (n = 3 independent measurements). B and C) Immunofluorescence analysis was performed using HuLM (B) and UTSM (C) cells cultured on sterile glass cover slips. Cells were incubated with anti-fibronectin antibody, followed by staining with CY3 (red)-conjugated specific secondary antibody. Staining signals for fibronectin (red) was monitored using a fluorescent microscopy. Nuclei of cells were staining with 4′,6 diamidino-2-phenylindole. D) Immunofluorescence analysis was performed as described above, and pictures were captured using laser confocal microscopy. Original magnification ×200 (B–D).
1,25(OH)2D3 Reduced PAI-1 Protein Expression in Cultured HuLM Cells
A TGF-β target gene, PAI-1 is known to be associated with the process of tissue fibrosis. To test whether the PAI-1 protein levels are affected by 1,25(OH)2D3 in uterine fibroid cells, we performed Western blot analysis using lysates from HuLM cells treated with different concentrations of 1,25(OH)2D3 for 48 h. Equal amounts of each lysate from control and 1,25(OH)2D3-treated HuLM cells were analyzed for PAI-1 protein expression using anti-PAI-1 antibody. We found that at the 10 nM concentration, 1,25(OH)2D3 significantly reduced PAI-1 protein expression in HuLM cells compared to control (P < 0.05) (Fig. 5A). Furthermore, 1,25(OH)2D3 reduced PAI-1 protein expression in HuLM cells in a concentration-dependent manner (Fig. 5A). To further test the effect of 1,25(OH)2D3 on PAI-1 protein expression, we performed similar Western blot analysis using lysates from 1,25(OH)2D3-treated UTSM cells. We found that 1,25(OH)2D3 reduced PAI-1 protein in a concentration-dependent manner in UTSM cells, whereas this reduction was not as strong as HuLM cells (Fig. 5B). These results suggest the potential of 1,25(OH)2D3 in regulating the TGF-β target PAI-1 gene expression in HuLM cells.
FIG. 5.
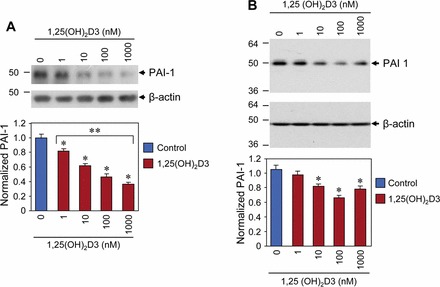
Effect of 1,25(OH)2D3 on protein expression of PAI-1 in HuLM and UTSM cells. Both HuLM (A) and UTSM (B) cells were serum starved and treated with increasing concentrations of 1,25(OH)2D3 (0, 1, 10, 100, and 1000 nM) for 48 h. Equal amounts of each cell lysate were examined by Western blot analysis using anti-PAI-1 antibody. Western blot analysis with anti-β-actin antibody was used as a loading control. The intensity of each protein band was quantified and normalized to the corresponding β-actin, and then the signal ratio (PAI-1:β-actin) was used to generate the graphs. *P < 0.05 when compared to control, **P < 0.05 when compared between 1,25(OH)2D3-treated data points. Data are presented as the mean ± SD (n = 3 independent measurements).
1,25(OH)2D3 Reduced mRNA and Protein Expressions of Proteoglycans in Cultured HuLM Cells
Proteoglycans are glycosylated proteins, which are important components in the ECM of connective tissues [48]. The small, leucine-rich proteoglycans such as fibromodulin, biglycan, and versican play major roles in ECM organization, and these proteins are overexpressed during the development of diseases. To test whether 1,25(OH)2D3 can reduce expression of those proteoglycans, we performed Western blot analysis using lysates from HuLM cells treated with increasing concentrations of 1,25(OH)2D3. We found that 1,25(OH)2D3 significantly reduced fibromodulin expression in HuLM cells (P < 0.05) (Fig. 6A). The levels of biglycan in HuLM cells were also verified by Western blot analysis, and these levels were significantly reduced by 1,25(OH)2D3 compared to untreated control (P < 0.05) (Fig. 6C). Moreover, we observed concentration-dependent reduction of biglycan protein in HuLM cells after 1,25(OH)2D3 treatment. On the other hand, versican is a large ECM proteoglycan that act as a structure molecule in ECM and plays a role in key events during the development of uterine fibroids. We found that versican expression was significantly reduced at the 10 nM concentration of 1,25(OH)2D3 in HuLM cells. Moreover, 1,25(OH)2D3 reduced versican expression in a concentration-dependent manner (P < 0.05) (Fig. 6E). To further examine the effects of 1,25(OH)2D3 on mRNA expression of the above-mentioned proteoglycans, we performed quantitative real-time PCR analysis using total RNA from HuLM cells treated with increasing concentrations of 1,25(OH)2D3. Similar to protein expressions, 1,25(OH)2D3 treatment reduced mRNA expression of fibromodulin (Fig. 6B), biglycan (Fig. 6D), and versican (Fig. 6F) in cultured HuLM cells. These results suggest that 1,25(OH)2D3 has the potential to reduce the expression of proteoglycans in cultured HuLM cells.
FIG. 6.
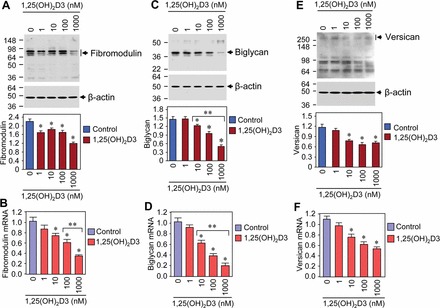
Effect of 1,25(OH)2D3 on mRNA and protein expressions of ECM-associated proteoglycans in HuLM cells. A, C, and E) Equal amounts of each lysate from HuLM cells treated with increasing concentrations of 1,25(OH)2D3 (0, 1, 10, 100, and 1000 nM) were examined by Western blot analysis using anti-fibromodulin (A), anti-biglycan (C), and anti-versican (E) antibodies. Western blot analysis with anti-β-actin antibody was used as a loading control. The intensity of each protein band was quantified and normalized to the corresponding β-actin, and then the signal ratio for individual proteins was used to generate the graphs. B, D, and F) Real-time PCR analysis for mRNA levels of fibromodulin, biglycan, and versican genes was performed using gene-specific sense and antisense primers as described in Materials and Methods. The mRNA levels of fibromodulin, biglycan, and versican were normalized to the glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and the normalized values were used to generate the graphs. *P < 0.05 when compared to control, **P < 0.05 when compared between 1,25(OH)2D3-treated data points. Data are presented as the mean ± SD (n = 3 independent measurements).
1,25(OH)2D3 Reduced Disorganized Structural Actin Fibers in Cultured HuLM Cells
We next evaluated whether 1,25(OH)2D3 has any effect on expression of structural smooth muscle actin fibers by performing immunofluorescence analysis using HuLM and UTSM cells treated with or without increasing concentrations of 1,25(OH)2D3. Cells were then fixed in formaldehyde solution, permeabilized, and stained with FITC-conjugated phalloidin to stain structural smooth muscle f-actin. Staining for f-actin was visualized by a fluorescent microscopy. We observed homogeneous parallel staining of actin fibers in UTSM cells, whereas disorganized accumulation of actin staining was visualized in HuLM cells (Fig. 7, A and B). Treatment with 1,25(OH)2D3 reduced the excessive deposition of disorganized actin fibers in HuLM cells but not in UTSM cells (Fig. 7, A and B). Laser confocal microscopy also revealed a significant reduction in staining of actin fibers by 1,25(OH)2D3 (Fig. 7C). We quantified the fluorescent staining using image-analysis software and found that 1,25(OH)2D3 significantly reduced (1.6- and 3.5-fold) fluorescent intensity of actin fibers in a concentration-dependent manner (P < 0.05) (Fig. 7C). We further used Western blot analysis to quantitatively measure the effect of 1,25(OH)2D3 on actin expression using lysates from HuLM and UTSM cells treated with or without increasing concentrations of 1,25(OH)2D3. At the 10 nM concentration, 1,25(OH)2D3 significant reduced the expression of actin fibers in HuLM cells (P < 0.05) (Fig. 7D). Furthermore, 1,25(OH)2D3 reduced α-actin levels in a concentration-dependent manner (Fig. 7D). On the other hand, 1,25(OH)2D3 did not markedly reduced α-actin levels in UTSM cells, and reduction of α-actin was not concentration-dependent (Fig. 7E). These results suggest that 1,25(OH)2D3 has the potential to reduce the synthesis and accumulation of disorganized actin fibers in HuLM cells.
FIG. 7.
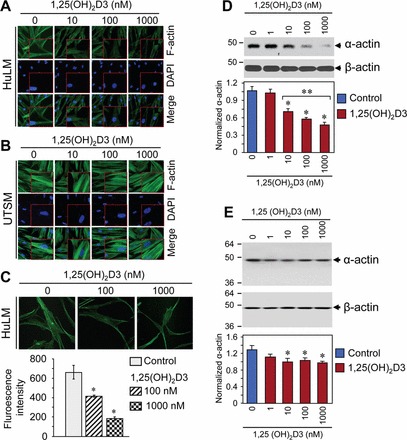
Effect of 1,25(OH)2D3 on expression and accumulation of structural actin fibers in HuLM and UTSM cells. A and B) Immunofluorescence analysis was performed after culturing HuLM (A) and UTSM (B) cells on sterile glass cover slips and treatment with increasing concentrations of 1,25(OH)2D3 (0, 10, 100, and 1000 nM) for 48 h. Treated cells were fixed, permeabilized, and stained with FITC-conjugated phalloidin. The smooth muscle f-actin staining (green) was monitored by a fluorescent microscopy. Nuclei of cells were stained with 4′,6 diamidino-2-phenylindole (blue). A high-magnification of the region is shown (bottom right). C) Immunofluorescence analysis was also performed as described above. The f-actin signals were captured and visualized using fluorescent laser confocal microscopy (top). Fluorescent staining was quantified by image-analysis software, and relative fluorescent intensity is shown. *P < 0.05 compared to control (bottom). D and E) Equal amounts of lysates from HuLM (D) and UTSM (E) cells treated with increasing concentrations of 1,25(OH)2D3 (0, 1, 10, 100, and 1000 nM) were examined by Western blot analysis using anti-α-actin antibody. The intensity of each protein signal was quantified and normalized to the corresponding β-actin, and then the signal ratio (α-actin:β-actin) was used to generate the graphs. *P < 0.05 compared to control, **P < 0.05 compared between 1,25(OH)2D3-treated data points. Data are presented as the mean ± SD (n = 3 independent measurements). Original magnification ×200 (A and B) and ×400 (inset in A, inset in B, and C).
DISCUSSION
In the present study, we examined the risk of reduced expression of VDR for the pathogenesis of human uterine fibroids. VDR is a nuclear receptor that modulates gene expression when interacting with its ligand, 1,25(OH)2D3, the biologically active form of vitamin D3. The cellular effects of VDR signaling include growth arrest, differentiation, and/or induction of apoptosis, demonstrating the participation of vitamin D signaling in the inhibition of cell growth. Herein, we determined the protein levels of VDR in human uterine fibroid tumors, and we compared these levels with those in the adjacent normal myometrium. Although we analyzed a total of 40 uterine fibroids and their adjacent normal myometrium, more than 60% of cases (25 of 40) showed reduced levels of VDR, as shown in the Supplemental Figure S1. We further determined that the reduced levels of VDR in these 25 uterine fibroid tumors were statistically significant (P = 0.0002) (Fig. 1). These results demonstrate that loss or inadequate expression of VDR might be a risk for the development of human uterine fibroids. The bioactive 1,25(OH)2D3 is known to function via binding to nuclear VDR for activation of further downstream signaling in cell systems. The reduced levels of VDR in uterine fibroid tumors suggest an association with the pathogenesis of uterine fibroids. On the other hand, we also observed that in approximately 40% of uterine fibroid cases, the levels of VDR protein were either upregulated or unchanged (Supplemental Fig. S1). However, it is unknown how the upregulation of VDR might be involved in uterine fibroid pathogenesis. We believe that in those cases, other factors such as somatic mutations in the mediator complex subunit 12 (MED12) gene might be the possible cause of fibroid pathogenesis. The MED12 gene somatic mutations have recently been demonstrated to be frequently associated with fibroid pathogenesis [49, 50]. Moreover, our recent unpublished observation also demonstrates frequent mutations in the MED12 gene in uterine fibroid tumors; thus, it is important to address these issues in further investigations.
In the present study, we next verified the biological function of 1,25(OH)2D3 through the induction of VDR expression in both HuLM and UTSM cells. HuLM cells serve as a suitable model of human uterine fibroids because these well-characterized cells represent similar human fibroid pathophysiology and characteristics [34]. Our results demonstrated that HuLM cells expressed detectable levels of VDR, whereas treatment with 1,25(OH)2D3 significantly induced nuclear VDR expression in a concentration-dependent manner (P < 0.05) (Fig. 2, A and B). Our Western blot data also showed that HuLM cells expressed lower levels of VDR compared to UTSM cells (Fig. 2C, right). Although UTSM cells are somewhat responsive to 1,25(OH)2D3 treatment to induce VDR levels, the response is quite weak compared to HuLM cells (Fig. 2C, left). Because UTSM cells were derived from normal human uterus [34] and these cells expressed comparatively higher levels of VDR (Fig. 2C, right), further treatment with 1,25(OH)2D3 could not considerably induce VDR levels in those cells. These results suggest that 1,25(OH)2D3 treatment can potentially sensitize HuLM cells while not significantly affecting UTSM cells. Moreover, 1,25(OH)2D3 exerts its cellular function by inducing nuclear VDR levels in HuLM cells. This result is consistent with our previously observation that 1,25(OH)2D3 can induce VDR expression in vitro cell culture [46] and in uterine fibroids tumors in an in vivo Eker rat model [29]. Moreover, this result is also consistent with previous observations where an analog of 1,25(OH)2D3 functions via the induction of VDR in both breast and prostate cancer cells [51]. An analog of vitamin D3 (paricalcitol), also known as Zemplar, has also been shown to function through the activation/induction of VDR and to inhibit cardiac fibrosis in a mouse model [52]. Our findings suggest a possible mechanism through which 1,25(OH)2D3 can function in uterine fibroid cells by inducing/activating the nuclear VDR levels, which can further interact with retinoid X receptor alpha (RXR-α) to form a VDR-RXR-α complex. This VDR-RXR-α heterodimeric complex further binds to specific VDREs in the promoter regions of vitamin D target genes, including genes that are associated with the process of fibrosis.
Next, we examined the effects of 1,25(OH)2D3 on ECM-associated proteins because the ECM plays an important role in the process of tissue fibrosis. It has been demonstrated previously that the excessive synthesis and accumulation of ECM leads to the process of tissue fibrosis [53]. Herein, we evaluated the role of 1,25(OH)2D3 in the regulation of ECM-associated protein expression in human uterine fibroid cells. We observed significant reduction of collagen type 1 and fibronectin proteins following 1,25(OH)2D3 treatment (Figs. 3 and 4), suggesting a potential role of 1,25(OH)2D3 in the regulation of key fibrotic proteins. Using immunofluorescence staining, we observed that collagen type 1 was localized primarily in the cytoplasm of both HuLM and UTSM cells (Figs. 3 and 4). However, fibronectin was localized around UTSM cells, whereas it was localized primarily in the cytoplasm of HuLM cells (Fig. 4). These findings are, to some extent, consistent with the observation by Stewart et al. [17] that fibronectin was localized primarily around individual smooth muscle cells and that collagen type 1 was distributed across the ECM and in the cytoplasm of smooth muscle cells. The limitation of this study is that we analyzed ECM using a 2D rather than a 3D cell culture model system that can mimic the actual living tumor environment and can produce abundant ECM [54]. Thus, it is important to use an established 3D cell culture system that produces abundant ECM. Nevertheless, in the present study, we examined the role of 1,25(OH)2D3 on reduction of ECM production in cultured HuLM cells. Our results support the view that uterine fibroid cells can undergo molecular alterations that may increase the fibrotic phenotype of these tumor cells by overproduction and accumulation of fibrotic proteins. These alterations in ECM-associated protein expression can be effectively suppressed by 1,25(OH)2D3 in uterine fibroid cells.
Collagen is a family of proteins that are the main components of the ECM. Although several different types of collagenic proteins exist, type I and type III comprise approximately 90% of total collagen [55]. The overall balance of ECM deposition can be affected by TGF-β through a variety of biological activities. Studies have shown that TGF-β expression is elevated in a wide array of fibrotic processes, including pulmonary fibrosis, liver cirrhosis, scleroderma, and keloids [56–59].
The TGF-β has been shown to induce ECM production and to stimulate expression of type 1 collagen (COL1A1) and PAI-1 [18]. TGF-β directly increases the expression of collagen type 1 and collagen type 2 transcripts [60, 61]. Uterine fibroids exhibit increased levels of ECM gene expression, and the ECM collagen fibril structure and orientation have been found to be disoriented and loosely packed in a nonparallel manner compared to matched normal myometrium [62, 63]. The deregulated accumulation of the ECM represents an imbalance between synthesis and dissolution and produces a fibroproliferative condition called keloids [9]. Figure 3 shows a concentration-dependent inhibition of collagen type 1 expression, demonstrating an important role of 1,25(OH)2D3 in the regulation of ECM deposition and that reduction of ECM deposition may ultimately lead to reduce uterine fibroid cell proliferation.
Another ECM-associated protein, fibronectin, forms fibrils that are associated with matrix components and promotes cell adhesion and migration, cytodifferentiation, phagocytosis, and cell growth [64]. It has been demonstrated that in adult lung, fibronectin is specifically localized at the basal membrane, around the smooth muscle cells and in the alveolar lining fluid [65]. The excessive synthesis and deposition of the collagenic protein was found in fibroplasia, a condition where fibronectin production is elevated [65]. We tested the effect of 1,25(OH)2D3 on fibronectin expression in uterine fibroid cells. Figure 4 shows that 1,25(OH)2D3 at 1–10 nM concentrations significantly reduced fibronectin expression in HuLM cells but not in UTSM cells, suggesting its regulatory role in ECM deposition in uterine fibroid cells. Moreover, PAI-1 is a TGF-β target gene known to be associated with the process of fibrosis [66]. Our results showed significant reduction of PAI-1 protein expression by 1,25(OH)2D3 treatment in HuLM cells (P < 0.05) (Fig. 5A), which demonstrates the potential role of 1,25(OH)2D3 in the regulation of fibrosis process.
Proteoglycans are important components in all ECM and are known to be elevated in uterine fibroids, so we also addressed whether 1,25(OH)2D3 can affect the expression of proteoglycans such as fibromodulin, biglycan, and versican in HuLM cells. Fibromodulin is a collagen-binding proteoglycan that plays a key role in fibrogenesis by regulating collagen fibril spacing and thickness. It has been shown that fibromodulin can promote tumor cells motility and invasion [67]. Fibromodulin mRNA and protein levels were found to be upregulated in uterine fibroids [38]. Figure 6, A and B, shows that 1,25(OH)2D3 reduced mRNA and protein levels of fibromodulin in HuLM cells, which suggests the potential role of 1,25(OH)2D3 in regulating the ECM. Furthermore, biglycan is a small, leucine-rich proteoglycan in ECM that influences differentiation and proliferation processes [68], and we tested whether 1,25(OH)2D3 can regulate biglycan expression in HuLM cells. Our results (Fig. 6, C and D) showed that mRNA and protein levels of biglycan were reduced by 1,25(OH)2D3 in a concentration-dependent manner in HuLM cells, suggesting ECM regulation by 1,25(OH)2D3. It has been demonstrated that versican is a large proteoglycan in ECM and is associated with cell proliferation and apoptosis [69]. The mRNA and protein levels of versican are overexpressed in uterine fibroids [19], and it has also been demonstrated that the aberrant production of excessive and disorganized ECM involves in the activation of the TGF-β signaling and that the excessive production of versican can lead to the fibroid phenotype [19]. Therefore, we next tested the effect of 1,25(OH)2D3 on the regulation of versican expression in both mRNA and protein levels in uterine fibroid cells. Our results showed reduction of mRNA and protein levels of versican in HuLM cells by 1,25(OH)2D3, and these results further indicate the role of 1,25(OH)2D3 in the regulation of ECM deposition.
The smooth muscle actin fibers act as structural support proteins for uterine fibroid cells. A previous study has shown that uterine fibroid cells produce excessive amounts of actin molecules and that structural changes in cell size occur due to the deposition and disorganization of structural actin fibers [70]. In the present study, we examined the effect of 1,25(OH)2D3 on expression of those smooth muscle actin proteins in HuLM cells. Figure 7 shows a significant reduction of structural smooth muscle f-actin by 1,25(OH)2D3 in HuLM cells but not in UTSM cells. Moreover, we observed a concentration-dependent reduction of α-actin in 1,25(OH)2D3-treated HuLM cells but not UTSM cells (Fig. 7, D and E). These findings further demonstrate that the function of 1,25(OH)2D3 is reducing the excessive synthesis and deposition of disorganized structural smooth muscle actin fibers, which ultimately can lead to the reduction of uterine fibroid phenotype.
In conclusion, we observed reduced levels of VDR in human uterine fibroids as compared to the adjacent normal myometrium. Treatment with 1,25(OH)2D3 significantly induced nuclear VDR expression in a concentration-dependent manner in HuLM cells. 1,25(OH)2D3 also significantly reduced the expression of ECM-associated collagen type 1, fibronectin, and PAI-1 in HuLM cells but not in UTSM cells. Moreover, 1,25(OH)2D3 reduced mRNA and protein levels of proteoglycans such as fibromodulin, biglycan, and versican in HuLM cells. These proteoglycans are the major components of ECM and are known to be upregulated in uterine fibroids. Furthermore, 1,25(OH)2D3 reduced excessive deposition and disorganized structural actin fibers in HuLM cells. Taken together, our findings suggest that human uterine fibroids expressed reduced levels of VDR and that reduction of VDR might be a risk factor for fibroid pathogenesis. In addition, treatment with 1,25(OH)2D3 can effectively reduce the expression of major ECM-associated proteins and structural actin fibers in HuLM cells. Thus, 1,25(OH)2D3 may have potential as an effective therapeutic agent for a safe, nonsurgical treatment option for human uterine fibroids.
Supplementary Material
ACKNOWLEDGMENT
We would like to thank Dr. Shawn J. Goodwin, Morphology Core Facility at Meharry Medical College, for his help in taking confocal images.
Footnotes
Supported by the Research Centers in Minority Institutions (RCMI) pilot grant 2G12RR003032-26 and in part by the Vanderbilt Clinical and Translational Science award (CTSA) grant UL1 RR024975 from the National Center for Research Resources/National Institutes of Health (NCRR/NIH; to S.K.H.) and R01 HD046228 (to A.A.H.) and Solvay Pharmaceuticals, Inc. (Case 09-CV-02261).
REFERENCES
- Farhi J, Ashkenazi J, Feldberg D, Dicker D, Orvieto R, Ben Rafael Z. Effect of uterine leiomyomata on the results of in vitro fertilization treatment. Hum Reprod 1995; 10: 2576–2578. [DOI] [PubMed] [Google Scholar]
- Surrey ES, Lietz AK, Schoolcraft WB. Impact of intramural leiomyomata in patients with a normal endometrial cavity on in vitro fertilization–embryo transfer cycle outcome. Fertil Steril 2001; 75: 405–410. [DOI] [PubMed] [Google Scholar]
- Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988–1990. Obstet Gynecol 1994; 83: 549–555. [DOI] [PubMed] [Google Scholar]
- Wilson EA, Yang F, Rees ED. Estradiol and progesterone binding in uterine leiomyomata and in normal uterine tissues. Obstet Gynecol 1980; 55: 20–24. [PubMed] [Google Scholar]
- Rein MS, Barbieri RL, Friedman AJ. Progesterone: a critical role in the pathogenesis of uterine myomas. Am J Obstet Gynecol 1995; 172: 14–18. [DOI] [PubMed] [Google Scholar]
- Baird DD, Dunson DB. Why is parity protective for uterine fibroids? Epidemiology 2003; 14: 247–250. [DOI] [PubMed] [Google Scholar]
- Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 2002; 76: 187–192. [DOI] [PubMed] [Google Scholar]
- Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science 2005; 308: 1589–1592. [DOI] [PubMed] [Google Scholar]
- Catherino WH, Leppert PC, Stenmark MH, Payson M, Potlog-Nahari C, Nieman LK, Segars JH. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosomes Cancer 2004; 40: 204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsibris JC, Segars J, Coppola D, Mane S, Wilbanks GD, O'Brien WF, Spellacy WN. Insights from gene arrays on the development and growth regulation of uterine leiomyomata. Fertil Steril 2002; 78: 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. The transforming growth factor-beta family. Annu Rev Cell Biol 1990; 6: 597–641. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997; 390: 465–471. [DOI] [PubMed] [Google Scholar]
- Schnaper HW, Hayashida T, Hubchak SC, Poncelet AC. TGF-beta signal transduction and mesangial cell fibrogenesis. Am J Physiol Renal Physiol 2003; 284: F243–F252. [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol 2002; 118: 211–215. [DOI] [PubMed] [Google Scholar]
- Arici A, Sozen I. Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril 2000; 73: 1006–1011. [DOI] [PubMed] [Google Scholar]
- Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J 2000; 19: 1745–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab 1994; 79: 900–906. [DOI] [PubMed] [Google Scholar]
- Ding L, Xu J, Luo X, Chegini N. Gonadotropin releasing hormone and transforming growth factor beta activate mitogen-activated protein kinase/extracellularly regulated kinase and differentially regulate fibronectin, type I collagen, and plasminogen activator inhibitor-1 expression in leiomyoma and myometrial smooth muscle cells. J Clin Endocrinol Metab 2004; 89: 5549–5557. [DOI] [PubMed] [Google Scholar]
- Norian JM, Malik M, Parker CY, Joseph D, Leppert PC, Segars JH, Catherino WH. Transforming growth factor beta3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod Sci 2009; 16: 1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. Receptors for the TGF-beta family. Cell 1992; 69: 1067–1070. [DOI] [PubMed] [Google Scholar]
- Barnard JA, Lyons RM, Moses HL. The cell biology of transforming growth factor beta. Biochim Biophys Acta 1990; 1032: 79–87. [DOI] [PubMed] [Google Scholar]
- Luo X, Ding L, Xu J, Chegini N. Gene expression profiling of leiomyoma and myometrial smooth muscle cells in response to transforming growth factor-beta. Endocrinology 2005; 146: 1097–1118. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D: A millenium perspective. J Cell Biochem 2003; 88: 296–307. [DOI] [PubMed] [Google Scholar]
- Ylikomi T, Laaksi I, Lou YR, Martikainen P, Miettinen S, Pennanen P, Purmonen S, Syvala H, Vienonen A, Tuohimaa P. Antiproliferative action of vitamin D. Vitam Horm 2002; 64: 357–406. [DOI] [PubMed] [Google Scholar]
- Tang XM, Dou Q, Zhao Y, McLean F, Davis J, Chegini N. The expression of transforming growth factor-beta s and TGF-beta receptor mRNA and protein and the effect of TGF-beta s on human myometrial smooth muscle cells in vitro. Mol Hum Reprod 1997; 3: 233–240. [DOI] [PubMed] [Google Scholar]
- Light BW, Yu WD, McElwain MC, Russell DM, Trump DL, Johnson CS. Potentiation of cisplatin antitumor activity using a vitamin D analogue in a murine squamous cell carcinoma model system. Cancer Res 1997; 57: 3759–3764. [PubMed] [Google Scholar]
- Sharan C, Halder SK, Thota C, Jaleel T, Nair S, Al-Hendy A. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil Steril 2011; 95: 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauer M, Rovio PH, Ylikomi T, Heinonen PK. Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertil Steril 2009; 91: 1919–1925. [DOI] [PubMed] [Google Scholar]
- Halder SK, Sharan C, Al-Hendy A. 1,25-dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model. Biol Reprod 2012; 86: 116, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder SK, Goodwin JS, Al-Hendy A. 1,25-Dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J Clin Endocrinol Metab 2011; 96: E754–E762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabry M, Halder SK, Allah AS, Roshdy E, Rajaratnam V, Al-Hendy A. Serum vitamin D3 level inversely correlated with uterine fibroid volume in different ethnic groups: a cross-sectional observational study. Int J Womens Health 2013; 5: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird DD, Hill MC, Schectman JM, Hollis BW. Vitamin D and the risk of uterine fibroids. Epidemiology 2013; 24: 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffoni A, Somigliana E, Vigano P, Benaglia L, Cardellicchio L, Pagliardini L, Papaleo E, Candiani M, Fedele L. Vitamin D status in women with uterine leiomyomas. J Clin Endocrinol Metab 2013; 98: E1374–E1378. [DOI] [PubMed] [Google Scholar]
- Carney SA, Tahara H, Swartz CD, Risinger JI, He H, Moore AB, Haseman JK, Barrett JC, Dixon D. Immortalization of human uterine leiomyoma and myometrial cell lines after induction of telomerase activity: molecular and phenotypic characteristics. Lab Invest 2002; 82: 719–728. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Kubota H, Yamamoto A, Kitamura A, Bachinger HP, Nagata K. Type I collagen in Hsp47-null cells is aggregated in endoplasmic reticulum and deficient in N-propeptide processing and fibrillogenesis. Mol Biol Cell 2006; 17: 2346–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder SK, Anumanthan G, Maddula R, Mann J, Chytil A, Gonzalez AL, Washington MK, Moses HL, Beauchamp RD, Datta PK. Oncogenic function of a novel WD-domain protein, STRAP, in human carcinogenesis. Cancer Res 2006; 66: 6156–6166. [DOI] [PubMed] [Google Scholar]
- Halder SK, Beauchamp RD, Datta PK. Smad7 induces tumorigenicity by blocking TGF-beta-induced growth inhibition and apoptosis. Exp Cell Res 2005; 307: 231–246. [DOI] [PubMed] [Google Scholar]
- Levens E, Luo X, Ding L, Williams RS, Chegini N. Fibromodulin is expressed in leiomyoma and myometrium and regulated by gonadotropin-releasing hormone analogue therapy and TGF-beta through Smad and MAPK-mediated signalling. Mol Hum Reprod 2005; 11: 489–494. [DOI] [PubMed] [Google Scholar]
- Honardoust D, Varkey M, Hori K, Ding J, Shankowsky HA, Tredget EE. Small leucine-rich proteoglycans, decorin and fibromodulin, are reduced in postburn hypertrophic scar. Wound Repair Regen 2011; 19: 368–378. [DOI] [PubMed] [Google Scholar]
- Attia M, Scott A, Duchesnay A, Carpentier G, Soslowsky LJ, Huynh MB, Van Kuppevelt TH, Gossard C, Courty J, Tassoni MC, Martelly I. Alterations of overused supraspinatus tendon: a possible role of glycosaminoglycans and HARP/pleiotrophin in early tendon pathology. J Orthop Res 2012; 30: 61–71. [DOI] [PubMed] [Google Scholar]
- Didangelos A, Mayr U, Monaco C, Mayr M. Novel role of ADAMTS-5 protein in proteoglycan turnover and lipoprotein retention in atherosclerosis. J Biol Chem 2012; 287: 19341–19345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi-Wen X, Renzoni EA, Kennedy L, Howat S, Chen Y, Pearson JD, Bou-Gharios G, Dashwood MR, du Bois RM, Black CM, Denton CP, Abraham DJ, et al. Endogenous endothelin-1 signaling contributes to type I collagen and CCN2 overexpression in fibrotic fibroblasts. Matrix Biol 2007; 26: 625–632. [DOI] [PubMed] [Google Scholar]
- Halder SK, Osteen KG, Al-Hendy A. Vitamin D3 inhibits expression and activities of matrix metalloproteinase-2 and −9 in human uterine fibroid cells. Hum Reprod 2013; 28: 2407–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I, Jakob M, Schafer D, Dick W, Spagnoli G, Heberer M. Quantitative analysis of gene expression in human articular cartilage from normal and osteoarthritic joints. Osteoarthritis Cartilage 2001; 9: 112–118. [DOI] [PubMed] [Google Scholar]
- Adesida AB, Mulet-Sierra A, Laouar L, Jomha NM. Oxygen tension is a determinant of the matrix-forming phenotype of cultured human meniscal fibrochondrocytes. PLoS ONE 2012; 7: e39339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artaza JN, Norris KC. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J Endocrinol 2009; 200: 207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordonez-Moran P, Larriba MJ, Palmer HG, Valero RA, Barbachano A, Dunach M, de Herreros AG, Villalobos C, Berciano MT, Lafarga M, Munoz A. RhoA-ROCK and p38MAPK-MSK1 mediate vitamin D effects on gene expression, phenotype, and Wnt pathway in colon cancer cells. J Cell Biol 2008; 183: 697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagishita M. Function of proteoglycans in the extracellular matrix. Acta Pathol Jpn 1993; 43: 283–293. [DOI] [PubMed] [Google Scholar]
- Makinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ, Gentile M, Yan J, Enge M, Taipale M, Aavikko M, Katainen R, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 2011; 334: 252–255. [DOI] [PubMed] [Google Scholar]
- McGuire MM, Yatsenko A, Hoffner L, Jones M, Surti U, Rajkovic A. Whole exome sequencing in a random sample of North American women with leiomyomas identifies MED12 mutations in majority of uterine leiomyomas. PLoS ONE 2012; 7: e33251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sintov AC, Berkovich L, Ben-Shabat S. Inhibition of cancer growth and induction of apoptosis by BGP-13 and BGP-15, new calcipotriene-derived vitamin D(3) analogs, in vitro and in vivo studies. Invest New Drugs 2013; 31: 247–255. [DOI] [PubMed] [Google Scholar]
- Meems LM, Cannon MV, Mahmud H, Voors AA, van Gilst WH, Sillje HH, Ruifrok WP, de Boer RA. The vitamin D receptor activator paricalcitol prevents fibrosis and diastolic dysfunction in a murine model of pressure overload. J Steroid Biochem Mol Biol 2012; 132: 282–289. [DOI] [PubMed] [Google Scholar]
- Norian JM, Owen CM, Taboas J, Korecki C, Tuan R, Malik M, Catherino WH, Segars JH. Characterization of tissue biomechanics and mechanical signaling in uterine leiomyoma. Matrix Biol 2012; 31: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik M, Catherino WH. Development and validation of a three-dimensional in vitro model for uterine leiomyoma and patient-matched myometrium. Fertil Steril 2012; 97: 1287–1293. [DOI] [PubMed] [Google Scholar]
- Calabresi C, Arosio B, Galimberti L, Scanziani E, Bergottini R, Annoni G, Vergani C. Natural aging, expression of fibrosis-related genes and collagen deposition in rat lung. Exp Gerontol 2007; 42: 1003–1011. [DOI] [PubMed] [Google Scholar]
- Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol 2007; 293: L525–L534. [DOI] [PubMed] [Google Scholar]
- Yata Y, Gotwals P, Koteliansky V, Rockey DC. Dose-dependent inhibition of hepatic fibrosis in mice by a TGF-beta soluble receptor: implications for antifibrotic therapy. Hepatology 2002; 35: 1022–1030. [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Mauviel A, Farge D. Transforming growth factor-beta signaling through the Smad proteins: role in systemic sclerosis. Autoimmun Rev 2006; 5: 563–569. [DOI] [PubMed] [Google Scholar]
- Bonniaud P, Margetts PJ, Ask K, Flanders K, Gauldie J, Kolb M. TGF-beta and Smad3 signaling link inflammation to chronic fibrogenesis. J Immunol 2005; 175: 5390–5395. [DOI] [PubMed] [Google Scholar]
- Ignotz RA, Massague J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem 1986; 261: 4337–4345. [PubMed] [Google Scholar]
- Sporn MB, Roberts AB, Wakefield LM, de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol 1987; 105: 1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert PC, Baginski T, Prupas C, Catherino WH, Pletcher S, Segars JH. Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil Steril 2004; 82: 1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol 2006; 195: 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberger DJ. Fibronectin. Int J Biochem Cell Biol 1997; 29: 939–943. [DOI] [PubMed] [Google Scholar]
- Limper AH, Fibronectin Roman J. A versatile matrix protein with roles in thoracic development, repair and infection. Chest 1992; 101: 1663–1673. [DOI] [PubMed] [Google Scholar]
- Samarakoon R, Higgins PJ. Integration of non-SMAD and SMAD signaling in TGF-beta1-induced plasminogen activator inhibitor type-1 gene expression in vascular smooth muscle cells. Thromb Haemost 2008; 100: 976–983. [PMC free article] [PubMed] [Google Scholar]
- Hu B, Thirtamara-Rajamani KK, Sim H, Viapiano MS. Fibulin-3 is uniquely upregulated in malignant gliomas and promotes tumor cell motility and invasion. Mol Cancer Res 2009; 7: 1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar A, Falus A, Koo E, Ujfalussy I, Sesztak M, Szuts I, Konrad K, Hodinka L, Bene E, Meszaros G, Ortutay Z, Farkas E, et al. Elevated levels of synovial fluid antibodies reactive with the small proteoglycans biglycan and decorin in patients with rheumatoid arthritis or other joint diseases. Rheumatology (Oxford) 2003; 42: 522–527. [DOI] [PubMed] [Google Scholar]
- Ohara N. A putative role of versican in uterine leiomyomas. Clin Exp Obstet Gynecol 2009; 36: 74–75. [PubMed] [Google Scholar]
- Rogers R, Norian J, Malik M, Christman G, Abu-Asab M, Chen F, Korecki C, Iatridis J, Catherino WH, Tuan RS, Dhillon N, Leppert P, et al. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol 2008; 198: 474 e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


