ABSTRACT
The dorsomedial nucleus (DMN) of the hypothalamus, the only site within the mediobasal hypothalamus of Syrian hamsters that both binds melatonin and has abundant concentrations of androgen receptors, has been proposed as a target tissue for induction of seasonal changes in brain sensitivity to steroid negative feedback. We tested whether DMN ablation, which does not interfere with pineal gland secretion of melatonin in short day lengths, prevents testicular regression by altering sensitivity to steroid negative feedback. Hamsters with DMN lesions, unlike control hamsters, failed to undergo testicular regression after transfer from a long (14 h light/day) to a short day length (8 h light/day); however, increased negative-feedback inhibition of follicle-stimulating hormone by testosterone was not compromised by ablation of the DMN, indicating that this tissue is not an essential mediator of seasonal changes in feedback sensitivity. We propose a redundant neural network comprised of multiple structures, each of which contributes to neuroendocrine mechanisms, that determines the effect of short days on gonadal function.
Keywords: FSH, hypothalamus, prolactin, seasonal reproduction, testosterone
Ablation of the hypothalamic dorsomedial nucleus prevents testicular regression in short day lengths, but not by eliminating increased negative-feedback sensitivity to testosterone.
INTRODUCTION
Many mammals manifest pronounced seasonal reproductive rhythms proximately controlled by day length (DL) [1–3]. Reproductive quiescence in hamsters is initiated by the gradual decline of DL in simulated natural photoperiods, and by the abrupt decrease in DL after transfer in a single day from long days (LDs) to short days (SDs) [4–7]. DL information is transduced by nocturnal pineal melatonin (Mel) secretion [6, 8–10], the duration of which is proportional to the length of the night [11]. Mel signals are decoded by hypothalamic and pituitary Mel-binding target tissues that control multiple seasonal rhythms [12, 13].
Pinealectomy, by eliminating Mel secretion, blocks the suppressive effects of SDs on the reproductive axis [6]. Several species-specific candidate sites for the reception of SD Mel signals have been identified, including the suprachiasmatic nucleus (SCN) in Siberian hamsters and the dorsomedial nucleus (DMN) of the hypothalamus in Syrian and Siberian hamsters [14–17].
In Syrian hamsters, ablation of the lateral aspect of the bed nucleus of the stria terminalis blocks gonadal involution in SDs without inducing hypersecretion of gonadotrophins [18]; however, the integrity of the SD pineal signal was not assessed, so disruption of Mel secretion may contribute to failure of gonadal regression, as is the case after lesions of the SCN [19, 20]. In both Siberian and Syrian hamsters, ablation of the DMN, or the DMN/ventromedial nucleus (VMN) complex of Syrian hamsters, blocks testicular responses to SDs [16, 17, 21, 22], without compromising Mel signaling [16, 17, 23]. Ablation of the DMN does not mask reproductive responses to SDs by inducing hypersecretion of gonadotrophins [17].
Negative-feedback sensitivity of GnRH and gonadotrophins to testosterone increases markedly in male Syrian hamsters several weeks after the transition from LDs to SDs. Testosterone-filled 4-mm Silastic capsules that substantially reduce blood luteinizing hormone (LH) and follicle-stimulating hormone (FSH) concentrations in SD males are completely ineffective in LD hamsters [24]. Testosterone concentrations of 0.5–3.0 ng/ml generated by these testosterone-filled capsules [25, 26] are within the range of the 2.0 ng/ml values of intact LD males (e.g., Ref. 27). Testosterone concentrations compatible with continued secretion of gonadotrophins in LD males inhibit LH and FSH secretion in SD males, thereby inducing testicular regression within 2 mo of SD treatment.
The sites of action at which Mel signaling increases feedback sensitivity to androgens are unspecified for the most commonly studied seasonal mammals (sheep and hamsters).
Maywood et al. [17] surmised that SD Mel signals in Syrian hamsters may directly alter GnRH neuronal activity, or indirectly influence negative-feedback sensitivity by acting on androgen-responsive neurons distinct from those that generate GnRH activity. In favoring the latter hypothesis, they pointed to the DMN as a likely target tissue; it is the only site within the mediobasal hypothalamus that both binds Mel and has abundant concentrations of androgen receptors. The DMN also contains RFamide-related peptides implicated in the control of gonadotrophin secretion [28, 29]. If the DMN is an essential component of the neural mechanism that controls seasonal changes in brain sensitivity to steroid negative feedback, then ablation of this structure should “hinder the induction of increased sensitivity to steroid negative feedback necessary for termination of the breeding season” [17]. The present study evaluated this hypothesis by testing whether the DMN is a necessary component of the mechanism that increases sensitivity of SD males to negative-feedback inhibition of FSH by testosterone.
MATERIALS AND METHODS
Male Syrian hamsters from a colony established from stock obtained from Harlan (Indianapolis, IN) were housed individually in translucent polypropylene cages (48 × 27 × 20 cm) on Tek-Fresh Lab Animal Bedding (Harlan Teklab, Madison, WI). Initially, a 14L:10D cycle (lights on at 0200 h PST) was in force, followed by an 8L:16D cycle (lights on at 0800 h PST), with room temperature at 22 ± 2°C. Harlan 8664 Teklab Rodent Diet and tap water were available ad libitum throughout testing. At regularly scheduled intervals, body mass (BM) and the length and width of the hamsters' right testes were measured externally under light anesthesia induced by isoflurane vapors. Estimated testis volume (ETV; testis length × width squared) is highly correlated with testis weight and reproductive competence [30]. All procedures were approved by the Animal Care and Use Committee of the University of California, Berkeley (institutional approval number R084-0911C) and were conducted in accordance with the Society for the Study of Reproduction's guidelines and standards.
Surgical Procedures
Hypothalamic lesions.
Surgeries were performed under ketamine cocktail anesthesia (21 mg ketamine, 2.4 mg xylazine, and 0.3 mg acepromazine/ml; 0.34 ml/100 g BM injected i.p.) supplemented with isoflurane vapors (Baxter Healthcare, Deerfield, IL) as necessary. Hamsters were injected s.c. with the analgesic 5% buprenorphine (0.2 ml/animal; Hospira Inc., Lake Forest, IL), perioperatively and 12 h postoperatively. Lesions of the DMN were placed using a Radionics Model RFG-4 A Research RF Lesion Generator system. Coordinates for the lesions were 0.53 mm posterior to bregma, 0.4 mm lateral to midline, and 7.8 mm ventral to dura mater, with the skull level between bregma and lambda. Radio frequency lesions were made by delivering 25 mV for 15 sec through an electrode insulated with epoxy, except for 0.5 mm at the electrode tip. For sham operations, the electrode was lowered to a depth of 1 mm above the DMN, but no current was passed.
Castrations.
Hamsters were anesthetized with isoflurane vapors and castrated through a midline incision in the abdominal cavity. Incisions were closed with sterile sutures and wound clips (Mikron Auto Clip, 9 mm). Hamsters were injected s.c. with the analgesic 5% buprenorphine perioperatively and 12 h postoperatively.
Blood sampling.
One blood sample (1.0 ml) was withdrawn from the retro-orbital sinus of each hamster between 1300 and 1500 h after 10, 12, and 15 wk of SD treatment, and assayed for serum concentrations of prolactin (PRL) or FSH. In each case, hamsters were anesthetized with isoflurane vapors and blood samples were centrifuged at 3500 rpm for 20 min; serum samples were stored at −80°C prior to radioimmunoassay.
Capsule construction.
Silastic capsules (Dow Corning, Midland, MI) were filled to a length of 4 mm with crystalline testosterone (Sigma, St. Louis, MO), and the ends of the capsules sealed with silicone rubber cement. Capsules of this size generate serum testosterone concentrations of 1–3 ng/ml [25, 26]. Capsules were incubated in saline for 24 h prior to s.c. placement in the interscapular area of anesthetized hamsters via a small incision that was closed with a wound clip.
Perfusions.
Hamsters were deeply anesthetized with sodium pentobarbital (200 mg/kg) and perfused transcardially with approximately 150 ml of 0.9% saline, followed by 300–400 ml of 4% paraformaldehyde in 0.1 M PBS (pH 7.3). Brains were sliced at 35-μm thickness and stained with cresyl violet to assess the extent of each lesion.
Radioimmunoassays
Follicle-stimulating hormone.
Serum was assayed for FSH using the rat FSH kit (previously validated for use in Syrian hamsters [31]) obtained from the National Hormone & Peptide Program (NHPP), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and Dr. A.F. Parlow. The primary antibody was rabbit anti-rat FSH S-11 and the standard rat FSH RP-2. Rat FSH-1 was used as the antigen for radioiodination, completed by the Chizzonite indirect method utilizing Pierce Pre-Coated Iodination Tubes (28601; Rockford, IL) and 125I from PerkinElmer Inc. (NEZ033A002MC; PerkinElmer Inc., Billerica, MA). The second antibody was a goat anti-rabbit gamma globulin. The sensitivity of the FSH assay was 1.4 ng/ml, and the intra- and interassay coefficients of variation were 4.0% and 6.0%, respectively.
Prolactin.
Serum was assayed using the hamster PRL kit obtained from the NHPP, NIDDK, and Dr. A. F. Parlow, and previously validated in Ref. 32. The primary antibody was guinea pig anti-hamster PRL (AFP2821591GP); hamster PRL (AFP10302E) was used as the standard for radioiodination, completed by the Chizzonite indirect method, as described above. The second antibody was a goat anti-guinea pig gamma globulin. The sensitivity of the PRL assay was 0.6 ng/ml, and the intra-assay coefficient of variation was 4.5%.
Statistics
Effects of photoperiodic treatment and time on ETV were assessed with repeated-measures ANOVA, followed by ANOVAs for individual data points, using the StatView program (Version 5.0.1; SAS Institute, Cary, NC) with significance level set to P = 0.05; t-tests evaluated the PRL and FSH data. StatView was used for all statistical tests. The intervention timeline is shown in Figure 1.
FIG. 1.

Experimental treatment sequence. Sham lesion, sham dorsomedial hypothalamic nucleus ablation.
RESULTS
Histological Analysis of Brain Lesions
Representative small and large lesions effective in blocking gonadal regression in SDs are shown in Figure 2 (A and B). Effective lesions extended from no more than 400 μm posterior of the termination of the PVN, bordering the dorsal termination of the VMN, and encompassed at least 75% of the DMN, centered around the third ventricle, and extended laterally from 75% through the VMN to slightly lateral of the termination of the VMN. Data from three hamsters with lesions that prevented gonadal regression were excluded from the analysis because they sustained damage to the PVN that compromised pineal function, as evidenced by failure to manifest the normal decrease in PRL concentrations in SDs.
FIG. 2.
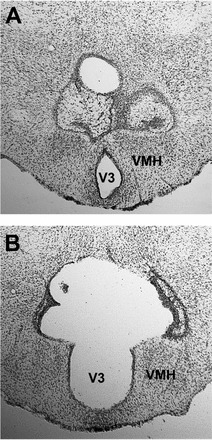
Subtotal (A) and complete (B) lesions of the DMN that prevented testicular regression in SDs and did not interfere with negative-feedback inhibition of FSH by testosterone (T). V3, third ventricle.
Effect of Lesions on Testicular Regression in Short DLs
Sham-operated hamsters underwent testicular regression after 10 wk of SD treatment (n = 12; P < 0.05 vs. Week 0 values [Fig. 3]), as was also the case for hamsters with ineffective lesions (not illustrated). Hamsters with effective DMN lesions did not manifest testicular regression during the SD challenge (n = 8; P > 0.05 compared to LD-sham males [n = 10]); males that remained in LDs sustained ETVs at Week 10 that were slightly lower, but did not differ significantly from baseline values.
FIG. 3.
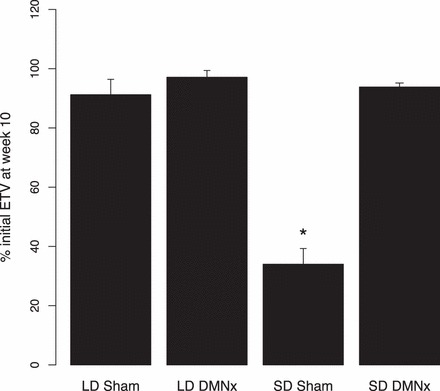
Testis values (mean + SEM) for hamsters in LDs (n = 11 for LD DMNx; n = 10 for LD sham) or after transfer to SDs for 10 wk. Week 10 values are plotted as percentage of ETVs at Week 0, when all groups were in LDs. The SD-sham group (n = 12) manifested substantial testicular regression (ETVs < 1500 mm3), which was blocked in SD DMNx hamsters (n = 8; ETVs > 3000 mm3). *Significantly different from the other three groups (P < 0.05).
PRL Concentrations
Males that remained in LDs generated high PRL concentrations (Fig. 4), whereas all hamsters transferred to SDs manifested marked decreases in PRL (P < 0.05 compared to LD sham and LD hamsters with ablation of the DMN [DMNx]). Because integrity of the pineal gland is required for SD suppression of PRL concentrations, this finding confirms that DMN damage did not interrupt neural innervation of the pineal gland [16, 23].
FIG. 4.
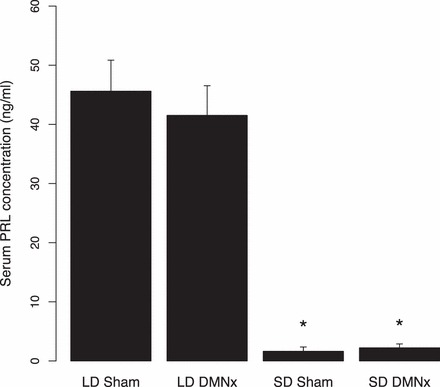
PRL concentrations (mean + SEM) for hamsters maintained in LDs or SDs for 10 wk. Designations and sample sizes are the same as in Figure 3. *Significantly different than both of the LD groups (P < 0.05).
FSH Concentrations During Baseline Testing and after a Testosterone Challenge
During baseline testing, after 12 wk of SD exposure, and 2 wk after castration, but prior to implantation of testosterone capsules, all groups had similarly high FSH concentrations (Fig. 5, black bars). At 3 wk after implantation of 4-mm testosterone capsules (Fig. 5, open bars), during which hamsters were maintained in their respective long and short DLs, sham and DMNx hamsters maintained in LDs sustained FSH concentrations that did not differ from baseline values. In sharp contrast, FSH concentrations were markedly lower in SD-sham hamsters. Most notably, FSH concentrations also were reduced in hamsters with DMN lesions that blocked testicular regression, differing from their own baseline values (P < 0.05), but not from values of SD-sham-operated hamsters (P > 0.05).
FIG. 5.
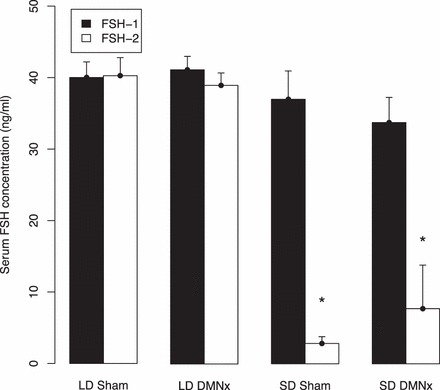
FSH concentrations (mean + SEM) for hamsters prior to (black bars; FSH-1 [see Fig. 1]) and after (open bars; FSH-2) treatment with 4-mm testosterone capsules. Designations and samples sizes are the same as in Figure 3. *Significantly different than corresponding values of both LD groups (P < 0.05).
DISCUSSION
The present findings indicate that, despite the requirement of the DMN for reproductive inhibition in SDs, this neural tissue is not necessary to sustain increased negative-feedback sensitivity of FSH to testosterone in short DLs. Thus, the failure of SD DMNx hamsters to undergo testicular regression is not attributable to lesion-induced abrogation of increased sensitivity to negative-feedback effects of testosterone, a possibility suggested by Maywood et al. [17]. The apparently normal SD response of FSH to a testosterone challenge confirms and extends observations that DMH ablation does not compromise the generation of the long-duration Mel signals that induce the SD neuroendocrine phenotype. This conclusion is buttressed by the normal decline in PRL concentrations in SD DMNx and control hamsters in the present and earlier studies [16, 23]. Some responses to SDs are eliminated in DMNx hamsters (e.g., testicular regression), whereas others (decreased PRL secretion, increased testosterone negative-feedback sensitivity) are unaffected, indicating that distinct mechanisms subserve the several traits that comprise the SD phenotype.
Our testosterone implants generated testosterone concentrations closely approximating those of intact male hamsters in LDs (2 ng/ml). These implants fail to inhibit gonadotropin secretion in LD males. After several weeks of SD treatment, however, sensitivity to negative-feedback effects of testosterone increases, and 2 ng testosterone/ml inhibits FSH and LH secretion in neurologically intact males; eventually, the gonads regress and secretion of testosterone declines. By challenging hamsters with a physiological testosterone dose, we established that loss of increased sensitivity to testosterone likely is not responsible for the failure of DMNx males to undergo gonadal regression in SDs.
The present findings leave open the possibility that the DMN mediates increased negative feedback to testosterone in neurologically intact hamsters, perhaps as part of a neural network in which multiple structures each independently increase sensitivity to feedback effects of testosterone. A distributed system of hypothalamic and thalamic sites mediates SD reproductive responses of Siberian hamsters to Mel [14, 21, 33]. Mel implants in multiple sites each induce testicular regression in LDs; the subsequent development of refractoriness to Mel also occurs independently at each site. Whether a distributed system controls steroid feedback sensitivity in SDs is presently unknown, and may include structures with abundant concentrations of androgen receptors. Hileman et al. [34] suggested that the control of seasonal breeding involves a complex interaction of different hypothalamic structures with the GnRH system. We cannot completely discount the possibility that the failure of DMNx hamsters to undergo testicular regression in SDs reflects positive masking induced by the lesion, perhaps via neurochemical perturbations that override the inhibitory effects of testosterone on gonadotrophin secretion. The DMN contains high concentrations of NPY (e.g., Ref. 35), which has been implicated in the control of pituitary gonadotropin secretion of hamsters [36]. Hypersecretion of gonadotrophins has not been detected in DMNx Syrian hamsters [16], and is unlikely to contribute to testicular maintenance in SDs.
The failure to eliminate enhanced feedback responses to testosterone was obtained in hamsters with complete DMN ablation, as well as those with partial DMN lesions. The possibility remains that incomplete DMN lesions that prevent testicular regression in SDs may spare sufficient DMN neurons to mediate increased responsiveness to testosterone negative feedback; this argument, however, does not apply to hamsters with complete ablation of the DMN. Blockade of testicular regression by DMH lesions, therefore, cannot be attributed to elimination of the effect of SDs to potentiate negative-feedback effects of LD concentrations of testosterone upon FSH.
We consider it unlikely that recovery of function in tissue surrounding the lesion during the 5 wk between when gonadal size was measured and final concentrations of FSH were determined contributed to maintenance of increased feedback sensitivity in DMNx hamsters. Lewis et al. [23] found no evidence of recovery of reproductive function in hamsters tested for 22 wk in SDs after sustaining DMN damage.
Because we did not measure LH, or determine that it exhibits a pattern similar to that of FSH, we cannot discount the possibility that damage to the DMN counteracts increased feedback sensitivity of LH to testosterone, possibly by upregulation of activin, thereby contributing to testicular maintenance in SDs.
In male Syrian and Siberian hamsters, mRNA expression and neuropeptide-immunoreactivity for gonadotrophin-inhibitory hormone (GnIH; also known as RFamide-related-peptide-3) are markedly reduced in short DLs [28, 37]. This response is mediated by Mel, with pinealectomy abolishing SD-induced inhibition of GnIH and Mel administration to LD hamsters leading to inhibition of GnIH to values similar to those of SD hamsters. Given the pronounced inhibitory role of GnIH on the reproductive axis (reviewed in Ref. 38), and the putative role of GnIH in mediating sex steroid negative feedback in female Syrian hamsters [39], we expected that GnIH would be essential for SD-induced enhancement of negative feedback in the present investigation. Although we did not specifically label brains for GnIH, lesions that ablated the entire DMH, the only brain region containing GnIH cell bodies in Syrian hamsters [39], failed to alter seasonal changes in negative feedback. These findings suggest either that GnIH does not mediate negative feedback in male hamsters or that negative feedback is mediated by GnIH together with other neurochemical systems, allowing for the maintenance of negative feedback in the absence of this neuropeptide.
ACKNOWLEDGMENT
We are grateful to Betty Hansen for running the FSH and PRL assays in the Diagnostic Endocrinology Laboratory of the Animal Health Diagnostic Center at Cornell University.
Footnotes
Supported by National Institutes of Health grants MH 61171 and HD 050470.
REFERENCES
- Goldman BD. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms 2001; 16: 283 301. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Zucker I, Schwartz WJ. Tracking the seasons: the internal calendars of vertebrates. Philos Trans R Soc Lond B Biol Sci 2008; 363: 341 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Nelson RJ, Zucker I. Mammalian seasonal rhythms: Behavior and neuroendocrine substrates : Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R. (eds.), Hormones, Brain, and Behavior, vol. 2 San Diego: Elsevier Science Press; 2002: 93 156. [Google Scholar]
- Beery AK, Trumbull JJ, Tsao JM, Costantini RM, Zucker I. Sex differences in the onset of seasonal reproductive quiescence in hamsters. Proc Biol Sci 2007; 274: 281 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MP, Turner KW, Park JH, Schoomer EE, Zucker I, Gorman MR. Seasonal regulation of reproduction: altered role of melatonin under naturalistic conditions in hamsters. Proc Biol Sci 2010; 277: 2867 2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ. The pineal and its hormones in the control of reproduction in mammals. Endocr Rev 1980; 1: 109 131. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Goldman BD. Effects of photoperiod on cyclicity and serum gonadotropins in the Syrian hamster. Biol Reprod 1975; 12: 223 231. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res 1993; 15: 161 190. [DOI] [PubMed] [Google Scholar]
- Bittman EL, Dempsey RJ, Karsch FJ. Pineal melatonin secretion drives the reproductive response to daylength in the ewe. Endocrinology 1983; 113: 2276 2283. [DOI] [PubMed] [Google Scholar]
- Carter DS, Goldman BD. Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): duration is the critical parameter. Endocrinology 1983; 113: 1261 267. [DOI] [PubMed] [Google Scholar]
- Darrow JM, Goldman BD. Circadian regulation of pineal melatonin and reproduction in the Djungarian hamster. J Biol Rhythms 1986; 1: 39 54. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Bittman EL. Photoperiodism and reproduction in mammals : RJ Nelson, Denlinger DL. (eds.), Photoperiodism: the Biological Calendar. NY: Oxford University Press; 2010; 503 542. [Google Scholar]
- Prendergast BJ. MT1 melatonin receptors mediate somatic, behavioral, and reproductive neuroendocrine responses to photoperiod and melatonin in Siberian hamsters (Phodopus sungorus). Endocrinology 2010; 151: 714 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman DA, Zucker I. Refractoriness to melatonin occurs independently at multiple brain sites in Siberian hamsters. Proc Natl Acad Sci U S A 2001; 98: 6447 6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badura LL, Goldman BD. Central sites mediating reproductive responses to melatonin in juvenile male Siberian hamsters. Brain Res 1992; 598: 98 106. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Hastings MH. Lesions of the iodomelatonin-binding sites of the mediobasal hypothalamus spare the lactotropic, but block the gonadotropic response of male Syrian hamsters to short photoperiod and to melatonin. Endocrinology 1995; 136: 144 153. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Bittman EL, Hastings MH. Lesions of the melatonin- and androgen-responsive tissue of the dorsomedial nucleus of the hypothalamus block the gonadal response of male Syrian hamsters to programmed infusions of melatonin. Biol Reprod 1996; 54: 470 477. [DOI] [PubMed] [Google Scholar]
- Raitiere MN, Garyfallou VT, Urbanski HF. Lesions in the anterior bed nucleus of the stria terminalis in Syrian hamsters block short-photoperiod-induced testicular regression. Biol Reprod 1997; 57: 796 806. [DOI] [PubMed] [Google Scholar]
- Bittman EL, Crandell RG, Lehman MN. Influences of the paraventricular and suprachiasmatic nuclei and olfactory bulbs on melatonin responses in the golden hamster. Biol Reprod 1989; 40: 118 126. [DOI] [PubMed] [Google Scholar]
- Bittman EL, Goldman BD, Zucker I. Testicular responses to melatonin are altered by lesions of the suprachiasmatic nuclei in golden hamsters Biol Reprod 1979; 21: 647 656. [DOI] [PubMed] [Google Scholar]
- Leitner C, Bartness TJ. An intact dorsomedial hypothalamic nucleus, but not the subzona incerta or reuniens nucleus, is necessary for short-day melatonin signal-induced responses in Siberian hamsters. Neuroendocrinology 2011; 93: 29 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae HH, Mangels RA, Cho BS, Dark J, Yellon SM, Zucker I. Ventromedial hypothalamic mediation of photoperiodic gonadal responses in male Syrian hamsters. J Biol Rhythms 1999; 14: 391 401. [DOI] [PubMed] [Google Scholar]
- Lewis D, Freeman DA, Dark J, Wynne-Edwards KE, Zucker I. Photoperiodic control of oestrous cycles in Syrian hamsters: mediation by the mediobasal hypothalamus. J Neuroendocrinol 2002; 14: 294 299. [DOI] [PubMed] [Google Scholar]
- Ellis GB, Turek FW. Time course of the photoperiod-induced change in sensitivity of the hypothalamic-pituitary axis to testosterone feedback in castrated male hamsters. Endocrinology 1979; 104: 625 630. [DOI] [PubMed] [Google Scholar]
- Arteaga-Silva M, Márquez-Villanueva Y, Martínez-García R, Hernández-González M, Bonilla-Jaime H, Retana-Márquez S. Effects of hormonal replacement with androgens and estrogens on male sexual behavior and plasma levels of these steroids in gonadectomized golden hamsters (Mesocricetus auratus). Physiol Behav 2005; 85: 571 580. [DOI] [PubMed] [Google Scholar]
- Campbell CS, Finkelstein JS, Turek FW. The interaction of photoperiod and testosterone on the development of copulatory behavior in castrated male hamsters. Physiol Behav 1978; 21: 409 415. [DOI] [PubMed] [Google Scholar]
- Park JH, Spencer EM, Place NJ, Jordan CL, Zucker I. Seasonal control of penile development of Siberian hamsters (Phodopus sungorus) by daylength and testicular hormones. Reproduction 2003; 125: 397 407. [DOI] [PubMed] [Google Scholar]
- Mason AO, Duffy S, Zhao S, Ubuka T, Bentley GE, Tsutsui K, Silver R, Kriegsfeld LJ. Photoperiod and reproductive condition are associated with changes in RFamide-related peptide (RFRP) expression in Syrian hamsters (Mesocricetus auratus). J Biol Rhythms 2010; 25: 176 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancel C, Bentsen AH, Sébert ME, Tena-Sempere M, Mikkelsen JD, Simonneaux V. Stimulatory effect of RFRP-3 on the gonadotrophic axis in the male Syrian hamster: the exception proves the rule. Endocrinology 2012; 153: 1352 1363. [DOI] [PubMed] [Google Scholar]
- Watson-Whitmyre M, Stetson MH. A mathematical method for estimating paired testes weight from in situ testicular measurements in three species of hamster. Anat Rec 1985; 213: 473 476. [DOI] [PubMed] [Google Scholar]
- Bast JD, Greenwald GS. Serum profiles of follicle-stimulating hormone, luteinizing hormone and prolactin during the estrous cycle of the hamster. Endocrinology 1974; 94: 1295 1299. [DOI] [PubMed] [Google Scholar]
- Soares MJ, Colosi P, Talamantes F. Development of a homologous radioimmunoassay for secreted hamster prolactin. Proc Soc Exp Biol Med 1983; 172: 379 381. [DOI] [PubMed] [Google Scholar]
- Leitner C, Bartness TJ. Distributed forebrain sites mediate melatonin-induced short-day responses in Siberian hamsters. Endocrinology 2010; 151: 3133 3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hileman SM, McManus CJ, Goodman RL, Jansen HT. Neurons of the lateral preoptic area/rostral anterior hypothalamic area are required for photoperiodic inhibition of estrous cyclicity in sheep. Biol Reprod 2011; 85: 1057 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi S, Kim YJ, Zheng F. Dorsomedial hypothalamic NPY and energy balance control. Neuropeptides 2012; 46: 309 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woller MJ, Campbell GT, Blake CA., Neuropeptide Y. and luteinizing hormone releasing hormone synergize to stimulate the development of cellular follicle-stimulating hormone in the hamster adenohypophysis. J Neuroendocrinol 1995; 7: 733 736. [DOI] [PubMed] [Google Scholar]
- Revel FG, Saboureau M, Pévet P, Simonneaux V, Mikkelsen JD. RFamide-related peptide gene is a melatonin-driven photoperiodic gene. Endocrinology 2008; 149: 902 912. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Ubuka T, Bentley GE, Kriegsfeld LJ. Gonadotropin-inhibitory hormone (GnIH): discovery, progress and prospect. Gen Comp Endocrinol 2012; 177: 305 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A 2006; 103: 2410 2415. [DOI] [PMC free article] [PubMed] [Google Scholar]


