ABSTRACT
The circadian clock in the suprachiasmatic nucleus (SCN) of the hypothalamus is the central pacemaker driving rhythms in endocrine physiology. Gonadal steroid hormones affect behavioral rhythms and clock gene expression. However, the impact of fluctuating ovarian steroid levels during the estrous cycle on internal circadian organization remains to be determined. Further, it is not known if steroid hormone depletion, as in menopause, affects the timing system. To determine the influence of estrous cycle stage and steroid depletion on circadian organization, we measured clock gene expression in the SCN and peripheral tissues from cycling and ovariectomized (OVX) period1-luciferase (per1-luc) transgenic rats. The estrous cycle had modest effects on mean phase and phase distribution of per1-luc expression in the SCN. Surprisingly, peak per1-luc expression in the SCN was widely distributed mainly at night, regardless of cycle stage, an effect eliminated by OVX. Treatment of SCN tissue explants with ovarian steroids did not significantly affect per1-luc expression, suggesting that brain regions outside the SCN mediate the phasic effects of steroids. Our data demonstrate that estrous cycle stage has tissue-dependent effects on the phase of per1-luc expression, phase synchrony among oscillators, and the phase relationship between some peripheral clocks and the light-dark cycle. They also reveal that steroid hormone depletion following OVX alters the timing system, suggesting that the decline in hormone levels, common during the transition to menopause, may be associated with irregular internal circadian organization. This effect on the timing system could contribute to the behavioral and physiological changes associated with this transition.
Keywords: circadian clock, estrous cycle, ovariectomy, period1, rat
The timing of circadian gene expression in the brain and periphery is affected by estrous cycle stage and ovarian-steroid hormone depletion following ovariectomy.
INTRODUCTION
Circadian rhythms of endocrine physiology and behavior are regulated by the central circadian pacemaker in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus [1]. In rodents, behavioral patterns vary during the 4- to 5-day estrous cycle [2, 3]. There is a transient increase in the level of activity and a modest advance of activity onset on the day of estrus, associated with a substantial rise in serum estradiol (E2) [4]. Gonadectomy (GDX) causes a significant change in the circadian rhythm of activity in rats, hamsters, and degus that is reversed by gonadal steroid hormone replacement [2, 5–10]. In blind GDX hamsters, estradiol treatment results in a small but significant shortening of the free-running period of wheel-running activity [11]. In rats who have undergone ovariectomy (OVX), lengthening of the free-running period of locomotor activity is attenuated by steroid hormone replacement [10]. In mice, testis removal has significant effects on the period and amplitude of locomotor activity that are normalized following testosterone replacement [12, 13]. These data demonstrate that gonadal steroids have direct and robust effects on circadian rhythms of behavior. However, a mechanism for these effects in terms of the molecular and physiological actions of steroid hormones in either the brain or periphery has not been determined.
At the cellular level, circadian rhythms are generated by an autoregulatory negative feedback loop of transcriptional regulators, including the enhancer bmal1 (arntl) and the repressors period (per) and cryptochrome (cry; for review see [14]). Oscillations of the molecular clock are also dependent on the timing of posttranscriptional and posttranslational modifications [15]. The clock is known to regulate the timing of gene expression and physiology in SCN pacemaker neurons and in peripheral tissues, including those of the digestive, cardiovascular, and reproductive systems [16–19]. The coordinated timing of oscillators in the central nervous system and the periphery has been referred to collectively as the circadian timing system [20]. It has long been suggested that the precision of the circadian timing system is a critical feature of adaptive physiological function, though evidence supporting this notion is scarce [21]. That said, a growing body of literature supports the conclusion that altering temporal relationships within the timing system can have serious health consequences [22–24].
Limited evidence supports a direct link between steroid receptor activation and clock gene expression. We have recently shown that estradiol shortens the period of clock gene expression in the mouse uterus in an estrogen receptor (ER)-dependent manner [25]. Ovarian steroids modulate the expression of gap junction proteins and have modest effects on the timing of clock gene expression in SCN neurons [26, 27]. Recently it was reported that SCN neurons in the mouse possess both androgen receptor (AR) and ER immunoreactivity [28, 29]. Further, ovarian steroid hormones can alter the pattern of clock gene expression in extra-SCN oscillators, including those in the limbic forebrain [30]. Rhythms of per1, per2, and bmal1 mRNA in the ovary, liver, and uterus are affected by estrous cycle stage [31]. Direct actions of nuclear hormone receptors at clock gene promoter sequences have been reported, including interactions with the classical AR, ER, and glucocorticoid receptors (GR) [25, 32–35]. Binding of progesterone receptors (PR) to clock, per1, cry1, and npas2 (neuronal pas-domain containing protein 2) promoter regions was also recently reported [36]. Many of these promoter regions lack canonical PR binding sites, suggesting that activated PR may function as part of a steroid-activated binding complex to regulate clock gene expression [36]. Together, these data suggest that dynamic variation in gonadal steroid hormones (GSH) or depletion of these hormones could have significant impact on the circadian timing system.
Fluctuating levels of ovarian steroid hormone secretion during the estrous cycle may affect the timing of clock gene expression in tissues of the female reproductive tract [25, 31]. We have previously reported the effects of the estrous cycle stage on the timing of clock gene expression in the SCN, ovary, and uterus in rats and mice [25]. The effects of fluctuating steroid hormones during the estrous cycle on clock function are in all probability not limited to tissues of the hypothalamo-pituitary-gonadal (HPG) axis. Ovarian steroids may well act to modulate internal circadian organization by adjusting peripheral clocks in a tissue-specific manner. To test this, we measured rhythms of per1-luciferase (per1-luc) gene expression in the SCN and peripheral tissues, including the liver, kidney, lung, white adipose tissue (WAT), cornea, oviduct, ovarian follicles, and pituitary, from cycling and OVX rats. We also examined the effects of exposure to ovarian steroids on the timing of per1-luc expression in isolated SCN tissue explants. Here, we report that internal circadian organization varies as a function of estrous cycle stage. We observed significant effects of OVX on the timing of per1-luc expression in multiple tissues, including the SCN. However, our data show that in vitro exposure of SCN slice cultures to steroid hormones at physiological levels does not significantly affect the timing and amplitude of per1-luc expression. These data imply that the influence of ovarian steroids on behavior may be indirect and could be mediated by steroid-sensitive regions outside of the SCN. Overall, our data suggest that fluctuating steroid hormone levels during the estrous cycle and the steroid depletion that follows OVX can have significant effects on internal circadian organization by altering rhythms of clock gene expression in both central and peripheral oscillators.
MATERIALS AND METHODS
Animals
All procedures and standards of care were approved by and carried out according to the guidelines of the University of Virginia Institutional Animal Care and Use Committee, the University of Rochester Committee for Animal Resources, and the National Institutes of Health Guidelines for the Use of Experimental Animals. Adult (2- to 5-mo old) female and male per1-luc transgenic rats (from a breeding colony at the University of Virginia) were used for all experiments. Rats were individually housed under conditions of controlled lighting, temperature, and humidity. Animals were provided food and water ad libitum. Staging of the estrous cycle was determined by daily vaginal cytology. Only rats displaying at least two consecutive 4-day estrous cycles with clear delineation between stages were used in these experiments.
Bilateral Ovariectomy
Female rats were ovariectomized by the dorsal approach through the muscle of the back, lateral to the spinal column. Ovaries and oviducts were isolated from the surrounding perigonadal adipose tissue and removed, leaving the uterine horns intact. Incisions were closed following a two-stage procedure with an absorbable suture through the muscle wall and sterile surgical staples through the skin. All animals were treated with ketoprofen (2–5 mg/kg) as a postoperative analgesic and allowed to recover on a heated pad before replacing them in light-tight boxes under a 12L:12D cycle with food and water available ad libitum. All surgical procedures were conducted according to the University of Virginia Institutional Animal Care and Use Committee Guidelines for Anesthesia and Aseptic Surgery. Rats were euthanized for tissue culture a minimum of 10 days (mean = 10.8, range = 10–23 days) after OVX, when the concentration of ovarian steroids reaches a nadir of <8 pg/ml (as previously measured in [37, 38]).
Tissue Culture and Luminescence Recording
Culture procedures are identical to those described in [39]. Animals were euthanized within the 3 h before lights off (ZT9–Z12; ZT is relative zeitgeber time in hours, where ZT0 = time of lights on and ZT12 = lights off) by overdose with CO2 anesthesia followed by cervical dislocation. Tissues collected on diestrus afternoon would have been exposed to a slight rise in serum estrogen, but not the peak of hormone levels occurring on the afternoon of proestrus [40, 41]. Tissues collected on proestrus would have been exposed to elevated estrogen and progestin levels at or near the onset of the gonadotropin surges but prior to ovulation (late night between proestrus and estrus). Tissues, including brain, liver, kidney, lung, pituitary, cornea, perigonadal WAT, whole ovary, and oviduct were removed and placed in chilled Hanks buffered salt solution. Though not a tissue of the HPG or metabolic axis, the cornea was selected because it is a robust peripheral oscillator known to express steroid hormone receptors. The brain was sectioned in the coronal plane with a vibrating microtome at a thickness of 300 μm. A section containing the bilateral SCN near the midpoint of the rostrocaudal extent was recovered, and a minimum of tissue including the paired SCN was removed. The cornea was removed from the eye and flattened. The ovary was isolated from the oviduct, and a single (3–5 mm in diameter) follicle was isolated. For all other tissues, individual 5-mm2 tissue explants were collected. Tissues (except for the follicles and WAT) were placed at the liquid interface on membranes (Millicell-CM; PICM030-50; Millipore). Follicles and WAT were rinsed thoroughly with clean culture medium and cultured free floating. All tissues were placed in 35-mm dishes containing 1.2 ml of medium (serum-free, low-sodium bicarbonate, no phenol red, Dulbecco modified Eagle medium [Invitrogen]) supplemented with 10 mM HEPES (pH 7.2), B27 (2%; Invitrogen), 0.1 mM luciferin (beetle luciferin, potassium salt; Promega), and antibiotics (25 U/ml penicillin, 25 mg/ml streptomycin; Invitrogen). All dishes were sealed with a cover glass using vacuum grease and placed inside a LumiCycle recording system (Actimetrics) within a light-tight 35°C environmental chamber. Bioluminescence from each tissue was counted for 1 min every 10 min for a minimum of 5 days. Robust circadian rhythms of per1-luc expression were readily measured in explants of each tissue (see Supplemental Fig. S1, all Supplemental Data are available online at www.biolreprod.org). Only those cultures displaying at least two clear cycles of per1-luc expression in vitro were considered rhythmic and included in subsequent analyses.
Ovarian Steroid Hormone Treatment of SCN Tissue Explants
Cyclo-β-dextran sugar-caged E2 and/or progesterone (P4) were diluted in sterile Millipore ddH20 and added directly to the recording media at the time of culture (Sigma). Cyclo-β-dextran-coated steroids are efficient due to their ability to dissolve readily in culture media. To control for the effects of sugar coating, SCN cultures were treated with cyclo-β-dextran sugar (100 μM, 500 μM, or 1 mM) without steroid. Following the addition of steroid or vehicle, dishes were sealed with a cover glass using vacuum grease and placed inside the LumiCycle within a light-tight 35°C environmental chamber. Bioluminescence from each tissue was counted for 1 min every 10 min for a minimum of 5 days.
Data Analysis
Luminescence from each culture was normalized by subtraction of the 24-h running average from the raw data and then smoothed with a 2-h running average (Origin Pro 8.5; OriginLab [42]). The peak time of per1-luc expression was determined on the first full 24-h cycle in culture and is expressed as ZT in hours (Origin Pro 8.5). Peak phase (ZT) was converted to angles and plotted on a Rayleigh graph. Phase distribution for a given tissue as a function of treatment (estrous stage, OVX) was measured with the Rayleigh uniformity test (Oriana; Kovac Computing Systems). An increase in phase distribution constitutes a decline in phase synchrony. A vector representing the arithmetic mean (hereafter referred to as mean phase) of the multiple angles was plotted as an arrow traveling away from the center of each plot. A dotted inner circle indicates the threshold for significance of the Rayleigh test; any vector crossing this line indicates significant clustering around the mean (see [43, 44]). Watson-Williams F-test (WW F-test) was used to determine the effects of estrous cycle stage or OVX on the mean phase within each tissue (e.g., liver on metestrus vs. liver on diestrus or liver from OVX, etc.; Oriana). All statistical tests were considered significant at P < 0.05.
For experiments examining the effects of ovarian steroid hormones in vitro, data were smoothed and detrended as described above. Data were then imported into the LumiCycle and analyzed with a chi-square periodogram (significance threshold = P < 0.001). A minimum of 5 days of luminescence data was included in the analyses. Group means for period (τ) were compared with a one-way ANOVA followed by Student-Neuman-Keul post-hoc test. All data are reported as means ± SEM and are considered significant at P < 0.05.
RESULTS
Rhythms of Per1-Luciferase Expression in Liver, Kidney, Lung, and WAT are Differentially Affected by Estrous Cycle Stage and OVX
We have previously reported that, in rats, the amplitude of per1 mRNA expression was lower on estrus than on any other day of the cycle and that peak bmal1 mRNA shifted between diestrus and proestrus [31]. These data suggest that the increase in ovarian estradiol on diestrus produces a significant phase shift of liver bmal1 mRNA and a precipitous drop in the amplitude of period1 mRNA on estrus. We have also previously reported that estrous cycle stage does not affect the mean phase of PER2::luciferase expression in mouse liver, though there was clearly a small difference (∼2 h) in phase between proestrus and metestrus in that study [31]. In the current study, we observed a small advance in the mean phase of per1-luc expression on proestrus relative to both diestrus and metestrus (Fig. 1A). Further, we detected a significant increase in phase synchrony from diestrus to proestrus that appeared to decline as the cycle progressed, approaching the limits of significance again by metestrus (Fig. 1A). There was also an effect of OVX on the liver, resulting in a significant delay of the mean phase (ZT0.8 ± 3.1 h) relative to both proestrus (ZT17.7 ± 0.86 h; P < 0.05) and estrus (ZT18.5 ± 1.64 h; P < 0.05).
FIG. 1.
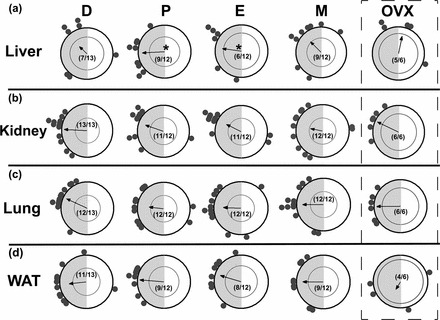
Rhythms of per1-luciferase expression in liver, kidney, lung, and WAT are differentially affected by estrous cycle stage and OVX. Rayleigh plots of peak per1-luc expression in individual liver (a), kidney (b), lung (c), and WAT (d) tissue cultures across the estrous cycle or from OVX rats. In a–d, the arrow (vector) in each plot represents the arithmetic mean phase. The vector length indicates synchrony around the mean, which is significant by the Rayleigh uniformity test if the tip of the vector crosses the inner dotted circle in each plot (P < 0.05). The numbers in parentheses indicate the number of rhythmic cultures/total cultures. Asterisks in each plot indicate significant difference in phase distribution between tissues collected on a specific cycle day and those from OVX rats (P < 0.05). D, diestrus; P, proestrus; E, estrus; M, metestrus; OVX, ovariectomy.
We did not observe a significant effect of estrous cycle stage or OVX on the phase of peak per1-luc expression in the kidney (Fig. 1B), though we did see a significant decline in synchrony across the cycle (Fig. 1B). That is, phase synchrony was greatest on diestrus and gradually diminished through proestrus and estrus, reaching a nadir on metestrus (P > 0.05 by Rayleigh test; Fig. 1B). Note, however, that this was largely due to one or two outliers in each case. We also did not detect a significant effect of cycle stage or OVX on the mean phase of per1-luc expression in lung tissue or synchrony among lung explants (Fig. 1C).
Similarly, there was no significant influence of the cycle on the timing of per1-luc expression in WAT (Fig. 1D). However, steroid hormone depletion following OVX did produce a significant decline in phase synchrony among WAT explant cultures (Fig. 1D).
Rhythms of Per1-Luciferase Expression in Ovarian Follicles, Oviduct, and Pituitary Gland Are Differentially Affected by Estrous Cycle Stage and OVX
In rats, we have shown that the amplitude of per1 mRNA expression in the ovary declines on estrus relative to proestrus but is recovered by diestrus [31]. Further, we reported that the phase of per1 mRNA in the ovary advanced on the day of estrus relative to proestrus, with a gradual delay and return to the original phase by diestrus [31]. Our current data lend qualified support to this finding, as we observed a small advance of the mean phase of per1-luc expression in isolated ovarian follicles on estrus (ZT13.73 ± 0.64 h) and metestrus (ZT12.72 ± 0.87 h) relative to proestrus (ZT15.69 ± 0.64 h; Fig. 2A). However, this effect was not significant by WW F-test (P > 0.05 for both comparisons). Though we did not observe an impact of cycle stage on mean phase, we did see an effect on phase synchrony among follicular cultures, marked by a dramatic increase in synchrony during the transition from diestrus to estrus (Fig. 2A). In fact, this was the most striking effect we observed on phase synchrony in all of the peripheral tissues we examined. The mean phase of per1-luc expression in the oviduct was not affected by cycle stage, though phase synchrony was significantly lower on estrus relative to both proestrus and metestrus (Fig. 2B).
FIG. 2.
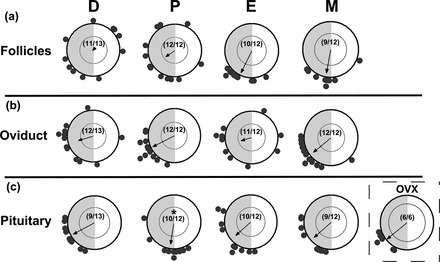
Rhythms of per1-luciferase expression in ovarian follicles, oviduct, and pituitary gland are differentially affected by estrous cycle stage and OVX. Rayleigh plots of peak per1-luc expression from individual follicular (a), oviduct (b), and pituitary (c) tissue cultures from each day of the estrous cycle or OVX rats. The asterisk in c indicates significant differences between the mean phase of per1-luc expression in pituitary gland explants collected on proestrus versus tissues collected on diestrus or estrus or from OVX rats (P < 0.05). All abbreviations and conventions are as described in Figure 1.
In the pituitary gland there was an effect of estrous cycle stage on mean phase of per1-luc expression; there was a significant phase advance on proestrus (ZT12.58 ± 0.86 h) compared with diestrus (ZT16.11 ± 0.70 h; P < 0.01; Fig. 2C). This advance was transient, as there was a phase delay by estrus (ZT15.25 ± 1.04 h; P < 0.05 vs. proestrus) that persisted through metestrus (ZT15.25 ± 0.92 h; P < 0.05 vs. proestrus). Although there was no effect of OVX on phase synchrony among pituitary cultures, the phase of per1-luc expression in pituitary tissue from OVX rats (ZT15.41 ± 0.65 h) was delayed relative to tissues collected on proestrus (P < 0.05; Fig. 2C) and closer to the phase we observed in tissues collected on estrus (ZT15.25 ± 1.04 h) and metestrus (ZT15.24 ± 0.92 h). It is worth noting that ovarian steroid hormone levels in rats peak on the day of proestrus and are at their lowest levels on the days of estrus and metestrus.
Rhythms of Per1-Luciferase Expression in the SCN and Cornea Are Differentially Affected by Estrous Cycle Stage and OVX
Though we have previously reported that mean PER2::LUC expression in mouse SCN was not affected by estrous cycle stage, phase synchrony was not determined in that study [31]. To our surprise, the peak phase of per1-luc expression in SCN explant cultures was widely distributed regardless of estrous cycle stage (Fig. 3A). Further, phase synchrony among SCN cultures increased following OVX (Fig. 3A). SCN explants from OVX rats showed robust phase clustering (P < 0.001) around the expected mean for the SCN (ZT9.2 ± 0.63 h) as determined in previous experiments (∼ZT9.5 in [45]). In addition to the increased synchrony, we also detected a significant phase advance of mean per1-luc expression in SCN tissue from OVX animals relative to tissue collected from cycling rats on diestrus (ZT13.24 ± 1.33 h; P < 0.05), proestrus (ZT15.74 ± 1.38 h; P < 0.01), and estrus (ZT14.36 ± 2.40, P < 0.05; Fig. 3A). Further, the phase of per1-luc expression in SCN tissue from OVX females was nearly identical to that of intact male rats (∼ZT9; Fig. 3A, OVX, vs. 3C).
FIG. 3.
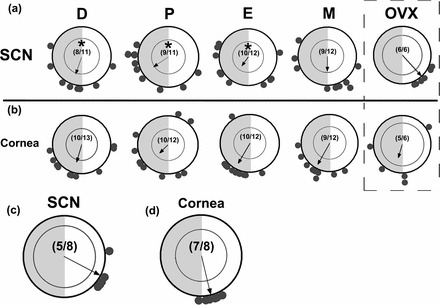
Rhythms of per1-luciferase expression in the SCN and cornea are differentially affected by estrous cycle stage and OVX. Rayleigh plots of peak per1-luc expression from individual SCN (a) and cornea (b) tissue cultures from each day of the estrous cycle and from OVX rats. c) Rayleigh plot of peak per1-luc expression in SCN tissue cultures from intact male rats. d) Rayleigh plot of peak per1-luc expression in individual cornea tissue cultures from intact male rats. All abbreviations and conventions are as described in Figure 1.
In the cornea we did not see an effect of cycle stage on mean phase, though we did observe a significant decline in phase synchrony on proestrus relative to diestrus that recovered by estrus (Fig. 3B). Desynchrony among corneas in female rats is in stark contrast to the highly stable and robust synchrony among corneal explants from intact male rats (Fig. 3B vs. Fig. 3D). OVX actually reduced phase synchrony among corneal explants, also in direct contrast with the effects of OVX on per1-luc expression in SCN tissue (Fig. 3A vs. Fig. 3B). There also appears to be a modest sex difference in the mean phase of per1-luc expression in corneal explants, with male corneas peaking slightly earlier than female corneas, regardless of cycle day (Fig. 3B vs. 3D).
Rhythms of Per1-Luciferase Expression in SCN Explants from OVX Rats Are Not Affected by Physiologically Relevant Concentrations of Ovarian Steroid Hormones
To further explore the influence of ovarian steroid hormones on per1-luc expression in the SCN, we treated SCN explants from OVX per1-luc rats with E2, P4, or E2+P4 (Fig. 4). Steroids were added at the time of culture and remained in the media for the duration of the recording. As shown in Figure 4A (and supplemental Fig. S1F), SCN explants (vehicle treated) produced a stable rhythm of per1-luc expression for up to 7 days in culture. We did not observe a significant effect of the cyclo-β-dextran sugar molecule (Supplemental Fig. S2A) on the period of per1-luc expression, and it was included in all control or untreated cultures. Rhythms of per1-luc expression in SCN explants were analyzed for changes in period as a function of steroid treatment. We found a significant lengthening of the period of per1-luc expression in cultures treated with high concentrations of P4 (P < 0.0001, WW F-test = 22.59) and combined E2+P4 (P < 0.001, WW F-test = 11.59), but not in cultures treated with high concentrations of E2 alone (P > 0.05, WW F-test = 0.45; Fig. 4, B–D). Post-hoc analysis within steroid treatment as a function of concentration shows that E2 treatment did not affect period at any of the concentrations tested (200—500 μM vs. vehicle controls; Fig. 4E). P4 treatment at concentrations of 10 μM (τ = 25.49 ± 0.4; P < 0.05), 100 μM (τ = 26.33 ± 0.48; P < 0.001), and 500 μM (τ = 28.71 ± 0.55; P < 0.001) resulted in a significant lengthening in period when compared with vehicle-treated controls (τ = 24.39 ± 0.13; Fig. 4F). Combined E2+P4-treatment also resulted in a significant lengthening in period as a function of dose, but only at high concentrations (100 μM [τ = 26.16 ± 0.37; P < 0.001] and 500 μM [τ = 26.62 ± 0.44; P < 0.001]; Fig. 4, G and H).
FIG. 4.
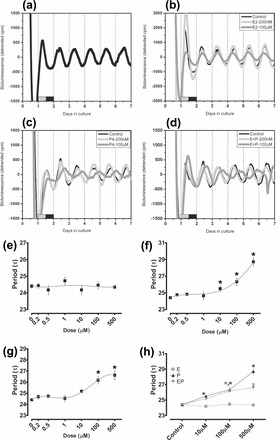
Ovarian steroid hormones at physiological levels do not affect the period of per1-luciferase expression in SCN tissue cultures. a) Recording of per1-luc luminescence in a vehicle-treated SCN tissue culture (peak phase ∼ZT9, τ = 24.2). b) Per1-luc expression in SCN explants treated with low (200 nM; light gray trace) and high (100 μM; dark gray trace) concentrations of E2 or vehicle (black trace) control. c) Per1-luc expression in SCN explants treated with low (200 nM; light gray trace) and high (100 μM; dark gray trace) concentrations of P4 or vehicle (black trace). d) Per1-luc expression in SCN explants treated with low (200 nM; light gray trace) and high (100 μM; dark gray trace) concentrations of E2+P4 or vehicle (black trace). e) Treatment with E2 alone (200 nM–500 μM) did not affect the period of per1-luc expression in the SCN. f) Treatment with P4 at or above 10 μM lengthened the period of per1-luc expression. g) Treatment with high concentrations of E2+P4 also lengthened the period of per1-luc expression. h) Although P4 (black triangles) and E2+P4 (inverted gray triangles) lengthened the period of per1-luc expression, the effects of P4 were slightly attenuated by the addition of E2 (gray squares). In a–d, the black trace is the vehicle control shown in a. In all figures the light-dark bar on the x-axis represents the light-dark cycle on the day of tissue collection. In e–h, *P < 0.05 versus vehicle control. In h, gray asterisks = P < 0.05 for E2+P4 treatment versus E2, and black asterisks = P < 0.05 for P4 treatment versus E2 treatment. All data in e–h are mean ± SEM.
DISCUSSION
Numerous investigators over the last 40 yr have endeavored to characterize the influence of GSH on circadian rhythms of mammalian physiology and behavior [2, 3, 11–13, 28, 46, 47]. In humans, evidence suggests that fluctuating GSH during the menstrual cycle can alter the timing of physiology and behavior [48, 49]. While it has been shown that GSH can alter circadian rhythms of activity, their impact on the molecular clock in central and peripheral tissues is poorly understood. A limited number of reports describe the effects of ovarian steroid hormones on gene expression in the SCN [25, 27]. There is also very limited evidence for an effect of GSH on the timing of the molecular clock in peripheral oscillators [31, 36, 50]. To date there have been no attempts to determine the effects of estrous cycle stage or steroid hormone depletion after OVX on internal circadian organization. In the present study, we have determined the impact of estrous cycle stage on internal circadian organization.
The most surprising—and perhaps most significant—result in the current study is the robust effect of ovarian factors on the mean phase and synchrony of the female SCN. The existing literature, based almost exclusively on data from males [44], leads one to expect tightly clustered rhythms peaking in the second half of the light portion of the light-dark cycle (i.e., ZT6–ZT12). This expectation is fulfilled in our study by data from males. Furthermore, in our experiments SCN data from OVX females are not different from the data from males, confirming that the very different distribution of SCN phases seen in all estrus cycle stages of cycling females is the result of ovarian influence. These influences could well be ovarian steroids, though the fact that they do not vary systematically across the cycle indicates that other factors may be involved. That is, we cannot ignore the possible influence of other ovarian outputs, including the circulating peptide hormones activin and inhibin. Moreover, it is possible that the intensity of these effects may be linearly dependent on the duration of steroid depletion. While 10 days has certainly been deemed adequate for steroid levels to reach a nadir in circulation, it may not be long enough for residual effects of steroid receptor activation to dissipate [37, 38]. Nonetheless, these data point to the existence of robust sex differences in basic parameters of the central circadian pacemaker and require further exploration. Though early reports suggested that SCN neurons did not contain the primary estrogen receptor ERα [51], more recent work has refuted that claim, revealing both ERα and ERβ expression in SCN neurons [29]. Further, relatively sparse expression of PRs has been observed in SCN pacemaker cells [52, 53]. Our current findings suggest that some of the effects of steroid hormones on circadian function are mediated indirectly through brain areas outside of the SCN. This suggestion is supported by the fact that steroid-responsive brain regions, including the basal forebrain, can influence SCN-driven behavioral rhythms [30]. There are significant synaptic connections between the SCN and several nuclei in these brain regions [54, 55]. Of particular interest are the results of Perrin et al. [30[, which suggest that the changing steroid hormone levels during the estrous cycle alter the rhythm of PER2 expression in the central nucleus of the medial amygdala and the oval nucleus of the bed nucleus of the stria terminalis, but not the SCN. In these regions, the amplitude of the PER2 rhythm significantly increased on proestrus, followed by a gradual decline in amplitude and delay in peak phase between proestrus and diestrus. A similar increase in amplitude was seen in OVX rats treated with estradiol benzoate [30]. These data suggest that the rising titer of ovarian E2 and/or P4 on the day of proestrus adjusts the timing of PER2 expression in frontal brain regions, which could in turn affect the timing of activity in SCN pacemaker neurons.
Recently we reported that the phase and amplitude of PER2 expression in the mouse SCN was not affected by the transition from proestrus to metestrus, suggesting that ovarian steroid hormones do not directly affect the SCN [31]. Those experiments did not examine the phase of clock gene expression in the SCN across the entire reproductive cycle, nor did they determine the impact of steroid hormone depletion by OVX. To ascertain the direct influence of ovarian steroids on the SCN and by extension on rhythms of behavior, we measured the response of per1-luc expression rhythms in SCN explants to ovarian steroid hormones. In vitro exposure of SCN tissue from OVX rats to E2, P4, or E2+P4 at high physiological levels (200 nM–1 μM) did not significantly alter the period of per1-luc expression, although higher concentrations (10 μM or greater) did lengthen the period of per1-luc expression in the SCN. Thus, at physiological levels ovarian steroids do not directly affect the timing of per1-luc expression in SCN neurons. These data are somewhat surprising, given the presence of E2 and P4 receptors on SCN pacemaker neurons and the established effects of ovarian hormones on behavior [29]. It is possible that the pulsatile nature of hormone secretion, which is not replicated in our experiments, is responsible for the influence of steroid hormones on the SCN in vivo. Given the dynamic nature of ovarian steroid levels, it is possible that, though they did not affect the period of per1-luc expression when applied chronically, acute fluctuation or pulses of ovarian steroids may have considerable influence on the phase or amplitude of clock gene expression in the SCN. That said, ovarian steroid hormone levels in the circulation change gradually, over several hours and even across several days. Our approach was intended to ascertain if this “sustained” (>12–24 h) period of elevated steroid hormone levels could effectively alter the timing of the circadian clock in the SCN. One would need a more concrete understanding of steroid hormone concentrations over time (days, hours) at the level of the SCN to truly address this issue. Future examination of phase-dependent exposure to acute ovarian steroid pulses, in the presence of steroid receptor antagonists, is warranted and could more completely define the impact of steroids on SCN pacemaker neurons.
Our results suggest that dynamic changes in ovarian steroid hormone levels associated with the cycle can affect the timing of the molecular clock in some peripheral tissues but not in others. Rhythms of per1-luc expression in peripheral oscillators involved in metabolic function, including the liver, kidney, lung, and WAT, were minimally affected by the reproductive cycle. However, in liver and WAT tissue, OVX resulted in a significant phase shift of per1-luc expression (liver) and/or a precipitous decline in phase synchrony (liver and WAT). Ovarian steroid hormones are known to regulate lipid metabolism, insulin sensitivity, and glucose tolerance in hepatocytes and adipose tissue [56–58]. Our data suggest that, in addition to their established impacts on metabolism, ovarian steroids may also modulate the timing of the clock. This notion is best supported by the effects of OVX we observed on the phase of per1-luc expression in these tissues. The lack of significant effects of estrous cycle stage or OVX on the mean phase of per1-luc expression and the synchrony among kidney and lung tissues suggest that the clock in these tissues is less responsive to changes in hormone levels across the cycle.
In our previous study, we did not see a significant effect of cycle stage on mean PER2::LUC expression in mouse ovarian tissue explants, though the phase distribution of isolated follicles was not reported [31]. As in that study, we did not observe a significant effect of cycle stage on the mean phase of per1-luc expression in isolated rat follicles. It is possible that the influence of cycle stage on phase synchrony among ovarian follicles reported here can be attributed to the development or maturation of the follicle. In the present study, efforts were made to collect only antral or preovulatory (Graafian) follicles [59]). Smaller preantral (or primary) follicles do not show significant rhythms of clock gene expression (our unpublished observation and [60, 61]). That said, it is possible that slight variation in follicular development (e.g., small antral to Graafian) between metestrus and proestrus could affect the phase of per1-luc expression. In fact, our results suggest that early in follicular development (late metestrus to diestrus) the follicles are synchronized, but the process of maturation increases phase distribution. It is possible that increased phase distribution among follicles is an indicator of variation in development that is normalized following stimulation with gonadotropins. This speculation is supported by the recent finding that stimulation of granulosa cells from gonadotropin-primed mice with luteinizing hormone (LH) increases expression of the gap junction protein Connexin 43 in conjunction with an increase in the amplitude of PER2 expression [62]. This effect may also depend on ovarian steroid hormones, since estrogen has been shown to stimulate Connexin gene expression in other cells, including SCN neurons [63]. Thus, increased robustness of circadian oscillators in individual granulosa (or theca) cells may lead to increased coordination among developing follicles at the onset of the preovulatory LH surge. Phase synchrony of per1-luc expression in isolated oviduct explants was modestly reduced from proestrus to estrus. It may be that the precipitous decline in circulating ovarian steroids following ovulation leads to a decline in phase coordination within the oviduct. That would not be surprising, given the density of steroid hormone receptors in the oviduct and its role in the process of ovulation [64]. It is also possible that various cell populations in the wall of the oviduct or cells at different locations along its length may have functionally disparate phases, although it is unclear how this phase variation might impact the process of ovulation, oocyte maturation, and fertilization [65, 66].
Of all the peripheral tissues we examined, the pituitary showed the most robust and persistent phase coherence. Interestingly, there was no effect of estrous cycle stage or OVX on phase synchrony among pituitary explants. However, there was a significant effect on the mean phase of per1-luc expression; there was a significant advance of peak per1-luc expression between diestrus and proestrus, followed by a significant delay on estrus. It is possible that steroid hormones indirectly affect the pituitary clock by regulating neuroendocrine-releasing factors, which facilitate the timing of pituitary hormone secretion [67–69].
We measured the phase of per1-luc expression in the cornea to determine the impact of estrous cycle stage and OVX on a particularly robust peripheral oscillator outside of the reproductive axis. As with the ovarian follicles, oviduct, liver, and kidney, the phase distribution of per1-luc expression in corneal cultures was only modestly affected by estrous cycle stage. However, steroid depletion following OVX significantly reduced phase synchrony among corneal explants. ER and PR are expressed at high density in corneal epithelial cells from rats, mice, rabbit, and humans [70–72]. Given the density of these receptors, it is reasonable to hypothesize that ovarian steroid hormones directly affect clock gene expression in the cornea. It is also possible that ovarian steroids affect the cornea indirectly, by modulating the level of serum glucocorticoids [73, 74]. Glucocorticoids, including corticosterone (CORT), are known to affect the timing of clock gene expression in the cornea [44], and their level is significantly affected by the estrous cycle [73]. Changes in CORT secretion could contribute to the increase in phase synchrony among cultures collected on estrus [73]. Elimination of this steroid-dependent effect on serum CORT may also explain the decline in phase coherence among corneal explants following OVX.
In summary, our data indicate that the rodent estrous cycle has differential effects on the timing of per1-luc expression in central and peripheral oscillators. The phase of per1-luc expression in several tissues, including the liver, WAT, and SCN, was significantly affected by ovarian steroid depletion (OVX). Because the physiological function of the circadian clock in many of these tissues remains poorly defined, it is difficult to draw strong conclusions regarding the impact of increased (or decreased) phase synchrony among the various tissues across the cycle. Fluctuations in phase synchrony may represent the normal condition for a tissue (e.g., ovarian follicles), simply being a function of changes in cellular development and maturation. However, in other tissues, reduced synchrony may represent a pathological state permissive for disease. A salient feature of menopause in women is a significant decline in circulating ovarian steroid hormones [75, 76], and reduced hormone levels have been linked to increased risk of cardiovascular disease, osteoporosis, chronic kidney disease, and stroke [77, 78]. While our data do not directly link any of these pathologies to clock function in any one tissue, the known impact of circadian disruption on fertility and metabolism suggest that a general decline in internal circadian organization or circadian misalignment (e.g., shift work and jet lag) represent a considerable risk factor for disease [23, 79]. Thus, it is possible that near complete steroid hormone depletion in aging postmenopausal women could severely disrupt the timing system. These effects, arising during the premenopausal transition, might be a component of the decline in reproductive function and a contributing factor to the increased disease risk seen in middle-age women.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge the valuable technical assistance of Lindsay A. Marchetti, Pratik Patel, Hing-Kiu Chan, and Denise T. Holmes.
Footnotes
Supported by National Institutes of Health RO1 grant MH56647 (to M.M.). Presented in part at the 45th Annual Meeting of the Society for the Study of Reproduction, August 12–15, 2012, State College, Pennsylvania.
REFERENCES
- Kriegsfeld LJ, Silver R. The regulation of neuroendocrine function: timing is everything. Horm Behav 2006; 49: 557 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science 1977; 196: 305. [DOI] [PubMed] [Google Scholar]
- Wollnik F, Turek FW. Estrous correlated modulations of circadian and ultradian wheel-running activity rhythms in LEW/Ztm rats. Physiol Behav 1988; 43: 389 396. [DOI] [PubMed] [Google Scholar]
- Wollnik F. Physiology and regulation of biological rhythms in laboratory animals: an overview. Lab Anim 1989; 23: 107 125. [DOI] [PubMed] [Google Scholar]
- Morin LP. Effect of ovarian hormones on synchrony of hamster circadian rhythms. Physiol Behav 1980; 24: 741. [DOI] [PubMed] [Google Scholar]
- Morin LP, Cummings LA. Splitting of wheelrunning rhythms by castrated or steroid treated male and female hamsters. Physiol Behav 1982; 29: 665. [DOI] [PubMed] [Google Scholar]
- Jechura TJ, Walsh JM, Lee TM. Testicular hormones modulate circadian rhythms of the diurnal rodent, Octodon degus. Horm Behav 2000; 38: 243 249. [DOI] [PubMed] [Google Scholar]
- Hummer DL, Jechura TJ, Mahoney MM, Lee TM. Gonadal hormone effects on entrained and free-running circadian activity rhythms in the developing diurnal rodent Octodon degus. Am J Physiol Regul Integr Comp Physiol 2007; 292: R586 R597. [DOI] [PubMed] [Google Scholar]
- Albers HE, Gerall AA, Axelson JF. Effect of reproductive state on circadian periodicity in the rat. Physiol Behav 1981; 26: 21. [DOI] [PubMed] [Google Scholar]
- Albers HE. Gonadal hormones organize and modulate the circadian system of the rat. Am J Physiol 1981; 241: R62. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Menaker M. Interaction of estradiol and progesterone: effects on circadian locomotor rhythm of female golden hamsters. Am J Physiol 1980; 239: R497. [DOI] [PubMed] [Google Scholar]
- Daan S, Damassa D, Pittendrigh CS, Smith ER. An effect of castration and testosterone replacement on a circadian pacemaker in mice (Mus musculus). Proc Natl Acad Sci U S A 1975; 72: 3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, Wang A, Sasanian J, Silver R. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology 2007; 148: 5487 5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron 2012; 74: 246 260. [DOI] [PubMed] [Google Scholar]
- Kojima S, Shingle DL, Green CB. Post-transcriptional control of circadian rhythms. J Cell Sci 2011; 124: 311 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest 2011; 121: 2133 2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellix MT, Menaker M. Circadian clocks in the ovary. Trends Endocrinol Metab 2010; 21: 628 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudic RD. Time is of the essence: vascular implications of the circadian clock. Circulation 2009; 120: 1714 1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell 2008; 134: 728 742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 2010; 72: 517 549. [DOI] [PubMed] [Google Scholar]
- Moore-Ede MC, Schmelzer WS, Kass DA, Herd JA. Internal organization of the circadian timing system in multicellular animals. Fed Proc 1976; 35: 2333 2338. [PubMed] [Google Scholar]
- Karatsoreos IN, Bhagat SM, Bowles NP, Weil ZM, Pfaff DW, McEwen BS. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology 2010; 151: 2117 2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol 2012; 349: 91 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomans CP, van den Berg SA, Lucassen EA, Houben T, Pronk AC, van der Spek RD, Kalsbeek A, Biermasz NR. Willems van Dijk K, Romijn JA, Meijer JH. The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes 2013; 62: 1102 1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TJ, Sellix MT, Menaker M, Block GD. Estrogen directly modulates circadian rhythms of PER2 expression in the uterus. Am J Physiol Endocrinol Metab 2008; 295: E1025 E1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TJ, Shinohara K, Funabashi T, Kimura F. Effect of estrogen on the expression of Cry1 and Cry2 mRNAs in the suprachiasmatic nucleus of female rats. Neurosci Res 2001; 41: 251. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Funabashi T, Nakamura TJ, Kimura F. Effects of estrogen and progesterone on the expression of connexin-36 mRNA in the suprachiasmatic nucleus of female rats. Neurosci Lett 2001; 309: 37. [DOI] [PubMed] [Google Scholar]
- Iwahana E, Karatsoreos I, Shibata S, Silver R. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Horm Behav 2008; 53: 422 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida B, Hrabovszky E, Kalamatianos T, Coen CW, Liposits Z, Kallo I. Oestrogen receptor alpha and beta immunoreactive cells in the suprachiasmatic nucleus of mice: distribution, sex differences and regulation by gonadal hormones. J Neuroendocrinol 2008; 20: 1270 1277. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Segall LA, Harbour VL, Woodside B, Amir S. The expression of the clock protein PER2 in the limbic forebrain is modulated by the estrous cycle. Proc Natl Acad Sci U S A 2006; 103: 5591 5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TJ, Sellix MT, Kudo T, Nakao N, Yoshimura T, Ebihara S, Colwell CS, Block GD. Influence of the estrous cycle on clock gene expression in reproductive tissues: effects of fluctuating ovarian steroid hormone levels. Steroids 2010; 75: 203 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall LA, Milet A, Tronche F, Amir S. Brain glucocorticoid receptors are necessary for the rhythmic expression of the clock protein, PERIOD2, in the central extended amygdala in mice. Neurosci Lett 2009; 457: 58 60. [DOI] [PubMed] [Google Scholar]
- Cao Q, Gery S, Dashti A, Yin D, Zhou Y, Gu J, Koeffler HP. A role for the clock gene per1 in prostate cancer. Cancer Res 2009; 69: 7619 7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia KA, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, Downes M, Evans RM. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 2011; 480: 552 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Nakahata Y, Tanaka M, Yoshida M, Soma H, Shinohara K, Yasuda A, Mamine T, Takumi T. Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. J Biol Chem 2005; 280: 42036 42043. [DOI] [PubMed] [Google Scholar]
- Rubel CA, Lanz RB, Kommagani R, Franco HL, Lydon JP, Demayo FJ. Research resource: genome-wide profiling of progesterone receptor binding in the mouse uterus. Mol Endocrinol 2012; 26: 1428 1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman ME, Sterman JR. Ovarian steroid modulation of prolactin surges in cervically-stimulated ovariectomized rats. Endocrinology 1978; 102: 1915. [DOI] [PubMed] [Google Scholar]
- Gerhold LM, Horvath TL, Freeman ME. Vasoactive intestinal peptide fibers innervate neuroendocrine dopaminergic neurons. Brain Res 2001; 919: 48. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science 2000; 288: 682. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology 1974; 94: 1704. [DOI] [PubMed] [Google Scholar]
- Nequin LG, Alvarez J, Schwartz NB. Measurement of serum steroid and gonadotropin levels and uterine and ovarian variables throughout 4 day and 5 day estrous cycles in the rat. Biol Reprod 1979; 20: 659 670. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Poole AS, Yamazaki S, Menaker M. Is the food-entrainable circadian oscillator in the digestive system? Genes Brain Behav 2003; 2: 32 39. [DOI] [PubMed] [Google Scholar]
- Pezuk P, Mohawk JA, Yoshikawa T, Sellix MT, Menaker M. Circadian organization is governed by extra-SCN pacemakers. J Biol Rhythms 2010; 25: 432 441. [DOI] [PubMed] [Google Scholar]
- Pezuk P, Mohawk JA, Wang LA, Menaker M. Glucocorticoids as entraining signals for peripheral circadian oscillators. Endocrinology 2012; 153: 4775 4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J Neurosci 2002; 22: 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW, Swann J, Earnest DJ. Role of the circadian system in reproductive phenomena. Recent Prog Horm Res 1984; 40: 143. [DOI] [PubMed] [Google Scholar]
- Axelson JF, Gerall AA, Albers HE. Effect of progesterone on the estrous activity cycle of the rat. Physiol Behav 1981; 26: 631. [DOI] [PubMed] [Google Scholar]
- Morofushi M, Shinohara K, Kimura F. Menstrual and circadian variations in time perception in healthy women and women with premenstrual syndrome. Neurosci Res 2001; 41: 339. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Uchiyama M, Okawa M, Saito K, Kawaguchi M, Funabashi T, Kimura F. Menstrual changes in sleep, rectal temperature and melatonin rhythms in a subject with premenstrual syndrome. Neurosci Lett 2000; 281: 159 162. [DOI] [PubMed] [Google Scholar]
- He PJ, Hirata M, Yamauchi N, Hattori MA. Up-regulation of Per1 expression by estradiol and progesterone in the rat uterus. J Endocrinol 2007; 194: 511 519. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence for the colocalization of estrogen receptor-β mRNA and estrogen receptor-α immunoreactivity in neurons of the rat forebrain. Endocrinology 1998; 139: 5267. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Wade GN. Progestin binding by brain and pituitary cell nuclei and female rat sexual behavior. Brain Res 1978; 140: 360. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, King JC, Toft DO, Turcotte J. Immunocytochemical localization of estrogen-induced progestin receptors in guinea pig brain. Brain Res 1988; 474: 1. [DOI] [PubMed] [Google Scholar]
- Leak RK, Moore RY. Topographic organization of suprachiasmatic nucleus projection neurons. J Comp Neurol 2001; 433: 312. [DOI] [PubMed] [Google Scholar]
- Saeb-Parsy K, Lombardelli S, Khan FZ, McDowall K, Au-Yong IT, Dyball RE. Neural connections of hypothalamic neuroendocrine nuclei in the rat. J Neuroendocrinol 2000; 12: 635. [DOI] [PubMed] [Google Scholar]
- Foryst-Ludwig A, Kintscher U. Metabolic impact of estrogen signalling through ERalpha and ERbeta. J Steroid Biochem Mol Biol 2010; 122: 74 81. [DOI] [PubMed] [Google Scholar]
- Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev 2004; 5: 197 216. [DOI] [PubMed] [Google Scholar]
- Hirao J, Nishimura M, Arakawa S, Niino N, Mori K, Furukawa T, Sanbuissho A, Manabe S, Nishihara M, Mori Y. Sex and circadian modulatory effects on rat liver as assessed by transcriptome analyses. J Toxicol Sci 2011; 36: 9 22. [DOI] [PubMed] [Google Scholar]
- Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil 1968; 17: 555 557. [DOI] [PubMed] [Google Scholar]
- Gras S, Georg B, Jorgensen HL, Fahrenkrug J. Expression of the clock genes Per1 and Bmal1 during follicle development in the rat ovary. Effects of gonadotropin stimulation and hypophysectomy. Cell Tissue Res 2012; 350: 539 548. [DOI] [PubMed] [Google Scholar]
- Karman BN, Tischkau SA. Circadian clock gene expression in the ovary: effects of luteinizing hormone. Biol Reprod 2006; 75: 624 632. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhao L, Kumazawa M, Yamauchi N, Shigeyoshi Y, Hashimoto S, Hattori MA. Down-regulation of core clock gene Bmal1 attenuates expression of progesterone and prostaglandin biosynthesis-related genes in rat luteinizing granulosa cells. Am J Physiol Cell Physiol 2013; 304: C1131 C1140. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Funabashi T, Mitushima D, Kimura F. Effects of estrogen on the expression of connexin32 and connexin43 mRNAs in the suprachiasmatic nucleus of female rats. Neurosci Lett 2000; 286: 107. [DOI] [PubMed] [Google Scholar]
- Okada A, Sato T, Ohta Y, Iguchi T. Sex steroid hormone receptors in the developing female reproductive tract of laboratory rodents. J Toxicol Sci 2005; 30: 75 89. [DOI] [PubMed] [Google Scholar]
- Sellix MT, Menaker M. Circadian clocks in mammalian reproductive physiology: effects of the “other” biological clock on fertility. Discov Med 2011; 11: 273 281. [PubMed] [Google Scholar]
- Boden MJ, Kennaway DJ. Circadian rhythms and reproduction. Reproduction 2006; 132: 379 392. [DOI] [PubMed] [Google Scholar]
- Resuehr HE, Resuehr D, Olcese J. Induction of mPer1 expression by GnRH in pituitary gonadotrope cells involves EGR-1. Mol Cell Endocrinol 2009; 311: 120 125. [DOI] [PubMed] [Google Scholar]
- Resuehr D, Wildemann U, Sikes H, Olcese J. E-box regulation of gonadotropin-releasing hormone (GnRH) receptor expression in immortalized gonadotrope cells. Mol Cell Endocrinol 2007; 278: 36 43. [DOI] [PubMed] [Google Scholar]
- Olcese J, Sikes HE, Resuehr D. Induction of PER1 mRNA expression in immortalized gonadotropes by gonadotropin-releasing hormone (GnRH): involvement of protein kinase C and MAP kinase signaling. Chronobiol Int 2006; 23: 143 150. [DOI] [PubMed] [Google Scholar]
- Kato H, Naito K, Katsu Y, Watanabe H, Ohta Y, Iguchi T. Ontogenic expression of estrogen receptor-alpha in female rat corneas. Ophthalmic Res 2006; 38: 361 365. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kinoshita Y, Tachibana M, Matsushima Y, Kobayashi Y, Adachi W, Sotozono C, Kinoshita S. Expression of sex steroid hormone receptors in human cornea. Curr Eye Res 2001; 22: 28 33. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Kasukabe T, Kobayashi Y, Suzuki T, Kinoshita S, Matsushima Y. Expression of estrogen receptor alpha and beta in the mouse cornea. Invest Ophthalmol Vis Sci 2000; 41: 668 670. [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. J Neuroendocrinol 2004; 16: 989 998. [DOI] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Bate E, Lightman SL, Ingram CD, Jessop DS, Harbuz MS. Gonadectomy reverses the sexually diergic patterns of circadian and stress-induced hypothalamic-pituitary-adrenal axis activity in male and female rats. J Neuroendocrinol 2004; 16: 516 524. [DOI] [PubMed] [Google Scholar]
- Burger HG. The endocrinology of the menopause. J Steroid Biochem Mol Biol 1999; 69: 31 35. [DOI] [PubMed] [Google Scholar]
- Davies KM, Heaney RP, Recker RR, Barger-Lux MJ, Lappe JM. Hormones, weight change and menopause. Int J Obes 2001; 25: 874. [DOI] [PubMed] [Google Scholar]
- Kim C. Does menopause increase diabetes risk? Strategies for diabetes prevention in midlife women. Women's Health 2012; 8: 155 167. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kondo K. Chronic kidney disease in postmenopausal women. Hypertens Res 2012; 35: 142 147. [DOI] [PubMed] [Google Scholar]
- Mahoney MM. Shift work, jet lag, and female reproduction. Int J Endocrinol 2010; 2010: 813764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


