ABSTRACT
In recent years, the study of mammalian acrosomal exocytosis has produced some major advances that challenge the long-held, general paradigms in the field. Principally, the idea that sperm must be acrosome-intact to bind to the zona pellucida of unfertilized eggs, based largely on in vitro fertilization studies of mouse oocytes denuded of the cumulus oophorus, has been overturned by experiments using state-of-the-art imaging of cumulus-intact oocytes and fertilization experiments where eggs were reinseminated by acrosome-reacted sperm recovered from the perivitelline space of zygotes. In light of these results, this minireview highlights a number of unresolved questions and emphasizes the fact that there is still much work to be done in this exciting field. Future experiments using recently advanced technologies should lead to a more complete and accurate understanding of the molecular mechanisms governing the fertilization process in mammals.
Keywords: acrosomal exocytosis, capacitation, fertilization, sperm
Unresolved questions concerning acrosomal exocytosis are highlighted; advanced technologies are anticipated to lead to a more complete understanding of the molecular mechanisms governing the fertilization process in mammals.
INTRODUCTION
The mammalian sperm is a highly polarized cell, consisting fundamentally of a head (containing the male's genetic material in the condensed nucleus) and a flagellum that provides the motive force to bring the sperm to the egg surface [1]. On the apical surface of the head is a secretory vesicle called the acrosome. Over the last several decades, acrosomal exocytosis (also called the “acrosome reaction”) has been recognized as playing an essential role in fertilization, but the nature of acrosomal function is still unclear. (Use of the terms “acrosome reaction” and “acrosomal exocytosis” can be confusing. For some time now, the Gerton laboratory has adapted the use of the term “acrosomal exocytosis” to refer to the composite set of events of the acrosomal secretory process, from membrane docking to fusion to release of soluble acrosomal materials to the dissolution and release of the particulate acrosomal matrix materials. For years, the term “acrosome reaction” paradigm was used to describe the acrosome as being in one of two “binary” states: “acrosome-intact” or “acrosome-reacted.” In contrast, we and others articulated the fact that acrosomal secretion is a continuous variable, i.e., an “analog” process [which we prefer to call “acrosomal exocytosis”] that involves intermediate steps that have functional consequences [2].)
This review highlights some of the unresolved questions surrounding acrosomal exocytosis. However, before we address the enigmas, let us briefly summarize features of the acrosome.
THE ACROSOME, A UNIQUE SECRETORY STRUCTURE
The acrosome of a mature sperm can be defined in terms of several structural compartments. Enclosing the lumen of the intact acrosomal vesicle is a continuous membrane generally recognized as having two zones, the outer acrosomal membrane (OAM) and the inner acrosomal membrane (IAM). Essentially, the OAM closely associates with the plasma membrane overlying the acrosome, and the IAM is laminated to the nuclear membrane [1]. The acrosomal lumen contains soluble components and a particulate structure called the acrosomal matrix [3–7]. Morphological subcompartments within the acrosomal matrix can be distinguished as regions of differing density by electron microscopy or areas containing different acrosomal antigens as detected by immunoelectron microscopy [4, 6, 8]. During acrosomal exocytosis, the OAM and plasma membrane fuse to form hybrid membrane vesicles that are shed from the sperm, revealing the IAM as a new domain of the plasma membrane. Upon fusion of the OAM with the plasma membrane, the soluble components of the acrosome are free to diffuse away while the particulate acrosomal matrix remains exposed on the sperm surface until the matrix breaks down and its components are released [4, 5, 9–12].
Although acrosomal exocytosis has many molecular mechanisms in common with other secretory processes, there are some features that make this event a special type of regulated secretion: a) a single exocytotic granule per cell is involved; b) there is no membrane recycling; and c) rather than a single vesicle–plasma membrane union point, fusion between the outer acrosomal and the plasma membranes occurs at multiple sites to destabilize the structure of the acrosome [13].
The acrosome is critical for mammalian fertilization. Sperm must have properly formed acrosomes to be functional in the fertilization process; men or mice carrying mutations affecting the formation or function of the sperm acrosome are infertile or display severe subfertility [14–17]. Mammalian sperm must undergo acrosomal exocytosis to penetrate the zona pellucida (ZP), the extracellular matrix surrounding the egg [18]. Following exocytosis, the posterior region of the acrosome, known as the equatorial segment, engages in membrane fusion with the oocyte [19–21]. A critical protein for sperm-egg fusion is IZUMO1, which is localized on the acrosomal membrane. As a consequence of acrosomal exocytosis, this protein relocalizes to the surface of the equatorial region, and sperm become fusion-competent [22–24].
CHALLENGES TO STUDYING ACROSOMAL EXOCYTOSIS
The study of acrosomal exocytosis in mammalian sperm cells is not straightforward. Sperm in any given population exhibit highly variable physiological properties from cell to cell. Sperm from the epididymis or an ejaculate represent an asynchronous, stochastic sample of cells that mature at differing rates and times to ensure that there is a subpopulation of sperm capable of fertilization at any given time. Keeping in mind that only one sperm is needed to fertilize an egg and that the window of time when any given sperm is competent for fertilization is probably narrow, it appears that nature has evolved a mechanism to stagger the times that an individual sperm becomes capable of performing its ultimate task, thereby increasing the chances that at least one of millions of cells in an ejaculate will be ready and able when an egg is available for fertilization.
Another challenge in these investigations is that acrosomal exocytosis occurs at the level of a single cell, yet many studies of the process attempt to draw conclusions from population-based studies. The fact that the cells being studied are not truly synchronous may lead to misleading interpretations. It is also arduous to determine the acrosomal status of a given sperm at the time of interaction with the ZP of the egg. It is complicated to visualize the interaction of sperm with egg in a truly physiological setting (i.e., the female reproductive tract) because the site of fertilization is within the ampulla of the oviduct and the sperm may need to traverse the cumulus cells surrounding the egg prior to reaching the ZP. Finally, sperm are very small cells, and it is very problematic to resolve the acrosome visually without resorting to engineered cells expressing microscopically detectable labels such as green fluorescent protein (GFP) [25]. This is especially difficult if one is trying to visualize the sperm within the cumulus mass or the oviduct.
As a consequence of these experimental restrictions in mammals, the biochemical mechanisms of acrosomal exocytosis have generally been studied in sperm removed from their natural environment, that is, the male and female reproductive tracts. For decades, studies of mammalian sperm acrosomal exocytosis have been patterned after fertilization experiments using gametes from external fertilizers like invertebrates (sea urchin) or certain nonmammalian vertebrates (frog). Absent the environments of the uterus and oviduct, researchers have been using an approach whereby the complex process of mammalian fertilization has been reduced to the interactions of their basic parts, free-swimming sperm (isolated from the cauda epididymis or from an ejaculate) and eggs recovered from ovarian follicles. In most cases, the cumulus cells surrounding ovarian or ovulated oocytes have been removed to eliminate one more potentially confounding factor. This experimental paradigm also eliminates any natural contributions of the oviduct, including factors that may support sperm capacitation of oocyte receptiveness.
Using these external fertilization research models as the patterns for investigations, some researchers have concluded that sperm must bind to the mammalian egg investments before they undergo acrosomal exocytosis [26]. In the absence of the cumulus cells, the only investment is the ZP, leading to the concept that the ZP contains a molecule(s) that acts as an “inducer” or “stimulator” of acrosomal exocytosis. It is also important to distinguish between the use of particulate ZPs and solubilized ZP proteins. In particular, in the light of recent papers, it is possible that these two chemical states of the ZP have different effects on the process of acrosomal exocytosis in vitro [27].
LIFE AFTER TESTIS
The brevity of this review precludes a detailed discussion of the extracellular conditions and developmental properties that sperm experience prior to ejaculation (e.g., acrosomal biogenesis, maturation of the acrosome, and epididymal maturation). Upon coitus, sperm experience major changes in their environment as they are released from the cauda epididymis and vas deferens and are mixed with secretions from the seminal vesicles, prostate, and bulbourethral glands while passing through the remaining conduits of the male reproductive tract and into the vagina of the female. From there, the sperm must move through the cervix and into the uterus. In most, if not all, mammalian species, the sperm temporarily halt in the oviduct near the transition zone between the uterus and oviduct, known as the uterotubal junction (UTJ) [28].
The sperm remain in the oviductal reservoir until they are released in a gradual manner and make their way up the oviduct. In the ampulla, the sperm encounter unfertilized eggs being transported down toward the uterus. It is there that fertilization takes place. It also should be emphasized that the sperm-to-egg ratio in the ampulla is generally accepted as being very low, possibly in the single digits [29, 30].
CAPACITATION OCCURS IN THE FEMALE REPRODUCTIVE TRACT
A key feature of mammalian reproduction is capacitation, the maturational step whereby sperm gain the ability to undergo acrosomal exocytosis and become capable of fertilizing eggs [31, 32]. In natural procreation, capacitation occurs while the spermatozoa reside in the female reproductive tract. As shown in a classic study by Visconti et al. [33], requisite features of capacitation include activation of the sperm adenylyl cyclase by bicarbonate and calcium, which are secreted by the female reproductive tract, and the efflux of cholesterol from the sperm plasma membrane to uncharacterized acceptors in the female reproductive tract. Mouse sperm capacitation is accompanied by a time-dependent increase in protein tyrosine phosphorylation that is dependent on the presence of bovine serum albumin (BSA), Ca2+, and NaHCO3, all three of which are also required for this maturational event. In addition, the capacitation-associated increase in protein tyrosine phosphorylation is downstream of the activation of protein kinase A (PK-A). BSA participates in the removal of cholesterol from the sperm plasma membrane, a process that is hypothesized to modulate capacitation. For example, when mouse sperm are incubated in a medium containing BSA, cholesterol is lost from the sperm plasma membrane into the medium; release of this sterol does not occur in medium devoid of BSA or another cholesterol acceptor such as cyclodextrin. Cholesterol release then leads to changes in protein tyrosine phosphorylation. When the action of BSA is inhibited by adding cholesterol-SO4 to the BSA-containing medium, the increase in protein tyrosine phosphorylation as well as capacitation are blocked, presumably by competition for cholesterol-binding sites on the BSA. Addition of increasing concentrations of BSA at a given concentration of cholesterol-SO4 and addition of dibutyryl cAMP plus 3-isobutyl-1-methylxanthine (IBMX) can overcome this inhibition. Another cholesterol-binding protein, high-density lipoprotein (HDL), also fulfills the BSA role in protein tyrosine phosphorylation increase associated with capacitation through a cAMP-dependent pathway. However, proteins that do not interact with cholesterol have no effect. HDL also supports sperm capacitation, as assessed by fertilization in vitro. Furthermore, bicarbonate is necessary for the capacitation-associated increase in protein tyrosine phosphorylation and is downstream of the action of BSA. Overall then, cholesterol release correlates with a transmembrane signal transduction pathway involving PK-A and protein tyrosine phosphorylation, leading to functional maturation of the sperm. Unresolved issues include:
How does sperm cholesterol efflux promote fertilization?
What is the mechanism whereby the loss of cholesterol facilitates acrosomal exocytosis?
Recently, it was demonstrated that phospholipase B is activated in response to sterol removal and stimulates acrosome exocytosis in murine sperm [34]. The authors proposed that this mechanism provides a basic model for exocytosis in which membrane fusions occur during capacitation/transit through the cumulus prior to any physical contact between the sperm and the oocyte's ZP. Other studies have shown that removing glycosylphosphatidylinositol (GPI)-anchored proteins from porcine sperm causes a reorganization of lipid microdomains on the cell surface [35]. Moreover, the authors reported that when they removed the GPI-anchored plasma membrane proteins with phospholipase C, they observed acrosomal swelling. Clearly, the link between these two phenomena, lipid microdomain reorganization and acrosomal swelling during capacitation, needs further investigation.
During capacitation, there are remarkable changes in ion homeostasis, such as increase in pH and K+ permeability, elevation of Ca2+, and decrease in the intracellular levels of Na+ observed in mammalian sperm [36], that lead to a sperm plasma membrane potential hyperpolarization in mouse, rabbit, bovine, and horse [36–38]. Capacitation-associated hyperpolarization has been related to the capacity of sperm to generate a transient Ca2+ elevation during acrosomal exocytosis [39]. Recently, de la Vega et al. [40] made the important discovery that hyperpolarization of the mouse sperm membrane potential is necessary and sufficient to prepare sperm for acrosomal exocytosis. To demonstrate this, sperm were incubated under conditions that did not support capacitation. The authors found that the cAMP/PK-A pathway activation and the concomitant increase in tyrosine phosphorylation did not occur when cells were hyperpolarized by drugs. In this situation, sperm acquired the ability to undergo acrosomal exocytosis in response to calcium ionophore, solubilized ZP, or a K+-induced depolarization. A persistent question is:
What is the molecular mechanism through which a change in the membrane potential is transduced into a gain of the ability to undergo exocytosis?
The plasma membrane and the outer acrosomal membrane are primed to fuse during capacitation. Recently, Zanetti and Mayorga [41] made striking observations of membrane attachments at the edge of outer acrosomal membrane invaginations present in swollen acrosomes. They discovered that during capacitation, the acrosomal swelling caused changes in distance between the OAM and the plasma membrane (PM), and that the probable contact between these two membranes could establish the docking or formation of fusion pores. Fundamental questions remaining to be answered are:
How does capacitation trigger the priming of the acrosome for exocytosis?
How do the OAM and PM go from being nonfusogenic to fusogenic?
THE OVIDUCTAL ENVIRONMENT
With the advent of human in vitro fertilization in 1978, interest in understanding the physiology of the fallopian tube waned because dysfunction or obstruction of the oviducts could be circumvented by this artificial reproductive technology. However, as various proteins thought to be involved in the later phases of sperm–egg interactions were examined using gene knockout technologies in mice, it became apparent that when genes encoding several of the proteins thought to be involved in the latter steps of fertilization were disrupted, the defects manifested themselves earlier in the female tract. For example, sperm from male mice lacking CLGN [42], ACE [43], ADAM1A, ADAM2 [44], or ADAM3 [45] were motile but could not pass through the UTJ. ADAM3 is commonly absent from or mislocated in the plasma membrane in all five lines of mutant mice, suggesting a key role in this process [29, 46]. Thus, the proteins encoded by these genes appear to bestow the capacity for sperm to migrate from the UTJ or be released from the oviductal reservoir into the oviduct ampulla [29], leading one to ponder:
How is the migration of the sperm into the ampulla regulated?
On the other hand, it is very significant that, in the context of whole animal studies, the sperm do not have the ability to bind to the ZP in vitro. This raises another question:
Is the acrosome involved?
Overall, the female reproductive tract possesses two basic features that promote and regulate sperm function. The lumenal environment includes the fluids and mucus that sperm must penetrate. The fluid is a rich source of bicarbonate, which promotes sperm capacitation, and the mucus provides a viscous environment the sperm must penetrate in their journey to the egg. Sperm encounter various female reproductive tract epithelial cells from the vagina to the ampulla of the oviduct. Perhaps the epithelium of the UTJ or early oviduct is most critical as this is the region where the sperm reside for a period of time prior to reinitiating movement up the oviduct to encounter the egg. The interactions occurring at the surfaces of the UTJ and sperm have still to be completely elucidated, as follows, but may be tied into capacitation:
Other than the requirements for oviductal calcium and bicarbonate for capacitation, do additional agents exist in the female reproductive tract that promote acrosomal exocytosis?
THE SITE OF ACROSOMAL EXOCYTOSIS
A key issue regarding the acrosome is:
Where does acrosomal exocytosis take place in mammalian fertilization?
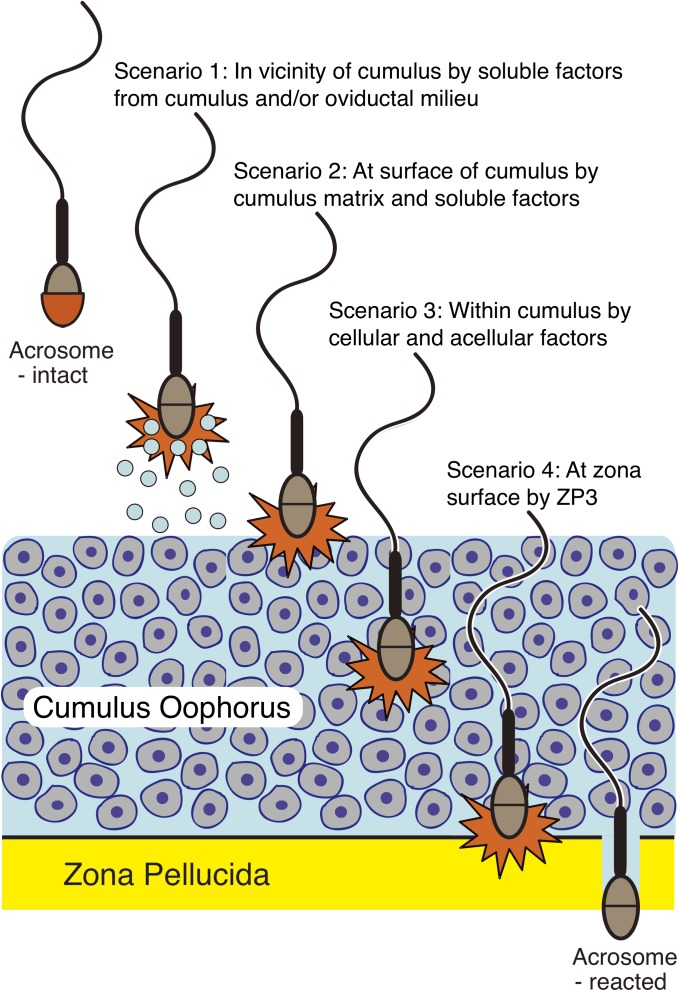
For over 30 years (and largely based upon laboratory experiments using in vitro fertilization of oocytes denuded of their cumulus cells), one of the central dogmas of the fertilization process in mammals has been that capacitated, acrosome-intact sperm bind to the ZP and then undergo acrosomal exocytosis [26]. Penetration of the ZP would then occur following acrosomal exocytosis and the release of zona-hydrolyzing enzymes [47]. The concept has been that, following acquisition of hyperactivated motility and the liberation of acrosomal contents, the fertilizing sperm gains the ability to penetrate the ZP and reach the perivitelline space between the ZP and the oolemma (Fig. 1, scenario 4). Baibakov et al. [27] claimed that sperm binding to ZP is not sufficient to induce acrosomal exocytosis. They and others observed that when transgenic mouse sperm carrying GFP in their acrosomes became bound to ZPs of unfertilized eggs expressing wild-type mouse zonae or human ZP2 instead of murine protein, the sperm retained GFP for extended periods of time, and they interpreted this to mean that sperm binding to ZP was not sufficient to induce acrosomal exocytosis [27, 48]. As a consequence of this and other elegant biochemical and mutant mouse studies, Baibakov et al. [27] challenged the prevalent model of sperm binding to a carbohydrate ligand of the ZP from an unfertilized egg. Interpretations from these studies were in direct contrast to a model favored by other studies in which, after fertilization, a ZP carbohydrate ligand for sperm was removed with a glycosidase released from the egg cortical granules by exocytosis, thereby precluding sperm from binding to zona from fertilized eggs or early embryos [49, 50]. Instead, Baibakov et al. [27] proposed a mechanosensory mechanism that involved 1) the binding of acrosome-intact sperm to the zona surface followed by 2) the loss of the acrosome as the sperm penetrate the ZP.
FIG. 1.
Where do sperm undergo acrosomal exocytosis? Scenarios 1 to 4 illustrate where sperm may undergo acrosomal exocytosis while traveling to the site of fertilization. The combination of existing and new, powerful live imaging techniques should help to solve this problem in the near future.
However, other studies challenged the need for sperm to be acrosome-intact when binding to the zona. Previous observations in rabbit demonstrated that acrosomes were absent from spermatozoa recovered from the perivitelline space but that these acrosome-reacted sperm were still capable of penetrating the zonae surrounding another set of unfertilized eggs [51]. In mouse, Jin et al. [25] recently made very provocative observations, using their imaging studies of spermatozoa fertilizing cumulus-enclosed oocytes. By very carefully imaging the loss of GFP from the acrosomes of fluorescent sperm, these researchers demonstrated that fertilizing mouse spermatozoa initiated acrosome exocytosis prior to contacting the ZP of cumulus-enclosed oocytes (Fig. 1, scenario 3). They did not demonstrate that exocytosis took place in the cumulus mass. Others have claimed that cumulus cell mass may be the site of acrosomal exocytosis, a concept supported by recent studies showing that syndecan-1 is present on cumulus cells and possesses the ability to induce exocytosis in vivo [52]. Inoue et al. [53] later supported these results by using sperm from mice with a targeted mutation in the gene encoding IZUMO1, a protein required for sperm binding and fusion with the oolemma [54]. These mutant sperm penetrated the ZP but accumulated in the perivitelline space from where they could be recovered following in vitro fertilization. The retrieved sperm had all undergone acrosomal exocytosis (as evident by the loss of the acrosomal GFP marker). When used to inseminate a second set of unfertilized eggs, the recovered, acrosome-reacted perivitelline sperm were again able to bind and penetrate the zonae of a second set of unfertilized eggs. There are many unresolved questions raised by these studies:
If sperm do not initiate acrosomal exocytosis upon contact with the ZP, where and when does this event take place under physiologically normal circumstances?
Does acrosomal exocytosis occur in response to ligand binding of a receptor or is there another mechanism, such as an internal physiological change, that reaches a threshold state and propels the process forward?
What is (are) the signal(s) that triggers exocytosis?
Is the process conserved among all mammalian species?
Do cumulus cells influence the process of acrosomal exocytosis?
Are there agents in the female reproductive tract that promote acrosomal exocytosis?
As sperm travel up the oviduct, they come closer to the ovary and ovulated oocytes with their associated cumulus cells, which secrete progesterone. This steroid hormone has gained attention as a possible physiological inducer of exocytosis as well as a chemoattractant for sperm [55, 56]. Progesterone interacts and activates CATSPER channels in the tail of human sperm [57, 58], opening the possibility that progesterone modulates calcium levels in the sperm and, thus, may indirectly stimulate exocytosis via these channels. However, the authors limited their assignment of the action of CATSPER to the function of the human sperm flagellum and did not draw a parallel with acrosomal exocytosis. Ren and Xia [59] recently demonstrated that mouse sperm treated with solubilized ZP proteins exhibited an influx of CATSPER-dependent calcium that progressed from the distal regions of the sperm tail toward the head. In a similar manner, mouse sperm containing GFP in their acrosomes lost the fluorescent probe when the sperm were treated with solubilized ZP proteins, in an anterograde manner from the posterior margin of the acrosome toward the anterior limit [60], raising the possibility that a calcium wave originating in the sperm tail may move forward through the sperm to elicit acrosomal exocytosis. In the case of humans, another possibility is that progesterone induces acrosomal exocytosis via its interaction with progesterone receptors located in the flagellar midpiece-neck region of the sperm [61]. What it is still not understood is:
What is the detailed spatiotemporal relationship between calcium changes in the flagellum and progesterone and/or other agonist effects on acrosomal exocytosis?
Because the acrosomal status of sperm being examined by patch-clamping and other procedures is not always known, another important question is:
How do the electrophysiological properties of sperm differ before and after acrosomal exocytosis?
Without knowing the exact locations where acrosomal exocytosis occurs during the course of normal fertilization (Fig. 1), a role for the ZP in stimulating or inducing this sperm secretory event cannot be discarded. During the biogenesis of the ZP within the ovarian follicle, it is likely that ZP proteins diffuse into the extracellular matrix of the cumulus cells surrounding the oocyte, either by not being incorporated into the particulate zona during assembly or by the slight degradation of ZP proteins after insertion into the zona. Further experimentation is necessary to investigate this possibility.
EMERGING THEMES IN ACROSOMAL EXOCYTOSIS
Following the tethering/docking of the OAM and PM, the fusion machinery needs to be assembled for exocytosis to occur. Different research groups have identified proteins in sperm that participate in exocytosis in somatic cells. The list includes Rab3A [62], the SNARE family [63], α-SNAP [64], NSF [65], complexin [66, 67], the calcium-binding protein synaptotagmin [68, 69], and calmodulin [70]. Other proteins that participate in exocytosis, such as dynamin, have also been identified [71]. Key components of the fusion machinery are the members of the SNARE family [63]. SNAREs are membrane-associated proteins that form stable hetero-oligomeric complexes consisting of a bundle of four parallel helices. When the plasma membrane and OAM are tethered, assisted by several factors including the participation of the cytoskeleton, SNAREs form trans complexes that bring the two bilayers in close proximity. SNAREs are classified as R or Q based on the identity of a highly conserved residue. Depending on combinations of SNAREs, they can efficiently assemble in complexes. A large set of proteins interacts with individual SNAREs (or with the assembled complex) and participates in the normal fusion mechanism [13].
The success of acrosomal exocytosis relies on the coordinated interaction of participating signaling molecules and the multi-PDZ domain protein MUPP1, a membrane raft-associated molecular organizer previously identified in a spatially organized, sequential signaling pathway mammalian spermatozoa [72]. MUPP1 controls the initial tethering and docking of the acrosomal vesicle, whereas syntaxin-2, a SNARE protein also expressed in the acrosomal cap of mammalian spermatozoa, appears to take part in the final process of acrosomal fusion. After the opening of the store-operated channels, the intracellular calcium increases and promotes the production of cAMP [73]. The increase of cAMP, communicated through RAPGEF (EPAC) activation, has the ability to drive the whole cascade of events necessary to bring exocytosis to completion. However, the final steps of exocytosis are triggered by the release of calcium from intracellular stores through IP3-sensitive calcium channels [74]. Other investigators have shown that several ZP-binding molecules are also present in sperm lipid rafts, suggesting that sperm lipid rafts may be the platforms on the sperm plasma membrane for ZP interaction and signaling associated with acrosomal exocytosis [75]. Curiously, several ZP-binding proteins have been altered or deleted by gene-targeting techniques in mouse, yet the sperm from these mutant mice are still fertile [76–79].
INTERMEDIATE STAGES OF EXOCYTOSIS
Over the years, investigators have provided evidence that acrosomal exocytosis is not an all-or-none event. In other words, between the extremes of fully “acrosome-intact” and “acrosome-reacted,” sperm representing intermediate stages of acrosomal exocytosis are formed. Consistent with the idea of intermediate stages of exocytosis, previous reports have defined four basic patterns of sperm staining with chlortetracycline dye [80]. Noncapacitated mouse sperm show the N pattern of staining. Capacitated, acrosome-intact mouse spermatozoa display a banded (B) pattern, progress to an intermediate spotty (S) pattern, and then proceed to the fully acrosome-reacted (AR) pattern (the N→B→S→AR transition). In later studies, transmission electron microscopy of human sperm identified six stages of acrosomal exocytosis, some of which involve the decondensation of the acrosomal matrix material while the OAM and PM appear to retain their integrity [81, 82]. More recently, multiple groups have shown that morphological and biochemical steps of hybrid vesicle formation occur prior to complete exocytosis. They have reported that during capacitation, acrosomal swelling causes changes in the distance between the OAM and the PM and that the probable contact between these two membranes could establish the docking or formation of the fusion pores [41, 83, 84]. In mouse and guinea pig sperm, there is a temporal relationship among the four stages of acrosomal exocytosis that represents successive transitional stages leading to the complete release of acrosomal components [9–11, 85]. These studies showed that, as capacitation progresses and spontaneous acrosomal exocytosis ensues, the exposure and release of soluble and acrosomal matrix proteins are not synchronous. Several questions are unresolved concerning this multistep process:
How is the creation of these intermediates of acrosomal exocytosis managed?
How is the differential release of the acrosomal components regulated?
What role does the differential release of the acrosomal components play in fertilization?
THE FUTURE AND CONCLUDING REMARKS
Understanding the role of the acrosome in fertilization is dependent on two overriding concerns. The previous paragraph hinted at these issues. The first is
What are the roles of the acrosomal components themselves?
There are various enzymatic activities present in the acrosomal lumen, but the roles, if any, of these components are unknown. For many years, it was believed that proteolytic digestion of the ZP was facilitated by the trypsin-like protease acrosin. However, knockout of the acrosin gene does not affect fertility, although it may provide a competitive advantage to wild-type relative to acrosin-null mouse sperm by promoting dispersion of the acrosomal matrix [77, 86, 87]. Additional studies must be performed to determine the function and fate of other acrosomal components. As shown from the acrosin knockout studies, a given protein may not be essential for fertilization, but it may endow sperm with a competitive advantage over those lacking a given acrosomal component. In fact, another way to state this question is:
"How do sperm penetrate the zona matrix?"
Acrosin is clearly not the requisite zona lysin [77, 86]:
Does another acrosomal hydrolase digest a path for the fertilizing sperm or does that cell pass through the zona by a mechanical mechanism?
The second area of interest is the mechanism of acrosomal exocytosis. For example, it has been known for almost 4 decades that calcium ionophores induce acrosomal exocytosis in mammalian spermatozoa [88]. However, these reagents still remain powerful tools for dissecting the chain of events leading to acrosomal exocytosis. For example, in a recent investigation by Tateno et al. [89], mouse spermatozoa treated with the calcium ionophore A23187 were found to fertilize eggs without activation of the cAMP/PK-A signaling pathway. From these experiments, the investigators concluded that the cAMP/PK-A pathway is upstream of the increase in intracellular calcium required for acrosomal exocytosis and hyperactivation of spermatozoa under normal in vitro conditions. Under these conditions, most sperm lose their acrosomes and are able to fertilize oocytes, which is consistent with the previous observations suggesting that acrosome-reacted sperm are able to penetrate and fertilize oocytes [25, 53]. An important question in this matter is:
What are the molecular mechanisms or targets that are activated downstream from the massive calcium influx and are essential for acquiring fertilizing capacity?
As can be seen from this brief review, the study of acrosomal exocytosis is far from over, and there are many unanswered questions. In fact, we maintain that this is a time for renewed vigor in understanding how capacitation prepares the sperm for exocytosis and determining the mechanisms regulating the membrane fusion events and protein release process that are so important. Further insights concerning acrosomal exocytosis may uncover new ways to address infertility and may open up novel avenues for the development of contraceptive approaches. The unique properties of the acrosomal vesicle could also be exploited to study exocytosis in general, with the end result that this information could be applied to other secretory processes. With the application of recently advanced technologies, the coming years should provide some exciting and significant discoveries concerning the roles and mechanisms of acrosomal exocytosis.
Supplementary Material
Footnotes
Supported by U.S. National Institutes of Health grants R01HD051999, R01HD057144, and P30ES013508 to G.L.G. and NIH grant R01TW008662 and Agencia Nacional de Promoción Cientifica y Tecnológica grant PICT 2012-1175 to M.G.B.
REFERENCES
- Eddy EM. The Spermatozoon. : Knobil E. (ed.), Knobil and Neill's Physiology of Reproduction. Boston: Elsevier; 2006; 3–54. [Google Scholar]
- Gerton GL. Function of the sperm acrosome : Hardy DM. (ed.), Fertilization. San Diego: Academic Press; 2002; 265–302. [Google Scholar]
- Buffone MG, Foster JA, Gerton GL. The role of the acrosomal matrix in fertilization. Int J Dev Biol 2008; 52 (5–6): 511–522. [DOI] [PubMed] [Google Scholar]
- Foster JA, Friday BB, Maulit MT, Blobel C, Winfrey VP, Olson GE, Kim KS, Gerton GL. AM67, a secretory component of the guinea pig sperm acrosomal matrix, is related to mouse sperm protein sp56 and the complement component 4-binding proteins. J Biol Chem 1997; 272 (19): 12714–12722. [DOI] [PubMed] [Google Scholar]
- Kim KS, Cha MC, Gerton GL. Mouse sperm protein sp56 is a component of the acrosomal matrix. Biol Reprod 2001; 64 (1): 36–43. [DOI] [PubMed] [Google Scholar]
- Olson GE, Winfrey VP. Structure of acrosomal matrix domains of rabbit sperm. J Struct Biol 1994; 112 (1): 41–48. [DOI] [PubMed] [Google Scholar]
- Westbrook-Case VA, Winfrey VP, Olson GE. Sorting of the domain-specific acrosomal matrix protein AM50 during spermiogenesis in the guinea pig. Dev Biol 1995; 167 (1): 338–349. [DOI] [PubMed] [Google Scholar]
- Westbrook-Case VA, Winfrey VP, Olson GE. A domain-specific 50-kilodalton structural protein of the acrosomal matrix is processed and released during the acrosome reaction in the guinea pig. Biol Reprod 1994; 51 (1): 1–13. [DOI] [PubMed] [Google Scholar]
- Buffone MG, Kim K-S, Doak BJ, Rodriguez-Miranda E, Gerton GL. Functional consequences of cleavage, dissociation and exocytotic release of ZP3R, a C4BP-related protein, from the mouse sperm acrosomal matrix. J Cell Sci 2009; 122 (Pt 17): 3153–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-S, Gerton GL. Differential release of soluble and matrix components: evidence for intermediate states of secretion during spontaneous acrosomal exocytosis in mouse sperm. Dev Biol 2003; 264 (1): 141–152. [DOI] [PubMed] [Google Scholar]
- Kim K-S, Foster JA, Kvasnicka KW, Gerton GL. Transitional states of acrosomal exocytosis and proteolytic processing of the acrosomal matrix in guinea pig sperm. Mol Reprod Dev 2011; 78 (12): 930–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noland TD, Friday BB, Maulit MT, Gerton GL. The sperm acrosomal matrix contains a novel member of the pentaxin family of calcium-dependent binding proteins. J Biol Chem 1994; 269 (51): 32607–32614. [PubMed] [Google Scholar]
- Mayorga LS, Tomes CN, Belmonte SA. Acrosomal exocytosis, a special type of regulated secretion. IUBMB Life 2007; 59 (4–5): 286–292. [DOI] [PubMed] [Google Scholar]
- Dam AHDM, Feenstra I, Westphal JR, Ramos L. Golde RJT van, Kremer Ja M. Globozoospermia revisited. Hum Reprod Update 2007; 13 (1): 63–75. [DOI] [PubMed] [Google Scholar]
- Kang-Decker N, Mantchev GT, Juneja SC, McNiven MA, van Deursen JM. Lack of acrosome formation in Hrb-deficient mice. Science 2001; 294 (5546): 1531–1533. [DOI] [PubMed] [Google Scholar]
- Lin Y-N, Roy A, Yan W, Burns KH, Matzuk MM. Loss of zona pellucida binding proteins in the acrosomal matrix disrupts acrosome biogenesis and sperm morphogenesis. Mol Cell Biol 2007; 27 (19): 6794–6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotomayor RE, Handel MA. Failure of acrosome assembly in a male sterile mouse mutant. Biol Reprod 1986; 34 (1): 171–182. [DOI] [PubMed] [Google Scholar]
- Ikawa M, Wada I, Kominami K, Watanabe D, Toshimori K, Nishimune Y, Okabe M. The putative chaperone calmegin is required for sperm fertility. Nature 1997; 387 (6633): 607–611. [DOI] [PubMed] [Google Scholar]
- Moore HDM, Bedford JM. Ultrastructure of the equatorial segment of hamster spermatozoa during penetration of oocytes. J Ultrastruct Res 1978; 62 (2): 110–117. [DOI] [PubMed] [Google Scholar]
- Oura C, Toshimori K. Ultrastructural studies on the fertilization of mammalian gametes. Int Rev Cytol 1990; 122: 105–151. [DOI] [PubMed] [Google Scholar]
- Wassarman PM. The biology and chemistry of fertilization. Science 1987; 235 (4788): 553–560. [DOI] [PubMed] [Google Scholar]
- Satouh Y, Inoue N, Ikawa M, Okabe M. Visualization of the moment of mouse sperm–egg fusion and dynamic localization of IZUMO1. J Cell Sci 2012; 125 (21): 4985–4990. [DOI] [PubMed] [Google Scholar]
- Sosnik J, Miranda PV, Spiridonov NA, Yoon S-Y, Fissore RA, Johnson GR, Visconti PE. Tssk6 is required for Izumo relocalization and gamete fusion in the mouse. J Cell Sci 2009; 122 (15): 2741–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnik J, Buffone M, Visconti PE. Analysis of CAPZA3 localization reveals temporally discrete events during the acrosome reaction. J Cell Physiol 2010; 224 (3): 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci U S A 2011; 108 (12): 4892–4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saling PM, Sowinski J, Storey BT. An ultrastructural study of epididymal mouse spermatozoa binding to zonae pellucidae in vitro: sequential relationship to the acrosome reaction. J Exp Zool 1979; 209 (2): 229–238. [DOI] [PubMed] [Google Scholar]
- Baibakov B, Gauthier L, Talbot P, Rankin TL, Dean J. Sperm binding to the zona pellucida is not sufficient to induce acrosome exocytosis. Development 2007; 134 (5): 933–943. [DOI] [PubMed] [Google Scholar]
- Suarez SS. Regulation of sperm storage and movement in the mammalian oviduct. Int J Dev Biol 2008; 52 (5–6): 455–462. [DOI] [PubMed] [Google Scholar]
- Okabe M. The cell biology of mammalian fertilization. Development 2013; 140 (22): 4471–4479. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. Mammalian fertilization : Knobil E, Neill JD. (eds.), The Physiology of Reproduction. New York: Raven Press; 1994. [Google Scholar]
- Austin CR. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B 1951; 4 (4): 581–596. [DOI] [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 1951; 168 (4277): 697–698. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Ning X, Fornés MW, Alvarez JG, Stein P, Connors SA, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev Biol 1999; 214 (2): 429–443. [DOI] [PubMed] [Google Scholar]
- Asano A, Nelson-Harrington JL, Travis AJ. Phospholipase B is activated in response to sterol removal and stimulates acrosome exocytosis in murine sperm. J Biol Chem 2013; 288 (39): 28104–28115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerke A, van der Lit J, Lolicato F, Stout TAE, Helms JB, Gadella BM. Removal of GPI-anchored membrane proteins causes clustering of lipid microdomains in the apical head area of porcine sperm. Theriogenology 2014; 81 (4): 613–624. [DOI] [PubMed] [Google Scholar]
- Escoffier J, Krapf D, Navarrete F, Darszon A, Visconti PE. Flow cytometry analysis reveals a decrease in intracellular sodium during sperm capacitation. J Cell Sci 2012; 125 (Pt 2): 473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-González EO, Sosnik J, Edwards J, Acevedo JJ, Mendoza-Lujambio I, López-González I, Demarco I, Wertheimer E, Darszon A, Visconti PE. Sodium and epithelial sodium channels participate in the regulation of the capacitation-associated hyperpolarization in mouse sperm. J Biol Chem 2006; 281 (9): 5623–5633. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Clark EN, Florman HM. Sperm membrane potential: hyperpolarization during capacitation regulates zona pellucida-dependent acrosomal secretion. Dev Biol 1995; 171 (2): 554–563. [DOI] [PubMed] [Google Scholar]
- Arnoult C, Kazam IG, Visconti PE, Kopf GS, Villaz M, Florman HM. Control of the low voltage-activated calcium channel of mouse sperm by egg ZP3 and by membrane hyperpolarization during capacitation. Proc Natl Acad Sci U S A 1999; 96 (12): 6757–6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Vega-Beltran JL, Sánchez-Cárdenas C, Krapf D, Hernandez-González EO, Wertheimer E, Treviño CL, Visconti PE, Darszon A. Mouse sperm membrane potential hyperpolarization is necessary and sufficient to prepare sperm for the acrosome reaction. J Biol Chem 2012; 287 (53): 44384–44393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti N, Mayorga LS. Acrosomal swelling and membrane docking are required for hybrid vesicle formation during the human sperm acrosome reaction. Biol Reprod 2009; 81 (2): 396–405. [DOI] [PubMed] [Google Scholar]
- Ikawa M, Nakanishi T, Yamada S, Wada I, Kominami K, Tanaka H, Nozaki M, Nishimune Y, Okabe M. Calmegin is required for fertilin alpha/beta heterodimerization and sperm fertility. Dev Biol 2001; 240 (1): 254–261. [DOI] [PubMed] [Google Scholar]
- Inoue N, Kasahara T, Ikawa M, Okabe M. Identification and disruption of sperm-specific angiotensin converting enzyme-3 (ACE3) in mouse. Plos One 2010; 5 (4): e10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, Kim E, Nakanishi T, Baba T. Possible function of the ADAM1a/ADAM2 fertilin complex in the appearance of ADAM3 on the sperm surface. J Biol Chem 2004; 279 (33): 34957–34962. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R, Muro Y, Isotani A, Tokuhiro K, Takumi K, Adham I, Ikawa M, Okabe M. Disruption of ADAM3 impairs the migration of sperm into oviduct in mouse. Biol Reprod 2009; 81 (1): 142–146. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R, Yamagata K, Ikawa M, Moss SB, Okabe M. Aberrant distribution of ADAM3 in sperm from both angiotensin-converting enzyme (Ace)- and calmegin (Clgn)-deficient mice. Biol Reprod 2006; 75 (5): 760–766. [DOI] [PubMed] [Google Scholar]
- Gaddum P, Blandau RJ. Proteolytic reaction of mammalian spermatozoa on gelatin membranes. Science 1970; 170 (3959): 749–751. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Ikawa M, Yamada S, Parvinen M, Baba T, Nishimune Y, Okabe M. Real-time observation of acrosomal dispersal from mouse sperm using GFP as a marker protein. FEBS Lett 1999; 449 (2–3): 277–283. [DOI] [PubMed] [Google Scholar]
- Shur BD, Rodeheffer C, Ensslin MA, Lyng R, Raymond A. Identification of novel gamete receptors that mediate sperm adhesion to the egg coat. Mol Cell Endocrinol 2006; 250 (1–2): 137–148. [DOI] [PubMed] [Google Scholar]
- Wassarman PM. Contribution of mouse egg zona pellucida glycoproteins to gamete recognition during fertilization. J Cell Physiol 2005; 204 (2): 388–391. [DOI] [PubMed] [Google Scholar]
- Kuzan FB, Fleming AD, Seidel GE., Jr. Successful fertilization in vitro of fresh intact oocytes by perivitelline (acrosome-reacted) spermatozoa of the rabbit. Fertil Steril 1984; 41 (5): 766–770. [DOI] [PubMed] [Google Scholar]
- Joshi CS, Khan SA, Khole VV. Regulation of acrosome reaction by Liprin α3, LAR and its ligands in mouse spermatozoa. Andrology 2014; 2 (2): 165–174. [DOI] [PubMed] [Google Scholar]
- Inoue N, Satouh Y, Ikawa M, Okabe M, Yanagimachi R. Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc Natl Acad Sci U S A 2011; 108 (50): 20008–20011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 2005; 434 (7030): 234–238. [DOI] [PubMed] [Google Scholar]
- Jaiswal BS, Tur-Kaspa I, Dor J, Mashiach S, Eisenbach M. Human sperm chemotaxis: is progesterone a chemoattractant? Biol Reprod 1999; 60 (6): 1314–1319. [DOI] [PubMed] [Google Scholar]
- Meizel S, Turner KO, Nuccitelli R. Progesterone triggers a wave of increased free calcium during the human sperm acrosome reaction. Dev Biol 1997; 182 (1): 67–75. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011; 471 (7338): 387–391. [DOI] [PubMed] [Google Scholar]
- Strunker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 2011; 471 (7338): 382–386. [DOI] [PubMed] [Google Scholar]
- Ren D, Xia J. Calcium signaling through CatSper channels in mammalian fertilization. Physiol (Bethesda) 2010; 25 (3): 165–175. [DOI] [PubMed] [Google Scholar]
- Buffone MG, Rodriguez-Miranda E, Storey BT, Gerton GL. Acrosomal exocytosis of mouse sperm progresses in a consistent direction in response to zona pellucida. J Cell Physiol 2009; 220 (3): 611–620. [DOI] [PubMed] [Google Scholar]
- Thomas P, Tubbs C, Garry VF. Progestin functions in vertebrate gametes mediated by membrane progestin receptors (mPRs): identification of mPR[alpha] on human sperm and its association with sperm motility. Steroids 2009; 74 (7): 614–621. [DOI] [PubMed] [Google Scholar]
- Lopez CI, Belmonte SA, De Blas GA, Mayorga LS. Membrane-permeant Rab3A triggers acrosomal exocytosis in living human sperm. FASEB J 2007; 21 (14): 4121–4130. [DOI] [PubMed] [Google Scholar]
- De Blas GA, Roggero CM, Tomes CN, Mayorga LS. Dynamics of SNARE assembly and disassembly during sperm acrosomal exocytosis. PLoS Biol 2005; 3 (10): e323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez F, Bustos MA, Zanetti MN, Ruete MC, Mayorga LS, Tomes CN. α-SNAP prevents docking of the acrosome during sperm exocytosis because it sequesters monomeric syntaxin. Plos One 2011; 6 (7): e21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomes CN, De Blas GA, Michaut MA, Farré EV, Cherhitin O, Visconti PE. Mayorga LS. alpha-SNAP and NSF are required in a priming step during the human sperm acrosome reaction. Mol Hum Reprod 2005; 11 (1): 43–51. [DOI] [PubMed] [Google Scholar]
- Roggero CM, De Blas GA, Dai H, Tomes CN, Rizo J, Mayorga LS. Complexin/synaptotagmin interplay controls acrosomal exocytosis. J Biol Chem 2007; 282 (36): 26335–26343. [DOI] [PubMed] [Google Scholar]
- Zhao L, Burkin HR, Shi X, Li L, Reim K, Miller DJ. Complexin I is required for mammalian sperm acrosomal exocytosis. Dev Biol 2007; 309 (2): 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutt DM, Baltz JM, Ngsee JK, Synaptotagmin VI. and VIII and syntaxin 2 are essential for the mouse sperm acrosome reaction. J Biol Chem 2005; 280 (21): 20197–20203. [DOI] [PubMed] [Google Scholar]
- Michaut M, De Blas G, Tomes CN, Yunes R, Fukuda M, Mayorga LS. Synaptotagmin VI participates in the acrosome reaction of human spermatozoa. Dev Biol 2001; 235 (2): 521–529. [DOI] [PubMed] [Google Scholar]
- Yunes R, Tomes C, Michaut M, De Blas G, Rodriguez F, Regazzi R, Mayorga LS. Rab3A and calmodulin regulate acrosomal exocytosis by mechanisms that do not require a direct interaction. FEBS Lett 2002; 525 (1–3): 126–130. [DOI] [PubMed] [Google Scholar]
- Reid AT, Lord T, Stanger SJ, Roman SD, McCluskey A, Robinson PJ, Aitken RJ, Nixon B. Dynamin regulates specific membrane fusion events necessary for acrosomal exocytosis in mouse spermatozoa. J Biol Chem 2012; 287 (45): 37659–37672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann F, Zitranski N, Borth H, Buech T, Gudermann T, Boekhoff I. CaMKIIα interacts with multi-PDZ domain protein MUPP1 in spermatozoa and prevents spontaneous acrosomal exocytosis. J Cell Sci 2009; 122 (24): 4547–4557. [DOI] [PubMed] [Google Scholar]
- Branham MT, Bustos MA, De Blas GA, Rehmann H, Zarelli VEP, Treviño CL, Darszon A, Mayorga LS, Tomes CN. Epac activates the small G proteins Rap1 and Rab3A to achieve exocytosis. J Biol Chem 2009; 284 (37): 24825–24839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Peña MJ, Castillo Bennett JV, Soler OM, Mayorga LS, Michaut MA. MARCKS protein is phosphorylated and regulates calcium mobilization during human acrosomal exocytosis. PLoS ONE 2013; 8 (5): e64551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerke A, Tsai PS, Garcia-Gil N, Brewis IA, Gadella BM. Capacitation-dependent reorganization of microdomains in the apical sperm head plasma membrane: Functional relationship with zona binding and the zona-induced acrosome reaction. Theriogenology 2008; 70 (8): 1188–1196. [DOI] [PubMed] [Google Scholar]
- Baba D, Kashiwabara S, Honda A, Yamagata K, Wu Q, Ikawa M, Okabe M, Baba T. Mouse sperm lacking cell surface hyaluronidase PH-20 can pass through the layer of cumulus cells and fertilize the egg. J Biol Chem 2002; 277 (33): 30310–30314. [DOI] [PubMed] [Google Scholar]
- Baba T, Azuma S, Kashiwabara S, Toyoda Y. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J Biol Chem 1994; 269 (50): 31845–31849. [PubMed] [Google Scholar]
- Muro Y, Buffone MG, Okabe M, Gerton GL. Function of the acrosomal matrix: zona pellucida 3 receptor (ZP3R/sp56) is not essential for mouse fertilization. Biol Reprod 2012; 86 (1): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif S, Wilson MD, Wagner R, Hunt P, Gertsenstein M, Nagy A, Lobe C, Koop BF, Hardy DM. Zonadhesin is essential for species specificity of sperm adhesion to the egg's zona pellucida. J Biol Chem 2010; 285 (32): 24863–24870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MA, Storey BT. Evidence for plasma membrane impermeability to small ions in acrosome-intact mouse spermatozoa bound to mouse zonae pellucidae, using an aminoacridine fluorescent pH probe: time course of the zona-induced acrosome reaction monitored by both chlortetracycline and pH probe fluorescence. Biol Reprod 1985; 33 (1): 235–246. [DOI] [PubMed] [Google Scholar]
- Stock CE, Fraser LR. The acrosome reaction in human sperm from men of proven fertility. Hum Reprod 1987; 2 (2): 109–119. [DOI] [PubMed] [Google Scholar]
- Yudin AI, Gottlieb W, Meizel S. Ultrastructural studies of the early events of the human sperm acrosome reaction as initiated by human follicular fluid. Gamete Res 1988; 20 (1): 11–24. [DOI] [PubMed] [Google Scholar]
- Tsai P-S, Garcia-Gil N, van Haeften T, Gadella BM. How pig sperm prepares to fertilize: stable acrosome docking to the plasma membrane. PLoS ONE 2010; 5 (6): e11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai P-SJ, Brewis IA, van Maaren J, Gadella BM. Involvement of complexin 2 in docking, locking and unlocking of different SNARE complexes during sperm capacitation and induced acrosomal exocytosis. PLoS ONE 2012; 7 (3): e32603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-S, Foster JA, Gerton GL. Differential release of guinea pig sperm acrosomal components during exocytosis. Biol Reprod 2001; 64 (1): 148–156. [DOI] [PubMed] [Google Scholar]
- Adham IM, Nayernia K, Engel W. Spermatozoa lacking acrosin protein show delayed fertilization. Mol Reprod Dev 1997; 46 (3): 370–376. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Murayama K, Okabe M, Toshimori K, Nakanishi T, Kashiwabara S, Baba T. Acrosin accelerates the dispersal of sperm acrosomal proteins during acrosome reaction. J Biol Chem 1998; 273 (17): 10470–10474. [DOI] [PubMed] [Google Scholar]
- Summers RG, Talbot P, Keough EM, Hylander BL, Franklin LE. Ionophore A23187 induces acrosome reactions in sea urchin and guinea pig spermatozoa. J Exp Zool 1976; 196 (3): 381–385. [DOI] [PubMed] [Google Scholar]
- Tateno H, Krapf D, Hino T, Sánchez-Cárdenas C, Darszon A, Yanagimachi R, Visconti PE. Ca2+ ionophore A23187 can make mouse spermatozoa capable of fertilizing in vitro without activation of cAMP-dependent phosphorylation pathways. Proc Natl Acad Sci U S A 2013; 110 (46): 18543–18548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.