ABSTRACT
Bisphenol A (BPA) is an endocrine-disrupting chemical (EDC) widely used in common consumer products containing polycarbonate plastics and epoxy resins. Previous studies indicate that other EDCs have species-dependent effects. Furthermore, some EDCs are known to have different effects in different strains within the same species. Little information, however, is known about whether the effects of BPA on the ovary differ by strain. Previous studies have shown that BPA inhibits follicle growth, induces atresia, and inhibits steroidogenesis and expression of steroidogenic enzymes in antral follicles from adult FVB mice. Thus, this study was designed to expand previous work by testing the hypothesis that mouse strain may differentially affect the susceptibility of adult antral follicles to BPA-induced toxicity. To test this hypothesis, antral follicles were mechanically isolated from adult FVB, CD-1, and C57BL/6 mice, individually cultured for 6–120 h and treated with either vehicle control (dimethylsulfoxide) or various concentrations of BPA (1.0 μg/ml, 10 μg/ml, or 100 μg/ml). After culture, media were subjected to measurements of hormone production via ELISA, and follicles were subjected to real-time PCR for analysis of genes known to regulate steroidogenesis, the cell cycle, and atresia. Overall, BPA inhibited follicle growth and steroidogenesis in all tested strains, but CD-1 follicles were slightly more sensitive to BPA at early time points than FVB and C57BL/6 follicles. These data suggest that CD-1, FVB, and C57BL/6 mice can all be used to investigate the effects of BPA on ovarian follicles.
Keywords: bisphenol A, follicle growth, steroidogenesis, strain
Mouse strain does not influence BPA-induced inhibition of follicle growth or steroidogenesis.
INTRODUCTION
Bisphenol A (BPA) is an endocrine-disrupting and high-volume-production chemical. It is present in a multitude of commonly used consumer products such as polycarbonate plastics, the epoxy resin linings of food and beverage cans, dental sealants, and receipt paper. Importantly, BPA can leach out from these products and, once released, can readily enter the body via ingestion, inhalation, or through dermal absorption [1]. Human exposure to BPA is continuous and widespread, with much of the exposure occurring via ingestion [2]. Once in the body, BPA can have widespread effects on many tissues, including the ovaries.
Many studies have investigated the effects of BPA on the reproductive system. Although some effects such as disrupted uterine implantation and pregnancy maintenance are supported by strong evidence [3–8], other effects such as the effects on steroidogenesis are only partially supported by existing data [4, 6, 9–15]. The ambiguity of these studies may be due to the study design, specifically the strain of animal used in the experiments.
Use of appropriate animal models is important in toxicology studies to decrease or avoid factors that could confound studies, introduce unnecessary variation in results, or improperly interpret the results [16, 17]. Use of the appropriate strain of animal model is important as well [18]. Genetic backgrounds of animal models determine the metabolic rate of a chemical, mediating how that chemical is absorbed, distributed, metabolized, and excreted among different species [19–21]. Ultimately, this may affect how sensitive strains are to the same chemical under the same exposure conditions [22]. For example, some studies have shown that outbred mouse strains, such as CD-1 mice, are less sensitive than inbred mouse strains to endocrine-disrupting chemicals [17]. CD-1 mice have been shown to be less sensitive to estrogen-induced inhibition of spermatogenesis than C57BL/6 mice [23]. However, other studies have shown that C57BL/6 mice are more sensitive to estrogen-induced alterations in oocyte development than CD-1, FVB, or Oct4-GFP mice [24]. Ideally, the most sensitive strain should be used in studies of endocrine-disrupting chemicals to determine the potentially most adverse effects of a particular chemical [17].
Previous studies have shown that strain may mediate the effects of BPA on the female reproductive system. BPA exposure increased expression of stimulated by retinoic acid 8 homolog (Stra8) and a variety of meiotic genes in gestationally exposed C57BL/6 mice [25]. However, in gestationally exposed CD-1 mice, BPA down-regulated expression of the Stra8, deleted in azoospermia-like (Dazl), and newborn ovary homeobox (Nobox) genes [26]. Additionally, perinatal BPA exposure more profoundly increased the sensitivity of mammary glands from prepubertal CD-1 mice to estradiol stimulus compared to mammary glands from C57BLl/6 mice [22]. Furthermore, BPA dose-dependently increased DNA synthesis in the vaginal epithelium of Fischer 344 inbred rats but did not affect the vaginal epithelium in Sprague-Dawley outbred rats [27].
Previous studies from our laboratory have shown that BPA inhibits mouse antral follicle growth and steroidogenesis [9, 10, 28]. The follicle is the functional unit of the ovary and it is responsible for the majority of steroidogenesis, which is required for normal reproductive function and overall female health. Although strain has been documented as a potential confounder of BPA studies, no studies have investigated the effect of strain in BPA-induced mouse antral follicle toxicity. Thus, the goal of this study was to investigate how strain mediates BPA-induced inhibition of antral follicle growth and steroidogenesis. We specifically tested the hypothesis that mouse strain differentially affects the sensitivity of adult antral follicles to BPA-induced toxicity.
MATERIALS AND METHODS
Chemicals
BPA powder (99%) was purchased from Sigma-Aldrich. A stock solution of BPA was dissolved and diluted in dimethylsulfoxide (DMSO; Sigma-Aldrich) to achieve various BPA treatment concentrations (1.3 mg/ml, 13.3 mg/ml, and 133 mg/ml) for final working concentrations of 1.0 μg, 10 μg, and 100 μg of BPA per milliliter of culture medium. Using these treatment concentrations allowed each working concentration to contain the same volume of chemical and vehicle. The concentrations chosen were based on those described in previous studies, which indicated that BPA (100 μg/ml) inhibited follicle growth and induced atresia and that BPA (10 μg/ml and 100 μg/ml) decreased sex steroid hormone levels [9, 28].
Animals
Adult, cycling, female FVB and C57BL/6 mice were purchased from Jackson Laboratory, and CD-1 mice were purchased from Charles River. The mice were allowed to acclimate to the facility for at least 5 days before use. The mice were housed at the University of Illinois at Urbana-Champaign, Veterinary Medicine Animal Facility. Food (Teklad product no. 8626; Harlan Laboratories) and water were provided for ad libitum consumption. Temperature was maintained at 22°C ± 1°C, and animals were subjected to 12L:12D cycles. The Institutional Animal Use and Care Committee at the University of Illinois at Urbana-Champaign approved all procedures involving animal care, euthanasia, and tissue collection.
In Vitro Time Course Follicle Culture
Female FVB, CD-1, and C57BL/6 mice were euthanized on postnatal days 32–35, and their ovaries were removed using aseptic technique. Antral follicles were mechanically isolated from the ovary based on relative size (250–400 μm), cleaned of interstitial tissue using fine watchmaker forceps [29, 30], individually placed in wells of a 96-well culture plate, and covered with unsupplemented α-minimal essential medium (α-MEM) prior to treatment. The numbers of antral follicles sufficient for statistical power were isolated from unprimed mouse ovaries of each strain; follicles from 2–3 mice were isolated per experiment, providing approximately 20–40 antral follicles from each mouse. Each experiment contained a minimum of 8–12 follicles per treatment group. Concentrations of vehicle control (DMSO) and BPA (1.0 μg/ml, 10 μg/ml, and 100 μg/ml) were individually prepared in supplemented α-MEM. Supplemented α-MEM was prepared with 1% ITS (10 ng/ml insulin, 5.5 ng/ml transferrin, 5.5 ng/ml selenium), 100 U/ml penicillin, 100 mg/ml streptomycin, 5 IU/ml human recombinant follicle-stimulating hormone (Dr. AF Parlow, National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA), and 5% fetal calf serum (Atlanta Biologicals). An equal volume of chemical was added for each dose to control for the amount of vehicle in each preparation (0.75 μl/ml of medium for BPA treatments; 1.0 μl/ml of medium for DMSO treatments). Antral follicles were cultured for up to 120 h in an incubator supplying 5% CO2 at 37°C. After each culture, follicles were snap-frozen in liquid nitrogen and subjected to quantitative PCR (qPCR) as described below. Samples of medium were collected and stored at −80°C until subjected to hormone assays as described below.
Analysis of Follicle Growth
Follicle growth was examined at 24-h intervals beginning with the initial measurement at time zero (0 h). To compare follicle sizes at collection, the average follicle size was determined by measuring follicle diameter on perpendicular axes with an inverted microscope equipped with a calibrated ocular micrometer. Average follicle sizes were compared among the strains based on treatment group at 0 h. To evaluate follicle growth over time within each strain, follicles were measured along perpendicular axes on subsequent days at 24-h intervals. These diameters were recorded in micrometers, averaged among treatment groups per 24-h intervals, and then converted to percent change at each time point. Percent change was determined by dividing the average diameter of the follicles at each 24-h interval per treatment group with the initial measurement (0 h) of that treatment group.
Analysis of Gene Expression by qPCR
Mouse antral follicles isolated from FVB, CD-1, and C57BL/6 mice were cultured and frozen as described above. Total RNA was extracted from follicles using the RNeasy micro kit (Qiagen) according to the manufacturer's protocol. Reverse transcriptase generation of cDNA was performed with 0.3–0.5 μg of total RNA, using an iScript RT kit (Bio-Rad Laboratories). Quantitative PCR was conducted using CFX96 real-time PCR detection system (Bio-Rad Laboratories) and accompanying software (CFX Manager Software) according to the manufacturer's instructions. CFX96 software quantifies the amount of PCR product generated by measuring a dye (SsoFast EvaGreen Supermix; Bio-Rad Laboratories) that fluoresces when bound to double-stranded DNA. A standard curve was generated from five serial dilutions of a combination of the samples, thus allowing analysis of the amount of cDNA in the exponential phase. Quantitative PCR analysis was performed using 2 μl of cDNA, forward and reverse primers (5 pmol) for cyclin-dependent kinase 4 (Cdk4; NM_009870.3), cyclin E1 (Ccne1; NM_007633.2), transformation-related protein 53 (Trp53; NM_011640.3), B-cell lymphoma 2 (Bcl2; NM_009741.3), Bcl2-associated X protein (Bax; NM_007527.3), steroidogenic acute regulator protein (Star; NM_011485.4), cytochrome P450 side-chain cleavage (Cyp11a1; NM_019779.3), or beta-actin (Actb; NM_007393), in conjunction with a SsoFast EvaGreen Supermix qPCR kit (Bio-Rad Laboratories). An initial incubation of 95°C for 10 min was followed by denaturing at 94°C for 10 sec, annealing at 60°C for 10 sec, and extension at 72°C for 10 sec for 40 cycles, followed by final extension at 72°C for 10 min. A melting curve was generated at 55°C–90°C to monitor the generation of a single product. The software also generated a standard curve. Actb was used as the reference gene for each sample. Final values were calculated and expressed as the ratio normalized to Actb expression. All analyses were performed in duplicate for at least three separate experiments.
Analysis of Hormone Levels
Samples of medium were collected at 24-h intervals of culture and subjected to ELISA (DRG Instruments GmbH) for measurement of estradiol, testosterone, androstenedione, and progesterone levels. Assays were run using the manufacturer's instructions. All samples were run in duplicate and all intra- and interassay coefficients of variability were less than 10%.
Statistical Analyses
Data are means ± SEM, and multiple comparisons among experimental groups were made using a general linear model univariate analysis, ANOVA, or a Student independent t-test, followed by the Tukey post hoc comparison test when appropriate. Tests for trend were analyzed using linear regression analyses for the overall effect of BPA concentration when appropriate. At least three separate experiments were conducted for each treatment prior to data analysis. Statistical significance was assigned at a P value of ≤0.05.
RESULTS
Effect of Strain on Antral Follicle Size at Initial Collection
To determine whether initial follicle size differed by strain at the time of collection, we measured follicle sizes from each strain at 0 h (Fig. 1). Follicle sizes did not differ among FVB, C57BL/6, and CD-1 mice at 0 h in any treatment group.
FIG. 1.
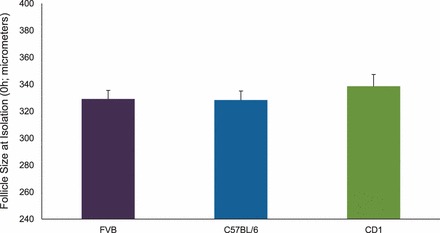
Effect of strain on antral follicle size at isolation (0 h). Antral follicles were mechanically isolated from FVB, C57BL/6, and CD-1 mice when the mice were 32–35 days old. Antral follicles were characterized by observation of an antral space in the follicles and those follicles between 250 and 400 μm average diameter at 0 h. Graph values are mean ± SEMs (n = 8–16 follicles per treatment group per experiment from at least three separate experiments; P ≤ 0.05).
Effect of Strain on Inhibited Follicle Growth with BPA
In previous studies, we have shown that BPA (100 μg/ml) inhibits follicle growth in antral follicles isolated from FVB mice, beginning at 72 h and continuing until 120 h in culture (Supplemental Fig. S1; all supplemental data are available online at www.biolreprod.org) [9, 28]. However, we did not know whether BPA (100 μg/ml) would inhibit follicle growth in other strains of mice as well. Therefore, to test how strain mediated follicle growth inhibition, we measured the growth of follicles isolated from C57BL/6 and CD-1 mice over time (Fig. 2). Similar to its effect on FVB follicles [9], BPA (100 μg/ml) significantly inhibited growth of C57BL/6 follicles compared to DMSO beginning at 72 h and continuing until 120 h in culture. However, BPA (100 μg/ml) significantly inhibited growth of CD-1 follicles compared to DMSO beginning at 48 h and continuing until 120 h in culture. Similar to its effect on FVB follicles [9], BPA (1.0 μg/ml and 10 μg/ml) did not significantly inhibit follicle growth in CD-1 or C57BL/6 follicles compared to controls at any time point.
FIG. 2.
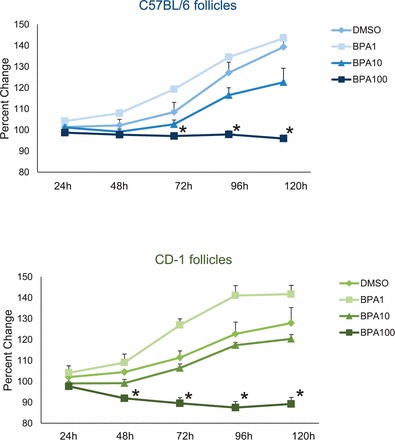
Effect of strain on inhibited follicle growth with BPA. Antral follicles were mechanically isolated from C57BL/6 and CD-1 mice and exposed in vitro to DMSO or BPA (1, 10, or 100 μg/ml). Growth of follicles was monitored during culture, recorded in micrometers, and reported as percent change at each subsequent time point compared to 0 h, of each appropriate treatment group. Graph values are means ± SEMs from at least three separate experiments. *Significantly different from DMSO controls (n = 8–16 follicles per treatment per experiment from at least three separate experiments; P ≤ 0.05).
Effect of Strain on the Severity of Inhibited Follicle Growth with BPA
Although follicle growth was inhibited sooner in CD-1 follicles than in FVB and C57BL/6 follicles, we did not know whether the inhibition was more severe in CD-1 follicles than in FVB and C57BL/6 follicles. Therefore, we compared the amount of follicle growth inhibition in BPA-treated (100 μg/ml) follicles among the strains. BPA (100 μg/ml) significantly inhibited growth of CD-1 follicles more than it inhibited growth of FVB and C57BL/6 follicles, beginning at 48 h and continuing until 120 h in culture (Fig. 3). Inhibition of follicle growth was not different between FVB and C57BL/6 follicles at any time point.
FIG. 3.
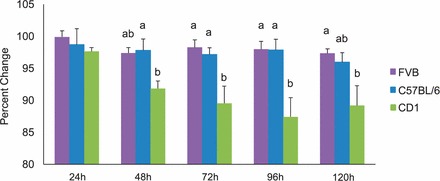
Effect of strain on the severity of follicle growth inhibition with BPA. Growth rates of antral follicles treated with BPA (100 μg/ml) were compared directly among the three strains over time. Growth of follicles was monitored during culture, recorded in micrometers, and reported as percent change at each subsequent time point compared to 0 h of BPA (100 μg/ml). Graph values are means ± SEMs from at least three separate experiments. Bars with differing letters are significantly different from each other (n = 8–16 follicles per treatment per experiment from at least three separate experiments; P ≤ 0.05).
Effect of Strain on Aberrant Induction of Cell Cycle Regulators and Atresia Factors with BPA
Follicle growth is regulated by proteins that control the cell cycle such as CDK4, CCNE1, TRP53, and atretic factors such as BAX and antiatretic factors such as BCL2. In previous studies, we have shown that BPA (100 μg/ml) aberrantly induces expression of cell cycle regulators and atresia factors in FVB follicles, which may lead to the inhibition of follicle growth (Supplemental Figs. S2 and S3) [28]. To test how strain mediates the induction of these factors in follicles isolated from CD-1 and C57BL/6 mice, we subjected the follicles to qPCR at 24-h intervals and measured expression of Cdk4, Ccne1, Trp53, Bax, and Bcl2 over time (Figs. 4 and 5). Similar to effects on FVB follicles [28], BPA (100 μg/ml) significantly induced the expression of Cdk4, Ccne1, Trp53, Bax, and Bcl2 in CD-1 and C57BL/6 follicles beginning at 24 h and continuing until 96 h in culture. BPA (1.0 and 10 μg/ml) did not affect the expression of cell cycle regulators or atresia factors in CD1 or C57BL/6 follicles compared to that in controls at any time point.
FIG. 4.
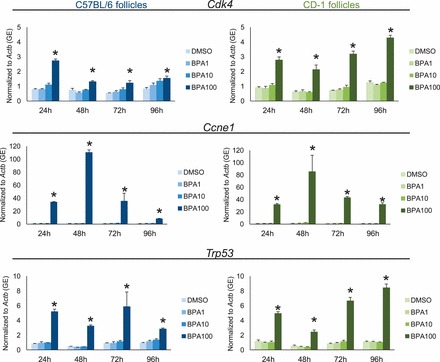
Effect of strain on aberrant induction of cell cycle regulators with BPA. After exposure of antral follicles from C57BL/6 and CD-1 mice to DMSO control or BPA (1–100 μg/ml) for 24–96 h in vitro, the follicles were collected and subjected to qPCR analysis for Cdk4, Ccne1, and Trp53 mRNA expression levels. All values were normalized to those of beta-actin (Actb) as a loading control. Graph values are means ± SEMs from at least three separate experiments. *P ≤ 0.05 compared to DMSO control. GE = genomic equivalent.
FIG. 5.
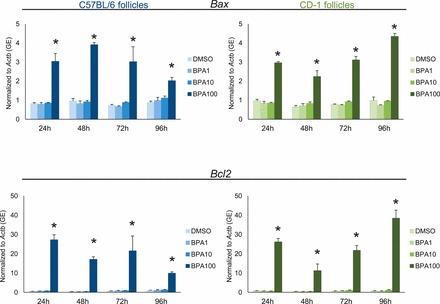
Effect of strain on aberrant induction of atresia factors with BPA. After exposure of antral follicles from C57BL/6 and CD-1 mice to DMSO control or BPA (1–100 μg/ml) for 24–96 h in vitro, the follicles were collected and subjected to qPCR analysis for Bax and Bcl2 mRNA expression levels. All values were normalized to those of beta-actin (Actb) as a loading control. Graph values are means ± SEMs from at least three separate experiments. *P ≤ 0.05 compared to DMSO control. GE = genomic equivalent.
Effect of Strain on Decreased Hormone Production with BPA
Previously, we have shown that BPA (10 and 100 μg/ml) inhibits estradiol, testosterone, and androstenedione levels beginning at 72 h in culture and inhibits progesterone levels beginning at 24 h in culture in CD-1 mice (Supplemental Fig. S4) [10]. To test whether strain affects this BPA-induced inhibition of hormone production, we collected medium from cultures of FVB and C57BL/6 follicles and measured estradiol, testosterone, androstenedione, and progesterone levels from 24 to 120 h in culture (Fig. 6). Similar to effects on CD-1 follicles [10], BPA (10 and 100 μg/ml) significantly decreased estradiol, testosterone, and androstenedione levels in FVB and C57BL/6 follicles compared to DMSO beginning at 72 h and continuing throughout culture (Fig. 6). In contrast to CD-1 follicles [10], BPA (1 μg/ml) did not significantly decrease progesterone levels in FVB and C57BL/6 follicles compared to DMSO at 24 h (Fig. 6). However, similar to CD-1 follicles [10], BPA (10 and 100 μg/ml) significantly decreased progesterone levels in FVB and C57BL/6 follicles compared to DMSO beginning at 48 h and continuing throughout culture. BPA (1.0 μg/ml) did not significantly inhibit estradiol, testosterone, androstenedione, or progesterone compared to DMSO at any time point in FVB or C57BL/6 follicles.
FIG. 6.
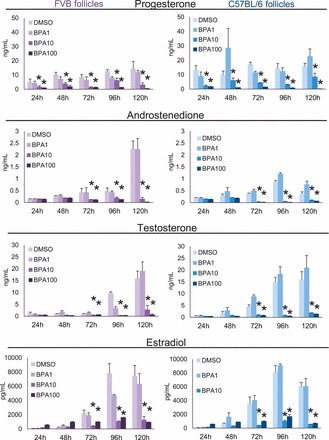
Effect of strain on decreased hormone production with BPA. After exposure of antral follicles from FVB or C57BL/6 mice to DMSO control or BPA (1–100 μg/ml) for 24–96 h in vitro, medium samples were collected at each time point, pooled per treatment group, and subjected to ELISA for progesterone, androstenedione, testosterone, and estradiol. Graph values are means ± SEMs from at least three separate experiments. *P ≤ 0.05 compared to DMSO control.
Effect of Strain on Decreased Expression of Star and Cyp11a1 with BPA
STAR and CYP11A1 control steroidogenesis in antral follicles. STAR catalyzes the transport of cholesterol from the outer mitochondrial membrane where CYP11A1 metabolizes cholesterol into pregnenolone, the sex steroid hormone precursor for steroidogenesis. Previously, we have shown that BPA (10 μg/ml) inhibits the expression of Star and Cyp11a1 expression beginning at 72 h and 18 h, respectively, in cultured CD-1 follicles (Supplemental Figs. S5 and S6) [10]. In this study, we tested how strain affects this inhibition of Star and Cyp11a1 (Figs. 7 and 8). Similar to effects on CD-1 follicles, BPA (10 μg/ml) significantly decreased the expression of Star in FVB and C57BL/6 follicles compared to that in controls beginning at 72 h and continuing throughout culture. Furthermore, BPA (10 μg/ml) significantly decreased the expression of Cyp11a1 in FVB and C57BL/6 follicles compared to that in controls beginning at 18 h and continuing throughout culture.
FIG. 7.
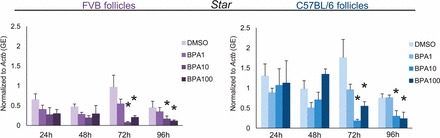
Effect of strain on decreased expression of Star with BPA. After exposure of antral follicles from FVB or C57BL/6 mice to DMSO control or BPA (1–100 μg/ml) for 24–96 h in vitro, the follicles were collected and subjected to qPCR analysis for Star mRNA expression levels. All values were normalized to those of beta-actin (Actb) as a loading control. Graph values are means ± SEMs from at least three separate experiments. *P ≤ 0.05 compared to DMSO control. GE = genomic equivalent.
FIG. 8.
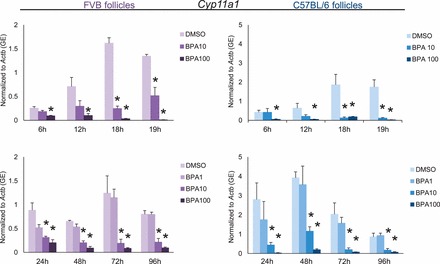
Effect of strain on decreased expression of Cyp11a1 with BPA. After exposure of antral follicles from FVB or C57BL/6 mice to DMSO control or BPA (1–100 μg/ml) for 24–96 h in vitro, the follicles were collected and subjected to qPCR analysis for Cyp11a1 mRNA expression levels. All values were normalized to those of beta-actin (Actb) as a loading control. Graph values are means ± SEMs from at least three separate experiments. *P ≤ 0.05 compared to DMSO control. GE = genomic equivalent.
DISCUSSION
Mouse strain did not alter the overall toxicity of BPA in antral follicles. BPA significantly inhibited follicle growth in FVB, C57BL/6, and CD-1 follicles compared to DMSO-treated controls. In CD-1 follicles, growth was inhibited sooner and more severely than in FVB and C57BL/6 follicles; however, there were no differences in BPA-induced dysregulation of cell cycle regulators and atresia factors among the three strains tested. BPA also significantly inhibited the production of progesterone, androstenedione, testosterone, and estradiol in FVB, C57BL/6, and CD-1 follicles compared to DMSO in controls. In CD-1 follicles, BPA inhibited progesterone production at a lower concentration than in FVB or C57BL/6 follicles, although this effect was not sustained over time. Furthermore, there were no differences between the strains in the BPA-induced down-regulation of Cyp11a1 and Star. Overall, these data indicate that BPA inhibits follicle growth and steroidogenesis similarly in all three strains tested, although CD-1 follicles may be slightly more sensitive to BPA at early time points than the other tested strains.
Follicle growth is required for normal fertility. Disruptions in follicle growth can lead to improper oocyte development and quality, impaired steroidogenesis, and impaired ovulation [31, 32]. Follicle growth is regulated by a series of checks and balances. Cell cycle regulators are responsible for promoting and controlling cell proliferation in the granulosa and theca cells [33]. These factors include the cyclins (e.g., CCNE1) and cyclin-dependent kinases (e.g., CDK4) that are responsible for ensuring that proper cell replication and proliferation occur. Complementary to the cell cycle regulators are atresia factors that promote cell survival (e.g., BCL2) or cell death (e.g., BAX). In a normal system, these factors work together via TRP53 to promote healthy cell proliferation or promote cell death, thereby preventing replication of cell defects, aberrant replication, and disease [31]. BPA exposure resulted in up-regulation of these factors, and, most importantly, this up-regulation occurred similarly in each strain tested. Although it is plausible that BPA increases global gene expression, we have previously found that BPA down-regulates genes in the steroidogenic pathway, indicating that BPA does not globally increase gene expression [28]. In recent studies using CD-1 follicles, we have found that Bax increases very early in culture and prior to Bcl2 (Peretz and Flaws, unpublished results), substantiating the hypothesis that BPA triggers the atretic pathway, leading to inhibited follicle growth and that the follicle responds by up-regulating factors controlling cell survival and cell proliferation, such as those in the cell cycle [28].
Normal sex steroid hormone production is also important for fertility as well as overall female health. BPA inhibited steroidogenesis similarly in all strains tested. At 24 h, BPA inhibited steroidogenesis in CD-1 follicles at a lower concentration than it did in FVB or C57BL/6 follicles. However, this effect was not sustained during culture. BPA also inhibited expression of Cyp11a1 and Star similarly in each strain. Thus, while it is possible that genetic variation between the strains mediates BPA-induced inhibition of steroidogenesis, the strain differences were not maintained during the culture and, thus, may not be physiologically relevant to the toxicity of BPA in antral follicles.
FVB and C57BL/6 mice are inbred strains of mice, whereas CD-1 mice are outbred mice and are considered to better represent the genetic diversity of the human population than the inbred strains of mouse. Although CD-1 mice are generally used for toxicology studies because of low cost, large litter sizes, and high fertility rates in laboratory settings [20, 31], FVB and C57BL/6 mice are mice commonly used in toxicology studies as well, each with its own varying sensitivity to endocrine disruptors, most likely due to genetic differences among the strains [17, 20]. Chemical idiosyncrasy is a genetically abnormal reaction to a chemical that may not be easily characterized by a genetic marker or enzymatic difference in an individual's ability to detoxify a chemical [34]. With chemical idiosyncrasy, animals may have the same overall reaction or response to a chemical, but some will be more sensitive to the chemical than others [34]. Although it is crucial to emphasize that all three strains of mouse experienced drastically inhibited follicle growth and steroidogenesis in response to BPA exposure, the slight sensitivity of CD-1 follicles compared to follicles from other strains may be due to a chemically idiosyncratic response to BPA.
Although no studies to our knowledge have investigated what specific genetic differences may alter the sensitivity of different mouse strains to a chemical, previous studies have documented how strain has equivocally mediated the toxicity of estrogenic chemicals. Conversely to the findings from our study, estradiol has been shown to suppress testes weight more significantly in juvenile C57BL/6 males than in CD-1 males (60% vs. 30%, respectively) [20, 23]. Estradiol also inhibited spermatogenesis more in C57BL/6 males than in CD-1 males [20, 23]. In another study, neonatal C57BL/6 ovaries had slower primary to secondary follicle transitions and better protected oocytes from cell death than FVB, CD-1, or Oct4-GFP mice after estradiol stimulation [24]. Furthermore, CD-1 mice and FVB mice had similar numbers of oocytes present at postnatal day 4 after exposure to estradiol in utero, although CD-1 mice had a faster rate of loss than FVB mice [24]. In contrast, adult female CD-1 and C57BL/6 mice showed no differences in uterotrophic response to estradiol [22]. In previous studies, we have shown the BPA does not induce antral follicle toxicity via a classical estrogenic pathway [28]. Thus, studies detailing how strain mediates the toxicity of estrogenic chemicals may not indicate why strain was not a factor in the overall toxicity of BPA in antral follicles in this study.
Interestingly, strain has affected the toxicity of BPA in studies of other tissues and species. Furthermore, these strain effects are recognized by the National Toxicology Program (NTP) [35]. For example, Charles-River Sprague-Dawley rats have low sensitivity to BPA. The Charles-River Sprague-Dawley rat has also been shown to be less sensitive to BPA than the Fischer 344 rat and the CF-1 mouse in a variety of reproductive endpoints [1, 35]. In another study, BPA was shown to differentially affect sperm count and motility in Long Evans and Wistar rats compared to Holtzman rats [7, 8]. Additionally, contrary to the decreased sensitivity to estrogenic compounds of CD-1 mice compared to that of C57BL/6 mice [20, 22, 23], gestational BPA exposure inhibited testosterone levels in CD-1 mice but not in C57BL/6 mice [12, 36, 37]. In females, perinatal exposure to BPA increased the number of terminal end buds in mammary tissues of CD-1 mice exposed to estradiol later in life without affecting the terminal end buds in mammary tissues of C57BL/6 mice [22]. Furthermore, BPA increased the weight of offspring born to gestationally exposed ICR and adult CD1 Swiss mice, decreased the weight of offspring born to neonatally exposed ICR mice, but did not affect the weight of offspring born to gestationally and neonatally exposed C57BL/6J mice [15, 38–40]. Although timing of exposure may also play a role in birth weight following exposure to BPA, future studies should directly compare BPA effects in various strains to account for other potential confounders.
This study is important for determining which mouse strain should be used for studies evaluating the toxicity of BPA on antral follicles. This study is novel because, to our knowledge, no prior studies have investigated how strain mediates the effects of BPA toxicity in mouse antral follicles. As a guide for future toxicological studies investigating BPA, the NTP has called for more studies evaluating mechanism of action and pharmacokinetic studies among various strain models [35]. Furthermore, the NTP concluded that future studies should choose an appropriate animal model for the chemical being tested, not just for convenience [35]. Thus, our study suggests that CD-1, FVB, and C57BL/6 mice can all be used to investigate the effects of BPA on ovarian antral follicles, but CD-1 mice may be slightly more sensitive to BPA at early time points than FVB and C57BL/6 mice, possibly due to chemical idiosyncrasy.
Supplementary Material
Footnotes
Supported by National Institutes of Health grants R01ES019178 and P20ES018163 and Environmental Protection Agency grant RD-83459301.
REFERENCES
- vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol a shows the need for a new risk assessment. Environ Health Perspect 2005; 113: 926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 2005; 113: 391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Diao H, Smith MA, Song X, Ye X. Preimplantation exposure to bisphenol A (BPA) affects embryo transport, preimplantation embryo development, and uterine receptivity in mice. Reprod Toxicol 2011; 32: 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger RG, Shaw J, deCatanzaro D. Impact of acute bisphenol-A exposure upon intrauterine implantation of fertilized ova and urinary levels of progesterone and 17b-estradiol. Reprod Toxicol 2008; 26: 94–99. [DOI] [PubMed] [Google Scholar]
- Berger RG, Foster WG, deCatanzaro D. Bisphenol-A exposure during the period of blastocyst implantation alters uterine morphology and perturbs measures of estrogen and progesterone receptor expression in mice. Reprod Toxicol 2010; 30: 393–400. [DOI] [PubMed] [Google Scholar]
- Varayoud J, Ramos JG, Bosquiazzo VL, Lower M, Munoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A alters rat uterine implantation-associated gene expression and reduces the number of implantation sites. Endocrinol 2011; 152: 1101–1111. [DOI] [PubMed] [Google Scholar]
- Salian S, Doshi T, Vanage G. Neonatal exposure of male rats to bisphenol A impairs fertility and expression of Sertoli cell junctional proteins in the testis. Toxicology 2009; 265: 56–67. [DOI] [PubMed] [Google Scholar]
- Salian S, Doshi T, Vanage G. Perinatal exposure of rats to bisphenol A affects the fertility of male offspring. Life Sci 2009; 85: 742–752. [DOI] [PubMed] [Google Scholar]
- Peretz J, Gupta RK, Singh J, Hernandez-Ochoa I, Flaws JA. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and down-regulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sci 2011; 119: 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz J, Flaws JA. Bisphenol A down-regulates rate-limiting Cyp11a1 to acutely inhibit steroidogenesis in cultured mouse antral follicles. Toxicol Appl Pharmacol 2013; 271: 249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez M, Bourguignon N, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ Health Perspect 2010; 118: 1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi W, Lee CK, Yeung WS, Giesy JP, Wong MH, Zhang X, Hecker M, Wong CK. Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus-pituitary-gonadal axis of CD-1 mice. Reprod Toxicol 2011; 31: 409–417. [DOI] [PubMed] [Google Scholar]
- Rivera OE, Varayoud J, Rodriguez HA, Munoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod Toxicol 2011; 32: 304–312. [DOI] [PubMed] [Google Scholar]
- Mendoza-Rodriguez CA, Garcia-Guzman M, Baranda-Avila N, Morimoto S, Perrot-Applanat M, Cerbon M. Administration of bisphenol A to dams during perinatal period modifies molecular and morphological reproductive parameters of the offspring. Reprod Toxicol 2011; 31: 177–183. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Kubota H, Ohtani K, Hojo R, Miyagawa M. Lack of effects for dietary exposure of bisphenol A during in utero and lactational periods on reproductive development in rat offspring. J Toxicol Sci 2012; 37: 565–573. [DOI] [PubMed] [Google Scholar]
- Yoshiki A, Moriwaki K. Mouse phenome research: implications of genetic background. ILAR J 2006; 47: 94–102. [DOI] [PubMed] [Google Scholar]
- Stokes WS. Selecting appropriate animal models and experimental designs for endocrine disruptor research and testing studies. ILAR J 2004; 45: 387–393. [DOI] [PubMed] [Google Scholar]
- Wood MW, Hart LW. Selecting appropriate animal models and strains: making the best use of research, information and outreach. Japanese Society for Alternatives to Animal Experiments 2007; 14: 303–306. [Google Scholar]
- Goodman JE, Sipes G, McConnell EE, Witorsch RJ, Slayton TM, Yu CJ, Lewis AS, Rhomberg LR. An updated weight of the evidence evaluation of reproductive and developmental effects of low doses of bisphenol A. Crit Rev Toxicol 2006; 36: 387–457. [DOI] [PubMed] [Google Scholar]
- Spearow JL, Doemeny P, Sera R, Leffler R, Barkley M. Genetic variation in susceptibility to endocrine disruption by estrogen in mice. Science 1999; 285: 1259–1261. [DOI] [PubMed] [Google Scholar]
- Spearow JL, Barkley M. Genetic control of hormone-induced ovulation rate in mice. Biol Reprod 1999; 61: 851–856. [DOI] [PubMed] [Google Scholar]
- Wadia PR, Vandenberg LN, Schaeberle CM, Rubin BS, Sonnenschein C, Soto AM. Perinatal bisphenol A exposure increases estrogen sensitivity of the mammary gland in diverse mouse strains. Environ Health Perspect 2007; 115: 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearow JL, O'Henley P, Doemeny P, Sera R, Leffler R, Sofos T, Barkley M. Genetic variation in physiological sensitivity to estrogen in mice. APMIS 2001; 109: 356–364. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Sundman EA, Patterson NL, Gephardt GW, Medico L, Jr, , Wilson KI. Differences in oocyte development and estradiol sensitivity among mouse strains. Reproduction 2010; 139: 349–357. [DOI] [PubMed] [Google Scholar]
- Lawson C, Gieske M, Murdoch B, Ye P, Li Y, Hassold T. Gene expression in the fetal mouse ovary is altered by exposure to low doses of bisphenol A. Biol Reprod 2011; 84: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Zhang LJ, Feng YN, Chen B, Feng YM, Liang GJ, Li L, Shen W. Bisphenol A exposure modifies DNA methylation of imprint genes in mouse fetal germ cells. Mol Biol Rep 2012; 39: 8621–8628. [DOI] [PubMed] [Google Scholar]
- Long X, Steinmetz R, Ben Jonathan N, Caperell-Grant A, Young PC, Nephew KP, Bigsby RM. Strain differences in vaginal responses to the xenoestrogen bisphenol A. Environ Health Perspect 2000; 108: 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz J, Craig ZR, Flaws JA. Bisphenol A inhibits follicle growth and induces atresia in cultured mouse antral follicles independently of the genomic estrogenic pathway. Biol Reprod 2012; 87: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol Sci 2006; 93: 382–389. [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Greenfield CR, Babus JK, Flaws JA. Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicol Sci 2005; 88: 213–221. [DOI] [PubMed] [Google Scholar]
- Crozet N, Ahmed-Ali M, Dubos MP. Developmental competence of goat oocytes from follicles of different size categories following maturation, fertilization and culture in vitro. J Reprod Fertil 1995; 103: 293–298. [DOI] [PubMed] [Google Scholar]
- Khatir H, Anouassi A, Tibary A. Effect of follicular size on in vitro developmental competence of oocytes and viability of embryos after transfer in the dromedary (Camelus dromedarius). Anim Reprod Sci 2007; 99: 413–420. [DOI] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. The cell cycle and programmed cell death. : Alberts B. (ed.), Molecular Biology of the Cell, 4th ed. New York: Garland Science; 2002; 983–10287. [Google Scholar]
- Eaton DL, Gilbert SG. Principles of toxicology : Klaassen CD. (ed.), Casarett and Doull's Toxicology: the Basic Science of Poisons, vol. 1, 8th ed. New York: McGraw-Hill Education; 2013: 13–48. [Google Scholar]
- Melnick R, Lucier G, Wolfe M, Hall R, Stancel G, Prins G, Gallo M, Reuhl K, Ho SM, Brown T, Moore J, Leakey J, et al. Summary of the National Toxicology Program's report of the endocrine disruptors low-dose peer review. Environ Health Perspect 2002; 110: 427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi W, Wan HT, Zhao YG, Wong MH, Giesy JP, Wong CK. Effects of perinatal exposure to bisphenol A and di(2-ethylhexyl)-phthalate on gonadal development of male mice. Environ Sci Pollut Res Int 2011; 19: 2515–2527. [DOI] [PubMed] [Google Scholar]
- LaRocca J, Boyajian A, Brown C, Smith SD, Hixon M. Effects of in utero exposure to Bisphenol A or diethylstilbestrol on the adult male reproductive system. Birth Defects Res B Dev Reprod Toxicol 2011; 92: 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama M, Choi EK, Wakitani S, Tachibana T, Khan H, Kusakabe KT, Kiso Y. Bisphenol-A (BPA) affects reproductive formation across generations in mice. J Vet Med Sci 2011; 73: 1211–1215. [DOI] [PubMed] [Google Scholar]
- Tyl RW, Myers CB, Marr MC, Sloan CS, Castillo NP, Veselica MM, Seely JC, Dimond SS, Van Miller JP, Shiotsuka RN, Beyer D, Hentges SG, et al. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol Sci 2008; 104: 362–384. [DOI] [PubMed] [Google Scholar]
- Nah WH, Park MJ, Gye MC. Effects of early prepubertal exposure to bisphenol A on the onset of puberty, ovarian weights, and estrous cycle in female mice. Clin Exp Reprod Med 2011; 38: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


