ABSTRACT
Cryopreservation of oocytes is becoming a valuable method for fertility preservation in women. However, various unphysiological alterations occur in the oocyte during the course of cryopreservation, one of which is the disappearance of the meiotic spindle. Fortunately, the meiotic spindle does regenerate after thawing the frozen oocytes, which enables completion of meiosis and further development after fertilization. Nonetheless, the mechanistic understanding of the meiotic spindle regeneration after cryopreservation is still scarce. Here, to gain insight into the mechanisms of the spindle disappearance and regeneration, we examined the status of spindle microtubules as well as the key components of the microtubule-organizing center (MTOC), specifically gamma-Tubulin, NEDD1, and Pericentrin, in mature (metaphase II) mouse oocytes at different steps of vitrification, a major cryopreservation technique. We found that the configuration of the spindle microtubules dynamically changed during the process of vitrification and that spindle regeneration was preceded by excessive microtubule polymerization, followed by reduction into the normal size and shape. Also, all three MTOC components exhibited disappearance and reappearance during the vitrification process, although Pericentrin appeared to regenerate in earlier steps compared to the other components. Furthermore, we found that the localization of the MTOC components to the spindle poles persisted even after depolymerization of spindle microtubules, suggesting that the MTOC components are impacted by vitrification independently from the integrity of the microtubules. The present study would set the stage for future investigations on the molecular mechanisms of the meiotic spindle regeneration, which may contribute to further improving protocols for oocyte cryopreservation.
Keywords: assisted reproductive technology, cryopreservation, meiotic spindle, oocyte, vitrification
The integrity of the meiotic spindle in the metaphase II oocyte, including the localization of microtubule bundles and distribution of the MTOC components, is dynamically altered during the course of the vitrification process.
INTRODUCTION
While assisted reproductive technologies (ARTs) have become the standard of care for many infertile individuals, there are still very limited options for female fertility preservation. By establishing cryopreservation of oocytes as the standard of care, it would give women a useful tool to preserve their fertility, including those who contract diseases or undergo procedures, like chemotherapy or radiation therapy for cancer, which may damage oocytes [1–4]. Oocyte cryopreservation would also benefit women who desire to postpone childbearing by cryopreserving intact oocytes that are retrieved at a younger age [2, 5–7]. Furthermore, this technology allows for the emergence of egg banks, easing and regulating the process of egg donations [2, 5, 8]. Currently, two types of cryopreservation methods are employed: slow freezing and vitrification. In slow freezing, oocytes are cooled down slowly for several hours with reduced concentrations of cryoprotectants to minimize their toxic effect [2, 9, 10]. In contrast, vitrification solidifies oocytes to a glass-like state through rapid cooling rates in the presence of high concentrations of cryoprotectants [2, 9, 10]. Many studies have suggested that vitrification is more advantageous than slow freezing for several reasons. Compared to slow freezing, vitrification can be performed using inexpensive equipment and less complicated manual techniques in a shorter amount of time [2, 6, 11]. Also, higher rates of oocyte survival, fertilization, embryo development, and pregnancy have been observed with vitrified oocytes compared to slow-frozen oocytes in various studies [2, 11–16]. Nonetheless, comparable numbers of live human births have been reported from both methods [17].
Due to the large size and high water content, oocytes are subjected to physical damage during cryopreservation, which may be inflicted by the formation of intracellular ice crystals, the solution effects, and osmotic shock [2, 9]. Such physical damages, together with the toxic impact from cryoprotectants, could severely impair survival, fertilization, and the developmental potential of cryopreserved oocytes [2, 9]. Furthermore, intracellular calcium concentration is aberrantly elevated after cryopreservation, which could trigger cortical granule release and cause premature hardening of the zona pellucida and parthenogenetic activation [2, 11, 14]. Also, DNA fragmentation, chromosome disorganization, aberrant gene expression, and damage to intracellular organelles, such as mitochondria, endoplasmic reticulum, and lysosomes, have been observed in the oocytes after cryopreservation [2, 10, 11, 14, 18]. Consequently, oocyte cryopreservation is technically more challenging compared to cryopreservation of sperm and embryos, which has already been adopted as standard procedures in most in vitro fertilization (IVF) clinics [19–21]. Oocyte cryopreservation was classified as an experimental procedure by the American Society for Reproductive Medicine until very recently [22], and further research endeavors are pivotal to enhance safety and consistency of the procedure [5, 6, 23].
One of the most prominent alterations caused by oocyte cryopreservation is the transient disappearance of the meiotic spindle. The meiotic spindle consists of bundles of microtubules that emanate from two acentriolar poles and hold chromosomes along the metaphase plate in mature oocytes that are arrested at metaphase II (MII) of meiosis. Upon fertilization, meiosis resumes, and the meiotic spindle segregates sister chromatids equally between the pronucleus and the second polar body. Thus, the meiotic spindle plays a critical role in generating a fertilized egg that contains the correct number and set of chromosomes [24, 25]. Many studies have found that microtubule bundles of the meiotic spindle depolymerize prior to or during the freezing process but reappear during the thawing and rehydration process of oocyte vitrification [2, 9–11, 14]. However, a recent report claims that the meiotic spindle does not disappear at any step of the vitrification process [26]. Some studies have shown increased incidence of chromosome misalignment and aneuploidy after oocyte vitrification [27–29], whereas other studies found no significant increase in such abnormalities [2, 14, 30]. Thus, controversy exists regarding how temporary disappearance of the meiotic spindle impacts chromosome stability, fertilization and embryonic development, and further investigation is warranted [7, 9, 11, 14, 15, 31]. Importantly, the mechanism of meiotic spindle regeneration after oocyte cryopreservation is essentially unknown. During normal oocyte maturation, assembly of the meiotic spindle takes place in concert with the progression of the meiotic cell cycle and is regulated by components of the microtubule-organizing centers (MTOCs), such as γ-Tubulin, Pericentrin, and NEDD1 proteins [32]. However, as vitrified oocytes are arrested at MII, it is unclear whether regeneration of the meiotic spindle after vitrification proceeds using the same machinery that constructs the meiotic spindle during normal meiosis.
Here, to gain insight into the mechanisms behind the meiotic spindle disappearance and regeneration, we examined the status of spindle microtubules and MTOC components in mouse MII oocytes at different steps of the vitrification procedure.
MATERIALS AND METHODS
Experimental Design
The purpose of the present study is to investigate the status of meiotic spindle during the process of oocyte vitrification, using the laboratory mouse as a model. First, in order to formulate proper fixation condition of oocytes for this study, we investigated the impact of microtubule-polymerizing agents D2O and Taxol, which are included in the fixative used in the previous study [26]. Second, oocytes were fixed at each step of the vitrification procedures, including dehydration, thawing, and warming steps, and then analyzed for the status of spindle microtubules and MTOC components by immunocytochemistry in order to determine structural changes in the meiotic spindle that may occur during the procedure. Finally, we tested whether the localization of MTOC components to the spindle poles was dependent on the integrity of spindle microtubules by depolymerizing microtubules at cold temperature.
Animals and Oocyte Collection
Outbred mice CD1 (Charles River Laboratories) and NIH Swiss (National Cancer Institute) were maintained within a controlled barrier facility in the University of Hawaii School of Medicine Biosciences Building in accordance with the policies of University of Hawaii's Institutional Animal Care and Use Committee. To collect mature oocytes at the metaphase II stage, 8- to 12-wk-old female mice were superovulated with intraperitoneal injections of 5 IU equine chorionic gonadotropin (Calbiochem) and then 48 h later with 5 IU human chorionic gonadotropin (hCG; Calbiochem). The mice were killed 16–17 h after the hCG injection, and their oviducts were collected and placed in a Petri dish with EmbryoMax FHM Hepes Buffered Medium (FHM; Millipore). The swollen ampullae of the oviducts were torn to release the cumulus-oocyte mass, which was incubated in FHM containing 75 U/ml hyaluronidase for 5–10 min, and aspirated through a narrow glass pipette to mechanically dissociate the cumulus cells. The abnormal-looking oocytes were discarded based on their morphology, such as presence of prominent and large cytoplasmic granules as well as excessive space between the oocyte and the zona pellucida. Only MII oocytes in good quality, judged by the presence of the first polar body, were used for the experiments. The oocytes deemed normal were incubated in EmbryoMax KSOM with 1/2 amino acids, glucose, and phenol red (KSOM; Millipore) at 37°C with 5% CO2 humidified air for about 20 min and then used for further experiments. Deuterium oxide (D2O) and paclitaxel (Taxol) were obtained commercially (Sigma). Parthenogenetic activation was induced by the method described previously, using the activation medium, that is, KSOM supplemented with 5 mM SrCl2 and 5 mM EGTA [33].
Oocyte Vitrification
All procedures were conducted at ambient temperature (22–24°C) unless noted otherwise, following the protocol reported previously [34]. Vitrification and devitrification was performed using the S3 system (Tyho-Galileo Research Laboratories), which utilizes glycerol and ethylene glycol as the major cryoprotectants. The oocytes were first placed in V1 for 5 min, then in V2 for 2 min, and finally in V3 for 2 min. Oocytes in V3 were aspirated into a denuding pipette (275–300 μm; Cook Medical), which was further encased in a CBS embryo straw (0.3 ml; Irvine Scientific) and then plunged into liquid nitrogen for freezing and storage [34]. For devitrification, the pipette containing vitrified oocytes was immersed in a 1 M sucrose solution at 37°C for 5 sec (this step is hereafter referred to as V3 postvitrification or V3PV), and contents were immediately transferred into T1. After incubation in T1 for 5 min, the oocytes were transferred into successive devitrification solutions (T2, T3, and T4) for 5 min each. The oocytes were then placed in T5 at 37°C for 5 min, followed by incubation in KSOM at 37°C with 5% CO2 humidified air for 2 h.
Immunocytochemistry
The oocytes that were unmanipulated or after the final 2-h incubation of the vitrification procedure (i.e., in KSOM) were fixed in 4% paraformaldehyde (PFA) in PBS for 20–30 min. To fix oocytes that were in the vitrification or devitrification solution (i.e., V1, V2, V3, T1, T3, or T5), 4% PFA was dissolved in the corresponding solution in order to minimize osmotic change upon fixation. The oocytes were washed in PBS containing 0.1% Tween20 (PBSw), permeabilized in 0.5% TritonX-100 in PBS for 15–20 min, and then incubated in PBSw containing 5% bovine serum albumin and 5 μg/ml RNaseA for 30 min. After a brief rinse in PBSw, the oocytes were incubated in PBSw containing one of the four primary antibodies for either 2 h at the ambient temperature or overnight at 4°C. The four antibodies used were mouse monoclonal anti-β-Tubulin antibody (TUB2.1; Sigma), mouse monoclonal anti-γ-Tubulin antibody (GTU-88; Sigma), mouse monoclonal anti-Pericentrin antibody (clone 30; BD Biosciences), and mouse monoclonal anti-NEDD1 antibody (7D10; Novus Biologicals). The oocytes were washed in PBSw and then incubated with AlexaFluor488-conjugated goat anti-mouse IgG (Invitrogen) for either 2 h at the ambient temperature or overnight at 4°C. The oocytes were washed in PBSw and then mounted in Vectashield mounting medium containing propidium iodide (PI; Vector Laboratories). The stained oocytes were observed under Zeiss Axiovert200 inverted fluorescent microscope, using optical filters for FITC and TRITC to visualize the Alexa-488 and PI signals, respectively. The images were captured using a Zeiss Digital Camera MRm and the Axio Vision program.
Time-Lapse Videomicroscopy
For time-lapse recording of activated oocytes, up to 20 oocytes were placed in a 20-μl drop of the activation medium in a Petri dish covered with mineral oil. The Petri dish was placed in Heating Insert P (PeCon), whose temperature and CO2 concentration were regulated by Tempcontrol 37-2 and CO2-Controller (PeCon), respectively. The Heating Insert P was enclosed in Incubator XL-3 (PeCon), attached to the inverted microscope with Hoffman Modulation Contrast optics. Images were captured every 10 min, starting from 3 min after the initial activation, using AxioCam MRm, controlled by the AxioVision software (Carl Zeiss).
Statistical Analysis
Student t-test was performed to compare distributions of spindle grades between two groups of samples, as indicated in the figure legends, and chi-square test was performed to compare ratio/percentage between two groups of samples, as specified in the text. P-values less than 0.05 were deemed significant.
RESULTS
Formation of Spindle-Like Microtubule Bundles Induced by Deuterium Oxide and Taxol
While loss of the meiotic spindle during oocyte cryopreservation has been reported in various studies [2, 9–11, 14], a recent study suggests that the meiotic spindle remains intact throughout the vitrification procedure [26]. However, we noticed that the fixative used in their study contains deuterium oxide (D2O) and Taxol [26]. Because these agents are known to promote microtubule polymerization [35–38], the actual status of spindle microtubules in the vitrified oocytes may not be accurately reflected in their observations. Indeed, D2O has been shown to stabilize spindle microtubules during cooling of mouse and human oocytes [39, 40]. To examine whether D2O and Taxol can cause microtubule polymerization after complete disappearance of the meiotic spindle, we conducted the following experiment. First, MII oocytes were incubated in FHM on ice for 45 min to depolymerize spindle microtubules, as microtubules are highly susceptible to cold temperatures [9, 14, 41–43]. This treatment completely depolymerized spindle microtubules in all the oocytes examined (n = 23), as shown by immunocytochemistry for β-Tubulin (Fig. 1, A and B). Cold-treated oocytes were then incubated in FHM, with or without D2O and Taxol, at the ambient temperature (22–24°C) for 30 min. After incubation in FHM without D2O or Taxol, no microtubules were detected around the oocyte chromosomes (n = 15; Fig. 1D). In contrast, the oocytes that were incubated in FHM containing D2O and Taxol exhibited distinct bundles of microtubules around the oocyte chromosomes, adopting an appearance similar to the meiotic spindle (n = 13; Fig. 1C). Thus, D2O and Taxol can induce formation of spindle-like microtubule bundles around the chromosomes even at the ambient temperature. We also investigated the impact of D2O and Taxol individually and found that each component can induce polymerization at ambient temperature. Taxol appeared more potent than D2O because D2O induced microtubule polymerization only around spindle poles (n = 9), whereas Taxol also induced transverse microtubules (n = 9). Therefore, either of these polymerizing agents should not be included in any steps for investigation of spindle microtubule status to avoid potential misinterpretation.
FIG. 1.
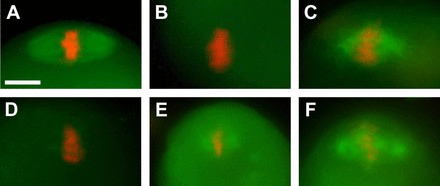
Formation of spindle-like microtubule bundles around the metaphase chromosomes induced by D2O and Taxol. Microtubules (green) are detected by immunocytochemistry for β-Tubulin, and chromosomes (red) are visualized with propidium iodide. A) Meiotic spindle in an unmanipulated MII oocyte. B) Depolymerization of spindle microtubules after incubation on ice for 45 min. C) Repolymerization of microtubule bundles around the metaphase chromosomes in a cold-treated oocyte after incubation in the presence of D2O (50%) and Taxol (1 μM) for 30 min. D) A cold-treated oocyte after incubation for 30 min in the absence of D2O or Taxol. E) Repolymerization of microtubule bundles around the metaphase chromosomes in a cold-treated oocyte by D2O alone. F) Repolymerization of microtubule bundles around the metaphase chromosomes in a cold-treated oocyte by Taxol alone. Bar = 10 μm.
Dynamic Disappearance and Reappearance of Spindle Microtubules During Oocyte Vitrification Procedure
In the present study, vitrification was performed using the protocol described in Materials and Methods. In our hands, this protocol yielded an 86.3% survival rate of oocytes (n = 95) through the devitrification and rehydration procedure up to the T5 step, and 96.2% of these oocytes remained viable during the subsequent 2 h of incubation in KSOM at 37°C (n = 26). To investigate alterations in the meiotic spindle during the vitrification procedure, oocytes were fixed at eight different steps, specifically, V1, V2, V3, V3PV, T1, T3, T5, and after the final incubation in KSOM and then examined by immunocytochemistry for β-Tubulin. A fixative was tailored for each step, namely, paraformaldehyde dissolved in the corresponding vitrification or devitrification solution, to minimize drastic change in osmotic pressure or chemical compositions at the beginning of fixation process (see Materials and Methods). The spindle microtubules exhibited varying degrees of morphological alteration at certain steps; therefore, we scored oocytes using a grading system from 0 to 5 based on the extent of microtubule polymerization or depolymerization (Fig. 2A and Table 1). Oocytes containing a spindle that was indistinguishable in shape and size from unmanipulated MII oocytes were scored grade 3. Grade 2 was assigned when the spindle was slightly but distinctly smaller in size (approximately 50% of the normal spindle or larger), whereas grade 1 was given to a severely diminished spindle that was markedly smaller in size (less than 50% of the normal spindle). An oocyte with no detectable spindle microtubules was scored grade 0. Some oocytes exhibited features of excessive microtubule polymerization, namely, widening of the spindle poles, presence of astral microtubules emanating from the spindle poles, and astral microtubules from cytoplasmic foci (Fig. 2A). We scored an oocyte with one or two of these features as grade 4 and that with all three of these features together as grade 5.
FIG. 2.
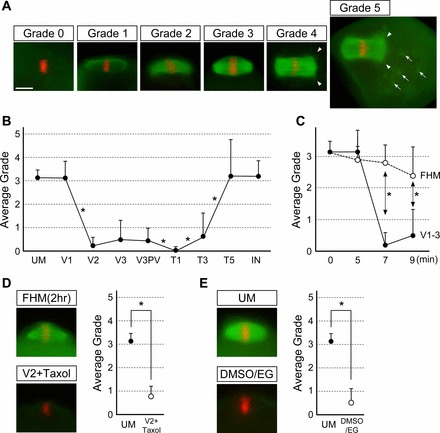
Disappearance and reappearance of spindle microtubules during the oocyte vitrification process. A) Grades of spindle microtubules: grade 0 = no detectable spindle microtubules; grade 1 = severely diminished spindle that is less than 50% of the normal spindle in size; grade 2 = mildly diminished spindle that is larger than 50% of the normal spindle; grade 3 = equivalent to the normal spindle in size and shape; grade 4 = spindle with excessive microtubule polymerization, namely, widening of the spindle poles, presence of astral microtubules emanating from the spindle poles (arrowheads), or astral microtubules from cytoplasmic foci (arrows); grade 5 = oocyte with all three features of excessive microtubule polymerization. B) A graph showing average grades of spindle microtubules at different steps of the vitrification procedure. Error bars represent standard deviations. Numbers of oocyte examined for each step are UM (unmanipulated; n = 22), V1 (n = 58), V2 (n = 42), V3 (n = 40), V3PV (postvitrification; n = 33), T1 (n = 35), T3 (n = 37), T5 (n = 29), IN (incubation for 2 h at 37°C; n = 27). Distributions of grades are compared between two adjacent groups by Student t-test, and asterisks represent statistically significant changes in average grade (P < 0.05). C) A graph showing comparison of average grades of spindle microtubules between oocytes treated with the vitrification solutions (corresponding to V1, V2, and V3 in B) and those incubated in FHM for the same durations. Error bars represent standard deviations. Distributions of grades are compared between the two treatments at the same incubation time point by Student t-test, and asterisks represent statistically significant differences (P < 0.05). D, top) Robust presence of spindle microtubules around the meiotic chromosomes after incubation in FHM at the ambient temperature for 2 h. D, bottom) Disappearance of spindle microtubules after the V2 step even in the presence of Taxol (1 μM). D, right) A graph showing comparison of average grades of spindle microtubules between unmanipulated (UM) oocytes and those in V2 containing Taxol. Error bars represent standard deviations. Distributions of grades are compared between two groups by Student t-test. Asterisks represent statistically significant difference (P < 0.05). E, left) Robust disappearance of spindle microtubules in oocytes that are treated with the vitrification solution consisting of DMSO and EG. E, right) A graph showing comparison of average grades of spindle microtubules between the two groups, and distributions of grades are compared by Student t-test. Error bars represent standard deviations. Asterisks represent statistically significant difference (P < 0.05). Bar = 10 μm.
TABLE 1.
Grading system to score the polymerization and depolymerization state of spindle microtubules.
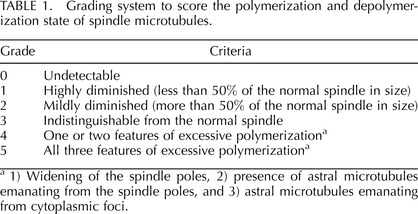
1) Widening of the spindle poles, 2) presence of astral microtubules emanating from the spindle poles, and 3) astral microtubules emanating from cytoplasmic foci.
Using this grading system, the status of spindle microtubules during the vitrification procedure is scored, as summarized in Figure 2B. Spindle microtubules were mostly unaffected in V1, whereas they were dramatically diminished in V2. The strikingly low average grade (0.19) in V2 was due to the absence of spindle microtubules in the majority of the oocytes examined (34 out of 42). Similarly, spindle microtubules were mostly absent in V3, although some oocytes (13 out of 40) exhibited detectable microtubules (grade 1 or higher). To determine whether the disappearance of spindle microtubules in V2 and V3 was caused by a prolonged exposure to the ambient temperature rather than to the vitrification solutions, we investigated the meiotic spindle in oocytes that were incubated in three successive FHM media drops using the identical times used during vitrification solution exposure at ambient temperature. Most of the oocytes still had an intact spindle even after the third FHM incubation (Fig. 2C). Surprisingly, incubation in FHM at the ambient temperature up to 2 h did not diminish spindle microtubules (n = 12; Fig. 2D). This suggests that the vitrification solutions (particularly V2) and not temperature alone largely contributed to depolymerization of the spindle microtubules. Spindle microtubules were also markedly diminished even in the presence of Taxol in V1 and V2 (Fig. 2D), indicating that the vitrification solutions strongly impact the integrity of meiotic spindle. Furthermore, to test whether disappearance of spindle microtubules was caused specifically by the vitrification solution used in this study (i.e., composed of ethylene glycol [EG] and glycerol), we examined the impact of another formula of vitrification solution that contains EG and dimethyl sulfoxide (DMSO) [26]. This formula also resulted in disappearance of spindle microtubules (n = 13; Fig. 2E).
We then examined the spindle microtubule status during the devitrification and rehydration process. Vitrified oocytes in V3 were rapidly warmed by immersing the carrier straw in a sucrose solution at 37°C for 5 sec. At this point (V3PV), spindle microtubules were still largely undetectable, although some exhibited detectable spindle microtubule bundles, similar to their appearance in V3 before vitrification. Interestingly, however, in the following T1, the average score further declined, as spindle microtubules were absent in essentially all oocytes examined (34 out of 35). In contrast, spindle microtubules were slightly but significantly repolymerized in T3 and much more so in T5. After the final incubation in KSOM, spindle microtubules were mostly restored, similar to those in the unmanipulated oocytes. Notably, even though the average grade for the T5 step (3.21) was very close to the average grade after the subsequent KSOM incubation (3.19), the incidence of grade 5 oocytes was significantly higher in the former (10 out of 29) than in the latter (2 out of 27; P < 0.01, chi-square test). This suggests that spindle microtubules actually polymerize excessively in T5 but then reduce to normal size during the final incubation.
In summary, spindle microtubules dynamically changed during the vitrification procedure. First, they were dramatically diminished in V2, which persisted in V3 before and after vitrification and warming. Spindle microtubules continued to diminish further in T1. Microtubule repolymerization first began in T3 and continued through T5. However, microtubule polymerization was first excessive in T5 but then normalized in size and shape during the final incubation at 37°C.
Incubation in KSOM After Thawing and Warming Is Crucial for Oocyte Survival
As described above, spindle microtubules were excessively polymerized in some of the oocytes at the end of T5, but they were adjusted to the normal size and shape after the 2-h incubation in KSOM. A question is whether oocytes before the final incubation are capable of resuming meiosis or whether additional incubation time is essential to rebuild the normal spindle that is competent for meiotic resumption. To test this, we measured timing and frequency of the second polar body emission using time-lapse videomicroscopy following parthenogenetic activation of oocytes before and after the final incubation (Fig. 3). We found that duration of incubation markedly impacted the rate of oocyte survival in response to activation. Namely, the survival rate of oocytes after the 2-h incubation was comparable to that of unmanipulated oocytes. In contrast, over 70% of the oocytes that were placed in KSOM after T5 only briefly (for about 1 min, indicated as “no incubation” in Fig. 3) died within 1 h after activation. Incubation for 20 min in KSOM significantly increased the survival rate (Fig. 3B; “No” vs. “20min” at 1 h postactivation, P < 0.001, chi-square test), but it was still significantly lower than the 2-h incubation (Fig. 3B, “2hr” vs. “20min” at 1 h postactivation, P < 0.001, chi-square test). This suggests that sufficient length of incubation in KSOM is important for survival of oocytes. Interestingly, however, regardless of length of incubation time, those oocytes that survived extruded the second polar body at similar timing and efficiency (Fig. 3C). Thus, the final incubation may be more crucial for oocyte survival in response to activation than acquisition of competence to resume meiosis.
FIG. 3.
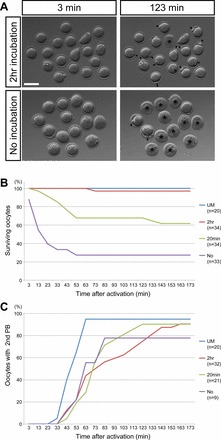
Time-lapse videomicroscopy of vitrified oocytes after parthenogenetic activation. A) Snapshot images of time-lapse recording of activated oocytes that have been vitrified and thawed. The top row shows images of activated oocytes that have been incubated for 2 h in KSOM after T5, and the bottom row shows images of those that have been activated soon after T5 without incubation. Asterisks indicate dead oocytes, and arrowheads point to the second polar body. Bar = 100 μm. B) Time course of survival of activated oocytes that have been incubated in KSOM for different durations. C) Time course of the second polar body (second BP) emission in surviving oocytes after activation. UM, unmanipulated oocytes. No, no incubation or less than 1 min of incubation.
Behavior of MTOC Components During Vitrification Process
To gain insight into the mechanisms of meiotic spindle disappearance and reappearance, we examined the localization of MTOC components, namely, γ-Tubulin, NEDD1, and Pericentrin, during the vitrification and warming processes. In unmanipulated MII oocytes, γ-Tubulin was intensely localized at the spindle poles and also along the spindle microtubules near the spindle poles (Fig. 4A). In contrast, NEDD1 and Pericentrin were tightly localized to the spindle poles in a distinct C shape or as a cluster of dots (Fig. 4, B and C).
FIG. 4.
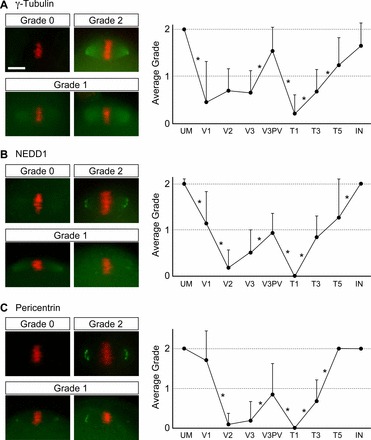
Disappearance and reappearance of MTOC components during oocyte vitrification process. A) γ-Tubulin. B) NEDD1. C) Pericentrin. Localizations of each MTOC component are scored based on their grades: grade 0 = no detectable localization, grade 1 = diminished and/or diffused localization, grade 2 = normal distribution. Representative images for each grade are shown on the left (green for the MTOC components and red for chromosomes). Graphs showing average grades of each MTOC component are shown on the right. Error bars represent standard deviations. Numbers of oocyte examined for each step are: UM (unmanipulated; n = 25, 53 and 36 [for γ-Tubulin, NEDD1, and Pericentrin, respectively]), V1 (n = 9, 16, and 7), V2 (n = 16, 23, and 22), V3 (n = 28, 32, and 39), V3PV (postvitrification; n = 13, 17, and 13), T1 (n = 25, 25, and 24), T3 (n = 27, 25, and 20), T5 (n = 13, 16, and 13), IN (incubation for 2 h at 37°C; n = 20, 16, and 12). Distributions of grades are compared between two adjacent steps by Student t-test, and asterisks represent statistically significant changes (P < 0.05). Bar = 10 μm.
The localization of the MTOC components noticeably diminished during the vitrification procedure. Thus, we scored the state of each component as follows (Fig. 4): grade 2: MTOC components were clear and indistinguishable from the unmanipulated oocytes; grade 0: the components were undetectable; grade 1: detectably weak or diffuse staining intermediate between 0 and 2.
The localization for γ-Tubulin was significantly diminished in V1 and remained low in V2 and V3 (Fig. 4A). In V3, 35.7% of oocytes (n = 28) had no detectable γ-Tubulin staining (grade 0). Interestingly, in V3PV, the γ-Tubulin staining was slightly but significantly increased, and none of the oocytes (n = 13) examined was grade 0. However, the γ-Tubulin was significantly diminished again in T1. In T3 and T5, the γ-Tubulin level gradually increased, and at the end of the final incubation in KSOM, 65.0% (n = 20) had the γ-Tubulin localization that was indistinguishable from normal oocytes (grade 2).
Similar to γ-Tubulin, NEDD1 started to diminish in V1. In V2, 82.6% of oocytes (n = 23) were grade 0. The NEDD1 localization was also transiently increased in V3PV, but it became undetectable in T1 in all oocytes (n = 25). NEDD1 localization was gradually restored in the following steps, and after the final incubation in KSOM, all oocytes (n = 16) were grade 2.
The dynamics of Pericentrin localization during vitrification were, overall, similar to the other MTOC components. However, Pericentrin also exhibited two unique behaviors. First, Pericentrin localization was not significantly altered in V1 but was significantly diminished in V2. Second, reappearance of Pericentrin took place earlier than the other two components, as all oocytes in T5 (n = 13) exhibited the normal localization pattern (grade 2).
In summary, all three MTOC components exhibited dynamic behaviors during vitrification, involving disappearance and reappearance similar to spindle microtubules.
Reappearance of Meiotic Spindle Components Is Not Dependent on Protein Synthesis
To test whether the reappearance of meiotic spindle requires synthesis of new tubulin or other proteins, protein synthesis was pharmacologically inhibited in oocytes during the thawing and warming steps by adding cycloheximide (CHX) to T1, T2, T3, T4, and T5. At the end of the T5 step, all of CHX-treated oocytes (n = 17) regenerated robust arrays of spindle microtubules that were indistinguishable from the control (n = 15). Also, distinct Pericentrin localization was observed at the spindle poles in all of CHX-treated oocytes (n = 17; Fig. 5A). These results suggest that regeneration of meiotic spindle components is due mainly to polymerization or reassembly of existing protein components rather than their new synthesis.
FIG. 5.
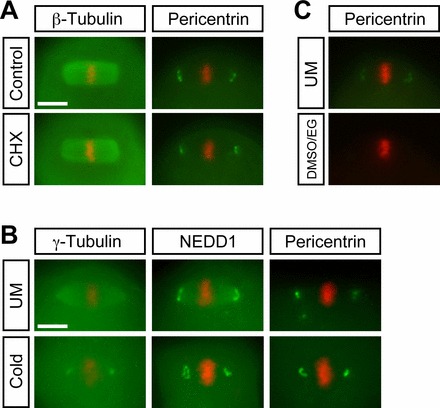
A) Protein synthesis-independent reappearance of spindle microtubules and Pericentrin during thawing and warming. CHX, cycloheximide (10 μM). B) Microtubule-independent localization of the MTOC components, γ-Tubulin, NEDD1, and Pericentrin, to the spindle poles. Distributions of the MTOC components (green) and chromosomes (red) are examined in unmanipulated (UM) and cold-treated (Cold) oocytes. C) Disappearance of Pericentrin localization in the vitrification solution consisting of DMSO and EG. Bar = 10 μm.
Localization of the MTOC Components to the Spindle Poles Is Not Dependent on Microtubule Integrity
Many studies, including ours, have shown that spindle microtubules are vulnerable to the vitrification process [2, 9–11, 14]. As shown above, the localization of the MTOC components to the spindle poles was also diminished by vitrification. Because the dynamics of disappearance and reappearance of microtubules and the MTOC components were similar, their integrity may be dependent on each other. Thus, we investigated whether the loss of spindle microtubules can cause disappearance of the MTOC components. MII oocytes were incubated in FHM on ice for 45 min to depolymerize spindle microtubules, and then the localizations of γ-Tubulin, NEDD1, and Pericentrin were assessed. The cold treatment depolymerized spindle microtubules in all oocytes examined, as described above. However, all three MTOC components were clearly localized to the presumptive spindle poles in all oocytes examined (Fig. 5B; n = 9 for γ-Tubulin, n = 17 for NEDD1, n = 11 for Pericentrin). The localization patterns of NEDD1 and Pericentrin in cold-treated oocytes were essentially indistinguishable from the unmanipulated oocytes. Interestingly, distribution of γ-Tubulin along the spindle microtubules was eliminated by cold treatment, whereas the localization to the poles was still intact, suggesting that the former but not the latter is dependent on spindle microtubules. Thus, the localization of all the MTOC components to the spindle poles was undisturbed by cold treatment, whereas spindle microtubules were depolymerized. This result indicates that vitrification diminishes the MTOC components at the spindle poles independently from the integrity of microtubules.
DISCUSSION
In the present study, we investigated the impact of vitrification on spindle microtubules and several MTOC components in order to obtain insight into the mechanisms of the meiotic spindle disappearance and regeneration during the vitrification process. Spindle microtubules were diminished in the vitrification solutions before oocyte freezing but gradually reappeared after warming, independently from new protein synthesis. Excessive polymerization of microtubules was observed in the last devitrification solution T5, but the spindle microtubules restored to a normal appearance after 2 h of incubation in KSOM at 37°C. This final incubation appeared to be critical for oocyte survival in response to activation. The MTOC components, namely, γ-Tubulin, NEDD1, and Pericentrin, also exhibited a dynamic behavior of disappearance and reappearance during the vitrification process. We also showed that the localization of MTOC components to the spindle poles was not dependent on spindle microtubules. Thus, the integrity and distribution of spindle microtubules as well as MTOC components dynamically change during the vitrification process.
Various studies have reported disappearance of the spindle microtubules during oocyte cryopreservation. Some observations were based on polarized light microscopy, which enables visualization of highly ordered molecules, namely, bundles of spindle microtubules, in live oocytes [31, 43–45]. Other observations were made by immunostaining, which allows detection of spindle microtubules in much higher resolutions, although it requires fixation of specimens [2, 9–11, 14]. While a recent immunostaining study reported that spindle microtubules are present throughout the oocyte vitrification procedure, we noticed that the fixative used in the study contains D2O and Taxol [26]. These two agents are known to promote microtubule polymerization [46–49] and indeed induced formation of spindle-like microtubule bundles in oocytes, where microtubules had been depolymerized by cold treatment, as shown in the present study. Thus, it is likely that inclusion of D2O and Taxol in the fixative may have caused aberrant polymerization of microtubules during fixation in the previous study [26]. Disappearance of the meiotic spindle during cryopreservation is also consistent with the cold-labile nature of microtubules [43, 50], and exposure even to room temperature causes abnormal spindle configuration in mouse and human oocytes [9, 14, 42]. Importantly, however, the present study showed that disappearance of spindle microtubules in the vitrification solutions are not solely due to exposure to room temperature, as incubation of oocytes in FHM at the ambient temperature for the duration comparable to the V1 to V3 treatments or even for 2 h did not significantly diminish the meiotic spindle. It is possible that cryoprotectants in the vitrification solutions enhance destabilization of spindle microtubules. Paradoxically, however, some cryoprotectants, such as DMSO and propanediol, have been shown to exhibit protective effects against low-temperature-induced depolymerization of microtubules [14]. Nonetheless, the vitrification solution containing DMSO still caused disappearance of meiotic spindles, as shown in the present study. Thus, further studies are needed to examine the varying effects of cryoprotectants, at different concentrations as well as different combinations with other compounds, in vitrification solutions on the meiotic spindle.
Various studies have also reported that the meiotic spindle is restored in the cryopreserved oocytes after thawing and incubation at 37°C, although the timing of recovery varies markedly among studies [9, 14, 42]. The present study showed that the process of spindle restoration is highly dynamic and complex. Oocytes at the T5 step exhibited excessive polymerization of microtubules, exemplified by widening of the spindle poles and formation of astral microtubules from the spindle poles, as well as cytoplasmic foci. These microtubule irregularities were reduced during the following warming step, and the spindle in most oocytes adopted a normal size and shape by the end of the final incubation period. It is of particular importance to examine whether oocytes with an enlarged meiotic spindle and excessive astral microtubules yield abnormalities in chromosome segregation and embryonic development after fertilization. Many oocytes at the end of T5 died in response to parthenogenetic activation, whereas surviving oocytes resumed meiosis with efficiency similar to 2-h-incubated oocytes. Currently, it is not clear whether there is correlation between survivability of oocytes and normalcy of regenerated meiotic spindle. However, it is likely that the final incubation is also crucial for regenerating other aspects of oocyte integrity that are essential for survival during the process of oocyte activation, as it involves dynamic changes in cortical cytoskeleton and organelles [51, 52].
The MTOC components, such as γ-Tubulin, NEDD1, and Pericentrin, play essential roles in generation of the spindle during meiosis, as demonstrated by knockdown experiments [32, 53]. To our knowledge, the present study is the first to examine behaviors of the MTOC components during oocyte vitrification process. All three MTOC components were diminished by the V2 step, which coincided with disappearance of spindle microtubules. Whether the loss of MTOC components contributed to depolymerization of microtubules is unknown. However, it is unlikely that the disappearance of microtubules diminished the MTOC components in the vitrification solutions because the MTOC components were stably localized to the spindle poles even after cold-induced depolymerization of spindle microtubules, as shown in the present study. After warming of vitrified oocytes, the MTOC components reappeared, which also coincided with reappearance of spindle microtubules. Considering the essential roles of the MTOC components in the spindle formation during meiosis, it is possible that reappearance of the MTOC may be a prerequisite for polymerization of spindle microtubules. In the present study, Pericentrin exhibited the speediest recovery after thawing, as its normal configuration was fully restored by the T5 step, as compared to the other MTOC components, which were fully recovered only later after the final incubation. Interestingly, Pericentrin plays the key role in recruiting other components, including γ-Tubulin and NEDD1, to MTOC during mitosis and meiosis [32, 53]. Thus, Pericentrin may also play a key role in assembling functional MTOC proteins after warming of vitrified oocytes.
In light of human ART, examination of meiotic spindle dynamics during the vitrification process in human oocytes is of utmost importance. The present study exploited the mouse as a model animal to investigate the behavior of meiotic spindle, focusing on spindle microtubules as well as the MTOC components, during the vitrification, thawing, and warming processes. This was possible because laboratory mice can supply a large number of high-quality oocytes in a consistent manner, which was crucial to conduct all experiments presented in this study. In general, it is extremely challenging to obtain a large number of high-quality human MII oocytes to conduct the same types of experiments. Thus, studies using oocytes of model animals could still be beneficial to gain insight into the impact of vitrification procedure on oocytes. However, one needs to be careful about interpretations of experimental outcomes of model animals because there may be significant differences in physiological and structural properties in human oocytes [54]. The American Society for Reproductive Medicine (ASRM) removed the “experimental” label from mature oocyte cryopreservation from their guideline very recently [22]. Even though spindle abnormalities in cryopreserved oocytes were originally of concern, the incidence of chromosomal abnormalities in human embryos derived from cryopreserved oocytes is not statistically different from that of control embryos [30], which is reflected in the new ASRM guideline. Nonetheless, the disappearance of the meiotic spindle and its later reappearance are highly unphysiological events, and continuous research on these processes at the molecular levels is essential to assess oocyte cryopreservation protocols more comprehensively.
ACKNOWLEDGMENT
The authors are grateful to Dr. Vernadeth B. Alarcon for discussion and continuous encouragement throughout this study. Ms. Aileen Y. Tanaka participated in a pilot study, which later led to the Taxol experiments presented in the present study. The authors also thank Ms. Dana Ann A. Tamashiro, Dr. Yukiko Yamazaki, Dr. Scott Lozanoff, and the Developmental and Reproductive Biology Graduate Program at the University of Hawaii for various supports.
Footnotes
Supported by the National Institutes of Health (R03 HD068648).
REFERENCES
- Lobo RA. Potential options for preservation of fertility in women. N Engl J Med 2005; 353 (1): 64–73. [DOI] [PubMed] [Google Scholar]
- Jain JK, Paulson RJ. Oocyte cryopreservation. Fertil Steril 2006; 86 (suppl 4): 1037–1046. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Sheehan CB, Rienzi L, Katz-Jaffe M, Larman MG. Analysis of oocyte physiology to improve cryopreservation procedures. Theriogenology 2007; 67 (1): 64–72. [DOI] [PubMed] [Google Scholar]
- Küçük M. Fertility preservation for women with malignant diseases: ethical aspects and risks. Gynecol Endocrinol 2012; 28 (12): 937–940. [DOI] [PubMed] [Google Scholar]
- De Melo-Martin I, Cholst IN. Researching human oocyte cryopreservation: ethical issues. Fertil Steril 2008; 89 (3): 523–528. [DOI] [PubMed] [Google Scholar]
- Homburg R, van der Veen F, Silber SJ. Oocyte vitrification—women's emancipation set in stone. Fertil Steril 2009; 91 (suppl 4): 1319–1320. [DOI] [PubMed] [Google Scholar]
- Noyes N, Knopman J, Labella P, McCaffrey C, Clark-Williams M, Grifo J. Oocyte cryopreservation outcomes including pre-cryopreservation and post-thaw meiotic spindle evaluation following slow cooling and vitrification of human oocytes. Fertil Steril 2010; 94 (6): 2078–2082. [DOI] [PubMed] [Google Scholar]
- Cobo A, Remohí J, Chang CC, Nagy ZP. Oocyte cryopreservation for donor egg banking. Reprod Biomed Online 2011; 23 (3): 341–346. [DOI] [PubMed] [Google Scholar]
- Koutlaki N, Schoepper B, Maroulis G, Diedrich K, Al-Hasani S. Human oocyte cryopreservation: past, present and future. Reprod Biomed Online 2006; 13 (3): 427–436. [DOI] [PubMed] [Google Scholar]
- Martínez-Burgos M, Herrero L, Megías D, Salvanes R, Montoya MC, Cobo AC, Garcia-Velasco JA. Vitrification versus slow freezing of oocytes: effects on morphologic appearance, meiotic spindle configuration, and DNA damage. Fertil Steril 2011; 95 (1): 374–377. [DOI] [PubMed] [Google Scholar]
- Varghese AC, Nagy ZP, Agarwal A. Current trends, biological foundations and future prospects of oocyte and embryo cryopreservation. Reprod Biomed Online 2009; 19 (1): 126–140. [DOI] [PubMed] [Google Scholar]
- Lane M, Gardner DK. Vitrification of mouse oocytes using a nylon loop. Mol Reprod Dev 2001; 58 (3): 342–347. [DOI] [PubMed] [Google Scholar]
- Kuleshova LL, Lopata A. Vitrification can be more favorable than slow cooling. Fertil Steril 2002; 78 (3): 449–454. [DOI] [PubMed] [Google Scholar]
- Gook DA, Edgar DH. Human oocyte cryopreservation. Hum Reprod Update 2007; 13 (6): 591–605. [DOI] [PubMed] [Google Scholar]
- Huang JY, Chen HY, Park JY, Tan SL, Chian RC. Comparison of spindle and chromosome configuration in in vitro- and in vivo-matured mouse oocytes after vitrification. Fertil Steril 2008; 90 (suppl 4): 1424–1432. [DOI] [PubMed] [Google Scholar]
- Edgar DH, Gook DA. A critical appraisal of cryopreservation (slow cooling versus vitrification) of human oocytes and embryos. Hum Reprod Update 2012; 18 (5): 536–554. [DOI] [PubMed] [Google Scholar]
- Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online 2009; 18 (6): 769–776. [DOI] [PubMed] [Google Scholar]
- Monzo C, Haouzi D, Roman K, Assou S, Dechaud H, Hamamah S. Slow freezing and vitrification differentially modify the gene expression profile of human metaphase II oocytes. Hum Reprod 2012; 27 (7): 2160–2168. [DOI] [PubMed] [Google Scholar]
- Bunge RG, Sherman JK. Frozen human semen. Fertil Steril 1954; 5 (2): 193–194. [DOI] [PubMed] [Google Scholar]
- Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature 1983; 305 (5936): 707–709. [DOI] [PubMed] [Google Scholar]
- Zeilmaker GH, Alberda AT, van Gent I, Rijkmans CM, Drogendijk AC. Two pregnancies following transfer of intact frozen-thawed embryos. Fertil Steril 1984; 42 (2): 293–296. [DOI] [PubMed] [Google Scholar]
- The Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril 2013; 99 (1): 37–43; DOI:10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- Albertini DF. The dawning of a new ice age for human oocyte cryopreservation. J Assist Reprod Genet 2011; 28 (12): 1141–1142. DOI:10.1007/s10815-011-9692-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde S, Heald R. Mechanisms and molecules of the mitotic spindle. Curr Biol 2004; 14 (18): R797–R805. [DOI] [PubMed] [Google Scholar]
- Dumont J, Desai A. A centrosomal spindle assembly and chromosome segregation during oocyte meiosis. Trends Cell Biol 2012; 22 (5): 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Lin CJ, Sung LY, Kort HI, Tian XC, Nagy ZP. Impact of phase transition on the mouse oocyte spindle during vitrification. Reprod Biomed Online 2011; 22 (2): 184–191. [DOI] [PubMed] [Google Scholar]
- Kola I, Kirby C, Shaw J, Davey A, Trounson A. Vitrification of mouse oocytes results in aneuploid zygotes and malformed fetuses. Teratology 1988; 38 (5): 467–474. [DOI] [PubMed] [Google Scholar]
- Huang JY, Chen HY, Tan SL, Chian RC. Effect of choline-supplemented sodium-depleted slow freezing versus vitrification on mouse oocyte meiotic spindles and chromosome abnormalities. Fertil Steril 2007; 88 (suppl 4): 1093–1100. [DOI] [PubMed] [Google Scholar]
- Coticchio G, Bromfield JJ, Sciajno R, Gambardella A, Scaravelli G, Borini A, Albertini DF. Vitrification may increase the rate of chromosome misalignment in the metaphase II spindle of human mature oocytes. Reprod Biomed Online 2009; 19 (suppl 3): 29–34. [DOI] [PubMed] [Google Scholar]
- Forman EJ, Li X, Ferry KM, Scott K, Treff NR, Scott RT Jr. Oocyte vitrification does not increased the risk of embryonic aneuploidy or diminish the implantation potential of blastocysts created after intracytoplasmic sperm injection: a novel, paired randomized controlled trial using DNA fingerprinting. Fertil Steril 2012; 98 (3): 644–649. [DOI] [PubMed] [Google Scholar]
- Gomes CM, Silva CA, Acevedo N, Baracat E, Serafini P, Smith GD. Influence of vitrification on mouse metaphase II oocyte spindle dynamics and chromatin alignment. Fertil Steril 2008; (suppl 4): 1396–1404. [DOI] [PubMed]
- Ma W, Baumann C, Viveiros MM. NEDD1 is crucial for meiotic spindle stability and accurate chromosome segregation in mammalian oocytes. Dev Biol 2010; 339 (2): 439–450. [DOI] [PubMed] [Google Scholar]
- Kishigami S, Wakayama T. Efficient strontium-induced activation of mouse oocytes in standard culture media by chelating calcium. J Reprod Dev 2007; 53 (6): 1207–1215. [DOI] [PubMed] [Google Scholar]
- Schiewe MC, Fahy GM. Validation of a simple and effective sterile vitrification system for embryos. Fertil Steril 2009; 90 (suppl 1): S288. [Google Scholar]
- Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods CM. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res 1995; 55 (11): 2325–2333. [PubMed] [Google Scholar]
- Chakrabarti G, Kim S, Gupta ML Jr, Barton JS, Himes RH. Stabilization of tubulin by deuterium oxide. Biochemistry 1999; 38 (10): 3067–3072. [DOI] [PubMed] [Google Scholar]
- Panda D, Chakrabarti G, Hudson J, Pigg K, Miller HP, Wilson L, Himes RH. Suppression of microtubule dynamic instability and treadmilling by deuterium oxide. Biochemistry 2000; 39 (17): 5075–5081. [DOI] [PubMed] [Google Scholar]
- Jordan MA. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr Med Chem Anticancer Agents 2002; 2 (1): 1–17. [DOI] [PubMed] [Google Scholar]
- Liu L, Inoue S, Trimarchi JR, Plante BJ, Keefe DL. Deuterium oxide (D2O) stabilizes meiotic spindles during cooling. Fertil Steril 2005; 84 (suppl 1): S381. [Google Scholar]
- Plante BJ, Liu L, Trimarchi JR, Jurema M, Keefe DL. Deuterium oxide (D2O) stabilizes meiotic spindles in living human oocytes during cooling. Fertil Steril 2005; 84 (suppl 1): S63–S64. [Google Scholar]
- Pickering SJ, Johnson MH. The influence of cooling on the organization of the meiotic spindle of the mouse oocytes. Hum Reprod 1987; 2 (3): 207–216. [DOI] [PubMed] [Google Scholar]
- Larman MG, Minasi MG, Rienzi L, Gardner DK. Maintenance of the meiotic spindle during vitrification in human and mouse oocytes. Reprod Biomed Online 2007; 15 (6): 692–700. [DOI] [PubMed] [Google Scholar]
- Gomes C, Merlini M, Konheim J, Serafini P, Motta EL, Baracat EC, Smith GD. Oocyte meiotic-stage-specific differences in spindle depolymerization in response to temperature changes monitored with polarized field microscopy and immunocytochemistry. Fertil Steril 2012; 97 (3): 714–719. [DOI] [PubMed] [Google Scholar]
- Chen CK, Wang CW, Tsai WJ, Hsieh LL, Wang HS, Soong YK. Evaluation of meiotic spindles in thawed oocytes after vitrification using polarized light microscopy. Fertil Steril 2004; 82 (3): 666–672. [DOI] [PubMed] [Google Scholar]
- Sereni E, Sciajno R, Fava L, Coticchio G, Bonu MA, Borini A. A. PolScope evaluation of meiotic spindle dynamics in frozen-thawed oocytes. Reprod Biomed Online 2009; 19 (2): 191–197. [DOI] [PubMed] [Google Scholar]
- Lamprecht J, Schroeter D, Paweletz N. Derangement of microtubule arrays in interphase and mitotic PtK2 cells treated with deuterium oxide (heavy water). J Cell Sci 1991; 98 (pt 4): 463–473. [DOI] [PubMed] [Google Scholar]
- Horwitz SB. Taxol (paclitaxel): mechanisms of action. Ann Oncol 1994; 5 (suppl 6): S3–S6. [PubMed] [Google Scholar]
- Krendel M, Inoué S. Anaphase spindle dynamics under D2O-enhanced microtubule polymerization. Biol Bull 1995; 189 (2): 204–205. [DOI] [PubMed] [Google Scholar]
- Abal M, Andreu JM, Barasoain I. Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr Cancer Drug Targets 2003; 3 (3): 193–203. [DOI] [PubMed] [Google Scholar]
- Sathananthan AH, Kirby C, Trounson A, Philipatos D, Shaw J. The effects of cooling mouse oocytes. J Assist Reprod Genet 1992; 9 (2): 139–148. [DOI] [PubMed] [Google Scholar]
- Brunet S, Maro B. Cytoskeleton and cell cycle control during meiotic maturation of the mouse oocyte: integrating time and space. Reproduction 2005; 130 (6): 801–811. [DOI] [PubMed] [Google Scholar]
- Miao YL, Williams CJ. Calcium signaling in mammalian egg activation and embryo development: the influence of subcellular localization. Mol Reprod Dev 2012; 79 (11): 742–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman WC, Sillibourne J, Rosa J, Doxsey SJ. Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol Biol Cell 2004; 15 (8): 3642–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers MC, Biggers JD. Chemically defined media and the culture of mammalian preimplantation embryos: historical perspective and current issues. Hum Reprod Update 2003; 9 (6): 557–582. [DOI] [PubMed] [Google Scholar]


