ABSTRACT
The four isoforms of serine/threonine phosphoprotein phosphatase 1 (PP1), derived from three genes, are among the most conserved proteins known. The Ppp1cc gene encodes two alternatively spliced variants, PP1 gamma1 (PPP1CC1) and PP1 gamma2 (PPP1CC2). Global deletion of the Ppp1cc gene, which causes loss of both isoforms, results in male infertility due to impaired spermatogenesis. This phenotype was assumed to be due to the loss of PPP1CC2, which is abundant in testis. While PPP1CC2 is predominant, other PP1 isoforms are also expressed in testis. Given the significant homology between the four PP1 isoforms, the lack of compensation by the other PP1 isoforms for loss of one, only in testis, is surprising. Here we document, for the first time, expression patterns of the PP1 isoforms in postnatal developing and adult mouse testis. The timing and sites of testis expression of PPP1CC1 and PPP1CC2 in testis are nonoverlapping. PPP1CC2 is the only one of the four PP1 isoforms not detected in sertoli cells and spermatogonia. Conversely, PPP1CC2 may be the only PP1 isoform expressed in postmeiotic germ cells. Deletion of the Ppp1cc gene in germ cells at the differentiated spermatogonia stage of development and beyond in Stra8 promoter-driven Cre transgenic mice results in oligo-terato-asthenozoospermia and male infertility, thus phenocopying global Ppp1cc null (−/−) mice. Taken together, these results confirm that spermatogenic defects observed in the global Ppp1cc knockout mice and in mice expressing low levels of PPP1CC2 in testis are due to compromised functions of PPP1CC2 in meiotic and postmeiotic germ cells.
Keywords: CRE, conditional knockout, gametogenesis, knock down, male fertility, male infertility, phosphatases, phosphoprotein phosphatases, promoter, serine/threonine phosphatase, spermatogenesis, transgenic
PPP1CC1 expression in Sertoli cells and premeiotic germ cells does not substitute for the loss of the PPP1CC2 isoform in developing germ cells.
INTRODUCTION
Steady-state levels of protein phosphorylation are due to the combined action of kinases and phosphatases. Protein kinases and phosphatases form major intracellular signaling mechanisms that are involved in a variety of cellular and physiological processes. The isoforms of phospho-protein phosphatase 1 (PP1) catalytic subunit (PPP1C) belong to the family of serine/threonine phosphatases. Other members of the family include PP2A, PP2B, PP4, PP5, PP6, and PP7. Based on data from genome databases, about 3% of eukaryotic genes are protein kinases, whereas the percentage is considerably lower in phosphatases in general, serine/threonine phosphatase in particular [1]. Consequently, the diversity and function of the phosphatases and of the PP1 catalytic subunits are controlled by myriad regulatory subunits [1, 2]. PP1 has been implicated in various eukaryotic cell functions, including metabolism, cell cycle regulation, and apoptosis [1, 3, 4]. In eukaryotes, three genes, Ppp1ca, Ppp1cb, and Ppp1cc, encode four PP1 isoforms, PP1 alpha (PPP1CA), PP1 beta (PPP1CB), PP1 gamma1 (PPP1CC1), and PP1 gamma2 (PPP1CC2), but only one gene (Glc7) in the yeast S. cerevisiae [5]. The isoforms PPP1CC1 and PPP1CC2 are from differentially spliced transcripts of Ppp1cc gene [5]. Mammalian PP1 isoforms share a high degree of amino acid sequence homology (40–297 amino acid, ∼90% idenity) and are among the most evolutionarily conserved proteins [6]. The PP1 isoforms differ only at the extreme N- and C-termini that are thought to play roles in binding to diverse regulatory subunits [2]. This remarkably high degree of conservation of PP1 proteins enables substitution of the S. cerevisiae PP1 (Glc7p), which is essential for its viability, with any of the mammalian isoforms [7].
While PPP1CA, PPP1CB, and PPP1CC1 are ubiquitous, PPP1CC2 is present at high levels in adult testis and spermatozoa [2, 6, 8–10]. It is notable that Ppp1cc2 is derived by alternate splicing of the Ppp1cc1 transcript, a splicing event that occurs only in mammals. Mammalian PPP1CC2 differs from PPP1CC1 and other PP1 isoforms in having a unique ∼22-amino acid segment. Whether this C-terminus confers specific biochemical properties for PPP1CC2 is not known [2, 5, 11]. It is significant that PPP1CC2 is the sole PP1 isoform detected in mature spermatozoa even though all four isoforms are expressed in testis. The PPP1CC2 is detected in sperm from all mammalian species tested [12].
Our laboratory was the first to show that PPP1CC2 plays a key role in sperm motility [13, 14]. High protein phosphatase activity is inversely correlated with sperm motility such that inhibition of PP1 catalytic activity results in motility initiation in immotile sperm and increased kinetic activity of motile bovine and monkey epididymal and human ejaculated spermatozoa [14, 15]. It was subsequently found that targeted disruption of the Ppp1cc gene, which eliminates both splice variants PPP1CC1 and PPP1CC2, results in male infertility due to impaired spermatogenesis [16]. Females appear normal, suggesting that PPP1CA and PPP1CB can substitute for the absence of PPP1CC isoforms in all tissues except testis [16]. It is also notable that global deletion of PPP1CA has no recognizable phenotype, suggesting that other PP1 isoforms can substitute for its loss (Nairn, unpublished data). The lack of compensation by other PP1 isoforms for the loss of PPP1CC isoforms only in testis is intriguing.
The fact that high levels of PPP1CC2 are expressed in testis led to the tentative conclusion that the loss of PPP1CC2, and not PPP1CC1, is responsible for the impaired spermatogenesis and male infertility of Ppp1cc-null mice [16, 17]. However, the possibility that the phenotype of Ppp1cc-null mice could be due to the combined loss of both PPP1CC1 and PPP1CC2 cannot yet be ruled out. We attempted to address this issue by determining if PPP1CC2, transgenically expressed in developing spermatogenic cells, could completely restore spermatogenesis and male fertility in Ppp1cc-null mice [18]. In these rescue studies, PPP1CC2 transgene expression in meiotic and postmeiotic cells was able to restore sperm numbers, normal sperm morphogenesis, and motility in Ppp1cc-null mice only when its expression level was equal to or above 50% of that in the testis of heterozygous (Ppp1cc+/−) mice. At lower PPP1CC2 levels, significant sperm production occurred, but sperm were malformed with low motility and, consequently, males remained infertile [18]. This apparent stoichiometric requirement of an enzyme such as PP1 for normal sperm morphogenesis to occur, in a manner similar to what would be expected for a structural protein, is intriguing. Therefore, the puzzle of why other PP1 isoforms cannot substitute for PPP1CC2 either in the global knockout mice or in Ppp1cc-null mice transgenically expressing low levels of PPP1CC2 remains.
Experiments reported here first examine expression of the four PP1 isoforms in developing and adult mouse testis. In the next set of experiments, we used a conditional knockout approach to eliminate the Ppp1cc gene in testis starting from differentiating spermatogonia using Stra8-Cre trangenic mice. This approach was undertaken to determine whether the phenotype of this selective knockout would resemble the global knockout.
We show that sites of expression in mouse testis of the four PP1 isoforms, of which PPP1CC1 and PPP1CC2 are non-overlapping. Expression of PPP1CC1 is essentially unaltered in the testis of conditional knockout compared to wild-type mice, suggesting that PPP1CC1 is present only in testicular somatic cells and spermatogonia and that PPP1CC2 is present exclusively in postmeiotic cells. High levels of Ppp1cc2 mRNA also suggest that both high promoter activity and efficient splicing mechanisms operate to generate abundant levels of Ppp1cc2 exclusively in meiotic and postmeiotic germ cells. We document, for the first time, the virtual absence of PPP1CC2 in Sertoli cells. Male infertility is the only phenotype resulting from the selective mutation of the Ppp1cc gene. Deletion of Ppp1cc only alters testis levels of PPP1CC2, but not PPP1CC1; this confirms that the loss of PPP1CC2 is responsible for impaired sperm generation and male infertility. The three isoforms PPP1CC1, PPP1CA, and PPP1CB, perhaps due to their presence largely in somatic and premeiotic germ cells in testis, cannot compensate for the loss of PPP1CC2 in meiotic and haploid germ cells. Finally, the conditional knockout studies also support our previous observation that adequate levels of PPP1CC2 are required for normal spermatogenesis and male fertility.
MATERIALS AND METHODS
Ethics Statement
Mice used in this study were housed and used at the Kent State University animal facility. Housing and handling was in accordance with the Kent State Institutional Animal Care and Use Committee under protocol 268DK 09-09 approved by the National Institute of Environmental Health Sciences Animal Care and Use Committee.
RNA Isolation and Probe Selection for Northern Blot Analysis
Total RNA from the testis of adult mice (8 wk or older) was isolated using TRI reagent (Sigma Aldrich) following the manufacturer's protocol. For total RNA isolation, testes were dissected and pooled from littermate male pups at ages 5, 7, 10, 15, 21, and 30 days. A restriction-digested and gel-purified fragment spanning the full length of the Ppp1cc2 cDNA (which should hybridize to messages corresponding to both Ppp1cc isoforms) was used as a probe. Ppp1cc1 mRNA is ∼2.3 kb, whereas Ppp1cc2 is ∼1.4 kb. A 327-bp probe from the unique 3′UTR region of Ppp1ca was PCR amplified from the Ppp1ca cDNA clone (from ATCC #7490695) using the forward 5′-CCTCCATGTGCTGCCCTTCTG-3′ and reverse 5′-GAGAATCCAGCTTTGACCTTTATTC-3′ primers. Similarly a ∼410-bp size probe was generated from the unique 3′UTR of the Ppp1cb isoform from the cDNA clone (from ATCC #10698998) using the forward 5′-TAAGGGTTAGCATTAACAAATG-3′ and the reverse 5′-TTACTAAATGAGAAAACTAC-3′ primers. Ppp1ca and Ppp1cb mRNAs are ∼1.3 kb and ∼4.3 kb, respectively. Northern blotting was performed as previously described [19]. The blots were reprobed with a β-actin probe to control for RNA loading. Total RNA (25 μg) was used for each lane for all samples studied.
Generation of Ppp1cc Germ Cell Knockout Mice
Mice bearing a Ppp1cc floxed allele, Ppp1cc+/flx (exon 2 and 3 were flanked by Lox-P sites), were generated in collaboration with Lexicon Genetics. The mice were bred to be homozygous for the floxed allele (Ppp1cc flx/flx). The germ cell expressing CRE transgenic mice driven by the 1.4-kb genomic region of the mouse Str8 promoter was purchased from Jackson Laboratories (008208 STOCK Tg [Stra8-cre] 1Reb/J). These mice have been characterized to show that the 1.4-kb promoter region is able to drive CRE expression only in males from 3 days postpartum (dpp) to preleptotene spermatocytes and that no CRE expression was detected in ovaries of female mice [20]. We crossed the Ppp1cc flx/flx mice with TgStra8-Cre mice to get Ppp1cc+/Δflx; TgStra8-Cre males in the first generation. In the subsequent generation, Ppp1cc+/Δflx; TgStra8-Cre males were crossed with Ppp1cc flx/flx female mice to produce offspring where 25% of the progeny have the genotype Ppp1cc flx/Δflx; TgStra8-Cre. Of which only the males undergo recombination in the germ cells to produce gametes where the Ppp1cc gene is knocked out. Since the Ppp1cc flx/Δflx; TgStra8-Cre females bred normally, they were crossed to Ppp1cc flx/flx males to maintain a steady supply of germ cell knockout (GcKO) animals. This maintenance cross yields two types of male pups, with genotypes Ppp1cc flx/flx; TgStra8-Cre and Ppp1cc flx/Δflx; TgStra8-Cre. Mice with the genotype Ppp1cc flx/Δflx; TgStra8-Cre that already had one floxed allele that underwent deletion (Δflx) were used to enhance the CRE-mediated excision rate of the remaining floxed allele. All transgene transmission followed the expected Mendelian ratios.
Genotyping and RT-PCR
Genotyping was performed by PCR with genomic DNA isolated from ear punches by alkaline lysis. The following primers were used for genotyping to distinguish between floxed and wild-type alleles: 5′-CTAACATAGCTTGAAGTATACAACTG-3′, 5′-CATATCTTGAGTGGTGCTTC-3′, and 5′-GAGACAGCTGACTCTACAC-3′. Animals heterozygous for the floxed allele (Ppp1cc+/flx) are distinguished from wild-type animals in having bands at positions 750 bp and 600 bp, respectively. Animals homozygous for the floxed allele (Ppp1cc flx/flx) show a single band of size 750 bp corresponding to the floxed allele, in contrast to wild-type animals, which show a single band of ∼600 bp. The primer pair used for detection of the Stra8-Cre transgene was forward primer 5′-GTGCAAGCTGAACAACAGGA-3′ and reverse primer 5′- AGGGACACAGCATTGGAGTC-3′. The transgene was detected as a ∼179-bp band on 1.5% agarose gel. RNA isolated from testis, brain, spleen, liver, and kidney was reverse transcribed using the QuantiTect Reverse Transcription kit (from Qiagen) following the manufacturer's protocol. The primer pair used for detection of the Cre cDNA was forward 5′-GTGCAAGCTGAACAACAGGA-3′ and reverse 5′- GTCTTGGTCCTGCCAATGTG-3′ [20]. The primer pair used for actin was 5′-GGTACCACCAGACAGCAC-3′ and 5′-TCTAGACTTCGAGCAGGAG-3′.
Preparation of Mouse Whole Testis and Sertoli Cell Protein Extracts
Adult, male mice 8–12 wk old were used for tissue and sperm extract preparation as described [18]. Sertoli cell isolation was performed as described previously [21], and Sertoli cells were isolated from 20-day-old Sprague-Dawley rats and cultured in serum-free media. Briefly, decapsulated testes were digested with collagenase (0.5 mg/ml, 33°C, 12 min) in enriched Krebs-Ringer bicarbonate (EKRB) buffer followed by three washes in EKRB medium to isolate seminiferous tubules. Tubules were digested with trypsin (0.5 mg/ml, 33°C, 12 min). An equal volume of Dulbecco modified Eagle medium (DMEM; Gibco, Life Technologies) containing 10% fetal calf serum was added to the Sertoli cells, which were then pelleted (100 × g, 5 min) and resuspended in serum-free medium containing 50% DMEM, 50% Ham F-12 (Mediatech), 5 mg/ml insulin, 5 mg/ml transferrin, 10 ng/ml epidermal growth factor, 1 mM sodium pyruvate, 200 units/ml penicillin, and 200 mg/ml streptomycin. Sertoli cells were cultured on Matrigel (BD Bioscience)-coated dishes (32°C, 5% CO2) or Matrigel-coated cover slips in dishes. The cells were washed with PBS on Day 2 and cultured further in serum-free media. The cultures were found to be routinely >95% pure as determined by phase microscopy and immunofluorescence staining with antiserum against intermediate filament protein Vimentin.
Spermatogonial Stem Cell Isolation and Culture
Spermatogonial stem cells (SSCs) were isolated and cultured as described previously [22]. Testes were collected from 6- to 8-day-old pups from the DBA/2 inbred mouse strain, decapsulated, and digested enzymatically with DNAase-I ([Sigma Aldrich], 5 min; 7 mg/ml in Hanks Balanced Salt Solution; Gibco, Life Technologies) and Trypsin/ethylenediaminetetraacetic acid (0.25%) for 5 min at 37°C. The digestion was stopped by adding fetal bovine serum, and the mixture was resuspended multiple times to break cell clumps and filtered through a 40-μM cell strainer (BD Biosciences) to remove remaining cell clumps. The cell suspension was centrifuged at 700 × g for 7 min, and the pellet was resuspended in 1× Dulbecco Phosphate-Buffered Saline (DPBS; Gibco, Life Technologies). To enrich germ cells, the cell suspension was layered gently on 2-ml 30% percoll gradient and centrifuged at 700 × g for 7 min. These enriched germ cell suspensions were incubated with Thy1+ beads (Miltenyi Biotec) and subjected to magnetic activated cell sorting (Miltenyi Biotec) to select Thy1+ cells to enrich SSCs. SSCs were eluted and washed with mouse SSC serum-free medium (mSFM) and plated onto STO (SIM mouse embryo-derived thioguanine and ouabain resistant) feeders in mSFM supplemented with 20 ng/ml recombinant human Glial Derived Nerve Growth Factor (Peprotech Inc.), 150 ng/ml rat recombinant GFRα1/Fc Chimera (R&D Systems), and 1 ng/ml recombinant human basic Fibroblast Growth Factor (BD Biosciences). SSC cultures were maintained at 37°C and 5% CO2, subcultured by blowing off SSCs from feeder cells, and replated at 1:2 to 1:3 ratios onto fresh STO feeders every 7 days. To make protein extracts, colonies of SSCs were detached from STO feeder cells by blow-off method. Supernatant containing colonies of SSCs was centrifuged at 700 × g for 7 min, and the SSC pellet was resuspended in boiling 1× SDS sample buffer.
Western Blot Analysis
Western blotting was performed as previously described [18]. The primary antibodies, anti-PPP1CC1 and anti-PPP1CC2, were commercially prepared (Zymed Laboratories), and their ability to recognize respective isoforms is well documented [18, 19, 23]. The antibodies were diluted as follows: for PLZF, 1:1000 (R & D Systems, catalog #AF2944); for GATA-4, 1:500 (Santa Cruz, catalog #SC-1237); for VASA, 1:10 000 (Abcam, catalog #Ab13480); and for Actin, 1:20 000 (Sigma, catalog #A2228).
Morphological Assessment of Spermatozoa from Cauda Epididymis and Vas Deferens
Sperm collection was done following the steps as outlined previously [18]. For counting, sperm were diluted (1:5 or 1:10) in ddH2O, and 10 μl of the diluted sperm was loaded onto a new improved Neubauer haematocytometer (Reichert). For formaldehyde fixation, sperm pellets were resuspended in freshly prepared 3.75% paraformaldehyde in 1× PBS and incubated on ice for 1 h. Fixed spermatozoa were mounted on clean slides and sealed under a cover slip. Sperm morphology was analyzed under 20× and 60× objectives with a 1 × 70 Olympus microscope using differential interference contrast (DIC) optics. From each slide, randomly selected nonoverlapping fields (≥10) were observed, and the numbers of normal and defective sperm were counted.
Histology and Immunohistochemistry
All steps, including testis collection, fixation, paraffin embedding, sectioning followed by deparaffinization, rehydration, dehydration and antigen retrieval for hematoxylin staining, and IHC, were done as described previously [18]. However, for PPP1CC1 immunostaining, 10–μm-thick sections were used. Sections were incubated with aforementioned anti-PPP1CC1 and anti-PPP1CC2 primary antibodies at 1:250 dilutions at 4°C overnight, followed by three brief washes in 1× PBS. Negative control sections were incubated in blocking solution lacking the primary antibody. Sections were subsequently incubated with corresponding Cy3-conjugated secondary antibodies for 1 h at room temperature. Finally, slides were washed a couple of times for 10 min each in 1× PBS and mounted with Vectashield (Vector Laboratories) mounting media (with or without 4′,6-diamidino-2-phenylindole [DAPI]), then examined using a Fluo View 1000 Confocal Fluorescence Microscope (Olympus).
Sperm Motility Analysis
Sperm motility analysis was performed using computer-assisted sperm analysis (CASA) with the CEROS sperm analysis system (software version 12.3; Hamilton Thorne Biosciences) following the steps outlined previously [18]. Motility was analyzed using the default Mouse-2 settings from Hamilton Thorne with minor adjustments. The settings for analysis were: 60 frames per second, 90 frames acquired; minimum contrast: 30; default cell size: 13 pixels; minimum cell size: four pixels; default cell intensity: 75; cells progressive if VAP (average path velocity) > 50 μm/sec and STR > 50%, slow cells were counted as motile; low VAP cut off: 10 μm/sec; low VSL (straight line velocity) cutoff = 0 μm/sec. For each chamber, 10–12 random nonoverlapping fields were recorded for analysis purposes. For the germ cell knockout and control groups, motility was recorded independently from n = 4 and n = 3 animals, respectively. The values for each motility parameter were expressed as the mean ± SEM.
Protein Concentration Estimation
Protein concentration of samples was estimated by the Bradford method using a DC Protein Assay Kit (Bio-Rad). Each sample (including standards) was measured in triplicate, and the resulting mean absorbance was used for developing a standard curve and determining subsequent protein concentration.
Statistics
Northern blots membranes were exposed to phosphor-imager cassettes overnight and were subsequently scanned and developed with a Typhoon scanner (GE Healthcare). The relative signal intensities were determined using ImageJ software (http://rsbweb.nih.gov/ij/) from at least three independent blots within a linear range of intensities. Results were analyzed by two-way ANOVA using a Tukey multiple comparison test at a 5% significance level utilizing GraphPad Prism 4.3 (GraphPad Software). Statistical analysis for Table 1 and for sperm motility parameters was performed using Sigma Plot software version 11.0.
TABLE 1.
Comparison of sperm number, morphology, and fertility between GcKO and control mice.*

A t-test was performed to compare testis weight and sperm number between test and control groups using the Shapiro-Wilk normality test, and differences were considered statistically significant if P < 0.05 at a confidence interval of 95%.
n denotes sample size.
Denotes no significant differences.
Denotes significant differences.
RESULTS
Ppp1cc2 Is the Only PP1 Isoform Highly Expressed in Developing Germ Cells in Testis
Previous work has demonstrated the tissue distribution of the two PP1 isoforms, PPP1CC1 and PPP1CC2. These studies showed that PPP1CC2 is predominant in the nuclei of germ cells in rats [10], whereas PPP1CC1 is a ubiquitous somatic isoform [8]. However, the exact cellular localization of PPP1CC1 in testis is not known. A documentation of the time and site of expression of the two PPP1CC isoforms is also lacking. To understand the specific functional roles of the two PPP1CC isoforms in testis, we set out to identify their relative abundance and expression patterns in testis and other tissues. We first used Northern blot for this purpose. A probe corresponding to the full length of Ppp1cc2 cDNA and capable of detecting both Ppp1cc isoforms was used. This allowed us to compare the relative intensities of the signals for the two isoforms and to ascertain the levels of the messages in other tissues. Ppp1cc2 message levels were significantly higher in testis compared to that in all other tissues included in the study (Fig. 1a). The Ppp1cc2 mRNA level was found to be ∼13-fold higher compared to Ppp1cc1 mRNA in testis (Fig. 1b). The Ppp1cc2 mRNA level in testis was approximately 98-, 127-, 48-, and 40-fold higher compared to that in brain, spleen, kidney, and liver, respectively (Fig. 1b).
FIG. 1.

Differential expression of Ppp1c isoforms in testis. a) Northern blot showing the differential expression pattern of Ppp1cc isoforms. Messenger RNA for the Ppp1cc1 isoform corresponding to ∼2.3 kb was detected in all tissues at low levels; in contrast, the Ppp1cc2 message (∼1.4 kb) was most abundant in the testis. Twenty-five micrograms of total RNA was loaded in each lane for each tissue. Br, brain; Spl, spleen; Kid, kidney; Liv, liver. b) Densitometry was performed using ImageJ, and its values were analyzed statistically using two-way ANOVA and plotted as relative expression levels. All values were normalized against the Ppp1cc1 mRNA intensity value in brain. Ppp1cc2 message levels were significantly higher compared to those of Ppp1cc1 in testis (∼13 fold, denoted by *) and Ppp1cc2 in other tissues. The error bar denotes the SEM. c) Northern blots showing the temporal expression pattern of all four PP1 isoforms in postnatal developing testis. Twenty-five micrograms of total RNA was loaded in each lane for each developmental time point. High levels of Ppp1cc2 expression coincided with the onset of meiosis in developing germ cells, and expression remained elevated thereafter. The Ppp1cc1 and Ppp1ca expression pattern appears to be restricted to Sertoli cells, spermatogonia, and preleptotene spermatocytes (Day 5–10). Ppp1cb was detected in low amounts in all the stages. β-Actin was used as a loading control.
In order to document the temporal expression of the Ppp1cc isoforms in the testis, we determined the levels of mRNA for each of the PP1 isoforms in the postnatal developing testis (Fig. 1c). The expression patterns of the Ppp1ca and Ppp1cc1 isoforms are similar: relatively high levels are observed in testes from 5- and 10-day-old mice, when Sertoli cells, Leydig cells, and spermatogonia are present, but when meiotic and haploid germ cells are absent. As shown in Figure 1b, messenger RNA levels for these isoforms appear to decline from Day 15, coinciding with the emergence of primary spermatocytes. The message for Ppp1cb is present at roughly the same levels at all stages of the developing testis. The expression pattern for Ppp1cc2 is distinct compared to that of all other PP1 isoforms. A low level of expression, comparable to levels in other tissues (spleen, kidney, and liver), was observed between 5 and 10 dpp testes. At 15 dpp, when pachytene spermatocytes emerge [24], there was a dramatic increase in expression of the mRNA for Ppp1cc2. Thereafter, levels of the message continued to coincide with the emergence of late pachytene spermatocytes and round and elongating spermatids (Day 21). The level reached a maximum in 30 dpp testes and was maintained at this high level in testes of adult mice.
PPP1CC1 Expression Is Restricted to Sertoli Cells and Premeiotic Germ Cells
The timing of the expression of mRNA by Northern blot analysis in developing testis suggested that Ppp1cc1 may be restricted to premeiotic germ cells and Sertoli cells, since its levels do not rise when spermiogenesis and spermiation occur. In order to directly confirm these findings, extracts were made from Sertoli cells isolated from 20-day-old rat testis and subject to Western blot analysis. As seen in Figure 2a, only three PP1 isoforms, PPP1CC1, PPP1CA, and PPP1CB, but not PPP1CC2, were detected in Sertoli cell extracts. To detect the pattern of expression of the PP1 isoforms in premeiotic germ cells, extracts were made from mouse primary culture of SSCs isolated from neonatal pups. Western blot analysis of these extracts detected the presence of all other PP1 isoforms except PPP1CC2 (Fig. 2b). In the same blot, we used 15P1 cells, an immortalized cell line derived from mouse Sertoli cells, to detect the presence of PP1 isoforms (Fig. 2b). PPP1CC2 is the only PP1 isoform absent in these cell extracts, confirming our finding with Sertoli cells isolated from rat testis (Fig. 2b, lane 2). Next, we performed immunofluorescence with sections of adult mouse testis to detect PPP1CC1 using an antibody specific for this isoform. Data from IHC showed localized PPP1CC1 expression in Sertoli cells, as evident from staining in the radial spoke-like structures radiating from the periphery toward the lumen of the seminiferous epithelium (Fig. 2c). At higher magnification, these characteristic radial spikes can be identified as Sertoli cell processes that branch through the germ cells at various stages of development (Fig. 2, e and f). PPP1CC1 staining was also evident along the periphery of the seminiferous tubules in spermatogonia and presumably in preleptotene spermatocytes, whereas staining was absent in developing germ cells starting from early pachytene spermatocytes (Fig. 2d, yellow arrow). In contrast, PPP1CC2 expression is detected in pachytene spermatocytes and in all subsequent stages of postmeiotic developing germ cells. These staining patterns confirm observations from earlier studies [17, 18].
FIG. 2.

Differential distribution of PPP1CC isoforms in testis. a) Western blot analysis showing expression of all four PP1 isoforms in purified rat Sertoli cells isolated from Day 20 neonatal testis. About 20 μl per lane of Sertoli cell extracts representing ∼1 × 106 cells were separated by SDS-PAGE. Western blotting was done using specific antibodies against each PP1 isoform as mentioned in the Materials and Methods section. Lane 1: among the four isoforms, PPP1CC1, PPP1CA, and PPP1CB could be clearly detected in Sertoli cells, while PPP1CC2 was absent in this cell type; lane 2: testis extracts were used as positive control for the presence of all four isoforms; lane 3: COS (kidney cell derived from African green monkey) cell extracts were used as a control for the absence of the PPP1CC2 isoform. Vimentin was used as a Sertoli cell marker. b) Immunoblot showing the expression of all four PP1 isoforms in mouse primary cultured SSCs derived from neonates (lane 3) and in 15P1 cells (lane 2). It is to be noted that PPP1CC2 is the only isoform absent in these cell types. GATA4 was used as a marker for Sertoli cells, while PLZF and VASA were used as control for the SSC marker. β-Actin was used as loading control for the lanes. c–f) Immunofluorescence detection of the PPP1CC1 isoform in paraffin-embedded testis sections of adult mice. c) At 20× magnification, specific Sertoli cell staining could be seen as radial spokes within the testis sections; the small, boxed region (white) is magnified and shown as an inset (large white box on bottom left corner) to show a PPP1CC1 presence in premeiotic cells, i.e., spermatogonia and preleptotene spermatocytes (yellow arrows). d) The corresponding DIC image. e) At 60× magnification, the radial spokes are clearly seen as Sertoli cell processes (white arrows). f) The corresponding superimposed DAPI counterstained image. The small, boxed region (white) was further zoomed and shown as an inset (bottom left corner) to clearly view the Sertoli cells and the adjacent germ cells. Sc, Sertoli cells. For immunodetection, the aforementioned PPP1CC1-specific antibody was used. All images are representative of observations from multiple sections in different animals.
Selective Deletion of the Ppp1cc Gene in Developing Germ Cells to Create Germ Cell Knockout Mice (Ppp1ccΔflx/Δflx; TgStra8-Cre+)
Global knockout of the Ppp1cc gene resulted only in male infertility, while females were normal and fertile. This suggests that all other PP1 isoforms, namely, PPP1CA and PPP1CB, can compensate for the loss of both PPP1CC isoforms in all other tissues except testis. Among the possible reasons for the lack of compensation by PPP1CA and PPP1CB are (a) a complete absence of these isoforms in the meiotic and postmeiotic germ cells (b) a possible requirement for Ppp1cc isoforms in other testicular cell types besides germ cells, i.e., Sertoli and or Leydig cells. In order to determine if Ppp1cc is required exclusively only in developing germ cells, we selectively ablated the Ppp1cc gene at the differentiated spermatogonia/preleptotene stage to obtain germ cell-specific Ppp1cc knockout mice. Mice homozygous for the mutant Ppp1cc alleles in germ cells, with the genotype Ppp1ccΔflx/Δflx; TgStra8-Cre, are hereafter denoted as GcKO. RT-PCR was performed to confirm the expression of the message for Cre in testis. As anticipated, Cre was detected in testis, but it is absent in all other tissues examined (brain, spleen, kidney, and liver; Fig. 3b). For all our analyses, adult male GcKO mice, 8 wk or older, were used and compared with age-matched littermate controls (Ppp1cc flx/flx). In order to determine changes in mRNA levels for the Ppp1cc isoforms, we performed Northern blot analysis to confirm efficacy of CRE-mediated deletion of the Ppp1cc floxed allele. As shown in Figure 3c, CRE-mediated excision resulted in a marked reduction of the Ppp1cc2 mRNA compared to control (Fig. 3c, single headed arrow). However, Ppp1cc1 mRNA remained unaltered in GcKO mice compared to control (Fig. 3c). In GcKO testis, mRNA species of ∼1.0 kb was seen, which could correspond to the truncated message arising from the mutant alleles (Fig. 3d, double-headed arrow). A truncated mRNA could be generated as a result of splicing occurring between exons 1 and 4 in the mutated allele.
FIG. 3.

Knockdown of the Ppp1cc gene in differentiating spermatogonia/early spermatocytes by the Cre-Lox method. a) Genomic PCR showing detection of the floxed allele. Lane 1: absence of the floxed allele is identified by a single 600-bp band in wild-type mice; lane 2: the presence of the homozygous floxed allele is detected as a single 750-bp band; lane 3: mice heterozygous for the floxed allele (the center band is a nonspecific PCR amplification product). The schematic exon-intron organization of the Ppp1cc wild-type allele and the floxed allele are denoted by arrows corresponding to their respective PCR bands. b) RT-PCR confirming the specificity of expression of the Cre transgene driven by the Str8-Cre promoter. For all the tissues included in the study, Cre message could only be detected in testis. Br, brain; Spl, spleen; Kid, kidney; Liv, liver; Ts, testis. c) Northern blot analysis confirming the targeted deletion of the Ppp1cc gene in the testis is shown. Ppp1cc2 mRNA was drastically reduced in Ppp1ccΔflx/Δflx; TgStr8-Cre mice compared to control (single-headed black arrow). The double-headed arrow shows the presence of a ∼1.0-kb truncated message produced as a result of splicing occurring between exon 1 and exon 4 (the putative splicing event is shown below the panel as schematic). It is to be noted that Ppp1cc1 mRNA remained almost unaltered in both GcKO and control mice.
Conditional Deletion of the Ppp1cc Gene Significantly Reduces Testis PPP1CC2 Levels, Whereas PPP1CC1 Expression Remains Unaltered
CRE-mediated knockdown of Ppp1cc resulted in a drastic reduction, but not a complete loss, of PPP1CC2 in testis (Fig. 4a). To obtain an estimate of levels of PPP1CC2 in GcKO mice, varying amounts of protein extracts from GcKO animals (20–50 μg of protein) were compared with a serially diluted testis extract from control mice. PPP1CC2 levels varied in the GcKO animals; however, PPP1CC2 levels in all the GcKO animals were significantly lower compared to those of control (Fig. 4, a and b). Based on comparison of the band intensities of serially diluted extracts, it appears that PPP1CC2 levels in GcKO animals were approximately 10-fold lower compared to those of controls. We also compared PPP1CC2 levels in testis between the GcKO and the transgenic rescue lines, which expressed varying levels of transgenic PPP1CC2 (Fig. 4c) [18]. The band intensities of the GcKO lane were comparable to those of the rescue lines eTg-F10 and pTg-M3, which were determined to have PPP1CC2 levels about 12.5% of that of the Ppp1cc+/− used as control [12]. As expected, PPP1CC2 expression in brain extracts from GcKO mice remained unaltered (Fig. 4d).
FIG. 4.

PPP1CC2 level was drastically reduced in GcKO mice. Western blot confirming the reduced expression of PPP1CC2 in the testis of Ppp1ccΔflx/Δflx; TgStr8-Cre mice. Serially diluted testis extracts from different sets of GcKO animals were compared to that of control. a) Forty micrograms and 20 μg of total protein from different GcKO mice (except GcKO#3, where only 20 μg was used) were loaded on to each lane and compared to that of control (20 μg, 10 μg, 5.0 μg, and 2.5 μg). b) A different set of GcKO animals, using 50 and 25 μg of total protein, was compared with that of control. PPP1CC2 levels varied among knockouts but were significantly lower than that of control. c) To quantify the relative levels of PPP1CC2 in GcKO animals, we compared them with those of transgenic lines for which PPP1CC2 levels are known [18]. PPP1CC2 levels in GcKO animals were comparable to transgenic lines eTg-F10 and pTg-M3, for which PPP1CC2 levels are 12.5% that of Ppp1cc+/− animals. d) To determine the PPP1CC2 levels in brain, ∼40 μg of total protein from GcKO#1 and 2 and control brain extracts were loaded. It is to be noted that PPP1CC2 levels remained unchanged in GcKO and control brain extracts, further confirming the testis-specific deletion of the Ppp1cc gene.
Next, we performed IHC to detect PPP1CC2 within the GcKO testis. PPP1CC2 exhibited a mosaic expression in GcKO testis cross sections (Fig. 5, a–c); a vast majority of the tubules were negative for the antigen, while there was a patchy signal in some of the tubules (Fig. 5, a–c and e–g). The reduced expression of PPP1CC2 as observed in the IHC verified the Western blot data. There was a variation within tubules in the intensity of staining for PPP1CC2. Immunostaining could be observed in a quarter to three quarters of the cross section of some of the tubules (Fig. 5, e–g). In testis sections from control animals, staining for PPP1CC2 was uniform within seminiferous tubules starting from early pachytene spermatocytes up to elongating spermatids (Fig. 5, d and h).
FIG. 5.

Incomplete deletion of the Ppp1cc gene in testis led to mosaic expression of the PPP1CC2 isoform within testis and seminiferous tubules. Immunohistochemistry (IHC) and confocal imaging was performed to observe the PPP1CC2 expression pattern within the testis of GcKO animals. a, b, and c) In a vast majority of the tubules, the PPP1CC2 signal was absent, as observed under 20× magnification. However, it is to be noted that among a small population of tubules, the PPP1CC2 signal (denoted by white arrowhead) was retained, giving rise to a mosaic pattern of expression in the GcKO testis. Magnification at 40× (e, f, and g) reveals a patchy expression of PPP1CC2 within these tubules derived from clonal populations of germ cells that escaped Ppp1cc deletion, compared to control (d and h). Arc (arrowhead) denotes the region of germinal epithelium that is negative or has a very low level of PPP1CC2 signal. Depending on the efficacy of the recombination, these negatively stained regions vary from three quarters (e) to one quarter (f) to half (g) of the tubule. d, d', h, and h') It is to be noted that control animals had uniform PPP1CC2 expression from early pachytene spermatocytes to elongating spermatids (denoted by double-headed arrows). a', b', c', and d') The corresponding DIC images of a, b, c, d, respectively. e', f', g', and h') These images are derived by overlaying the corresponding DAPI-counterstained images on e, f, g, and h, respectively. All images are representative of multiple sections from different animals.
Data from Western blot analysis (Fig. 6, a–c) show that PPP1CC1 levels, unlike those of PPP1CC2, are essentially unaltered in the testis of GcKO compared to control mice. To further confirm this, we performed immunohistochemistry of testis sections from GcKO mice. In testis sections from GcKO mice, PPP1CC1 could be clearly seen within the Sertoli cells and in premeiotic germ cells (Fig. 6, d and e), comparable to the staining pattern seen in wild-type testis (Fig. 2, e and f).
FIG. 6.

Expression of the PPP1CC1 isoform remains unaltered in the testis of GcKO mice. In order to detect whether selective deletion of the Ppp1cc gene in developing germ cells affects PPP1CC1 expression, Western blotting was used to compare testis extracts from different GcKO mice and control wild-type mice. a and b) Panel showing that PPP1CC1 levels in different GcKO animals (lanes 1–3, 4–8) were comparable to controls, suggesting its exclusion from meiotic and postmeiotic germ cells. c) Western blot comparing PPP1CC1 levels in both testis and brain of GcKO with that of control. It is to be noted that PPP1CC1 levels in brain remained unchanged with respect to those of control. β-Actin was used as a loading control. d and e) Immunohistochemistry was performed to show PPP1CC1 localization within the seminiferous tubules of GcKO animals. It can be seen that PPP1CC1 was localized in a manner comparable to that of wild-type control (Fig. 2, c and e). The PPP1CC1 signal can be clearly identified in Sertoli cells (denoted by arrows) and toward the periphery of the lumen as in wild type (Fig. 2, c and e).
GcKO Mice Are Infertile Due to Severe Oligozoospermia
We next tested the fertility of the GcKO male mice as measured by their ability to sire pups. To assess fertility, 8-wk-or-older GcKO mice and age-matched control littermates (Ppp1cc flx/flx) were paired with adult wild-type CD1 (+/+) females. The data in Table 1 show that all GcKO males tested (n = 22) were infertile, whereas females were normal in their ability to sire pups. The females were used to propagate the germ cell knockout line (see Materials and Methods). Control male mice (n = 7) were all fertile and sired pups with an average litter size of 8 ± 0.7 pups per litter (Table 1). To ascertain the possible reasons for the lack of fertility in the GcKO male mice, we examined the number and morphology of mature caudal sperm from these animals. The average number of spermatozoa isolated from GcKO male mice was 0.4 × 107/ml (±0.08), significantly lower compared to those isolated from control (4.0 × 107/ml [±0.08]). The lumen of a majority of the seminiferous tubules in GcKO mice were devoid of spermatozoa (Fig. 7). However, a few tubules contained spermatozoa in their lumen, indicating normal spermatogenesis (Fig. 7, a and b). Some of these tubules seemed to contain few spermatozoa, whereas others contained what appears to be normal levels (Fig. 7, c–e) resembling wild-type tubules (Fig. 7f). This observation is consistent with the IHC data that showed a mosaic pattern of PPP1CC2 expression in testis sections of GcKO mice. We also examined cross sections of caput and caudal regions of the epididymis. A vast majority of the tubular cross sections in the caput region are devoid of sperm (Fig. 8a), while others contain prematurely released round spermatids and spermatozoa (Fig. 8, b and c). Consistent with this finding in the caput region, the content of the caudal tubules was a mixture of mostly round cells (Fig. 8, d and e) and mature spermatozoa (Fig. 7, f–h).
FIG. 7.
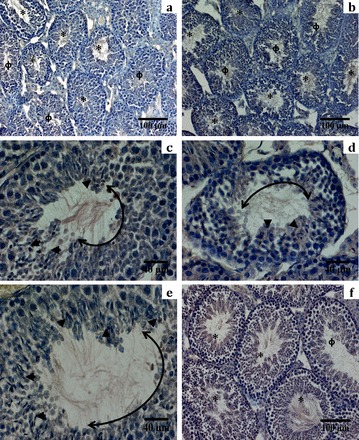
Hematoxylin-stained sections of GcKO testis compared to that of control. a and b) A majority of the tubules obtained from GcKO testis were devoid of spermatozoa (denoted by Φ). However, in a small population of seminiferous tubules in these testes, spermatozoa could be seen at varying numbers (denoted by *) compared to the control group, in which the vast majority of the tubules were filled with spermatozoa (f). c–e) Closer examination of some of these tubules at 40× magnification revealed that the alignment of mature spermatozoa along the lumen of the tubule was not uniform. The spermatozoa heads (identified as darkly stained spots) are denoted by arrowheads, were localized in patch, and were excluded from other parts of the lumen of the same tubule. The length of the germinal epithelium directly overlaying the part of the lumen that lacks any spermatozoa is denoted by the double-headed arc. In comparison, tubules containing sperm from the control groups have spermatozoa uniformly distributed along the periphery of the lumen (f). This pattern of emergence of mature spermatozoa correlates with the mosaic expression of PPP1CC2 seen in Figure 5. All images are representative of multiple sections obtained from different animals from a given group.
FIG. 8.

Histological sections of different regions of the epididymis of GcKO mice compared to that of control. Hematoxylin-stained sections of the caput region of the vasa differentia (at 10× and 20× magnification) reveal three broad categories of tubules: (a) common ones are those that are completely devoid of spermatozoa, (b) followed by the ones that are filled with prematurely released round spermatids (denoted by broken arrow), and (c) less-common ones that are filled with spermatozoa and round spermatids (denoted by arrowheads). However, the relative number and proportion of these tubules varied among the different GcKO animals. It is to be noted that the corresponding regions from littermate control animals (f, g) had tubular lumen mostly filled with released mature spermatozoa (denoted by blue arrowheads; original magnification ×20). d and e) Sections obtained at 20× magnification along the caudal region of the vasa further revealed a similar pattern and were filled mostly with prematurely released round cells that were mixed with fewer numbers of spermatozoa. h) In comparison, the controls had their caudal lumen compacted with mature spermatozoa (denoted by blue arrow). All images shown here are from the different regions of both epididymis of a single individual mutant mouse and are representative of similar observations from other GcKO mice.
GcKO Mice Are Teratozoospermic
A range of abnormalities was observed in caudal epididymal spermatozoa in the GcKO infertile males (Fig. 9), including a bending of the head between the midpiece and principal pieces, a deformity of the mitochondrial sheath (stunted or thinned), a kink at the annulus between the midpiece and principle piece, and a deformed or malformed head. The deformities of the mitochondrial sheath and the bent heads were the most frequently observed phenotype. The percentages of defective compared to normal sperm in GcKO mice were 66% ± 6.5% and 34% ± 6.5%, respectively, compared to 93% ± 1.2% morphologically normal sperm from the epididymis of wild-type testis (Table 1). Western blot analysis showed that GcKO sperm have considerably lower PPP1CC2 compared to those of control sperm (Fig. 9). These low levels are comparable to the levels of PPP1CC2 found in morphologically defective sperm from rescue mice expressing low levels of transgenic PPP1CC2 [18].
FIG. 9.

Morphological abnormalities of mature caudal spermatozoa as observed in GcKO male mice with low levels of PPP1CC2 in testis and sperm. Top panel (A) showing varying ranges of structural abnormalities of mature caudal spermatozoa observed in GcKO animals. a and b) Spermatozoa with aberrant bending of head between the capitulum and the proximal midpiece. Another commonly observed phenotype includes different types of malformed or deformed head (c, d, f, and g). Mitochondrial defects that are frequently observed are stunted mitochondria (f and g) and a region of thinning (f and h). Significant populations of morphologically normal sperm (Table 1) were also observed in these mutant animals (e). All micrographs were photographed under DIC optics with an Olympus 70 light microscope. Abnormal regions of the spermatozoa are denoted by white arrows, and the corresponding normal regions are denoted by black arrows in the normal sperm (e). Bars = 50 μm. These observations are representative of multiple samples from cauda epididymal preparations of different GcKO animals. B) Western blot data comparing PPP1CC2 levels present in 1% SDS whole-sperm extract among different animals. Protein extract from 2 × 106 sperm was loaded in each lane from different GcKO mice (4, 5, and 6) and compared to PPP1CC2 transgenic rescue lines and also to littermate controls.
Next, we quantified the motility parameters of caudal epididymal sperm from GcKO animals. Results of CASA are summarized in Figure 10. Both percentage of motility (50% ± 7.9%) and percentage of progressive motility (10.5% ± 2.3%) in the GcKO animals were significantly lower than that of control (85.7% ± 2.7% and 35.7% ± 2.3%, respectively). Other sperm motility parameters—VAP (54.0 ± 5.8 μm/sec), VSL (32.3 ± 4.1 μm/sec), and VCL (curvilinear velocity; 106.4 ± 9.6 μm/sec)—were also significantly reduced compared to control (VAP: 96.2 ± 4.5 μm/sec; VSL: 58.6 ± 2.7 μm/sec; and VCL: 185.1 ± 6.0 μm/sec).
FIG. 10.

Comparison of motility characteristics of mature caudal sperm between GcKO and control animals. Reduction of PPP1CC2 levels affected all motility characteristics of GcKO sperm. a) Total percent motility (black bar) and progressive motility (grey bar) of mature epididymal sperm were significantly reduced in GcKO animals compared to control, with P = 0.014 and 0.001, respectively. b) Mean values of velocity parameters VAP, VSL, and VCL also scored significant differences between GcKO (black) and littermate controls (grey), with P values of 0.003, 0.005, and 0.001, respectively. All statistical comparisons between the GcKO animal and its littermate control were done using a t-test at a confidence interval of 95%, using the Shapiro-Wilk normality test. Differences were considered significant if P < 0.05. * denotes motility parameters for which significant differences from positive control values were observed. For each animal, ≥10 nonoverlapping fields were recorded for analysis. The error bar atop denotes SEM. All statistics were done on data collected from n = 4 GcKO animals and n = 3 control animals.
DISCUSSION
The documented infertility of male mice due to the global deletion of the Ppp1cc gene raises many questions that remain unanswered. It has been assumed that loss of PPP1CC2, the predominant isoform in testis, is responsible for this phenotype. Impaired spermatogenesis and male infertility could also be due to the combined loss of both PPP1CC isoforms in testicular somatic cells and developing germ cells. Another puzzling observation is that the other PP1 isoforms, given their significant homology at the amino acid level, could not compensate for the loss of PPP1CC1 and PPP1CC2 in testis.
The conditional knockout approach was undertaken to answer some of these questions. Though we anticipated complete deletion of the Ppp1cc floxed allele in spermatogonia, only a partial deletion occurred, perhaps due to low penetrance of the CRE expression [25]. Thus, due to incomplete deletion, a mosaic pattern of expression of PPP1CC2 remained in a few of the seminiferous tubules of the testis of GcKO mice. The incomplete deletion may have resulted in some undifferentiated spermatogonia escaping disruption of the Ppp1cc gene. Consequently, these cells continued to express PPP1CC2 at normal levels in all differentiating cells derived from them. These clones escaping Ppp1cc gene disruption likely underwent normal development and differentiation to give rise to mature spermatozoa that were morphologically normal. This explains the presence of a markedly reduced number, but not a complete absence, of mature sperm in the epididymis of GcKO animals (mean sperm count 0.4 × 107/ml ± 0.08) compared to the conventional knockout [16, 17]. A significant proportion of spermatozoa also appeared morphologically normal (about 34% ± 6.5%) compared to deformed testicular spermatozoa, as seen in the Ppp1cc−/− knockout [17]. However, in comparison to transgenic rescue lines with low levels of PPP1CC2 (eTg-F10, pTg-M3: PPP1CC2 levels about 12.5% of that of Ppp1cc+/−), there were significantly lower numbers of mature caudal spermatozoa in GcKO mice [18]. A likely explanation is that in the rescue lines, PPP1CC2 levels were uniformly low within the tubules, whereas in the testis of GcKO mice, a majority of the seminiferous tubules lacked PPP1CC2. Our previous study [18] showed that low PPP1CC2 levels in meiotic and haploid germ cells resulted in the generation of morphologically abnormal spermatozoa and in reduced motility. The reason for the presence of morphologically defective spermatozoa in GcKO mice is puzzling. We speculate that random deletion of PPP1CC2 occurred only in some of the preleptotene spermatocytes within a clonal syncytium. This would have then been accompanied by diffusion of the PPP1CC2 protein throughout the cell population (syncytium) by cytoplasmic bridges, which would have resulted in reduction of the levels of PPP1CC2 below a threshold required for normal sperm morphogenesis. Spermatozoa arising from these developing germ cells with low levels of PPP1CC2 may have led to morphologically defective spermatozoa. The Ppp1cc−/− knockout males were infertile due to an almost complete lack of spermatozoa, whereas the GcKO males were infertile because of oligo-terato asthenozoospermia due to the loss of PPP1CC2 in the majority of the seminiferous tubules. These findings, along with the recently reported transgenic rescue studies [18], clearly suggest that PPP1CC2 at adequate levels is essential for spermatogenesis.
We determined the expression levels of the mRNAs for the two alternatively spliced Ppp1cc isoforms in mouse testis compared to other tissues. Messenger RNA for both Ppp1cc isoforms were detected in all tissues, whereas in adult testis, Ppp1cc2 was more abundant compared to Ppp1cc1 (Fig. 1a), an observation that was first reported by Shima et al. [10] in rats. Northern blot analysis showed that starting from Day 15 to adult testis, the only transcript from the Ppp1cc gene was the alternately spliced form, Ppp1cc2. This increased expression of Ppp1cc2 in testis coincided with the emergence of early pachytene spermatocytes at Postnatal Day 15 [24]. On the other hand, Northern blot data and the presence of PPP1CC1 but not PPP1CC2 in Western blots show that the transcript from Ppp1cc did not undergo alternate splicing in Sertoli cells and in spermatogonia. This indicates that high levels of Ppp1cc2 in testis are due to both high promoter activity and efficient splicing mechanisms, which are specific and restricted to meiotic and postmeiotic germ cells.
With respect to the putative Ppp1cc promoter, we have shown that a genomic region spanning 2.6 kb upstream of the transcription start site could recapitulate the wild-type expression pattern of PPP1CC2, with high levels in testis and minimal expression in other tissues [18]. Analyses of the genomic sequence of the promoter region showed that is has high nucleotide sequence homology (+272 bp to −200 bp) across various species and contains conserved binding sites for the transcription factors Spz1, a-Myb (Mybl1), and Sp1 [26]. Of these, Spz1 and a-Myb are testes specific. Overexpression of Spz1 [27] or deletion of a-Myb leads to male infertility [28]. Studies are underway to determine if the 0.5-kb region upstream of the transcription start site and the region in the 5′UTR containing the potential Spz1 binding site can direct germ cell-specific expression of the EGFP reporter gene.
An intriguing question is why the other PP1 isoforms do not compensate for the loss of PPP1CC2 in the testis of GcKO or Ppp1cc−/− mice. Immunohistochemistry of testis sections with PPP1CA antibody suggests that the increase in PPP1CA in null compared to wild-type testis could be localized to Sertoli cells and premeiotic germ cells [8]. Results from the more detailed characterization of the PPP1CC isoforms in Sertoli and spermatogonia show that Ppp1cc1 is primarily restricted to Sertoli cells, spermatogonia, and possibly preleptotene spermatocytes. Western blot analysis of extracts from Sertoli cells and SSCs clearly showed that PPP1CC1 was the only PPP1CC isoform present in these cell types. The lack of a decline in PPP1CC1 levels in GcKO testis also confirms that this isoform is excluded from developing spermatocytes and spermatids. This almost complete lack of detectable levels of PPP1CC2 in Sertoli cells and spermatogonia and of PPP1CC1 in postmeiotic germ cells is remarkable. It is noteworthy that in tissues other than testis, such as in brain, there appears to be compensation by the other isoforms for the loss of one or more of the PP1 isoforms (Nairn, unpublished data). Western blot analysis of testis extracts showed that PPP1CA expression in Ppp1cc−/− null mice was upregulated [17, 29]. While it is possible that this upregulation may not be sufficient to overcome the loss of PPP1CC2, it is more likely that this increased expression of PPP1CA does not occur in postmeiotic developing germ cells where PPP1CC2 is absent. The exclusion of three of the four isoforms in postmeiotic testicular cells may be one of the reasons why PPP1CC2 is the only PP1 isoform found in spermatozoa. Based on these observations, we suggest that PP1 isoforms other than PPP1CC2 are almost completely excluded particularly in postmeiotic germ cells. This exclusion of all three of the four PP1 isoforms could be the reason why they cannot compensate for the loss of the fourth, PPP1CC2.
Analysis of genomic databases revealed that splicing of the Ppp1cc pre-mRNA to yield Ppp1cc2 should occur only in mammals [26]. For example, based on the annotated database, the Xenopus genome contains the orthologue of PPP1CC1 virtually identical to that found in mammals, but only Ppp1cc1 should be generated, since splice sites for generation of the Ppp1cc2 transcript are absent [30]. It is notable that spermatozoa from Xenopus contain only PPP1CC1 [12]. That mammals have evolved testis-specific alternate splicing of the Ppp1cc transcript to generate the Ppp1cc2 isoform while excluding Ppp1cc1 in spermatocytes and spermatids is intriguing. Is it possible that the highly conserved C-terminus of PPP1CC2 confers biochemical properties required for sperm morphogenesis mechanisms and aspects of sperm function unique to mammals? Would spermatogenesis occur if other PP1 isoforms are expressed transgenically or by targeted knock-in to substitute for PPP1CC2 in developing germ cells at levels comparable to PPP1CC2? Studies are underway to answer these questions by the transgenic expression of PPP1CC1 isoform in the meiotic and postmeiotic germ cells of Ppp1cc-null mice.
In summary, our data for the first time provide a detailed characterization of the abundance and distribution of the two PPP1CC isoforms within testis. We show that PPP1CC1 and PPP1CC2 expression is mutually nonoverlapping in testis. PPP1CC1 is present in Sertoli cells, spermatogonia, and possibly in preleptotene spermatocytes but essentially absent in meiotic and postmeiotic germ cells. Selective deletion of the Ppp1cc gene affected only PPP1CC2 levels, while levels of PPP1CC1 were unaltered in GcKO mice. Our targeted knockdown further demonstrates the specific requirement for Ppp1cc and its gene product PPP1CC2, only in meiotic and postmeiotic germ cells, for normal sperm development and male fertility. The targeted deletion also confirmed the determination that PPP1CC1 is present in Sertoli cells and premeiotic germ cells and possibly also in Leydig cells. That is, PPP1CC2 is excluded from somatic cells and spermatogonia, whereas PPP1CC1, and possibly PPP1CA and PPP1CB, are excluded in postmeiotic germ cells. Finally, data from this study also corroborates the transgenic rescue experiments in which we found that high levels of PPP1CC2 levels are essential for normal spermatogenesis [18]. That is, reduction in the levels of PPP1CC2 reduces sperm output and causes defects in sperm morphology and motillity. The reasons for this isoform-specific requirement of adequate quantities of PPP1CC2 and the role of the unique C-terminus of PPP1CC2 for male fertility in mammals remain to be determined.
ACKNOWLEDGMENT
We are thankful to Mike Model for his help with confocal and bright field microscopy. We are grateful to the National Institutes of Health for funding the work.
Footnotes
Supported by the National Institutes of Health grants R01HD038520-08 and DA 10044.
REFERENCES
- Cohen PT. Protein phosphatase 1–targeted in many directions. J Cell Sci 2002; 115: 241–256. [DOI] [PubMed] [Google Scholar]
- Bollen M, Peti W, Ragusa MJ, Beullens M. The extended PP1 toolkit: designed to create specificity. Trends Biochem Sci 2010; 35: 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K, Erdodi F, Patton JG, Hartshorne DJ. Interaction of protein phosphatase type 1 with a splicing factor. FEBS Lett 1996; 389: 191–194. [DOI] [PubMed] [Google Scholar]
- Garcia A, Cayla X, Guergnon J, Dessauge F, Hospital V, Rebollo MP, Fleischer A, Rebollo A. Serine/threonine protein phosphatases PP1 and PP2A are key players in apoptosis. Biochimie 2003; 85: 721–726. [DOI] [PubMed] [Google Scholar]
- Okano K, Heng H, Trevisanato S, Tyers M, Varmuza S. Genomic organization and functional analysis of the murine protein phosphatase 1c gamma (Ppp1cc) gene. Genomics 1997; 45: 211–215. [DOI] [PubMed] [Google Scholar]
- Lin Q, Buckler ES, Muse SV, Walker JC. Molecular evolution of type 1 serine/threonine protein phosphatases. Mol Phylogenet Evol 1999; 12: 57–66. [DOI] [PubMed] [Google Scholar]
- Gibbons JA, Kozubowski L, Tatchell K, Shenolikar S. Expression of human protein phosphatase-1 in Saccharomyces cerevisiae highlights the role of phosphatase isoforms in regulating eukaryotic functions. J Biol Chem 2007; 282: 21838–21847. [DOI] [PubMed] [Google Scholar]
- da Cruz e Silva EF, Fox CA, Ouimet CC, Gustafson E, Watson SJ, Greengard P. Differential expression of protein phosphatase 1 isoforms in mammalian brain. J Neurosci 1995; 15: 3375–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa Y, Sasaki K, Shima H, Shibuya M, Sugimura T, Nagao M. Protein phosphatases possibly involved in rat spermatogenesis. Biochem Biophys Res Commun 1990; 171: 230–235. [DOI] [PubMed] [Google Scholar]
- Shima H, Haneji T, Hatano Y, Kasugai I, Sugimura T, Nagao M. Protein phosphatase 1 gamma 2 is associated with nuclei of meiotic cells in rat testis. Biochem Biophys Res Commun 1993; 194: 930–937. [DOI] [PubMed] [Google Scholar]
- Barker HM, Craig SP, Spurr NK, Cohen PT. Sequence of human protein serine/threonine phosphatase 1 gamma and localization of the gene (PPP1CC) encoding it to chromosome bands 12q24.1-q24.2. Biochim Biophys Acta 1993; 1178: 228–233. [DOI] [PubMed] [Google Scholar]
- Chakrabarti R, Cheng L, Puri P, Soler D, Vijayaraghavan S. Protein phosphatase PP1 gamma 2 in sperm morphogenesis and epididymal initiation of sperm motility. Asian J Androl 2007; 9: 445–452. [DOI] [PubMed] [Google Scholar]
- Smith GD, Wolf DP, Trautman KC. da Cruz e Silva EF, Greengard P, Vijayaraghavan S. Primate sperm contain protein phosphatase 1, a biochemical mediator of motility. Biol Reprod 1996; 54: 719–727. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Stephens DT, Trautman K, Smith GD, Khatra B. da Cruz e Silva EF, Greengard P. Sperm motility development in the epididymis is associated with decreased glycogen synthase kinase-3 and protein phosphatase 1 activity. Biol Reprod 1996; 54: 709–718. [DOI] [PubMed] [Google Scholar]
- Smith GD, Wolf DP, Trautman KC, Vijayaraghavan S. Motility potential of macaque epididymal sperm: the role of protein phosphatase and glycogen synthase kinase-3 activities. J Androl 1999; 20: 47–53. [PubMed] [Google Scholar]
- Varmuza S, Jurisicova A, Okano K, Hudson J, Boekelheide K, Shipp EB. Spermiogenesis is impaired in mice bearing a targeted mutation in the protein phosphatase 1cgamma gene. Dev Biol 1999; 205: 98–110. [DOI] [PubMed] [Google Scholar]
- Chakrabarti R, Kline D, Lu J, Orth J, Pilder S, Vijayaraghavan S. Analysis of Ppp1cc-null mice suggests a role for PP1gamma2 in sperm morphogenesis. Biol Reprod 2007; 76: 992–1001. [DOI] [PubMed] [Google Scholar]
- Sinha N, Pilder S, Vijayaraghavan S. Significant expression levels of transgenic PPP1CC2 in testis and sperm are required to overcome the male infertility phenotype of Ppp1cc null mice. PLoS One 2012; 7: e47623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Pilder S, Nairn AC, Ramdas S, Vijayaraghavan S. PP1gamma2 and PPP1R11 are parts of a multimeric complex in developing testicular germ cells in which their steady state levels are reciprocally related. PLoS One 2009; 4: e4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadate-Ngatchou PI, Payne CJ, Dearth AT, Braun RE. Cre recombinase activity specific to postnatal, premeiotic male germ cells in transgenic mice. Genesis 2008; 46: 738–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri P, Walker WH. The tyrosine phosphatase SHP2 regulates Sertoli cell junction complexes. Biol Reprod 2013; 88: 59. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem 2007; 282: 25842–25851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri P, Myers K, Kline D, Vijayaraghavan S. Proteomic analysis of bovine sperm YWHA binding partners identify proteins involved in signaling and metabolism. Biol Reprod 2008; 79: 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellve AR, Cavicchia JC, Millette CF, O'Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol 1977; 74: 68–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Ma HY, Schuster A, Lin YM, Yan W. Incomplete cre-mediated excision leads to phenotypic differences between Stra8-iCre; Mov10l1(lox/lox) and Stra8-iCre; Mov10l1(lox/Delta) mice. Genesis 2013; 51: 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N. Mammal specific protein phosphatase isoform, PPP1CC2, is essential for sperm function and male fertility. Kent, OH: Kent State University; 2012. Thesis. [Google Scholar]
- Hsu SH, Hsieh-Li HM, Li H. Dysfunctional spermatogenesis in transgenic mice overexpressing bHLH-Zip transcription factor, Spz1. Exp Cell Res 2004; 294: 185–198. [DOI] [PubMed] [Google Scholar]
- Toscani A, Mettus RV, Coupland R, Simpkins H, Litvin J, Orth J, Hatton KS, Reddy EP. Arrest of spermatogenesis and defective breast development in mice lacking A-myb. Nature 1997; 386: 713–717. [DOI] [PubMed] [Google Scholar]
- Terry-Lorenzo RT, Carmody LC, Voltz JW, Connor JH, Li S, Smith FD, Milgram SL, Colbran RJ, Shenolikar S. The neuronal actin-binding proteins, neurabin I and neurabin II, recruit specific isoforms of protein phosphatase-1 catalytic subunits. J Biol Chem 2002; 277: 27716–27724. [DOI] [PubMed] [Google Scholar]
- The Ensembl Genomic Databases For Mouse (GRCm38), Mouse Comparative Genomics. Cambridge, UK: Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus; http://www.ensembl.org/Mus_musculus/. Accessed March 1, 2012. [Google Scholar]


