ABSTRACT
Recent data from human and mouse studies strongly support an indispensable role for steroid receptor coactivator-2 (SRC-2)—a member of the p160/SRC family of coregulators—in progesterone-dependent endometrial stromal cell decidualization, an essential cellular transformation process that regulates invasion of the developing embryo into the maternal compartment. To identify the key progesterone-induced transcriptional changes that are dependent on SRC-2 and required for endometrial decidualization, we performed comparative genome-wide transcriptional profiling of endometrial tissue RNA from ovariectomized SRC-2flox/flox (SRC-2f/f [control]) and PRcre/+/SRC-2flox/flox (SRC-2d/d [SRC-2-depleted]) mice, acutely treated with vehicle or progesterone. Although data mining revealed that only a small subset of the total progesterone-dependent transcriptional changes is dependent on SRC-2 (∼13%), key genes previously reported to mediate progesterone-driven endometrial stromal cell decidualization are present within this subset. Along with providing a more detailed molecular portrait of the decidual transcriptional program governed by SRC-2, the degree of functional diversity of these progesterone mediators underscores the pleiotropic regulatory role of SRC-2 in this tissue. To showcase the utility of this powerful informational resource to uncover novel signaling paradigms, we stratified the total SRC-2-dependent subset of progesterone-induced transcriptional changes in terms of novel gene expression and identified transcription factor 23 (Tcf23), a basic-helix-loop-helix transcription factor, as a new progesterone-induced target gene that requires SRC-2 for full induction. Importantly, using primary human endometrial stromal cells in culture, we demonstrate that TCF23 function is essential for progesterone-dependent decidualization, providing crucial translational support for this transcription factor as a new decidual mediator of progesterone action.
Keywords: decidualization, endometrium, human, microarray, mouse, progesterone, steroid receptor coactivator-2, transcription factor 23
Induction of transcription factor 23 by progesterone requires steroid receptor coactivator-2 and is essential for endometrial decidualization.
INTRODUCTION
To establish placentation, the endometrial stromal cell layer must first decidualize to enable successful invasion of the developing embryo into the maternal compartment [1–3]. While endometrial decidualization requires the coordinated actions of ovarian estradiol (E2) and progesterone (P4), increasing evidence from human and mouse studies supports a crucial role for transcriptional coregulators (coactivators and corepressors) in this cellular transformation process [4–8]. One such coregulator is steroid receptor coactivator-2 (SRC-2; also known as NCOA-2, GRIP-1, and TIF-2), which has been shown to play a pivotal role in progesterone-dependent endometrial decidualization [9–11].
A member of the p160/SRC family of coactivators, SRC-2 (as with SRC-1 and -3) exerts potent pleiotropic controls on a wide spectrum of physiological systems, ranging from metabolic homeostasis to male reproductive biology to mammary gland morphogenesis [9, 12, 13]. In keeping with this pleiotropy, clinical and mouse studies have shown that dysregulation of SRC-2 is causally linked to myriad clinicopathologies, including glycogenopathy [14], prostate cancer [15], and childhood leukemias [16, 17] (the latter due to chromosomal translocations at the SRC-2 locus). In female reproductive biology, studies have implicated a requirement for SRC-2 in a number of normal cellular processes of the uterus as well as in the etiopathogenesis of this tissue [9, 11, 18–20]. In normal endometrial function, we have revealed that endometrial SRC-2 plays a central role during the early stages of P4-dependent stromal cell decidualization [11]. Specifically, ablation of SRC-2 in cells of the murine uterus or knockdown in primary human endometrial stromal cells (hESCs) in culture results in a block in P4-dependent transformation of a stromal fibroblast cell into a polygonal epithelioid decidual cell, an essential underpinning of the decidualization progression program at the cellular level.
As a first step toward gaining mechanistic insight into SRC-2's involvement in this cellular process, our recent metabolome profiling reveals that SRC-2 in the endometrial stromal cell is essential for acceleration of the glycolytic flux by P4 [11], a prerequisite metabolic reprogramming step that fuels the decidual response. While this profiling approach provides essential metabolic insight into SRC-2's role in endometrial stromal cell function, information cannot be obtained on P4-responsive transcriptional changes that are dependent on SRC-2 but distinct from metabolic signaling. To address this issue, we applied high-density oligonucleotide microarray analysis to identify an acute P4-responsive transcriptome in the murine uterus that is both dependent on SRC-2 function and critical for decidualization. Representing less than 13% of the genome-wide P4-responsive transcriptome, we identified a subset of P4-responsive genes that are dependent on SRC-2 expression. Remarkably, many genes previously reported to mediate P4-driven decidualization are contained within this SRC-2-dependent transcriptome, underscoring the importance of SRC-2 in this endometrial process. Mining this transcriptome for novel genes—and, in particular transcription factors—we reveal transcription factor 23 (TCF23) as a critical basic-helix-loop-helix (bHLH) transcription factor required for human endometrial stromal cell decidualization.
MATERIALS AND METHODS
Mice and Hormone Treatments
Mice were housed within a vivarium maintained at Baylor College of Medicine with a 12L:12D recurrent photocycle. Mice were treated humanely and surgical procedures performed in accordance Institutional Animal Care and Use Committee. The PRCre/+SRC-2f/f (SRC-2d/d) bigenic mouse, in which SRC-2 expression is abrogated selectively in progesterone receptor (PR)-positive cells, was generated as previously described [9, 21]. Control mice used in these studies included SRC-2flox/flox (SRC-2f/f) siblings in which the SRC-2 allele is floxed [13]; progesterone receptor knockout (PRKO) mice served as additional controls [22]. To detect early transcriptional responses to P4, 6-wk-old mice were ovariectomized before resting for 2 wk to remove ovarian-derived steroid hormones. Following 2 wk of rest, mice received a single subcutaneous injection of 1 mg of P4 (Sigma-Aldrich) in 100 μl of sesame oil; corresponding vehicle controls received 100 μl of sesame oil. Six hours following the P4 or vehicle injection, mice were euthanized for tissue collection.
Microarray Analysis
Microarray analysis was performed using 18 SRC-2f/f (controls) and 18 SRC-2d/d (SRC-2-depleted) mice. Hormone treatments were performed as explained above. Six hours postinjection, uteri from each mouse group were collected for total RNA isolation using the RNeasy total RNA isolation kit (Qiagen Inc.). Total RNA from three mice per genotype per treatment were pooled and assigned as one sample (three samples per genotype per treatment). The integrity and quality of all RNA samples were analyzed with a Bioanalyzer 2100 (Agilent Technologies) before microarray hybridization. Fragmented cRNA was hybridized to Affymetrix Mouse Genome 430 2.0 Arrays (Affymetrix). The quality of the arrays was assessed using Microarray Suite version 5.0 software (MAS 5.0; Affymetrix); array data have been deposited in the Gene Expression Omnibus database (www-ncbi-nlm-nih-gov.ezproxyhost.library.tmc.edu/geoprofiles [GSE31406]). Affymetrix CEL files were processed using dChip software (www.dchip.org) based on a perfect match and mismatch model. Probe sets with an average signal of <40 units across profiles were filtered from the analysis. Two-sided t-tests and fold changes (using log-transformed values) were carried out to define differentially expressed genes [23]. Genes were clustered using supervised methods previously described [23–26]. Java Treeview software was used for representing changes in gene expression patterns as color maps [23, 27].
Human Endometrial Stromal Cell Isolation
Following receipt of written informed consent, endometrial tissue was biopsied from healthy patients during the proliferative phase of their menstrual cycle in accordance with the guidelines of the Declaration of Helsinki and the Institutional Review Board at Baylor College of Medicine [28]; hESCs were isolated from endometrial tissue biopsies and cultured as previously described [11]. Briefly, endometrial biopsy tissue was digested in DMEM/F12 medium containing collagenase (2.5 mg/ml [Sigma-Aldrich]) and DNase I (0.5 mg/ml [Sigma-Aldrich]) for 1.5 h at 37°C. Following tissue digestion, lymphocytes were removed and hESCs isolated using the Ficoll-Paque reagent layer (GE Healthcare Biosciences). Collected hESCs were then resuspended in DMEM/F-12 media containing 10% FBS, 100 units/ml penicillin, and 0.1 mg/ml streptomycin (hESC media) and cultured as per standard tissue culture procedures [11, 29]. Studies were carried out using primary hESCs derived from at least three individual subjects, and only hESCs propagated through no more than four passages were used in these experiments.
Quantitative Real-Time PCR
Total RNA was isolated from mouse uteri or hESCs using the RNeasy total RNA isolation kit (Qiagen). One microgram of total RNA was reverse transcribed to cDNA using the TaqMan Reverse Transcription kit (Applied Biosystems) following the PCR reaction conditions: 10 min at 25°C, 30 min at 48°C, and 5 min at 95°C. Quantitative real-time PCR analysis was performed using ABI Prism 7700 sequence detection system with TaqMan 2× master mix and validated primers (Applied Biosystems). Ribosomal RNA (18S) was used as an internal control for gene-specific primers and validated primers. Primer sequences used in these studies are shown in Table 1.
TABLE 1.
Sequences of quantitative real-time PCR primers used to assess transcript levels of human and mouse uterine genes.
Decidualization of hESCs and siRNA Transfection
All siRNA transfections were carried out in triplicate when hESCs reached 60%–70% confluency per well of a six-well culture plate. Smart pools of siRNAs targeting SRC-2 (NCOA-2 [L-020159-00-0005]) and TCF23 (L-005643-00-0005) were used in these studies (Thermo Scientific, Dharmacon RNAi Technologies); nontargeting siRNA (D-001810-10-05) was used as a negative control. Each siRNA was transfected in 1× Opti-MEM I reduced-serum media (Invitrogen Corporation) using Lipofectamine 2000 reagent, and after 5 h, transfection media was replaced with hESC media. Forty-eight hours later, in vitro decidualization of hESCs was initiated by treating hESCs with decidualization or EPC media (1× Opti-MEM I reduced-serum media containing 2% fetal bovine serum [FBS], 17β-estradiol [E2; 100 nM], medroxyprogesterone acetate [MPA; 10 μM; Sigma-Aldrich], and 8-bromo cAMP [50 μM; Sigma-Aldrich]). [29]. The day that hESCs first received EPC media was assigned as Day 0, and EPC media was renewed every 2 days. At appropriate time points as per experimental conditions, total RNA was isolated and subjected to quantitative real-time PCR. Transcript levels of established decidualization markers, prolactin (PRL) and insulin-like growth factor binding protein-1 (IGFBP-1), were determined as molecular determinants of decidualization [29].
Statistical Analysis
Statistical analyses of data were performed using the two-tailed Student t-test with the Instat Tool package version 3.0 (GraphPad Inc.); results were considered statistically significant with a P-value <0.05.
RESULTS
Identification of a Progesterone-Responsive Transcriptome in the Murine Endometrium That Is Dependent on SRC-2
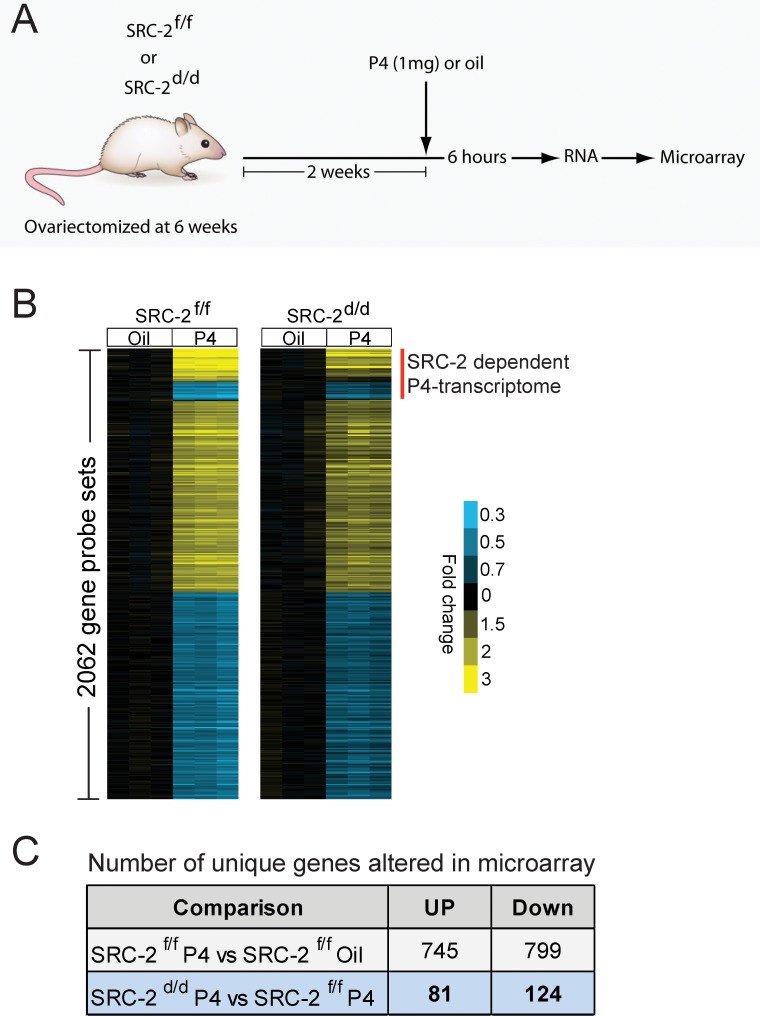
To advance endometrial decidualization, correct P4 responsiveness of the endometrial stromal cell requires SRC-2 function [9, 11]. Therefore, to identify the key P4-responsive transcriptional changes that require SRC-2 to drive complete stromal decidualization, we conducted a comparative microarray analysis of whole uterine tissue derived from ovariectomized SRC-2f/f (control) and SRC-2d/d (SRC-2-depleted) mice acutely treated with P4. Previously, we have used variations of this hormone treatment model to uncover decidual genes acutely responsive to P4 [30–32]. For this microarray study, total RNA was prepared from whole uterine horns dissected from 8-wk-old SRC-2f/f and SRC-2d/d mice 6 h after receiving a subcutaneous injection of P4 (1 mg); all mice were ovariectomized at 6 wk of age (Fig. 1A). To serve as vehicle controls, total uterine RNA was prepared from both genotype groups 6 h following an injection of sesame oil (see Materials and Methods). Microarray analysis was performed on total RNA from each genotype and treatment group using the Affymetrix GeneChip Mouse Genome 430 2.0 Array. The GeneChip contains an array of more than 45 000 probe sets covering over 34 000 verified mouse genes. Extensive bioinformatic analysis revealed the following expression data matrices of uterine genes: 1) unique genes induced or repressed in control SRC-2f/f mice by P4 alone (the P4-responsive transcriptome; Supplemental Tables S1 and S2; Supplemental Data are available online at www.biolreprod.org), and 2) unique P4-regulated genes induced or repressed in a SRC-2-dependent manner (the P4-responsive transcriptome dependent on SRC-2 [red vertical line represents ∼13% of the P4-responsive transcriptome; Fig. 1B; Tables 2 and 3; Supplemental Tables S3 and S4]). In accordance with the known transcriptional induction and repression responses elicited by P4 exposure in the murine uterus and other tissues [23, 31], an equivalent number of uterine genes were induced (745 unique genes) or repressed (799 unique genes) by P4 in the SRC-2f/f uterus. Surprisingly, the transcript levels of only a small subset of P4-regulated genes (∼13%) were significantly changed with SRC-2 depletion (205 unique genes, which includes induced and repressed; Fig. 1C; Tables 2 and 3; Supplemental Tables S3 and S4). Importantly, although the number of genes in this subset is small, a significant number of key genes previously linked to P4-dependent decidualization are present in this subset [11, 33, 34] (Tables 2 and 3). The divergent signals projected by these established target genes, along with their importance to P4-dependent decidualization and their dependency on SRC-2 function, support SRC-2 as a pleiotropic coregulator in this uterine process at the molecular level [9, 11, 35].
FIG. 1.
A subset of progesterone transcriptional responses in the murine endometrium requires SRC-2. A) Schematic of the experimental design for the microarray study. Six-week-old SRC-2f/f (control) and SRC-2d/d (SRC-2-depleted) mice were ovariectomized and rested for 2 wk. Two weeks postsurgery, mice were treated with either sesame oil (vehicle control) or P4 for 6 h before uterine RNA was processed for microarray analysis. B) Colorgram of differential gene expression in uteri from vehicle control or P4-treated ovariectomized SRC-2f/f and SRC-2d/d mice. The extent of gene expression changes is represented by a blue-yellow color scale (blue: high expression; yellow: low expression; black: no change). C) Table shows the number of unique endometrial genes that are acutely induced or repressed by P4 (SRC-2f/f P4 treatment vs. SRC-2f/f Oil treatment) and the number of unique endometrial genes that are induced or repressed by P4, which are dependent on SRC-2 expression (SRC-2d/d P4 treated vs. SRC-2f/f P4 treated).
TABLE 2.
The top 30 genes with significantly decreased transcript levels in the P4-treated SRC-2d/d mouse uterus compared to P4-treated SRC-2f/f mouse uterus (P < 0.05).a
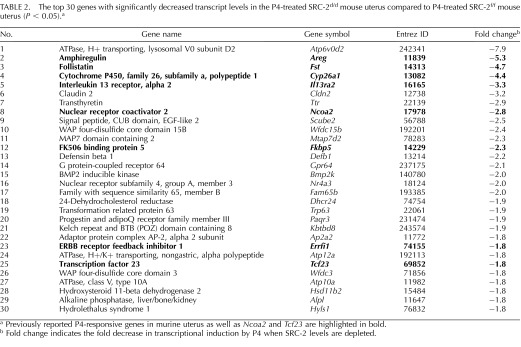
Previously reported P4-responsive genes in murine uterus as well as Ncoa2 and Tcf23 are highlighted in bold.
Fold change indicates the fold decrease in transcriptional induction by P4 when SRC-2 levels are depleted.
TABLE 3.
The top 30 genes with significantly increased transcript levels in the P4-treated SRC-2d/d mouse uterus compared to P4-treated SRC-2f/f mouse uterus (P < 0.05).a
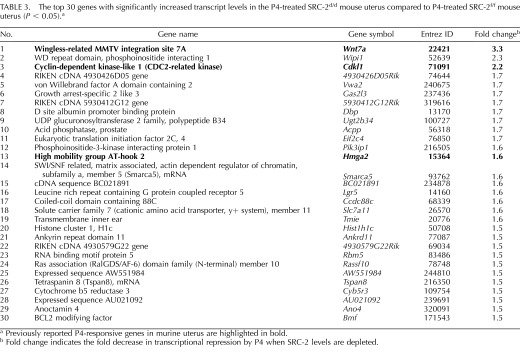
Previously reported P4-responsive genes in murine uterus are highlighted in bold.
Fold change indicates the fold decrease in transcriptional repression by P4 when SRC-2 levels are depleted.
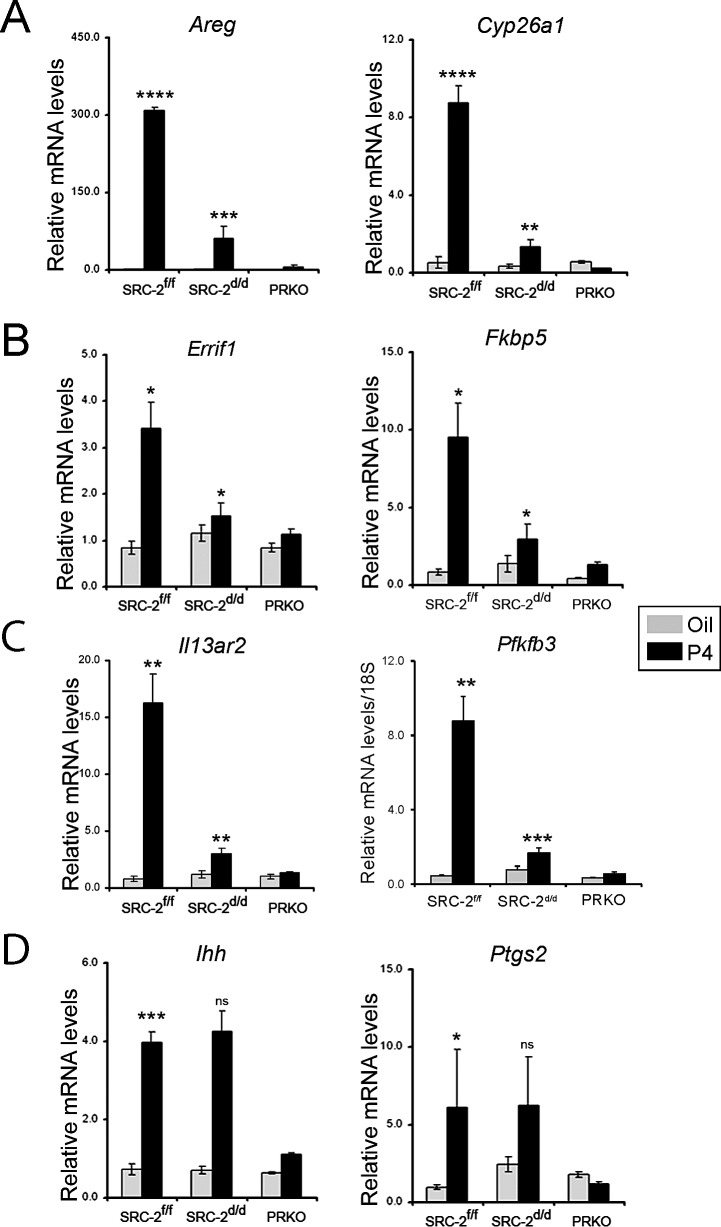
To validate our microarray analysis, we determined the transcript levels of a number of these previously reported P4 targets using uterine total RNA isolated from a separate cohort of ovariectomized SRC-2f/f and SRC-2d/d mice treated for 6 h with P4 or vehicle. Total uterine RNA from similarly treated PRKO mice was included as a negative control (Fig. 2). As validation of our microarray analysis, previously reported P4-responsive genes in murine uterus, such as Amphiregulin (Areg) [36], Cytochrome P450, family 26, subfamily a, polypeptide 1 (Cyp26a1) [31], ERBB receptor feedback inhibitor 1 [37], FK506 binding protein 5 (Fkbp5) [38], Interleukin 13 receptor, alpha 2 (Il13ra2) [37], 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase3 (Pfkfb3) [11], are significantly induced by P4 in the uterus of the SRC-2f/f control mouse (Fig. 2). In contrast, P4 induction of these genes is markedly attenuated in the SRC-2d/d uterus. It is noteworthy that P4 induction of Indian hedgehog (Ihh) and Prostaglandin-endoperoxide synthase 2 (Ptgs2) is not altered in the uterus of the SRC-2d/d mouse as compared to the SRC-2f/f control (Fig. 2), indicating that not all previously reported P4 targets involved in peri-implantation biology are controlled by SRC-2.
FIG. 2.
Postmicroarray validation at the transcriptional level. Quantitative real-time PCR analysis of transcript levels of known P4-responsive genes (Areg, Cyp26a1, Errfi1, Fkbp5, II13ra2, and Pfkfb3) in uterine total RNA derived from SRC-2f/f, SRC-2d/d, and PRKO mice, previously treated with either sesame oil (vehicle control) or P4 for 6 h. Transcript levels of P4-responsive genes, Ihh and Ptgs2, were not altered by SRC-2 depletion in the murine endometrium. Error bars represent the mean standard error (±SEM); *P < 0.05, **P < 0.01; ***P < 0.001; and ****P < 0.0001. Note: the statistically insignificant value is indicated as ns (not significant).
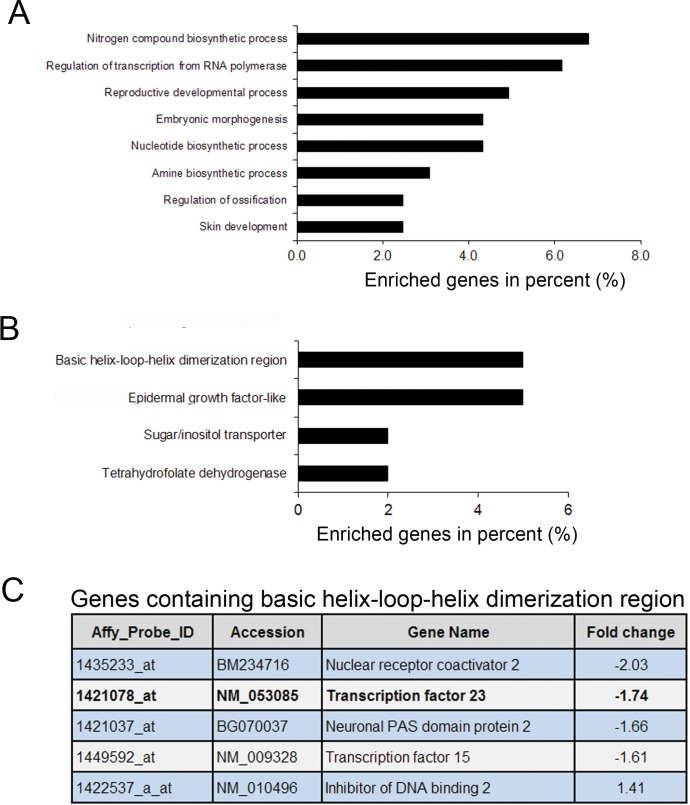
Having demonstrated a crucial role for SRC-2 in the induction of established P4 targets in the murine uterus, we next mined the SRC-2-dependent P4 transcriptome for novel genes required for endometrial decidualization. Mining for significantly enriched gene ontology (GO) terms, we revealed functional gene classes involved in a plethora of physiological processes, ranging from the biosynthesis of nucleotides and amino acids to reproductive development (Fig. 3A). Interestingly, analysis of gene clusters with similar protein domain(s) revealed a significant enrichment for genes that contain the bHLH DNA binding domain (Fig. 3B). As bHLH proteins belong to a family of transcription factors that play a major role in development and differentiation [39], we focused further on this protein class. Intriguingly, transcription factor 23 (Tcf23) is the most significantly down-regulated bHLH gene in the P4-responsive transcriptome when SRC-2 is depleted (Fig. 3C). Because of the specific transcription of Tcf23 in the murine ovary, uterus, and testis (Tcf23, also known as OUT) [40, 41], we further evaluated this transcription factor in terms of its responsiveness to P4, dependency on SRC-2, and importance to endometrial stromal cell decidualization.
FIG. 3.
Functional annotation of SRC-2-dependent progesterone responsive genes in the murine uterus. A) Unique SRC-2-dependent genes that are induced or repressed by P4 were analyzed for annotated gene function and similarities using the functional annotation and classification tools provided by DAVID Bioinformatics Resources, 6.7 (NIAID/NIH). Comparative enrichment of GO terms in the SRC-2-dependent P4 gene list is shown. The X-axis represents the percentage of genes enriched for individual GO term (P < 0.01). B) List of genes grouped based on the presence of similar protein domains. The X-axis represents the percentage of genes classified for each group (P < 0.05). C) Table illustrating unique genes that have bHLH protein domain is shown. The fold change indicates the fold reduction (−) in P4 induction of each gene when SRC-2 expression levels are markedly diminished.
Progesterone Induction of Tcf23 Transcription in the Murine Endometrium Requires SRC-2
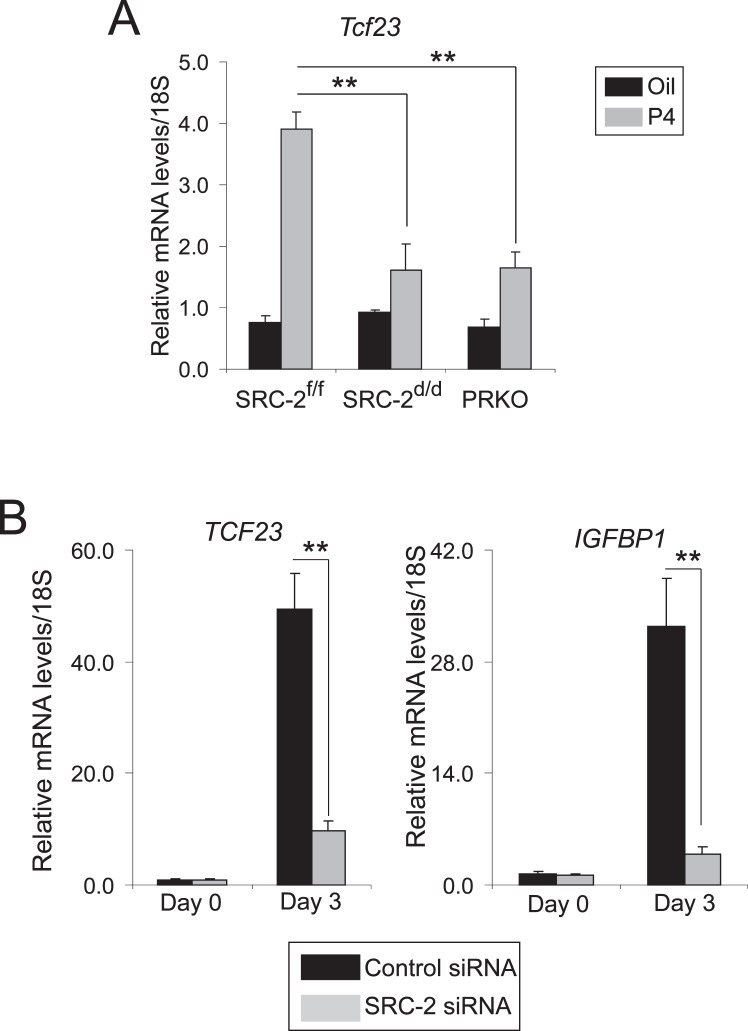
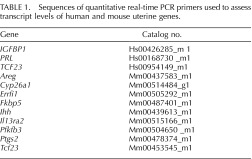
To determine if Tcf23 transcription in the murine endometrium is induced by P4 and dependent on SRC-2, transcript levels of Tcf23 were determined in uteri from ovariectomized SRC-2f/f, SRC-2d/d, and PRKO mice treated for 6 h with P4. As shown in Figure 4A, P4 significantly induced the transcription of Tcf23 in the uterus of the SRC-2f/f; however, this induction is markedly reduced in the SRC-2d/d and PRKO mouse. These results reveal that P4 induction of Tcf23 transcription requires SRC-2 in the murine endometrium. Importantly, TCF23 transcription also is significantly induced during P4-dependent decidualization of hESCs; again, TCF23 induction requires SRC-2 expression in this cell type (Fig. 4B). However, given that diverse set of factors, including micro-RNAs, have been implicated in progesterone action in female reproductive biology, it is fascinating to test whether micro-RNAs can potentially target Tcf23 in uterus [42].
FIG. 4.
Progesterone induction of Tcf23 transcription in the murine endometrium or human endometrial stromal cells requires SRC-2. A) Relative transcript levels of Tcf23 in uteri from SRC-2f/f, SRC-2d/d, and PRKO mice treated with sesame oil or progesterone for 6 h. B) Comparative transcript levels of IGFBP-1 and TCF23 in hESCs previously transfected with control siRNA or SRC-2 siRNA and cultured in EPC cocktail at the indicated time points. Error bars represent the ±SEM from triplicates; **P < 0.01.
Human Endometrial Stromal Cell Decidualization Requires TCF23
Because a knockout mouse model for Tcf23 is not available, we used an established hESC culture system [11] to test the functional importance of TCF23 in P4-dependent decidualization. While primary hESCs decidualize over a 6-day culture period in response to EPC media [11], TCF23 knockdown resulted in a block in hormone-dependent hESC decidualization at the cell and molecular levels (Fig. 5). Compared to control cells, hESCs with knockdown of TCF23 fail to transform from a fibroblastic to an epithelioid morphology that typifies full hESC decidualization [43] (Fig. 5A). The difference in cell morphology is reflected at the molecular level by a significant reduction in the induction of the PRL and IGFBP-1 decidual differentiation markers when TCF23 levels are reduced (Fig. 5, B and C). Collectively, these findings highlight an evolutionary conserved regulation of TCF23 by SRC-2 in the endometrial stromal cell and an indispensable role for this transcription factor in the endometrial decidual progression program. Moreover, these results underscore the utility of the SRC-2-dependent P4-responsive murine endometrial transcriptome as a powerful informational resource to prioritize new coregulator-dependent molecular signals that are critical for endometrial stromal cell decidualization.
FIG. 5.
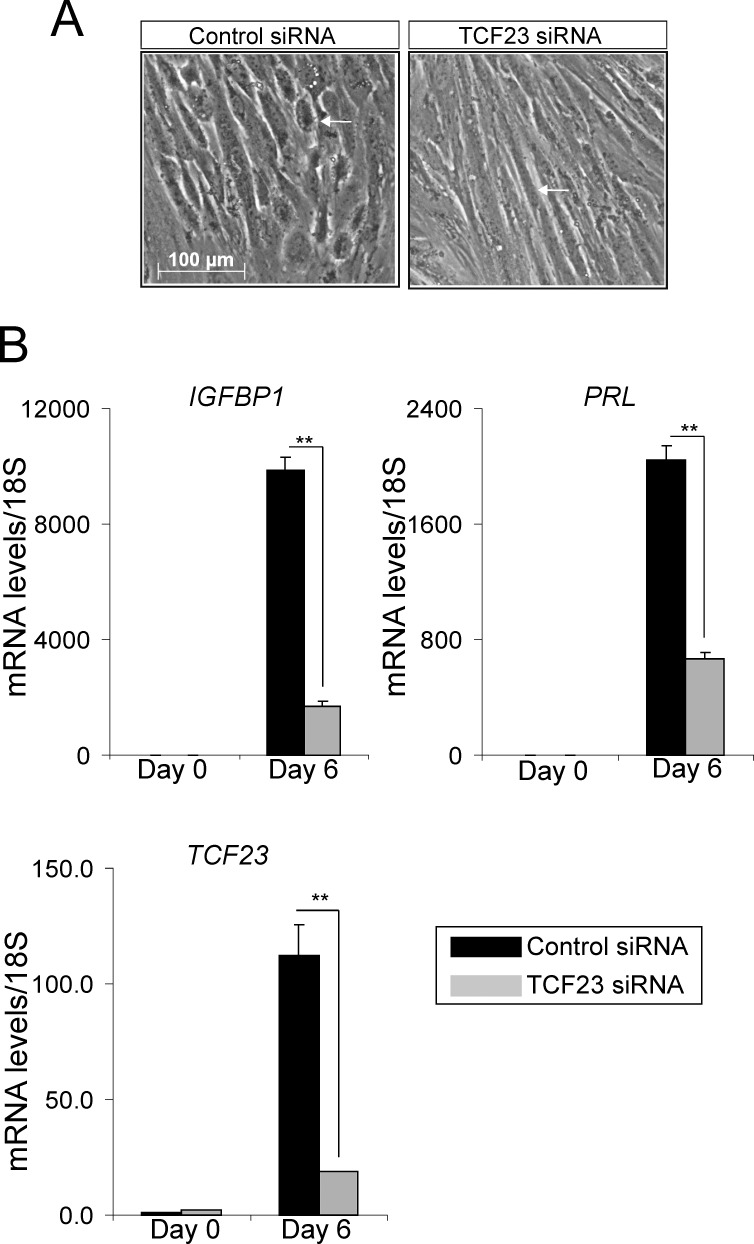
TCF23 is essential for hESC decidualization. A) Morphology of hESCs transfected with control siRNA or TCF23 siRNA, following 6 days of culture in the presence of EPC media. White arrowhead indicates the expected cobble stone appearance of the epithelioid stromal cells in the presence of the control siRNA (left panel) as well as the hESCs that remain fibroblastic in appearance when cultured in the presence of TCF23 siRNA (right panel). B) Transcript levels of IGFBP-1, PRL, and TCF23 in hESCs transfected with control siRNA or TCF23 siRNA and cultured in the presence of EPC media at the time points indicated. Error bars represent the ±SEM from triplicates; **P < 0.01.
DISCUSSION
Prior to hemochorial placentation, the endometrial stromal compartment undergoes a type of mesenchymal-to-epithelial transition, termed decidualization, in which stromal fibroblasts transform into transient epithelioid decidual cells [1]. Apart from providing a protective and immunotolerant environment for embryo development, the decidualized stromal cell layer is thought to regulate invasion of the embryonic trophoblast to a sufficient depth to enable fetoplacental circulation between the chorionic villi and the maternal intervillus space [44]. Therefore, because of the importance of the decidualized stroma to the establishment of the maternofetal interface, inadequate endometrial decidualization is considered a critical—yet understudied—causal factor for recurrent implantation failure and early embryo miscarriage in the human [45, 46]. Currently, our understanding of the key molecular underpinnings of endometrial stromal cell decidualization is rudimentary, a knowledge gap that threatens to undercut future improvements in the diagnosis and treatment of a nonreceptive uterus or a uterus with a poor capacity for decidualization.
In previous reports, we demonstrated that progesterone-driven decidualization requires SRC-2 [11], a member of a coregulator class known to act as potent modulators of transcriptional signaling cascades initiated by both nuclear receptor and nonnuclear receptor transcription factors [47]. The present study reveals that endometrial SRC-2 is essential for the full induction by P4 of a transcriptional profile in which a number of the downstream signals have been previously shown to be required for endometrial stromal cell decidualization. Given that the majority of genes in this SRC-2-dependent transcriptional profile have not been previously reported to be involved in P4-dependent decidualization (Supplemental Table S4), we used functional and structural domain analytic software to further subdivide this transcriptional profile in terms of transcription factors for which induction by P4 requires SRC-2 activity. This analytic approach revealed that members of the bHLH family of transcription factors are significantly represented compared to other transcription factor family members for which P4 induction is dependent on SRC-2. Further refinement of this analysis uncovered Tcf23 as the most prominent bHLH transcription factor based on P4 responsiveness and dependency on SRC-2.
This finding is significant since Tcf23 (or OUT) was previously shown to be transcriptionally expressed exclusively in the ovary, uterus, and testis of the adult mouse [40], suggesting a potential role for this bHLH transcription factor in female and male reproductive biology. In keeping with its induction by P4, transcriptional studies demonstrated that Tcf23 is induced to its highest levels in the endometrium of the virgin mouse during the diestrus phase of the estrous cycle [40], a stage of the cycle in which the ovarian corpus luteum synthesizes and secretes P4. While animal models and suitable antibodies are not currently available to study Tcf23 function and spatiotemporal expression dynamics in vivo, past in vitro investigations show that Tcf23 may control the functional activities of other bHLH-containing transcription factors [40].
Sequence alignment shows that Tcf23 displays a high degree of sequence homology (exceeding 40% amino acid sequence identity in the bHLH domain) to bHLH factors that are expressed in tissues of mesodermal origin that exert potent regulatory controls on cell-fate determination and differentiation [39]. Surprisingly, unlike the majority of bHLH transcription factors, Tcf23 fails to bind cognate E-box DNA sequences (CANNTG) but can form heterodimeric complexes with other E-box-binding bHLH transcription factors [40]. For example, MyoD (a myogenic tissue-specific class B bHLH transcription factor) and E12 (a ubiquitously expressed class A bHLH transcription factor) are prevented from forming functional homo- and heterodimers in the presence of Tcf23, leading to a block in E-box-mediated transactivation [40]. In the context of these studies, the data suggest that Tcf23 may act as a repressive bHLH factor similar to Twist or Mist1 [48, 49]. Notwithstanding these findings, key questions remain in terms of the role of Tcf23 in endometrial stromal cell decidualization: 1) Does endometrial Tcf23 bind a DNA response element other than an E-box? 2) In controlling gene expression during the decidualization process, does Tcf23 act as an activator, repressor, or both? 3) What is the interacting partner(s) of Tcf23 in the endometrial stromal cell? For instance, can Tcf23 interact with Tcf15 (Fig. 3C) and/or heart and neural crest derivatives expressed 2 (Hand2)? A recently described bHLH transcription factor required for decidualization [50, 51], Hand2 has yet to be matched with an endometrial interacting partner. 4) Does abrogation of Tcf23 in the mouse uterus cause a block in decidualization? An answer to this question awaits the generation of a suitable knockout mouse model.
Despite these unanswered questions, our findings here provide proof of principle that identification of a new decidual gene from this SRC-2-dependent transcriptome not only will provide new perspectives on the mechanistic underpinnings of decidual SRC-2 function but also may furnish novel biomarkers or therapeutic targets in the future diagnosis or treatment of a nonreceptive uterus.
Supplementary Material
ACKNOWLEDGMENT
The technical expertise of Jie Li, Yan Ying, Rong Zhao, and Yiqun Zhang is gratefully acknowledged.
Footnotes
This research was funded by grants from the National Institutes of Health (RO1 HD-07857, HD-042311, CA-77530, and U54 HD-0077495 to B.W.O., F.J.D., and J.P.L.).
REFERENCES
- Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. Embryo implantation. Dev Biol 2000; 223: 217–237. [DOI] [PubMed] [Google Scholar]
- Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med 2012; 18: 1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med 1999; 340: 1796–1799. [DOI] [PubMed] [Google Scholar]
- Aghajanova L, Velarde MC, Giudice LC. The progesterone receptor coactivator Hic-5 is involved in the pathophysiology of endometriosis. Endocrinology 2009; 150: 3863–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe J, Li Q, Mussi P, Liao L, Lydon JP, DeMayo FJ, Xu J. Nuclear receptor coactivator-6 attenuates uterine estrogen sensitivity to permit embryo implantation. Dev Cell 2012; 23: 858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabona JM, Simmen FA, Nikiforov MA, Zhuang D, Shankar K, Velarde MC, Zelenko Z, Giudice LC, Simmen RC. Kruppel-like factor 9 and progesterone receptor coregulation of decidualizing endometrial stromal cells: implications for the pathogenesis of endometriosis. J Clin Endocrinol Metab 2012; 97: E376–E392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Yoon S, Zhao Y, Park SE, Liao L, Xu J, Lydon JP, DeMayo FJ, O'Malley BW, Bagchi MK, Katzenellenbogen BS. Uterine development and fertility are dependent on gene dosage of the nuclear receptor coregulator REA. Endocrinology 2012; 153: 3982–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Park S, Bagchi MK, Taylor RN, Katzenellenbogen BS. The coregulator, repressor of estrogen receptor activity (REA), is a crucial regulator of the timing and magnitude of uterine decidualization. Endocrinology 2013; 154: 1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Soyal SM, Fernandez-Valdivia R, Gehin M, Chambon P, Demayo FJ, Lydon JP, O'Malley BW. Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol Cell Biol 2006; 26: 6571–6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Amato P, Allred DC, Fernandez-Valdivia R, Nguyen J, O'Malley BW, DeMayo FJ, Lydon JP. Steroid receptor coactivator 2 is essential for progesterone-dependent uterine function and mammary morphogenesis: insights from the mouse–implications for the human. J Steroid Biochem Mol Biol 2006; 102: 22–31. [DOI] [PubMed] [Google Scholar]
- Kommagani R, Szwarc MM, Kovanci E, Gibbons WE, Putluri N, Maity S, Creighton CJ, Sreekumar A, DeMayo FJ, Lydon JP, O'Malley BW. Acceleration of the glycolytic flux by steroid receptor coactivator-2 is essential for endometrial decidualization. PLoS Genet 2013; 9: e1003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra AR, Kommagani R, Saha P, Louet JF, Salazar C, Song J, Jeong J, Finegold M, Viollet B, DeMayo F, Chan L, Moore DD, et al. Cellular energy depletion resets whole-body energy by promoting coactivator-mediated dietary fuel absorption. Cell Metab 2011; 13: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehin M, Mark M, Dennefeld C, Dierich A, Gronemeyer H, Chambon P. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol Cell Biol 2002; 22: 5923–5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra AR, Louet JF, Saha P, An J, Demayo F, Xu J, York B, Karpen S, Finegold M, Moore D, Chan L, Newgard CB, et al. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke's disease. Science 2008; 322: 1395–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010; 18: 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carapeti M, Aguiar RC, Goldman JM, Cross NC. A novel fusion between MOZ and the nuclear receptor coactivator TIF2 in acute myeloid leukemia. Blood 1998; 91: 3127–3133. [PubMed] [Google Scholar]
- Liang J, Prouty L, Williams BJ, Dayton MA, Blanchard KL. Acute mixed lineage leukemia with an inv(8)(p11q13) resulting in fusion of the genes for MOZ and TIF2. Blood 1998; 92: 2118–2122. [PubMed] [Google Scholar]
- Gregory CW, Wilson EM, Apparao KB, Lininger RA, Meyer WR, Kowalik A, Fritz MA, Lessey BA. Steroid receptor coactivator expression throughout the menstrual cycle in normal and abnormal endometrium. J Clin Endocrinol Metab 2002; 87: 2960–2966. [DOI] [PubMed] [Google Scholar]
- Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci U S A 2003; 100: 9518–9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P, Lin Z, Reierstad S, Wu J, Ishikawa H, Marsh EE, Innes J, Cheng Y, Pearson K, Coon JSt, JJ Kim, Chakravarti D, et al. Transcription factor KLF11 integrates progesterone receptor signaling and proliferation in uterine leiomyoma cells. Cancer Res 2010; 70: 1722–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 2005; 41: 58–66. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, , Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 1995; 9: 2266–2278. [DOI] [PubMed] [Google Scholar]
- Fernandez-Valdivia R, Mukherjee A, Creighton CJ, Buser AC, DeMayo FJ, Edwards DP, Lydon JP. Transcriptional response of the murine mammary gland to acute progesterone exposure. Endocrinology 2008; 149: 6236–6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A 1998; 95: 14863–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci U S A 2001; 98: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadt EE, Li C, Ellis B, Wong WH. Feature extraction and normalization algorithms for high-density oligonucleotide gene expression array data. J Cell Biochem Suppl 2001; (suppl 37): 120–125. [DOI] [PubMed]
- Saldanha AJ. Java Treeview–extensible visualization of microarray data. Bioinformatics 2004; 20: 3246–3248. [DOI] [PubMed] [Google Scholar]
- WMA Declaration of Helsinki serves as guide to physicians. Calif Med 1966; 105: 149–150. [PMC free article] [PubMed] [Google Scholar]
- Brosens JJ, Hayashi N, White JO. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology 1999; 140: 4809–4820. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Lee KY, Han SJ, Aronow BJ, Lydon JP, O'Malley BW, DeMayo FJ. The p160 steroid receptor coactivator 2, SRC-2, regulates murine endometrial function and regulates progesterone-independent and -dependent gene expression. Endocrinology 2007; 148: 4238–4250. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Lee KY, Kwak I, White LD, Hilsenbeck SG, Lydon JP, DeMayo FJ. Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology 2005; 146: 3490–3505. [DOI] [PubMed] [Google Scholar]
- Rubel CA, Lanz RB, Kommagani R, Franco HL, Lydon JP, DeMayo FJ. Research resource: genome-wide profiling of progesterone receptor binding in the mouse uterus. Mol Endocrinol 2012; 26: 1428–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia HF, Ma JJ, Sun J, Yang Y, Peng JP. Retinoic acid metabolizing enzyme CYP26A1 is implicated in rat embryo implantation. Hum Reprod 2010; 25: 2985–2998. [DOI] [PubMed] [Google Scholar]
- Kaiser M, Gibori G, Mayo KE. The rat follistatin gene is highly expressed in decidual tissue. Endocrinology 1990; 126: 2768–2770. [DOI] [PubMed] [Google Scholar]
- Fernandez-Valdivia R, Mukherjee A, Amato P, Allred DC, Nguyen J, DeMayo FJ, Lydon JP. Progesterone-action in the murine uterus and mammary gland requires steroid receptor coactivator 2: relevance to the human. Front Biosci 2007; 12: 3640–3647. [DOI] [PubMed] [Google Scholar]
- Das SK, Chakraborty I, Paria BC, Wang XN, Plowman G, Dey SK. Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol 1995; 9: 691–705. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Lee HS, Lee KY, White LD, Broaddus RR, Zhang YW, Vande Woude GF, Giudice LC, Young SL, Lessey BA, Tsai SY, Lydon JP, et al. Mig-6 modulates uterine steroid hormone responsiveness and exhibits altered expression in endometrial disease. Proc Natl Acad Sci U S A 2009; 106: 8677–8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Dai D, Lee KY, Rubel CA, Roop D, Boerboom D, Jeong JW, Lydon JP, Bagchi IC, Bagchi MK, DeMayo FJ. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J 2011; 25: 1176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. An overview of the basic helix-loop-helix proteins. Genome Biol 2004; 5: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumi O, Mori S, Boku S, Tsuji Y, Hashimoto N, Nishikawa S, Yokota Y. OUT. a novel basic helix-loop-helix transcription factor with an Id-like inhibitory activity. J Biol Chem 2000; 275: 3510–3521. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Narumi O, Muguruma K, Yamamoto I, Shinkai Y, Yokota Y. Genomic organization and chromosomal mapping of the basic helix-loop-helix factor OUT (Tcf23/TCF23). Cytogenet Cell Genet 2001; 94: 23–25. [DOI] [PubMed] [Google Scholar]
- Hawkins SM, Buchold GM, Matzuk MM. Minireview: the roles of small RNA pathways in reproductive medicine. Mol Endocrinol 2011; 25: 1257–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med 2007; 25: 445–453. [DOI] [PubMed] [Google Scholar]
- Wright C, Sibley CP. Placental transfer in health and disease. : The Placenta. New York: Wiley-Blackwell; 2011: 66–74. [Google Scholar]
- Salker M, Teklenburg G, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, Roelen BA, Quenby S, et al. Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One 2010; 5: e10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teklenburg G, Salker M, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, Roelen BA, Quenby S, et al. Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS One 2010; 5: e10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wu RC, O'Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer 2009; 9: 615–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemercier C, To RQ, Carrasco RA, Konieczny SF. The basic helix-loop-helix transcription factor Mist1 functions as a transcriptional repressor of myoD. EMBO J 1998; 17: 1412–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q, Xu Y, He T, Qin C, Xu J. Normal and disease-related biological functions of Twist1 and underlying molecular mechanisms. Cell Res 2012; 22: 90–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, Srivastava D, Bagchi MK, Bagchi IC. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 2011; 331: 912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyen DV, Bany BM. Evidence for a conserved function of heart and neural crest derivatives expressed transcript 2 in mouse and human decidualization. Reproduction 2011; 142: 353–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.