ABSTRACT
The preimplantation period is a time of reprogramming that may be vulnerable to disruption. This question has wide clinical relevance since the number of children conceived by in vitro fertilization (IVF) is rising. To examine this question, outbred mice (CF1 × B6D2F1) conceived by IVF and cultured using Whitten medium and 20% O2 (IVFWM group, less optimal) or K simplex optimized medium with amino acids and 5% O2 (IVFKAA group, more optimal and similar to conditions used in human IVF) were studied postnatally. We found that flushed blastocysts transferred to recipient mice provided the best control group (FB group), as this accounted for the effects of superovulation, embryo transfer, and litter size. We observed that many physiological parameters were normal. Reassuringly, IVFKAA offspring did not differ significantly from FB offspring. However, male IVFWM mice (but not females) were larger during the first 19 wk of life and exhibited glucose intolerance. Male IVFWM mice also showed enlarged left heart despite normal blood pressure. Expression of candidate imprinted genes (H19, Igf2, and Slc38a4) in multiple adult tissues did not show differences among the groups; only Slc38a4 was down-regulated following IVF (in both culture conditions) in female adipose tissue. These studies demonstrate that adult metabolism is affected by the type of conditions encountered during the preimplantation stage. Further, the postnatal growth trajectory and glucose homeostasis following ex vivo manipulation may be sexual dimorphic. Future work on the long-term effects of IVF offspring should focus on glucose metabolism and the cardiovascular system.
Keywords: ART, DOHaD, embryos
Increased disturbance during the preimplantation period alters adult phenotype.
INTRODUCTION
Since the original observation of an increase in cardiovascular disease and diabetes in adults who were subjected to famine conditions for a defined time in utero [1, 2], the developmental origin of health and disease hypothesis (DOHaD) hypothesis has spurred investigation on the delayed effects of the prenatal environment on adult physiology, behavior, and disease [3]. Importantly, individuals who appear normal at birth may develop a disease or phenotype in adulthood that was in fact caused by events that occurred in utero [1, 2].
However, the possibility that metabolic abnormalities may develop in adults following in vitro preimplantation culture has not been extensively studied. This is important since the practice of in vitro fertilization (IVF) and other assisted reproductive technologies (ART) has become well established and today accounts for 1%–5% of live births in the Western world [4]. During the process of IVF, both gametes and the preimplantation embryo are exposed to laboratory conditions that do not perfectly recapitulate the normal environment of the Fallopian tube and uterus [5]. Within this artificial environment, crucial developmental events occur, including fertilization itself, the establishment of the embryonic axes, the first cell divisions, the establishment of the trophoblast cell lineage, and changes in the epigenome of the entire embryo [6, 7].
Fortunately, epidemiologic data show that IVF offspring are healthy; however, there is a statistically significant increase in birth defects (from 3%–4% in the general population to 4%–5% for ART newborns) [8, 9]. In addition, there is also a small but significant increase in imprinting disorders, including Angelman and Beckwith-Wiedemann syndromes [10]. While some studies of children conceived by ART showed no clear metabolic abnormalities [11, 12], others have shown statistically significant differences in fat deposition, increased blood pressure, and increased fasting glucose [13–15].
In a study using a mouse model, various sex-specific effects of IVF on postnatal growth, body composition, and glucose clearance were found [16]. The mice were followed until 8 wk of age. In addition, different groups discovered that preimplantation in vitro culture had a significant effect on the behavior of adult mice [17, 18].
Animal models studying the adult consequences of IVF are particularly helpful in separating the effects of parental infertility versus the effects of embryo manipulation and culture conditions itself, as normal gamete donors can be used. The shorter generation time of most experimental (i.e., rodents) and farm animals is another advantage of animal models. In addition to indicating which physiological systems might be at risk in IVF-conceived adults, animal models are useful for optimizing IVF conditions. For example, most clinics are now culturing embryos using 5% oxygen (O2) rather than atmospheric O2 (20%) because animal models showed that gene expression in embryos cultured with 5% O2 were more similar to normal embryos than those cultured with 20% O2 [19].
The present study describes the effects of IVF, in two different culture media, on many aspects of postnatal physiology, including growth, glucose clearance, body composition, cardiovascular parameters, sexual development, and endocrinology, from birth to 30 wk of age. Many parameters fell within normal limits, but there were sex-specific and culture medium-specific effects of IVF on growth and metabolism. In addition, this study tested and confirmed the importance of using mice that were conceived in vivo but subjected to embryo transfer as the proper controls for this and future experiments on the influence of IVF on development and adult function.
MATERIALS AND METHODS
In Vitro Fertilization and Embryo Transfer
Mice were kept in the University of California at San Francisco (UCSF) animal facility with controlled temperature conditions of 23°C, a 12L:12D cycle, and ad libitum access to water and mouse chow (20% protein, 9% fat; LabDiet). In vitro fertilization was performed as previously described [20]. Briefly, 6-wk-old CF1 female mice were superovulated by injecting 5 IU PMSG and 42–46 h later 5 IU hCG. Oocytes were collected from the ampullae 13 h after hCG injection. Sperm were obtained from the cauda epididymis of B6D2F1/J males.
Gametes were coincubated in Whitten medium (WM) containing 15 mg/ml BSA for 4 h. Fertilized oocytes were washed and cultured to the blastocyst stage under mineral oil in a 37°C humidified atmosphere in modular incubators. Two conditions were used—WM with 20% O2 (IVFWM group) or K modified simplex optimized medium + amino acids (KSOM+AA) under 5% O2 (IVFKAA group)—to examine whether culture conditions could affect the outcome. Using KSOM+AA with 5% O2 is generally considered to be more optimal than WM with 20% O2 [21].
Naturally ovulating female CF1 mice, mated with vasectomized CD1 males, were used as recipients for embryo transfers. Mating was confirmed by the presence of a vaginal plug the following morning. Blastocysts transfers were performed 2.5 days postcoitum. Late-cavitating blastocysts of similar morphology (n = 11–19 embryos, split between both horns) were then transferred to the uterine horns of pseudopregnant recipients [22]. It is important to note that a similar numbers of embryos was transferred in all experimental groups.
Control mice were generated as follows:
-
1)
In vivo group: Animals were allowed to conceive naturally without superovulation. One B6D2F1/J male and one CF1 female mouse were housed together overnight; the presence of a vaginal plug, checked in the early morning after mating, was considered evidence of mating.
-
2)
Flushed blastocysts (FB) group: CF1 female mice were superovulated as described above and housed together with a B6D2F1/J male. Late-cavitating blastocysts were flushed from the uterus on Day 3.5 at 1500 h, that is, 87 h postfertilization [23], and immediately transferred to the uterine horns of pseudopregnant CF1 recipients.
Initial experiments were performed comparing IVFWM versus control mice, while a second cohort of IVFKAA mice was generated with a 6-wk delay. This was done because WM is considered a more stressful medium, and if no difference in phenotype had been observed between IVFWM and FB mice, experiments would not have been repeated in the more optimal IVFKAA group. For example, since differences were found between FB and IVFWM with the glucose tolerance test, this test was done with IVFKAA mice, but sexual maturation was not examined in the IVFKAA mice, as it was identical in FB and IVFWM mice.
The protocol for animal handling and treatment procedures was reviewed and approved by the Animal Care Facility at UCSF.
Morphometric Data and Postnatal Growth
Morphometric characteristics were measured at birth (weight, length, biparietal diameter [BPD], and anogenital distance). All pups in litter (of any size) were used to generate birth data (Table 1). However, only animals from litters with 4–10 pups were used in the postnatal study.
TABLE 1.
Litter characteristics for this study.
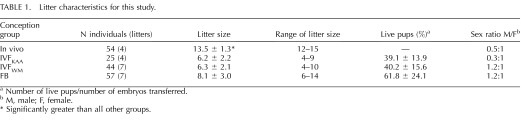
Number of live pups/number of embryos transferred.
M, male; F, female.
Significantly greater than all other groups.
Weight was measured weekly until 30 wk. At that time, some of the animals were sent for insulin clamp and cardiovascular testing. Pubertal development (testicular descent and preputial separation in males and vaginal opening in females) was assessed daily, as described [24].
Dual-Energy X-Ray Absorption Scan
Total body lean and fat mass was evaluated using dual-energy X-ray absorption (DEXA) scanning. Mice were tested shortly after puberty (8 wk) and in adulthood (28 wk) for the lean and fat composition of the body. Mice were anesthetized (ketamine 100 mg/kg; zylaxine 10 mg/kg) and scanned three times (with repositioning between scans) using a Lunar PIXImus densitometer (software version 1.42.006.010; Lunar Corp.). All mice were fasted for 3 h before the DEXA measurements [25]. The intraindividual coefficient of variation (CV) was determined for each animal using the three DEXA scans.
Intraperitoneal Glucose Tolerance Test
The intraperitoneal glucose tolerance test (IPGTT) was performed at 19 wk of age. Mice were fasted for 6 h prior to the test. Whole-blood glucose levels were measured using a handheld glucometer (Roche Diagnostics), and 20–50 μl of blood were collected from the tail vein to determine insulin levels. Glucose (2 mg/g) was given injected intraperitoneally, and glucose and insulin were measured 0, 15, 30, 60, and 120 min postinjection. Food was immediately returned to the mice following the last measurement.
The insulinogenic index was calculated by dividing the area under the curve for insulin (t = 0–30 min) by the area under the curve for glucose (t = 0–30 min). The insulin resistance index was calculated using fasting levels of glucose and insulin (HOMA-IR = glucose [mg/dl] × insulin [ng/ml]/ 405) [26].
3-3H-D-Glucose and 14C-2-Deoxyglucose Hyperinsulinemic-Euglycemic Clamp
This procedure was performed at the Mouse Metabolic Phenotyping Center (MMPC) of Vanderbilt University, following the protocol of Ayala et al. [27]. Only FB and IVFWM male mice, at 38 wk of age, were used (FB; n = 9, from three litters; IVFWM n = 10, from four litters). A 2-h hyperinsulinemic-euglycemic clamp was performed after a 5-h fast in awake males. Mice were catheterized at least 5 days before the experiment. A 5-μCi bolus of (3-3H) glucose was given 90 min before the insulin infusion, followed by a 0.05-μCi/min infusion for 90 min. Blood samples were obtained with an arterial catheter. Basal glucose-specific activity was determined from blood samples obtained 15 and 5 min before the test. Fasting insulin levels were determined from blood samples taken 5 min before the test. The clamp was begun at t = 0 min with a continuous infusion of human insulin (Humulin R, 4 mU/kg/min; Eli Lilly). The (3-3H) glucose infusion was increased to 0.2 μCi/min for the remainder of the experiment. Euglycemia (100–120 mg/dl) was maintained by measuring blood glucose every 10 min starting at t = 0 min and infusing 50% dextrose as necessary. Mice received saline-washed erythrocytes from donors throughout the clamp (5–6 μl/min) to prevent their hematocrit from falling by more than 5%. A 12-μCi bolus of 2 (14C) deoxyglucose (2-14C-DG) was given at t = 78 min to assess insulin-stimulated glucose uptake in tissue. Blood samples (80–240 μl) were taken every 10 min from t = 80 to t = 120 min and processed to determine plasma (3-3H) glucose and 2-14C-DG. Clamp insulin was determined at t = 100 and 120 min. At t = 120 min, mice were anesthetized with sodium pentobarbital. The soleus, gastrocnemius, superficial vastus lateralis, diaphragm, heart, adipose tissue, and brain were excised, immediately frozen, and stored at −80°C until analyzed.
Rates of basal hepatic glucose production and insulin-stimulated whole-body and tissue glucose uptake were determined [28] as follows. After deproteinization with barium hydroxide (Ba [OH]2, 0.3 N) and zinc sulfate (ZnSO4, 0.3 N), plasma (3-3H)glucose and 2-14C-DG radioactivity was determined by liquid scintillation counting (Packard TRI-CARB 2900TR) with Ultima Gold (Packard) as scintillant to measure basal hepatic glucose production. Tissue samples were weighed and homogenized in 0.5% perchloric acid. Homogenates were centrifuged and neutralized with KOH. One aliquot was counted directly to determine 2-14C-DG and 2-14C-DG-6-phosphate (2-14C-DGP) radioactivity. A second aliquot was treated with Ba(OH)2 and ZnSO4 to remove 2-14C-DGP and any tracer incorporated into glycogen and then counted to determine 2-14C-DG radioactivity. The 2-14C-DGP is the difference between the two aliquots. In all experiments, the accumulation of 2-14C-DGP was normalized to tissue weight.
Blood Pressure
This procedure was performed at the MMPC of Vanderbilt University. Plethysmography was performed using a tail-cuff BP apparatus (BP-2000; Visitech Systems, Inc.). Only FB and IVFWM male mice at 38 wk of age were used: (FB n = 13 from three litters; IVFWM n = 11 from three litters). This technology is noninvasive and has a good concordance with direct BP measurements. Blood pressure and pulse measurements were recorded by the same investigator at the same time each day. Each day, 20 measurements of blood pressure and pulse were made, of which the final 10 were averaged to obtain the daily blood pressure reading for that animal. Prior to recording experimental data, mice were subjected to a 10-day training period.
Echocardiography
This procedure was performed at the MMPC of Vanderbilt University. Only male mice at 38 wk of age were used (FB, n = 13 from three litters; IVFWM, n = 10 from three litters). Transthoracic echocardiography was performed using a system (Sonos 5500; Agilent) with a 15-MHz high-frequency linear transducer at a frame rate of 100 frames/sec, as described [29]. All images were acquired at a depth setting of 20 mm. Wall thickness and chamber dimension were determined from M-mode tracings in conscious mice. Left-ventricular (LV) wall thickness was evaluated in the interventricular septum (IVS) and the LV posterior wall (LVPW). End-diastolic measurements (IVSd, LVPWd, and LV internal dimension [LVIDd]) were obtained at the point of maximal LV diastolic dimension. LV end-systolic dimensions (IVSs, LVPWs, and LVIDs) were obtained at the time of most anterior systolic excursion of the LVPW associated with minimal chamber dimension. The FS%, a measure of LV systolic performance, was calculated from M-mode-derived LV dimensions using the formula (LVIDd − LVIDs)/LVIDd × 100%. The standard M-mode formula for ejection fraction was used: ejection fraction = (LVIDd3 − LVIDs3)/LVIDd3 × 100% [29].
Quantitative Real-time PCR
RNA was extracted from three tissues (liver, fat, and muscle) of IVFWM, IVFKAA, and FB mice, and quantitative real-time PCR was conducted as described previously [22]. Tissues from individual animals (females, eight per group; males, FB n = 4; IVFWM and IVFKAA n = 5) were assayed in triplicate. Total RNA was extracted by manually homogenizing samples in Trizol reagent and isolated using RNeasy Mini Kit with DNase digestion to remove residual DNA (Qiagen). Reverse transcription was accomplished using a commercially available first-strand cDNA synthesis kit according to manufacturer's protocol (iScript cDNA Synthesis Kit; Bio-Rad Laboratories). Quantification of gene transcripts were performed using SyBr green PCR Supermix (Bio-Rad) with 10 ng of cDNA. Amounts of H19, Igf2, and Slc38a4 were normalized to levels of H2A (forward: 5′-ACATGGCGGCGGTGCTGGAGT; reverse: 5′-CGGGATGATGCGCGTCTTCTTGTT). Primer sequences can be found in Bloise et al. [22].
H19 was selected because of its known alteration following preimplantation embryo culture [30]. Igf2 is important in somatic growth [31]. Slc38a4 was chosen because we have found differences in Slc38a4 expression in IVF placentae [22].
Insulin Measurement
To obtain insulin levels, 5–10 μl of serum or culture supernatant was assayed using an ultrasensitive insulin ELISA kit (Alpco).
Corticosterone Assay
Corticosterone was measured in male mice at approximately 25 wk of age. Approximately 40 μl of blood were collected from the tail vein of each mouse around 1400–1600 h, when corticosterone (CORT) levels are near their circadian peak [32]. Since elevated CORT levels can be detected in circulation just 2 min from the initial cage disturbance [33], blood collections were completed within the 2 min from the initial cage handling. Samples were centrifuged at 4°C for 10 min, and plasma was collected and transferred into new tubes. Plasma samples were diluted in 1:100 assay buffer, and approximately two 100-μl aliquots for each sample were collected to perform radioimmunoassay (RIA) in duplicate using GammaCoat cortisol I-125 coated-tube RIA kit (INCSTAR Corp.) as previously described [34]. These samples were analyzed by Dr. Charles Wilkinson, University of Washington.
Statistics
All data are presented as the mean ± SD unless otherwise specified. Either a one-way analysis of variance (ANOVA) or a two-tailed Student t-test was used for statistical analysis as appropriate. Tukey post hoc test was applied to test for differences between groups when a one-way ANOVA was significant using the Prism software package (Graphpad). A P-value of <0.05 was considered significant. The reported number represents the number of mice; the number of litters is given in parentheses. The single mouse is the experimental unit [35].
RESULTS
Litter Size and the Choice of a Control Group
The original, most intuitive control group for these studies consisted of nonmanipulated, naturally mated and gestated controls (in vivo group). However, it soon became apparent that the litter size in the in vivo group was significantly larger (13.5 ± 1.3, n = 4 litters) than that of the IVF groups (IVFKAA, 6.2 ± 2.2, n = 4 litters; IVFWM, 6.3 ± 2.1, n = 7 litters; Table 1). Correspondingly, the birth weights of the pups in the in vivo group were significantly smaller (1.44 ± 0.12) than those of either of the IVF groups (IVFKAA, 1.73 ± 0.20; IVFWM, 1.73 ± 0.23). Within groups, male and female mice did not differ in their birth weights. There was an equal distribution of litter size among the FB, IVFKAA, and IVFWM groups, and we did not find significant differences in any parameters when assessing animals originating from the smaller or larger litters.
These results led us to add a second control group, called the FB group, in which blastocysts from superovulated females were immediately transferred to recipient mice. This strategy has been adopted before [36]. The litter size from this group was statistically the same as the IVF groups (8.1 ± 3.0, n = 7 litters; Table 1), and the birth weights of the FB mice (1.66 ± 0.16) were also not different from the IVF groups. Unless otherwise noted, the FB group was used as the control for the rest of the study.
There were no significant differences in the percent of live births (number of pups divided by the number of embryos transferred) or sex ratio among the FB and IVF groups (Table 1). The length of gestation was also measured for all of the groups. For the in vivo group, this was a calculation of the date of birth minus the plug date (19 days, n = 4 litters). Two perspectives could be used for measuring the gestational length for groups that had undergone embryo transfer: time from the detection of the vaginal plug in the recipient to the time of delivery (dam's perspective) and the time from fertilization to delivery (the embryo's perspective). The time from vaginal plug to birth was 19.0 ± 0.6 days (n = 7) for FB, 19.0 ± 0.0 (n = 4) for IVFKAA, and 19.1 ± 0.4 (n = 7) for IVFWM. For the FB group, the donor and recipient dams were at the same stage, that is, 2 days from the detection of a vaginal plug, so the pregnancy length was the same from both perspectives. Since the IVF embryos were cultured for 4.5 days prior to transfer to a 2.5-day pregnant female, the timing from this perspective is 2 days longer, roughly 21 days, for IVF pregnancies. Gestational length from the dam's perspective most closely corresponds to the length of an in vivo pregnancy.
Growth and Development
There was no difference between IVF groups and the FB group in any of the parameters measured at birth (body weight, body length, biparietal diameter [BPD], or anogenital distance; Supplemental Table S1; all Supplemental Data are available online at www.biolreprod.org). However, the anogenital distance was significantly greater in the in vivo group as compared to the groups in which the embryos had been transferred. This was true for both males and females. Comparing the anogenital index (anogenital distance/body weight) among groups accentuated the difference between in vivo and transferred groups, as pups in the in vivo group were smaller (Table 2).
TABLE 2.
Anogenital (AG) distance at birth.
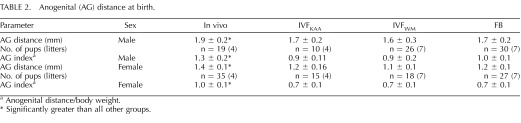
Anogenital distance/body weight.
Significantly greater than all other groups.
In general, male and female mice within each group had the same mean body weights each week from birth to weaning (3 wk), after which time the males in all groups became significantly heavier (Supplemental Table S2; Supplemental Figure S1).
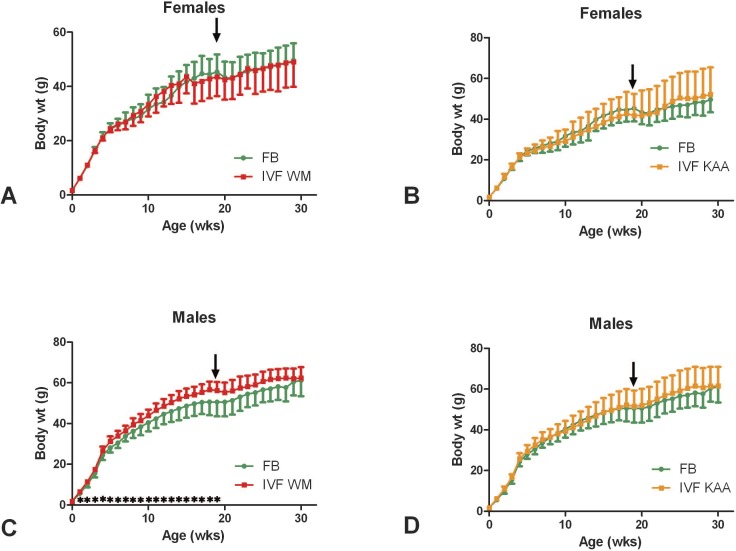
Female IVF mice had the same pattern of growth as those in the FB group (Fig. 1, A and B). A time-point-by-time-point comparison showed no statistical difference except at 2 wk of age, at which time IFVKAA females were larger than FB.
FIG. 1.
Growth curves. Female (A, B) and male (C, D) IVFWM and IVFKAA mice were compared separately to FB control mice. The IVFWM males (C) were significantly larger (*) than their FB counterparts for most of their growth. Performing the GTT (arrows) appeared to temporarily disrupt the growth of the mice. N for each time point are given in Supplemental Table S2. Values are means ± SD.
Although they weighed the same at birth, male IVFWM mice were heavier than FB males from 1 to 19 wk (Fig. 1C). The specific growth rate (change in weight/weight) of IVFWM was significantly greater than FB between birth and 1 wk of age (FB, 0.698 ± 0.027; IVFWM, 0.734 ± 0.025) and then again between 17 and 18 wk of age (FB, 0.003 ± 0.038; IVFWM, 0.023 ± 0.015), when the FB growth curve began to flatten out. Conducting the IP-GTT and associated insulin measurements at approximately 19 wk appeared to affect the body weights of the mice, causing a temporary slowing of growth (Fig. 1). By 20 wk of age the FB mice had attained the same weight as the IVFWM, and the two groups maintained the same adult weight. The IVFKAA males had the same growth pattern as the FB control males (Fig. 1D).
Percent body fat and bone mineral density (BMD) were measured by DEXA scanning at 8 and 28 wk of age. At 8 wk of age, the percent body fat of male and female mice and among treatment groups was not different (22%; Supplemental Table S1).
Percent body fat increased significantly between 8 and 28 wk of age in both males and females, with older animals having more body fat. Females in each group had more adipose tissue, on average, than males. The difference between males and females was statistically significant in the FB and the IVFKAA groups but not the IVFWM group (Supplemental Table S1). There were no differences between IVF and FB animals, comparing males and females separately, in percent body fat at 28 wk of age (Supplemental Table S1).
BMD was significantly greater in males than in females at both 8 and 28 wk of age (Supplemental Table S1). At 8 wk of age, IVF males had greater BMD than FB males (FB, 0.0566 ± 0.0028 g/cm2; IVFKAA, 0.0615 ±.0041 g/cm2; IVFWM, 0.0593 ± 0.0030 g/cm2; Supplemental Figure S2), while females did not differ in BMD (Supplemental Table S1). At 28 wk, there were no BMD differences between FB and either of the IVF groups for either males or females (Supplemental Table S1).
The sexual development of IVF male and female mice appeared to be similar to FB controls. The age at which the testes descended, 22–23 days, and the age at which preputial separation occurred, 24 days, were identical in IVFWM and the FB group (Supplemental Table S1). For females, vaginal opening was detected at the same ages, 26 days, in FB and IVFWM mice.
Corticosterone Levels
Serum corticosterone levels, measured in males at 25 wk, were similar in mice from the FB and IVFWM groups (Supplemental Table S1).
Glucose Homeostasis
In all groups analyzed and under all conditions, serum glucose levels in female mice were significantly lower than in males (Fig. 2). In female 19-wk-old mice, under 6-h fasting conditions, the level of glucose did not differ among the groups. The pattern of the IP-GTT response was identical for females in the IVF groups and the FB control (Fig. 2A). Point-by-point comparisons and an analysis of the area under the curve (AUC) showed no difference among FB, IVFKAA, and IVFWM females (Fig. 2C).
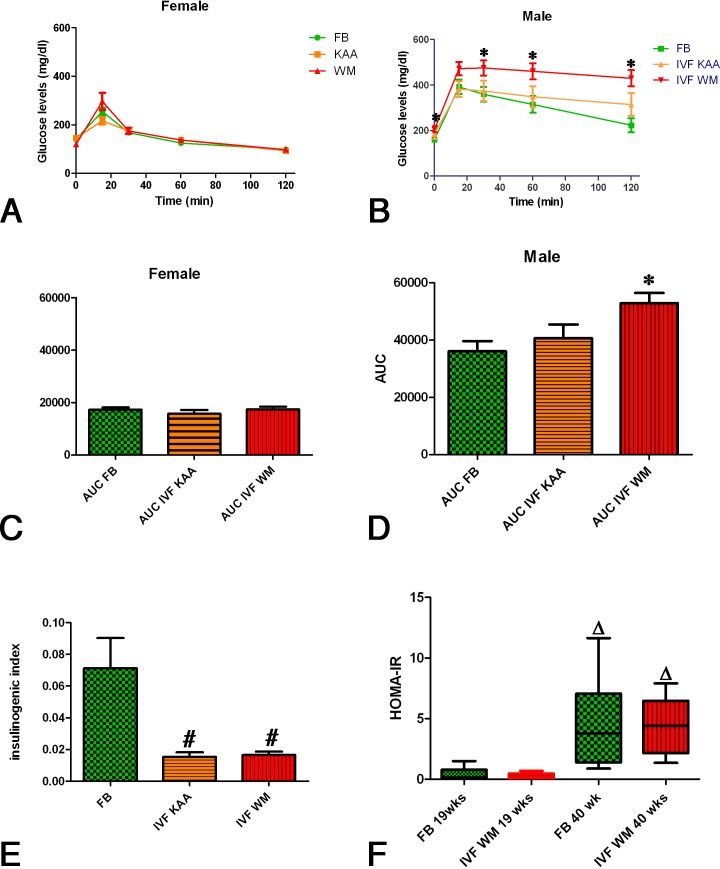
FIG. 2.
Glucose tolerance test. Female IVF mice (A) had a normal glucose tolerance as evidenced by glucose levels over time and by the calculation of the AUC (C). Male IVFWM mice, however, showed glucose intolerance, maintaining significantly higher levels of serum glucose (*) after intraperitoneal glucose injection (B). This was also reflected in the significantly higher AUC for male IVFWM mice (D). The insulinogenic index (E) was significantly lower (#) in IVF mice. The insulin resistance index (HOMA-IR; F) for males was not different between FB and IVFWM groups at either 19 or 40 wk (n = 9; three litters; same mice at both time points) but increased significantly (Δ) with time. Values are means ± SD.
Male IVFWM showed evidence of glucose intolerance. In particular, fasting glucose levels were significantly greater in the IVFWM group than in the in the FB group. During the IP-GTT. the peak glucose levels were reached at 15 min for the FB and IVFKAA group, but levels were still climbing at that time in the IVFWM group and remained statistically higher for the duration of the test (Fig. 2C). The AUC for glucose in IVFWM males was significantly greater than for FB (Fig. 2D). At the time of the IP-GTT, IVFWM males weighed significantly more than FB males. The AUC correlated positively with body weight for male FB and IVFWM mice but not IVFKAA (Supplemental Figure S3A). There was no correlation of body weight with AUC in any group for the female mice (Supplemental Figure S3B).
For both males and females at 19 wk of age, fasting insulin levels, the insulin curve during the IP-GTT, and AUC for insulin were similar in IVF groups and in the FB group (Supplemental Figure S4). Overall, females had insulin levels that were approximately half of those seen in males (Supplemental Figure S4). Interestingly, while females had a normal response to glucose infusion and insulin peaked at 15 min postinjection, the insulin curves in males were relatively flat in all groups (Supplemental Figure S4).
The insulinogenic index, the amount of insulin secreted for a given glucose stimulus (AUCinsulin0–30/AUCglucose0–30), was significantly greater in FB males than in either IVFWM or IVFKAA males (Fig. 2E). A calculation of the insulin resistance index, HOMA-IR, showed no difference among the three groups (FB, 2.80 ± 2.87; IVFKAA, 2.68 ± 3.05; IVFWM, 3.77 ± 2.49). At 40 wk of age, basal insulin and glucose levels were measured after an overnight fast in a subset of males from the FB and IVFWM groups. The HOMA-IR calculated from these values was significantly elevated compared to the values at 19 wk of age, but there was still no difference between the FB and the IVFWM groups (Fig. 2F).
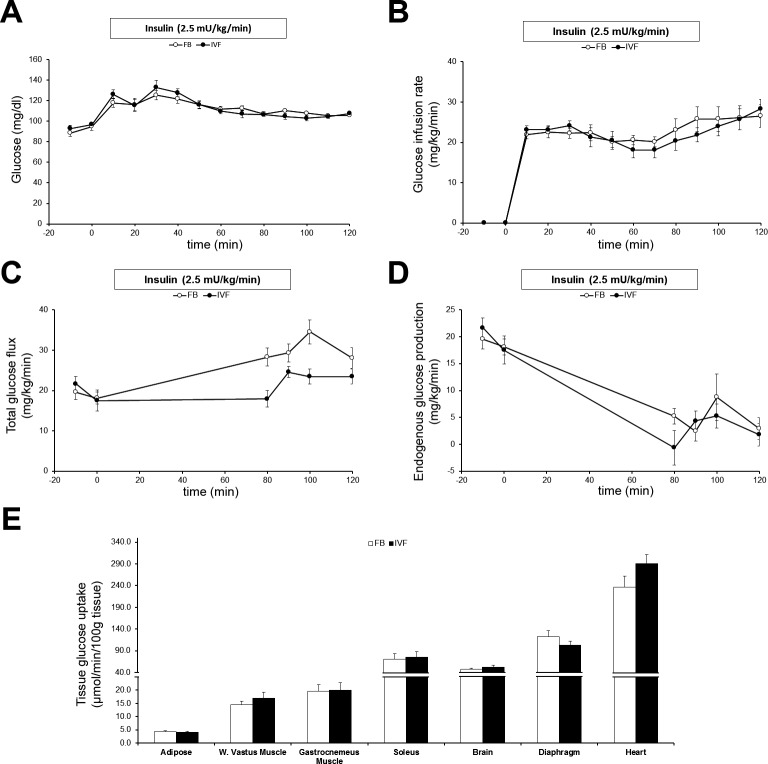
To further investigate whether the elevated levels of glucose found during the IP-GTT were due to peripheral insulin resistance, IFVWM and FB males were tested using the hyperinsulinemic-euglycemic clamp technique. Overall, both FB and IVFWM mice had unusually high baseline insulin levels but no difference in insulin resistance. Basal glucose levels, basal insulin levels, insulin levels at clamp, peptide C levels, and glucose infusion rate were similar in the two groups (Supplemental Table S3; Fig. 3, A and B). Whole-body glucose utilization (Fig. 3C) was equal at baseline and only different at clamp, when it was significantly higher in the FB group. Suppression of liver glucose production (Fig. 3D) was not different between the two groups. There was no statistically significant difference in insulin sensitivity in any peripheral tissue tested (Fig. 3E), although the higher glucose accumulation in the heart of IVFWM mice was nearly so (P = 0.09), suggesting a trend toward increased insulin sensitivity in this organ.
FIG. 3.
Hyperinsulinemic-euglycemic clamp. A) Glucose levels were maintained in both FB and IVFWM males at approximately 100 ng/dl at clamp. B) Glucose infusion rate was similar in both groups. C) Total glucose flux at baseline was not statistically different between IVFWM mice and control FB mice, but at clamp (the average of the 100- and 120-min values), it was significantly lower in IVFWM mice (Supplemental Table S3; n = 9–11 per group from three litters per treatment). D) The endogenous glucose production rate was similar in both groups at both baseline and clamp. E) Glucose uptake measured in various organs was also not different. Values are means ± SD.
Cardiovascular Phenotype
The cardiovascular anatomy and physiology of a subset of FB and IVFWM males were analyzed (Table 3). Systemic systolic blood pressure was significantly lower in the IVFWM mice, but diastolic and mean blood pressures were not different between the groups. Both the end-systolic volume (10 ± 1 FB vs. 14 ± 4 IVFWM) and the end-diastolic volumes were greater in the IVFWM mice (57 ± 10 FB vs. 70 ± 14 IVFWM). The total heart weights of the groups were not different, but both the LV mass, calculated with the formula of Troy et al. [37], and the LVPW and interseptal wall dimensions at diastole, were greater in the IVFWM hearts (Table 3).
TABLE 3.
Cardiovascular phenotype.
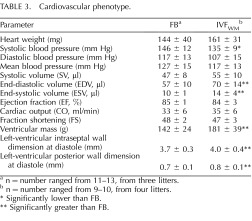
n = number ranged from 11–13, from three litters.
n = number ranged from 9–10, from four litters.
Significantly lower than FB.
* Significantly greater than FB.
Expression of Imprinted Genes
The mRNA levels of H19, Igf2, and Slc38a4 genes were measured in fat, muscle, and liver from female (n = 8) and male (FB, n = 4; IVFKAA and IVFWM, n = 5) adult mice. Only Slc38a4 showed any differences in expression, and these were seen solely in female fat tissue, with a 6.7-fold decrease in the IVFKAA and an 8.6-fold decrease in the IVFWM group compared to FB. There was no difference in expression for any other imprinted genes in any of the remaining tissues tested (Supplemental Figure S5).
DISCUSSION
This article establishes a model for studying the metabolic long-term health effects of IVF using outbred mice. We found that preimplantation disturbances may result in adult metabolic differences, indicating that the preimplantation period constitutes a window of development sensitive to reprogramming. The differences that we did find were culture-medium specific. Reassuringly, mice generated using optimal culture conditions were normal in most aspects of development and adult physiology measured. However, suboptimal culture conditions resulted in a clear adult phenotype. Adult male mice generated in vitro using WM and 20% O2 manifested perturbations in growth, glucose tolerance, and cardiac size.
Among the most striking effects following culture in suboptimal conditions was an alteration of the growth curve of male IVFWM mice. These mice grew faster between birth and 1 wk of age, then gained weight at a rate parallel to that of controls so that their growth curve stayed shifted to the left until 20 wk of age. Unfortunately, the IPGTT interfered with weight gain in all the mice, right at the time that growth rates were slowing so that it is difficult to determine exactly when the mice would have normally leveled off (Fig. 1). This difference in growth pattern can be best understood when prenatal growth is also taken into consideration. We have previously shown that IVFWM blastocysts are made up of significantly fewer cells [20] and are smaller at Embryonic Day 12.5 [38]. However, their growth velocity increases in the latter part of gestation so that their weight at birth is the same as and then greater than that of the control mice. This pattern of accelerated fetal/neonatal growth may be an indicator of prenatal stress and may be linked to metabolic changes.
Other animal studies have shown that culture media affect in utero growth [39] and birth weight [40]. Jimenez-Chillaron et al. [41] have shown that mice subjected to intrauterine growth retardation will manifest a postnatal “catch-up” growth and will develop glucose intolerance later in life. Evidence in humans is yet inconclusive, with some authors finding an effect of culture medium on birth weight [42] and others not [43].
A second important finding is that IVFWM male mice were clearly glucose intolerant, as shown by the IPGTT test (Fig. 2). The insulinogenic index [44] for IVF males, under both culture conditions, was significantly lower than FB, while the HOMA-IR calculation for peripheral insulin resistance [26] was not different, pointing to a β-cell problem rather than a defect in peripheral tissues. This hypothesis was not disproven by the hyperinsulinemic-euglycemic clamp experiments. If anything, the IVFWM mice seemed to be slightly more sensitive to insulin. Unfortunately, the clamp studies were complicated by the unusually high insulin levels found at this age in the particular outbred strain used. In fact, at 38 wk of life, when the insulin clamp was performed, the basal insulin levels were very high, approximately three times the normal levels seen at 19 wk of age. The very high peptide C levels confirmed an increase in insulin production rather than a decrease in insulin clearance. The HOMA-IR at 40 wk was significantly elevated in both groups compared to what was observed at 19 wk of age (Fig. 2F), but there was no difference between IVFWM and FB. Given these findings, it is possible that elevated serum insulin levels may have interfered with our ability to accurately detect differences in the degree of insulin resistance between the FB and IVFWM males, as the experimental infusion of insulin may not have appeared to be sufficiently hyperinsulinemic to these mice. Given these findings, the use of the outbred cross CF1 × B6D2F1 mice is not suggested for future long-term metabolic studies.
An alternative cause for glucose intolerance could be a pancreatic defect with altered insulin production. In support of this point, we found that the insulinogenic index was reduced in both types of IVF mice at 19 wk of age compared to control. A decrease in the insulinogenic index suggests a reduced ability to secrete insulin in response to glucose, likely because of beta cell insensitivity to glucose [45]. Further experiments are needed to investigate this hypothesis.
The third important finding of this study is related to the hemodynamic testing. IVFWM male mice had lower systolic blood pressure than control mice. Although these results are reassuring, they are contrary to the several results in the literature [36]. For example, Watkins et al. [36] cultured two-cell embryos to the blastocyst stage and found that embryo culture and, to a lesser extent, embryo transfer led to an enhanced systolic blood pressure at 21 wk in both male and females. Rexhaj et al. [46] performed IVF in FVB mice and found that male offspring had increased mean blood pressure at 14 wk of age. The most likely reasons for these differences in results are variation in the strain of mice used, the length of culture, and type of culture medium used.
In apparent contrast to the lowered blood pressure, echocardiographic analysis showed hypertrophy of the left heart. In fact, although cardiac weight was not statistically heavier, the LV mass calculated with the Troy formula was higher in IVF mice [37]. Further, the end-systolic and end-diastolic volumes were also increased. This enlargement of the left heart, both in the myocardium and of blood volume, in the absence of increased exercise and high blood pressure suggests that this phenomenon was secondary to stress in utero [47–49]. A link between embryonic or fetal stress and abnormal cardiac development has been described before. Impairment of placental function has been shown to predispose offspring to cardiovascular disease in both humans and animals [50]. Indeed, we have found that the placenta of IVF mice is larger and less efficient [22]. In future studies, we plan to test whether IVFKAA or female mice display a cardiac phenotype.
Importantly, female IVFWM mice did not show evidence of any specific alteration of growth or metabolic parameters suggesting a sexually dimorphic phenotype. Sexual dimorphic effects of in utero stress have been described before [51, 52]. Remarkably, male and female blastocysts show already remarkable differences in gene expression [53]; it is therefore not surprising that disturbance of physiologic signals because of culture in vitro during the preimplantation period will generate different health effects in the two sexes.
Reassuringly, both male and female IVFKAA mice had a growth and metabolic phenotype that was similar to control FB mice. This finding is important since modification of KSOM medium with amino acids is the basis for a commercially available medium, used commonly in human IVF [54]. Among the few differences observed, male IVFKAA mice had a greater anogenital distance at birth than control mice, but that difference was not significant when anogenital index was calculated (Supplemental Table S1). Further, the insulinogenic index, the amount of insulin secreted for a given glucose stimulus (Fig. 2E), was significantly lower in IVFKAA males than in control FB males.
Our study also shows that each culture condition has a specific effect on growth and development. Overall, WM and 20% O2 appear to have a more adverse effect on growth and metabolic health, at least in male mice; by contrast, KSOM medium with amino acids and 5% O2 was associated with no or only minor metabolic adult health alteration compared to control. This is not surprising since we have found that blastocysts cultured in WM and 20% O2 have more than 1000 genes different from in vivo control blastocysts, while only 29 genes were different between KSOM+AA and 5% O2 and control blastocysts [19, 55]. It remains to be established whether a “dose-response” effect is present following preimplantation stress (WM being more stressful and KSOM+AA being more physiologic) or whether each culture condition might have its own particular effect that must be evaluated independently. The differences in anogenital distance found in IVFKAA mice and not IVFWM would point to the second explanation. Further, there might be health effects that follow culture in vitro that are independent of the conditions used. For example, Ecker et al. [17] found that similar behavioral changes were present in two-cell embryos cultured with either WM or KSOM+AA.
The length of gestation, when calculated according to the dam's reproductive cycle, was normal even with IVF embryos. However, the IVF blastocysts took 2 days longer to develop from fertilization to birth than did normally conceived (in vivo and FB) mice. All this extra time was spent in vitro, growing from zygote to blastocyst. It is intriguing to speculate that this extra period of development in vitro resulted in molecular/ mechanistic changes responsible for the observed adult phenotype. Future studies should be designed to define which particular constituent(s) in the culture medium or what oxygen concentration is responsible for causing the observed phenotypes.
One possible mechanism by which changes in the preimplantation environment could affect adult growth and physiology is through alteration of the epigenome. Imprinted genes are epigenetically regulated, and there is evidence that preimplantation culture alters expression of imprinted genes [30]. To ascertain if preimplantation culture affects adult imprinted gene expression, we examined the expression of three candidate imprinted genes (H19, Igf2, and Slc38a4) in multiple tissues of adult animals. Overall, we found no difference in expression among the groups, suggesting that there are no widespread alterations in imprinted genes in these animals. Interestingly, Slc38a4 was down-regulated following IVF (in both culture conditions) only in female adipose tissue. The reason for this tissue-specific and sexual dimorphic result is unclear, and it is not associated with an observed postnatal phenotype. Of note, however, we have found that IVF placenta display reduced transport of neutral amino acids and down-regulation of Slc38a4 at Embryonic Day 18.5 [22].
One significant, general finding of our article is the establishment of the proper control group for studying the long-term effects of IVF. In particular, the use of the in vivo mice for comparison is inappropriate, at least to assess a metabolic phenotype. In fact, the larger litter size found in the in vivo group can, by itself, result in a different growth pattern in offspring. Therefore, the FB group is a more suitable control, as it accounts for superovulation, litter size, and the embryo transfer procedure. Indeed, the embryo transfer procedure alone has been shown to alter expression of imprinted genes [56].
One potential limitation of this study is sample size. We have based sample size calculation on having at least 25 animals per group (range 25–54) originating from at least four litters (range four to seven) to follow over time. This number is consistent with other published reports; for example, Fernandez-Gonzales [35] analyzed 35 and 43 offspring and Ecker [17] 42–50 offspring with no specification of how many litters the animal originated. The lowest sample size used was nine male animals from three litters for the insulin clamp experiments.
In summary, we have found that IVF and subsequent culture of preimplantation embryos is associated with metabolic reprogramming, depending on the culture conditions used. Therefore, we can conclude that the preimplantation period represents a window of reprogramming and that stressful conditions during this delicate period of growth can lead to adult onset of metabolic diseases, as postulated by the DOHaD hypothesis [3]. Our data are particularly valuable because culture in vitro is routinely used to treat patients with infertility, and as of today more than 5 million children have been conceived with these technology [4]. Future studies should be directed at confirming these observations in different strains of mice and at understanding the molecular mechanism underlying the described findings.
Supplementary Material
Footnotes
Supported by a National Institute of Child Health and Human Development (NICHD) grant (RO1:HD 062803-01A1) and American Diabetes Association grant to P.F.R.
REFERENCES
- Painter RC, de Rooij SR, Bossuyt PM, Simmers TA, Osmond C, Barker DJ, Bleker OP, Roseboom TJ. Early onset of coronary artery disease after prenatal exposure to the Dutch famine. Am J Clin Nutr 2006; 84: 322–327. quiz 466–327. [DOI] [PubMed] [Google Scholar]
- de Rooij SR, Painter RC, Roseboom TJ, Phillips DI, Osmond C, Barker DJ, Tanck MW, Michels RP, Bossuyt PM, Bleker OP. Glucose tolerance at age 58 and the decline of glucose tolerance in comparison with age 50 in people prenatally exposed to the Dutch famine. Diabetologia 2006; 49: 637–643. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Mothers, Babies and Health in Later Life. Glasgow: Churchill Livingstone; 1998. [Google Scholar]
- Adamson DG, Zegers-Hochschild F, Ishihara O, Sullivan E, Mansour R, Nygren KG, Banker M, Dyer S, de Mouzon J. ICMART world report: preliminary 2008 data. Hum Reprod 2012; 27(suppl 2): ii38–ii39.
- Feuer SK., Rinaudo P. Preimplantation stress and development. Birth Defects Res (pt C) 2012; 96: 299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature 2007; 447: 425–432. [DOI] [PubMed] [Google Scholar]
- Cockburn K, Rossant J. Making the blastocyst: lessons from the mouse. J Clin Invest 2010; 120: 995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Bower C, Milne E, de Klerk N, Kurinczuk JJ. Assisted reproductive technologies and the risk of birth defects—a systematic review. Hum Reprod 2005; 20: 328–338. [DOI] [PubMed] [Google Scholar]
- Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol 2004; 103: 551–563. [DOI] [PubMed] [Google Scholar]
- Batcheller A, Cardozo E, Maguire M, DeCherney AH, Segars JH. Are there subtle genome-wide epigenetic alterations in normal offspring conceived by assisted reproductive technologies? Fertil Steril 2011; 96: 1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mau Kai C, Main KM, Andersen AN, Loft A, Skakkebaek NE, Juul A. Reduced serum testosterone levels in infant boys conceived by intracytoplasmic sperm injection. J Clin Endocrinol Metab 2007; 92: 2598–2603. [DOI] [PubMed] [Google Scholar]
- Mau Kai C, Main KM, Nyboe Andersen A, Loft A, Chellakooty M, Skakkebaek NE, Juul A. Serum insulin-like growth factor-I (IGF-I) and growth in children born after assisted reproduction. J Clin Endocrinol Metab 2006; 91 (11): 4352–4360. [DOI] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Vermeiden JP, van Leeuwen FE, Delemarre-van de Waal HA. Pubertal development in children and adolescents born after IVF and spontaneous conception. Hum Reprod 2008; 23: 2791–2798. [DOI] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Vermeiden JP, van Leeuwen FE, Delemarre-van de Waal HA. Cardiometabolic differences in children born after in vitro fertilization: follow-up study. J Clin Endocrinol Metab 2008; 93: 1682–1688. [DOI] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Vermeiden JP, van Leeuwen FE, Delemarre-van de Waal HA. Growth and development of children born after in vitro fertilization. Fertil Steril 2008; 90: 1662–1673. [DOI] [PubMed] [Google Scholar]
- Scott KA, Yamazaki Y, Yamamoto M, Lin Y, Melhorn SJ, Krause EG, Woods SC, Yanagimachi R, Sakai RR, Tamashiro KL. Glucose parameters are altered in mouse offspring produced by assisted reproductive technologies and somatic cell nuclear transfer. Biol Reprod 2010; 83: 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker DJ, Stein P, Xu Z, Williams CJ, Kopf GS, Bilker WB, Abel T, Schultz RM. Long-term effects of culture of preimplantation mouse embryos on behavior. Proc Natl Acad Sci U S A 2004; 101: 1595–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R. Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behavior. Proc Natl Acad Sci 2004; 101: 5880–5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaudo PF, Giritharan G, Talbi S, Dobson AT, Schultz RM. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil Steril 2006; 86: 1252–1265. [DOI] [PubMed] [Google Scholar]
- Giritharan G, Talbi S, Donjacour A, Di Sebastiano F, Dobson AT, Rinaudo PF. Effect of in vitro fertilization on gene expression and development of mouse preimplantation embryos. Reproduction 2007; 134: 63–72. [DOI] [PubMed] [Google Scholar]
- Perin PM, Maluf M. Nicolosi Foltran Januário DA, Nascimento Saldiva PH. Comparison of the efficacy of two commercially available media for culturing one-cell embryos in the in vitro fertilization mouse model. Fertil Steril 2008; 90: 1503–1510. [DOI] [PubMed] [Google Scholar]
- Bloise E, Lin W, Liu X, Simbulan R, Kolahi KS, Petraglia F, Maltepe E, Donjacour A, Rinaudo P. Impaired placental nutrient transport in mice generated by in vitro fertilization. Endocrinology 2012; 153: 3457–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy AGM, Vintersten K, Behringer R. Manipulating the Mouse Embryo: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press; 2003: 161–208. [Google Scholar]
- Zambrano E, Rodriguez-Gonzalez GL, Guzman C, Garcia-Becerra R, Boeck L, Diaz L, Menjivar M, Larrea F, Nathanielsz PW. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J Physiol 2005; 563: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy TR, Clair AL. Precision and accuracy of dual-energy X-ray absorptiometry for determining in vivo body composition of mice. Obes Res 2000; 8: 392–398. [DOI] [PubMed] [Google Scholar]
- Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 2008; 294: E15–E26. [DOI] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 2006; 55: 390–397. [DOI] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, Hansotia T, Flock G, Seino Y, Wasserman DH, Drucker DJ. Insulin action in the double incretin receptor knockout mouse. Diabetes 2008; 57: 288–297. [DOI] [PubMed] [Google Scholar]
- Rottman JN, Ni G, Brown M. Echocardiographic evaluation of ventricular function in mice. Echocardiography 2007; 24: 83–89. [DOI] [PubMed] [Google Scholar]
- Doherty AS, Mann MR, Tremblay KD, Bartolomei MS, Schultz RM. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod 2000; 62: 1526–1535. [DOI] [PubMed] [Google Scholar]
- Angiolini E, Coan PM, Sandovici I, Iwajomo OH, Peck G, Burton GJ, Sibley CP, Reik W, Fowden AL, Constancia M. Developmental adaptations to increased fetal nutrient demand in mouse genetic models of Igf2-mediated overgrowth. Faseb J 2011; 25: 1737–1745. [DOI] [PubMed] [Google Scholar]
- Montano MM, Wang MH, Even MD, vom Saal FS. Serum corticosterone in fetal mice: sex differences, circadian changes, and effect of maternal stress. Physiol Behav 1991; 50: 323–329. [DOI] [PubMed] [Google Scholar]
- Davidson JM, Jones LE, Levine S. Feedback regulation of adrenocorticotropin secretion in “basal” and “stress” conditions: acute and chronic effects of intrahypothalamic corticoid implantation. Endocrinology 1968; 82: 655–663. [DOI] [PubMed] [Google Scholar]
- Wilkinson CW, Raff H. Comparative evaluation of a new immunoradiometric assay for corticotropin. Clin Chem Lab Med 2006; 44: 669–671. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Moreira P, Bilbao A, Jimenez A, Perez-Crespo M, Ramirez MA, Rodriguez De Fonseca F, Pintado B, Gutierrez-Adan A. Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behavior. Proc Natl Acad Sci U S A 2004; 101: 5880–5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins AJ, Platt D, Papenbrock T, Wilkins A, Eckert JJ, Kwong WY, Osmond C, Hanson M, Fleming TP. Mouse embryo culture induces changes in postnatal phenotype including raised systolic blood pressure. Proc Natl Acad Sci U S A 2007; 104: 5449–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy BL, Pombo J, Rackley CE. Measurement of left ventricular wall thickness and mass by echocardiography. Circulation 1972; 45: 602–611. [DOI] [PubMed] [Google Scholar]
- Delle Piane L, Lin W, Liu X, Donjacour A, Minasi P, Revelli A, Maltepe E, Rinaudo PF. Effect of the method of conception and embryo transfer procedure on mid-gestation placenta and fetal development in an IVF mouse model. Hum Reprod 2010; 25: 2039–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer C, Esteves TC, Arauzo-Bravo MJ, Le Gac S, Nordhoff V, Schlatt S, Boiani M. ART culture conditions change the probability of mouse embryo gestation through defined cellular and molecular responses. Hum Reprod 2012; 27 (9): 2627–2640. [DOI] [PubMed] [Google Scholar]
- Young LE, Sinclair KD, Wilmut I. Large offspring syndrome in cattle and sheep. Rev Reprod 1998; 3: 155–163. [DOI] [PubMed] [Google Scholar]
- Jimenez-Chillaron JC, Hernandez-Valencia M, Lightner A, Faucette RR, Reamer C, Przybyla R, Ruest S, Barry K, Otis JP, Patti ME. Reductions in caloric intake and early postnatal growth prevent glucose intolerance and obesity associated with low birthweight. Diabetologia 2006; 49: 1974–1984. [DOI] [PubMed] [Google Scholar]
- Nelissen EC, Van Montfoort AP, Coonen E, Derhaag JG, Geraedts JP, Smits LJ, Land JA, Evers JL, Dumoulin JC. Further evidence that culture media affect perinatal outcome: findings after transfer of fresh and cryopreserved embryos. Hum Reprod 2012; 27: 1966–1976. [DOI] [PubMed] [Google Scholar]
- Vergouw CG, Kostelijk EH, Doejaaren E, Hompes PG, Lambalk CB, Schats R. The influence of the type of embryo culture medium on neonatal birthweight after single embryo transfer in IVF. Hum Reprod 2012; 27: 2619–2626. [DOI] [PubMed] [Google Scholar]
- Sone H, Kagawa Y. Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia 2005; 48: 58–67. [DOI] [PubMed] [Google Scholar]
- Tura A, Kautzky-Willer A, Pacini G. Insulinogenic indices from insulin and C-peptide: comparison of beta-cell function from OGTT and IVGTT. Diabetes Res Clin Pract 2006; 72: 298–301. [DOI] [PubMed] [Google Scholar]
- Rexhaj E, Paoloni-Giacobino A, Rimoldi SF, Fuster DG, Anderegg M, Somm E, Bouillet E, Allemann Y, Sartori C, Scherrer U. Mice generated by in vitro fertilization exhibit vascular dysfunction and shortened life span. J Clin Invest 2013; 123: 5052–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks KL, McMullen JR. The athlete's heart vs. the failing heart: can signaling explain the two distinct outcomes? Physiology (Bethesda) 2011; 26: 97–105. [DOI] [PubMed] [Google Scholar]
- Hein S, Arnon E, Kostin S, Schonburg M, Elsasser A, Polyakova V, Bauer EP, Klovekorn WP, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation 2003; 107: 984–991. [DOI] [PubMed] [Google Scholar]
- Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 1975; 56: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornburg KL, O'Tierney PF, Louey S. Review: The placenta is a programming agent for cardiovascular disease. Placenta 2010; 31 (suppl): S54–S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaudo P, Wang E. Fetal programming and metabolic syndrome. Annu Rev Physiol 2012; 74: 107–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. Am J Hum Biol 2010; 22: 330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Rizos D, Lonergan P, Gutierrez-Adan A. Transcriptional sexual dimorphism during preimplantation embryo development and its consequences for developmental competence and adult health and disease. Reproduction 2011; 141: 563–570. [DOI] [PubMed] [Google Scholar]
- Wiemer KE, Anderson AR, Kyslinger ML, Weikert ML. Embryonic development and pregnancies following sequential culture in human tubal fluid and a modified simplex optimized medium containing amino acids. Reprod Biomed Online 2002; 5: 323–327. [DOI] [PubMed] [Google Scholar]
- Rinaudo P, Schultz RM. Effects of embryo culture on global pattern of gene expression in preimplantation mouse embryos. Reproduction 2004; 128: 301–311. [DOI] [PubMed] [Google Scholar]
- Rivera RM, Stein P, Weaver JR, Mager J, Schultz RM, Bartolomei MS. Manipulations of mouse embryos prior to implantation result in aberrant expression of imprinted genes on day 9.5 of development. Hum Mol Genet 2008; 17: 1–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.