ABSTRACT
Zinc is an essential nutrient for optimal fertility, but the effects of preconception zinc deficiency on postimplantation development are not known. Female mice were fed a control or a zinc-deficient diet (ZDD) for 4–5 days before ovulation (preconception). Embryonic and/or placental development were evaluated on Days 3.5, 6.5, 10.5, 12.5, and 16.5 of pregnancy. The findings show a decrease in embryo length (31%, Day 10.5; 13%, Day 12.5; 10%, Day 16.5) and weight (23%, Day 16.5) in embryos from mothers fed a ZDD preconception. Zinc deficiency also caused a high incidence of pregnancy loss (46%, Day 10.5; 34%, Day 12.5; 51%, Day 16.5) compared to control (2%, Day 10.5; 7%, Day 12.5; 9%, Day 16.5). ZDD embryos transferred to normal recipients were 38% smaller and implantation rate was only 10% compared to 40% for controls. Trophoblast cell differentiation and implantation on Day 6.5 of pregnancy were compromised by preconception zinc deficiency. On Day 12.5 of pregnancy, placenta weight and area of fetal placenta were decreased 37% and 31%, respectively, by preconception zinc deficiency. Consistent with a smaller fetal placenta, expression of key placental transcripts, including Ar, Esx1, Syna, Tfeb, Dlx3, and Gcm1 mRNA, but not Ctsq mRNA, were decreased 30%–70% in the ZDD group. Preconception zinc deficiency caused 41%–57% of embryos to exhibit delayed or aberrant neural tube development, as examined by light microscopy and magnetic resonance imaging. Collectively, the findings provide evidence for the importance of preconception zinc in promoting optimal fertility and oocyte developmental potential.
Keywords: fetal development, oocyte, zinc
Preconception zinc deficiency causes significant defects in fetal and placental development.
INTRODUCTION
Oocyte quality plays a key role in determining the success of fertilization, pregnancy, and postnatal health. The ovarian follicular environment, in which oocytes grow and mature, is the prime determinant of oocyte quality, but the conditions that promote an optimal follicular environment are not completely defined. It has been known for some time that a suboptimal supply of nutrients during pregnancy can impair embryo and fetal development and postnatal health by altering epigenetic modifications of the chromatin [1–5]. The mineral zinc acts as a catalytic, structural, and signaling factor in the regulation of a diverse array of cellular pathways involving hundreds of enzymes and proteins [6, 7]. Given these wide-ranging roles, it is not surprising that insufficient zinc during pregnancy causes developmental defects in many species [8–13]. However, the role of zinc during the preconception period in promoting later development during pregnancy is not clearly understood. Previously, we reported that a diet very low in zinc (<1 mg/kg) fed for 3–10 days before ovulation causes a range of fertility defects. For example, a 3-day treatment with a zinc-deficient diet (ZDD) decreased the ability of the oocyte to complete the first meiotic division [14]. This is in agreement with a seminal study in this area showing that zinc increases in the oocyte during in vitro maturation, and that restricting zinc with a chelator causes meiotic failure in vitro [15]. In addition to regulating meiosis, preconception zinc is also important for promoting preimplantation embryo development. A 5-day preconception treatment with a ZDD causes not only decreased meiotic maturation, but also decreased fertilization and preimplantation development to the blastocyst stage [16]. These meiotic, fertilization, and preimplantation development defects were associated with reduced chromatin methylation and increased expression of repetitive elements in zinc-insufficient oocytes [16]. Indeed, supplementing zinc-depleted oocytes with a methyl donor (s-adenosylmethionine) during in vitro maturation restored chromatin methylation and partially rescues fertilization, confirming that zinc depletion affects epigenetic programming of the oocyte. Given that preconception zinc status is an important determinant of maturation, fertilization, and preimplantation development [14, 16], we now evaluate the impact of preconception zinc deficiency on postimplantation development. The findings show that preconception zinc deficiency has long-lasting detrimental effects on both embryo and placenta development, with important implications for postnatal health.
MATERIALS AND METHODS
Animal Model of Zinc Deficiency
Female CD1 mice (Mus musculus) were obtained from the research colony of the investigators. Newly weaned 18-day-old mice were housed on wire-bottom racks in polycarbonate cages and fed a control diet (29 mg zinc/kg) based on AIN76 (MP Biomedicals, Solon, OH) or a ZDD, based on the control diet, but containing <1 mg zinc/kg. This is the same diet as the control diet, but with zinc omitted from the mineral mix. Diets were fed for 4 or 5 days before ovulation. At 2 days before ovulation, animals were primed with 5 IU eCG (National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases). Ovulation was induced with 5 IU hCG given 48 h after eCG. At the time of hCG, some animals were bred to a fertile male. Only animals with a mating plug on the morning after mating were used for subsequent analyses. Importantly, animals receiving a ZDD were switched back to a control diet at the time of hCG and for the duration of the experiment. Animals were maintained according to the Guide for the Care and Use of Laboratory Animals (Institute for Learning and Animal Research). All animal use was reviewed and approved by the Institutional Animal Care and Use Committee at Pennsylvania State University.
Postimplantation Development
To study effects of acute in vivo zinc deficiency before ovulation on postimplantation embryonic development, female mice were fed a control or ZDD for 4 or 5 days. This treatment period was chosen because longer treatments with a ZDD cause a decrease in ovulation [14]. In the first experiment, animals received dietary treatments for 5 days before ovulation, as we have done previously [16]. Embryos were collected shortly after implantation is complete, on Day 10.5 of pregnancy. Implantation sites were counted and embryos with a heartbeat were considered viable. Embryonic loss was calculated as the proportion of embryos without a heartbeat divided by the total number of implantation sites. In subsequent experiments, later stages of embryo development were examined on Days 12.5 and 16.5 of pregnancy. However, for these experiments, dietary treatments were applied for 4 days because of the high pregnancy loss observed on Day 10.5. Fetal length and fetal loss were determined for each group, and fetal weight was determined on Day 16.5. Fetal length was determined by measuring the greatest distance from the crown of the head to the posterior end of each embryo (crown-to-rump length).
Blastocyst Outgrowth Assay
To determine if trophoblast cell differentiation and proliferation were compromised, blastocyst embryos from control and ZDD groups were flushed from the uterus on Day 3.5 after mating. Individual embryos were placed in single wells of a 96-well dish coated with collagen (1 mg/ml) and were cultured in Dulbecco modified Eagle medium with 5% serum for up to 72 h. At 48 and 72 h, each embryo was photographed. Embryos with trophoblast cells attached to the culture dish with large cytoplasmic vacuolar structures of a trophoblast giant cell were scored as differentiated. Area of the outgrowth was determined at 72 h using a DP-20 color camera and software (Olympus, New Castle, PA).
Implantation and Placental Development
To determine if implantation and placental development were compromised by preconception zinc deficiency, female mice were fed control or ZDD for 4 days before ovulation, induced to ovulate, and bred to a fertile male, as described above. On Day 6.5 of pregnancy, implantation sites were counted and individual sites were fixed in 4% paraformaldehyde. Tissue sections (6 μm) were stained with hematoxylin and eosin using standard methods. The largest cross-sectional area from 10–14 implantation sites was measured in each group. On Day 12.5 of pregnancy, the placenta was carefully removed from the uterus, weighed, and either frozen for mRNA analysis or fixed in 4% paraformaldehyde for 12 h and embedded in paraffin. Tissue sections (6 μm) were stained with hematoxylin and eosin using standard methods. The areas of the fetal (labyrinth and spongiotrophoblast) and maternal decidua were determined in three to six midline sections of randomly selected placenta from three control and four ZDD mice.
Embryo Transfer
To determine if development could be improved by transferring embryos to a normal recipient, female mice were fed a control diet or ZDDs for 5 days, superovulated, and bred to a fertile male, as described above. Blastocyst embryos were flushed from the reproductive tract on Day 3.5 of pregnancy. The 8-wk-old recipient female mice were superovulated with eCG/hCG and bred to vasectomized males to induce pseudopregnancy. Only recipients with a mating plug were used for embryo transfer. Morphologically normal Day-3.5 blastocysts (11–12 blastocysts/transfer) from control and ZDD animals were transferred to recipient mice ∼72 h after mating using a Non-Surgical Embryo Transfer (NSET; ParaTechs, Lexington, KY) device, as described previously [17]. The NSET device provides a convenient and rapid method of embryo transfer, and is a modified flexible tip that fits onto standard pipette dispensers (20 μl). The procedure itself is very simple. The blastocyst embryos are loaded into the NSET tip in 2 μl of media. A speculum is placed in the vaginal opening, the tip is passed through the cervix, and the embryos are expelled. The procedure is fast and there is no need for anesthesia or analgesia. It is important to transfer embryos no later than 72 h after induction of pseudopregnancy to prevent closure of the cervix. On Day 10.5 of pregnancy, implantation rate, pregnancy loss, and morphological development of embryos were evaluated.
Magnetic Resonance Imaging
Fixed embryos (two control and two ZDD) collected on Day 16.5 of pregnancy were used for magnetic resonance imaging (MRI). The embryos were immersed into a PBS solution containing 1.5% of Magnevist (Bayer Healthcare, Germany), an MRI contrast agent, for 5 days to remove any free paraformaldehyde from the specimens and to reduce the MRI time. All MRI sessions were conducted on a 14.1 tesla Agilent microimaging system equipped with 1 T/m gradients using a home-built 18-mm ID loop gap resonator. A standard spin echo sequence with a repetition time of 70 ms, an echo time of 12.4 ms, and an average of 4 repetitions per session yielded a total scan time of 3 h and 13 min. The achieved resolution was 40 μm isotropic (field of view, 18 × 11 × 8 mm3; matrix, 450 × 207 [75% partial Fourier] × 200). Due to zerofilling the pixel resolution was 20 μm isotropic. Data reconstruction was conducted using Matlab (The MathWorks, Natick, MA). Image segmentation was performed using Avizo (Vsg3D, Burlington, MA).
Isolation of Total RNA and Quantitative PCR
Total RNA was isolated from placentas (9–13/group) randomly selected from six to nine different litters using the RNAeasy mini kits (Qiagen, Valencia, CA). Total RNA was reverse transcribed into cDNA, as described previously [18], using the Quantitek cDNA synthesis kit (Qiagen). Quantification of Ar, Esx1, Gcm1, Syna, Tfeb, Ctsq, and Dlx3 mRNAs was conducted using gene-specific primers (Table 1) and Rpl19 mRNA as the normalizer, as described previously [14, 19]. Only one product of the appropriate size was identified for each set of primers, and all amplification products were sequenced to confirm specificity.
TABLE 1.
Primer sequences used for quantitative PCR.

Statistical Analysis
Results for fetal length, fetal weight, quantitative PCR, placental weight, fetal placenta area (%), implantation area, outgrowth area, fetal/maternal ratio, fetal loss, and implantation rate were analyzed by Student t-test. Proportional data were transformed (arcsine) before analysis. The JMP 7.1 statistical analysis software (SAS, Cary, NC) was used for all analyses.
RESULTS
Effect of Preconception Zinc Deficiency on Embryonic Development on Day 10.5 of Pregnancy
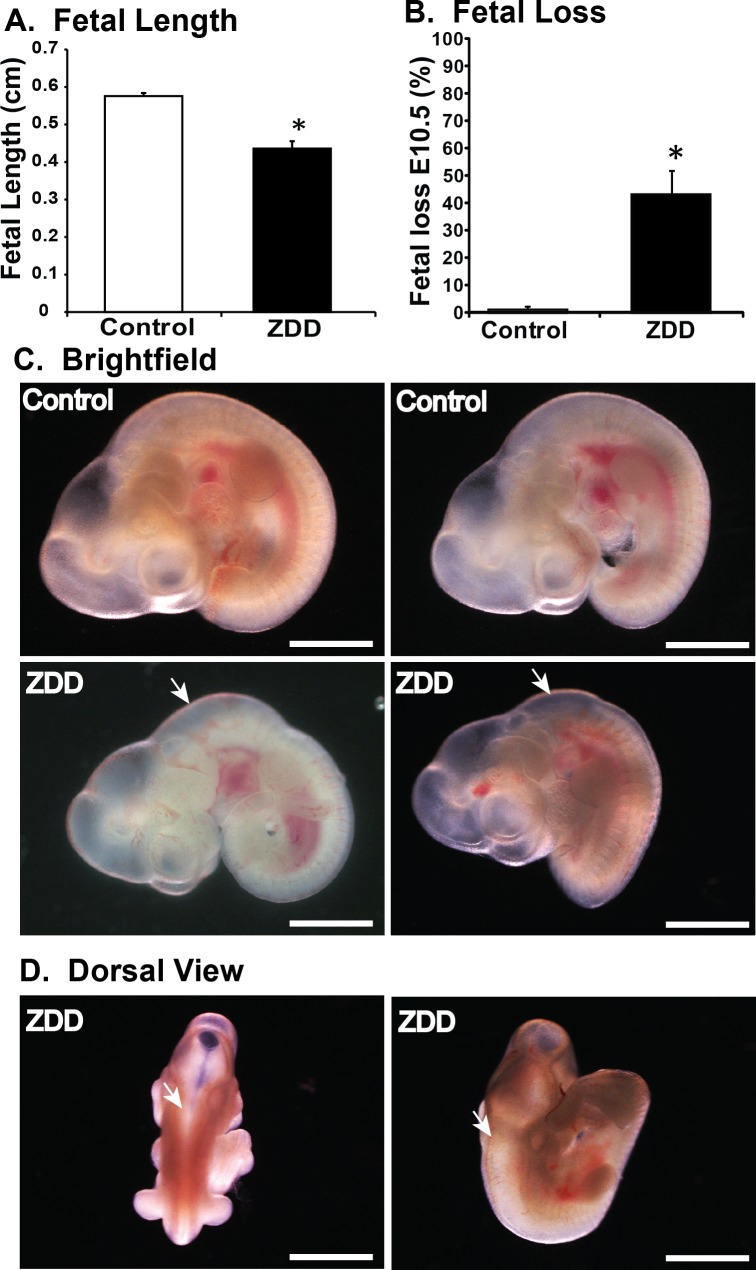
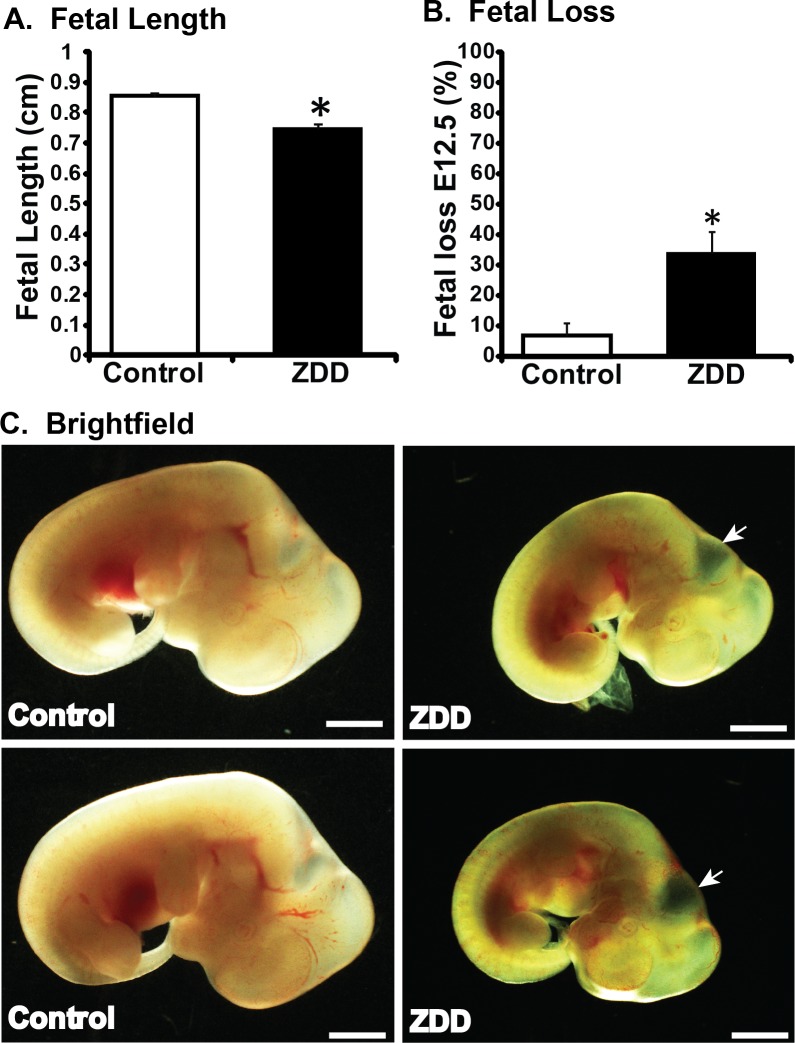
Zinc is an important factor required for fertilization and preimplantation development [16]. To further elucidate the function of zinc during postimplantation development, embryos were collected on Day 10.5 of pregnancy from animals fed a control diet or ZDD for 5 days before ovulation (preconception). Embryo length (crown-to-rump length) was reduced by 31% in ZDD embryos compared to control (Fig. 1A). Moreover, there was a 46% increase in the proportion of implantation sites containing either no embryo or an embryo without a heartbeat in the ZDD group compared to only 2% of sites in the control group (Fig. 1B). In addition to overall smaller size, 41% of ZDD embryos showed delayed closure of the neural tube compared to the control group (Fig. 1C, white arrows).
FIG. 1.
Effect of zinc deficiency on fetal development on Day 10.5 of pregnancy. Fetal length (A) and fetal loss (B) on Day 10.5 of pregnancy in animals that were fed a control diet or ZDD for 5 days before ovulation. C) Representative brightfield images of fetal development from control and ZDD (5 days) groups. D) Dorsal view of ZDD showing open neural tube. White arrows indicate the presence of open neural tube. *Significant difference by Student t-test, P < 0.05, n = 4–5 litters. Values are mean ± SEM. Bar = 100 μm.
Effect of Embryo Transfer on Embryonic Development of Zinc-Deficient Embryos on Day 10.5 of Pregnancy
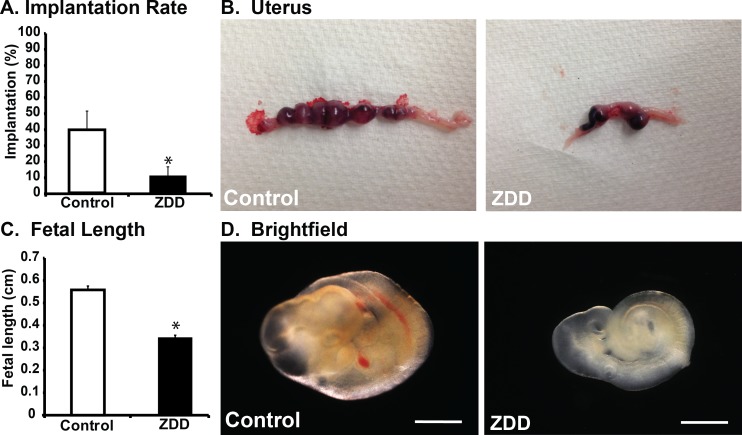
To determine if developmental defects and implantation failure in ZDD animals were the result of defects in the embryo itself or an inability of the uterus to support implantation, blastocyst embryos from animals fed a control diet and ZDD for 5 days preconception were transferred to pseudopregnant recipient females. Blastocysts of similar size were flushed from the donor uteri and transferred (10 or 11 embryos/transfer) within 1 h to pseudopregnant females. Implantation rate and embryo development were assessed on Day 10.5 of pregnancy. The implantation rate was very low in the ZDD group, with only 10% of embryos successfully implanting compared to 40% of control embryos (Fig. 2, A and B). However, even if implantation did occur, embryos in the ZDD group were 38% smaller than control embryos (Fig. 2, C and D).
FIG. 2.
Embryo transfer. A) Proportion of transferred embryos that implanted and survived to Day 10.5 of pregnancy in animals that were fed a control diet or ZDD for 5 days before ovulation. B) Representative images from control and ZDD uteri. Fetal length (C) and representative images (D) of embryos from control and ZDD groups on Day 10.5 of pregnancy. *Significant difference by Student t-test, P < 0.05, n = 4–5 litters. Values are mean ± SEM. Bar = 100 μm.
Effect of Preconception Zinc Deficiency on Trophoblast Differentiation
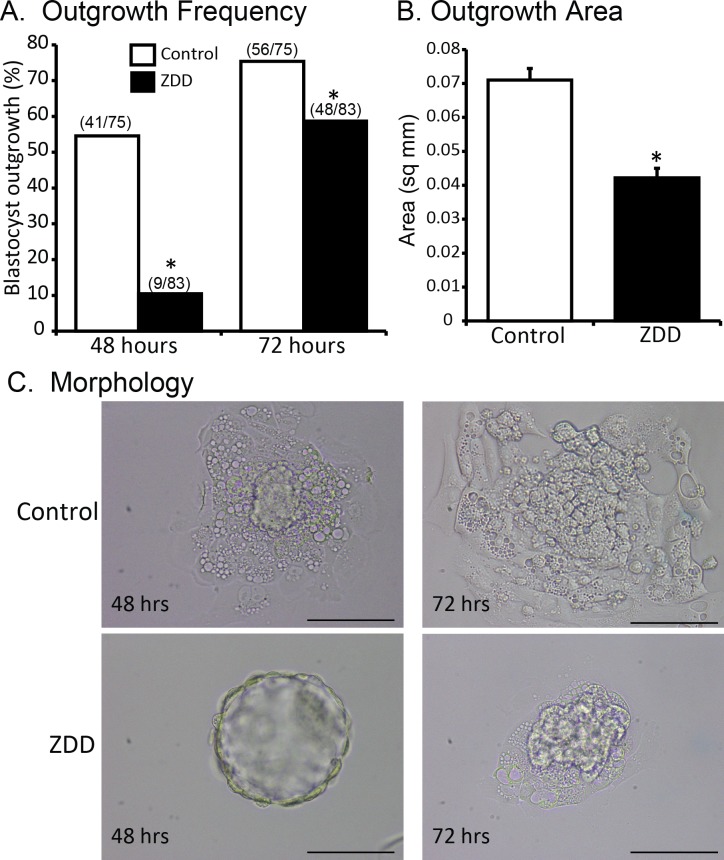
Previous work showed that preconception zinc deficiency compromised development to the blastocyst stage [20], which suggests that trophoblast cell differentiation may be compromised. To test this idea, a blastocyst outgrowth assay was performed. Blastocysts were cultured for 72 h. At 48 h, the frequency of blastocyst outgrowth was 55% in the control group compared to 11% in the ZDD group (Fig. 3A). This increased to 75% and 56%, respectively, after 72 h, although the ZDD group was still significantly lower than the control group (Fig. 3A). At 72 h, the area occupied by differentiating trophoblast cells was 40% greater in the control group (0.071 ± 0.003 mm2) compared to the ZDD group (0.042 ± 0.003 mm2), suggesting a defect in trophoblast cell proliferation (Fig. 3, B and C).
FIG. 3.
Effect of zinc deficiency on trophoblast outgrowth. A) Frequency of trophoblast outgrowth at 48 and 72 h after culture of blastocyst embryos from animals that were fed a control diet or ZDD for 5 days before ovulation and collected on Day 3.5 of pregnancy. B) Outgrowth area in control and ZDD groups 72 h after culture. C) Representative images of control and ZDD blastocysts 48 and 72 h in culture. *Significant difference by Student t-test, P < 0.05. Values are mean ± SEM. Bar = 100 μm.
Effect of Preconception Zinc Deficiency on Early Implantation
To determine if early implantation was affected by preconception zinc deficiency, implantation sites were counted on Day 6.5 of pregnancy. In control animals, there were significantly more implantation sites compared to the ZDD group (29 ± 2.5 vs. 17 ± 2.9; P < 0.05; Fig. 4, A and B). Histological examination of these implantation sites revealed a 36% smaller cross-sectional area in the ZDD group compared to the control group (3.17 ± 0.25 control; 2.03 ± 0.21 mm2; P < 0.05; Fig. 4, C and D).
FIG. 4.
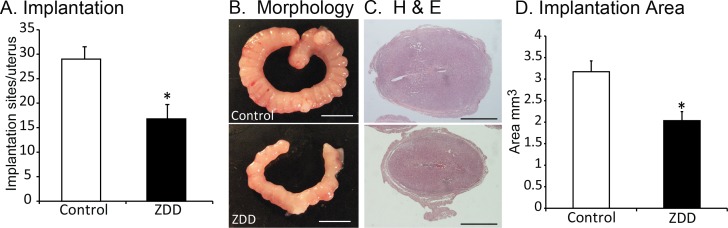
Effect of zinc deficiency on early implantation. A) Number of implantations per animal on Day 6.5 of pregnancy in animals that were fed a control diet or ZDD for 4 days before ovulation. B) Representative uterus images from control and ZDD animals. C) Histological sections of implantation sites from control and ZDD animals. D) Cross-sectional area of implantation sites in control and ZDD groups. *Significant difference by Student t-test, P < 0.05. Values are mean ± SEM. Bars = 10 mm (B) and 1 mm (C).
Effect of Preconception Zinc Deficiency on Placental Development
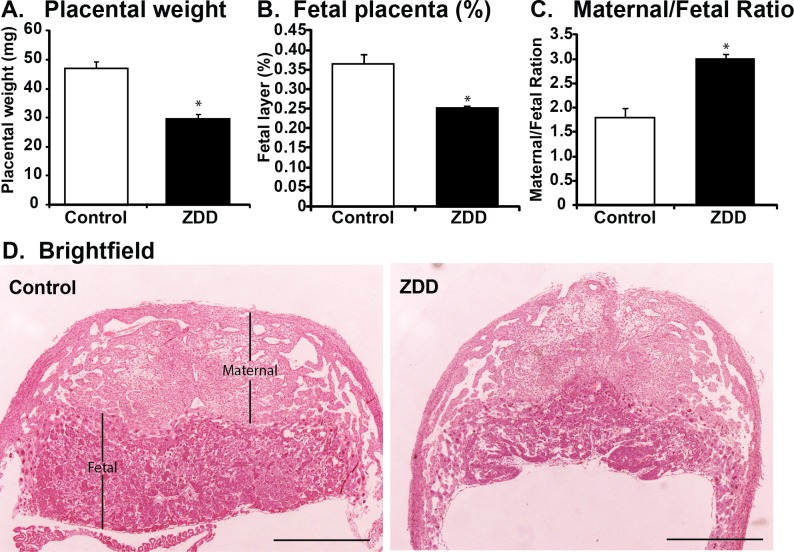
Decreased growth of ZDD embryos on Day 12.5 of pregnancy could be caused by defective placental development. To test this idea, placentas were collected on Day 12.5 of pregnancy from animals fed a control diet and ZDD for 4 days preconception and bred to fertile males. Placental weight was significantly reduced by 37% in ZDD animals compared to control animals (Fig. 5A). Histological examination revealed that the decrease in placental weight was due, in large part, to a 31% decrease in the area of the labyrinth and spoingiotrophoblast layers (Fig. 5B). Due to the reduced fetal contribution to the placenta, the ratio of maternal decidua to fetal placenta was increased by 40% in ZDD compared to control (Fig. 5C).
FIG. 5.
Effect of zinc deficiency on placental development. Placental weight (A), area of fetal placenta (% of total area) (B), and ratio of maternal (decidua) placental to fetal placental (C) in placenta collected on Day 12.5 of pregnancy from animals that were fed a control diet or ZDD for 4 days before ovulation. D) Representative hematoxylin and eosin (H&E) -stained sections of placenta from control and ZDD groups. *Significant difference by Student t-test, P < 0.05, n = 5–7. Values are mean ± SEM. Bar = 1 mm.
Effect of Preconception Zinc Deficiency on Placental Gene Expression
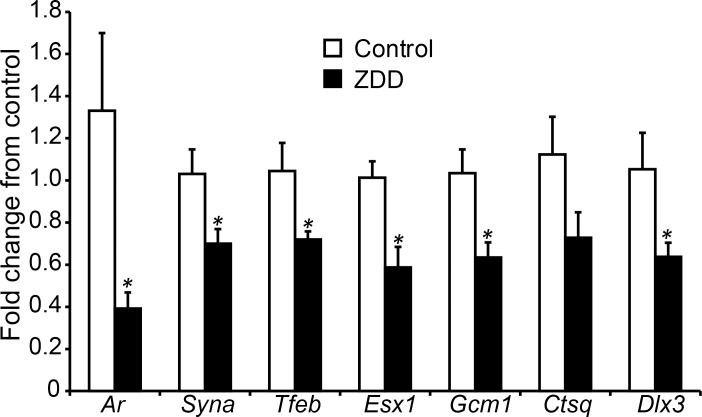
The compromised placental development on Day 12.5 of pregnancy was associated with a decrease in several key placental transcripts. The relative fold change for Ar, Syna, Tfeb, Esx1, Gcm1, and Dlx2 decreased by 70%, 32%, 31%, 42%, 38%, and 39% in ZDD placenta compared to control, while Ctsq mRNA did not change (Fig. 6).
FIG. 6.
Effect of zinc deficiency on placenta gene expression. Relative expression (fold change) of Ar, Syna, Tfeb, Esx1, Gcm1, Ctsq, and Dlx3 mRNA in placentas collected on Day 12.5 of pregnancy from animals that were fed a control diet or ZDD for 4 days before ovulation. *Significant difference by Student t-test, P < 0.05, n = 9–13. Values are mean ± SEM.
Effect of Preconception Zinc Deficiency on Embryonic Development on Day 12.5 of Pregnancy
As the 5-day treatment resulted in high pregnancy loss on Day 10.5, a 4-day dietary treatment was used to examine developmental effects on Day 12.5 (and 16.5). Female mice were fed a ZDD or control diet for 4 days preconception and bred to a fertile male. Embryos were collected on Day 12.5 of pregnancy. Embryos in the ZDD group were growth restricted, with a 13% decrease in the crown-to-rump length compared to controls (Fig. 7A). Moreover, 34% of implantation sites contained no embryo or a nonviable embryo compared to 7% in the control group (Fig. 7B). Similar to the previous experiment, there was a developmental delay in the ZDD embryos, which had shorter limb buds, characteristic of growth retardation. Significantly, ZDD embryos had a larger fourth ventricle, consistent with neural tube defects (Fig. 7C, arrows).
FIG. 7.
Effect of zinc deficiency on fetal development on Day 12.5 of pregnancy. Fetal length (A), fetal loss (B), and representative images of embryos (C) collected on Day 12.5 of pregnancy from animals that were fed a control diet or ZDD for 4 days before ovulation. *Significant difference by Student t-test, P < 0.05, n = 4–5 litters. Values are mean ± SEM. Bar = 100 μm.
Effect of Preconception Zinc Deficiency on Embryonic Development on Day 16.5 of Pregnancy
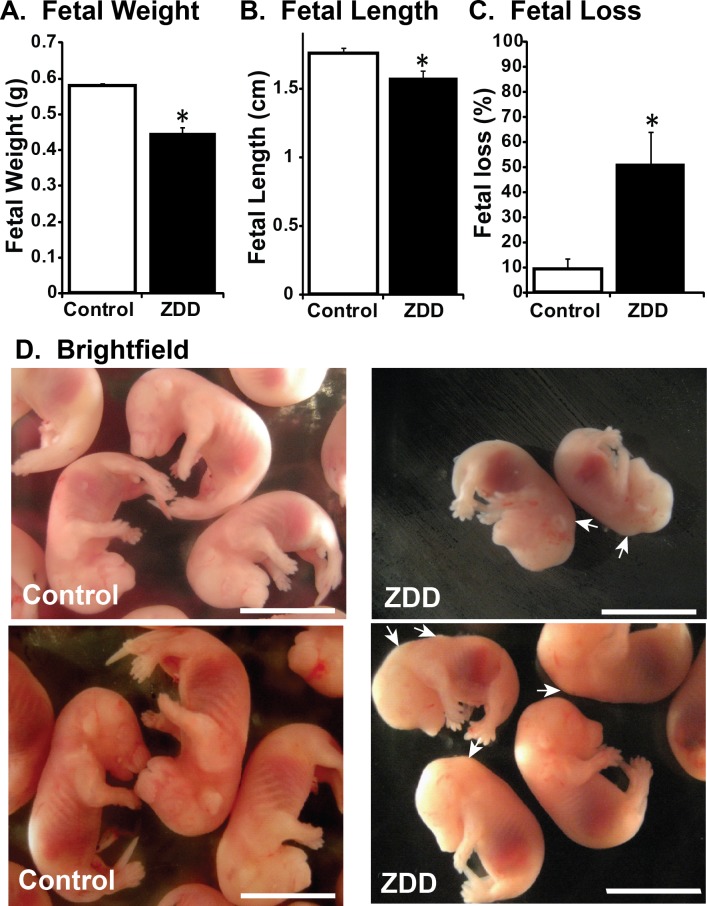
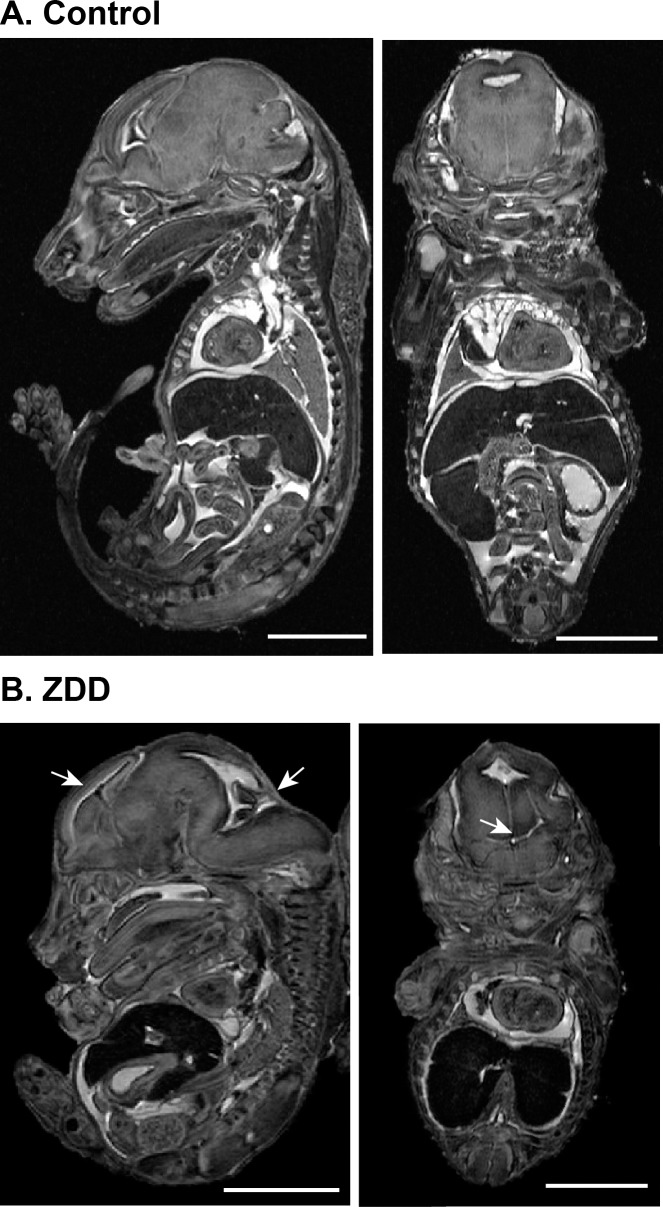
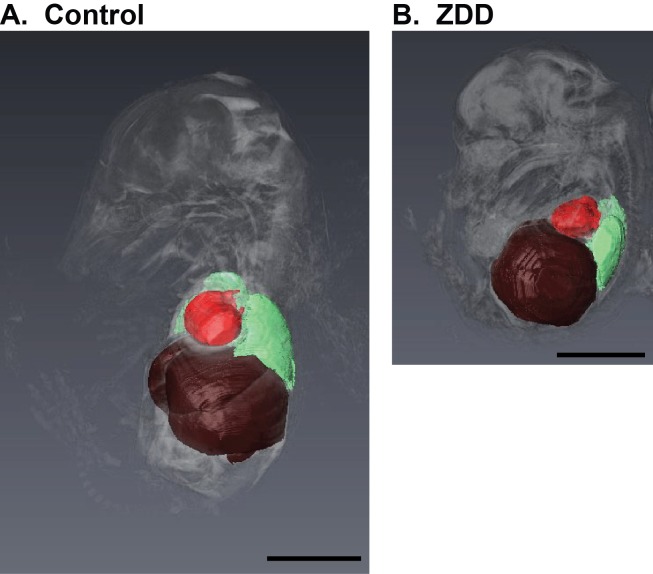
To determine whether preconception zinc depletion has detrimental effects on embryonic development in late pregnancy, female mice were fed control diet or ZDD for 4 days preconception and embryos were collected on Day 16.5 of pregnancy. Embryo weight (Fig. 8A) and length (Fig. 8B) were decreased by 23% and 10%, respectively, compared to control embryos. In addition, a 4-day preconception zinc deficiency caused significant postimplantation embryo loss. More than half (51%) of implantation sites in the ZDD animals contained either no embryo or a nonviable embryo. In contrast, only 9% of implantations were lost in the control animals (Fig. 8C). Consistent with smaller size, embryos from ZDD animals were not as developed, as indicated by splayed digits compared to the parallel arrangement of digits in control embryos (Fig. 8D). However, ZDD embryos were also developmentally compromised. Consistent with neural tube defects earlier in development, 57% of ZDD embryos had a pronounced bump on the back of the head at the base of the brain on Day 16.5 of pregnancy (Fig. 8D, arrows). To gain a more detailed understanding of the neural defects in ZDD embryos, high-field MRI was used for detailed comparison of morphological differences in two representative control and ZDD embryos. The use of MRI for early embryo imaging is emerging as a very useful technique in the field of developmental biology [21]. Control embryos showed normal closure of the neural tube, as indicated by a well-formed cerebellum and brain stem (Fig. 9). However, consistent with observed neural tube defects observed on Days 10.5 and 12.5, ZDD embryos on Day 16.5 of pregnancy showed open neural folds in the anterior and posterior brain regions (Fig. 9B, arrows). After organ segmentation analysis, three-dimensional reconstructions of the heart, liver, and lungs showed much more compacted abdominal and thoracic compartments in the ZDD embryos compared to control embryos, reflecting the overall smaller size of the ZDD embryos (Fig. 10).
FIG. 8.
Effect of zinc deficiency on fetal development on Day 16.5 of pregnancy. Fetal weight (A), fetal length (B), and fetal loss (C) on Day 16.5 of pregnancy from animals that were fed a control diet or ZDD for 4 days before ovulation. D) Brightfield images of fetal development from control and ZDD animals. *Significant difference by Student t-test, P < 0.05, n = 4–5 litters. Values are mean ± SEM. Bar = 1 cm.
FIG. 9.
Morphological assessment of fetal development by MRI. Sagittal and coronal MRI images from fetuses collected on Day 16.5 of pregnancy from animals that were fed a control diet (A) or ZDD (B) for 4 days before ovulation. Bar = 100 μm.
FIG. 10.
Morphological assessment of organ position by MRI. Space-filling reconstruction sagittal images of MRI data showing heart (red), lungs (green), and liver (brown) in fetuses collected on Day 16.5 of pregnancy from animals that were fed a control diet (A) or ZDD (B) for 4 days before ovulation. Bar = 100 μm.
DISCUSSION
The requirement for zinc during pregnancy has been known for some time. Chronic maternal zinc deficiency leads to abnormal fetal development, birth defects, and low birth weight in various species, including humans [8–13]. Early studies in rodents suggested that a 3-day dietary zinc deficiency centered around fertilization caused slower embryo development in vitro [22]. More recently, zinc depletion in vivo during the preconception period or in vitro with a metal chelator has been shown to severely disrupt oocyte maturation, fertilization, and preimplantation development [14–16, 23]. However, the specific requirement for zinc during the preconception period for postimplantation embryo and placental development is not well understood. The present findings show that acute preconception zinc deficiency causes severe growth delay and defects in embryonic and placental development. Collectively, these effects lead to a very high rate of pregnancy loss. Our findings underscore the importance of optimal preconception nutrition, including providing sufficient zinc, to optimize fertility and pregnancy outcomes.
In previous studies focused on the effects of zinc deficiency on pregnancy, dietary treatments were prolonged, marginal, given during pregnancy, or encompassed both pre- and postconception periods [8–13]. Thus, the treatments did not target the preconception period specifically, and it was not possible to separate the preconception requirement for zinc from the requirement during pregnancy. In our previous studies, we began to address the importance of preconception zinc nutrition on ovarian function and fertility [14, 16]. The animal model developed in these previous studies was designed to specifically target the period of antral follicular development with dietary zinc deficiency. This was accomplished by beginning dietary treatments at weaning on Postnatal Day 18. At this time, there is a cohort of follicles on the ovary poised to become antral follicles. These follicles are part of a prepubertal follicular wave that begins growth at birth. During the 3- to 5-day period of zinc deficiency, follicles are stimulated to grow into preovulatory follicles by injecting mice with eCG, followed 48 h later by hCG to stimulate ovulation. At the time of hCG, the dietary treatments were discontinued so that all animals received the same zinc-replete diet for the remainder of the experiment [14, 16]. Using this model, we have previously shown that completion of meiosis, fertilization, and preimplantation development were severely disrupted by as little as a 3-day period of dietary zinc deficiency, but the effects on later development were not examined.
A main finding of this study was that preconception zinc depletion for 4–5 days caused postimplantation developmental delay. This occurred even though animals receiving ZDD were switched back to a zinc-replete diet at the time of the hCG injection. In the first experiment, embryos were collected on Day 10.5 of pregnancy. A 5-day treatment period was chosen based on our previous results showing effects on preimplantation development [16]. Strikingly, ZDD embryos were 31% smaller than control embryos on Day 10.5. This represents a significant developmental delay that is still present on Days 12.5 and 16.5 of pregnancy, when embryo length was decreased by 13% and 10%, respectively, and embryo weight was decreased by 23% on Day 16.5. In addition to growth retardation, there was also a high rate of pregnancy loss (34%–50%) in the ZDD groups compared to controls (2%–9%). Developmental delay in ZDD animals could be the result of poor oocyte/embryo quality and/or compromised uterine function. However, results from embryo transfer experiment support an effect of zinc depletion on oocyte/embryo quality rather than altered uterine receptivity. In this experiment, a known number of blastocyst embryos (11–12) were transferred from treated animals (control or ZDD) to untreated recipients induced into pseudopregnancy. This allowed a more precise calculation of embryo loss. Blastocyst embryos were transferred instead of two-cell embryos because, in a previous study, only a small proportion of two-cell embryos from ZDD animals progressed to the blastocyst stage by Day 3.5 of pregnancy [16]. For the current experiment, only morphologically normal blastocyst embryos of similar size were transferred. Clearly, the findings indicate that the apparently “normal” ZDD blastocyst embryos were of inferior developmental capacity, because only 10% successfully implanted compared to 40% for control embryos. Moreover, even ZDD embryos that did implant after embryo transfer were 40% smaller than control embryos on Day 10.5 of pregnancy. Thus, preconception zinc deficiency causes defects in the embryos themselves rather than the uterine environment. However, effects of ZDD on the oviduct could also contribute to the developmental delay, because embryos were collected on Day 3.5 of pregnancy, shortly after the blastocysts leave the oviduct and enter the uterus. Further research is needed to clarify the role of zinc in the function of the oviduct and how this might affect embryo development. Nevertheless, these findings provide strong evidence that preconception zinc is essential for producing oocytes and early embryos of high developmental potential.
One likely explanation for the developmental defects and pregnancy loss in ZDD animals could be compromised placental development or function. Placental defects begin with compromised trophoblast cell differentiation, as indicated by lower frequency and smaller area in the trophoblast outgrowth assay. The compromised trophoblast cell differentiation in ZDD embryos is likely responsible for the decreased number of implantations on Day 6.5 of pregnancy, which is chronologically equivalent to the 72-h time point of the outgrowth assay. Not only were there fewer implantation sites in the ZDD group on Day 6.5 of pregnancy, but the size of the implantations were smaller in the ZDD group. This probably means that the ZDD embryos did not efficiently induce the decidualization process in the recipient endometrium, although this remains to be established with specific decidualization assays. Nevertheless, the fetal portion of the placenta continues to be compromised on Day 12.5 of pregnancy. At this time, placentas from ZDD animals were much smaller than control animals, mainly due to reduced development of the fetal spongiotrophoblast and labyrinth layers. Consistent with a decrease in development of the fetal placenta, expression of key genes expressed in the trophoblast-derived cells of the fetal placenta, including Gcm1 [24], Esx1 [25], Ctsq [26], Tfeb [27], and Syna [28], were decreased by zinc deficiency. Deletion of the trophoblast marker, Gcm1, causes complete lack of labyrinth development and placental failure [24]. Moreover, lower expression of Gcm1 is associated with development of pre-eclampsia [29]. Zinc deficiency is also associated with increased risk for pre-eclampsia [30, 31]. Thus, the preconception zinc deficiency could have significant implications for the occurrence of pre-eclampsia in women, but this idea remains to be tested directly. It is also not clear if zinc deficiency affects placental development by directly reducing expression of these critical placental transcripts or though some other mechanism that then results in decreased gene expression. Nevertheless, preconception zinc deficiency results in multiple programing defects in the trophoblast cell lineage.
These placental defects likely account for the dramatically delayed fetal development in ZDD animals due to reduced surface area for maternal-fetal exchange, leading to growth retardation. The mechanism by which preconception zinc deficiency alters placental function or development is not known. However, one contributing factor is that programing of the maternal genome is compromised in the oocytes of zinc-deficient animals. Previous studies clearly demonstrate that preconception zinc deficiency causes a decrease in global chromatin (histone and DNA) methylation in oocytes from preovulatory follicles [16]. The decreased methylation is associated with an increase in expression of repetitive elements, which may contribute to genome instability [32] and affect later placental and/or embryo development. Developmental defect may also result from decrease methylation of imprint control regions (ICRs) in the maternal genome. Similar to our findings, embryos lacking methylation of maternal ICRs (imprint free) also show developmental delay around the same time in pregnancy (Day 9.5) and neural tube defects [33]. Whether maternal ICRs are demethylated in zinc-deficient embryos is not known, but previous research shows that ZDD blastocysts express lower amounts of Igf2 and H19 transcripts than control embryos [16]. This suggests that zinc depletion may disrupt imprinting of the Igf2/H19 locus. However, this remains to be determined. As shown previously, the supplementation of a methyl donor, s-adenosylmethionine, during in vitro maturation restores chromatin methylation and partially reverses the fertilization defects in ZDD oocytes [16]. In vivo, supplementation with a methyl-rich diet has also been shown to increase DNA methylation and alter gene expression at specific loci [34]. It is tempting to speculate that dietary supplementation with methyl donors, such as s-adenosylmethionine (SAM), could prevent or reverse the effects of zinc deficiency in vivo on postimplantation embryo and placental development. If this concept is correct, SAM may be a useful reagent for improving the epigenetic characteristics of oocytes used for in vitro fertilization. These hypotheses are currently being tested.
Apart from a global developmental delay in ZDD embryos discussed earlier, the findings demonstrate that preconception zinc deficiency can cause developmental defects. In particular, there is a lack or delay in the closure of the neural tube. This was clearly seen in ZDD embryos on Day 10.5 of pregnancy, when 41% of embryos exhibited a delay in complete closure of the neural tube comprising the spinal cord, and on Day 16.5, when 57% of embryos had a pronounced protrusion at the top of the spinal cord. MRI of Day 16.5 ZDD embryos showed clearly that the bump at the back of the head was caused by open neural folds. This phenotype is similar to that observed during folate deficiency [35]. This is not surprising, since folate also participates in the synthesis of the methyl donor, SAM [36], and folate has recently been shown to accumulate in the oocyte during follicular development [37]. Previously, we have shown that SAM rescues methylation defects in ZDD oocytes and partially restores fertilization potential [16]. These observations suggest that, along with folate, sufficient zinc is important for preventing birth defects resulting from disrupted maternal epigenetic programing.
We have shown that preconception zinc deficiency severely affects postimplantation development of the fetus and placenta. These findings have significant implications for human health, because they suggest that zinc deficiency need not be prolonged to have an impact on subsequent development, even after normal dietary zinc is restored. Moreover, the findings indicate that the fetal placenta is an important tissue affected by preconception zinc deficiency, but the mechanisms responsible for disrupted placental development remain unclear. Collectively, the findings add to a growing body of evidence showing that zinc is acutely important for ovarian function and the production of fertile oocytes and embryos.
Footnotes
Supported by start-up funds from the College of Agricultural Sciences at Pennsylvania State University and National Institutes of Health grant HD057283 to F.J.D.
REFERENCES
- Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal nutrition and fetal development. J Nutr 2004; 134: 2169–2172. [DOI] [PubMed] [Google Scholar]
- Kim KC, Friso S, Choi SW. DNA methylation, an epigenetic mechanism connecting folate to healthy embryonic development and aging. J Nutr Biochem 2009; 20: 917–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr 2002; 132: 2393S–2400S. [DOI] [PubMed] [Google Scholar]
- Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol 2003; 23: 5293–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdge GC, Hanson MA, Slater-Jefferies JL, Lillycrop KA. Epigenetic regulation of transcription: a mechanism for inducing variations in phenotype (fetal programming) by differences in nutrition during early life? Br J Nutr 2007; 97: 1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onagbesan OM, Metayer S, Tona K, Williams J, Decuypere E, Bruggeman V. Effects of genotype and feed allowance on plasma luteinizing hormones, follicle-stimulating hormones, progesterone, estradiol levels, follicle differentiation, and egg production rates of broiler breeder hens. Poult Sci 2006; 85: 1245–1258. [DOI] [PubMed] [Google Scholar]
- Falchuk KH, Montorzi M. Zinc physiology and biochemistry in oocytes and embryos. Biometals 2001; 14: 385–395. [DOI] [PubMed] [Google Scholar]
- Blamberg DL, Blackwood UB, Supplee WC, Combs GF. Effect of zinc deficiency in hens on hatchability and embryonic development. Proc Soc Exp Biol Med 1960; 104: 217–220. [DOI] [PubMed] [Google Scholar]
- Jameson S. Zinc status in pregnancy: the effect of zinc therapy on perinatal mortality, prematurity, and placental ablation. Ann N Y Acad Sci 1993; 678: 178–192. [DOI] [PubMed] [Google Scholar]
- Shah D, Sachdev HP. Effect of gestational zinc deficiency on pregnancy outcomes: summary of observation studies and zinc supplementation trials. Br J Nutr 2001; 85 (Suppl 2): S101–S108. [DOI] [PubMed] [Google Scholar]
- Keen CL, Clegg MS, Hanna LA, Lanoue L, Rogers JM, Daston GP, Oteiza P, Uriu-Adams JY. The plausibility of micronutrient deficiencies being a significant contributing factor to the occurrence of pregnancy complications. J. Nutr 2003; 133: 1597S–1605S. [DOI] [PubMed] [Google Scholar]
- Uriu-Adams JY, Keen CL. Zinc and reproduction: effects of zinc deficiency on prenatal and early postnatal development. Birth Defects Res B Dev Reprod Toxicol 2010; 89: 313–325. [DOI] [PubMed] [Google Scholar]
- Apgar J. Zinc and reproduction. Annu Rev Nutr 1985; 5: 43–68. [DOI] [PubMed] [Google Scholar]
- Tian X, Diaz FJ. Zinc depletion causes multiple defects in ovarian function during the periovulatory period in mice. Endocrinology 2012; 153: 873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AM, Vogt S, O'Halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol 2010; 6: 674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Diaz FJ. Acute dietary zinc deficiency before conception compromises oocyte epigenetic programming and disrupts embryonic development: preconception zinc and oocyte quality. Dev Biol 2013; 376: 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M, Bass S, Spear B. A device for the simple and rapid transcervical transfer of mouse embryos eliminates the need for surgery and potential post-operative complications. Biotechniques 2009; 47: 919–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz FJ, O'Brien MJ, Wigglesworth K, Eppig JJ. The preantral granulosa cell to cumulus cell transition in the mouse ovary: development of competence to undergo expansion. Dev Biol 2006; 299: 91–104. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-[delta][delta]CT method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Tian X, Diaz FJ. Acute dietary zinc deficiency before conception compromises oocyte epigenetic programming and disrupts embryonic development. Dev Biol 2013; 376: 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J, Böse J, Bamforth S, Gruber A, Broadbent C, Clarke K, Neubauer S, Lengeling A, Bhattacharya S. Identification of cardiac malformations in mice lacking Ptdsr using a novel high-throughput magnetic resonance imaging technique. BMC Dev Biol 2004; 4: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Wiley LM, Zidenberg-Cherr S, Keen CL. Influence of short-term maternal zinc deficiency on the in vitro development of preimplantation mouse embryos. Proc Soc Exp Biol Med 1991; 198: 561–568. [DOI] [PubMed] [Google Scholar]
- Kim AM, Bernhardt ML, Kong BY, Ahn RW, Vogt S, Woodruff TK, O'Halloran TV. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem Biol 2011; 6: 716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson-Cartwright L, Dawson K, Holmyard D, Fisher SJ, Lazzarini RA, Cross JC. The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat Genet 2000; 25: 311–314. [DOI] [PubMed] [Google Scholar]
- Li Y, Lemaire P, Behringer RR. Esx1, a novel X chromosome-linked homeobox gene expressed in mouse extraembryonic tissues and male germ cells. Dev Biol 1997; 188: 85–95. [DOI] [PubMed] [Google Scholar]
- Simmons DG, Fortier AL, Cross JC. Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev Biol 2007; 304: 567–578. [DOI] [PubMed] [Google Scholar]
- Steingrimsson E, Tessarollo L, Reid SW, Jenkins NA, Copeland NG. The bHLH-Zip transcription factor Tfeb is essential for placental vascularization. Development 1998; 125: 4607–4616. [DOI] [PubMed] [Google Scholar]
- Dupressoir A, Marceau G, Vernochet C, Bénit L, Kanellopoulos C, Sapin V, Heidmann T. Syncytin-A and syncytin-B, two fusogenic placenta-specific murine envelope genes of retroviral origin conserved in Muridae. Proc Natl Acad Sci U S A 2005; 102: 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge SA, Minhas A, Whiteley KJ, Qu D, Sled JG, Kingdom JCP, Adamson SL. Effects of reduced Gcm1 expression on trophoblast morphology, fetoplacental vascularity, and pregnancy outcomes in mice. Hypertension 2012; 59: 732–739. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim YJ, Lee R, Moon JH, Jo I. Serum levels of zinc, calcium, and iron are associated with the risk of preeclampsia in pregnant women. Nutr Res 2012; 32: 764–769. [DOI] [PubMed] [Google Scholar]
- Jain S, Sharma P, Kulshreshtha S, Mohan G, Singh S. The role of calcium, magnesium, and zinc in pre-eclampsia. Biol Trace Elem Res 2010; 133: 162–170. [DOI] [PubMed] [Google Scholar]
- Hedges DJ, Deininger PL. Inviting instability: transposable elements, double-strand breaks, and the maintenance of genome integrity. Mutat Res 2007; 616: 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Proudhon C, Bestor TH, Woodfine K, Lin C-S, Lin S-P, Prissette M, Oakey RJ, Bourc'his D. The parental non-equivalence of imprinting control regions during mammalian development and evolution. PLoS Genet 2010; 6: e1001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A 2007; 104: 13056–13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safi J, Joyeux L, Chalouhi GE. Periconceptional folate deficiency and implications in neural tube defects. J Pregnancy 2012; 2012: 295083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson OS, Sant KE, Dolinoy DC. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J Nutr Biochem 2012; 23: 853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra M, Trasler JM, Baltz JM. Folate transport in mouse cumulus-oocyte complexes and preimplantation embryos. Biol Reprod 2013; 89: 63. [DOI] [PubMed] [Google Scholar]