Abstract
Vagal tone is a measure of cardiovascular function that facilitates adaptive responses to environmental challenge. Low vagal tone is associated with poor emotional and attentional regulation in children and has been conceptualized as a marker of sensitivity to stress. We investigated whether the associations of a wide range of psychosocial stressors with internalizing and externalizing psychopathology were magnified in adolescents with low vagal tone. Resting heart period data were collected from a diverse community sample of adolescents (ages 13–17; N =168). Adolescents completed measures assessing internalizing and externalizing psychopathology and exposure to stressors occurring in family, peer, and community contexts. Respiratory sinus arrhythmia (RSA) was calculated from the interbeat interval time series. We estimated interactions between RSA and stress exposure in predicting internalizing and externalizing symptoms and evaluated whether interactions differed by gender. Exposure to psychosocial stressors was associated strongly with psychopathology. RSA was unrelated to internalizing or externalizing problems. Significant interactions were observed between RSA and child abuse, community violence, peer victimization, and traumatic events in predicting internalizing but not externalizing symptoms. Stressors were positively associated with internalizing symptoms in adolescents with low RSA but not in those with high RSA. Similar patterns were observed for anxiety and depression. These interactions were more consistently observed for male than female individuals. Low vagal tone is associated with internalizing psychopathology in adolescents exposed to high levels of stressors. Measurement of vagal tone in clinical settings might provide useful information about sensitivity to stress in child and adolescent clients.
The autonomic nervous system plays a central regulatory function of maintaining homeostasis through coordinated influences on multiple organ systems, including the heart, lungs, salivary glands, kidneys, sweat glands, and many others. Autonomic nervous system activation occurs in response to a variety of changes in both the internal and external environment that require adaptation by the organism, including experiences of psychosocial stress (Berntson, Cacioppo, & Quigley, 1993a; Lucini, Di Fede, Parati, & Pagani, 2005; Lucini, Norbiato, Clerici, & Pagani, 2002; Porges, 1995a; Sloan et al., 1994). Changes in autonomic nervous system function occur rapidly following exposure to a stressor or another change in the environment, typically within milliseconds to seconds, and mediate cardiovascular and hemodynamic responses to stress. For this reason, the autonomic nervous system has long been conceptualized as a central physiological marker of stress reactivity and sensitivity (Porges, 1992, 1995a, 2007). Individual differences in autonomic nervous system function have been linked to a variety of physical and mental health outcomes in both children and adults (Beauchaine, 2001; Boyce et al., 2001; Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996; Thayer, Friedman, & Borkovec, 1996). Although measures of autonomic nervous system function are frequently used as clinical markers of disease risk, they have not typically been employed as risk markers by mental health clinicians. In the current report, we examine the extent to which specific aspects of autonomic nervous system function might provide valuable information to clinicians about sensitivity to stress—the propensity to experience negative outcomes following exposure to stressors—and, potentially, risk for psychopathology in children and adolescents.
Specifically, we examine whether vagal tone interacts with psychosocial stress exposure to predict psychopathology in adolescents. Vagal tone is a measure of parasympathetic nervous system control over heart rate (Allen, Chambers, & Towers, 2007; Berntson et al., 1997; Porges, 1992, 1995a, 2007). The parasympathetic nervous system is involved in functions that promote growth and restoration. During conditions of rest, the parasympathetic nervous system facilitates digestion, bodily repair, and energy conservation (Porges, 1995a, 1995b, 2007). Following exposure to a stressor, the parasympathetic nervous system typically functions to inhibit sympathetic nervous system activation, reduce heart rate and metabolic output, and return the body to homeostasis once the stressor has ended (Berntson et al., 1997; Porges, 1992, 1995a, 1995b, 2007). Respiratory sinus arrhythmia (RSA) is a noninvasive measure of parasympathetic influences on heart rate used to estimate vagal tone (Berntson, Cacioppo, & Quigley, 1993b; Grossman & Taylor, 2007; Porges, 1992, 1995a). RSA reflects a coupling of heart rate and respiration that leads to systematic variability in heart rate during inhalation as compared to exhalation (Allen et al., 2007; Berntson et al., 1993b), and a variety of studies indicate that RSA is a measure of parasympathetic nervous system functioning that reflects vagal influences on heart rate (Cacioppo et al., 1994; Grossman, Stemmler, & Meinhardt, 1990; Kollai & Mizsei, 1990; Porges, 2007). Because of the central role the parasympathetic nervous system plays in inhibiting arousal and promoting recovery following environmental challenges, vagal tone has been conceptualized as an important marker of self-regulation that has implications for social behavior (Appelhans & Luecken, 2006; Porges, 1995b, 2001b, 2007; Thayer & Lane, 2000).
Indeed, resting vagal tone has been associated with both attentional regulation and emotion regulation in youths (Beauchaine, 2001; Porges, 2007). Low resting vagal tone has been associated with heightened emotional reactivity, poor attentional and inhibitory control, and deficits in multiple aspects of emotion regulation in a variety of studies in children and adolescents (Blandon, Calkins, Keane, & O’Brien, 2008; Calkins & Keane, 2004; Huffman et al., 1998; Mezzacappa, Kindlon, Saul, & Earls, 1998; Stifter & Fox, 1990; Stifter, Fox, & Porges, 1989; Suess, Porges, & Blude, 1994). Poor vagal tone has also been associated with child psychopathology. Children with internalizing psychopathology have been observed to have lower resting vagal tone (Boyce et al., 2001; Forbes, Fox, Cohn, Galles, & Kovacs, 2006; Shannon, Beauchaine, Brenner, Neuhaus, & Gatzke-Kopp, 2007), and other metrics of low heart rate variability have also been documented in adults with anxiety disorders (Friedman & Thayer, 1998; Thayer et al., 1996). A meta-analysis reported low resting vagal tone in adults with major depression (Rottenberg, 2007). Low resting vagal tone (Beauchaine, Gatzke-Kopp, & Mead, 2007; Mezzacappa et al., 1997; Porges et al., 1996) and low heart rate variability (Pine et al., 1998) have also been documented frequently in children with aggression and externalizing problems. However, the relationship between vagal tone and internalizing psychopathology has been somewhat inconsistent across studies, with important moderators of this association emerging. For example, one study reported an association between low resting vagal tone and depressive symptoms only in children with a parental history of early-onset depression (Forbes et al., 2006). In another study, low resting vagal tone was most strongly associated with internalizing psychopathology among children who also exhibited high levels of vagal withdrawal during a social stressor (Hinnant & El-Sheikh, 2009).
One possibility is that vagal tone influences the relationship between environmental adversity and child psychopathology. Given the central role the parasympathetic nervous system plays in inhibiting sympathetic activation and promoting physiological recovery and flexibility in response to stressors (Porges, 2007), individual differences in vagal tone might be most strongly associated with psychopathology following exposure to psychosocial stressors. Specifically, individuals with low vagal tone might experience prolonged physiological activation and slower emotional and physiological recovery following stressors (Lane, Adcock, & Burnett, 1992), which, in turn, might be associated with heightened risk for psychopathology. The degree to which low vagal tone is associated with heightened vulnerability to psychopathology following exposure to psychosocial stressors has been examined in previous studies of children. These studies have each shown that vagal tone moderates the relationship between marital conflict and child adjustment, such that children with poor vagal tone are at increased risk for internalizing and externalizing problems and other adverse functional outcomes in the context of high marital conflict (El-Sheikh, Harger, & Whitson, 2001; El-Sheikh & Whitson, 2006; Katz & Gottman, 1995, 1997; Leary & Katz, 1997; Whitson & El-Sheikh, 2003). Together, these findings suggest that vagal tone influences the associations between environmental adversity and child outcomes.
In the current study, we expand the existing literature on the role of vagal tone as a moderator of the relationship between environmental adversity and youth psychopathology in three ways. First, the vast majority of studies have focused on vagal tone as a moderator of the relationship between marital conflict or other aspects of maladaptive family functioning and child outcomes (El-Sheikh et al., 2001; El-Sheikh & Whitson, 2006; Katz & Gottman, 1995, 1997; Leary & Katz, 1997; Whitson & El-Sheikh, 2003). Although parental interactions and relationship quality are important determinants of child adjustment, youths are embedded in a variety of other social contexts that have relevance for developmental outcomes (Bronfenbrenner, 1986). Here, we investigate the degree to which vagal tone influences the associations of psychosocial stressors occurring in the family, peer, and community contexts with child psychopathology. Exposure to traumatic stressors, including child maltreatment, community violence, and other types of traumatic events, is a potent risk factor for the onset of both internalizing and externalizing psychopathology in youths (Cohen, Brown, & Smailes, 2001; Copeland, Keeler, Angold, & Costello, 2007; Margolin & Gordis, 2000; McLaughlin et al., 2012). Victimization by peers is another developmentally salient stressor for children and adolescents that is associated with symptoms of anxiety and depression (Hawker & Boulton, 2000; Storch, Masia-Warner, Crisp, & Klein, 2005; Vernberg, Abwender, Ewell, & Beery, 1992) and predicts the onset of internalizing and externalizing disorders (Coie, Lochman, Terry, & Hyman, 1992). Identifying factors associated with heightened psychopathology among youths who have experienced these types of psychosocial stressors could provide valuable information for clinicians working with children and adolescents as it may allow for earlier and more targeted intervention and prevention efforts. Specifically, clinicians could measure vagal tone in order to identify clients who might be particularly likely to develop psychopathology following experiences of psychosocial stress. This information would provide clinicians with information about which clients are particularly likely to benefit from an intensification of clinical intervention following experiences of stress and adversity. To our knowledge, previous research has not examined whether vagal tone moderates the relationship between multiple forms of psychosocial stress exposure and youth psychopathology.
Second, prior studies examining vagal tone as a moderator of the relationship between environmental adversity and psychopathology have focused exclusively on children. We extend prior research by examining this question in adolescents. Adolescence is an important period in which to examine these associations. Marked developmental changes in physiological stress response systems occur during adolescence, such that adolescents exhibit greater reactivity to social and performance stressors than children (Gunnar, Wewerka, Frenn, Long, & Griggs, 2009; Stroud et al., 2009). Adolescents are also more emotionally vulnerable to the effects of stressors, as stressful events become more closely linked to the emergence of negative affect during adolescence (Larson & Ham, 1993; Larson, Moneta, Richards, & Wilson, 2002). Peer relationships become increasingly important during this period, meaning that social stressors occurring in the peer context might have a particularly strong influence on psychopathology for adolescents. Adolescence is also a period of heightened vulnerability for the development of psychopathology (Hankin et al., 1998b; Lewinsohn, Striegel-Moore, & Seeley, 2000; Twenge & Nolen-Hoeksema, 2002). In addition, the associations of vagal tone with child outcomes have been found to vary across development, with stronger relationships observed between low vagal tone and psychopathology among older children and adolescents as compared to young children (Beauchaine, 2001; Beauchaine et al., 2007).
Finally, we examine gender differences in the extent to which vagal tone is associated with psychopathology following psychosocial stressors. Substantial gender differences in the development of physiological stress responses systems emerge during adolescence (Stroud, Papandonatos, Williamson, & Dahl, 2004; Stroud, Salovey, & Epel, 2002), and sex hormones can influence numerous aspects of stress response system development during this period (Oldehinkel & Bouma, 2011). During adolescence, girls exhibit heightened physiological reactivity to social stressors relative to boys, whereas boys exhibit greater reactivity to achievement-related stressors (Stroud et al., 2002). Gender differences in stress reactivity have also been observed in the relationship between stressors and depressive symptoms, such that girls are more likely than boys to develop depressive symptoms following stress exposure in adolescence (Cyranowski, Frank, Young, & Shear, 2000; Hankin, Mermelstein, & Roesch, 2007; Rudolph & Hammen, 1999). Given the heightened sensitivity to psychosocial stress observed among girls during the adolescent transition, we expected that the association between low vagal tone and psychopathology would be particularly pronounced in girls as compared to boys.
We examine whether vagal tone moderates the association between psychosocial stress exposure and psychopathology in a diverse community-based sample of adolescents in order to determine whether vagal tone might be a useful marker of stress sensitivity for mental health clinicians working with adolescents. Specifically, we examine whether low vagal tone magnifies the associations of multiple psychosocial stressors—including child abuse, community violence, peer victimization, and traumatic events—with internalizing and externalizing psychopathology in adolescents. We hypothesized that the association between psychosocial stressors and psychopathology would be stronger in adolescents with low vagal tone than in adolescents with high vagal tone. Because previous research suggests that the association of vagal tone with youth depressive symptoms varies according to the presences of adverse environmental factors (Forbes et al., 2006), we examined symptoms of depression and anxiety separately to determine whether vagal tone interacted with psychosocial stressors to predict particular types of internalizing psychopathology. In addition, we determine whether the moderating role of vagal tone in the association of stress exposure with adolescent psychopathology varies by gender. We anticipated that vagal tone would moderate the association between stress exposure and internalizing psychopathology more strongly in girls than in boys.
METHODS
Sample
A community-based sample of 168 adolescents aged 13 to 17 was recruited for participation in the study. Adolescents were recruited using flyers at schools, after-school programs, general medical clinics, and the general community in Boston and Cambridge, Massachusetts. Recruitment efforts were targeted at recruiting a sample with high racial/ethnic diversity as well as variability in exposure to adversity. The sample was 56.0% female (n =94) and had a mean age of 14.9 years (SD =1.36). Racial/ethnic composition of the sample was as follows: 40.8% White (n =69), 18.34% Black (n =31), 17.8% Hispanic (n =30), 7.7% Asian (n =13), and 14.8% Biracial or Other (n =25). Approximately one third of the sample (38.1%, n = 64) was from single-parent households. Adolescents taking medications known to influence autonomic function were excluded (n =4). Equipment malfunctions resulted in loss of autonomic data from 8 participants. An additional 3 participants were excluded from analysis due to presence of a heart murmur (n =1), severe cognitive impairment (n =1), and presence of a pervasive developmental disorder (n =1). The final analytic sample included 157 participants.
Physiological Measures
Continuous cardiac and hemodynamic measures were recorded noninvasively according to accepted guidelines (Sherwood et al., 1990). Electrocardiogram (ECG) recordings were obtained with a Biopac ECG amplifier (Goleta, CA) using a modified Lead II configuration (right clavicle, left lower torso, and right leg ground). Cardiac impedance recordings were obtained with a Bio-Impedance Technology model HIC-2500 impedance cardiograph (Chapel Hill, NC). One pair of Mylar electrode tapes were placed on the neck, and another pair were placed on the torso. Biopac MP150 hardware and Acknowledge software was used to integrate and acquire the ECG and impedance cardiography data, both of which were sampled at 1.0 kHz. ECG and impedance cardiograph were scored by trained personal following acquisition. All signals were visually inspected and scored using Mindware Heart Rate Variability (HRV) Software (Mindware Technologies, Gahanna, OH).1
RSA was calculated from the interbeat interval time series using spectral analysis implemented in Mindware HRV Software. RSA was calculated for the frequency band 0.12–0.40 Hz. Based on evidence suggesting that control for respiration rate is necessary for RSA to represent a valid measure of purely parasympathetic cardiac control (Berntson et al., 1997; Grossman, Karemaker, & Wieling, 1991; Grossman & Taylor, 2007), we controlled for respiration rate in all analysis. Respiration rate was derived from the basal cardiac impedance signal.
Psychosocial Stress Exposure
Adolescents completed measures assessing exposure to a wide range of stressors and traumatic events occurring in family, peer, and community contexts.
Child abuse was assessed using the Childhood Trauma Questionnaire (CTQ; Bernstein, Ahluvalia, Pogge, & Handelsman, 1997; Bernstein et al., 2003). The CTQ is a 28-item scale that assesses the frequency of exposure to maltreatment during childhood and adolescence. Three types of abuse are assessed: physical abuse, sexual abuse, and emotional abuse. Respondents indicate how often each experience occurred on a 5-point Likert scale ranging from 1 (never true) to 5 (very often true). The CTQ is among the most commonly used measures of child maltreatment and has excellent psychometric properties including internal consistency, test–retest reliability, and convergent and discriminant validity with both interview measures and clinician reports of maltreatment (Bernstein et al., 1997; Bernstein, Fink, Hondelsman, Foote, & Lovejoy, 1994). We created an abuse composite by summing items from the Physical Abuse, Sexual Abuse, and Emotional Abuse subscales. The abuse composite demonstrated good reliability in our sample (α =0.88).
Community Violence Exposure was assessed using the Screen for Adolescent Violence Exposure (SAVE; Hastings & Kelley, 1997). The SAVE is a 32-item measure assessing violence exposure in school, home, and neighborhood contexts. Only items assessing school and neighborhood violence were considered to avoid overlap with the CTQ with regard to experiences of child abuse. Respondents rate the frequency of exposure to indirect violence (e.g., “I have heard about someone getting shot”) as well as being the victim of violence (e.g., “Someone has pulled a knife on me”) on a 5-point Likert scale ranging from 1 (never) to 5 (almost always). The SAVE has demonstrated good reliability and validity in prior studies of adolescents (Hastings & Kelley, 1997). The SAVE total violence exposure scale demonstrated excellent internal consistency in this sample (α =0.89).
Peer Victimization experiences were assessed using the Revised Peer Experiences Questionnaire (RPEQ; Prinstein, Boergers, & Vernberg, 2001). The RPEQ was developed from the Peer Experiences Questionnaire (Vernberg, Jacobs, & Hershberger, 1999) and assesses overt, relational, and reputational victimization by peers. The questionnaire includes 18 items that ask participants to rate how often an aggressive behavior was directed toward them in the past year on a 5-point Likert scale ranging from 1 (never) to 5 (a few times a week). Example items include “A kid threatened to hurt or beat me up” (overt); “To get back at me, another kid told me that he or she would not be my friend” (relational); and “A kid gossiped about me so that others would not like me” (reputational). The original and revised measure has demonstrated good test-retest reliability, internal consistency, and convergent validity (Prinstein et al., 2001; Vernberg, Fonagy, & Twemlow, 2000). We created a total victimization score are by summing all victimization items. The RPEQ total victimization scale demonstrated good internal consistency in this sample (α =0.87).
Other Lifetime Traumatic Events were assessed using the trauma assessment included in the posttraumatic stress disorder section of the Composite International Diagnostic Interview (Kessler & Üstun, 2004), which has been adapted for use in national samples of adolescents (McLaughlin et al., 2013). Other traumatic events assessed within this module included interpersonal violence (e.g., physical assaults by a romantic partner; items querying child maltreatment were removed to avoid overlap with other measures), accidents and injuries (e.g., natural disasters), and network events (e.g., sudden unexpected death of a loved one). A trauma score was created by summing the total number of distinct traumatic events that occurred in the respondent’s lifetime.
Psychopathology
Internalizing and externalizing psychopathology was assessed using the Youth Self Report form of the Child Behavior Checklist (CBCL), and externalizing psychopathology was also assessed using the parent-report CBCL (Achenbach, 1991; Achenbach & Rescorla, 2001). The CBCL scales are among the most widely used measures of youth emotional and behavioral problems and use extensive normative data to generate age-standardized estimates of the severity of internalizing and externalizing psychopathology. The broad-scale internalizing and externalizing scales as well as the internalizing syndrome subscales have demonstrated validity in discriminating between youths with and without psychopathology (Achenbach, 1991; Achenbach, Dumenci, & Rescorla, 2003; Chen, Faraone, Biederman, & Tsuang, 1994; Ebesutani et al., 2010; Kendall et al., 2007; Seligman, Ollendick, Langley, & Baldacci, 2004). We examined interactions between RSA and psychosocial stress exposure in predicting the externalizing composite and the following dimensions of the internalizing composite: anxiety/depression and depression/ withdrawal. Because parents have been shown to provide unique information with regards to youth externalizing problems (Bird, Gould, & Staghezza, 1992; Grills & Ollendick, 2002), we examined both youth-reported and parent-reported externalizing problems.
Procedure
Cardiac data were collected from all adolescent participants prior to completion of questionnaires about stress exposure and psychopathology. Heart period data were acquired during a 10-min period in which participants were asked to sit quietly without moving. Adolescents then completed questionnaire measures. A parent or guardian completed questionnaires in a separate room. Informed consent was obtained from the parent or guardian who attended the session with the participant, and assent was provided by all adolescents. Adolescents were paid $50 for participation.
Statistical Analysis
We first examined the associations of RSA and stress exposure variables with psychopathology outcomes. Next, we evaluated the hypothesis that low RSA would be more strongly associated with psychopathology among adolescents exposed to psychosocial stressors. To do so, we created interaction terms between RSA and each of our four stress exposure variables. All variables were standardized prior to creating interaction terms and conducting regression analysis, and main effect terms for RSA and stress exposure were included in interaction models. Procedures outlined by Aiken and West (1991) were used to evaluate significant interactions. We controlled for age and gender in all analyses and for respiration rate in models including RSA. Finally, we examined the potential moderating role of gender by creating three-way interaction terms between RSA, psychosocial stressors, and gender in predicting psychopathology. Significant interactions were followed up by examining the two-way interaction between RSA and psychosocial stressors separately for male and female participants.
RESULTS
Descriptive Statistics
Table 1 provides the distribution of RSA, psychosocial stress variables, and psychopathology in the sample, separately for male and female participants. Resting RSA was normally distributed in both male (M =6.44, SD =1.14) and female (M =6.82, SD =0.99) participants. Exposure to psychosocial stressors varied widely across participants.
TABLE 1.
Distribution of Resting RSA, Psychosocial Stressors, and Psychopathology by Sex
| Male
|
Female
|
t Value | p Value | |||
|---|---|---|---|---|---|---|
| M | (SD) | M | (SD) | |||
| Male | ||||||
| Resting RSA | 6.44 | (1.14) | 6.82 | (0.99) | 2.24 | .027 |
| Child Abuse | 3.75 | (6.50) | 4.65 | (7.03) | 1.45 | .148 |
| Community Violence | 42.97 | (12.17) | 42.17 | (10.39) | −0.87 | .385 |
| Peer Victimization | 8.37 | (7.57) | 8.09 | (7.11) | −0.45 | .655 |
| Other Trauma | 4.18 | (3.24) | 4.08 | (2.95) | −0.36 | .717 |
| YSR Anxious/Depressed | 55.61 | (7.07) | 56.16 | (6.72) | 0.50 | .616 |
| YSR Depressed/Withdrawn | 56.00 | (6.64) | 56.05 | (6.45) | 0.05 | .958 |
| YSR Externalizing | 51.54 | 10.19 | 49.94 | (11.22) | 0.75 | .456 |
| CBCL Externalizing | 46.31 | (8.83) | 52.67 | (9.14) | 2.21 | .029 |
Note. N =157.
RSA and Psychopathology
RSA was not associated with youth-reported symptoms of anxiety/depression (β =.0.08, p =.348), depression/ withdrawal (β =0.07, p =.450), or externalizing problems (β =0.10, p = .25), or with parent-reported externalizing problems (β =0.04, p =.67), in models controlling for age, gender, and respiration rate.
Psychosocial Stressors and Psychopathology
Associations between psychosocial stressors and internalizing and externalizing psychopathology are shown in Table 2. Each of the domains of stress exposure—child abuse, community violence, peer victimization, and other traumatic events—was positively and significantly associated with youth-reported anxiety/ depression and depression/withdrawal, and with youth-and parent-reported externalizing problems.
TABLE 2.
Associations Between Psychosocial Stressors and Youth Internalizing Psychopathologya
| YSR Anxiety/Depression
|
YSR Depression/Withdrawal
|
YSR Externalizing
|
CBCL Externalizing
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | t(153) | p | β | t(153) | p | β | t(153) | p | β | t(153) | p | |
| Child Abuse | 0.29* | 3.75 | <.001 | 0.31* | 4.02 | <.001 | 0.38* | 4.92 | <.001 | 0.22* | 2.71 | .007 |
| Community Violence | 0.17* | 2.13 | .035 | 0.24* | 3.07 | .003 | 0.43* | 5.94 | <.001 | 0.17* | 2.16 | .032 |
| Peer Victimization | 0.48* | 7.11 | <.001 | 0.33* | 4.49 | <.001 | 0.49* | 7.07 | <.001 | 0.24* | 3.13 | .002 |
| Other Trauma | 0.30* | 3.94 | <.001 | 0.29* | 3.76 | <.001 | 0.49* | 7.06 | <.001 | 0.25* | 3.16 | .002 |
Note. N =157. YSR =Youth Self-Report; CBCL =Child Behavior Checklist.
All psychosocial stress variables were standardized; analyses control for age and gender.
p < .05, two-sided test.
Interactions Between RSA and Psychosocial Stressors
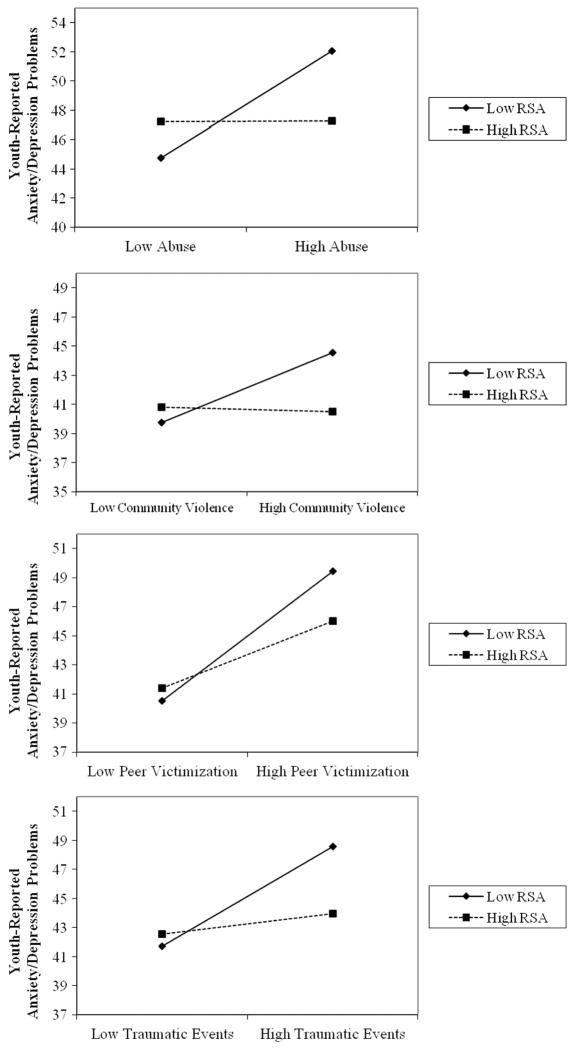
We next examined whether RSA moderated the association between psychosocial stress exposure and internalizing psychopathology. We found significant interactions between RSA and each of the four domains of stress in predicting anxiety/depression problems based on the Youth Self-Report (see Figure 1). RSA interacted with child abuse (β =−0.22, p = .006), community violence exposure (β =−0.20, p =.013), peer victimization (β =−0.17, p =.017), and traumatic events (β =−0.21, p =.008), in predicting anxiety/depression problems.
FIGURE 1.
Interactions between respiratory sinus arrhythmia (RSA) and psychosocial stressors in predicting youth-reported anxiety/depression problems. Note. Figures depict low RSA and low exposure to psychosocial stressors as 1 SD below the mean and high RSA and high exposure to psychosocial stressors as 1 SD above the mean. The y-axis is scaled to 15 points.
For significant interactions, we next evaluated the simple slope of the association between childhood adversity and youth-reported internalizing problems at high and low levels of RSA (i.e., 1 SD above and below the mean). The positive association between child abuse and anxiety/depression was significant for adolescents with low RSA (β = 3.64), t(145) =4.61, p < .001, but was not significant for adolescents with high RSA (β =0.02), t(145) =0.03, p =.980. A similar pattern was observed for community violence and exposure to other traumatic events. Community violence exposure and anxiety/depression were positively associated for adolescents with low RSA (β =2.41), t(145) =3.44, p < .001, but not for adolescents with high RSA (β =−0.82), t(145) =0.17, p =.863. Similarly, the positive association between traumatic events and anxiety/ depression was significant for adolescents with low RSA (β =3.42), t(145) =4.99, p < .001, but not for adolescents with high RSA (β =0.71), t(145) =0.93, p =.355. Finally, the slope of the relationship between peer victimization and anxiety/depression problems was strongest among youths with low RSA (β =4.47), t(145) =6.97, p < .001, but was still positive and significant among youths with high RSA (β =2.31), t(145) =3.59, p < .001.
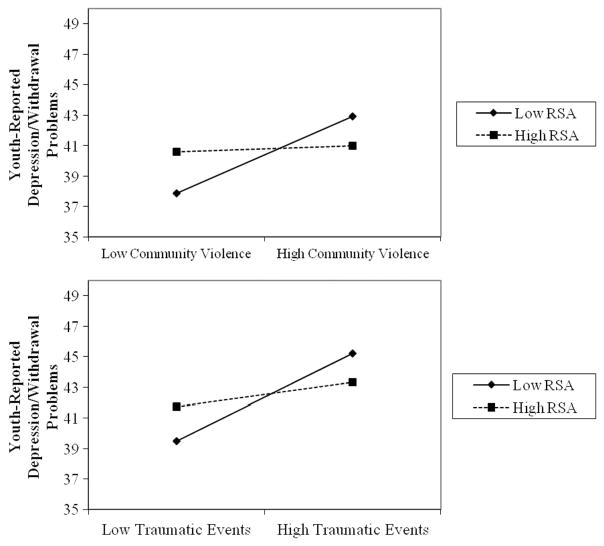
RSA interacted with two of the four domains of psychosocial stress in predicting problems with depression/ withdrawal: community violence (β =−0.19, p =.019), and other traumatic events (β =−0.20, p =.016; see Figure 2). The pattern of these interactions mirrored what was found for anxiety/depression problems. The positive association between community violence and depression/withdrawal was significant for adolescents with low RSA, (β =2.54), t(145) =3.71, p < .001, but was not significant for adolescents with high RSA (β =0.20), t(145) =0.25, p =.801. Similarly, traumatic events were positively associated with depression/ withdrawal problems among adolescents with low RSA (β =2.87), t(145) = 4.16, p < .001, but not among adolescents with high RSA (β = 0.78), t(145) =1.02, p =.311.
FIGURE 2.
Interaction between respiratory sinus arrhythmia (RSA) and psychosocial stressors in predicting youth-reported depression/withdrawal problems. Note. Figures depict low RSA and low exposure to psychosocial stressors as 1 SD below the mean and high RSA and high exposure to psychosocial stressors as 1 SD above the mean. The y-axis is scaled to 15 points.
Next, we examined interactions between RSA and psychosocial stressors in predicting both youth-reported and parent-reported externalizing problems. None of these interactions were significant.
Gender Differences
We found evidence for a gender difference in the moderating role of RSA on the relationship between psychosocial stress exposure and some forms of internalizing symptomatology. In all cases, RSA moderated the association between stressors and internalizing symptoms for male but not for female participants.
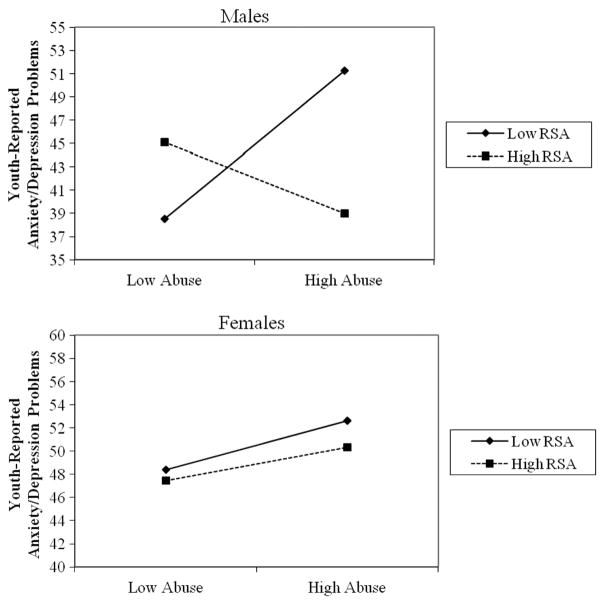
The three-way interaction between gender, RSA, and psychosocial stress exposure was significant in predicting anxiety/depression problems in three of the four models, including for child abuse (β =0.42, p < .001), community violence (β =0.26, p =.017), and other traumatic events (β =0.21, p =.027). The interaction between RSA and stress exposure in predicting anxiety/depression was significant for child abuse (β = −0.52, p < .001), community violence exposure (β = −0.46, p < .001), and traumatic events (β =−0.39, p < .001), for male participants but was not significant for any domain of stressor for female participants (see Figure 3). Among female participants, higher stress exposure was associated with higher anxiety/depression problems, regardless of the level of RSA.
FIGURE 3.
Gender differences in the interaction between respiratory sinus arrhythmia (RSA) and child abuse in predicting youth-reported anxiety/ depression. Note. Figures depict low RSA and child abuse as 1 SD below the mean and high RSA and high child abuse as 1 SD above the mean. The y-axis is scaled to 20 points.
No interactions with gender were observed in predicting problems with depression/withdrawal.
DISCUSSION
Exposure to psychosocial stressors is a potent risk factor for the onset of mental disorders in children and adolescents (Cohen et al., 2001; McLaughlin et al., 2012; Rudolph & Hammen, 1999). Identifying those youths at high risk of experiencing psychopathology following stress exposure is important in order to effectively target preventive interventions and to provide clinicians with greater ability to predict which youths are most likely to experience negative mental health consequences after exposure to stress and adversity. RSA is an inexpensive and easy to acquire marker of vagal tone that assesses parasympathetic nervous system control over heart rate (Beauchaine, 2001; Berntson et al., 1993b; Porges, 1995a, 2007). In the current report, we provide novel evidence indicating that low vagal tone is a potentially useful clinical marker of adolescent stress sensitivity associated with internalizing psychopathology following a wide range of psychosocial stressors. Our findings suggest that the association between psychosocial stressors and internalizing, but not externalizing, problems is moderated by vagal tone, such that stressors are associated strongly with internalizing psychopathology among adolescents with low vagal tone but not among those with high vagal tone. This interaction is present across multiple domains of stressors occurring in family, community, and peer contexts and predicts symptoms of anxiety and depression. Gender differences are present in the extent to which vagal tone moderates the relationship between stressors and internalizing psychopathology, such that low vagal tone is associated with heightened stress-related vulnerability particularly among male individuals. Together, these findings suggest that incorporating measures of vagal tone into clinical assessments might provide useful information for clinicians regarding stress sensitivity in adolescent clients.
The interaction between vagal tone and psychosocial stressors indicates that adolescents with low vagal tone are particularly likely to exhibit internalizing problems following exposure to stressors and that high vagal tone may serve as a buffer against the negative mental health consequences of multiple types of stressors. Indeed, the well-established relationship between adverse environmental events and internalizing psychopathology was only significant among adolescents with low vagal tone, particularly among male adolescents. These findings are consistent with several previous reports documenting a protective role of high resting vagal tone among children living in families with high levels of marital conflict (El-Sheikh et al., 2001; El-Sheikh et al., 2009; Katz & Gottman, 1995). These studies find that children with high vagal tone are less likely to develop internalizing problems, externalizing problems, and poor physical health outcomes in the context of high marital conflict than children with low vagal tone. We extend these previous findings by documenting that vagal tone moderates the relationship between multiple domains of stressors and internalizing psychopathology during adolescence, a developmental period in which associations of vagal tone, stressors, and psychopathology has not previously been examined. This pattern suggests that low vagal tone may represent a diathesis to psychopathology that is activated following exposure to stressors. This relationship was observed for child abuse and peer victimization in predicting anxiety/depression and for community violence exposure and other traumatic events in predicting both anxiety/depression and depression/withdrawal problems.
Why might high vagal tone protect against the development of internalizing problems following exposure to psychosocial stressors? The parasympathetic nervous system returns the body to homeostasis following stressors and promotes physiological recovery through antagonistic influences on sympathetic arousal (Beauchaine, 2001; Porges, 1992, 1995a, 2007). Speeded physiological recovery following psychosocial stressors is one potential mechanism through which high vagal tone might buffer youths from stress-related internalizing psychopathology. In a previous report, we found that adolescents with high vagal tone exhibited greater heart rate deceleration and sympathetic nervous system recovery in the immediate aftermath of a laboratory-based social-evaluative stressor (McLaughlin, Alves, & Sheridan, 2013), suggesting that high vagal tone is associated with improved physiological recovery following stress. A similar pattern of faster heart rate adaptation to psychosocial stress (Lane et al., 1992) and following trauma reminders among those with PTSD (Sack, Hopper, & Lamprecht, 2004) has been reported among adults with high vagal tone. In addition, vagal tone is associated with a variety of dispositional characteristics that may influence emotional recovery following exposure to stressors. In children, high resting vagal tone is associated with positive emotionality, social competence, use of adaptive emotion regulation skills, and good performance on tasks of attentional regulation (Eisenberg et al., 1995; Fabes, Eisenberg, & Eisenbud, 1993; Fabes, Eisenberg, Karbon, Troyer, & Switzer, 1994; Suess et al., 1994). Youths with high vagal tone may therefore be better able to adaptively modulate and recover from negative emotions that are elicited by experiences of psychosocial stress. Future research is needed to delineate the precise mechanisms through which high vagal tone might buffer children and adolescents from the adverse mental health consequences of exposure to psychosocial stressors.
The interaction between vagal tone and stress exposure in predicting internalizing psychopathology for was stronger for male than for female adolescents in predicting anxiety/depression problems. Female adolescents were more likely to exhibit these types of internalizing problems than males, and the association between psychosocial stressors and internalizing psychopathology was strong and positive regardless of vagal tone. In contrast, male adolescents exhibited a consistent pattern of interaction between vagal tone and psychosocial stress in predicting psychopathology such that the positive relationship between psychosocial stress exposure and internalizing problems was observed only for male adolescents with low vagal tone. Gender differences in the association of vagal reactivity with developmental outcomes in children have been observed previously, although the pattern of findings is inconsistent across studies. One study observed that high vagal reactivity—an index of flexible vagal responding—was associated with better academic outcomes in boys but not girls (Obradovic, Bush, Stamperdahl, Adler, & Boyce, 2010) whereas another found that low vagal reactivity predicted internalizing problems for boys but not girls (El-Sheikh & Whitson, 2006). Mechanisms underlying these gender differences are unknown. However, one possibility is that the moderating influence of vagal tone on the association between stressors and psychopathology varies by gender due to differences in sympathetic nervous system function. Beauchaine (2001) argued that vagal tone is a marker of emotional stability and regulation, whereas sympathetic nervous system function is a marker of behavioral inhibition and activation, and that risk for psychopathology is dependent on patterns of activation across both of these systems. Gender differences in sympathetic nervous system function may moderate the degree to which high vagal tone provides a buffer against psychopathology in the context of environmental stressors. Future research is needed to evaluate this possibility empirically. Regardless of the underlying mechanisms, it is possible that the gender differences observed in this study play some role in contributing to the elevated prevalence of internalizing psychopathology among female adolescents as compared to male that emerges during adolescence (Hankin et al., 1998a; Nolen-Hoeksema & Twenge, 2002). Whereas male adolescents with low vagal tone experienced elevated internalizing psychopathology specifically in the context of exposure to psychosocial stressors, female adolescents exposed to high levels of stressors had elevated internalizing psychopathology regardless of their level of vagal tone. This is consistent with evidence suggesting that female individuals experience greater mental health consequences following psychosocial stressors, particularly maltreatment, than males, which may contribute to gender differences in adolescent internalizing psychopathology (Cutler & Nolen-Hoeksema, 1991; Hankin & Abramson, 2001; MacMillan et al., 2001).
Vagal tone has the potential to be a clinically useful marker of stress sensitivity and risk of internalizing psychopathology following stressors. Identifying children and adolescents who are most likely to develop internalizing problems after exposure to psychosocial stressors would allow clinicians to adjust intervention intensity accordingly for clients who have recently experienced stressors. Measures of vagal tone are relatively simple and inexpensive to acquire using equipment that may already by available at most medical centers, clinics, or university health centers, increasing the feasibility of using vagal tone to inform clinical practice. An ECG is the only piece of equipment needed to acquire information on heart rate variability. Free software that can be used to transform the ECG raw signal into an interbeat interval time series and generate metrics of heart rate variability, including RSA, is available from multiple sources (Allen et al., 2007; Niskanen, Tarvainen, Rantaaho, & Karjalainen, 2004; Rodriguez-Linares, Vila, Mendez, Lado, & Olivieri, 2008). Of importance, vagal tone can be measured either while children and adolescents sit quietly before or after a session, as was done in the current study, or during sessions involving challenging clinical material, including exposure sessions, role plays, or discussion of emotionally evocative topics. Vagal reactivity to laboratory-based manipulations has also been linked to stress-related vulnerability to psychopathology (El-Sheikh & Whitson, 2006; Leary & Katz, 2004; Obradovic, Bush, & Boyce, 2011), and it is possible that measuring vagal reactivity to challenging portions of therapeutic interventions could also provide clinically useful information. Incorporating psychophysiological markers of stress vulnerability into clinical practice also provides advantages over standard methods based on clinical interviews and self-report measures. The use of psychophysiological markers has the benefit of reducing clinician reliance on youth-reported and parent-reported information about dispositional characteristics underlying vulnerability to psychopathology following stress exposure for which self-awareness may be poor and reporting biases are prominent, such as emotionality, emotion regulation skills, and responses to stress (Robinson & Clore, 2002; Thomas & Deiner, 1990). Psychophysiological measurements are free from these sorts of biases. In clinical settings were an ECG is available, collection of resting heart period data could be incorporated relatively easily into a standard intake battery to supplement self-reported information.
In addition to providing information about stress sensitivity, measures of vagal tone also might be a useful target of intervention. It is possible that interventions that increase vagal tone would have positive influences on stress sensitivity and vulnerability to internalizing psychopathology among youths exposed to trauma or experiencing high degrees of social adversity. Although individual differences in vagal tone are evident by early infancy and are stable over periods of several months to a year (Fracasso, Porges, Lamb, & Rosenberb, 1994; Huffman et al., 1998; Stifter et al., 1989), vagal tone appears to be malleable. Relaxation training and mindfulness have both shown promise in increasing vagal tone in small studies of adults (Ditto, Eclache, & Goldman, 2006; Sarang & Telles, 2006; Wu & Lo, 2008). Determining whether these intervention strategies lead to improvements in vagal tone among children and adolescents is an important goal for future research. Because of the ease with which it is acquired, vagal tone is also a promising process measure of improvement during the course of clinical intervention. Heart period data can feasibly be collected at multiple time points across the course of treatment so that vagal tone can be examined as a potential mechanism of intervention effects.
Notable strengths of the study include assessment of multiple domains of psychosocial stressors known to be both common and strongly associated with adolescent psychopathology, recruitment of a racially and ethnically diverse community sample of participants, and our focus on adolescence, a developmental period in which the interaction of vagal tone and psychosocial stressors has not previously been examined. However, study findings should be interpreted in light of several limitations. The most notable limitation is the cross-sectional nature of our data, which does not allow us to establish the temporal ordering of exposure to psychosocial stressors and psychopathology. As a result, it is possible that internalizing psychopathology in some youths predated or even contributed to the occurrence of stressors. Children and adolescents with internalizing disorders have been shown to engage in a variety of interpersonal behaviors that generate stressors in their lives (Rudolph & Hammen, 1999; Rudolph et al., 2000), and these behaviors result in a bidirectional association between stress exposure and internalizing psychopathology. Although the pattern observed here is consistent with prior longitudinal studies that focused explicitly on marital conflict (El-Sheikh & Whitson, 2006), replication of our findings in prospective studies is necessary. Second, our analyses examined the interaction between vagal tone and psychosocial stress exposure in predicting youth-reported but not parent-reported psychopathology. We elected to focus exclusively on youth-reported internalizing psychopathology because parent-reported information about adolescent internalizing problem is only weakly related to information provided by adolescents themselves, and adolescents are considered to be more valid reporters of internalizing problems than their parents (Achenbach, McConaughy, & Howell, 1987; Cantwell, Lewinsohn, Rohde, & Seeley, 1997). Finally, our assessment of psychosocial stress exposure was limited by reliance on self-report questionnaires associated with reporting biases (Raphael, Cloitre, & Dohrenwend, 1991) as opposed to a stressor interview, which captures more objective indices of stressors, their timing, and the level of threat associated with these stressors (Hammen, 2008). Replication of these findings in studies utilizing interview-based methods for assessing stressors is therefore warranted.
Vagal tone moderates the associations of a wide range of psychosocial stressors with internalizing psychopathology in adolescents, particularly for male adolescents, such that stress exposure is associated more strongly with symptoms of anxiety and depression in adolescents with low vagal tone. Exposure to psychosocial stressors is unassociated with internalizing problems in adolescents with high vagal tone. Incorporating measures of vagal tone into clinical practice might provide clinicians with useful information regarding stress sensitivity in child and adolescent clients. Moreover, interventions targeted at improving vagal tone in children and adolescents at high risk of exposure to psychosocial stressors might provide protection against the negative mental health consequences of these experiences.
Footnotes
Biopac and Mindware software, which are not free software packages, were used to acquire and score the heart period data in the current study. However, multiple free software packages are available for analyzing heart period data, which we describe in greater detail in the Discussion section.
Contributor Information
Katie A. McLaughlin, Department of Psychology, University of Washington
Leslie Rith-Najarian, Department of Psychology, University of California at Los Angeles.
Melanie A. Dirks, Department of Psychology, McGill University
Margaret A. Sheridan, Developmental Medicine Center, Boston Children’s Hospital, Harvard Medical School
References
- Achenbach TM. Integrative guide for the 1991 CBCL/4-18, YSR and TRF Profiles. Burlington: Department of Psychiatry, University of Vermont; 1991. [Google Scholar]
- Achenbach TM, Dumenci L, Rescorla L. DSM-oriented and empirically based approaches to constructing scales from the same item pool. Journal of Clinical Child and Adolescent Psychology. 2003;32:328–340. doi: 10.1207/S15374424JCCP3203_02. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, McConaughy SH, Howell CT. Child/adolescent behavioral and emotional problems: Implications of cross informant correlations for situational specificity. Psychological Bulletin. 1987;101:213–232. [PubMed] [Google Scholar]
- Achenbach TM, Rescorla L. The manual for the ASEBA school-age forms and profiles. Burlington: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Allen JB, Chambers AS, Towers DN. The many metrics of cardiac chronotropy: A pragmatic primer and a brief comparison of metrics. Biological Psychology. 2007;74:243–262. doi: 10.1016/j.biopsycho.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Review of General Psychology. 2006;10:229–240. [Google Scholar]
- Beauchaine TP. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Hondelsman L, Foote J, Lovejoy M. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse and Neglect. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik, van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Cardiac psychophysiology and autonomic space in humans: Empirical perspectives and conceptual implications. Psychological Bulletin. 1993a;114:296–322. doi: 10.1037/0033-2909.114.2.296. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993b;30:183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Bird HR, Gould MS, Staghezza B. Aggregating data from multiple informants in child psychiatry epidemiological research. Journal of the American Academy of Child & Adolescent Psychiatry. 1992;31:78–85. doi: 10.1097/00004583-199201000-00012. [DOI] [PubMed] [Google Scholar]
- Blandon AY, Calkins SD, Keane SP, O’Brien M. Individual differences in trajectories of emotion regulation processes: The effects of maternal depressive symptomatology and children’s physiological regulation. Developmental Psychology. 2008;44:1110–1123. doi: 10.1037/0012-1649.44.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, Quas JA, Abbey A, Smider NA, Essex MJ, Kupfer DJ. Autonomic reactivity and psychopathology in middle childhood. British Journal of Psychiatry. 2001;179:144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U. Ecology of the family as a context for human development: Research perspectives. Developmental Psychology. 1986;22:723–742. [Google Scholar]
- Cacioppo JT, Berntson GG, Binkley PF, Quigley KS, Uchino BN, Fieldstone A. Autonomic cardiac control. II. Noninvasive indices and basal response as revealed by autonomic blockades. Psychophysiology. 1994;31:586–598. doi: 10.1111/j.1469-8986.1994.tb02351.x. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Keane SP. Cardiac vagal regulation across the preschool period: Stability, continuity, and implications for childhood adjustment. Developmental Psychobiology. 2004;45:101–112. doi: 10.1002/dev.20020. [DOI] [PubMed] [Google Scholar]
- Cantwell D, Lewinsohn PM, Rohde P, Seeley JR. Correspondance between adolescent report and parent report of psychiatric diagnostic data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:610–619. doi: 10.1097/00004583-199705000-00011. [DOI] [PubMed] [Google Scholar]
- Chen W, Faraone SV, Biederman J, Tsuang MT. Diagnostic accuracy of the Children Behavior Checklist scales for attention-deficit hyperactivity disorder. Journal of Consulting and Clinical Psychology. 1994;62:1017–1025. doi: 10.1037/0022-006X.62.5.1017. [DOI] [PubMed] [Google Scholar]
- Cohen P, Brown J, Smailes E. Child abuse and neglect and the development of mental disorders in the general population. Development and Psychopathology. 2001;13:981–999. [PubMed] [Google Scholar]
- Coie JD, Lochman JE, Terry R, Hyman C. Predicting early adolescent disorder from childhood aggression and peer rejection. Journal of Consulting and Clinical Psychology. 1992;60:783–792. doi: 10.1037//0022-006x.60.5.783. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Keeler G, Angold A, Costello EJ. Traumatic events and posttraumatic stress in childhood. Archives of General Psychiatry. 2007;64:577–584. doi: 10.1001/archpsyc.64.5.577. [DOI] [PubMed] [Google Scholar]
- Cutler SE, Nolen-Hoeksema S. Accounting for sex differences in depression through female victimization: Childhood sexual abuse. Sex Roles. 1991;24:425–438. [Google Scholar]
- Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression: A theoretical model. Archives of General Psychiatry. 2000;57:21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- Ditto B, Eclache M, Goldman N. Short-term autonomic and cardiovascular effects of mindfulness body scan meditation. Annals of Behavioral Medicine. 2006;32:277–234. doi: 10.1207/s15324796abm3203_9. [DOI] [PubMed] [Google Scholar]
- Ebesutani C, Bernstein A, Nakamura BJ, Chorpita BF, Higa-McMillan CK, Weisz JR. Concurrent validity of the Child Behavior Checklist DSM-oriented scales: Correspondance with DSM diagnoses and comparison to syndreom scales. Journal of Psychopathology and Behavioral Assessment. 2010;32:373–384. doi: 10.1007/s10862-009-9174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Murphy BC, Maszk P, Smith M, Karbon M. The role of emotionality and regulation in children’s social functioning: A longitudinal study. Child Development. 1995;66:1360–1384. [PubMed] [Google Scholar]
- El-Sheikh M, Harger J, Whitson SM. Exposure to inter-parental conflict and children’s adjustment and physical health: The moderating role of vagal tone. Child Development. 2001;72:1617–1636. doi: 10.1111/1467-8624.00369. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Kouros CD, Erath S, Cummings EM, Keller P, Staton L. Marital conflict and children’s externalizing behavior: Interactions between parasympathetic and sympathetic nervous system activity. Monographs for the Society for Research in Child Development. 2009;74:1–79. doi: 10.1111/j.1540-5834.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Whitson SA. Longitudinal relations between marital conflict and child adjustment: Vagal regulation as a protective factor. Journal of Family Psychology. 2006;20:30–39. doi: 10.1037/0893-3200.20.1.30. [DOI] [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N, Eisenbud L. Behavioral and physiological correlates of children’s reactions to others in distress. Developmental Psychology. 1993;29:655–663. [Google Scholar]
- Fabes RA, Eisenberg N, Karbon M, Troyer D, Switzer G. The relations of children’s emotion regulation to their vicarious emotional responses and comforting behaviors. Child Development. 1994;65:1678–1693. doi: 10.1111/j.1467-8624.1994.tb00842.x. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Fox NA, Cohn JF, Galles SF, Kovacs M. Children’s affect regulation during a disappointment: Psychophysiological responses and relation to parent history of depression. Biological Psychology. 2006;71:264–277. doi: 10.1016/j.biopsycho.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Fracasso MP, Porges SW, Lamb ME, Rosenberb AA. Cardiac activity in infancy: Reliability and stability of individual differences. Infant Behavior and Development. 1994;17:277–284. [Google Scholar]
- Friedman BH, Thayer JF. Autonomic balance revisited: Panic anxiety and heart rate variability. Journal of Psychosomatic Research. 1998;44:133–151. doi: 10.1016/s0022-3999(97)00202-x. [DOI] [PubMed] [Google Scholar]
- Grills AE, Ollendick T. Issues in parent–child agreement: The case of structured diagnostic interviews. Clinical Child and Family Psychology Review. 2002;5:57–83. doi: 10.1023/a:1014573708569. [DOI] [PubMed] [Google Scholar]
- Grossman P, Karemaker J, Wieling W. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: The need for respiratory control. Psychophysiology. 1991;28:201–216. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Stemmler G, Meinhardt E. Paced respiratory sinus arrhythmia as an index of cardiac parasympathetic tone during varying behavioral tasks. Psychophysiology. 1990;27:404–416. doi: 10.1111/j.1469-8986.1990.tb02335.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology. 2007;74:263–285. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Development and Psychopathology. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Stress exposure and stress generation in adolescent depression. In: Nolen-Hoeksema S, Hilt LM, editors. Handbook of depression in adolescents. New York, NY: Routledge; 2008. pp. 305–344. [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: An elaborated cognitive vulnerability-transactional stress model. Psychological Bulletin. 2001;127:773–796. doi: 10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998a;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998b;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Mermelstein R, Roesch L. Sex differences in adolescent depression: Stress exposure and reactivity models. Child Development. 2007;78:279–295. doi: 10.1111/j.1467-8624.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- Hastings TL, Kelley ML. Development and validation of the Screen for Adolescent Violence Exposure (SAVE) Journal of Abnormal Child Psychology. 1997;25:511–520. doi: 10.1023/a:1022641916705. [DOI] [PubMed] [Google Scholar]
- Hawker DSJ, Boulton MJ. Twenty years’ research on peer victimization and psychosocial maladjustment: A meta-analytic review of cross-sectional studies. Journal of Child Psychology and Psychiatry. 2000;41:441–455. [PubMed] [Google Scholar]
- Hinnant JB, El-Sheikh M. Children’s externalizing and internalizing symptoms over time: The role of individual differences in patterns of RSA responding. Journal of Abnormal Child Psychology. 2009;37:1049–1061. doi: 10.1007/s10802-009-9341-1. [DOI] [PubMed] [Google Scholar]
- Huffman LC, Bryan YE, del Carmen R, Pedersen FA, Doussard-Roosevelt JA, Porges SW. Infant temperament and cardiac vagal tone: Assessments at twelve weeks of age. Child Development. 1998;69:624–635. [PubMed] [Google Scholar]
- Katz LF, Gottman JM. Vagal tone protects children from marital conflict. Development and Psychopathology. 1995;7:83–92. [Google Scholar]
- Katz LF, Gottman JM. Buffering children from marital conflict and dissolution. Journal of Clinical Child Psychology. 1997;26:157–171. doi: 10.1207/s15374424jccp2602_4. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Puliafico AC, Barmish AJ, Choudhury MS, Henin A, Treadwell KS. Assessing anxiety with the child behavior checklist and the teacher report form. Journal of Anxiety Disorders. 2007;21:1004–1015. doi: 10.1016/j.janxdis.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Üstun TB. The World Mental Health (WHM) survey initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) International Journal of Methods in Psychiatric Research. 2004;13:93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollai M, Mizsei G. Respiratory sinus arrhythmia is a limited measure of cardiac parasympathetic control in man. Journal of Physiology. 1990;424:329–342. doi: 10.1113/jphysiol.1990.sp018070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane JD, Adcock RA, Burnett RE. Respiratory sinus arrhythmia and cardiovascular responses to stress. Psychophysiology. 1992;29:461–470. doi: 10.1111/j.1469-8986.1992.tb01720.x. [DOI] [PubMed] [Google Scholar]
- Larson R, Ham M. Stress and “storm and stress” in early adolescence: The relationship of negative events with dysphoric affect. Developmental Psychology. 1993;29:130–140. [Google Scholar]
- Larson R, Moneta G, Richards MH, Wilson S. Continuity, stability, and change in daily emotional experience across adolescence. Child Development. 2002;73:1151–1165. doi: 10.1111/1467-8624.00464. [DOI] [PubMed] [Google Scholar]
- Leary A, Katz LF. Coparenting, family-level processes, and peer outcomes: The moderating role of vagal tone. Development and Psychopathology. 1997;16:593–608. doi: 10.1017/s0954579404004687. [DOI] [PubMed] [Google Scholar]
- Leary A, Katz LF. Coparenting, family-level processes, and peer outcomes: The moderating role of vagal tone. Development and Psychopathology. 2004;16:593–608. doi: 10.1017/s0954579404004687. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Striegel-Moore RH, Seeley JR. Epidemiology and natural course of eating disorders in young women from adolescence to young adulthood. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:1284–1292. doi: 10.1097/00004583-200010000-00016. [DOI] [PubMed] [Google Scholar]
- Lucini D, Di Fede G, Parati G, Pagani M. Impact of chronic psychosocial stress on autonomic cardiovascular regulation in otherwise healthy subjects. Hypertension. 2005;46:1201–1206. doi: 10.1161/01.HYP.0000185147.32385.4b. [DOI] [PubMed] [Google Scholar]
- Lucini D, Norbiato G, Clerici M, Pagani M. Hemodynamic and autonomic adjustments to real life stress conditions in humans. Hypertension. 2002;39:184–188. doi: 10.1161/hy0102.100784. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Fleming JE, Streiner DL, Lin E, Boyle MH, Jamieson E, Beardslee W. Childhood abuse and lifetime psychopathology in a community sample. American Journal of Psychiatry. 2001;158:1878–1883. doi: 10.1176/appi.ajp.158.11.1878. [DOI] [PubMed] [Google Scholar]
- Margolin G, Gordis EB. The effects of family and community violence on children. Annual Review of Psychology. 2000;51:445–479. doi: 10.1146/annurev.psych.51.1.445. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Alves S, Sheridan MA. Vagal Regulation and Internalizing Psychopathology among Adolescents Exposed to Childhood Adversity. 2013. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky A, Kessler RC. Childhood adversities and first onset of psychiatric disorders in a national sample of adolescents. Archives of General Psychiatry. 2012;69:1151–1160. doi: 10.1001/archgenpsychiatry.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Koenen KC, Hill E, Petukhova M, Sampson NA, Zaslavsky A, Kessler RC. Trauma exposure and posttraumatic stress disorder in a US national sample of adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52:815–830. doi: 10.1016/j.jaac.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzacappa E, Kindlon D, Saul JP, Earls F. Executive and motivational control of performance task behavior, and autonomic heart-rate regulation in children: Physiologic validation of two-factor solution inhibitory control. Journal of Child Psychology and Psychiatry. 1998;39:525–531. [PubMed] [Google Scholar]
- Mezzacappa E, Tremblay RW, Kindlon D, Saul JP, Arseneault L, Seguin J, Earls F. Anxiety, antisocial behavior, and heart rate regulation in adolescent males. Journal of Child Psychology and Psychiatry. 1997;38:457–469. doi: 10.1111/j.1469-7610.1997.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Niskanen JP, Tarvainen MP, Rantaaho PO, Karjalainen PA. Sofware for advanced HRV analysis. Computer Methods and Programs in Biomedicine. 2004;76:73–81. doi: 10.1016/j.cmpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Twenge JM. Age, gender, race, socioeconomic status, and birth cohort difference on the children’s depression inventory: A meta-analysis. Journal of Abnormal Psychology. 2002;111:578–588. doi: 10.1037//0021-843x.111.4.578. [DOI] [PubMed] [Google Scholar]
- Obradovic J, Bush NR, Boyce WT. The interactive effect of marital conflict and stress reactivity on externalizing and internalizing symptoms: The role of laboratory stressors. Development and Psychopathology. 2011;23:101–114. doi: 10.1017/S0954579410000672. [DOI] [PubMed] [Google Scholar]
- Obradovic J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development. 2010;81:270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel AJ, Bouma EMC. Sensitivity to the depressogenic effect of stress and HPA-axis reactivity adolescence: A review of gender differences. Neuroscience and Biobehavioral Reviews. 2011;35:1757–1770. doi: 10.1016/j.neubiorev.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Pine DS, Wasserman MS, Miller L, Coplan JD, Bagiella E, Kovelenku P, Sloan RP. Heart period variability and psychopathology in urban boys at risk for delinquency. Psychophysiology. 1998;35:521–529. doi: 10.1017/s0048577298970846. [DOI] [PubMed] [Google Scholar]
- Porges SW. Vagal tone: A physiologic marker of stress vulnerability. Pediatrics. 1992;90:498–504. [PubMed] [Google Scholar]
- Porges SW. Cardiac vagal tone: A physiological index of stress. Neuroscience and Biobehavioral Reviews. 1995a;19:225–233. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology. 1995b;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, Boergers J, Vernberg EM. Overt and relational aggression in adolescents: Social-psychological adjustment of aggressors and victims. Journal of Clinical Child Psychology. 2001;30:479–491. doi: 10.1207/S15374424JCCP3004_05. [DOI] [PubMed] [Google Scholar]
- Raphael B, Cloitre M, Dohrenwend BP. Problems of recall and misclassification with checklist methods of measuring stressful life events. Health Psychology. 1991;10:62–74. doi: 10.1037//0278-6133.10.1.62. [DOI] [PubMed] [Google Scholar]
- Robinson MD, Clore GL. Belief and feeling: Evidence for an accessibility model of emotional self-report. Psychological Bulletin. 2002;128:934–960. doi: 10.1037/0033-2909.128.6.934. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Linares L, Vila X, Mendez A, Lado M, Olivieri D. RHRV: An R-based software package for heart rate variability analysis of ECG recordings. Paper presented at the 3rd Iberian Conference in Systems and Information Technologies (CISTI 2008); Vigo, Spain. 2008. Jun, [Google Scholar]
- Rottenberg J. Cardiac vagal control in depression: A critical anaysis. Biological Psychology. 2007;74:200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: A transactional perspective. Child Development. 1999;70:660–677. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C, Burge D, Lindberg N, Herzberg DS, Daley SE. Toward an interpersonal life-stress model of depression: The developmental context of stress generation. Development and Psychopathology. 2000;12:215–234. doi: 10.1017/s0954579400002066. [DOI] [PubMed] [Google Scholar]
- Sack M, Hopper JW, Lamprecht F. Low respiratory sinus arrhythmia and prolonged psychophysiological arousal in posttraumatic stress disorder: Heart rate dynamics and individual differences in arousal regulation. Biological Psychiatry. 2004;55:284–290. doi: 10.1016/s0006-3223(03)00677-2. [DOI] [PubMed] [Google Scholar]
- Sarang P, Telles S. Effects of two yoga based relaxation techniques on heart rate variability (HRV) International Journal of Stress Management. 2006;13:460–475. [Google Scholar]
- Seligman L, Ollendick T, Langley AK, Baldacci B. The utility of measures of child and adolescent anxiety: A meta-analytic review of the Revised Children’s Manifest Anxiety Scale, the State-Trait Anxiety Inventory for Children, and the Child Behavior Checklist. Journal of Clinical Child and Adolescent Psychology. 2004;33:557–565. doi: 10.1207/s15374424jccp3303_13. [DOI] [PubMed] [Google Scholar]
- Shannon KE, Beauchaine TP, Brenner SL, Neuhaus E, Gatzke-Kopp L. Familial and temperamental predictors of resilience in children at risk for conduct disorder and depression. Development and Psychopathology. 2007;19:701–727. doi: 10.1017/S0954579407000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Dooren LJP. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Sloan RP, Shapiro PA, Bagiella E, Boni SM, Paik M, Bigger JT, Jr, JM Effect of mental stress throughout the day on cardiac autonomic control. Biological Psychology. 1994;37:89–99. doi: 10.1016/0301-0511(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Stifter CA, Fox NA. Infant reactivity: Physiological correlates of newborn and 5-month temperament. Developmental Psychology. 1990;26:582–588. [Google Scholar]
- Stifter CA, Fox NA, Porges SW. Facial expressivity and vagal tone in 5- and 10-month-old infants. Infant Behavior and Development. 1989;12:127–137. [Google Scholar]
- Storch E, Masia-Warner C, Crisp H, Klein RG. Peer victimization and social anxiety in adolescence: A prospective study. Aggressive Behavior. 2005;31:437–452. [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Williamson D, Dahl R. Sex differences in the effects of pubertal development on responses to corticotrop in releasing hormone challenge. Annal of the New York Academy of Sciences. 2004;1021:348–351. doi: 10.1196/annals.1308.043. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: Social rejection versus achievement stress. Biological Psychiatry. 2002;52:318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Suess PE, Porges SW, Blude DL. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology. 1994;31:17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biological Psychiatry. 1996;39:255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thomas DL, Deiner E. Memory accuracy in the recall of emotions. Journal of Personality and Social Psychology. 1990;59:291–297. [Google Scholar]
- Twenge JM, Nolen-Hoeksema S. Age, gender, race, socioeconomic status, and birth cohort differences on the children’s depression inventory: A meta-analysis. Journal of Abnormal Psychology. 2002;111:578–588. doi: 10.1037//0021-843x.111.4.578. [DOI] [PubMed] [Google Scholar]
- Vernberg EM, Abwender DA, Ewell KK, Beery SH. Social anxiety and peer relationships in early adolescence: A prospective analysis. Journal of Clinical Child Psychology. 1992;21:189–196. [Google Scholar]
- Vernberg EM, Fonagy P, Twemlow S. Preliminary report of the topeka peaceful schools project. Topeka, KS: Menninger Clinic; 2000. [Google Scholar]
- Vernberg EM, Jacobs AK, Hershberger SL. Peer victimization and attitudes about violence during early adolescence. Journal of Clinical Child Psychology. 1999;28:386–395. doi: 10.1207/S15374424jccp280311. [DOI] [PubMed] [Google Scholar]
- Whitson SA, El-Sheikh M. Marital conflict and health: Processes and protective factors. Aggression and Violent Behavior. 2003;8:283–312. [Google Scholar]
- Wu S, Lo P. Inward-attention meditation increases parasympathetic activity: A study based on heart rate variability. Biomedical Research. 2008;29:245–250. doi: 10.2220/biomedres.29.245. [DOI] [PubMed] [Google Scholar]