Abstract
Introduction
Aggressive behavior can be a dangerous complication of schizophrenia. Hostility is related to aggression. This study aimed to compare the effects of olanzapine, perphenazine, risperidone, quetiapine, and ziprasidone on hostility in schizophrenia.
Methods
We used the data that were acquired in the 18-month Phase 1 of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study. We analyzed the scores of the Positive and Negative Syndrome Scale (PANSS) hostility item in a subset of 614 patients who showed at least minimal hostility (a score ≥ 2) at baseline.
Results
The primary analysis of hostility indicated an effect of difference between treatments (F4,1487 = 7.78, P<0.0001). Olanzapine was significantly superior to perphenazine and quetiapine at months 1, 3, 6, and 9. It was also significantly superior to ziprasidone at months 1, 3, and 6, and to risperidone at months 3 and 6.
Discussion
Our results are consistent with those of a similar post-hoc analysis of hostility in first-episode subjects with schizophrenia enrolled in the European First-Episode Schizophrenia Trial (EUFEST) trial, where olanzapine demonstrated advantages compared with haloperidol, quetiapine, and amisulpride.
Conclusion
Olanzapine demonstrated advantages in terms of a specific antihostility effect over the other antipsychotics tested in Phase 1 of the CATIE trial.
Keywords: schizophrenia, hostility, aggression, antipsychotics
Introduction
Aggressive behavior can be a dangerous complication of schizophrenia. Aggression is overt action intended to harm. This term describes animal and human behavior. The term aggression tends to be used in biomedical and psychological context. Aggressive behavior has been classified into 2 subtypes: impulsive or premeditated. Impulsive aggression is a hair-trigger aggressive response to provocation with loss of behavioral control. Premeditated aggression is a planned aggressive act that is neither spontaneous, nor committed in a an agitated state.
Violence denotes aggression among humans. The term is more commonly used in sociology and criminology (eg, violent crime). The terms violence and aggression are used interchangeably, depending on context.
Hostility denotes unfriendly attitudes. Overt irritability, anger, resentment, or aggression are behavioral manifestations of hostility. Hostility is defined operationally by rating scales. The clinical importance of hostility is in its close association with violence and nonadherence to treatment. A detailed discussion of the definitions of aggression, violence, and hostility can be found elsewhere.1
The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study2,3 found that at baseline, 18% of subjects had engaged in violent behavior in the previous 6 months; of these, 4% had committed serious acts of violence involving weapons or causing injury to another person.4 A Swedish study found that among 8003 schizophrenia patients, 1054 (13.2%) were convicted at least once for violent crime, compared with 5.3% of general population controls.5
Hostility, as defined by the Positive and Negative Syndrome Scale (PANSS)6 item, may include overt aggressive behavior among its manifestations. In the CATIE study, for each unit of increase on the rating of PANSS Hostility at baseline, the odds of serious violence during the preceding 6 months increased by a factor of 1.65 (P<.001).4 A recent meta-analysis of risk factors for violence in individuals diagnosed with psychosis has estimated that hostility during the study period as well as higher hostility scores significantly elevate violence risk [respective odds ratios 2.8 (95% CI 1.8-4.2) and 1.5 (95% CI 1.0-2.1)].7
The PANSS Hostility item has been used as a proxy measure for aggression in psychopharmacological studies.1 Recently, the efficacy of antipsychotics against hostility was examined by post-hoc analyses of the European First-Episode Schizophrenia Trial (EUFEST).8 In that randomized, open trial, haloperidol, amisulpride, olanzapine, quetiapine, and ziprasidone were compared regarding their effects on hostility. The scores on the hostility item of the PANSS were analyzed in a subset of 302 patients who showed at least minimal hostility (a score > 2) at baseline. The results indicated significant differences between treatments. Olanzapine was significantly superior to haloperidol, quetiapine, and amisulpride in reducing hostility in the first 3 months of treatment.
Here we present analogous post-hoc analyses of the hostility item implemented in Phase 1 of the CATIE. We hypothesized that the medications would differ in their effects on hostility, and that olanzapine’s effects on hostility would be superior to those of the other antipsychotics.
Methods
Study population and interventions
Phase 1 of the multicenter CATIE study enrolled 1493 patients with schizophrenia who were recruited at 57 clinical sites in the United States (16 university clinics, 10 state mental health agencies, 7 Veterans Affairs medical centers, 6 private nonprofit agencies, 4 private-practice sites, and 14 mixed-system sites). The study used broad inclusion and minimal exclusion criteria and allowed the enrollment of patients with coexisting conditions and those who were taking other medications. These features of the study make the results widely applicable.
The participants were randomly assigned to receive olanzapine (olanzapine (7.5 to 30 mg per day), perphenazine (8 to 32 mg per day), quetiapine (200 to 800 mg per day), risperidone (1.5 to 6.0 mg per day), or ziprasidone (40 to 160 mg per day) for up to 18 months in a double-blind trial.3 The ziprasidone treatment arm was added 1 year after the study began, once ziprasidone was approved for use by the U.S. Food and Drug Administration. Patients with current tardive dyskinesia were not assigned to perphenazine.
The participants were followed for up to 18 months as outpatients. Psychopathology was assessed with the PANSS at baseline and at months 1, 3, 6, 9, 12, 15, and 18. Hostility is one of the PANSS items. The hostility item score range is 0-7. A Hostility item score of 1 means “no hostility,” whereas 2 is a rating of “minimal” hostility, with the criterion being “questionable pathology; the patient may be at the upper extreme of normal limits.”9 A rating of 3, “mild,” has the descriptor “the patient shows indirect or restrained communication of anger, such as sarcasm, disrespect, hostile expressions and occasional irritability.” Ratings of 4 (“moderate”) and 5 (“moderate severe”) also do not require the presence of physically assaultive behaviors. The highest ratings of 6 (“severe”) and 7 (“extreme”) are more likely related to aggressive behaviors that would be considered serious, with the respective criteria of “uncooperativeness and verbal abuse or threats notably influence the interview and seriously impact upon the patient’s social relations; the patient may be violent and destructive but is not physically assaultive toward others” and “marked anger by the patient results in extreme uncooperativeness, precluding other interactions, or in a physical assault episode directed toward others.” Hostility is one of the positive items on the PANSS scale. The other positive items are delusions, conceptual disorganization, hallucinatory behavior, excitement, grandiosity, and suspiciousness/persecution.
The time to the discontinuation of treatment for any cause was the primary outcome variable to assess effectiveness.3 The study was approved by the institutional review board at each site, and written informed consent was obtained from the patients or their legal guardians.
Statistical procedures
Similar to the analyses of the hostility change in the EUFEST study, all analyses were based on the subsample of the modified intent-to-treat population from the parent study who displayed a baseline hostility score of at least 2 (“minimal hostility”). This criterion was needed for the exclusion of patients who did not have sufficient initial severity of hostility (ie, were rated 1, meaning “no hostility”), and therefore had no room for improvement as a result of treatment.
Two statistical approaches were adopted to analyze all available data: (1) the random regression hierarchical linear modeling (HLM), which allows the use of observations with incomplete data; and (2) the traditional analysis of covariance (ANCOVA) analysis of change over time [endpoint, or last observation carried forward analysis (LOCF) for observed change at study endpoint for each subject].
Random regression hierarchical linear modeling (HLM), a longitudinal data-analytic approach that permits the use of observations with incomplete repeated measures data (eg, patients who discontinue before completing the study), was adopted as the primary statistical model for the study. In the HLM analysis, change in PANSS Hostility over time across study visits served as the dependent variable. The independent factors included “treatment group” and “time.” Regarding treatment group, the 5 different treatments were applied as between-subject factors. Time (in months) from baseline served as a within-subject, random-effect factor. Interactions between the 2 independent factors were also included in the model. An unstructured covariance matrix was specified in the analyses in order to account for the time-structured nature of the data (serial correlations across time among assessments of efficacy).
Gender, age, and change in the PANSS positive scale were used as covariates in the HLM analyses. The latter variable was applied as a time-varying covariate to ensure that group differences among treatment groups in change over time identified in the study were specific with respect to hostility (ie, were independent of change in severity of positive symptoms over time). Change in positive symptoms was defined as the sum of changes on the items of the PANSS positive scale, excluding hostility. Accordingly, the following items were included: delusions, conceptual disorganization, hallucinatory behavior, excitement, grandiosity, and suspiciousness/persecution.
The model effects were tested by the F-statistic. The estimation of the degrees of freedom was based on the Satterthwaite approximation.10 If a significant main effect or interaction involving treatment group and time was detected, post-hoc analyses were performed to examine the direction of changes (time effect) or the differences in change over time among the treatment groups (interaction effect). An a-level of 0.05 (2-sided) was adopted for all analyses of statistical significance. The Tukey-Kramer method was used for adjustment for Type I error inflation due to the multiple comparisons.
ANCOVA using the LOCF approach was used for sensitivity analyses. Furthermore, similar to the approach used previously in this data set,11 we implemented analyses using statistical models that included covariates for whether the participant was recruited before or after the introduction of ziprasidone, whether the participant had tardive dyskinesia at baseline, and for site effects.
Change from baseline at the study endpoint for each individual patient was applied as a dependent variable, whereas treatment group was used as the principal independent variable of interest in the ANCOVA model. Similar to the primary HLM analyses, gender, age, and change in positive symptoms were included as covariates in the ANCOVA analyses. If a significant overall effect of treatment group was detected, post-hoc analyses with the Tukey-Kramer method for correction against alpha-inflation were performed to investigate the pairwise group differences in change over time among the treatment groups.
The Statistical Analysis System for Windows (version 9.2; SAS Institute, Cary, NC, USA) was used for the implementation of all statistical analyses, including the HLM (Proc Mixed) and ANCOVA (Proc GLM) analyses.
Findings
Descriptive statistics
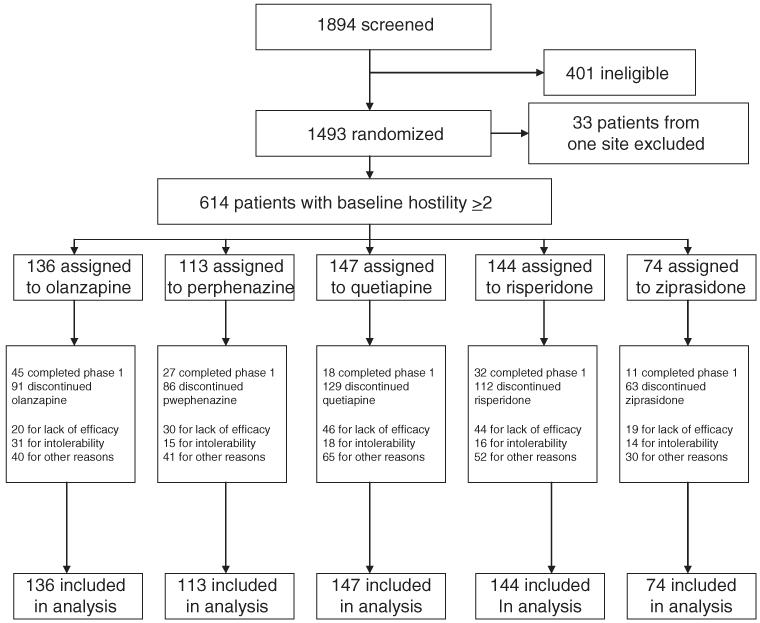
Demographic and clinical characteristics of the patients who were included in the current study because they had a hostility score ≥ 2 at baseline (N = 614) are listed in Table 1. At baseline, there were no statistically significant differences between treatment groups in hostility scores. The other baseline characteristics were similar between the treatment groups. They were also similar to the parent population. Patient disposition is shown in Figure 1.
TABLE 1. Sociodemographic and psychopathological characteristics at baseline.
| Olanzapine (N = 136) |
Perphenazine (N = 113) |
Quetiapine (N = 147) |
Risperidone (N = 144) |
Ziprasidone (N = 74) |
Total (N = 614) |
|
|---|---|---|---|---|---|---|
| Sociodemograhic characteristics | ||||||
| Age (years)a | 40.3 (10.4) | 38.7 (11.1) | 40.4 (11.6) | 39.9 (11.8) | 39.8 (11.1) | 39.9 (11.2) |
| Women | 41/136 (30%) | 24/113 (21%) | 41/147 (28%) | 36/144 (25%) | 25/74 (34%) | 167/614 (27%) |
| White | 85/136 (63%) | 64/113 (57%) | 102/147 (69%) | 99/144 (69%) | 42/74 (57%) | 392/614 (64%) |
| Psychopathology score (PANSS)b | ||||||
| Total | 82.2 (17.4) | 81.9 (17.5) | 81.3 (16.1) | 82.9 (15.9) | 85.5 (18.1) | 82.5 (16.8) |
| Positive | 20.2 (5.2) | 20.5 (5.5) | 21.4 (5.0) | 20.8 (4.9) | 21.9 (5.5) | 20.9 (5.2) |
| Negative | 21.6 (6.1) | 22.0 (6.0) | 20.0 (6.3) | 21.3 (6.4) | 22.0 (6.3) | 21.3 (6.2) |
| General | 40.4 (9.8) | 39.4 (9.7) | 39.9 (9.0) | 40.9 (8.5) | 40.6 (9.8) | 40.3 (9.3) |
| Hostility | 2.7 (0.8) | 2.6 (0.7) | 2.7 (0.9) | 2.6 (0.7) | 2.7 (0.9) | 2.7 (0.8) |
Data are n/N (%) or mean (SD), unless otherwise indicated. Denominators change because of incomplete data.
PANSS = positive and negative syndrome scale. For PANSS, theoretical scores range from 30–210 (total scale), 7–49 (positive scale), 7–49 (negative scale), 16–112 (general psychopathology scale), 1–7 (hostility). Higher scores indicate more severe psychopathology.
FIGURE 1.
Flowchart of patient disposition. The exclusion of data for all 33 patients from 1 site was caused by concern about the integrity of data from that site. Patients who showed no hostility at baseline (hostility score = 1; N = 790) were also excluded (see text).
Primary analysis of hostility change (HLM)
The results of our primary analysis of hostility change from baseline showed a statistically significant effect of treatment group (F4,1487 = 7.78, P<0.0001), indicating differential treatment effects on hostility.
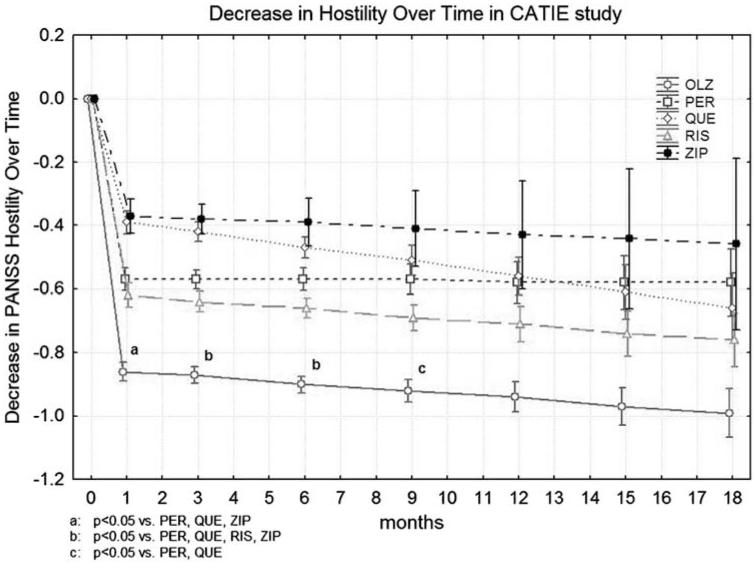
Post-hoc treatment group contrasts for change from baseline were computed for each subsequent time point and for overall change. Olanzapine was significantly superior to perphenazine and quetiapine at months 1, 3, 6, and 9. It was also significantly superior to ziprasidone at months 1, 3, and 6, and to risperidone at months 3 and 6. Corrected P values are shown in Figure 2. All medications produced statistically significant improvements in comparison with baseline at all time points, except for ziprasidone, which was not significant starting from month 9, and perphenazine at month 18.
FIGURE 2.
Decrease in PANSS Hostility rating over time in CATIE study. Corrected P values are shown.
We also present the results that were not adjusted for change in positive symptoms. These results showed a statistically significant effect of treatment group (F4,1498 = 7.40, P<0.0001). Olanzapine was significantly superior to perphenazine at months 1, 3, 6, and 9 (P≤0.0161). It was also superior to quetiapine at months 1, 3, 6, 9, and 12 (P≤0.0245); risperidone at months 3 and 6 (P≤0.0319); and ziprasidone at months 1, 3, and 6 (P≤0.0046).
The introduction of covariates for whether the participant was recruited before or after the introduction of ziprasidone, whether s/he had tardive dyskinesia at baseline, and for site effect had no substantial influence on principal results.
Sensitivity analysis (LOCF)
There was a statistically significant effect of treatment group (F = 5.48, P = 0.0002). Olanzapine was significantly superior to quetiapine (P = 0.0002) and to ziprasidone (P = 0.0048). Olanzapine’s superiority to risperidone was marginal (P = 0.0543).
The results of LOCF analysis not adjusted for change in positive symptoms showed a statistically significant effect of treatment group (F = 6.87, P<0.0001). Olanzapine was significantly superior to quetiapine (P<0. 0001), risperidone (P = 0.0143), and ziprasidone (P = 0.0007).
Discussion
The results supported our hypothesis that the medications would differ in their effects on hostility, and that olanzapine’s effects would be superior to those of other antipsychotics. Advantages were found for olanzapine compared with perphenazine, quetiapine, risperidone, and ziprasidone for a specific antihostility effect, independent of reduction of other positive symptoms of schizophrenia. Sensitivity analysis using LOCF was generally consistent with our primary HLM analysis. The trajectory of response for olanzapine, as measured by a decrease in PANSS Hostility over time (Figure 2), is consistent with the trajectories of PANSS total score and of Clinical Global Impressions—Severity score as reported for the entire sample in the primary CATIE report.3 Our results are also consistent with those of a similar post-hoc analysis of hostility in first-episode subjects with schizophrenia who were enrolled in the EUFEST trial,8 where olanzapine demonstrated advantages compared with haloperidol, quetiapine, and amisulpride. Our results are also consistent with those reported in a 12-week randomized double-blind clinical trial that was specifically designed to test specific antiaggressive effects of clozapine, olanzapine, and haloperidol.12 In that study, subjects who were assigned to clozapine had statistically significant lower endpoint aggression scores than patients assigned to either olanzapine or haloperidol. Patients in the olanzapine group had statistically significant lower endpoint aggression scores than patients in the haloperidol group. However, no differences were seen among the 3 groups in terms of reduction of psychopathology as measured by the PANSS total score, suggesting that clozapine’s and olanzapine’s advantages were related to a specific antiaggressive effect.
In addition to this report and that from EUFEST, our methods of assessing specific antihostility effects of second-generation antipsychotics have been previously used a priori,13 as well as in a number of other posthoc analyses.14-16 Similar techniques have been used post-hoc by others.17-19 Results have varied from superiority to haloperidol (compared to risperidone, olanzapine, quetiapine, and ziprasidone) to no difference from haloperidol (for aripiprazole) in terms of antihostility or antiaggressive effect. Compared with haloperidol, the second-generation antipsychotics were associated with fewer extrapyramidal effects and thus were considered more tolerable and overall more effective. Methodologies varied, however, and specific antihostility or antiaggressive effect was not always determined (for olanzapine17) or was inconsistently demonstrated (for quetiapine18,19).
The correlation of PANSS Hostility item scores and violent outcomes may not be consistent. Although we found that medications differed in their effects on hostility during Phase 1 of CATIE, there were no differences among treatments in their effects on violence during the same Phase.11 There are several potential explanations of this apparent discrepancy.
First, hostility and violence are different constructs that are assessed using different instruments. A detailed description of the PANSS Hostility item is presented in a preceding section (“Study Populations and Interventions”).
In the study by Swanson et al,11 the MacArthur Community Violence Interview was used to measure violent behavior and included minor violence, corresponding to battery without injury or weapon use; and serious violence, corresponding to any battery using a weapon or resulting in injury, any threat with a lethal weapon in hand, or any sexual assault. This level of violence differs qualitatively and quantitatively from the psychopathological symptom of hostility as measured by the PANSS.
Second, the time scale of the assessments of hostility in our study differed from that used in the Swanson study11 to assess violence. We used multiple PANSS ratings that were based partly on the patient’s behavior during the rating interview and partly on the information covering the behavior in the preceding week.9 Swanson et al conducted 2 interviews: one at baseline and another at 6 months. Finally, the 2 studies used different subsets of CATIE patients.
Although hostility is different from violence, it can be a significant barrier to discharge from a hospital and reintegration into the community20; in the clinical experience of the authors, hostility is a common treatment target that is identified in individual care plans. The principal concern about hostility as a symptom is the potential for escalation of aggressive behaviors. Hostile behavior is a dynamic risk factor that is strongly associated with increased violence risk in persons who are psychotic, as demonstrated in a meta-analysis of 110 studies that reported on 45,533 individuals.7
There are several limitations to our study. First, patients recruited for the CATIE study were not necessarily hostile, and those who were tended to display low levels of hostility. However, we note that the effect of olanzapine that was observed in the EUFEST study was very similar to the current results, although the EUFEST patients had higher baseline levels of hostility.8
Second, with 74% of subjects discontinuing Phase 1, the attrition rate was high, although this rate is generally consistent with those previously observed.21 The high discontinuation rate was partly due to study design, as subjects were eligible to discontinue Phase 1 and be re-randomized because of perceived lack of efficacy or tolerability with the original randomized medication.
Third, the CATIE was criticized because patients with tardive dyskinesia were not allowed to be randomized to perphenazine; because the maximal dose of olanzapine used was 30 mg per day (instead of the 20 mg per day recommended by the manufacturer), resulting in the average dose of 20 mg per day actually used; and for various other methodological problems.22
The problem with the olanzapine dose has been somewhat mitigated by a subsequent 8-week study that compared the efficacy of olanzapine 10, 20, and 40 mg per day.23 All dose groups showed improvement in PANSS total scores from baseline to endpoint without significant dose-response relationship. Thus, the higher dosing of olanzapine used in the CATIE is an unlikely explanation of its superior efficacy against hostility. It should be noted that similar effects of olanzapine against hostility were observed in the EUFEST study that used the recommended dose range of 5-20 mg per day.
Conclusion
Olanzapine demonstrated advantages in terms of a specific antihostility effect over the other antipsychotics tested in Phase 1 of the CATIE trial. This effect replicates, in long-term schizophrenia patients, the findings reported in patients in their first episode.8 In general, the present findings are consistent with what is already known about olanzapine.
The use of olanzapine in these circumstances needs to be considered within the context of the multiple causes of the hostile behavior and olanzapine’s potential for weight gain and changes in metabolic variables.24
Hostile and aggressive behavior in schizophrenia is etiologically heterogeneous.25 Violence among adults with schizophrenia may follow at least 2 distinct pathways: one associated with premorbid conditions, including antisocial conduct, and another associated with the acute psychopathology of schizophrenia.26 Schizophrenia patients who are violent may show comorbid psychopathic traits.27,28 The effectiveness of antipsychotics in such cases merits further study.
Clinical Implications.
-
■
Hostile and aggressive behavior may complicate care for patients with schizophrenia.
-
■
Antipsychotics differ in their efficacy against hostility.
-
■
The study suggests that olanzapine is superior to perphenazine, quetiapine, ziprasidone, and risperidone in its efficacy against hostility in schizophrenia.
Appendix
The CATIE Study Investigators Group included the following: L. Adler, Clinical Insights, Glen Burnie, MD; M. Bari, Synergy Clinical Research, Chula Vista, CA; I. Belz, Tri-County/Mental Health and Mental Retardation Services, Conroe, TX; R. Bland, Southern Illinois University School of Medicine, Springfield, IL; T. Blocher, Mental Health and Mental Retardation Authority of Harris County, Houston, TX; B. Bolyard, Cox North Hospital, Springfield, MO; A. Buffenstein, Queen’s Medical Center, Honolulu, HI; J. Burruss, Baylor College of Medicine, Houston, TX; M. Byerly, University of Texas Southwestern Medical Center at Dallas, Dallas, TX; J. Canive, Albuquerque Veterans Affairs Medical Center, Albuquerque, NM; S. Caroff, Behavioral Health Service, Philadelphia, PA; C. Casat, Behavioral Health Center, Charlotte, NC; E. Chavez-Rice, El Paso Community Mental Health and Mental Retardation Center, El Paso, TX; J. Csernansky, Washington University School of Medicine, St. Louis, MO; P. Delgado, University Hospitals of Cleveland, Cleveland, OH; R. Douyon, Veterans Affairs Medical Center, Miami, FL; C. D’Souza, Connecticut Mental Health Center, New Haven, CT; I. Glick, Stanford University School of Medicine, Stanford, CA; D. Goff, Massachusetts General Hospital, Boston, MA; S. Gratz, Eastern Pennsylvania Psychiatric Institute, Philadelphia, PA; G. T. Grossberg, Saint Louis University School of Medicine-Wohl Institute, St. Louis, MO; M. Hale, New Britain General Hospital, New Britain, CT; M. Hamner, Medical University of South Carolina and Veterans Affairs Medical Center, Charleston, SC; R. Jaffe, Belmont Center for Comprehensive Treatment, Philadelphia, PA; D. Jeste, University of California, San Diego, Veterans Affairs Medical Center, San Diego, CA; A. Kablinger, Louisiana State University Health Sciences Center, Shreveport, LA; A. Khan, Psychiatric Research Institute, Wichita, KS; S. Lamberti, University of Rochester, Medical Center, Rochester, NY; M. T. Levy, Staten Island University Hospital, Staten Island, NY; J. A. Lieberman, University of North Carolina School of Medicine, Chapel Hill, NC; G. Maguire, University of California-Irvine, Orange, CA; T. Manschreck, Corrigan Mental Health Center, Fall River, MA; J. McEvoy, Duke University Medical Center, Durham, NC; M. McGee, Appalachian Psychiatric Healthcare System, Athens, OH; H. Meltzer, Vanderbilt University Medical Center, Nashville, TN; A. Miller, University of Texas Health Science Center at San Antonio, San Antonio, TX; D. D. Miller, University of Iowa, Iowa City, IA; H. Nasrallah, University of Cincinnati Medical Center, Cincinnati, OH; C. Nemeroff, Emory University School of Medicine, Atlanta, GA; S. Olson, University of Minnesota Medical School, Minneapolis, MN; G. F. Oxenkrug, St. Elizabeth’s Medical Center, Boston, MA; J. Patel, University of Massachusetts Health Care, Worcester, MA; F. Reimherr, University of Utah Medical Center, Salt Lake City, UT; S. Riggio, Mount Sinai Medical Center-Bronx Veterans Affairs Medical Center, Bronx, NY; S. Risch, University of California-San Francisco, San Francisco, CA; B. Saltz, Mental Health Advocates, Boca Raton, FL; T. Simpatico, Northwestern University, Chicago, IL; G. Simpson, University of Southern California Medical Center, Los Angeles, CA; M. Smith, Harbor-UCLA Medical Center, Torrance, CA; R. Sommi, University of Missouri, Kansas City, MO; R. M. Steinbook, University of Miami School of Medicine, Miami, FL; M. Stevens, Valley Mental Health, Salt Lake City, UT; A. Tapp, Veterans Affairs Puget Sound Health Care System, Tacoma, WA; R. Torres, University of Mississippi, Jackson, MS; P. Weiden, SUNY Downstate Medical Center, Brooklyn, NY; J. Wolberg, Mount Sinai Medical Center, New York, NY.
Footnotes
Disclosures
Jan Volavka does not have anything to disclose. Leslie Citrome has the following disclosures: Alexza, consultant, consulting fees; Alkermes, consultant, consulting fees; Bristol-Myers Squibb, consulting, consultant fees; Bristol-Myers Squibb, speaker, speaker fees; Eli Lilly, consultant, consulting fees; Eli Lilly, speaker, speaker fees; Envivo, consultant, consulting fees; Forest, consultant, consulting fees; Genentech, consultant, consulting fees; Janssen, consultant, consulting fees; Lundbeck, consultant, consulting fees; Lundbeck, speaker, speaker fees; Merck, consultant, consulting fees; Merck, speaker, speaker fees; Mylan, consultant, consulting fees; Novartis, consultant, consulting fees; Novartis, speaker, speaker fees; Noven, consultant, consulting fees; Otsuka, consultant, consulting fees; Otsuka, speaker, speaker fees; Pfizer, consultant, consulting fees; Pfizer, speaker, speaker fees; Reckitt Benckiser, consultant, consulting fees; Sunovion, consultant, consulting fees; Sunovion, speaker, speaker fees; J & J, small number of shares and common stock; AstraZeneca, speaker, speaker fees. Pal Czobor does not have anything to disclose. Richard A. Van Dorn does not have anything to disclose.
REFERENCES
- 1.Volavka J. Neurobiology of Violence. 2nd ed. American Psychiatric Publishing, Inc.; Washington, DC: 2002. [Google Scholar]
- 2.Stroup TS, McEvoy JP, Swartz MS, et al. The National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: schizophrenia trial design and protocol development. Schizophr Bull. 2003;29(1):15–31. doi: 10.1093/oxfordjournals.schbul.a006986. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 4.Swanson JW, Swartz MS, Van Dorn RA, et al. A national study of violent behavior in persons with schizophrenia. Arch Gen Psychiatry. 2006;63(5):490–499. doi: 10.1001/archpsyc.63.5.490. [DOI] [PubMed] [Google Scholar]
- 5.Fazel S, Langstrom N, Hjern A, Grann M, Lichtenstein P. Schizophrenia, substance abuse, and violent crime. JAMA. 2009;301(19):2016–2023. doi: 10.1001/jama.2009.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatry. 1989;155(Suppl 7):59–65. [PubMed] [Google Scholar]
- 7.Witt K, van Dorn R, Fazel S. Risk factors for violence in psychosis: systematic review and meta-regression analysis of 110 studies. PLoS One. 2013;8(2):e55942. doi: 10.1371/journal.pone.0055942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volavka J, Czobor P, Derks EM, et al. Efficacy of antipsychotic drugs against hostility in the European First-Episode Schizophrenia Trial (EUFEST) J Clin Psychiatry. 2011;72(7):955–961. doi: 10.4088/JCP.10m06529. [DOI] [PubMed] [Google Scholar]
- 9.Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) rating manual. 1986. Unpublished work. [DOI] [PubMed] [Google Scholar]
- 10.Little RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. 2nd ed. SAS Press; Cary, NC: 2006. [Google Scholar]
- 11.Swanson JW, Swartz MS, Van Dorn RA, et al. Comparison of antipsychotic medication effects on reducing violence in people with schizophrenia. Br J Psychiatry. 2008;193(1):37–43. doi: 10.1192/bjp.bp.107.042630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krakowski MI, Czobor P, Citrome L, Bark N, Cooper TB. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622–629. doi: 10.1001/archpsyc.63.6.622. [DOI] [PubMed] [Google Scholar]
- 13.Citrome L, Volavka J, Czobor P, et al. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility in treatment-resistant patients with schizophrenia and schizoaffective disorder. Psychiatr Serv. 2001;52(11):1510–1514. doi: 10.1176/appi.ps.52.11.1510. [DOI] [PubMed] [Google Scholar]
- 14.Czobor P, Volavka J, Meibach RC. Effect of risperidone on hostility in schizophrenia. J Clin Psychopharmacol. 1995;15(4):243–249. doi: 10.1097/00004714-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Volavka J, Czobor P, Citrome L, et al. Efficacy of aripiprazole against hostility in schizophrenia and schizoaffective disorder: data from 5 double-blind studies. J Clin Psychiatry. 2005;66(11):1362–1366. doi: 10.4088/jcp.v66n1103. [DOI] [PubMed] [Google Scholar]
- 16.Citrome L, Volavka J, Czobor P, et al. Efficacy of ziprasidone against hostility in schizophrenia: post hoc analysis of randomized, open-label study data. J Clin Psychiatry. 2006;67(4):638–642. doi: 10.4088/jcp.v67n0415. [DOI] [PubMed] [Google Scholar]
- 17.Kinon BJ, Roychowdhury SM, Milton DR, Hill AL. Effective resolution with olanzapine of acute presentation of behavioral agitation and positive psychotic symptoms in schizophrenia. J Clin Psychiatry. 2001;62(Suppl 2):17–21. [PubMed] [Google Scholar]
- 18.Arango C, Bernardo M. The effect of quetiapine on aggression and hostility in patients with schizophrenia. Hum Psychopharmacol. 2005;20(4):237–241. doi: 10.1002/hup.686. [DOI] [PubMed] [Google Scholar]
- 19.Chengappa KN, Goldstein JM, Greenwood M, John V, Levine J. A post hoc analysis of the impact on hostility and agitation of quetiapine and haloperidol among patients with schizophrenia. Clin Ther. 2003;25(2):530–541. doi: 10.1016/s0149-2918(03)80094-2. [DOI] [PubMed] [Google Scholar]
- 20.Volavka J, Swanson JW, Citrome LL. Understanding and managing violence in schizophrenia. In: Lieberman JA, Murray RM, editors. Comprehensive Care of Schizophrenia: A Textbook of Clinical Management. 2nd ed. Oxford University Press; New York: 2012. pp. 262–290. [Google Scholar]
- 21.Geddes J, Freemantle N, Harrison P, Bebbington P. Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. BMJ. 2000;321(7273):1371–1376. doi: 10.1136/bmj.321.7273.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moller HJ. Do effectiveness (“real world”) studies on antipsychotics tell us the real truth? Eur Arch Psychiatry Clin Neurosci. 2008;258(5):257–270. doi: 10.1007/s00406-008-0812-0. [DOI] [PubMed] [Google Scholar]
- 23.Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder: a randomized, double-blind, fixed-dose study. J Clin Psychopharmacol. 2008;28(4):392–400. doi: 10.1097/JCP.0b013e31817e63a5. [DOI] [PubMed] [Google Scholar]
- 24.Citrome L, Holt RI, Walker DJ, Hoffmann VP. Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig. 2011;31(7):455–482. doi: 10.2165/11589060-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Volavka J, Citrome L. Heterogeneity of violence in schizophrenia and implications for long-term treatment. Int J Clin Pract. 2008;62(8):1237–1245. doi: 10.1111/j.1742-1241.2008.01797.x. [DOI] [PubMed] [Google Scholar]
- 26.Swanson JW, Van Dorn RA, Swartz MS, et al. Alternative pathways to violence in persons with schizophrenia: the role of childhood antisocial behavior problems. Law Hum Behav. 2008;32(3):228–240. doi: 10.1007/s10979-007-9095-7. [DOI] [PubMed] [Google Scholar]
- 27.Laajasalo T, Salenius S, Lindberg N, Repo-Tiihonen E, Hakkanen-Nyholm H. Psychopathic traits in Finnish homicide offenders with schizophrenia. Int J Law Psychiatry. 2011;34(3-4):324–330. doi: 10.1016/j.ijlp.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Abushua’leh K, bu-Akel A. Association of psychopathic traits and symptomatology with violence in patients with schizophrenia. Psychiatry Res. 2006;143(2-3):205–211. doi: 10.1016/j.psychres.2005.05.017. [DOI] [PubMed] [Google Scholar]