Abstract
Ventriculomegaly occurs when there is imbalance between creation and absorption of cerebrospinal fluid (CSF); even when treated, long-term behavioral changes occur. Kaolin injection in the cisterna magna of rats produces an obstruction of CSF outflow and models one type of hydrocephalus. Previous research with this model shows that neonatal onset has mixed effects on Morris water maze (MWM) and motoric performance; we hypothesized that this might be because the severity of ventricular enlargement was not taken into consideration. In the present experiment, rats were injected with kaolin or saline on postnatal day (P)21 and analyzed in subgroups based on Evan's ratios (ER) of the severity of ventricular enlargement at the end of testing to create 4 subgroups from least to most severe: ER0.4–0.5, ER0.51-0.6, ER0.61-0.7, and ER0.71-0.82, respectively. Locomotor activity (dry land and swimming), acoustic startle with prepulse inhibition (PPI), and MWM performance were tested starting on P28 (122 cm maze) and again on P42 (244 cm maze). Kaolin-treated animals weighed significantly less than controls at all times. Differences in locomotor activity were seen at P42 but not P28. On P28 there was an increase in PPI for all but the least severe kaolin-treated group, but no difference at P42 compared with controls. In the MWM at P28, all kaolin-treated groups had longer path lengths than controls, but comparable swim speeds. With the exception of the least severe group, probe trial performance was worse in the kaolin-treated animals. On P42, only the most severely affected kaolin-treated group showed deficits compared with control animals. This group showed no MWM learning and no memory for the platform position during probe trial testing. Swim speed was unaffected, indicating motor deficits were not responsible for impaired learning and memory. These findings indicate that kaolin-induced ventriculomegaly in rats interferes with cognition regardless of the final enlargement of the cerebral ventricles, but final size critically determines whether lasting locomotor, learning, and memory impairments occur.
Keywords: development, cognitive development, hydrocephalus, spontaneous locomotor activity, Morris water maze, acoustic startle response, prepulse inhibition
Introduction
Ventriculomegaly (VM) occurs when there is an imbalance between the production and absorption of cerebrospinal fluid (CSF). Coupled with a frequent increase of intracranial pressure, VM leads to impairments in widespread regions of the CNS (Air et al., 2010;Budde et al., 2008;Shirane et al., 1992;Tashiro and Drake, 1998;Yuan et al., 2009;Ding et al., 2001a;Ding et al., 2001b) (reviewed in (Del Bigio, 2004;McAllister et al., 1991)), especially the corpus callosum, periventricular white matter, fornix, internal and external capsules, as well as other white matter regions (Air et al., 2010;Chumas et al., 1994;Del Bigio et al., 1997b;Del Bigio and Zhang, 1998;Jiang et al., 2006;Richards et al., 1995;Shirane et al., 1992;Tashiro and Drake, 1998). White matter damage has been reported to underlie some of the poor behavioral outcomes in children with VM (Del Bigio et al., 2003;Fletcher et al., 1992;Fletcher et al., 1996), however, current clinical evaluation of such damage remains limited by the inability to quantify damage non-invasively in such a way that is sensitive, specific, and predictive. Animal models of VM have been developed to better understand the progression of damage and predict outcome.
While VM can be induced by a number of different processes, one way to induce it mechanically in animals is by kaolin injection in the cisterna magna. Kaolin-induced VM can cause a loss of dopamine neurons in the substantia nigra in adult rats and significantly reduce levels of other monoamines (Chovanes et al., 1988;Hwang et al., 2009;Lovely et al., 1989). These decreases in monoamines are concurrent with reductions in locomotor activity beginning 3 days after treatment and becoming more severe by 4 weeks (Hwang et al., 2009). Similarly, kaolin-treated adult rats spend more time in closed arms of an elevated plus-maze 3 days after injection, but spend less time in closed arms 4 weeks later (Hwang et al., 2011), suggesting that effects on anxiety are transient. In addition to reductions in dopaminergic neurons, kaolin treated rats show increases in cholecystokinin and decreases in neuropeptide-Y 3 days post-treatment, with no differences by 4 weeks. During active avoidance learning, kaolin-treated rats had increased reaction times to escape when classified as having moderate or severe VM compared with controls (Kuchiwaki et al., 1994).
Kaolin-induced VM on postnatal day 1 (P1) produces reductions in weight gain in rats classified as having severe VM by P19 compared with controls (Khan et al., 2006). Magnetic resonance imaging of VM severity on P7 and P21 in the Khan et al. (2006) study showed that enlargement of the ventricles was stable throughout the 14 days between scans, such that animals did not change from mild to severe VM during this time or vice versa. Furthermore, the kaolin-induced VM animals showed no developmental delays in a number of behaviors, although some impairment in Morris water maze (MWM) latency to reach the escape platform was observed in the most severely affected VM rats compared with controls (Khan et al., 2006). This increase in latency to find the platform may be explained by the slower swimming speeds of the VM animals reported in this study (Khan et al., 2006). Consistent with a lack of spatial learning changes, these authors were unable to demonstrate MWM deficits when animals were injected with kaolin on P21 (Khan et al., 2003). These kaolin-treated animals also showed no changes in body weight, locomotor activity, or swimming ability. Inconsistent differences in MWM learning (i.e., deficits on one trial of a day but not the last trial of the day) were observed in another study where animals received kaolin on P21 (Del Bigio et al., 1997a). The kaolin-treated animals showed body weight reductions suggesting a greater effect was achieved in this experiment. Previous reports have also demonstrated weight reductions with this model (Deren et al., 2009;Deren et al., 2010;McAllister et al., 1985),
While a number of reasons related to timing of injection or other procedures may explain the lack of effect following kaolin in some experiments, one major factor not accounted for above is the severity of VM. The VM induced by kaolin is not consistent between animals (McAllister et al., 1985;McAllister et al., 1991), therefore identifying the magnitude of VM in relation to outcome may improve the model and explain past inconsistencies. In order to characterize in vivo changes to white matter, diffusion tensor imaging (DTI) can be useful. VM increases astrogliosis and microgliosis (Deren et al., 2010;Miller and McAllister, 2007) and these changes correlate with abnormal DTI measures (Yuan et al., 2010). The abnormal DTI data in a series of WM regions of interest from a subset of the animals used in the present study have been reported previously (Yuan et al., 2012). In the present study, the behavioral outcome data were analyzed in association with the DTI data to explore the potential underpinnings of functional changes. It should be noted that the present study was designed to examine the behavioral consequences of kaolin-induced VM that begins at a time in brain development that correlates with human infancy based on comparative anatomy (Clancy et al., 2007), since the infantile period is when hydrocephalus most commonly develops clinically. We also accounted for the severity of VM after testing to address what appears to be inconsistencies identified in this area of research as noted above. In order to do this, we analyzed the behavioral data by Evan's ratio (ER) subgrouping.
Materials and Methods
Animals and Treatments
Male Sprague-Dawley rats (Charles River, Raleigh, NC) were obtained on P14 with their dams and allowed to acclimate to the housing conditions of the vivarium at Cincinnati Children's Research Foundation. The vivarium is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. The vivarium is pathogen free and uses the Modular Animal Caging System (Alternative Design, Siloam Spring, AR) with HEPA filtered air that is supplied via the Flex-Air System (Alternative Design, Siloam Spring, AR) at 30 air changes/h. Water was provided ad libitum with the Lixit automated system (SE Lab Group, Napa, CA). Each cage (polysulfonate cages 26 × 48 cm and 20 cm tall) had ad libitum food, contained woodchip bedding, and a semicircular stainless steel enclosure to provide environmental enrichment (see (Vorhees et al., 2008)). Animals were maintained on a 14 h light: 10 h dark cycle. All procedures were approved by the Institutional Animal Care and Use Committee of Cincinnati Children's Research Foundation and conform to animal use guidelines set forth by the National Institutes of Health.
On P21, the animals were weaned and divided into two groups: kaolin injected (VM, n = 30 with 2 deaths, final n = 28) or saline injected (SAL, n = 30 with 2 deaths, final n = 28). VM animals were anesthetized with isoflurane (1-4%) and injected with a solution of 30-50 μl kaolin (25% w/v in sterile saline) percutaneously over 10 s into the cisterna magna as described previously (Yuan et al., 2010;Yuan et al., 2012). For the SAL animals the same volume of sterile physiological saline was injected using the same procedures. Animals recovered all reflexes prior to being housed with a littermate for 1 week prior to the start of behavioral testing. All animals were weighed one day before surgery, daily for 1 week after surgery, and weekly thereafter (P35, 42, 49).
Behavioral Assessment
Animals had behavioral testing at two ages: firstly, one week following surgery (starting on P28) and the secondly, three weeks after surgery (starting on P42). All behavioral tests were conducted by a technician blinded to the condition of each animal, although VM was observable in some of the animals.
Locomotor Activity
On P28, animals were tested for spontaneous locomotor exploration/activity in 41 cm × 41 cm test chambers (Accuscan electronics, Columbus, OH). Animals were allowed to explore the chamber for 60 min and data were captured in 5 min intervals. The dependent measure was the amount of horizontal activity (measured in photobeam interruptions). Animals were retested in the same apparatus on P42. The chambers were cleaned with 70% ethanol between animals.
Acoustic Startle with Prepulse Inhibition (PPI)
Acoustic startle with prepulse inhibition was tested beginning at least 1 h after locomotor activity. Acoustic startle reactivity was measured in an SR-LAB apparatus (San Diego Instruments, San Diego, CA). Animals were placed in an acrylic cylindrical holder mounted on a platform with a piezoelectric accelerometer as the force transducer attached to the underside of the platform. The platform was located inside a sound-attenuated chamber. Each animal had a 5 min acclimation period prior to a 5 × 5 Latin square sequence of trials of 5 types: no stimulus, startle stimulus with no prepulse, or 70, 75, or 80 dB prepulse followed by the startle stimulus.
Each set of 25 trials was repeated 4 times with an intertrial interval of 8 s for a total of 100 trials. The startle signal was a 20 ms 110 dB sound pressure level (SPL) mixed frequency sound burst with a background of 67 dB and the startle recording window of 100 ms following startle signal onset. All prepulses preceded the startle-eliciting stimulus by 70 ms (onset to onset). The dependent measure was startle amplitude measured as voltage change (mV). Trials of the same type were averaged together. Chambers were cleaned with 70% ethanol between animals.
Straight Channel Swim
On P28 after PPI, animals were assessed in a 244 cm straight swimming channel (width 15 cm) filled with room temperature water to test for swimming ability and motivation to escape to a submerged platform at one end. This task also taught animals that escape was possible. Each animal was given 4 consecutive trials with a limit of 2 min/trial. All trials were started when the animal was placed in the water facing the end wall and ended when the animal found the platform located at the opposite end. Trials were timed by the experimenter.
Morris Water Maze Acquisition
Spatial learning in the MWM began on P29. At this age, animals were tested in a 122 cm diameter stainless steel tank, with a 10 cm diameter platform that was 1.5 cm below the water surface and positioned in the southwest quadrant of the tank (with south being closest to the experiment, not compass directions). The platform was not visible because it was colored to match the background color of the maze. Animals were given 6 days of learning trials with the platform and 1 memory (probe) trial with no platform on day 7. Four trials were administered on each day during the learning phase with quasi-randomized start positions (N, E, SE, NW) and a time limit of 2 min per trial with 15 s on the platform between trials as described (Vorhees and Williams, 2006). Data were collected using ANY-maze videotracking software (Stoelting Instruments, Wood Dale, IL). Animals that did not acquire the platform in the 2 min limit were removed and placed on the platform for 15 s. For the probe trial, animals were started from the NE position and allowed 60 s to investigate the tank with the platform removed. The dependent measures on platform trials were latency, path length, cumulative distance to the platform, and swim speed. On probe trials, measures assessed were average distance to the previous platform location and percent time in the target quadrant.
Behavioral Retesting (P42)
At P42 animals were retested in the locomotor apparatus as above for 60 min. Beginning at least 1 h after locomotor testing, animals were retested for PPI, and then retested in the straight channel swim procedure.
Morris Water Maze Relearning
On P43 animals began a relearning procedure in the MWM. Animals were now tested in a new room in a 244 cm tank. They again had 6 days of learning trials and 1 memory trial on day 7. The platform size remained the same (10 cm diameter) and was also positioned 2 cm below water surface in the NE quadrant with start positions of SW, S, W, and NW. The probe trial started from the SW position. All other parameters were the same as at the earlier age.
Morris Water Maze Cued
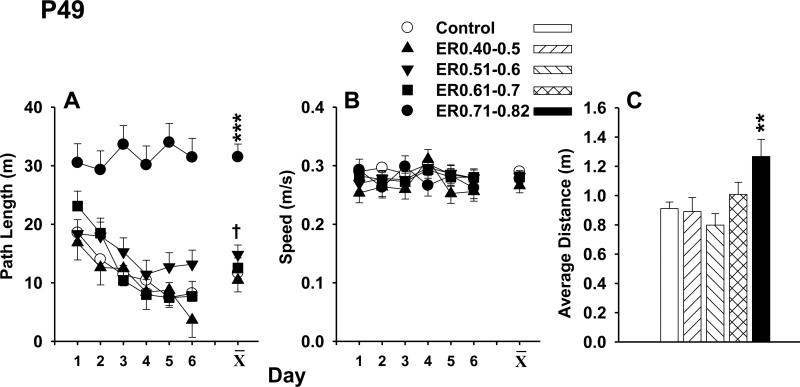
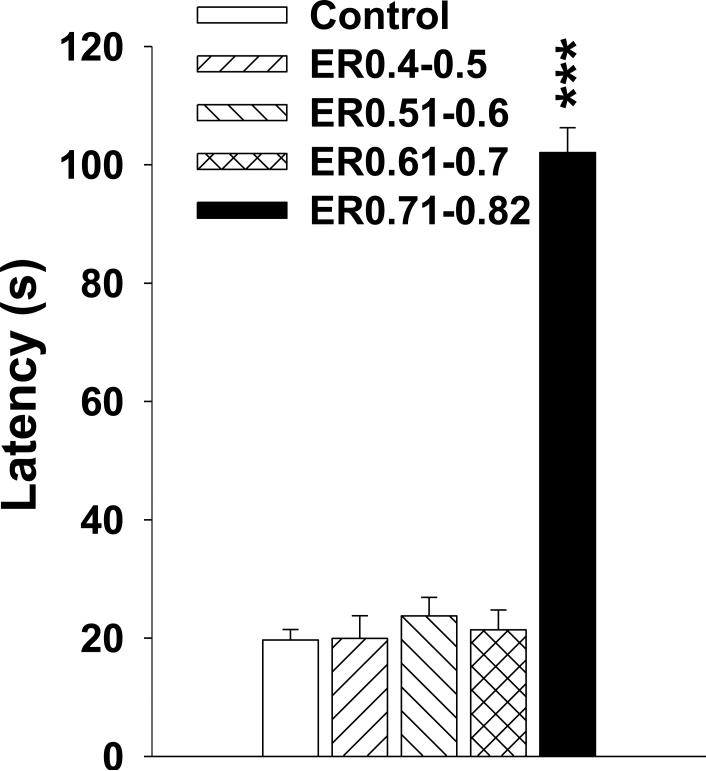
On P49, 1 h following the hidden MWM probe trial, animals were tested in a cued version of the MWM. For this test, the 10 cm diameter platform had a cue (orange ball) mounted on it that protruded 12 cm above the platform on a brass rod to demarcate the platform position. Animals were given 2 blocks of 4 trials with a minimum of 30 min between blocks. Time limit to locate the platform was 2 min and start and platform positions were randomized on every trial. Time to reach the platform was recorded.
Magnetic Resonance Imaging
After behavioral testing was finished, the rats were scanned on a 7T Bruker magnetic resonance imaging scanner (Bruker Biospec 70/30, Karlsruhe, Germany). The rats were anesthetized with 4% isoflurane during the scan as part of the standard procedure. It should be noted that the MRI scans were designed to occur at P49-P52, after all the behavioral tests were finished, to preclude any potential impact of necessary anesthetization on behavioral measures. The MR scan protocol included a T2-weighted RARE sequence for anatomical structures and ventricle size assessment for all the animals and a diffusion tensor imaging (DTI) sequence for quantification of WM anisotropic diffusion properties for a subset of the animals (12 VM rats and 6 SAL rats). The alterations in DTI found in the rats with VM and used in the current paper were reported (Yuan et al., 2012), therefore only a brief summary is provided for the specifications in the protocol. The DTI data were acquired with a 30-direction four-shot EPI spin echo diffusion sequence with the following parameters: TR/TE = 5,000/21 ms; FOV=32×32 mm; in-plan resolution=125×125 μm; slice thickness=0.5 mm; 19 slices; b value = 670 s/mm2; NSA = 6; scan time = 90 min. The 2D T2-weighted anatomical images were acquired using the same geometry setting as used in the DTI scan. Other scan specifications for the T2-w sequence included the following: TR/TE=4,000/54 ms; RARE factor=16; scanning time=17:04 min. DTI data were analyzed using standard procedures for pre-processing and tensor reconstruction and DTI measures fractional anisotropy (FA) and mean diffusivity (MD) were subsequently calculated in a series of WM regions of interest that included the corpus callosum, external capsule, internal capsule, and fornix. Evan's ratios (ER), the ratio between the widths of the lateral ventricles to that of the entire brain in a coronal section at the level of the Foraman of Monro, were calculated from the T2 weighted images. The details for the imaging data acquisition and data processing and analysis, including software, ROI delineation, and representative DTI images and ROIs, have been reported in our previous work (see Figure 1 for ROI identification in both representative VM and SAL rats and Figure 2 for ER determination in Yuan et al., 2012).
Figure 1.
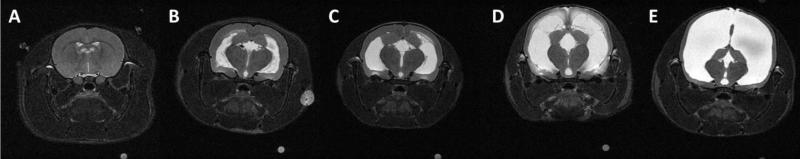
T2-weighted images showing representative ventricle sizes in SAL (A), ER0.4–0.5 (B), ER0.51-0.6 (C), ER0.61-0.7 (D), ER0.71-0.82 (E) rats. The Evan's ratio for each of these rats was as follows: 0.31, 0.46, 0.57, 0.68, and 0.82 in (A) to (E), respectively.
Figure 2.
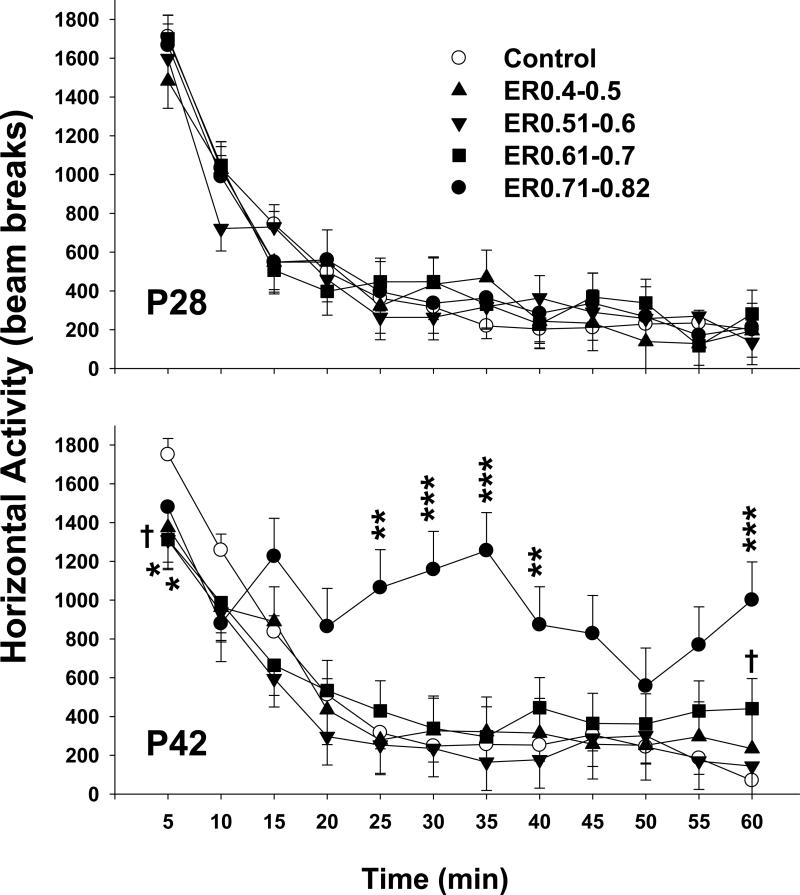
Locomotor activity on P28 (top panel) and P49 (bottom panel). On P28, there were no differences in horizontal activity among the 5 groups. On P42 when animals were retested for horizontal activity Group and Group x Interval were significant. Overall, the ER0.71-0.82 group showed increased activity relative to the SAL group. During the first 5 min, the ER0.51-0.60 and ER0.61-0.70 animals had decreased exploratory behavior compared with the SAL animals. The ER0.71-0.82 group had increased activity between 25-40 min compared with the SAL group and again at the 60 min interval. * p < 0.05, ** p < 0.01, *** p < 0.0001, † p < 0.1 versus SAL.
Following the MRI, animals in the kaolin group were divided into 4 groups by ER as follows: ER0.4–0.5, ER0.51-0.6, ER0.61-0.7, and ER0.71-0.82. The SAL animals had ERs less than or equal to 0.33. Representative T2-weighted images showing different ventricle sizes from SAL and the 4 VM groups are shown in Figure 1.
Statistics
Data were analyzed using mixed linear factorial analysis of variance (SAS v9.2, SAS Institute, Cary, NC). The between subjects factors were Group (ER0.4–0.5, ER0.51-0.6, ER0.61-0.7, ER0.71-0.82, or SAL) and the repeated measures factors were: Week (for body weights), Interval (for locomotor activity), Prepulse intensity (for PPI), Trial (for straight channel), Day (for MWM), and Block (for cued MWM). Significant interactions were further analyzed using slice-effect ANOVAs by the repeated measure and corrections for multiple comparisons used the FDR (false discovery rate) method. Degrees of freedom were calculated by the Kenward-Roger method. Significance was set at p ≤ 0.05 (1-tailed; only deficits were hypothesized); data are presented as least square (LS) means ± LS SEMs.
Results
Body weights
During the 4 weeks after surgery, all animals, regardless of group, showed an increase in body weight (p < 0.0001, Table 1). Nonetheless, the kaolin-treated animals weighed less than SAL controls regardless of ER (main effect of group (F(4, 54.6) = 50.46, p <0.0001), and group x week interaction (F(12, 152) = 44.79, p < 0.0001); all kaolin-treated animals weighed less than SAL controls, regardless of week).
Table 1.
Body Weights (g) of animals treated with saline (SAL) or kaolin (VM). The VM animals were further divided based on the Evan's ratio
| Treatment | N | P28 | P35 | P42 | P49 | |
|---|---|---|---|---|---|---|
| SAL | 28 | 104.9 ± 4.3 | 169.2 ± 4.3 | 231.6 ± 4.3 | 287.2 ± 4.3 | |
| ER 0.40-0.50 | 6 | 73.5 ± 9.2** | 106.0 ± 9.2*** | 130.2 ± 9.2*** | 148.5 ± 9.2*** | |
| ER 0.51-0.60 | 9 | 79.2 ± 7.5** | 113.0 ± 7.5*** | 137.7 ± 7.5*** | 156.4 ± 7.5*** | |
| ER 0.61-0.70 | 8 | 78.3 ± 8.0** | 109.3 ± 8.0*** | 129.2 ± 8.0*** | 143.9 ± 8.0*** | |
| ER 0.71-0.82 | 5 | 80.6± 10.1* | 112.1 ± 10.1*** | 142.8 ± 10.1*** | 162.0 ± 10.1*** |
p < 0.05
p < 0.01
p < 0.001 versus SAL at the same age.
Locomotor Activity
On P28 there were no differences in horizontal activity among the groups. All groups showed an initial exploratory period followed by habituation to the chamber (Interval, p < 0.0001; Figure 2, top). Examination of distance in the center, periphery, and number of rears all confirmed no differences among the groups (not shown).
On P42 when animals were retested for locomotor activity, there were significant group, F(4,51.3) = 2.82, P <0.05, and interval, p < 0.0001 main effects, and a group x interval interaction, F(44,513) = 2.22, p < 0.0001. Overall, the ER0.71-0.82 group showed increased activity relative to SAL controls. Examination of the interaction showed that during the first 5 min, the ER0.51-0.60 and ER0.61-0.70 animals had decreased exploratory behavior compared with SAL controls (Figure 2, bottom), whereas only a trend was observed for the ER0.40-0.50 animals. No difference was observed between the ER0.71-0.82 group and SAL group during the first interval or between the other groups and SAL on other intervals (with the exception of a nonsignificant trend at 60 min for the ER0.61-0.7 group). The ER0.71-0.82 group showed increased activity between 25-40 min compared with the SAL group and again at the 60 min interval. The central and peripheral distances traveled showed similar patterns among the groups as horizontal activity, although the ER0.71-0.82 group had more peripheral activity during more intervals compared with SAL controls (not shown).
Acoustic Startle/PPI
For PPI on P28, all kaolin-treated groups, with the exception of the ER0.4-0.5 group, had greater responses to the tone, regardless of prepulse, compared with SAL controls, F(4,51) = 3.84, p < 0.009 (Figure 3A). Prepulses inhibited the response for all animals (p < 0.0001), and there were no differential effects among the groups as a function of prepulse intensity. Examination of the percent inhibition following prepulses confirmed this, showing no difference between the kaolin and SAL-treated groups (Figure 3C).
Figure 3.
Acoustic startle with prepulse inhibition was run on P28 (panel A & C) and again on P42 (panel B & D). On p28 all the VM groups with the exception of the ER0.4-0.5 group had greater responses to the tone, regardless of the prepulse, compared with SAL animals, p < 0.05 (panel A). No group differences were observed on P42 for startle response (panel B). No differences were observed between the VM groups and the SAL group for percent inhibition (panel C and D). * p < 0.05, ** p < 0.01 versus SAL.
Unlike the increased startle reactivity observed on P28, no differences were observed on P42 for this test (Figure 3B). All animals responded to the prepulse with attenuated responses (p < 0.0001), but there was no interaction with prepulse or group. Examination of percent inhibition confirmed the amplitude findings; there was a trend for the group effect (p < 0.07) that was not analyzed further, and no interaction was noted (Figure 3D).
Straight Channel Swimming
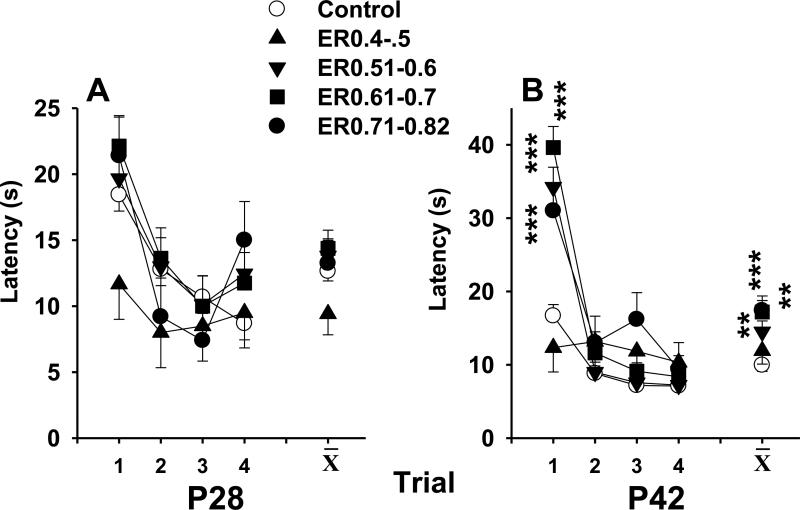
All animals improved their latency to reach the opposite end across trials on P28 (p < 0.0001), and there were no differences among groups or interaction with group (Figure 4A). On P42, with the exception of the ER0.4-0.5 group, the kaolin-treated animals had increased latencies (main effect: F(4,44.8) = 6.53, p < 0.0004). There was also a group x interval interaction (F(12,120) = 5.42, p < 0.0001) which was caused by an effect on only one trial (Figure 4B). Comparable latencies were observed on the other 3 trials. Rats in all groups reached asymptotic performance with a latency of 10 s or less on the last trial indicating no motor deficits.
Figure 4.
Straight channel swimming was assessed at P28 (panel A) and P42 (panel B). All animals improved their latency to reach the goal across trials on P28 (p < 0.0001), but there were no Group differences. On P42, with the exception of the ER0.4-0.5 group, the VM animals had increased latencies (p < 0.0004), although only trial 1 was significant when the Group x Interval interaction was examined (p < 0.0001; Figure 3B). ** p < 0.01, *** p < 0.0001, versus SAL.
Morris Water Maze Acquisition
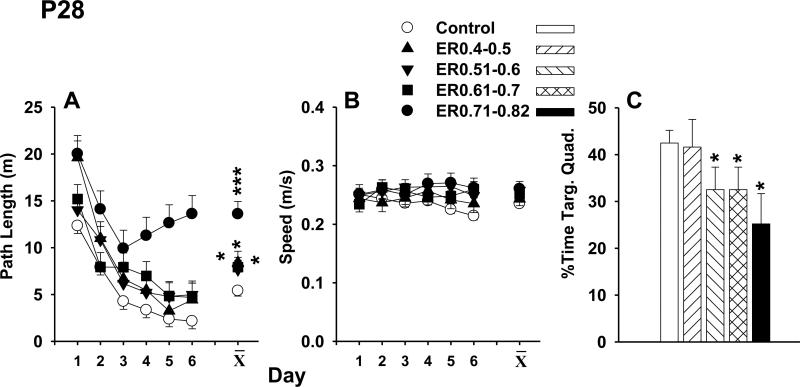
On P29, MWM learning was in the 122 cm maze. Because latencies, path lengths, and cumulative distances are highly correlated (see (Vorhees and Williams, 2006)) and since path lengths are impervious to changes in swim speed, path length data are presented. The path lengths of the kaolin-treated animals, regardless of ER subgroup, were longer than SAL controls, F(4,51.1) = 8.81, p < 0.0001 (Figure 5A). All animals showed a significant decline in path lengths over days (p < 0.0001), demonstrating that learning was not prevented in kaolin-treated groups but rather impaired. Similar effects were observed for latency and cumulative distance (not shown). Kaolin-treated animals did not differ from the SAL controls in swim speeds (Figure 5B).
Figure 5.
Morris water maze training started on P29 in a 122 cm water tank. The path lengths of the VM animals, regardless of Evans ratio, were longer than the SAL animals, p < 0.0001 (panel A), and all animals had shorter path lengths over days (p < 0.0001). No differences in swim speeds were observed (panel B). There was a significant reduction in the percent of time that the VM animals (with the exception of the ER0.4-0.5 group) spent in the target quadrant compared with the SAL animals (p < 0.04; panel C). * p < 0.05, *** p < 0.0001, versus SAL.
A single memory (probe) trial was given 24 h after the last training trial on day-7. For average distance from the platform, there was a non-significant trend (p = 0.10) for kaolin-treated animals to be further from the platform site than SAL controls. There was a significant reduction in the percent time kaolin-treated animals (with the exception of the ER0.4-0.5 group) spent in the target quadrant compared with SAL control animals (F(4,51) = 2.72, p < 0.04; Figure 5C). No differences were found between groups for swim speed during the probe trial.
Morris Water Maze Relearning
Animals were retested in the MWM beginning on P43. For this phase, the animals were tested in a new room in a larger 244 cm diameter maze. Unlike the P28 test, only the ER0.71-0.82 animals had longer path lengths than SAL controls (group main effect: F(4, 51.2) = 18.5, p < 0.0001). As seen in Figure 6A, there were no differences between SAL and the other kaolin-treated groups at this age, although it was noted that the ER0.51-0.6 group exhibited a trend (p = 0.10). There was a significant interaction of group x day as well, F(20, 223) =1.67, p < 0.05. Only the ER0.71-0.82 group was different from SAL controls when this interaction was analyzed further. Overall, path lengths decreased over days (p < 0.0001). Similar patterns were observed for latency and cumulative distance (not shown). Similar to the acquisition phase, no speed differences were detected (Figure 6B).
Figure 6.
Animals were retested in the MWM beginning on P43 in a 244 cm water tank. Only the ER0.71-0.82 animals had longer path lengths than the SAL animals (p < 0.0001; panel A), although the ER0.51-0.6 animals showed a trend (p = 0.1). No speed differences were detected during the relearning phase (panel B). For the memory trials, the ER0.71-0.82 animals had greater average distances compared with SAL animals (Figure 5C). ** p < 0.01, *** p < 0.0001, † p < 0.1 versus SAL.
For the probe trial, there was an effect on average distance from the former platform location, F(4,49) = 3.09, p < 0.03. This was attributable to deficits in the ER0.71-0.82 animals compared with SAL controls (Figure 6C). There were no other differences found on the probe trial (not shown).
Morris Water Maze Cued Learning
At least 1 h after the probe trial on P49, animals were tested for their ability to locate a cued platform. There was a main effect of group, F(4,51) = 85.64, P < 0.0001, and of block (p < 0.0001), but no interaction of the two. The ER0.71-0.82 animals were slower to reach the platform compared with SAL controls (Figure 7). The ER0.71-0.82 animals required almost the entire 2 min trial to locate the platform. None of the other kaolin-treated groups differed from SAL controls.
Figure 7.
Morris water maze cued platform was examined at least 1 h after the probe trial on P49. The ER0.71-0.82 animals were slower in locating the platform relative to the SAL animals (p < 0.0001). None of the other VM groups were different from the SAL animals. *** p < 0.0001, versus SAL.
Correlations with MRI data
A subset (n =6) of randomly selected controls and a subset of intentionally selected kaolin-treated animals (based on ventricle size being not so severe as to distort scans to the point that anatomical features could not be identified or DTI not performed) were scanned. The full MRI and DTI analysis of all these animals has been presented (Yuan et al., 2012). For the kaolin-treated group, only one animal was included from the ER0.71-0.82 set because the others were deemed too severe. In order to ensure that animals were representative of the groups of animals for behavioral learning, the data for the 18 animals used for DTI were reanalyzed with all kaolin-treated animals combined in a single group. For the P28 MWM test, the reconfigured kaolin-treated groups still had longer path lengths than SAL controls, F(1,16) = 9.26, p < 0.008), as found before. No differences were observed between the kaolin-treated animals and SAL controls at the later MWM testing age. This was not unexpected given that only one ER0.71-0.82 animal was included in the reconfigured kaolin-treated group.
A number of DTI parameters showed significant correlations with the path length during MWM acquisition using the subset of 18 rats. The corpus callosum genu and body fractional anisotropy (FA) values were negatively correlated (r = −0.52, p < 0.04 and r = −0.55, p < 0.02, respectively) and mean diffusivity (MD) positively correlated with path length (r = 0.63, p < 0.008 and r = 0.49, p < 0.04). No significant correlations were found for the splenium of the corpus callosum with FA or MD. For the anterior external capsule, FA was positively correlated with path length, r = 0.70, p < 0.002. No significant correlations were observed for the medial or posterior regions of the external capsule for FA or MD, and no significant correlation was observed for MD in the anterior external capsule. For the posterior internal capsule, both FA and MD were positively correlated with mean path length, r = 0.65, p < 0.004 and r = 0.67, p < 0.003, respectively. No significant correlations were observed for the anterior internal capsule or the fornix for FA or MD.
For the MWM retest, no significant correlations were observed for any region with path length. Since the most severely affected group (i.e., ER0.71-0.82) had only one animal represented, this is not unexpected.
Correlations of DTI and Evan's Ratio
All animals that received DTI were included in correlations for ER. In the corpus callosum both FA and MD showed correlations with ER. For FA, the genu was negatively correlated with ER, r = −0.77, p < 0.0005, as was the body of the corpus callosum, r = −0.89, p < 0.0001. MD was positively correlated with ER in the genu, r = 0.88, p < 0.0001, body, r = 0.81, p < 0.0001, and splenium, r = 0.50, p < 0.05. For the external capsule, only the anterior region showed a correlation with FA, r = 0.86, p < 0.0001, whereas none of the other external capsule regions correlated with ER for FA or MD. For the posterior internal capsule, FA was correlated with ER, r = 0.78, p < 0.001, as was MD, r = 0.46, p < 0.05. The fornix FA measurement was also correlated with ER, r = 0.61, p < 0.008. No other correlations were significant.
Discussion
This experiment examined the effects of VM induced by injection of kaolin in the cistern magna. It is recognized that VM can occur from a number of different causes, but we were interested in the outcome rather than the precipitating events. VM in humans is most likely to be diagnosed during infancy. Therefore we injected kaolin on P21, a period of brain development in the rat that is equivalent to human infancy brain development (Del Bigio et al., 1997b;Del Bigio and Zhang, 1998;Botfield et al., 2013). Consistent decreases in body weight over the length of the experiment were observed for all of the rats with induced VM, regardless of the severity of the enlargement. While body weights may be a good indicator of general health they did not have predictive value for cognitive ability. As shown in our data, the body weights of kaolin-injected rats were reduced both 1week and 3 weeks post VM induction when compared with SAL controls. While the decrease of MWM performance at 1 week post VM induction in kaolin injected rats was in line with the body weight change at the same time point, the change of MWM performance at 3 weeks post VM induction was variable among different groups: only rats in the ER0.71-0.82 group were found to be impaired in their ability to learn the MWM, whereas animals that had an ER of under 0.71 were able to perform as well as control animals, eliminating body weight as a cause of MWM deficits. Therefore, it appears that animals are more susceptible to negative behavioral effects during the early stages of ventricular enlargement. The reason for the more pervasive cognitive impairment at earlier time points may be the result of rate of change over time. It is known that serial lesions cause less cognitive impairment than single-stage lesions and that slow expanding lesions produce less functional loss than rapidly developing ones (Finger et al., 1973;Isseroff et al., 1976). Once the VM reaches maximum size and is no longer expanding by 3 weeks, only the group with the most severe VM showed lasting impairment, indicating that the other groups experienced functional recovery most likely through plasticity-mediated remodeling processes. It may be hypothesized that a rapid increase in intracranial pressure shortly after kaolin injection affected all groups similarly, but that over a longer period of time the intracranial pressure equilibrated. In this regard, it should be noted that others have demonstrated that VM remains relatively stable over a 2 week period following kaolin treatment (Khan et al., 2006). This may also explain the increased startle and PPI responses observed in animals at P28 with no differences in any of the animals at P42, including the ER0.71-0.82 group.
The animals with the largest Evan's ratio at the end of the study had the worst behavioral outcome. In particular the ER0.71-0.82 kaolin-treated animals showed no learning three weeks after surgery; an effect foreshadowed by the fact that this group started to learn at the earlier test age, then deteriorated (c.f., Figure 5). This is shown by the upturn of the learning curve after day 3, whereas animals in all other groups continued to decrease their path length to the platform. When the ER0.71-0.82 animals were retested 3 weeks after kaolin, they showed no learning curve. This striking effect may be the product of damage to WM pathways at this extreme degree of VM, and WM damage has been shown clearly by MRI in previous studies (Deren et al., 2010;Hale et al., 1992;Harris et al., 1996;Jones et al., 1993;Jones et al., 1995a;Jones et al., 1995b;Mangano et al., 1998;Miller and McAllister, 2007;Yuan et al., 2009;Yuan et al., 2010;Yuan et al., 2012). Sensorimotor connectivity impairments have been reported in infantile felines with kaolin-induced hydrocephalus (Eskandari et al., 2011) as have decreases in cortical norepinephrine levels via long periventricular white matter pathways (Lovely et al., 1989). A second possibility is that the hippocampus was damaged and therefore the most severe VM animals were unable to learn a spatial task such as the MWM. Ablation of the hippocampus produces severe deficits in learning and memory in the MWM (Morris et al., 1990;Morris et al., 1982) and deficits in spatial learning in rats after induction of infantile VM have been reported (Del Bigio and Massicotte, 2001;Jones et al., 1995b). These functional deficits correlate well with cellular changes observed in the hippocampal formation and fimbria. A third possibility is that the animals were visually impaired. We did not directly test the visual ability of animals, however, the findings of irreversible retinal ganglion cell degeneration in the cat (Williamson et al., 1992) suggests that our animals may not have fully intact vision. We tested the animals in the cued version of the MWM, where the platform location is marked by a cue immediately over the platform. The ER0.71-0.82 animals were unable to perform this task and required almost the entire 2 min time allowance to locate the platform, whereas all other animals completed this task in approximately 20 s. A test of visual capabilities should be included in future studies in order to determine if the visual cortex or pathways to the visual cortex are so compressed as to interfere with vision; proper vision being an absolute requirement for the performance of the MWM. The ER0.71-0.82 animals were also hyperactive especially at the later test age since they showed increased locomotor patterns compared with all other groups. Since the animals did not display habituation, this suggests that other neurological impairments may contribute to problems with performance in the task requirements of the MWM that are secondary to spatial learning per se. In agreement with this the ER0.71-0.82 animals took longer to locate the platform on trial 1 of the straight channel on P42 than animals in all other groups. Interestingly animals in the least severe group appeared to swim faster on the first trials of the straight channel compared with the other groups. In this study we did not hypothesize that there would be “beneficial” effects of VM and therefore only impairments from the kaolin injections were noted by the directional statistical testing. In regard to straight channel swimming, this is a control procedure to determine if affected animals have the same motivation to escape from water and/or swim differently than control animals. In this study, it appears the least severe VM group swam faster on the first couple of trials in the straight channel relative to all other groups. Importantly, no differences in swimming speed during learning in the MWM were detected among groups, including the most severely affected animals suggesting that the increased speed to finish the straight channel did not transfer to the MWM during learning.
DTI scans were performed on a subset of animals after the end of behavioral testing. Significant correlations with FA and MD for regions of the corpus collosum, internal capsule, and external capsule to path length during the first MWM test were found, whereas no significant correlations were observed for when the animals were retested at the later age. The latter absence of significant correlation is most likely the product of having only one ER0.71-0.82 animal scanned. The earlier age correlations suggest that compression of major white matter tracks that communicate between regions involved in neural circuits subserving complex cognitive abilities such as spatial learning and memory are severely disrupted when the nuclei that mediate these functions are uncoupled.
Limitations of the study include the inability to predict the degree of kaolin-induced VM thereby requiring ex post facto sorting of the data. Although the total amount of kaolin injected was kept the same for all VM rats, and we confirmed the location of kaolin accumulation at the pathway between the fourth ventricle and the spinal canal in most VM rats (in some rats this location was not included in the FOV during the scan), the amount of kaolin accumulated at this location is a possible factor for ventricle size. However, the imaging data (both T2-w and DTI) were acquired with a slice thickness of 0.5 mm, which did not allow for an accurate quantification to correlate kaolin volume and VM severity. The accuracy of ER is another factor that needs to be taken into consideration. In the present study, the measurement of ER was performed by an experienced operator and author (WY) with additional guidance from authors JPM and FTM. The test-retest error was controlled below 0.01-0.02 for this ratio. Therefore, it is not expected that human error in determining ER would significantly impact the overall results. If we were able to predict VM, group sizes could be better determined to maximize statistical sensitivity without having to resort to treating larger numbers of animals than would be necessary to ensure adequate numbers at each ER strata. Another consideration is that we only tested behavioral outcomes for 3 weeks following the kaolin treatment, therefore we do not know from these experiments whether there are longer-term impacts on cognition that would be manifest if the VM continued. Similarly, animals were tested only for allocentric learning in the MWM without examination of other types of learning (i.e., egocentric, object recognition, working memory, conditioned fear, etc.). While we examined locomotor activity and sensorimotor gating using PPI, we did not test vision, attention, discrimination, impulsivity, or other behaviors that might be affected by VM.
The data suggest that greater attention should be given to the rate of ventricular expansion as well as degree of expansion, as rate may predict at least short-term cognitive impairment. Future experiments should evaluate the rate of expansion and intracranial pressure beginning soon after kaolin injection and continuing through behavioral testing. In this experiment we did not want to confound behavioral outcomes with multiple exposures to anesthetic and therefore only assessed the ER at the end of testing. Future studies should also test the reversibility of the neurologic deficits once intracranial pressure is normalized by placing a shunt into the ventricles. The animals with the most severe change in ER also showed the greatest deficits in learning in the MWM. The ability to predict the severity of VM early in its progression would be beneficial to determine the requirement for shunt placement. This is especially important given that children's development occurs over longer time scales than rats, and therefore early learning deficits may be more profound, since unlike rodents, cumulative knowledge for later learning is essential in humans. The data also suggest that DTI may have predictive value for cognitive effects. While the present study was limited in this respect by the DTI data being at the end and hence could only be used retrospectively, the correlations suggest that a prospective use of DTI in such a study combined with behavioral assessment could provide new insights into the prognostic potential of DTI in hydrocephalus and VM. The current study lays the foundation for additional research evaluating the reversal of VM with CSF diversion using cognitive and radiologic factors as end points to allow us to gain further insight into the long-term effects of surgical treatment at the onset of VM versus treatment after VM has been extant for long periods of time. While it is clear that severe VM had effects that were long-lasting, future studies should not regard the least severe or moderate as having no effects especially considering the early deficits observed in even the least severe group in the early stages of VM. Cognitive testing of animals with or without treatment (i.e., shunts) in adulthood is an important next step in determining the long-term consequences of even the mildest forms of VM. Neurobehavioral testing and evaluation of specific MRI sequences to compare treatment results will be a significant advance in understanding hydrocephalus in a translational model.
Highlights.
Kaolin injected on postnatal day (P)21 to induce ventriculomegaly
Kaolin animals divided based on severity of ventriculomegaly
Kaolin animals had increased startle response at P28 except the least severe group
Kaolin animals had Morris water maze deficits when tested on P28
The most severe ventriculomegaly animals were unable to learn in the MWM on P42
Acknowledgements
The authors would like to acknowledge support from NIH training grant ES07051, the Division of Neurology at Cincinnati Children's Research Foundation, and the Department of Neurosurgery at the University of Utah.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Air EL, Yuan W, Holland SK, Jones BV, Bierbrauer K, Altaye M, Mangano FT. Longitudinal comparison of pre- and postoperative diffusion tensor imaging parameters in young children with hydrocephalus. J Neurosurg Pediatr. 2010;5:385–391. doi: 10.3171/2009.11.PEDS09343. [DOI] [PubMed] [Google Scholar]
- Botfield H, Gonzalez AM, Abdullah O, Skjolding AD, Berry M, McAllister JP, Logan A. Decorin prevents the development of juvenile communicating hydrocephalus. Brain. 2013;136:2842–2858. doi: 10.1093/brain/awt203. [DOI] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang HF, Russell JH, Cross AH, Song SK. Axonal injury detected by in vivo diffusion tensor imaging correlates with neurological disability in a mouse model of multiple sclerosis. NMR Biomed. 2008;21:589–597. doi: 10.1002/nbm.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chovanes GI, McAllister JP, Lamperti AA, Salotto AG, Truex RC., Jr. Monoamine alterations during experimental hydrocephalus in neonatal rats. Neurosurgery. 1988;22:86–91. doi: 10.1227/00006123-198801010-00014. [DOI] [PubMed] [Google Scholar]
- Chumas PD, Drake JM, Del Bigio MR, Da SM, Tuor UI. Anaerobic glycolysis preceding white-matter destruction in experimental neonatal hydrocephalus. J Neurosurg. 1994;80:491–501. doi: 10.3171/jns.1994.80.3.0491. [DOI] [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR. Cellular damage and prevention in childhood hydrocephalus. Brain Pathol. 2004;14:317–324. doi: 10.1111/j.1750-3639.2004.tb00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR, Crook CR, Buist R. Magnetic resonance imaging and behavioral analysis of immature rats with kaolin-induced hydrocephalus: pre- and postshunting observations. Exp Neurol. 1997a;148:256–264. doi: 10.1006/exnr.1997.6644. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR, Kanfer JN, Zhang YW. Myelination delay in the cerebral white matter of immature rats with kaolin-induced hydrocephalus is reversible. J Neuropathol Exp Neurol. 1997b;56:1053–1066. doi: 10.1097/00005072-199709000-00010. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR, Massicotte EM. Protective effect of nimodipine on behavior and white matter of rats with hydrocephalus. J Neurosurg. 2001;94:788–794. doi: 10.3171/jns.2001.94.5.0788. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR, Wilson MJ, Enno T. Chronic hydrocephalus in rats and humans: white matter loss and behavior changes. Ann Neurol. 2003;53:337–346. doi: 10.1002/ana.10453. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR, Zhang YW. Cell death, axonal damage, and cell birth in the immature rat brain following induction of hydrocephalus. Exp Neurol. 1998;154:157–169. doi: 10.1006/exnr.1998.6922. [DOI] [PubMed] [Google Scholar]
- Deren KE, Forsyth J, Abdullah O, Hsu EW, Klinge PM, Silverberg GD, Johanson CE, McAllister JP. Low levels of amyloid-beta and its transporters in neonatal rats with and without hydrocephalus. Cerebrospinal Fluid Res. 2009;6:4. doi: 10.1186/1743-8454-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deren KE, Packer M, Forsyth J, Milash B, Abdullah OM, Hsu EW, McAllister JP. Reactive astrocytosis, microgliosis and inflammation in rats with neonatal hydrocephalus. Exp Neurol. 2010;226:110–119. doi: 10.1016/j.expneurol.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Ding Y, McAllister JP, Yao B, Yan N, Canady AI. Axonal damage associated with enlargement of ventricles during hydrocephalus: a silver impregnation study. Neurol Res. 2001a;23:581–587. doi: 10.1179/016164101101199045. [DOI] [PubMed] [Google Scholar]
- Ding Y, Yao B, Lai Q, McAllister JP. Impaired motor learning and diffuse axonal damage in motor and visual systems of the rat following traumatic brain injury. Neurol Res. 2001b;23:193–202. doi: 10.1179/016164101101198334. [DOI] [PubMed] [Google Scholar]
- Eskandari R, Harris CA, McAllister JP. Reactive astrocytosis in feline neonatal hydrocephalus: acute, chronic, and shunt-induced changes. Childs Nerv Syst. 2011;27:2067–2076. doi: 10.1007/s00381-011-1552-4. [DOI] [PubMed] [Google Scholar]
- Finger S, Walbran B, Stein DG. Brain damage and behavioral recovery: serial lesion phenomena. Brain Res. 1973;63:1–18. doi: 10.1016/0006-8993(73)90072-3. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Bohan TP, Brandt ME, Brookshire BL, Beaver SR, Francis DJ, Davidson KC, Thompson NM, Miner ME. Cerebral white matter and cognition in hydrocephalic children. Arch Neurol. 1992;49:818–824. doi: 10.1001/archneur.1992.00530320042010. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Bohan TP, Brandt ME, Kramer LA, Brookshire BL, Thorstad K, Davidson KC, Francis DJ, McCauley SR, Baumgartner JE. Morphometric evaluation of the hydrocephalic brain: relationships with cognitive development. Childs Nerv Syst. 1996;12:192–199. doi: 10.1007/BF00301250. [DOI] [PubMed] [Google Scholar]
- Hale PM, McAllister JP, Katz SD, Wright LC, Lovely TJ, Miller DW, Wolfson BJ, Salotto AG, Shroff DV. Improvement of cortical morphology in infantile hydrocephalic animals after ventriculoperitoneal shunt placementI. Neurosurgery. 1992;31:1085–1096. doi: 10.1227/00006123-199212000-00015. [DOI] [PubMed] [Google Scholar]
- Harris NG, McAllister JP, Conaughty JM, Jones HC. The effect of inherited hydrocephalus and shunt treatment on cortical pyramidal cell dendrites in the infant H-Tx rat. Exp Neurol. 1996;141:269–279. doi: 10.1006/exnr.1996.0161. [DOI] [PubMed] [Google Scholar]
- Hwang YS, Shim I, Chang JW. The behavioral change of locomotor activity in a kaolin-induced hydrocephalus rat model: evaluation of the effect on the dopaminergic system with progressive ventricle dilatation. Neurosci Lett. 2009;462:198–202. doi: 10.1016/j.neulet.2009.07.039. [DOI] [PubMed] [Google Scholar]
- Hwang YS, Shim I, Chang JW. Anxiety responses and neurochemical changes in a kaolin-induced rat model of hydrocephalus. J Neurosurg Pediatr. 2011;7:401–407. doi: 10.3171/2011.1.PEDS10182. [DOI] [PubMed] [Google Scholar]
- Isseroff A, Leveton L, Freeman G, Lewis ME, Stein DG. Differences in the behavioral effects of single-stage and serial lesions of the hippocampus. Exp Neurol. 1976;53:339–354. doi: 10.1016/0014-4886(76)90076-5. [DOI] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Jones HC, Briggs RW, Harris NG. Inherited hydrocephalus in H-Tx rat pups: treatment monitored with magnetic resonance imaging. Eur J Pediatr Surg 3 Suppl. 1993;1:29–30. [PubMed] [Google Scholar]
- Jones HC, Harris NG, Briggs RW, Williams SC. Shunt treatment at two postnatal ages in hydrocephalic H-Tx rats quantified using MR imaging. Exp Neurol. 1995a;133:144–152. doi: 10.1006/exnr.1995.1017. [DOI] [PubMed] [Google Scholar]
- Jones HC, Rivera KM, Harris NG. Learning deficits in congenitally hydrocephalic rats and prevention by early shunt treatment. Childs Nerv Syst. 1995b;11:655–660. doi: 10.1007/BF00300725. [DOI] [PubMed] [Google Scholar]
- Khan OH, Enno T, Del Bigio MR. Tacrolimus and cyclosporine A are of no benefit to young rats with kaolin-induced hydrocephalus. Pediatr Neurosurg. 2003;39:309–313. doi: 10.1159/000075259. [DOI] [PubMed] [Google Scholar]
- Khan OH, Enno TL, Del Bigio MR. Brain damage in neonatal rats following kaolin induction of hydrocephalus. Exp Neurol. 2006;200:311–320. doi: 10.1016/j.expneurol.2006.02.113. [DOI] [PubMed] [Google Scholar]
- Kuchiwaki H, Nagasaka M, Inao S, Sugita K. Progression of kaolin-induced hydrocephalus and changes in performance of operant tasks by rats. J Neurol Sci. 1994;121:32–38. doi: 10.1016/0022-510x(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Lovely TJ, McAllister JP, Miller DW, Lamperti AA, Wolfson BJ. Effects of hydrocephalus and surgical decompression on cortical norepinephrine levels in neonatal cats. Neurosurgery. 1989;24:43–52. doi: 10.1227/00006123-198901000-00007. [DOI] [PubMed] [Google Scholar]
- Mangano FT, McAllister JP, Jones HC, Johnson MJ, Kriebel RM. The microglial response to progressive hydrocephalus in a model of inherited aqueductal stenosis. Neurol Res. 1998;20:697–704. doi: 10.1080/01616412.1998.11740586. [DOI] [PubMed] [Google Scholar]
- McAllister JP, Cohen MI, O'Mara KA, Johnson MH. Progression of experimental infantile hydrocephalus and effects of ventriculoperitoneal shunts: an analysis correlating magnetic resonance imaging with gross morphology. Neurosurgery. 1991;29:329–340. [PubMed] [Google Scholar]
- McAllister JP, Maugans TA, Shah MV, Truex RC., Jr. Neuronal effects of experimentally induced hydrocephalus in newborn rats. J Neurosurg. 1985;63:776–783. doi: 10.3171/jns.1985.63.5.0776. [DOI] [PubMed] [Google Scholar]
- Miller JM, McAllister JP. Reduction of astrogliosis and microgliosis by cerebrospinal fluid shunting in experimental hydrocephalus. Cerebrospinal Fluid Res. 2007;4:5. doi: 10.1186/1743-8454-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Schenk F, Tweedie F, Jarrard LE. Ibotenate Lesions of Hippocampus and/or Subiculum: Dissociating Components of Allocentric Spatial Learning. Eur J Neurosci. 1990;2:1016–1028. doi: 10.1111/j.1460-9568.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Richards HK, Bucknall RM, Jones HC, Pickard JD. Uncoupling of LCBF and LCGU in two different models of hydrocephalus: a review. Childs Nerv Syst. 1995;11:288–292. doi: 10.1007/BF00301762. [DOI] [PubMed] [Google Scholar]
- Shirane R, Sato S, Sato K, Kameyama M, Ogawa A, Yoshimoto T, Hatazawa J, Ito M. Cerebral blood flow and oxygen metabolism in infants with hydrocephalus. Childs Nerv Syst. 1992;8:118–123. doi: 10.1007/BF00298263. [DOI] [PubMed] [Google Scholar]
- Tashiro Y, Drake JM. Reversibility of functionally injured neurotransmitter systems with shunt placement in hydrocephalic rats: implications for intellectual impairment in hydrocephalus. J Neurosurg. 1998;88:709–717. doi: 10.3171/jns.1998.88.4.0709. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Herring NR, Schaefer TL, Grace CE, Skelton MR, Johnson HL, Williams MT. Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: effects of dose and rearing conditions. Int J Dev Neurosci. 2008;26:599–610. doi: 10.1016/j.ijdevneu.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson EC, Pearson HE, McAllister JP. Gliosis and ganglion cell death in the developing cat retina during hydrocephalus and after decompression. Brain Res Dev Brain Res. 1992;70:47–52. doi: 10.1016/0165-3806(92)90102-3. [DOI] [PubMed] [Google Scholar]
- Yuan W, Deren KE, McAllister JP, Holland SK, Lindquist DM, Cancelliere A, Mason M, Shereen A, Hertzler DA, Altaye M, Mangano FT. Diffusion tensor imaging correlates with cytopathology in a rat model of neonatal hydrocephalus. Cerebrospinal Fluid Res. 2010;7:19. doi: 10.1186/1743-8454-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Mangano FT, Air EL, Holland SK, Jones BV, Altaye M, Bierbrauer K. Anisotropic diffusion properties in infants with hydrocephalus: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2009;30:1792–1798. doi: 10.3174/ajnr.A1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, McAllister JP, Lindquist DM, Gill N, Holland SK, Henkel D, Rajagopal A, Mangano FT. Diffusion tensor imaging of white matter injury in a rat model of infantile hydrocephalus. Childs Nerv Syst. 2012;28:47–54. doi: 10.1007/s00381-011-1590-y. [DOI] [PubMed] [Google Scholar]