Abstract
Microtubules (MTs)1, cytoskeletal elements found in all mammalian cells, play a significant role in cell structure and in cell division. They are especially critical in the proper functioning of post-mitotic central nervous system neurons, where MTs serve as the structures on which key cellular constituents are trafficked in axonal projections. MTs are stabilized in axons by the MT-associated protein tau, and in several neurodegenerative diseases, including Alzheimer’s disease, frontotemporal lobar degeneration, and Parkinson’s disease, tau function appears to be compromised due to the protein dissociating from MTs and depositing into insoluble inclusions referred to as neurofibrillary tangles. This loss of tau function is believed to result in alterations of MT structure and function, resulting in aberrant axonal transport that likely contributes to the neurodegenerative process. There is also evidence of axonal transport deficiencies in other neurodegenerative diseases, including amyotrophic lateral sclerosis and Huntington’s disease, which may result, at least in part, from MT alterations. Accordingly, a possible therapeutic strategy for such neurodegenerative conditions is to treat with MT-stabilizing agents, such as those that have been used in the treatment of cancer. Here, we review evidence of axonal transport and MT deficiencies in a number of neurodegenerative diseases, and summarize the various classes of known MT-stabilizing agents. Finally, we highlight the growing evidence that small molecule MT-stabilizing agents provide benefit in animal models of neurodegenerative disease and discuss the desired features of such molecules for the treatment of these central nervous system disorders.
1.1 Microtubules and their role in neuronal axons
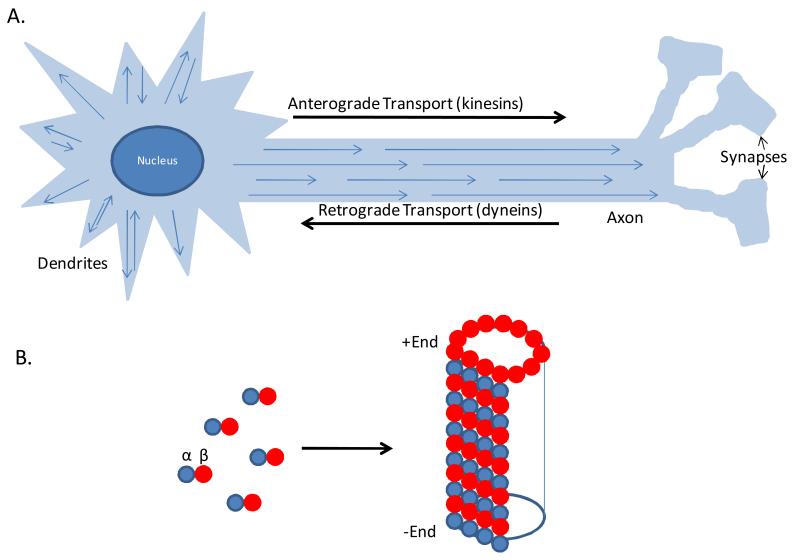
Microtubules (MTs) comprise a key cytoskeletal component of all eukaryotic cells, as they play an integral role in the process of mitosis through their involvement in the segregation of chromosomes along mitotic spindles in dividing cells1. In addition to their role in mitosis, MTs also provide structural and functional support in cells; this is particularly evident in the nervous system, where MTs play a fundamental role in the health of neurons2. The axons of neurons can extend great distances (up to 3 feet for certain motor neurons in humans), and thus vital cellular components, including nutrients, mitochondria, proteins, mRNA and growth factors, must be shuttled to and from the cell body along these axonal projections. The transport of these species is largely dependent on either fast or slow axonal transport that is mediated by molecular motors that move their associated cargo along the MTs within the axonal processes. In particular, the kinesin family of MT-associated motors are involved in anterograde transport (i.e., away from the cell body)3, whereas the dynein motors direct retrograde transport4 toward the cell body (Figure 1a).
Figure 1.
A. Schematic of a neuron with microtubules (MTs) within axonal and dendritic processes. Arrowheads represent the (+) end of MTs, with dendrites containing both (+)-end distal and (−)-end distal MTs. Distinct molecular motors transport cellular cargo in the anterograde (kinesins) and retrograde (dyneins) directions along MTs. B. MTs are comprised of aligned protofilaments comprised of α- and β-tubulin heterodimers, with exposed β-tubulin at the (+) end and α-tubulin at the (−) end.
MTs are typically composed of 13 aligned protofilaments, with each protofilament comprised of a polymer of repeating α- and β-tubulin heterodimers5, 6 (Figure 1b). There are a number of α and β tubulin isoforms in mammals which may confer subtle changes to MT structure or function, although the exact significance of these differing isotypes is largely unknown6. The assembly of tubulin heterodimers into MTs is typically initiated at microtubule organizing centers (MTOC), with the addition of α/β heterodimers that contain one GTP each per α and β tubulin subunit to a growing MT in an outward direction, such that β-tubulin is exposed at the “plus” end, whereas the MTOC-associated “minus” end has a terminal α-tubulin. Thus, in most cells the minus end is typically near the nucleus. However, in neurons, MTs are discontinuous along the axonal and dendritic processes, such that there are multiple minus and plus ends (Figure 1a) and a traditional MTOC may not persist as neurons mature7, 8. The plus end of MTs in neurons thus project outward along the axon towards the terminus9. MTs exhibit a feature known as “dynamic instability”, in which a given MT will undergo periods of growth followed by times of disassembly5. This results from the hydrolysis of GTP to GDP within β-tubulin subunits, as the conversion of the terminal plus-end β-tubulin GTP to GDP, prior to the addition of another GTP-containing heterodimer, can lead to MT depolymerization. Such disassembly occurs less frequently at the minus-end, presumably because this end is typically stabilized by a MTOC, or perhaps by alternative nucleation sites in neurons7, 8. MTs can also undergo a process referred to as “treadmilling”, in which growth at the plus-end is accompanied by shortening at the minus-end, and this behavior may be important during mitosis5, 6. In neurons, MTs appear to have greater stability than in many other cell types, and thus the extent of MT “dynamicity” is reduced. This MT stability is due, at least in part, to a number of MT-associated proteins (MAPs) that interact with MTs within neurons, with tau protein playing the predominate role in stabilizing MTs in axons6, 10-12.
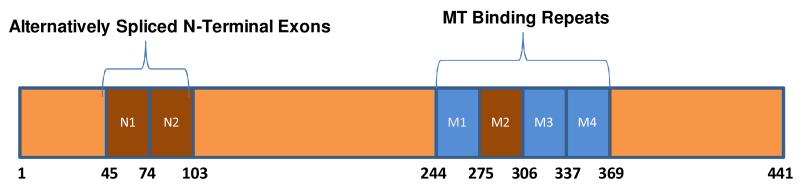
In humans, six isoforms of tau are generated via differential mRNA splicing that contain either 3 (3R) or 4 (4R) repeated (although non-identical) microtubule-binding domains13-15 (Figure 2). Perhaps unsurprisingly, 4R tau species appear to bind with greater avidity to MTs than do 3R isoforms16, and the balance of 4R-to-3R isoforms of tau seems to be tightly regulated such that in human neurons there are approximately equal concentrations of 4R and 3R isoforms as a group, but not individually with respect to each of the 6 isoforms17. In addition to the presumed role in providing stabilization to axonal MTs, tau also appears to regulate the interaction of kinesin with MTs. For example, tau over-expression in cultured neurons reduces kinesin engagement with MTs18, 19 and alters mitochondrial transport20. Likewise, physiological concentrations of tau can alter kinesin-mediated transport using a purified protein system21. There is also some evidence that tau may interact with dynactin22, which plays a role in dynein-mediated transport. Thus, it would appear that tau plays a critical role in axonal transport through both the stabilization of MTs as well as through the regulation of MT motor protein interactions.
Figure 2.
Schematic of human tau. The inclusion or exclusion of the second MT-binding repeat (M2) encoded by exon 10 of the tau gene results in 4R or 3R tau species. Additional isoforms are created by the inclusion or exclusion of two coding exons (N1 and N2) in the amino-terminal region of tau. Amino acid numbers refer to the longest tau isoform.
2.1 Evidence of MT and axonal transport dysfunction in neurodegenerative disease
The critical role of MTs in axonal transport suggests that any significant perturbation of MT structure or function could be highly detrimental to neurons. Similarly, alterations of normal MT motor function would compromise axonal transport and have negative consequences on neuronal physiology. In fact, transgenic (Tg) mice with altered dynactin23, 24 display a neurodegenerative phenotype, with targeted deletion of kinesin function also inducing axonal degeneration in mice25. Moreover, mutations that result in altered dynein or kinesin function have been implicated in a number of neurodegenerative diseases (reviewed in 26). These data point to the criticality of the axonal transport process to neuronal function and survival. Indeed, there is compelling evidence of MT and/or axonal transport deficiencies in a number of neurodegenerative diseases, as briefly reviewed below.
2.1.1 Alzheimer’s disease (AD) and related tauopathies
AD is the most common neurodegenerative condition in the world, with ~5 million cases in the United States. A key pathological hallmark within the AD brain is the presence of neurofibrillary tangles (NFTs) and neuropil threads comprised of fibrillar inclusions of tau protein within neuronal cell bodies and processes, respectively13. As noted, tau is a MAP that appears to stabilize MTs and modulate MT motor function in axons, and tau inclusions are also found in several additional “tauopathies”, such as frontotemporal lobar degenerative conditions that include Pick’s disease, corticobasal syndrome and progressive supranuclear palsy27, 28. Tau aggregates are thought to contribute to the neuronal loss observed in these diseases, a hypothesis substantially bolstered by the finding that mutations in tau can result in inherited frontotemporal degeneration with Parkinsonism linked to chromosome 17 (FTDP-17)17, 29. Many of the mutations in FTDP-17 promote tau disengagement from MTs30, 31 and some also enhance fibrillization of tau32, 33. Moreover, the hyperphosphorylation of tau that is observed in AD and the other tauopathies generally decreases the avidity of tau for MTs34-37. Increased phosphorylation at certain residues can also enhance tau fibrillization38, 39. Thus, there are two potentially detrimental consequences of tau disengagement from MTs and aggregation into NFTs and neuropil threads. First, misfolded oligomeric or fibrillar tau may exert a toxic effect within neurons40. In addition, the dissociation of tau from MTs, with subsequent sequestration into insoluble inclusions, likely results in a loss-of-function that causes MT and axonal transport abnormalities.
This latter hypothesis is supported by a number of observations in AD brain and in Tg mouse models of tauopathy. For example, there is evidence of a reduction in both the number and length of MTs in AD brain41, as well as a decrease in acetylated α-tubulin, which is considered a marker of stable MTs42. Similarly, the amount of isolated tau that is competent to bind to MTs is reduced in extracts from AD brain relative to control brain37. Moreover, a reduction in MT density has been observed in tau Tg mouse models in which tau inclusions form with age43, 44. Finally, a recent study has revealed an increase of MT dynamicity in two established Tg mouse models of tauopathy45.
2.1.2 Parkinson’s Disease (PD)
PD is a progressive neurodegenerative condition in which intracellular inclusions comprised of α-synuclein, referred to as Lewy bodies, accumulate within neurons. PD is characterized by motor deficits, with primary involvement of dopaminergic neurons within the substantia nigra, although other neuronal systems are also often affected46. There is evidence from both cellular and animal models of PD that neuronal function may be compromised as a result of MT and axonal transport deficits. PD is often modeled through the treatment of neuron cultures or rodents with environmental toxins such as rotenone, as these agents induce dopaminergic neuropathology that resembles certain aspects of PD47. Interestingly, rotenone appears to directly affect MT polymerization48-50, as do several other toxins used in PD models, including MPTP51, 52 and certain herbicides53, 54.
A number of recent reports also suggest a link between α-synuclein pathology and MT dysfunction. For example, there is evidence of impaired axonal transport in Tg mice expressing a mutant form of α-synuclein (A53T) that is found in inherited PD55. Interestingly, the effect of α-synuclein on axonal transport may be mediated by alterations in tau, as a reduction of normal tau function has been observed in both cellular and animal models of PD. For example, increased hyperphosphorylated tau has been reported in several brain regions of aged Tg mice expressing wild-type human α-synuclein, with a consequent reduction of MT-bound tau and an increase of depolymerized tubulin56, 57. Similarly, increased tau phosphorylation was observed in the striatum of a Tg mouse model expressing A53T mutant human α-synuclein; again, there was a decrease of MT-associated tau58. These studies follow earlier observations which revealed that Tg mice expressing A30P or A53T human α-synuclein developed hyperphosphorylated tau or tau inclusions that paralleled the accumulation of α-synuclein aggregates59, 60. It has also been suggested that the increases of tau phosphorylation observed in the α-syn Tg mice may result from α-synuclein-mediated activation of tau kinases, including glycogen synthase kinase 3β56, 58 and protein kinase A61. It is thus interesting that a recent study62 revealed that the kinase LRRK2, which is linked to inherited PD63, can phosphorylate MT-bound tau and reduce tau-MT interaction. Moreover, PD-associated LRRK2 mutations further increased this tau phosphorylation62. Notably, LRRK2 mutations that are found in PD appear to enhance the binding of LRRK2 to MTs64, perhaps providing an explanation for the increased tau phosphorylation that was observed upon expression of mutated LRRK262. Finally, cerebrospinal fluid tau levels are reduced in early PD in parallel with reduced levels of and α-synuclein and Aβ65, and a novel cerebrospinal fluid-based biomarker methodology suggests that there is a deficit of axonal transport in PD patients relative to non-PD control subjects55.
2.1.3 Amyotrophic Lateral Sclerosis (ALS)
ALS is a late-onset neurodegenerative disorder that affects motor neurons, typically with a rapid disease progression. Given the extreme length of motor neurons projecting to the extremities, axonal transport is particularly crucial to the functioning and health of these cells. The majority of ALS cases are sporadic, although ~10% are inherited, with multiple gene mutations linked to the disease66. The first mutations described in familial ALS were in the superoxide dismutase-1 (SOD1) gene, and several Tg mouse models have been created in which mutant SOD1 is expressed67. These mice develop motor neuron disease, with several reports revealing that axonal transport deficiencies develop relatively early in these models68-70. The mechanisms by which mutated SOD1 affects axonal transport are not fully understood, and there are likely multiple contributing factors, including ATP deficits, altered motor protein function, damage to transport cargo and/or MT abnormalities71. With regard to the latter possibility, there is evidence of increased MT dynamicity in SOD1 transgenic mice72. Although only ~2% of ALS patients have SOD1 mutations, axonal transport deficiencies may be a common feature found within other inherited and sporadic cases of ALS. For example, mutations in the dynactin subunit p150Glued can cause inherited ALS23, 24, directly implicating alterations in dynein/dynactin-mediated MT transport in the disease.
2.1.4 Huntington’s Disease (HD)
HD is an autosomal-dominant inherited neurodegenerative condition caused by the aggregation within neurons of mutated huntingtin protein containing polyglutamine repeats at the amino-terminus, which result from expanded CAG repeats in the first exon of the huntingtin gene73. There is evidence of altered axonal transport in cells that express mutated huntingtin, with a diminution of mitochondrial74, 75 and vesicular75 transport in primary neurons. Similarly, altered axonal transport has been measured in Tg mice that express mutated hutingtin, with an onset that precedes motor symptoms in the mice75. Notably, a similar alteration of axonal transport has been observed both in Drosophila76 and in mice in which expression of huntingtin was reduced75, suggesting that the protein may normally play a role in axonal transport and that the accumulation of insoluble huntingtin aggregates results in a loss of function. This is consistent with the findings that huntingtin interacts with the Huntingtin-Associated Protein 1 (HAP1), which binds the p150Glued subunit of dynactin77, 78. Moreover, HAP1 has also been reported to interact with kinesin light chain79. Interestingly, these proteins, along with tubulin, are found associated with insoluble htt in extracts from HD brain75, consistent with a sequestration of these components within huntingtin aggregates that may result in an alteration of axonal transport and perhaps MT structure.
2.1.5 Summary
Faulty axonal transport is a recurring theme in the neurodegenerative diseases discussed above, as well as in neurodegenerative conditions not discussed here, such as the demyelinating disorders Charcot-Marie Tooth disease71 and multiple sclerosis80. In general, these reductions of transport can be attributed to deficiencies in motor protein function and/or alterations of MT structure. Among the major neurodegenerative conditions discussed here, the evidence of MT deficiencies is arguably the greatest for the tauopathies and PD, followed by ALS. There is little evidence to support a fundamental defect in MT structure in HD, although the observation that tubulin can be found associated with huntingtin deposits75 might suggest the possibility of MT alterations in this disease.
The compelling data supporting a MT deficiency in tauopathies that results from a loss of tau function led to the hypothesis, first published nearly two decades ago81, that MT-stabilizing drugs may have utility in the treatment of these disorders. Given the accumulating evidence of possible MT deficits in other neurodegenerative diseases, it is possible that MT-stabilizing agents may have applicability beyond the tauopathies. MT-stabilizing drugs have been used for the treatment of cancers for some time, as exemplified by the taxane family members, paclitaxel and docetaxel82, 83. Below, we provide an overview of the various classes of known MT-stabilizing molecules, followed by a review of the scientific evidence that supports the potential utility of such compounds for the treatment of neurodegenerative disease.
3.1 An overview of MT-stabilizing molecules
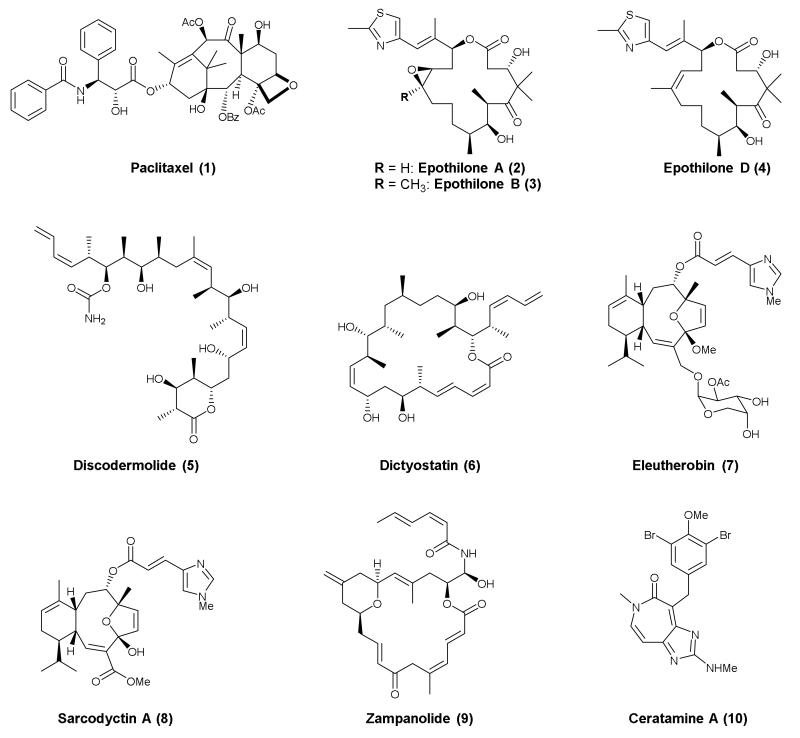
Since the discovery of paclitaxel (Taxol®, 1, Figure 3) in 196784, 85 and the subsequent elucidation of the MT-stabilizing properties of this natural product86, several additional classes of molecules, primarily natural products and derivatives thereof, have been identified that are functionally similar to paclitaxel in promoting MT stabilization87, 88. Paclitaxel stabilizes MTs by binding within the lumen of the MT at a site in the β-tubulin subunit, which is commonly referred to as the taxane site. The interaction of 1 with β-tubulin results in conformational changes in the M-loop of β-tubulin that ultimately stabilize lateral interactions of adjacent protofilaments89, 90. Representative compounds from the different classes of natural products that are found to interact within or in close proximity to the taxane site on MTs, producing taxol-like MT-stabilization, are shown in Figure 3. These include members of the epothilones91 [e.g., epothilone A (2), B (3), and D (4)], discodermolide92, 93 (5), dictyostatin94 (6), eleuthesides [e.g., eleutherobin95 (7) and sarcodyctin A96 (8)], zampanolide97, 98 (9) and ceratamines99 (e.g., ceratamine A, 10). For each of these classes, competition-binding experiments revealed that these compounds target binding sites that overlap with the taxane site found on β-tubulin. In addition, X-ray crystal structures of tubulin-bound 2 and 9 have confirmed that these compounds interact with the taxane binding site and promote the restructuring of the M-loop into a short helix structure98. Zampanolide, unlike 2, was found to bind covalently with the taxane binding site97. This is not the only example of a MT-stabilizing agent that covalently modifies tubulin, as cyclostreptin (11, Figure 4) was first reported as an alkylating MT-stabilizing agent. Notably, while 11 is also reported to compete with paclitaxel binding, the binding site of 11 has been localized at the surface of the MT at a site that may be important for the initial interaction of 1 with the MT, prior to its translocation to the luminal site100.
Figure 3.
Representative compounds from different classes of MT-stabilizing natural products that interact with the taxane binding site.
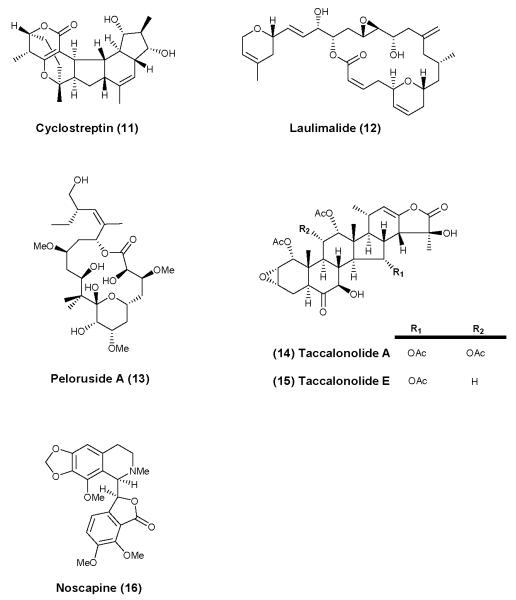
Figure 4.
Representative compounds from different classes of naturally occurring MT-stabilizing (11-15) or MT-modulating (16) agents that do not interact with the taxane binding site.
Among the MT-stabilizing natural products that do not interact with the taxane binding site, the most prominent examples are laulimalide (12) and peloruside (13), shown in Figure 4. Both of these compounds have been found to interact with β-tubulin at a shared site, localized at the surface of the MT101, that does not overlap with the taxane binding site. Synergistic effects on MT-stabilization have been described between drugs that interact with the taxane-binding site and 12 or 13102. An additional promising class of MT-stabilizing agents are the steroidal natural products, taccalonolides103. While the initially discovered taccalonolides A and E (14 and 15, respectively, Figure 4) were not highly potent MT-stabilizing agents, selected congeners have been identified which are potent both in cell-free and in cell-based assays104, 105. This compound class is still under investigation and the binding site for the taccalonolides has not yet been identified.
Finally, in addition to the abovementioned classes of natural products with MT-stabilizing activity, the opium alkaloid, noscapine (16, Figure 4), has been shown to modulate MT-dynamics, albeit without any significant impact on total MT mass. Although 16, which has been used for several years as an anti-tussive agent106, 107, does not seem to alter MT polymerization over a wide range of concentrations, more potent analogues have been identified which cause vinblastine-like depolymerization of MTs108. These findings indicate that 16 may be a weakly active MT-destabilizing agent, which mostly affects MTs at the level of dynamics rather than MT mass. Because of these properties and the favorable pharmacokinetic and safety features of noscapine, this compound has been investigated in the context of neurodegenerative diseases (vide infra)109.
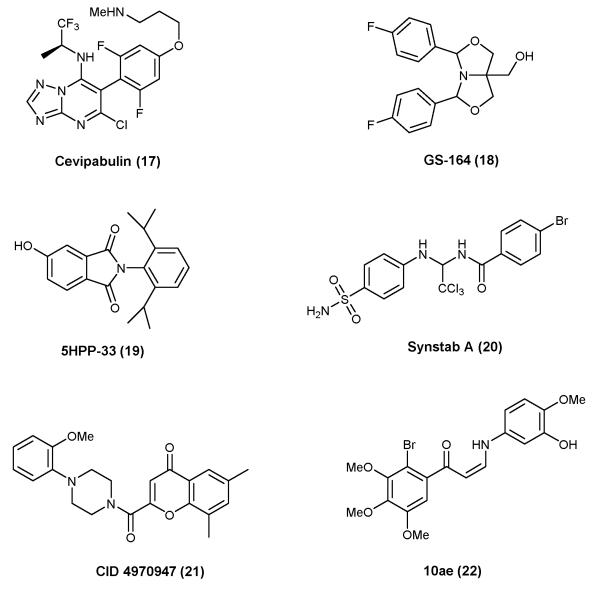
As summarized above, the vast majority of MT-stabilizing agents are naturally occurring compounds. However, significant progress has been made in the area of synthetic small molecules with MT-stabilizing properties. Among these, particularly interesting are the triazolopyrimidines, typified by cevipabulin110 (17, Figure 5). This compound has been reported to have a rather unique mode of action, as competition experiments revealed that 17 can displace vincristine, but not paclitaxel111. This observation suggests that 17 and possibly other related heterocyclic compounds112 bind to the vinca site on β-tubulin or to an allosteric binding site that when occupied may interfere with the binding of vincristine. Other interesting examples of synthetic small-molecule MT-stabilizing agents, shown in Figure 5, are GS-164 (18)113, phthalimide 5HPP-33 (19)114, Synstab (20)115, as well as the recently discovered molecules CID 4970947 (21)116 and the Z-1-Aryl-3-arylamino-2-propen-1-ones, such as 10ae (22)117.
Figure 5.
Representative examples of synthetic small molecule MT-stabilizing agents.
4.1 The potential of MT-stabilizing agents for the treatment of neurodegenerative disease
The concept of utilizing MT-stabilizing drugs for the treatment of neurodegenerative disease was first tested in a tau Tg mouse model in which NFT-like inclusions develop with age, primarily within neurons of the brain stem and spinal cord118. Administration of paclitaxel (1) to these mice resulted in an improvement of axonal transport and a reduction in the motor phenotype that develops as a result of tau inclusions within motor neurons, but did not attenuate tau pathology itself119. This study provided an important proof-of-principle that a MT-stabilizing agent could compensate for axonal transport deficits that presumably resulted from a destabilization of MTs after tau deposition into insoluble tangles. Moreover, as therapeutic benefit was achieved without a reduction of tau pathological burden, there data suggested that correcting a loss of tau function may be more critical than eliminating tau inclusions. However, 1, like many known MT-stabilizing agents, does not cross the blood-brain barrier effectively120, as would be required for treatment of human neurodegenerative disease, and presumably was efficacious in the aforementioned Tg mouse model because of uptake at neuromuscular junctions.
More recently, studies have demonstrated that the epothilone family of MT-stabilizing compounds are generally brain-penetrant120. Work from our laboratories demonstrated that epothilone D (4) is both efficacious and safe in Tg mouse models that develop tau inclusions within the brain when used at doses that are significantly lower than had been utilized in oncology clinical trials. Notably, 3 months of once-weekly administrations of 4 at doses that are ~1/100th the amounts utilized in cancer trials were found to increase MT density, reduce axonal dystrophy and improved cognitive performance in both preventative and interventional studies with tau Tg mice that develop NFT-like inclusions44, 121. Importantly, the intervention study demonstrated that 4 could improve axonal transport, reduce tau pathology and prevent the hippocampal neuron and synapse loss that is observed in these animals with age121. Another team has recently obtained similar results with 4 in two additional tau Tg mouse models45. Moreover, this group demonstrated that there was MT hyperdynamicity in these aged tau Tg mice, which was normalized by treatment with 4. Thus, the results obtained with 4 in these tau Tg mouse models provide important proof-of-principle that brain-penetrant MT-stabilizing agents have the potential for the treatment of tauopathies. Importantly, epothilone D (4) has since progressed to clinical testing in AD patients (http://clinicaltrials.gov/ct2/show/NCT01492374).
Epothilone D (4) has also recently undergone evaluation in an MPTP-induced mouse model of PD122. As noted previously, toxins such as rotenone and MPTP have been shown to affect MT dynamics48, 49, 51, 123, and rotenone-induced toxicity in dopaminergic midbrain neuron cultures can be abrogated by treatment with 1123. In this recent study, MPTP-treated mice showed an impairment of axonal transport in dopaminergic axons and changes in post-translational MT modifications that were normalized by treatment with 4122. Interestingly, MPTP treatment appeared to have a somewhat complicated effect on MTs, increasing the depolymerization of dynamic MTs while also causing enrichment of stable MTs, with a decrease in dynamicity 52, 122. Importantly, treatment with 4 partially prevented the decrease in dopamine levels and the loss of nigral dopaminergic neurons that was observed after MPTP treatment122. These data further extend the potential utility of 4 in neurodegenerative disease, and provide evidence of MT dysfunction in an animal model of PD. However, it will be important to further validate brain-penetrant MT-stabilizing agents in additional PD animal models, including Tg mice that express genes that are mutated in familial PD, such as α-synuclein and LRRK2.
There is also evidence that the MT-modifying agent, noscapine (16), can improve MT and axonal transport deficits in a mutant SOD1 Tg mouse model of ALS72. Interestingly, this study revealed that the MTs in axons from the spinal cord and sciatic nerve, as well as from the cortex, showed hyperdynamicity that appeared to manifest at an early age and increase with time. Moreover, 16 decreased the observed MT hyperdynamicity, improved axonal transport and delayed disease onset with an improvement of motor performance. As noted, 16 is reported to differ from typical MT-stabilizing compounds in that it does not promote MT polymerization, but rather modulates MT dynamics107. The change in MT dynamicity observed in the SOD1 Tg model bears resemblance to that found in Tg models of tauopathy45, where the MT-stabilizing agent 4 has proven effective44, 45, 121. This raises the question of whether the mechanism by which 4 improves outcomes in the various tauopathy models may in fact be more akin to the reported action of 16, with increases of MT-stabilization107, 108 perhaps being less important than a reduction of MT hyperdynamics. In this regard, doses of MT-modulating agents that are below those which promote MT assembly or disassembly are known to affect MT dynamics124. Thus, as discussed further below, an important feature of drugs to treat MT alterations in neurodegenerative disease may be to normalize dynamicity without over-stabilizing MTs.
5.1 Desired features of MT-stabilizing agents for neurodegenerative disease
As summarized above, there is growing evidence of the potential of MT-stabilizing compounds for the treatment of neurodegenerative disease; however, only a very limited number of example compounds have been tested in animal models of these diseases. As with nearly all therapeutics, MT-stabilizing agents are unlikely to fully compensate for the deficits observed in neurodegenerative disease. Rather, the hope is that drugs of this type will at least partially restore MT function, with meaningful improvements in patient outcomes. Ideal candidate compounds to treat MT alterations in neurodegenerative disease would be expected to: (A) cross the blood-brain barrier; (B) normalize MT dynamicity and perhaps also increase the stability of the MT system so as to restore effective axonal transport in diseased neurons; and (C) have little or no systemic toxicity at effective doses. In addition, it would be advantageous if MT-stabilizing agents with these properties could be administered orally for ease of administration to the generally elderly patients affected by neurodegenerative disease.
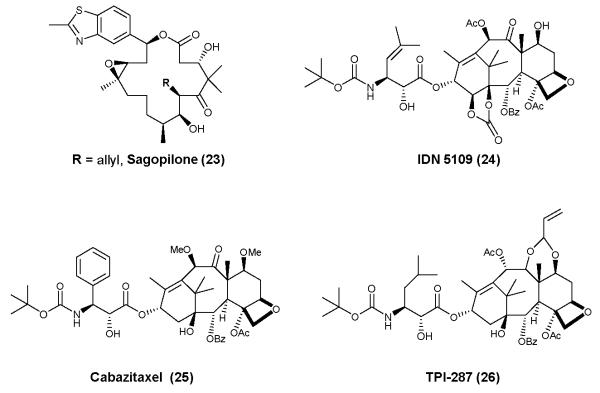
MT-stabilizing agents used to treat neurodegenerative diseases of the brain must be able to readily cross the blood-brain barrier so as to maintain a sufficient brain exposure to provide effective modulation of axonal MTs. Unfortunately, the majority of MT-stabilizing agents exhibit low or negligible brain exposure, due to unfavorable physical chemical properties that can hamper passive diffusion and/or to the molecules being P-glycoprotein substrates, such that they are actively transported back into the bloodstream. Nonetheless, several examples of brain-penetrant MT-stabilizing agents have been identified. In addition to different members of the epothilone class, such as 3, 4, and sagopilone (23, Figure 6)125, 126, selected paclitaxel derivatives have been developed which exhibit improved brain penetration. These include IDN-5109 (24)127, cabazitaxel (25)128, and TPI-287 (26)129 (Figure 6). Moreover, recent studies from our laboratories demonstrate that dictyostatin (6) crosses the blood-brain barrier in mice and maintains prolonged brain exposure and pharmacodynamic activity in a manner similar to epothilone D (4)130. In addition to good blood-brain barrier permeability, another potential desirable feature of MT-stabilizing drugs for the treatment of neurodegenerative disease is prolonged brain exposure relative to that in blood. For many CNS drugs, the pharmacokinetic profiles in the plasma and brain are similar, with comparable half-lives and clearance values. However, our studies with 444, 120 and more recently 6130 have demonstrated that these compounds show a brain retention that far exceeds their duration in the plasma. This differential between brain and plasma exposure for MT-stabilizing drugs may be advantageous, particularly in the context of neurodegenerative disease treatment. First, this property allows for less frequent drug administration, as exemplified by the once-weekly dosing of 4 in the studies conducted in tau Tg mice44, 121. In addition, extended brain exposure of MT-stabilizing drugs allows for lower overall doses and clearance of the drug from the blood and periphery, where dose-limiting side-effects are observed in cancer patients receiving drugs of this class.
Figure 6.
Selected brain-penetrant MT-stabilizing agents. These examples, like 3, 4 and 6 shown in Figure 3, have been reported to enter the brain.
In this regard, another important consideration is that the therapeutic regimens of MT-stabilizing agents for the treatment of neurodegenerative diseases are likely to be substantially different from those typically employed to treat cancer. Indeed, the objective would be to avoid triggering apoptosis of rapidly dividing cells, as in cancer treatment, so as to minimize side-effects and rather to normalize MTs and axonal transport in the axons of diseased neurons. As a result, optimal treatment of neurodegenerative diseases will likely require long-term administration of low doses of a MT-stabilizing drug. Although the safety and tolerability of chronic administration of low doses of MT-stabilizing agents has not yet been reported in humans, the absence of toxicities after multiple months of dosing in mice suggest that such treatments may not be associated with the severe side-effects caused by such drugs in cancer chemotherapy82, 131, 132. Nonetheless, it will be imperative to monitor the tolerability of low doses of MT-stabilizing drugs in patients upon long-term dosing, as there are still many unknown aspects of such a therapeutic strategy. This includes the effects that MT modulation might have on non-diseased cells within the brain, including glia and unaffected neurons. As with all therapeutics, the benefits of such treatments will have to be weighed against any observed side-effects. Finally, an interesting unresolved question is the relative importance of MT stabilization and increased MT mass vs. normalization of MT dynamics in the treatment of neurodegenerative disease. As noted, there is evidence of decreased MT mass in AD41, 42 and in a Tg mouse model of tauopathy44, as well as increased MT hyperdynamicity in similar tau Tg models45. Treatment with 4 resulted in both an improvement of MT density44, 121 and suppression of MT hyperdynamicity45 in these models. Similarly, there appears to be increased MT dynamicity in a mutant SOD1 model of ALS, with a normalization of MT dynamics after treatment with 1672. In contrast, neuron-like cells52 and mice122 treated with MPTP to model PD have been reported to have an increase in markers of stable MTs, and perhaps a decrease of MT dynamics. Notably, 4 seemed to normalize MTs in MPTP-treated mice, such that there was an attenuation of the toxin-induced neurodegeneration122. Taken together, these data might suggest that a disruption of MT dynamics is the common feature of these models of neurodegenerative disease. In cancer, it is believed that the suppression of MT dynamics, and not an overall change in MT mass, is the important therapeutic feature of both MT-stabilizing and MT-depolymerizing drugs124. If this is also true for the treatment of MT deficits in neurodegenerative conditions, it is possible that MT-directed molecules need not have dramatic effects on overall MT mass, and that normalization of dynamicity may be sufficient to improve outcomes in these diseases.
In conclusion, an increasing body of literature is pointing to axonal transport deficiencies as being a critical feature of a number of neurodegenerative diseases, and these transport problems may arise in several of these diseases through an alteration of MT stabilization and/or dynamicity. Accordingly, brain-penetrant MT-directed agents that can stabilize MTs and/or normalize MT dynamics hold considerable promise as therapeutics for these devastating conditions, and there is thus a need for the further characterization and development of such agents.
Acknowledgements
Financial support for this work was provided by NIH grants AG029213 and AG044332.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: AD, Alzheimer’s disease; ALS, amyotrophic lateral sclerosis; FTDP-17, frontotemporal degeneration with Parkinsonism linked to chromosome 17; HD, Huntington’s disease; MT, microtubules; MTOC, microtubule organizing center; NFT, neurofibrillary tangles; PD, Parkinson’s disease; Tg, transgenic; 3R, 3-microtubule binding repeat tau; 4R, 4-microtubule binding repeat tau
References
- 1.Dogterom M, Surrey T. Current opinion in cell biology. 2013;25:23. doi: 10.1016/j.ceb.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Roy S, Zhang B, Lee VMY, Trojanowski JQ. Acta Neuropathologica. 2005;109:5. doi: 10.1007/s00401-005-0999-3. [DOI] [PubMed] [Google Scholar]
- 3.Hirokawa N, Noda Y. Physiological reviews. 2008;88:1089. doi: 10.1152/physrev.00023.2007. [DOI] [PubMed] [Google Scholar]
- 4.Ishikawa T. Journal of structural biology. 2012;179:229. doi: 10.1016/j.jsb.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Desai A, Mitchison TJ. Annual review of cell and developmental biology. 1997;13:83. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 6.Wade RH. Methods in molecular medicine. 2007;137:1. doi: 10.2119/molecular%20medicine-2006-00038. [DOI] [PubMed] [Google Scholar]
- 7.Ori-McKenney KM, Jan LY, Jan YN. Neuron. 2012;76:921. doi: 10.1016/j.neuron.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stiess M, Maghelli N, Kapitein LC, Gomis-Ruth S, Wilsch-Brauninger M, Hoogenraad CC, Tolic-Norrelykke IM, Bradke F. Science. 2010;327:704. doi: 10.1126/science.1182179. [DOI] [PubMed] [Google Scholar]
- 9.Kuijpers M, Hoogenraad CC. Molecular and cellular neurosciences. 2011;48:349. doi: 10.1016/j.mcn.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Drechsel DN, Hyman AA, Cobb MH, Kirschner MW. Molecular Biology of the Cell. 1992;3:1141. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pryer NK, Walker RA, Skeen VP, Bourns BD, Soboeiro MF, Salmon EDJ. Cell Sci. 1992;103(Pt 4):965. doi: 10.1242/jcs.103.4.965. [DOI] [PubMed] [Google Scholar]
- 12.Trinczek B, Biernat J, Baumann K, Mandelkow EM, Mandelkow E. Molecular Biology of the Cell. 1995;6:1887. doi: 10.1091/mbc.6.12.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballatore C, Lee VMY, Trojanowski JQ. Nature Reviews Neuroscience. 2007;8:663. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 14.Brunden KR, Trojanowski JQ, Lee VMY. Nature Reviews Drug Discovery. 2009;8:783. doi: 10.1038/nrd2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Neuron. 1989;3:519. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 16.Panda D, Samuel JC, Massie M, Feinstein SC, Wilson L. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9548. doi: 10.1073/pnas.1633508100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI, Geschwind DH, Bird TD, McKeel D, Goate A, Morris JC, Wilhelmsen KC, Schellenberg GD, Trojanowski JQ, Lee VMY. Science. 1998;282:1914. doi: 10.1126/science.282.5395.1914. [DOI] [PubMed] [Google Scholar]
- 18.Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow EM. Journal of Cell Biology. 2002;156:1051. doi: 10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixit R, Ross JL, Goldman YE, Holzbaur ELF. Science. 2008;319:1086. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoothoff W, Jones PB, Spires-Jones TL, Joyner D, Chhabra E, Bercury K, Fan Z, Xie H, Bacskai B, Edd J, Irimia D, Hyman BT. J Neurochem. 2009;111:417. doi: 10.1111/j.1471-4159.2009.06316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vershinin M, Carter BC, Razafsky DS, King SJ, Gross SP. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:87. doi: 10.1073/pnas.0607919104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnani E, Fan J, Gasparini L, Golding M, Williams M, Schiavo G, Goedert M, Amos LA, Spillantini MG. The EMBO journal. 2007;26:4546. doi: 10.1038/sj.emboj.7601878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munch C, Rosenbohm A, Sperfeld AD, Uttner I, Reske S, Krause BJ, Sedlmeier R, Meyer T, Hanemann CO, Stumm G, Ludolph AC. Ann Neurol. 2005;58:777. doi: 10.1002/ana.20631. [DOI] [PubMed] [Google Scholar]
- 24.Puls I, Jonnakuty C, LaMonte BH, Holzbaur EL, Tokito M, Mann E, Floeter MK, Bidus K, Drayna D, Oh SJ, Brown RH, Jr., Ludlow CL, Fischbeck KH. Nature genetics. 2003;33:455. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- 25.Falzone TL, Stokin GB, Lillo C, Rodrigues EM, Westerman EL, Williams DS, Goldstein LSB. Journal of Neuroscience. 2009;29:5758. doi: 10.1523/JNEUROSCI.0780-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perlson E, Maday S, Fu MM, Moughamian AJ, Holzbaur EL. Trends Neurosci. 2010;33:335. doi: 10.1016/j.tins.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kidd M. Nature. 1963;197:192. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- 28.Lee VMY, Balin BJ, Otvos L, Trojanowski JQ. Science. 1991;251:675. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- 29.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JBJ, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Nature. 1998;393:702. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 30.Hasegawa M, Smith MJ, Goedert M. Febs Letters. 1998;437:207. doi: 10.1016/s0014-5793(98)01217-4. [DOI] [PubMed] [Google Scholar]
- 31.Dayanandan R, Van Slegtenhorst M, Mack TGA, Ko L, Yen SH, Leroy K, Brion JP, Anderton BH, Hutton M, Lovestone S. Febs Letters. 1999;446:228. doi: 10.1016/s0014-5793(99)00222-7. [DOI] [PubMed] [Google Scholar]
- 32.Nacharaju P, Lewis J, Easson C, Yen S, Hackett J, Hutton M, Yen SH. Journal of Neuropathology and Experimental Neurology. 1999;58:545. [Google Scholar]
- 33.Barghorn S, Zheng-Fischhofer Q, Ackmann M, Biernat J, von Bergen M, Mandelkow EM, Mandelkow E. Biochemistry. 2000;39:11714. doi: 10.1021/bi000850r. [DOI] [PubMed] [Google Scholar]
- 34.Alonso AD, GrundkeIqbal I, Iqbal K. Neurobiology of Aging. 1994;15:S37. [Google Scholar]
- 35.Wagner U, Utton M, Gallo JM, Miller CCJ. Journal of Cell Science. 1996;109:1537. doi: 10.1242/jcs.109.6.1537. [DOI] [PubMed] [Google Scholar]
- 36.Merrick SE, Trojanowski JQ, Lee VMY. Journal of Neuroscience. 1997;17:5726. doi: 10.1523/JNEUROSCI.17-15-05726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VMY. Neuron. 1993;10:1089. doi: 10.1016/0896-6273(93)90057-x. [DOI] [PubMed] [Google Scholar]
- 38.Alonso AD, GrundkeIqbal I, Iqbal K. Nature Medicine. 1996;2:783. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- 39.Necula M, Kuret J. Journal of Biological Chemistry. 2004;279:49694. doi: 10.1074/jbc.M405527200. [DOI] [PubMed] [Google Scholar]
- 40.Brunden K, Trojanowski JQ, Lee VMY. J.Alzheimers Dis. 2008;14:393. doi: 10.3233/jad-2008-14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cash AD, Aliev G, Siedlak SL, Nunomura A, Fujioka H, Zhu XW, Raina AK, Vinters HV, Tabaton M, Johnson AB, Paula-Barbosa M, Avila J, Jones PK, Castellani RJ, Smith MA, Perry G. American Journal of Pathology. 2003;162:1623. doi: 10.1016/s0002-9440(10)64296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hempen B, Brion JP. Journal of Neuropathology and Experimental Neurology. 1996;55:964. doi: 10.1097/00005072-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Ishihara T, Zhang B, Higuchi M, Yoshiyama Y, Trojanowski JQ, Lee VMY. American Journal of Pathology. 2001;158:555. doi: 10.1016/S0002-9440(10)63997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunden KR, Zhang B, Carroll J, Yao Y, Potuzak JS, Hogan AML, Iba M, James MJ, Xie SX, Ballatore C, Smith AB, III, Lee VMY, Trojanowski JQ. Journal of Neuroscience. 2010;30:13861. doi: 10.1523/JNEUROSCI.3059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barten DM, Fanara P, Andorfer C, Hoque N, Wong PYA, Husted KH, Cadelina GW, Decarr LB, Yang L, Liu V, Fessler C, Protassio J, Riff T, Turner H, Janus CG, Sankaranarayanan S, Polson C, Meredith JE, Gray G, Hanna A, Olson RE, Kim SH, Vite GD, Lee FY, Albright CF. Journal of Neuroscience. 2012;32:7137. doi: 10.1523/JNEUROSCI.0188-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foltynie T, Kahan J. J Neurol. 2013;260:1433. doi: 10.1007/s00415-013-6915-1. [DOI] [PubMed] [Google Scholar]
- 47.Cicchetti F, Drouin-Ouellet J, Gross RE. Trends Pharmacol Sci. 2009;30:475. doi: 10.1016/j.tips.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Brinkley BR, Barham SS, Barranco SC, Fuller GM. Experimental Cell Research. 1974;85:41. doi: 10.1016/0014-4827(74)90210-9. [DOI] [PubMed] [Google Scholar]
- 49.Marshall LE, Himes RH. Biochimica et Biophysica Acta. 1978;543:590. doi: 10.1016/0304-4165(78)90315-x. [DOI] [PubMed] [Google Scholar]
- 50.Choi WS, Palmiter RD, Xia Z. J Cell Biol. 2011;192:873. doi: 10.1083/jcb.201009132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cappelletti G, Surrey T, Maci R. FEBS Lett. 2005;579:4781. doi: 10.1016/j.febslet.2005.07.058. [DOI] [PubMed] [Google Scholar]
- 52.Cartelli D, Ronchi C, Maggioni MG, Rodighiero S, Giavini E, Cappelletti G. J Neurochem. 2010;115:247. doi: 10.1111/j.1471-4159.2010.06924.x. [DOI] [PubMed] [Google Scholar]
- 53.Rosso SB, Caceres AO, deDuffard AME, Duffard RO, Quiroga S. Toxicological Sciences. 2000;56:133. doi: 10.1093/toxsci/56.1.133. [DOI] [PubMed] [Google Scholar]
- 54.Holy J. Journal of Toxicology and Environmental Health-Part A. 1998;54:319. doi: 10.1080/009841098158872. [DOI] [PubMed] [Google Scholar]
- 55.Fanara P, Wong PY, Husted KH, Liu S, Liu VM, Kohlstaedt LA, Riiff T, Protasio JC, Boban D, Killion S, Killian M, Epling L, Sinclair E, Peterson J, Price RW, Cabin DE, Nussbaum RL, Bruhmann J, Brandt R, Christine CW, Aminoff MJ, Hellerstein MK. The Journal of clinical investigation. 2012;122:3159. doi: 10.1172/JCI64575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haggerty T, Credle J, Rodriguez O, Wills J, Oaks AW, Masliah E, Sidhu A. Eur.J Neurosci. 2011;33:1598. doi: 10.1111/j.1460-9568.2011.07660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaul T, Credle J, Haggerty T, Oaks AW, Masliah E, Sidhu A. BMC.Neurosci. 2011;12:79. doi: 10.1186/1471-2202-12-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wills J, Credle J, Haggerty T, Lee JH, Oaks AW, Sidhu A. PLoS.One. 2011;6:e17953. doi: 10.1371/journal.pone.0017953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frasier M, Walzer M, McCarthy L, Magnuson D, Lee JM, Haas C, Kahle P, Wolozin B. Experimental Neurology. 2005;192:274. doi: 10.1016/j.expneurol.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 60.Giasson BI, Forman MS, Higuchi M, Golbe LI, Graves CL, Kotzbauer PT, Trojanowski JQ, Lee VM. Science. 2003;300:636. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- 61.Qureshi HY, Paudel HK. J Biol Chem. 2011;286:5055. doi: 10.1074/jbc.M110.178905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawakami F, Yabata T, Ohta E, Maekawa T, Shimada N, Suzuki M, Maruyama H, Ichikawa T, Obata F. PLoS.One. 2012;7:e30834. doi: 10.1371/journal.pone.0030834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klein C, Westenberger A. Cold Spring Harb.Perspect.Med. 2012;2:a008888. doi: 10.1101/cshperspect.a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kett LR, Boassa D, Ho CC, Rideout HJ, Hu J, Terada M, Ellisman M, Dauer WT. Hum Mol Genet. 2012;21:890. doi: 10.1093/hmg/ddr526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang JH, Irwin DJ, Chen-Plotkin AS, Siderowf A, Caspell C, Coffey CS, Waligorska T, Taylor P, Pan S, Frasier M, Marek K, Kieburtz K, Jennings D, Simuni T, Tanner CM, Singleton A, Toga AW, Chowdhury S, Mollenhauer B, Trojanowski JQ, Shaw LM. and the Parkinson’s Progression Markers, I. JAMA neurology. 2013 [Google Scholar]
- 66.Robberecht W, Philips T. Nature reviews. Neuroscience. 2013;14:248. doi: 10.1038/nrn3430. [DOI] [PubMed] [Google Scholar]
- 67.Heiman-Patterson TD, Sher RB, Blankenhorn EA, Alexander G, Deitch JS, Kunst CB, Maragakis N, Cox G. Amyotrophic lateral sclerosis: official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. 2011;12:79. doi: 10.3109/17482968.2010.550626. [DOI] [PubMed] [Google Scholar]
- 68.De Vos KJ, Chapman AL, Tennant ME, Manser C, Tudor EL, Lau KF, Brownlees J, Ackerley S, Shaw PJ, McLoughlin DM, Shaw CE, Leigh PN, Miller CC, Grierson AJ. Hum Mol Genet. 2007;16:2720. doi: 10.1093/hmg/ddm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williamson TL, Cleveland DW. Nature neuroscience. 1999;2:50. doi: 10.1038/4553. [DOI] [PubMed] [Google Scholar]
- 70.Zhang B, Tu PH, Abtahian F, Trojanowski JQ, Lee VMY. Journal of Cell Biology. 1997;139:1307. doi: 10.1083/jcb.139.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Vos KJ, Grierson AJ, Ackerley S, Miller CC. Annu Rev Neurosci. 2008;31:151. doi: 10.1146/annurev.neuro.31.061307.090711. [DOI] [PubMed] [Google Scholar]
- 72.Fanara P, Banerjee J, Hueck RV, Harper MR, Awada M, Turner H, Husted KH, Brandt R, Hellerstein MK. J Biol Chem. 2007;282:23465. doi: 10.1074/jbc.M703434200. [DOI] [PubMed] [Google Scholar]
- 73.Clabough EB. The Yale journal of biology and medicine. 2013;86:217. [PMC free article] [PubMed] [Google Scholar]
- 74.Chang DT, Rintoul GL, Pandipati S, Reynolds IJ. Neurobiol Dis. 2006;22:388. doi: 10.1016/j.nbd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 75.Trushina E, Dyer RB, Badger JD, 2nd, Ure D, Eide L, Tran DD, Vrieze BT, Legendre-Guillemin V, McPherson PS, Mandavilli BS, Van Houten B, Zeitlin S, McNiven M, Aebersold R, Hayden M, Parisi JE, Seeberg E, Dragatsis I, Doyle K, Bender A, Chacko C, McMurray CT. Mol Cell Biol. 2004;24:8195. doi: 10.1128/MCB.24.18.8195-8209.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gunawardena S, Her LS, Brusch RG, Laymon RA, Niesman IR, Gordesky-Gold B, Sintasath L, Bonini NM, Goldstein LS. Neuron. 2003;40:25. doi: 10.1016/s0896-6273(03)00594-4. [DOI] [PubMed] [Google Scholar]
- 77.Engelender S, Sharp AH, Colomer V, Tokito MK, Lanahan A, Worley P, Holzbaur EL, Ross CA. Hum Mol Genet. 1997;6:2205. doi: 10.1093/hmg/6.13.2205. [DOI] [PubMed] [Google Scholar]
- 78.Li SH, Gutekunst CA, Hersch SM, Li XJ. J Neurosci. 1998;18:1261. doi: 10.1523/JNEUROSCI.18-04-01261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McGuire JR, Rong J, Li SH, Li XJ. J Biol Chem. 2006;281:3552. doi: 10.1074/jbc.M509806200. [DOI] [PubMed] [Google Scholar]
- 80.Neumann H. Current opinion in neurology. 2003;16:267. doi: 10.1097/01.wco.0000073926.19076.29. [DOI] [PubMed] [Google Scholar]
- 81.Lee VMY, Daughenbaugh R, Trojanowski JQ. Neurobiology of Aging. 1994;15:S87. doi: 10.1016/0197-4580(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 82.Bedard PL, Di Leo A, Piccart-Gebhart MJ. Nature Reviews Clinical Oncology. 2010;7:22. doi: 10.1038/nrclinonc.2009.186. [DOI] [PubMed] [Google Scholar]
- 83.Saloustros E, Mavroudis D, Georgoulias V. Expert Opinion on Pharmacotherapy. 2008;9:2603. doi: 10.1517/14656566.9.15.2603. [DOI] [PubMed] [Google Scholar]
- 84.Wall ME, Wani MC. In 153rd National Meeting of the American Chemical Society; Miami Beach, Fla. 1967. [Google Scholar]
- 85.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. J. Am. Chem. Soc. 1971;93:2325. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 86.Schiff PB, Fant J, Horwitz SB. Nature. 1979;277:665. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 87.Altmann KH. Curr. Opin. Chem. Biol. 2001;5:424. doi: 10.1016/s1367-5931(00)00225-8. [DOI] [PubMed] [Google Scholar]
- 88.Ballatore C, Brunden KR, Huryn DM, Trojanowski JQ, Lee VMY, Smith AB. J Med Chem. 2012;55:8979. doi: 10.1021/jm301079z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Amos LA, Lowe J. Chem. Biol. 1999;6:R65. doi: 10.1016/s1074-5521(99)89002-4. [DOI] [PubMed] [Google Scholar]
- 90.Amos LA. Org. Biomol. Chem. 2004;2:2153. doi: 10.1039/b403634d. [DOI] [PubMed] [Google Scholar]
- 91.Altmann K-H, Hofle G, Muller R, Mulzer J, K. P. Epothilones: An Outstanding Family of Anti-Tumor Agents: From Soil to The Clinic. Springer Wien; New York: 2009. [Google Scholar]
- 92.ter Haar E, Kowalski RJ, Hamel E, Lin CM, Longley RE, Gunasekera SP, Rosenkranz HS, Day BW. Biochemistry. 1996;35:243. doi: 10.1021/bi9515127. [DOI] [PubMed] [Google Scholar]
- 93.Hung DT, Chen J, Schreiber SL. Chem. Biol. 1996;3:287. doi: 10.1016/s1074-5521(96)90108-8. [DOI] [PubMed] [Google Scholar]
- 94.Isbrucker RA, Cummins J, Pomponi SA, Longley RE, Wright AE. Biochem. Pharmacol. 2003;66:75. doi: 10.1016/s0006-2952(03)00192-8. [DOI] [PubMed] [Google Scholar]
- 95.Long BH, Carboni JM, Wasserman AJ, Cornell LA, Casazza AM, Jensen PR, Lindel T, Fenical W, Fairchild CR. Cancer Res. 1998;58:1111. [PubMed] [Google Scholar]
- 96.Ciomei M, Albanese C, Pastori W, Grandi M, Pietra F, D’Ambrosio M, Guerriero A, Battistini C. Abstract 30. Proc. Am. Ass. Canc. Res. 1997;38:5. [Google Scholar]
- 97.Field Jessica J., Pera B, Calvo E, Canales A, Zurwerra D, Trigili C, Rodríguez-Salarichs J, Matesanz R, Kanakkanthara A, Wakefield SJ, Singh AJ, Jiménez-Barbero J, Northcote P, Miller John H., López Juan A., Hamel E, Barasoain I, Altmann K-H, Díaz José F. Chem. Biol. 2012;19:686. doi: 10.1016/j.chembiol.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prota AE, Bargsten K, Zurwerra D, Field JJ, Díaz JF, Altmann KH, Steinmetz MO. Science. 2013;339:587. doi: 10.1126/science.1230582. [DOI] [PubMed] [Google Scholar]
- 99.Karjala G, Chan Q, Manzo E, Andersen RJ, Roberge M. Cancer Res. 2005;65:3040. doi: 10.1158/0008-5472.CAN-04-4369. [DOI] [PubMed] [Google Scholar]
- 100.Buey RM, Calvo E, Barasoain I, Pineda O, Edler MC, Matesanz R, Cerezo G, Vanderwal CD, Day BW, Sorensen EJ, Lopez JA, Andreu JM, Hamel E, Diaz JF. Nat. Chem. Biol. 2007;3:117. doi: 10.1038/nchembio853. [DOI] [PubMed] [Google Scholar]
- 101.Bennett MJ, Barakat K, Huzil JT, Tuszynski J, Schriemer DC. Chem. Biol. 2010;17:725. doi: 10.1016/j.chembiol.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 102.Hamel E, Day BW, Miller JH, Jung MK, Northcote PT, Ghosh AK, Curran DP, Cushman M, Nicolaou KC, Paterson I. Mol. Pharmacol. 2006;70:1555. doi: 10.1124/mol.106.027847. [DOI] [PubMed] [Google Scholar]
- 103.Tinley TL, Randall-Hlubek DA, Leal RM, Jackson EM, Cessac JW, Quada JC, Jr., Hemscheidt TK, Mooberry SL. Cancer Res. 2003;63:3211. [PubMed] [Google Scholar]
- 104.Li J, Risinger AL, Peng J, Chen Z, Hu L, Mooberry SL. J. Am. Chem. Soc. 2011;133:19064. doi: 10.1021/ja209045k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peng J, Risinger AL, Fest GA, Jackson EM, Helms G, Polin LA, Mooberry SL. J. Med. Chem. 2011;54:6117. doi: 10.1021/jm200757g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ye K, Ke Y, Keshava N, Shanks J, Kapp JA, Tekmal RR, Petros J, Joshi HC. Proc Natl Acad Sci U S A. 1998;95:1601. doi: 10.1073/pnas.95.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Landen JW, Lang R, McMahon SJ, Rusan NM, Yvon AM, Adams AW, Sorcinelli MD, Campbell R, Bonaccorsi P, Ansel JC, Archer DR, Wadsworth P, Armstrong CA, Joshi HC. Cancer Res. 2002;62:4109. [PubMed] [Google Scholar]
- 108.Anderson JT, Ting AE, Boozer S, Brunden KR, Crumrine C, Danzig J, Dent T, Faga L, Harrington JJ, Hodnick WF, Murphy SM, Pawlowski G, Perry R, Raber A, Rundlett SE, Stricker-Krongrad A, Wang J, Bennani YL. J Med Chem. 2005;48:7096. doi: 10.1021/jm050674q. [DOI] [PubMed] [Google Scholar]
- 109.Fanara P, Banerjee J, Hueck RV, Harper MR, Awada M, Turner H, Husted KH, Brandt R, Hellerstein MK. J Biol Chem. 2007;282:23465. doi: 10.1074/jbc.M703434200. [DOI] [PubMed] [Google Scholar]
- 110.Zhang N, Ayral-Kaloustian S, Nguyen T, Afragola J, Hernandez R, Lucas J, Gibbons J, Beyer C. J. Med. Chem. 2007;50:319. doi: 10.1021/jm060717i. [DOI] [PubMed] [Google Scholar]
- 111.Beyer CF, Zhang N, Hernandez R, Vitale D, Lucas J, Nguyen T, Discafani C, Ayral-Kaloustian S, Gibbons JJ. Cancer. Res. 2008;68:2292. doi: 10.1158/0008-5472.CAN-07-1420. [DOI] [PubMed] [Google Scholar]
- 112.Zhang N, Ayral-Kaloustian S, Nguyen T, Hernandez R, Beyer C. Bioorg. Med. Chem. Lett. 2007;17:3003. doi: 10.1016/j.bmcl.2007.03.070. [DOI] [PubMed] [Google Scholar]
- 113.Shintani Y, Tanaka T, Nozaki Y. Cancer Chemother. Pharmacol. 1997;40:513. doi: 10.1007/s002800050695. [DOI] [PubMed] [Google Scholar]
- 114.Li PK, Pandit B, Sackett DL, Hu Z, Zink J, Zhi J, Freeman D, Robey RW, Werbovetz K, Lewis A, Li C. Mol. Cancer Ther. 2006;5:450. doi: 10.1158/1535-7163.MCT-05-0254. [DOI] [PubMed] [Google Scholar]
- 115.Haggarty SJ, Mayer TU, Miyamoto DT, Fathi R, King RW, Mitchison TJ, Schreiber SL. Chem Biol. 2000;7:275. doi: 10.1016/s1074-5521(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 116.Yang WS, Shimada K, Delva D, Patel M, Ode E, Skouta R, Stockwell BR. ACS Med Chem Lett. 2012;3:35. doi: 10.1021/ml200195s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reddy MV, Akula B, Cosenza SC, Lee CM, Mallireddigari MR, Pallela VR, Subbaiah DR, Udofa A, Reddy EP. J Med Chem. 2012;55:5174. doi: 10.1021/jm300176j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VMY. Neuron. 1999;24:751. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- 119.Zhang B, Maiti A, Shively S, Lakhani F, McDonald-Jones G, Bruce J, Lee EB, Xie SX, Joyce S, Li C, Toleikis PM, Lee VMY, Trojanowski JQ. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:227. doi: 10.1073/pnas.0406361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brunden KR, Yao Y, Potuzak JS, Ferrar NI, Ballatore C, James MJ, Hogan AL, Trojanowski JQ, Smith AB, 3rd, Lee VMY. Pharmacological Research. 2011;63:341. doi: 10.1016/j.phrs.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang B, Carroll J, Trojanowski JQ, Yao Y, Iba M, Potuzak JS, Hogan AL, Xie SX, Smith AB, III, Lee VMY, Brunden KR. Journal of Neuroscience. 2012;32:3601. doi: 10.1523/JNEUROSCI.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cartelli D, Casagrande F, Busceti CL, Bucci D, Molinaro G, Traficante A, Passarella D, Giavini E, Pezzoli G, Battaglia G, Cappelletti G. Scientific reports. 2013;3:1837. doi: 10.1038/srep01837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ren Y, Liu W, Jiang H, Jiang Q, Feng J. J Biol Chem. 2005;280:34105. doi: 10.1074/jbc.M503483200. [DOI] [PubMed] [Google Scholar]
- 124.Jordan MA, Wilson L. Nature reviews. Cancer. 2004;4:253. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 125.Klar U, Hoffmann J, Giurescu M. Expert Opin. Investig. Drugs. 2008;17:1735. doi: 10.1517/13543784.17.11.1735. [DOI] [PubMed] [Google Scholar]
- 126.Hoffmann J, Fichtner I, Lemm M, Lienau P, Hess-Stumpp H, Rotgeri A, Hofmann B, Klar U. Neuro. Oncol. 2009;11:158. doi: 10.1215/15228517-2008-072). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Laccabue D, Tortoreto M, Veneroni S, Perego P, Scanziani E, Zucchetti M, Zaffaroni M, D’Incalci M, Bombardelli E, Zunino F, Pratesi G. Cancer. 2001;92:3085. doi: 10.1002/1097-0142(20011215)92:12<3085::aid-cncr10150>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 128.Bouchet BP, Galmarini CM. Drugs Today (Barc) 2010;46:735. doi: 10.1358/dot.2010.46.10.1519019. [DOI] [PubMed] [Google Scholar]
- 129.Fitzgerald DP, Emerson DL, Qian YZ, Anwar T, Liewehr DJ, Steinberg SM, Silberman S, Palmieri D, Steeg PS. Molecular Cancer Therapeutics. 2012;11:1959. doi: 10.1158/1535-7163.MCT-12-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brunden KR, Gardner NM, James MJ, Yao Y, Trojanowski JQ, Lee V-MY, Paterson I, Ballatore C, Smith AB., III. ACS Med. Chem. Lett. 2013 doi: 10.1021/ml400233e. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cortes J, Baselga J. The Oncologist. 2007;12:271. doi: 10.1634/theoncologist.12-3-271. [DOI] [PubMed] [Google Scholar]
- 132.Cheng KL, Bradley T, Budman DR. Biologics: Targets & Therapy. 2008;2:789. doi: 10.2147/btt.s3487. [DOI] [PMC free article] [PubMed] [Google Scholar]