ABSTRACT
Tolerance of the maternal immune system in pregnancy is important for successful pregnancy because the semiallogeneic fetus may be subject to antifetal responses. We examined maternal tolerance to the fetus using a murine system in which a model paternally inherited antigen, ovalbumin (OVA), is expressed exclusively in the fetus and placenta. By employing T cell receptor (TCR) transgenic mice specific for major histocompatibility complex class I- or class II-restricted epitopes of OVA (OT-I and OT-II) as mothers, we investigated the fate of fetus-specific CD8+ and CD4+ T cells, respectively, during gestation. Both OVA-specific CD8+ and CD4+ T cells displayed an activated phenotype in the peripheral lymphoid tissues of OVA-bred OT-I and OT-II mice, consistent with their encounter of fetal antigen. Whereas a small percentage of OVA-specific CD4+ T cells were deleted in the periphery and thymus of OVA-bred OT-II mice, with evidence of TCR downregulation in the remaining T cells, deletion and TCR downregulation were not observed in OVA-bred OT-I mice. Both CD4+ and CD8+ T cells upregulated inducible costimulator expression in response to the fetal antigen, but only CD4+ T cells consistently upregulated the inhibitory receptors programmed cell death 1 and cytotoxic T lymphocyte antigen-4. More regulatory T cells (Tregs) were present in pregnant OVA-bred than in WT-bred OT-II mice, revealing that Tregs expanded specifically in response to the fetal antigen. These data indicate that several mechanisms tolerize fetal antigen-specific maternal CD4+ T cells, whereas tolerance of fetal antigen-specific CD8+ T cells is less effective. The importance of these mechanisms is underscored by the finding that fetal loss occurs in OVA-bred OT-I but not OT-II mice.
Keywords: fetal antigen, MHC class I, MHC class II, pregnancy, T cells
Maternal CD4+ T cell tolerance mechanisms are complete in this model of fetal neoantigen, whereas CD8+ T cell tolerance intermittently failed.
INTRODUCTION
During pregnancy, the mother sustains a fetus expressing foreign, paternally derived alloantigens, which constitutes an apparent immunological paradox. Epidemiological findings suggest that spontaneous abortion and preeclampsia are associated with activation of the maternal immune system, implying that maternal immune tolerance is required for the well-being of the fetus [1, 2]. Correlative studies and animal models have uncovered several regulatory mechanisms that are believed to cooperate in helping protect the fetus from potential rejection by the maternal immune system [3, 4]. Suppression of maternal leukocytes at the maternal-fetal interface may contribute to fetal tolerance, and multiple potential means of regulation have been identified at this site. These include the absence of most major histocompatibility complex (MHC) molecules on fetal trophoblasts, which limits the immunogenicity of the semiallogeneic fetus and the presence of immunoregulatory molecules on trophoblasts, including Fas ligand, CD274, human leukocyte antigen-G, and indoleamine 2,3-dioxygenase [5, 6]. In addition, regulatory T cells (Treg) are expanded during pregnancy and have been shown to be important for successful pregnancy [7–9].
It is now clear that contact between the mother and fetus is not restricted to the maternal-fetal interface. Maternal lymphocytes interact with fetal antigens within the highly organized networks of lymphoid tissues. Extrauterine fetal antigens likely arise from apoptotic trophoblasts and trophoblastic particles, which are shed into maternal blood as a normal part of placental growth [10, 11], as well as from fetal cells, which can persist for many years postpartum [12]. Studies using both B cell receptor (BCR) and T cell receptor (TCR)-transgenic mice, in which B and T cells monoclonally express an antigen receptor recognizing a defined model or natural antigen, demonstrate unequivocally that maternal lymphocytes are aware of fetal antigens, and the data are consistent with the postulate that the interaction between fetal antigen and lymphocytes results in tolerance within lymphoid tissues [13–19]. These studies indicate that the same mechanisms employed to mediate tolerance to self-antigens are used to regulate fetus-specific lymphocytes during pregnancy. Deletion, TCR and CD8 downregulation, and hyporesponsiveness have all been observed as tolerogenic responses to fetal antigen during gestation, with the specific mechanism of tolerance probably dependent on both the type of antigen (MHC antigens vs. minor histocompatibility antigen, mHAg) and the relative affinity of the BCR or TCR studied in each experimental system. Further characterization of maternal T cell responses to the fetus revealed that presentation of fetal antigen requires maternal antigen-presenting cells (APCs) and occurs in secondary lymphoid tissues and that these T cells have restricted access to the maternal-fetal interface [19–21].
The studies to date demonstrate that maternal lymphocytes interact with fetal antigens during gestation and that the outcome of this interaction is tolerogenic in nature. However, our knowledge on the fate of fetus-specific T cells is based largely on models of CD8+ T cells, with less known about fetus-specific CD4+ T cells. Tolerizing fetus-specific CD4 T cells may be important for regulating the responses of CD8+ T cells against the fetus by preventing the provision of help by the CD4 cells. Although CD4+ T cells have been shown to be activated during gestation [19, 20], the potential mechanisms that regulate fetus-specific CD4+ T cells are unknown. In addition, the ability of central tolerance to contribute to tolerance to the fetus has never been assessed. In mice, fetal antigen is believed to be released from the placenta into maternal blood once the maternal blood supply to the developing placenta is established [19]. A blood-borne source of fetal antigen could also traffic to the thymus and tolerize developing fetus-specific T cells in much the same manner as has been demonstrated in other models of T cell tolerance [22, 23].
We [24, 25] and others [19, 20, 26] have utilized a model system that employs chicken ovalbumin (OVA) as a paternally inherited fetal neoantigen. In this model, OVA simulates a mHAg to which the mother is immunologically naïve. To achieve this, females that lack endogenous OVA are bred to transgenic males that ubiquitously express membrane-bound OVA under the control of the actin promoter [27]. As a consequence, OVA is expressed exclusively in the fetus, mimicking a paternally derived mHAg to which the mother is immunologically naive [19]. Because OVA can be proteolytically processed and presented in the context of the class I and class II MHC molecules, H2Kb and I-Ab, respectively, dams expressing these molecules present the resulting fetally derived OVA peptides in a context that can be recognized by CD8+ and CD4+ T cells on the surface of their own APCs [19].
By employing TCR transgenic mice specific for either a MHC class I or class II-restricted epitope of OVA (OT-I and OT-II, respectively) as mothers to OVA-expressing fetuses, we compared the fate of fetus-specific CD4+ and CD8+ T cells during gestation. Our results suggest that fetus-specific CD4+ T cells are tolerized by several mechanisms, including deletion, TCR downregulation, and modulated expression of costimulatory and coinhibitory molecules. Evidence of deletion in the thymus was also detected. Surprisingly, no clear mechanism was apparent for maintaining tolerance of CD8+ T cells to the fetus using this model, and this observation correlated with a higher incidence of fetal loss.
MATERIALS AND METHODS
Mice
C57BL/6J (B6), C57BL/6-Tg (TcraTcrb)1100Mjb/J (OT-I), C57BL/6-Tg (TcraTcrb)425Cbn/J (OT-II), and C57BL/6-Tg (CAG-OVA)916Jen/J (OVA) mice were purchased from Jackson Laboratory. TCR transgenic OT-I and OT-II 6- to 10-wk-old females were bred with either B6 or OVA homozygous male mice and monitored daily for the appearance of a vaginal plug. The day of appearance of a copulation plug was designated Gestation Day 0.5 (gd0.5). Homozygous OVA male mice were identified by comparing the relative amount of OVA to apolipoprotein B (ApoB) by quantitative PCR on a 7500 RealTime PCR System using Power SYBR Green PCR Master Mix (Applied Biosystems). The primers used for quantitative PCR were OVA-F: 5′-CAG CCA AGC TCC GTG GAT T-3′, OVA-R: 5′-TCT CCC ACA GTC CTT TGA AGA CA-3′, ApoB-F: 5′-CAC GTG GGC TCC AGC ATT-3′, and ApoB-R: 5′-TCA CCA GTC ATT TCT GCC TTT G-3.′ Mice were housed in a specific pathogen-free facility, and the experiments were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
Flow Cytometry
Single cell suspensions were prepared from the thymus, spleen, paraaortic lymph nodes (paLN), inguinal LNs (iLN), and pooled axillary and brachial LNs (ax/bLN) of virgin, B6-bred, and OVA-bred OT-I and OT-II females. Single cell suspensions from the placentas of pooled healthy implantation sites and pooled resorption sites from individual mice at gd10.5 were also prepared. Cells were counted using a hemacytometer and trypan blue to determine the total cellularity of each tissue. Anti-CD16/32 (clone 2.4G2 or 93; 500 ng/stain) was incubated with 1–5 × 106 cells to block Fc receptors followed by staining with antibodies specific for phenotypic markers (Supplemental Table S1; all the Supplemental data are available online at www.biolreprod.org). Antibodies used included those against CD4 (clone RM4-5 or GK1.5; 12.5 ng/stain), CD8 (53-6.7; 25 ng/stain), Vα2 (B20.1; 12.5 ng/stain), Vβ5 (MR9-4; 62.5 ng/stain), CD24 (M1/69; 62.5 ng/stain), CD44 (IM7; 50 ng/stain), CD62L (MEL-14; 12.5 ng/stain), inducible costimulator (ICOS) (7E.17G9; 100 ng/stain), programmed cell death 1 (PDCD1; 125 ng/stain) (J43), CD25 (PC61.5; 12.5 ng/stain), CD28 (37.51; 100 ng/stain), CD69 (H1.2F3; 100 ng/stain), cytotoxic T lymphocyte associated protein-4 (CTLA-4) (UC10-4B9; 100 ng/stain), and forkhead box P3 (Foxp3) (FJK-16s; 100 ng/stain). Antibodies were purchased from either BD Biosciences or eBioscience. Following cell surface staining, cells were permeabilized with permeabilization/fixation buffer (eBioscience) before staining for CTLA-4 and Foxp3. Stained cells were analyzed using a LSR II cytometer and FACSDiva v5.0.2 software (BD Biosciences).
Identification of Fetal Implantation and Resorption Sites
Fetal implantation sites were identified at gd5.5 by intravenous injection of 1% Chicago blue dye to anesthetized mice immediately prior to euthanization as described previously [28]. Fetal resorption sites were identified at gd10.5, 13.5, and 17.5 by their hemorrhagic appearance and were most often smaller as compared with normal fetuses and placentas.
Proliferation Assay
Splenocytes were labeled with 2.5 μM carboxyfluorescein diacetate succinimidyl ester (Molecular Probes) for 10 min at 37°C. After washing, 5 × 105 carboxyfluorescein diacetate succinimidyl ester-labeled splenocytes from OT-I or OT-II mice were plated in 96-well round-bottom plates with various amounts of either OVA257-264 (SIINFEKL) (ProImmune) or OVA323-339 (ISQAVHAAHAEINEAGR) (GenScript) peptide, respectively. Proliferation was assessed by flow cytometry 3 or 4 days later for OT-I and OT-II cells, respectively, following staining with antibodies specific for CD8 or CD4. The proliferation index (PI) was calculated as described previously [20] using the formula: PI = (nun + nd1 + nd2 + ndn)/(nun/20 + nd1/21 + nd2/22 + ndn/2n), where n = cell number, un = undivided peak, d1 = division 1, d2 = division 2, etc.
Statistical Analyses
Comparisons between virgin and pregnant mice throughout gestation were made using one-way analysis of variance or Kruskal-Wallis ANOVA on ranks for nonparametric data. Post-hoc comparisons were performed using the Holm-Sidak (or Dunn for nonparametric data) test, with nonpregnancy serving as the control. To compare B6- versus OVA-bred females across gestation, the Mann-Whitney rank sum test was used. All the comparisons were made using SigmaStat software. Values were considered statistically different at P < 0.05.
RESULTS
Fetus-Specific CD4+ T Cells Are Activated and Deleted in Lymphoid Tissues
In C57Bl/6J mice, OVA can be proteolytically processed and presented in the context of class I and class II MHC by APCs. Specifically, the OVA-derived peptide SIINFEKL (OVA257-264) can be presented in the context of the class I molecule, H-2Kb, and OVA-derived ISQAVHAAHAEINEAGR (OVA323-339) can be presented in the context of the class II molecule, I-Ab. Here, we employed transgenic ACT-mOVA males bred to homozygosity or wild-type C57Bl/6 (B6) males as sires to either OT-I or OT-II TCR transgenic females. OT-I transgenic mice monoclonally express a Vα2+Vβ5+ TCR on CD8+ T cells that recognizes the H-2Kb/OVA257-264 epitope. Likewise, OT-II transgenic mice monoclonally express a Vα2+Vβ5+ TCR on CD4+ T cells that recognizes the I-Ab/OVA323-339 epitope. Using these transgenic animal models, we tracked the fate of fetal antigen-specific T cells during gestation.
To determine the fate of fetal antigen-specific CD4+ T cells during gestation, pregnant OVA- or B6-bred OT-II mice were sacrificed at gd0.5, 5.5, 10.5, 13.5, and 17.5. Total cellularity of central and peripheral lymphoid organs was determined together with the phenotype of the maternal CD4+ T cells within these organs. In B6-bred OT-II mice, the total number of cells in the thymus decreased 2-fold at gd13.5 and 3-fold by gd17.5 compared to virgin OT-II mice, whereas the cellularity of the spleen increased 1.5-fold at gd10.5 and 13.5 before returning to nonpregnant levels by gd17.5 (Fig. 1A). These observations are consistent with previous studies on the effects of pregnancy on lymphoid tissues [29, 30].
FIG. 1.
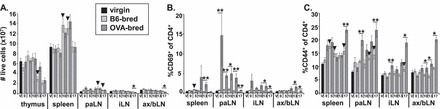
Fetal antigen-specific CD4+ T cells are activated in peripheral lymphoid tissues. Cells from the thymus, spleen, paraaortic lymph nodes (paLN), inguinal lymph nodes (iLN), and pooled axillary and brachial lymph nodes (ax/bLN) of OT-II mice were counted, then stained with antibodies to CD4, CD8, CD69, and CD44, and analyzed by flow cytometry. A) Total cellularity of lymphoid tissues. Mean percentage of CD4+CD8− cells that are CD69+ (B) and CD44+ (C) are shown. SEM is shown, and significant differences are indicated by symbols (▾P < 0.05 between virgin and B6-bred mice; *P < 0.05, **P < 0.005 between B6-bred and OVA-bred mice at same gestational day). For virgin mice, n = 8. For gd0.5, 5.5, 10.5, 13.5, and 17.5, n = 6, 7, 6, 6, and 7, respectively, for B6-bred OT-II mice, and n = 6, 7, 6, 6, and 8, respectively, for OVA-bred OT-II mice.
We next examined whether fetal antigen induced changes in the expression of activation markers (Supplemental Table S1) on the fetus-specific T cells by comparing the percentage of CD4+ T cells that were CD44hi, CD62Llo, CD28hi, CD69+, and CD25+ in the peripheral lymphoid tissues of OVA-bred and B6-bred OT-II mice. Because of the changes in cellularity during gestation described above that occurred independently of antigenic differences with few exceptions (P > 0.05), the percentage rather than absolute number of cells was analyzed to allow comparisons between the gestational time points. Upregulation of the early activation marker CD69 was observed in the paLN at all of the time points examined, including as early as the day after coitus; increases in the percentage of CD69+ CD4+ T cells in all peripheral lymphoid tissues examined were also observed later in gestation (Fig. 1B). Because CD69 upregulation following antigen stimulation is rapid but transient [31], this result suggests that OVA is presented in the uterus-draining lymph nodes to maternal T cells throughout gestation. To determine whether activated fetus-specific CD4+ T cells persist, we examined the expression of CD44. Although pregnancy alone resulted in upregulation of CD44 in maternal CD4+ T cells in the spleen and paLN beginning at gd5.5, further antigen-specific upregulation of CD44 due to embryonic OVA was evident as early as gd5.5 in the paLN and iLN, and was present in all peripheral lymphoid organs examined at gd17.5 (Fig. 1C). Similarly, the percentage of CD4+ T cells that are CD62Llo, CD25+, and CD28hi is also increased during gestation in an antigen-specific manner when CD4+ T cells from OVA-bred OT-II mice are compared to B6-bred OT-II mice, although changes in these activation markers generally were not observed until gd13.5 (Supplemental Fig. S1, A–C). Collectively, these results suggest that activation of maternal T cells by fetal antigen occurs as early as insemination in uterus-draining lymph nodes, with activated T cells present in all lymphoid tissues by midgestation, and that some markers (i.e., CD69 and CD44) may be more sensitive than others at tracking this activation.
Tolerance to OVA Is Complete in the CD4 Compartment
Despite utilizing a model in which virtually all the maternal T cells are specific for a fetal antigen recognized as foreign, tolerance to the fetus was maintained when OT-II females were bred to OVA males. No difference in either the average number of viable fetuses or resorbing fetuses was observed in OVA-bred compared to B6-bred OT-II mice (Fig. 2). This result suggests that the interaction of maternal T cells with fetal antigen in lymphoid organs results in tolerance induction.
FIG. 2.
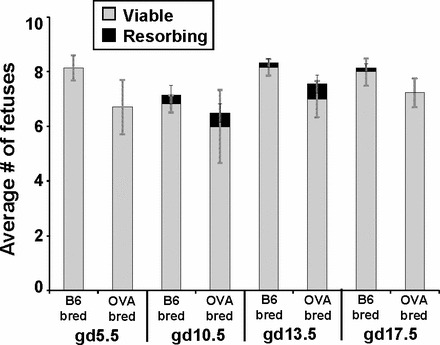
Fetal tolerance is maintained in OVA-bred OT-II mice. The average number of viable and resorbing fetuses in B6-bred and OVA-bred OT-II mice at gd5.5, 10.5, 13.5, and 17.5 is shown with the SEM. For gd5.5, 10.5, 13.5, and 17.5, n = 7, 6, 6, and 7, respectively, for B6-bred OT-II mice, and n = 7, 6, 9, and 8, respectively, for OVA-bred OT-II mice. Significant differences in the number of viable or resorbing fetuses between B6-bred and OVA-bred at the same gestational day were determined using a Student t-test (none detected).
Potential Mechanisms of Fetus-Specific CD4+ T Cell Regulation During Gestation
The fact that fetal viability is unaltered in OVA-bred OT-II mice despite activation of fetus-specific T cells, suggests that tolerance is the outcome of fetal-antigen recognition by maternal T cells. Therefore, several mechanisms of tolerance induction were assessed in this model, including deletion, TCR downregulation, expression of inhibitory molecules, and anergy. The percentage of CD4+ T cells in B6-bred and OVA-bred OT-II mice was compared throughout gestation to determine if deletion contributes to fetal tolerance in this model. Although the percentage of CD4+ T cells was reduced nonspecifically by pregnancy at gd5.5–13.5 in the spleen and at gd17.5 in the thymus, a further antigen-specific reduction was observed in all the peripheral lymphoid tissues of OVA-bred OT-II mice by gd17.5 or earlier when compared to B6-bred OT-II mice at the same time points (Fig. 3A). A decrease in the percentage of CD4+CD8−-single-positive T cells in OVA-bred compared to B6-bred OT-II mice was also observed in the thymus (Fig. 3A), suggesting that OVA is presented in the thymus and deletes developing thymocytes. This observation was supported by a significant reduction in the percentage of mature CD24lo CD4SP thymocytes in OVA-bred compared to B6-bred OT-II mice at gd13.5 and gd17.5 (Supplemental Fig. S1D).
FIG. 3.

Fetal antigen-specific CD4+ T cells are subject to deletion and TCR downregulation. Cells from the thymus, spleen, paraaortic lymph nodes (paLN), inguinal lymph nodes (iLN), and pooled axillary and brachial lymph nodes (ax/bLN) of OT-II mice were stained with antibodies to CD4, CD8, Vα2, Vβ5, ICOS, PDCD1, CTLA-4, and Foxp3 and analyzed by flow cytometry. A) Mean percentage of total cells that are CD4+CD8−. B–F) mean percentage of CD4+CD8− cells that are ICOS+ (B), PDCD1+ (C), Vα2hiVβ5hi (D), CTLA-4+ (E), and Foxp3+ (F). SEM is shown, and significant differences are indicated by symbols (▾P < 0.05 between virgin and B6-bred mice; *P < 0.05, **P < 0.005 between B6-bred and OVA-bred mice at same gestational day). For virgin mice, n = 8. For gd0.5, 5.5, 10.5, 13.5, and 17.5, n = 6, 7, 6, 6, and 7, respectively, for B6-bred OT-II mice, and n = 6, 7, 6, 6, and 8, respectively, for OVA-bred OT-II mice.
To determine if TCR downregulation occurs in response to fetal antigen, the percentage of CD4+ T cells expressing high levels of both the alpha and beta chain of the OT-II TCR was compared between OVA-bred and B6-bred OT-II mice during gestation. A reduced percentage of Vα2hiVβ5hi CD4+ T cells was found in OVA-bred compared to B6-bred OT-II mice in the ax/bLN at gd10.5–17.5, iLN at gd13.5–17.5, and in the spleen at gd17.5 (Fig. 3D), indicating that TCR downregulation is present in a subset of fetus-specific maternal CD4+ T cells during gestation. However, the mean fluorescence intensity of Vα2 and Vβ5 was not significantly different between either the Vα2hiVβ5hi subset or total CD4+ T cells from OVA-bred and B6-bred OT-II mice (data not shown), suggesting that the lower percentage of Vα2hiVβ5hi CD4+ T cells could also be due to preferential deletion of this subset of CD4+ T cells.
To determine the role of regulatory/inhibitory molecules in maintaining tolerance to paternally inherited antigens in the CD4+ T cell compartment during gestation, the expression of several key molecules (Supplemental Table S1) involved in T cell regulation was compared in virgin, B6-bred, and OVA-bred OT-II mice. The percentage of ICOS+ cells was significantly greater (2.5- to 8-fold) in CD4+ T cells in OVA-bred compared to B6-bred OT-II mice in all peripheral lymphoid tissues at both gd13.5 and 17.5 (Fig. 3B). Although ICOS expression may simply reflect T cell activation, increased ICOS levels in the presence of fetal antigen suggests that this molecule may play a role in immune deviation toward T helper 2 (Th2)-type responses in pregnancy. The percentage of CD4+ T cells expressing PDCD1 was also higher (4- to 6-fold) in all peripheral lymphoid tissues at both gd13.5 and 17.5 in OVA-bred compared to B6-bred OT-II mice, with significant upregulation first detected at gd5.5 in the paLN (1.7-fold) (Fig. 3C). This result suggests that inhibition of fetus-specific maternal CD4+ T cells may occur through PDCD1 by either CD274-expressing trophoblast cells at the maternal-fetal interface or by APCs in the periphery. Alternatively, increased expression of PDCD1 may be important in regulating CD4+ T cell conversion into CD4+CD25+ Tregs as has been suggested by others [32, 33]. The percentage of CD4+ T cells that were CTLA-4+ was similarly increased in OVA-bred OT-II mice starting in the paLN, with approximately twice the proportion of CD4+ T cells in the paLN from gd5.5–17.5 and in the spleen at gd17.5, and approximately four times the proportion of CD4+ T cells in the iLN and ax/bLN at gd17.5 expressing CTLA-4 in OVA-bred compared to B6-bred OT-II mice (Fig. 3E). CTLA-4 is a potent negative regulator of T cells, and its increased expression could play an important role in maintaining tolerance in the fetus-specific OT-II cells. However, most of the CD4+ T cells that were CTLA-4+ were also FoxP3+ (data not shown), suggesting that the CTLA-4+ population of CD4+ T cells consists primarily of Tregs.
With the established importance of Tregs in maintaining tolerance to alloantigen-expressing fetuses, we examined the fetal antigen-specific effects on this population in our model. As has been reported previously [8], pregnancy induced an expansion of Tregs, observed in our system as a 1.5- to 2-fold increase in the percentage of CD4+ T cells that are FoxP3+ in B6-bred compared to virgin OT-II mice at gd13.5 and 17.5 in all the peripheral lymphoid tissues (Fig. 3F). In addition to the antigen-independent expansion of Tregs during pregnancy, a 2-fold antigen-specific expansion of Tregs was observed in all lymphoid tissues of OVA-bred compared to B6-bred OT-II mice at gd17.5 (Fig. 3F).
In addition to phenotyping the fetal antigen-specific CD4+ T cells during gestation, the ability of these cells to proliferate ex vivo in response to fetal antigen was assessed. The proliferation indices of CD4+ T cells from virgin, B6-bred, and OVA-bred OT-II mice to OVA peptide was not significantly different (Fig. 4A). This result suggests that the function of fetus-specific CD4+ T cells, as measured by proliferation, is not significantly altered during gestation and that other mechanisms may be more important in maintaining tolerance to the fetus in this experimental system.
FIG. 4.
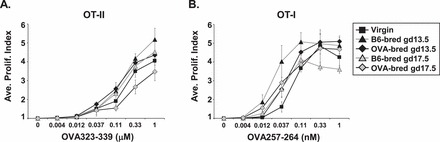
Proliferative capacity of fetus-specific T cells is unaltered during gestation. The average proliferation index (PI) of virgin, B6-bred, and OVA-bred OT-II (A) or OT-I (B) splenocytes to peptide stimulation as described in Materials and Methods is shown with the SEM. For virgin, B6-bred gd13.5, OVA-bred gd13.5, B6-bred gd17.5, and OVA-bred gd17.5 mice, n = 7, 3, 5, 7, and 5, respectively, for OT-II mice, and n = 7, 7, 6, 6, and 7, respectively, for OT-I mice. Significant differences in the PI at each peptide concentration between B6-bred and OVA-bred mice at the same gestational day or between virgin and pregnant mice were determined using a Student t-test (none detected).
Together, these data demonstrate that MHC class II-restricted maternal CD4+ T cells encounter fetal mHAg during gestation, resulting in modulation of activation markers and inhibitory molecules, downregulation of TCR, expansion of Tregs, and a lower percentage of fetus-specific T cells in lymphoid tissues; all of which may help maintain tolerance to the fetus.
Fetus-Specific CD8+ T Cells Are Activated but Not Deleted in Lymphoid Tissues
The effect of the model fetal mHAg OVA on maternal OVA-specific CD8+ T cells was assessed in the same fashion as for the CD4+ T cells, primarily by examining the phenotype of the fetus-specific CD8+ T cells during gestation. The expression of various markers (Supplemental Table S1) on CD8+ T cells from OVA-bred and B6-bred OT-I TCR transgenic mice was compared at gd0.5, 5.5, 10.5, 13.5, and 17.5. As with OT-II mice, alterations in total cellularity of lymphoid organs due to hormonal changes during pregnancy were observed, with fewer cells in the thymus (1.5-fold at gd10.5, 2.5-fold at gd13.5, and 4-fold at gd17.5) and more cells in the spleen (1.5-fold at gd10.5 and gd13.5) and paLN (3-fold at gd5.5, 1.7-fold at gd10.5, 1.4-fold at gd13.5, and 2-fold at gd17.5) of B6-bred OT-I mice compared to virgin OT-I mice (Fig. 5A). When OT-I female mice were bred with OVA males, we only detected significant upregulation of CD69 at gd13.5 in the iLN (1.6-fold change) of OVA-bred OT-I mice in relation to B6-bred OT-I mice (Fig. 5B). However, antigen-specific activation of the maternal CD8+ T cells to the OVA+ fetuses clearly occurred because a significantly greater percentage of CD8+ T cells from OVA-bred OT-I mice upregulated CD44 than in B6-bred OT-I mice. This was true at all gestational time points in the paLN, from gd5.5 onward in the iLN and ax/bLN, and from gd13.5 onward in the spleen (Fig. 5C). The activation of T cells in all the peripheral lymphoid tissues examined by gd13.5 indicates either that the fetus-specific CD8+ T cells encounter fetal OVA in all these tissues or that they have trafficked to all the lymphoid sites by gd13.5. This indication of T cell activation was supported by a significant increase in the percentage of CD28hi CD8+ T cells by gd13.5 in the spleen and paLN (2.5- to 3-fold) and in all the peripheral lymphoid tissues at gd17.5 (3- to 10-fold) in OVA-bred compared to B6-bred OT-I mice (Supplemental Fig. S2C).
FIG. 5.
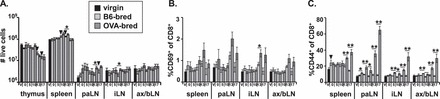
Fetal antigen-specific CD8+ T cells are activated and expand in peripheral lymphoid tissues. Cells from the thymus, spleen, paraaortic lymph nodes (paLN), inguinal lymph nodes (iLN), and pooled axillary and brachial lymph nodes (ax/bLN) of OT-I mice were counted, then stained with antibodies to CD4, CD8, CD69, and CD44, and analyzed by flow cytometry. A) Total cellularity of lymphoid tissues. Mean percentage of CD4−CD8+ cells that are CD69+ (B), and CD44+ (C). SEM is shown, and significant differences are shown by symbols (▾P < 0.05 between virgin and B6-bred mice; *P < 0.05, **P < 0.005 between B6-bred and OVA-bred mice at same gestational day). For virgin mice, n = 7. For gd0.5, 5.5, 10.5, 13.5, and 17.5, n = 6, 6, 6, 8, and 6, respectively, for B6-bred OT-I mice, and n = 6, 7, 7, 9, and 7, respectively, for OVA-bred OT-I mice.
The same potential tolerance mechanisms investigated for OVA-specific maternal CD4+ T cells were also assessed for the CD8+ T cells. In contrast to our observations with fetus-specific CD4+ T cells, it does not appear that deletion of OVA-specific CD8+ T cells occurred during gestation, with little change in the percentage of CD8+ T cells between OVA-bred and B6-bred OT-I mice in the thymus and peripheral lymphoid tissues (Fig. 6A). In fact, the opposite appeared true, as small (1.2- to 1.6-fold) but significant increases in the percentage of CD8+ T cells were detected in the paLN of OVA-bred compared to B6-bred OT-I mice at gd5.5, 13.5, and 17.5 (Fig. 6A). Another difference from our findings with fetus-specific CD4+ T cells was that when the level of TCR was assessed, no decrease in the percentage of CD8+ T cells that were Vα2hiVβ5hi between OVA-bred and B6-bred OT-I mice was observed in any lymphoid tissue during gestation (Fig. 6D). This result indicates that activation of the cells, as detected by modulation of CD44, is not accompanied by TCR downregulation.
FIG. 6.
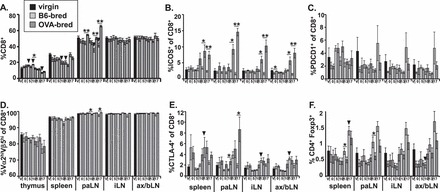
Fetal antigen-specific CD8+ T cells do not undergo regulatory mechanisms. Cells from the thymus, spleen, paraaortic lymph nodes (paLN), inguinal lymph nodes (iLN), and pooled axillary and brachial lymph nodes (ax/bLN) of OT-I mice were counted then stained with antibodies to CD4, CD8, Vα2, Vβ5, ICOS, PDCD1, CTLA-4, and Foxp3 and analyzed by flow cytometry. A) Total cellularity of lymphoid tissues. Mean percentage of CD4−CD8+ cells that are ICOS+ (B), PDCD1+ (C), Vα2hiVβ5hi (D), CTLA-4+ (E), and CD4+FoxP3+ (F) are shown. SEM is shown, and significant differences are shown by symbols (▾P < 0.05 between virgin and B6-bred mice; *P < 0.05, **P < 0.005 between B6-bred and OVA-bred mice at same gestational day). For virgin mice, n = 7. For gd0.5, 5.5, 10.5, 13.5, and 17.5, n = 6, 6, 6, 8, and 6, respectively, for B6-bred OT-I mice, and n = 6, 7, 7, 9, and 7, respectively, for OVA-bred OT-I mice.
Modulation of many of the T cell regulatory molecules examined in maternal fetus-specific CD8+ T cells during gestation with an allogeneic fetus was also distinct from the changes we observed in the CD4+ T cells. However, like CD4+ T cells, there was a striking increase in the proportion (2- to 9-fold) of CD8+ T cells from OVA-bred OT-I mice that were ICOS+ at both gd13.5 and 17.5 in all the peripheral lymphoid tissues compared with B6-bred OT-I mice (Fig. 6B). Although the role of ICOS in CD8+ T cells is less well understood than for CD4+ T cells, it is believed to provide costimulation to promote effector functions [34, 35]. Thus, expression of ICOS suggests that this population of CD8+ T cells in OVA-bred mice constitutes primed effector cells that could mediate damage to the fetus. Unlike CD4+ T cells, though, PDCD1 expression on CD8+ T cells from OVA-bred OT-I mice was unaltered compared to B6-bred OT-I mice during gestation (Fig. 6C), suggesting that fetus-specific CD8+ T cells are not regulated through this inhibitory receptor in this model. Others have also identified PDCD1 as a marker of regulatory CD8+ T cells [36]; thus, CD8+ Tregs do not appear to be generated in this model. This idea is supported by the absence of FoxP3 in the CD8+ T cells from OVA-bred OT-I mice (data not shown). Expression of another inhibitory molecule, CTLA-4, was mostly similar on CD8+ T cells between OVA-bred and B6-bred OT-I mice during gestation, suggesting that it does not play a crucial inhibitory role in these cells (Fig. 6E). Nevertheless, a function for CTLA-4 in tolerance induction cannot be ruled out because a higher percentage of CD8+ T cells from OVA-bred OT-I mice expressed CTLA-4 in the paLN at gd10.5 and 17.5 compared to B6-bred OT-I mice.
Although there are very few CD4+ T cells in OT-I mice (an average of 4% and 3% of the total splenic and LN cellularity, respectively, in virgin OT-I mice), a large proportion of these are FoxP3+ (20%–30% of CD4 cells in the peripheral lymphoid tissues), and thus constitute Tregs. Although there is a 2-fold increase in the percentage of CD4+FoxP3+ cells at gd17.5 in all peripheral lymphoid tissues due to pregnancy alone, this expansion is OVA antigen-independent because no difference is observed in the percentage of CD4+FoxP3+ cells between OVA-bred and B6-bred OT-I mice (Fig. 6F). Although a 1.7-fold reduction in the percentage of CD4+FoxP3+ cells was detected in the paLN of OVA-bred OT-I mice at gd13.5 and 17.5 compared to B6-bred OT-I mice (Fig. 6F), this finding likely reflects a reduction in the percentage of all CD4+ T cells in the paLN of OVA-bred mice due to a concomitant antigen-dependent increase in the percentage of CD8+ T cells at those time points (see Fig. 6A), rather than an influence of fetal antigen on Treg levels during pregnancy. This notion is supported by the fact that no difference was observed in the absolute number of CD4+FoxP3+ cells in the paLN of OVA-bred and B6-bred OT-I mice at those time points (data not shown).
Similar to our findings with fetus-specific CD4+ T cells, no effect on T cell responsiveness was observed with fetus-specific CD8+ T cells. OVA-specific CD8+ T cells from OVA-bred OT-I mice proliferated to the same extent as those from B6-bred OT-I mice to peptide in vitro at both gd13.5 and 17.5, indicating that the function of fetus-specific CD8+ T cells is not altered following encounter with fetal antigen during gestation (Fig. 4B).
Together, the data analyzing OVA-bred OT-I mice indicate that fetus-specific CD8+ T cells encounter fetal antigen in lymphoid tissues during pregnancy and become activated. However, the outcome of this encounter does not appear to result in deletion, receptor downregulation, or anergy. The fact that only about half the OVA-bred OT-I pregnancies examined consisted of more than three viable fetuses whereas all the B6-bred OT-I pregnancies yielded at least five viable fetuses is consistent with these observations (Fig. 7A). We found that OVA-bred OT-I mice had significantly more resorption sites than B6-bred OT-I mice at gd10.5, which correlated with a reduced average number of viable fetuses from gd10.5 onward (Fig. 7B). This result suggests that in some cases tolerance to fetal OVA is broken. When present, this antigen-specific fetal loss appears to occur by gd10.5 because the average number of resorption sites is not significantly different between OVA-bred and B6-bred OT-I mice after gd10.5, presumably due to complete resorption of those sites as gestation progresses. Likewise, the average number of viable fetuses does not decrease further after gd10.5 in OVA-bred OT-I mice, suggesting that antifetal responses begin by gd10.5 but after gd5.5 (Fig. 7B).
FIG. 7.

Fetal loss occurs in OVA-bred OT-I mice. A) The number of viable and resorbing fetuses in individual B6-bred and OVA-bred OT-I mice at gd10.5, 13.5, and 17.5 as well as at gd17.5 of a second pregnancy is shown. The percentages of B6-bred and OVA-bred mice with greater than three viable fetuses at each gestational day and for all time points combined (total) are indicated above each graph and at the bottom, respectively. B) The average number of viable and resorbing fetuses in B6-bred and OVA-bred OT-I mice at gd5.5, 10.5, 13.5, and 17.5 is shown with SEM. The average numbers of pups in the first litter and of viable and resorbing fetuses at gd17.5 of a second pregnancy are also shown. For gd5.5, 10.5, 13.5, 17.5, first litter, and gd17.5 of the second pregnancy, n = 6, 6, 8, 6, 6, and 6, respectively, for B6-bred OT-I mice; and n = 7, 7, 9, 7, 6, and 6, respectively, for OVA-bred OT-I mice. Significant differences in the number of viable or resorbing fetuses between B6-bred and OVA-bred at the same gestational day were determined using a Student t-test (*P < 0.05).
Consistent with an ongoing immune response against the fetus in some cases, the placentas of both resorbing and healthy implantation sites in OVA-bred OT-I mice at gd10.5 have on average 2- and 2.5-fold more total cells per site, respectively, than those in B6-bred OT-I mice (Fig. 8A). Although the percentage of CD8+ cells was not significantly higher in the implantation sites of OVA-bred compared to B6-bred OT-I mice (Fig. 8B), a higher percentage of the CD8+ cells in the OVA-bred implantation sites were ICOS+ (Fig. 8C), suggesting that more of the CD8 cells are phenotypically activated/effector cells when a fetal mHAg difference is present. Interestingly, the healthy implantation sites of OVA-bred OT-I mice contained significantly more CD8+ cells/site than those of B6-bred mice (Fig. 8D), which may indicate that some of the healthy sites in OVA-bred mice were being actively infiltrated and damaged by fetus-specific T cells and eventually would be resorbed. However, no difference was detected between viable fetuses from OVA-bred and B6-bred OT-I dams in either average crown-rump length (for OVA-bred vs. B6-bred, gd10.5: 6.8 ± 0.15 mm vs. 6.6 ± 0.13 mm, P = 0.25; gd13.5: 10.6 ± 0.11 mm vs. 10.3 ± 0.08 mm, P = 0.02; and gd17.5: 16.9 ± 0.17 mm vs. 17.1 ± 0.14 mm, P = 0.26) or neonatal weight (OVA-bred: 1.27 ± 0.026 g; B6-bred: 1.26 ± 0.015 g; P = 0.54), suggesting that the majority of fetuses that were deemed viable by appearance in OVA-bred mice were indeed healthy. Together these results indicate that fetal tolerance in OVA-bred OT-I mice is maintained to varying degrees in individual mice.
FIG. 8.
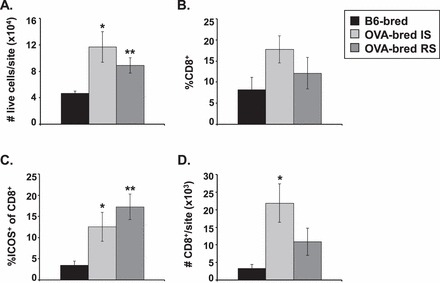
OVA+ implantation sites contain more OT-I T cells. Cells from pooled healthy implantation (IS) or resorbing (RS) placentas of individual B6-bred or OVA-bred OT-I mice at gd10.5 were counted then stained with antibodies to CD4, CD8, and ICOS and analyzed by flow cytometry. A) Total cellularity per implantation site. B) Mean percentage of total cells that are CD4−CD8+. C) Mean percentage of CD4−CD8+ cells that are ICOS+. D) Mean number of CD8+ cells per implantation site. SEM is shown and significant differences were determined using a Student t-test (*P < 0.05, **P < 0.005 between B6-bred and OVA-bred mice). For B6-bred, OVA-bred IS, and OVA-bred RS, n = 6, 5, and 5, respectively.
The observation of fetal loss in OVA-bred OT-I mice led us to ask whether fetus-specific CD8+ T cells are being primed or tolerized during their initial encounter with fetal antigen. To determine the effect that exposure to fetal mHAg has on subsequent pregnancy with fetuses expressing the same antigenic difference, the number of healthy and resorbing fetuses in OVA-bred and B6-bred mice were compared at gd17.5 of a second pregnancy. As with the first pregnancy, OT-I mice bred twice with OVA males had significantly fewer viable fetuses (as well as more resorption sites) at gd17.5 than those bred twice with B6 males (Fig. 7B). Although neither absolute tolerance nor priming of fetus-specific maternal T cells appears to result from the initial fetal antigen encounter during the first pregnancy, a greater percent of CD8 cells are CD44hi, CD62Llo, and ICOS+ in the second pregnancy, suggesting that some of these are memory cells primed during the first pregnancy (Supplemental Fig. S3). Interestingly, in contrast to our results with a single pregnancy, an antigen-specific increase (2- to 3-fold) in the percentage of CD4+FoxP3+ cells was detected in the LNs at gd17.5 of the second pregnancy (Supplemental Fig. S3K). The percentage of CD4+FoxP3+ cells in the LNs of OVA-bred mice was also 2-fold higher in the second pregnancy than in the first (Supplemental Fig. S3K), which is consistent with recent results seen by others [37]. This increase in CD4+FoxP3+ cells during the second pregnancy could not be attributed to the lower percentage of CD8+ cells in the LNs of 2× OVA-bred OT-I mice (Supplemental Fig. S3E) because the absolute number of CD4+FoxP3+ cells was also significantly higher (3- to 4-fold) in all LNs of 2× OVA-bred compared to 1× OVA-bred OT-I mice (data not shown). Despite this increased percent of Tregs in the second pregnancy, no significant difference was detected in either the average number of viable fetuses or the percent of OVA-bred OT-I mice that resorb fetuses between the first and second pregnancy at gd17.5 (Fig. 7B). Nonetheless, the concomitant increase of activated/memory CD8+ T cells in the second pregnancy without a compounding effect on fetal loss suggests that they may be important.
DISCUSSION
This study was undertaken to determine how maternal CD4+ and CD8+ T cells specific for a fetal mHAg respond to that antigen during gestation and the outcome of that response. Our experiments suggest initial contact of maternal CD4+ T cells with OVA occurs immediately after copulation. This early activation of the maternal immune system complements the findings that antigen derived from seminal fluid is presented this early and supports the notion that immune tolerance to fetal antigens commences as soon as the mother is exposed to paternal antigens following insemination [20]. Exposure to fetal antigen appears to occur in a sequential fashion from the regional lymph nodes and spleen early in pregnancy to the nondraining lymph nodes and even the thymus by the time a fully formed placenta has developed. The small, but significant, upregulation of CD69 on CD4+ T cells in OVA-bearing OT-II mice at all the time points examined suggests that fetal antigen is presented to maternal lymphocytes throughout gestation. Together, these results suggest a limited, local exposure to antigen early in gestation, with more widespread antigen presentation and/or recirculation of activated T cells as gestation progresses.
Differences in the expression of activation and inhibitory markers on OVA-specific CD4+ T cells in the presence of OVA-expressing fetuses were observed across gestation. While some of these differences may simply reflect T cell activation, our results offer insight into potential mechanisms by which maternal fetal antigen-specific CD4+ T cells are tolerized. For example, a clear fetal antigen-specific effect on maternal CD4+ T cells was the upregulation of ICOS at gd13.5 and 17.5. Increased ICOS expression in the presence of fetal antigen is consistent with suggestions of maternal immune deviation toward Th2-type responses [38]. Additionally, the upregulation of PDCD1 on the CD4+ T cells in OVA-bred OT-II mice suggests that this molecule may be important in inhibiting fetus-specific maternal CD4+ T cells that may subsequently encounter CD274-expressing trophoblast cells at the maternal-fetal interface and/or CD274-expressing maternal APCs. Although our previous work demonstrates a noncritical role for PDCD1/CD274 interactions in murine pregnancy [39, 40]; in experiments using human cells, expression of the ICOS and PDCD1 ligands, ICOSL and CD274, respectively, by trophoblasts and macrophages at the maternal-fetal interface suggest that these receptors function in cytokine modulation during pregnancy [39, 41–44]. Alternatively, increased expression of PDCD1 may be important in converting CD4+ T cells into Tregs as has been suggested by others [32, 33]. Indeed, nearly all the FoxP3+ CD4+ T cells we detected were also PDCD1+ (data not shown). The increased expression of CTLA-4 found on CD4+ T cells from OVA-bred OT-II mice provides yet another possible mechanism that can contribute to tolerance of fetal antigen-specific CD4+ T cells in this model. As with PDCD1 expression, most of the CD4+ T cells that were CTLA-4+ were also FoxP3+ (data not shown), implying that many of the CTLA-4+ cells could be accounted for by Tregs. Nevertheless, the fact that a subset of the CTLA-4+ CD4 cells does not express FoxP3, suggests that this potent negative modulator of T cells may have a direct role in inhibiting antifetal CD4+ T cell responses.
Tregs have been shown by others to play an important role in maintaining fetal tolerance [8, 37, 45–47]. Our data demonstrate that, in addition to the general expansion of Tregs during pregnancy, fetal antigen drives additional expansion of fetus-specific Tregs. This result is consistent with the induction of H-Y-specific Tregs to both model and natural fetal antigens in pregnancy [37, 48]. The antigen-specific expansion of Tregs we observed in OVA-bred mice was not detected until gd17.5, which is later in gestation than previous studies examining syngeneic and allogeneic breedings [8, 49]. The inability to detect OVA-specific Treg expansion earlier in gestation could reflect limitations of this model. Using intact TCR transgenic mice with a monoclonal T cell repertoire may make it difficult to detect small differences within the large antigen-specific cell population that are statistically significant (over the inherent background due to mouse to mouse variation), such that the increase in Tregs is not apparent until the cells have accumulated to appreciable levels. Similarly, deletion and TCR downregulation of OT-II cells were not detected until the second half of gestation in most tissues, even though T cell activation was apparent by gd0.5. Although TCR downregulation in this model may represent the transient phenotype of recently activated cells, the fact that we do not observe TCR downregulation in CD8+ T cells from OVA-bred OT-I mice despite a substantial amount of activation suggests that the TCR downregulation in the OT-II cells represents a bona fide mechanism of tolerance.
In contrast to the variety of potential mechanisms identified that may regulate OVA-specific CD4+ cells in OVA-bred OT-II mice, no consistent tolerogenic effect of fetal antigen on maternal CD8+ T cells was detected in OVA-bred OT-I mice despite clear evidence for activation of these cells. This result was surprising as it is generally accepted that the OT-I TCR has a higher functional affinity than the OT-II TCR; a notion supported by our in vitro proliferation data where OT-II cells require approximately 1000-fold more peptide than OT-I cells to generate a similar proliferative response (Fig. 4). These observations may signify less efficient in vivo processing and presentation of fetal OVA by maternal APCs on MHC class I molecules than on MHC class II molecules. Cross-presentation of exogenous fetal antigen by maternal APCs is likely required for generation of MHC class I-restricted fetal epitopes because others have shown that direct presentation of fetal mHAgs by fetal cells does not contribute to priming of maternal lymphocytes [19, 20]. Thus, it is likely that MHC class I-restricted epitopes of fetal antigen are less abundant than MHC class II-restricted epitopes because cross-presentation into the class I pathway is a process almost exclusive to dendritic cells [50], whereas, all MHC class II-expressing APCs could potentially generate MHC class II-restricted fetal epitopes. If presentation of MHC class II-restricted epitopes of fetal antigen is indeed more widespread than that of MHC class I-restricted epitopes, this may explain the differences in tolerance induction we observe between OVA-bred OT-I and OT-II mice.
Despite the lack of direct evidence for tolerance mechanisms in OVA-bred OT-I mice, they must in fact exist because approximately half the OVA-bred OT-I mice maintain healthy fetuses throughout gestation. The dramatic upregulation of CD44 demonstrates that the OT-I T cells in OVA-bred mice encounter fetal antigen during gestation and become activated, agreeing with previous results that maternal CD8+ T cells are exposed to and respond to fetal mHAgs [15, 19, 20]. With no detectable role for deletion, TCR downregulation, inhibitory molecules, or anergy in maintaining fetal tolerance of CD8+ T cells, events at the maternal-fetal interface may be important in regulating the fetus-specific T cells in this model. The lack of detectable tolerance mechanisms with OVA-bred OT-I mice is somewhat similar to a MHC-class I-restricted alloreactive model utilized by Zhou and Mellor [16]. Much like us, they found an expansion of fetus-specific CD8+ T cells during gestation, although in their model this expansion did not correlate with upregulation of activation markers. Regulatory mechanisms for fetus mHAg-specific CD8+ T cells have been identified in studies that examined tolerance to a MHC class I-restricted male mHAg. Deletion and anergy (but not TCR or coreceptor downregulation) were observed at gd14 in pregnant H-Y TCR transgenic mice compared to nonpregnant mice [15], and these effects were confirmed to be antigen-specific with the absence of deletion in H-Y mice carrying only female fetuses [51].
Another unexpected finding to arise from our studies of OVA-bred OT-I mice besides the lack of identifiable tolerance mechanisms was the fetal rejection observed in approximately half the OVA-bred OT-I mice. This is the first report in which spontaneous fetal loss occurred in a study using TCR transgenic mice specific for a fetally expressed mHAg or MHC difference. Even though we do not yet understand what tolerance mechanisms are critical in this model, the lack of differences in expression of tolerance markers between OVA-bred mice that did and did not resorb fetuses (data not shown) suggests that alternative/additional tolerance mechanisms than those investigated in this study are at play. Another question of this model is why half and not all of the OVA-bred OT-I dams experienced fetal loss. Nevertheless, these results are comparable to other murine models of alloantigen-associated fetal loss that also display well below 100% penetrance. For example, resorption rates in the abortion-prone murine model CBAxDBA are typically 20%–50% [52, 53]. Similarly, complete loss of allogeneic fetuses is not exhibited in mice in which tolerogenic molecules are rendered nonfunctional [54–56]. Additionally, disease penetrance in some models of both fetal loss and autoimmune disease are widely variable among and within institutions, possibly due to environmental stressors such as animal density and noise [57, 58].
While the mechanism of fetal loss is uncertain, the increased number of activated CD8+ T cells within the implantation sites of OVA-bred compared to B6-bred OT-I mice suggests that these cells may be directly involved. Recent data indicate that paternally inherited H-2Kb is expressed by trophoblast cells of the developing mouse placenta [59]. Although previous studies suggest that this expression is not sufficient for initial priming of CD8+ T cells [19, 20], it may be enough for trophoblasts to serve as targets in the face of activated CD8+ T cells. While this hypothesis is consistent with proximal tolerogenic events at the maternal-fetal interface being key, further experiments are required to confirm whether this in fact occurs.
The data presented here complement work in a parallel model previously used by us and others [19, 20, 39]. The cited studies utilized a model in which responses of adoptively transferred antigen-specific T cells were used as a readout of antigen presentation, while the current study tracks endogenous maternal antigen-specific CD4+ and CD8+ T cell responses across pregnancy and the effect that constant exposure to fetal antigen throughout gestation has on a population of fetal antigen-specific maternal T cells. The use of OT-I and OT-II dams not only made this possible, but also allowed us to assess the role of central tolerance in maternal T cell tolerance for the first time. Ongoing studies in our laboratory confirm that endogenous CD8+ T cell responses to paternally inherited fetal OVA occur and can be measured in pregnant OVA-bred B6 mice without the aid of TCR transgenic cells (Jasti and Petroff, unpublished data). This is also true for responses to the natural mHAg H-Y in both pregnant mice [17] and women carrying male fetuses [60–62]. While most published studies agree that activation markers and Treg are induced in response to fetal antigens, this study is the first to identify tolerance-inducing molecules on antigen-responsive cells.
In human (and mouse) pregnancy, there are likely hundreds or thousands of natural mHAg, only a fraction of which are defined [63, 64]. Presently, the principal defined natural murine mHAg for which tools and reagents are readily available are the H-Y antigens SMCY and UTY. As used in this study, OVA represents an alternative, autosomally encoded antigen that is inherited by all fetuses in a given pregnancy or with a given partnership. Thus, utilization of the OVA transgene as a model fetal mHAg, although artificial in nature, provides a mechanism to study maternal tolerance to a ubiquitously expressed fetal antigen. Differences in results between studies of different fetal antigens are expected and likely reflect differences in abundance, cellular expression, and MHC-binding strength between antigens as well as differences in the affinity of the TCR of the responding fetal antigen-specific maternal T cells for the corresponding MHC/peptide complex. Therefore, the maternal responses to various fetal antigens in women is likely diverse, and studies of Y-chromosome encoded, autosomal, and model antigens assist in a fuller understanding of the complexity of the maternal immune response to the fetus.
Although the use of TCR transgenic OT-I mothers (in which virtually all the CD8+ T cells can recognize the model fetal mHAg OVA) does not represent a real-world situation, the finding that fetal loss can occur in this setting nevertheless suggests that tolerance of maternal T cells specific for fetal antigens is important to establish and maintain. In women, maternal T cell responses to paternally inherited H-Y antigen of male fetuses occurs often during normal pregnancies [61], and responses to other antigens also occur as a result of pregnancy [60]. The clinical importance of these observations in pregnancy failure is uncertain because there is no direct evidence of antigen-specific T cell-mediated fetal loss in women. However, epidemiologic data suggests that idiopathic recurrent pregnancy loss subsequent to a normal pregnancy (secondary recurrent miscarriage) is associated with male sex of the firstborn [65]. The association is strongest when the mother carries the H-Y restricting class II HLA alleles, implying that tolerance of class II-restricted T cells may be particularly important [66]. Thus, while maternal/fetal mismatches in mHAg antigens and subsequent tolerance is clearly the norm in healthy pregnancy, whether unfavorable sequelae could result, for example, in cases of environmental stress or pathogen load, is a distinct possibility and requires further investigation.
In summary, this study investigated maternal T cell responses to fetal mHAg during pregnancy. Our findings of spontaneous fetal rejection in OVA-bred OT-I mice demonstrate that regulation of maternal responses to mHAgs can be critical for the well-being of the fetus. The ability to detect fetal antigen-specific responses in maternal lymphoid tissues suggests that tolerance to the fetus is likely established in lymphoid tissues through classical tolerance mechanisms as observed in OVA-bred OT-II mice. Although both minor and major histocompatibility antigen differences exist between fetus and mother, because of the limited expression of MHC molecules on trophoblast cells, it is unclear whether direct recognition of fetal MHC by maternal T cells poses a significant risk. Indeed, efforts to break tolerance in alloreactive BCR and TCR transgenic models have been unsuccessful even with targeted overexpression of paternal MHC in trophoblast cells [16, 67, 68]. Therefore, even though both alloreactive B cells and CD8+ T cells specific for fetal MHC are subject to tolerance induction [13, 14, 16], regulation of maternal responses to mHAg may be especially important in maintaining tolerance to the fetus. Although the events responsible for the spontaneous fetal rejection observed in OVA-bred OT-I mice are unknown, this observation underscores the importance of active tolerance induction to fetal antigens during pregnancy and warrants further studies in understanding the mechanisms by which tolerance to the fetus is normally upheld.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Joseph Juscius for assistance with genotyping of transgenic mice and Joyce Slusser and Richard Hastings for maintenance of the Flow Cytometry Core machinery.
Footnotes
This work was supported by NIH grants R01 HD045611, and P20 RR016443. A.L.P. was supported by NIH training grant T32HD007455. S.J. was supported in part by the University of Kansas Medical Center Biomedical Research Training Program.
REFERENCES
- Sehmsdorf US, Zenclussen AC, Arck P, Hertwig K, Joachim RA, Klapp B, Hildebrandt MO. Human miscarriage is associated with increased number of CD26+ decidual lymphocytes. Scand J Immunol 2004; 59: 400–407. [DOI] [PubMed] [Google Scholar]
- Saito S, Sakai M, Sasaki Y, Nakashima A, Shiozaki A. Inadequate tolerance induction may induce preeclampsia. J Reprod Immunol 2007; 76: 30–39. [DOI] [PubMed] [Google Scholar]
- Trowsdale J, Betz AG. Mother's little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol 2006; 7: 241–246. [DOI] [PubMed] [Google Scholar]
- Guleria I, Sayegh MH. Maternal acceptance of the fetus: true human tolerance. J Immunol 2007; 178: 3345–3351. [DOI] [PubMed] [Google Scholar]
- Petroff MG. Immune interactions at the maternal-fetal interface. J Reprod Immunol 2005; 68: 1–13. [DOI] [PubMed] [Google Scholar]
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998; 281: 1191–1193. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod 2004; 10: 347–353. [DOI] [PubMed] [Google Scholar]
- Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 2004; 5: 266–271. [DOI] [PubMed] [Google Scholar]
- Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, Kotsch K, Leber J, Volk H-D. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol 2005; 166: 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz B. The feto-maternal interface: setting the stage for potential immune interactions. Semin Immunopathol 2007; 29: 83–94. [DOI] [PubMed] [Google Scholar]
- Redman CWG, Sargent IL. Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta 2008; 29: S73–S77. [DOI] [PubMed] [Google Scholar]
- Johnson KL, Bianchi DW. Fetal cells in maternal tissue following pregnancy: what are the consequences? Hum Reprod Update 2004; 10: 497–502. [DOI] [PubMed] [Google Scholar]
- Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science 1995; 270: 630–633. [DOI] [PubMed] [Google Scholar]
- Ait-Azzouzene D, Gendron MC, Houdayer M, Langkopf A, Burki K, Nemazee D, Kanellopoulos-Langevin C. Maternal B lymphocytes specific for paternal histocompatibility antigens are partially deleted during pregnancy. J Immunol 1998; 161: 2677–2683. [PubMed] [Google Scholar]
- Jiang SP, Vacchio MS. Multiple mechanisms of peripheral T cell tolerance to the fetal “allograft”. J Immunol 1998; 160: 3086–3090. [PubMed] [Google Scholar]
- Zhou M, Mellor AL. Expanded cohorts of maternal CD8+ T-cells specific for paternal MHC class I accumulate during pregnancy. J Reprod Immunol 1998; 40: 47–62. [DOI] [PubMed] [Google Scholar]
- James E, Chai JG, Dewchand H, Macchiarulo E, Dazzi F, Simpson E. Multiparity induces priming to male-specific minor histocompatibility antigen, HY, in mice and humans. Blood 2003; 102: 388–393. [DOI] [PubMed] [Google Scholar]
- Seavey MM, Mosmann TR. Paternal antigen-bearing cells transferred during insemination do not stimulate anti-paternal CD8+ T cells: role of estradiol in locally inhibiting CD8+ T cell responses. J Immunol 2006; 177: 7567–7578. [DOI] [PubMed] [Google Scholar]
- Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest 2007; 117: 1399–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldenhauer LM, Diener KR, Thring DM, Brown MP, Hayball JD, Robertson SA. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J Immunol 2009; 182: 8080–8093. [DOI] [PubMed] [Google Scholar]
- Nancy P, Tagliani E, Tay C-S, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science 2012; 336: 1317–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann A, Zal T, Stockinger B. Antigen-presenting cells in the thymus that can negatively select MHC class II-restricted T cells recognizing a circulating self antigen. J Immunol 1997; 158: 693–706. [PubMed] [Google Scholar]
- Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol 2006; 7: 1092–1100. [DOI] [PubMed] [Google Scholar]
- Taglauer ES, Yankee TM, Petroff MG. Maternal PD-1 regulates accumulation of fetal antigen-specific CD8+ T cells in pregnancy. J Reprod Immunol 2009; 80: 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff MG. Review: fetal antigens—identity, origins, and influences on the maternal immune system. Placenta 2011; 32 (Suppl 2): S176–S181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldenhauer LM, Hayball JD, Robertson SA. Utilising T cell receptor transgenic mice to define mechanisms of maternal T cell tolerance in pregnancy. J Reprod Immunol 2010; 87: 1–13. [DOI] [PubMed] [Google Scholar]
- Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant 2003; 3: 1355–1362. [DOI] [PubMed] [Google Scholar]
- Deb K, Reese J, Paria BC. Methodologies to study implantation in mice. Methods Mol Med 2006; 121: 9–34. [DOI] [PubMed] [Google Scholar]
- Zoller AL, Schnell FJ, Kersh GJ. Murine pregnancy leads to reduced proliferation of maternal thymocytes and decreased thymic emigration. Immunology 2007; 121: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton MT, Fortner KA, Bizargity P, Bonney EA. Pregnancy alters the proliferation and apoptosis of mouse splenic erythroid lineage cells and leukocytes. Biol Reprod 2009; 81: 457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Jung LKL, Bjorndahl JM, Fu SM. Human T cell activation. III. Rapid induction of a phosphorylated 28kD/32kD disulfide-linked early activation antigen (EA 1) by 12-o-tetradecanoyl phorbol-13-acetate, mitogens and antigens. J Exp Med 1986; 164: 1988–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habicht A, Dada S, Jurewicz M, Fife BT, Yagita H, Azuma M, Sayegh MH, Guleria I. A link between PDL1 and T regulatory cells in fetomaternal tolerance. J Immunol 2007; 179: 5211–5219. [DOI] [PubMed] [Google Scholar]
- Wafula PO, Teles A, Schumacher A, Pohl K, Yagita H, Volk H-D, Zenclussen AC. PD-1 but not CTLA-4 blockage abrogates the protective effect of regulatory T cells in a pregnancy murine model. Am J Reprod Immunol 2009; 62: 283–292. [DOI] [PubMed] [Google Scholar]
- Wallin JJ, Liang L, Bakardjiev A, Sha WC. Enhancement of CD8+ T cell responses by ICOS/B7h costimulation. J Immunol 2001; 167: 132–139. [DOI] [PubMed] [Google Scholar]
- Schenk AD, Gorbacheva V, Rabant M, Fairchild RL, Valujskikh A. Effector functions of donor-reactive CD8 memory T cells are dependent on ICOS induced during division in cardiac grafts. Am J Transplant 2009; 9: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Wan N, Zhang S, Moore Y, Wan F, Dai Z. Cutting edge: programmed death-1 defines CD8+CD122+ T cells as regulatory versus memory T cells. J Immunol 2010; 185: 803–807. [DOI] [PubMed] [Google Scholar]
- Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 2012; 490: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, Cardenas I, Abrahams VM, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci 2011; 1221: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglauer ES, Trikhacheva AS, Slusser JG, Petroff MG. Expression and function of PDCD1 at the human maternal-fetal interface. Biol Reprod 2008; 79: 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff MG, Perchellet A. B7 family molecules as regulators of the maternal immune system in pregnancy. Am J Reprod Immunol 2010; 63: 506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff MG, Chen L, Phillips TA, Azzola D, Sedlmayr P, Hunt JS. B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol Reprod 2003; 68: 1496–1504. [DOI] [PubMed] [Google Scholar]
- Petroff MG, Kharatyan E, Torry DS, Holets L. The immunomodulatory proteins B7-DC, B7-H2, and B7-H3 are differentially expressed across gestation in the human placenta. Am J Pathol 2005; 167: 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu T, Barrier BF, Schust DJ. The regulation of T-cell cytokine production by ICOS-B7-H2 interactions at the human fetomaternal interface. Immunol Cell Biol 2011; 89: 417–425. [DOI] [PubMed] [Google Scholar]
- Nagamatsu T, Schust DJ, Sugimoto J, Barrier BF. Human decidual stromal cells suppress cytokine secretion by allogenic CD4+ T cells via PD-1 ligand interactions. Hum Reprod 2009; 24: 3160–3171. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlström AC, Care AS. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod 2009; 80: 1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenclussen AC. CD4(+)CD25+ T regulatory cells in murine pregnancy. J Reprod Immunol 2005; 65: 101–110. [DOI] [PubMed] [Google Scholar]
- Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, Saito S. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol 2010; 85: 121–129. [DOI] [PubMed] [Google Scholar]
- Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci U S A 2010; 107: 9299–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JX, Zeng YY, Liu Y. Fetal alloantigen is responsible for the expansion of the CD4(+)CD25(+) regulatory T cell pool during pregnancy. J Reprod Immunol 2007; 75: 71–81. [DOI] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P. G-IS, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 2002; 17: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacchio MS, Hodes RJ. CD28 costimulation is required for in vivo induction of peripheral tolerance in CD8 T cells. J Exp Med 2003; 197: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouat G. Assal Meliani A, Martal J, Raghupathy R, Elliott JF, Elliot J, Mosmann T, Wegmann TG. IL-10 prevents naturally occurring fetal loss in the CBA x DBA/2 mating combination, and local defect in IL-10 production in this abortion-prone combination is corrected by in vivo injection of IFN-tau. J Immunol 1995; 154: 4261–4268. [PubMed] [Google Scholar]
- Schumacher A, Heinze K, Witte J, Poloski E, Linzke N, Woidacki K, Zenclussen AC. Human chorionic gonadotropin as a central regulator of pregnancy immune tolerance. J Immunol 2013; 190: 2650–2658. [DOI] [PubMed] [Google Scholar]
- Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, Tirado I, Markert UR, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med 2007; 13: 1450–1457. [DOI] [PubMed] [Google Scholar]
- Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, Noelle RJ, Coyle A, Mellor AL, Khoury SJ, Sayegh MH. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med 2005; 202: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riella LV, Dada S, Chabtini L, Smith B, Huang L, Dakle P, Mfarrej B, D'Addio F, Adams L-T, Kochupurakkal N, Vergani A, Fiorina P, et al. B7h (ICOS-L) maintains tolerance at the fetomaternal interface. Am J Pathol 2013; 182: 2204–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DA, Banwatt D, Chaouat G. Stress-triggered abortion in mice prevented by alloimmunization. Am J Reprod Immunol 1993; 29: 141–147. [DOI] [PubMed] [Google Scholar]
- Arck PC, Merali FS, Manuel J, Chaouat G, Clarke DA. Stress-triggered abortion: inhibition of protective suppression and promotion of tumor necrosis factor-alpha release as a mechanism triggering resorptions in mice. Am J Reprod Immunol 1995; 33: 74–80. [DOI] [PubMed] [Google Scholar]
- Madeja Z, Yadi H, Apps R, Boulenouar S, Roper SJ, Gardner L, Moffett A, Colucci F, Hemberger M. Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc Natl Acad Sci U S A 2011; 108: 4012–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdijk RM, Kloosterman A, Pool J, van de Keur M, Naipal AM, van Halteren AG, Brand A, Mutis T, Goulmy E. Pregnancy induces minor histocompatibility antigen-specific cytotoxic T cells: implications for stem cell transplantation and immunotherapy. Blood 2004; 103: 1961–1964. [DOI] [PubMed] [Google Scholar]
- Lissauer D, Piper K, Goodyear O, Kilby MD, Moss PAH. Fetal-specific CD8+ cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. J Immunol 2012; 189: 1072–1080. [DOI] [PubMed] [Google Scholar]
- Piper KP, McLarnon A, Arrazi J, Horlock C, Ainsworth J, Kilby MD, Martin WL, Moss PA. Functional HY-specific CD8+ T cells are found in a high proportion of women following pregnancy with a male fetus. Biol Reprod 2007; 76: 96–101. [DOI] [PubMed] [Google Scholar]
- Linscheid C, Petroff MG. Minor histocompatibility antigens and the maternal immune response to the fetus during pregnancy. Am J Reprod Immunol 2013; 69: 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulmy E. Minor histocompatibility antigens: from transplantation problems to therapy of cancer. Human Immunol 2006; 67: 433–438. [DOI] [PubMed] [Google Scholar]
- Svarre Nielsen H, Nybo Andersen A-M, Kolte AM, Christiansen OB. A firstborn boy is suggestive of a strong prognostic factor in secondary recurrent miscarriage: a confirmatory study. Fertil Steril 2008; 89: 907–911. [DOI] [PubMed] [Google Scholar]
- Nielsen HS, Steffensen R, Varming K, Van Halteren AGS, Spierings E, Ryder LP, Goulmy E, Christiansen OB. Association of HY-restricting HLA class II alleles with pregnancy outcome in patients with recurrent miscarriage subsequent to a firstborn boy. Hum Mol Genet 2009; 18: 1684–1691. [DOI] [PubMed] [Google Scholar]
- Rogers AM, Boime I, Connolly J, Cook JR, Russell JH. Maternal-fetal tolerance is maintained despite transgene-driven trophoblast expression of MHC class I, and defects in Fas and its ligand. Eur J Immunol 1998; 28: 3479–3487. [DOI] [PubMed] [Google Scholar]
- Ait-Azzouzene D, Caucheteux S, Tchang F, Wantyghem J, Moutier R, Langkopf A, Gendron MC, Kanellopoulos-Langevin C. Transgenic major histocompatibility complex class I antigen expressed in mouse trophoblast affects maternal immature B cells. Biol Reprod 2001; 65: 337–344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


