ABSTRACT
Proper regulation of trophoblast proliferation, differentiation, and function are critical for placenta development and function. The RNA-binding protein, LIN28A, has been well characterized as a potent regulator of differentiation in embryonic stem cells; however, little is known about the function of LIN28A in the placenta. We assessed LIN28A in vitro using mouse trophoblast stem (mTS) cells and human trophoblast cells (ACH-3P). We observed that LIN28A decreased and let-7 miRNA increased when mTS cells were induced to differentiate into mouse trophoblast giant cells (mTGCs) upon the removal of FGF4, heparin and conditioned medium. Similarly, we observed that LIN28A decreased in ACH-3P cells induced to syncytialize with forskolin treatment. To assess LIN28A in vivo we examined Embryonic Day 11.5 mouse placenta and observed abundant LIN28A in the chorioallantoic interface and labyrinth layer, with little LIN28A staining in spongiotrophoblast or differentiated mTGCs. Additionally, shRNA-mediated LIN28A knockdown in ACH-3P cells resulted in increased spontaneous syncytialization, and increased levels of syncytiotrophoblast markers hCG, LGALS13, and ERVW-1 mRNA. Additionally, targeted degradation of LIN28A mRNA increased responsiveness to forskolin-induced differentiation. In contrast, targeted degradation of Lin28a mRNA in mTS cells did not alter cell phenotype when maintained under proliferative culture conditions. Together, these data establish that LIN28A has a functional role in regulating trophoblast differentiation and function, and that loss of LIN28A in human trophoblast is sufficient to induce differentiation, but does not induce differentiation in the mouse.
Keywords: differentiation, LIN28A, placenta, syncytiotrophoblast, trophoblast
LIN28A regulates trophoblast differentiation.
INTRODUCTION
The initial differentiation event during embryo development is the segregation of pluripotent cells into either trophoblast or inner cell mass cell lineages. Placenta establishment and development originates and depends upon trophoblast stem (TS) cell differentiation into specialized trophoblast subtypes. Disruption of temporal or molecular programs regulating the differentiation of TS cells can result in insufficient sublineage trophoblast populations, and ultimately in compromised invasion or utero-placental vascular remodeling, poor metabolic exchange, and/or abnormal endocrine function. In the human, trophoblast dysfunction is thought to be an underlying cause of the development of increased intervillus blood pressure, perfusion velocity, and the development of global hypertension and inflammation associated with placental diseases, such as pre-eclampsia (PE) and intrauterine growth restriction (IUGR) [1–5]. Assessments of placental tissue taken from PE and IUGR pregnancies are associated with lower cytotrophoblast fusion indices, a higher rate of syncytial apoptosis, and insufficient extravillous trophoblast invasion and villus development [6–8]. Immediate implications of placenta dysfunction include fetal growth restriction, fetal distress, preterm delivery, and, in acute cases, death. However, research also indicates that placenta dysfunction contributes to long-term physiological programing of the offspring, manifesting in increased risk of hypertension, cardiac disease, diabetes, and obesity [9–12]. Therefore, understanding the mechanisms regulating trophoblast differentiation is key to developing means for efficient diagnoses and treatment of placenta dysfunction.
LIN28A, an RNA binding protein, has been well characterized as a potent posttranscriptional regulator of differentiation in embryonic stem cells (ESCs), with the ability to enhance retrodifferentiation of somatic cells into induced pluripotent stem cells (iPSC) [13–16]. Additionally, reactivation of LIN28A in terminally differentiated tissue has been implicated in oncogenic transformation and poor prognostic outcome [17–20]. To date, LIN28A has been described to have two distinct regulatory mechanisms for maintaining pluripotency: inhibition of let-7 miRNA maturation [18] and direct posttranscriptional regulation of target mRNA [21]. LIN28A blocks let-7 miRNA maturation in undifferentiated cells by recruiting terminal uridylyl transferase [14, 22, 23]. The human let-7 miRNA family consists of 10 different mature let-7 miRNA sequences produced from 13 precursor sequences on nine different chromosomes. Biological function of the let-7 miRNAs is determined by the conserved let-7 seed sequence targeting mRNA. Although the different family members likely have an overlapping set of targets, it is possible that different family members have different functions in the same cell [24, 25]. While there has been extensive research into the role of LIN28A in ESC differentiation [16, 21, 26, 27], there are few data on whether LIN28A regulates TS cell differentiation important for the establishment and function of trophoblast sublineages critical for placenta health. Yang and Moss [28] observed LIN28A in Embryonic Day (E) 7.5 mouse trophoblast, and Vogt et al. [29] reported a role for LIN28A at the two-cell stage in the mouse, concluding that LIN28A regulates the maturation of nucleoli required for the transition between maternal and embryonic genome control. Additionally, Vogt et al. [29] reported finding LIN28A isolated to the outer blastomeres in marmoset blastocysts, suggesting a role for LIN28A in early primate trophectoderm development.
The aim of this study was to determine whether LIN28A is important for modulating trophoblast differentiation, and ultimately to determine whether disruption of LIN28A would impact trophoblast differentiation and/or function.
MATERIALS AND METHODS
All animal experiments were performed in accordance with protocols approved by the Colorado State University Institutional Animal Care and Use Committee.
Cell Lines
Mouse TS (mTS) cells were derived from blastocyst-stage embryos at 3.5 Days Postcoitum from naturally bred Black Swiss female mice, using techniques previously described [30, 31]. Briefly, mouse blastocysts were collected and cultured on a feeder layer of mitomycin-C-treated mouse embryonic fibroblasts. TS cell colonies were isolated from blastocyst outgrowths and separated from feeder fibroblasts through serial passage. Isolated mTS cells were maintained in 70% mouse embryonic fibroblast conditioned medium and 30% TS medium (RPMI 1640, 2 mM L-glutamine, 30% FBS, 1 mM sodium pyruvate, 100 μM β-mercaptoethanol, antibiotic-antimycotic solution containing 10 000 IU/ml penicillin, 10 000 μg/ml streptomycin, 25 μg/ml amphotericin) supplemented with 25 ng/ml FGF4 and 1 μg/ml heparin. Mouse TS cell differentiation into mouse trophoblast giant cells (mTGCs) was induced by removal of conditioned medium, FGF4, and heparin for 6 days.
ACH-3P cells (a generous gift from Ursula Hiden, Medical University of Graz, Austria), a cell line derived from the fusion of AC1-1 cells with primary first-trimester human trophoblast cells [32], were grown in F-12 Medium (10% FBS, 2 mM L-glutamine, antibiotic-antimycotic solution containing 10 000 IU/ml penicillin, 10 000 μg/ml streptomycin, 25 μg/ml amphotericin). ACH-3P cells were induced to differentiate into syncytiotrophoblast by treatment with 40 μM forskolin for 48 h; forskolin is known to induce morphological fusion of cultured trophoblast cells, which closely resembles morphology of natural syncytiotrophoblast [33].
Real-Time RT-PCR
Total RNA was extracted from cells using miRNA Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's directions. For mRNA analysis, cDNA was generated from 1 μg of total cellular RNA using qScript cDNA Supermix (product no. 95048; Quanta Biosciences, Gaithersburg, MD) and quantitative real-time RT-PCR (qPCR) of mRNA was performed as described previously [30]. Briefly, each 1× 20-μl qPCR reaction consisted of 10 μl LightCycler 480 Probes Master mix (product no. 04707494001; Roche, Mannheim, Germany), 1 μl of 150 nM TaqMan Gene Expression Assay (Applied Biosystems, Carlsbad, CA), and 9 μl of cDNA template diluted to 90 μl. Quantitative PCR was performed using the Light Cycler 480 thermal cycler (Roche) with the following parameters: 10-min preincubation at 95°C, 45 cycles of amplification, which included denaturation at 95°C for 10 sec, annealing at 60°C for 30 sec and extension at 72°C for 1 sec, followed by a final cooling cycle at 40°C for 5 min. Normalization of mRNA levels in mTS cells was calculated using levels of glyceraldehyde-3-phosphate dehydrogenase (Gapdh). Normalization of mRNA in human cells was accomplished using the geometric mean of TATA box binding protein mRNA (TBP) and mitochondrial ribosomal protein S15 mRNA (MRPS15).
For miRNA analysis, cDNA was generated from 500 ng of total cellular RNA using miScript II RT Kit (product no. 218161; Qiagen). The 1× 10-μl miRNA qPCR reaction consisted of 5 μl LightCycler 480 SYBR Green I Master mix (product no. 04707516001; Roche), 1 μl of forward primer, 1 μl universal reverse primer (SBI System Biosciences, Mountain View, CA for mouse let-7 miRNA primers, Qiagen miScript for human let-7 miRNA primers), and 8 μl of cDNA template diluted to a volume of 150 μl. MicroRNA qPCR was performed using the Light Cycler 480 thermal cycler (Roche) with the following cycling parameters: 15-min enzyme activation step at 95°C, followed by 45 cycles of amplification, which included denaturation at 94°C for 15 sec, annealing at 55°C for 30 sec, and extension at 70°C for 30 sec, followed by a melting curve analysis to confirm amplification quality and specificity. Normalization of let-7 miRNA levels in mTS cells was calculated using the geometric mean of ncRNA (U6) snRNA and small nucleolar RNA, C/D box 43 (RNU43) snoRNA. Normalization of let-7 miRNA levels in human cells was calculated using the geometric mean of small nucleolar RNA, C/D box 41 (SNORD41), and small nucleolar RNA, C/D box 44 (SNORD44) miRNAs. Relative expression was determined for all qPCR data using the comparative Ct method [34].
Statistics.
We used Student t-test to compare relative expression of genes in wild-type trophoblast, nontarget controls, LIN28A knockdown (KD), and forskolin-treated cells from each of the experimental models. We used the Newman-Keul multiple-comparison test with an alpha of 0.05 to compare let-7 miRNA levels between mTS Lin28a KD and nontarget control (see Fig. 3C).
FIG. 3.
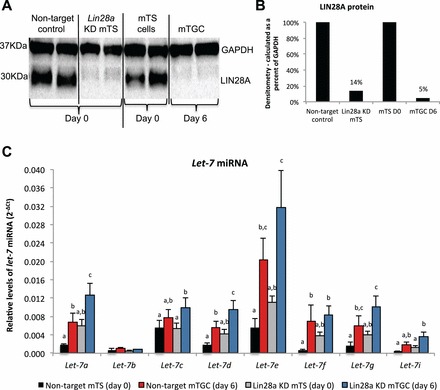
Short-hairpin RNA-mediated Lin28a KD in mTS cells results in increased levels of let-7 miRNA. A) LIN28A protein levels in Lin28a KD and nontarget control mTS cells (columns 1–4) in relation to mTS cells at Day 0 and after 6 days in differentiation medium (columns 5–8). B) Densitometry analysis of LIN28A protein bands (calculations made as a percent of GAPDH protein). C) Levels of let-7 miRNA in Lin28a KD and nontarget control mTS cells, and mTS cells collected at Day 0 and after 6 days in differentiation medium. Error bars represent SEM. Different letters indicate P < 0.05.
Western Blot
Cellular protein was assessed by Western blot analysis, similar to procedures described previously [30, 35, 36]. Briefly, cells were lysed in RIPA buffer (20 mM Tris, 137 mM NaCl, 10% glycerol, 1% nonidet P-40, 3.5 mM SDS, 1.2 mM sodium deoxycholate, 1.6 mM EDTA, pH 8) supplemented with 10% protease/phosphatase inhibitor cocktail (product no. P8340; Sigma-Aldrich, St. Louis, MO), and 1 mM phenylmethanesulfonyl fluoride. Protein concentration was determined using the Bradford assay method [37]; absorbance was measured at λ 595 nm using the Biotek Synergy 2 Microplate Reader (Biotek, Winooski, VT). Protein was electrophoresed through 4%–12% Bis-Tris gels, then transferred to 0.45-μm pore nitrocellulose membrane at a constant 100 V on ice for 1 h. Membranes were blocked in 10% milk-TBST (50 mM Tris, 150 mM NaCl, 0.05% Tween 20, pH 7.6) overnight at 4°C to reduce nonspecific binding, then incubated with primary antibody at ambient temperature for 2 h. Membranes were washed in TBST, and then incubated with a horseradish peroxidase-conjugated secondary antibody for 1 h at ambient temperature. For assessment of LIN28A protein in human and mouse whole-cell lysate, a polyclonal antibody to LIN28A (1:1000 dilution; product no. ab63740; Abcam, Inc., Cambridge, MA) was used in conjunction with a horseradish peroxidase-conjugated secondary (1:2000 dilution; product no. sc-2004; Santa Cruz Biotechnology, Inc., Dallas, TX). An antibody to beta-actin (1:2000 dilution; product no. sc-47778; Santa Cruz Biotechnology), or an antibody to GAPDH (1:2000 dilution; product no. ab37168; Abcam, Inc.), was used in conjunction with horseradish peroxidase-conjugated secondary as loading control and to normalize LIN28A protein in cell lysates (1:2000 dilution; product no. 1858413; Pierce, Rockford, IL; or 1:2000 dilution; product no. sc-2004; Santa Cruz Biotechnology). Membranes were developed using ECL Western Blotting Detection Reagent chemiluminescent kit (product no. RPN2132; Amersham, Pittsburgh, PA), and membranes were imaged using the ChemiDoc XRS+ chemiluminescence system (Bio-Rad, Hercules, CA). Densitometry calculations were performed using Image Lab Software (Bio-Rad). Fold change was calculated as a percent of nontarget control protein after normalization.
Immunohistochemistry
Mouse placentas were collected on E11.5 from naturally bred Black Swiss female mice. Placenta tissue was fixed in 4% paraformaldehyde and paraffin embedded. Immunohistochemistry was performed as previously described [35]. Briefly, 6-μm sections were cut, dewaxed in xylene, and rehydrated through serial ethanol dilutions, then treated with 3% H2O2 to reduce endogenous peroxidase activity. Sections were blocked in SuperBlock Blocking Buffer (product no. 37580; ThermoScientific, Waltham, MA) overnight at 4°C to reduce nonspecific binding. Cells were incubated with an antibody to LIN28A overnight at 4°C (1:200 dilution; product no. ab63740; Abcam, Inc.). For negative controls, primary antibody was preabsorbed with antibody-specific synthetic antigen (2:1 ratio; product no. ab64578; Abcam, Inc.). The VectaStain Elite ABC Kit (rabbit IgG) biotinylated anti-rabbit secondary antibody was used according to manufacturer's directions, and positive immunostaining was visualized using the avidin-biotin complex (ABC) system (product no. PK-6101; Vector Laboratories, Burlingame, CA). Diaminobenzedine was used as the final chromogen (product no. SK-4100; Vector Laboratories) and Harris modified hematoxylin was used to counterstain.
LIN28A Knockdown
Stably transduced Lin28a mRNA KD and nontarget control cell lines were created using commercially available MISSON shRNA Lentiviral Transduction Particles, lentiviral-based pLKO.1-puro vectors (Sigma-Aldrich). The pLKO.1-puro vectors used contained an shRNA sequence cassette downstream of a modified U6 shRNA promoter and a puromycin resistance gene for selection downstream of a human phosphoglycerate kinase eukaryotic promoter. The lentiviral-based pLKO.1-puro vector used to knock down Lin28a in mTS cells contains an shRNA sequence designed to target mouse Lin28a mRNA for degradation (CCGGCAAAGGAGACAGGTGCTACAACTCGAGTTGTAGCACCTGTCTCCTTTGTTTTTG; clone no. TRCN0000102578; Sigma-Aldrich).
The lentiviral-based pLKO.1-puro vector used to knock down LIN28A in ACH-3P cells contains an shRNA sequence designed to target human LIN28A mRNA for degradation (CCGGACCTACTTTCGAGAGGAAGAACTCGAGTTCTTCCTCTCGAAAGTAGGTTTTTT; clone no. TRCN0000021802; Sigma-Aldrich). Both ACH-3P and mTS nontarget control cell lines were created using a pLKO.1-puro vector with an shRNA sequence designed to target no known mammalian genes (CCGGGCGCGATAGCGCTAATAATTTCTCGAGAAATTATTAGCGCTATCGCGCTTTT; product no. SHC002V; Sigma-Aldrich). Mouse TS cells were infected in three replicate experiments with either Lin28a mRNA-targeted particles or nontarget particles at a multiplicity of infection (MOI) of 100 viral particles per cell. Infected mTS cells were selected by treatment with 2 μg/ml puromycin for 14 days or until all untreated control cells were dead. ACH-3P cells were infected in three replicate experiments with either the LIN28A mRNA-targeted particles or the nontarget control particles at an MOI of 500 viral particles per cell. Infected ACH-3P cells were selected by treatment with 8 μg/ml puromycin for 14 days or until all untreated control cells were dead. The degree of LIN28A mRNA and protein KD was determined by qPCR and Western blot, as described previously here.
Enzyme-Linked Immunosorbent Assay
ACH-3P nontarget and LIN28A KD cells were plated in six-well culture dishes with 25 000 cells per well and six replicates per treatment. Cell culture medium (1.5 ml/replicate) was collected after 48 h, and cells were collected and counted. Concentrations of human chorionic gonadotropin (hCG) were quantified using an hCG ELISA kit utilizing a mouse monoclonal anti-hCG (specific to beta-hCG) conjugated to horseradish peroxidase according to the manufacturer's instructions (product no. 25-HCGHU-E01; ALPCO Diagnostics, Salem NH).
Experimental replicates were assayed in triplicate, and absorbance was measured at λ 450 nm. Soluble hCG concentrations (mIU/ml) were calculated by plotting sample mean absorbance values against the mean values calculated from duplicate standard curve values. The standard curve consisted of 0, 5, 20, 50, 150, and 300 mIU/ml hCG standards. Soluble hCG concentrations were then normalized to the number of cells at the time of collection. We used the Student t-test to compare hCG concentrations between nontarget controls and LIN28A KD ACH-3P cell medium. P values of less than 0.05 were considered to be statistically significant.
Immunofluorescence
ACH-3P nontarget and LIN28A KD cells were plated on 18-mm glass coverslips in six-well culture dishes with 10 000 cells per well and six replicates per treatment. To determine ACH-3P cells positive for hCG, immunofluorescence was performed in a manner similar to that described previously [38]. Briefly, cells were grown for 48 h, medium was aspirated, cells were rinsed with PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4) and then fixed in 100% ice-cold acetone for 10 min at −20°C. Cells were rinsed with PBS and then blocked in 10% goat serum in PBS-0.1% Tween for 20 min at ambient temperature. Cells were incubated with anti-beta-hCG primary antibody (1:50 dilution; product no. RB-059-A; ThermoScientific) overnight at 4°C with gentle agitation. Cells were rinsed with PBS-0.1% Tween and incubated for 1 h at ambient temperature with AlexFluor 488-conjugated secondary antibody (1:1000 dilution; product no. A11008; Invitrogen, Carlsbad, CA). Cells were incubated with a plasma membrane stain (CellMask Orange; Invitrogen) according to the manufacturer's directions, rinsed, and then incubated with 5 μg/ml Hoechst 33342 for 10 min at ambient temperature to visualize cell nuclei.
To assess ACH-3P cell fusion, ACH-3P cells were plated on 18-mm glass coverslips in six-well culture dishes with 10 000 cells per well and six replicates per treatment. Cells were rinsed with PBS and then incubated with the plasma membrane stain, Concanavalin A, AlexaFluor 488 conjugate for 10 min at ambient temperature (1:1000 dilution; product no. C11252; Invitrogen). Cells were fixed in 100% ice-cold acetone for 10 min at −20°C, rinsed in PBS, and then treated with 500 nM propidium iodide for 10 min at ambient temperature.
RESULTS
Levels of LIN28A and let-7 miRNA in mTS Cells
Mouse TS cells were used to determine if levels of LIN28A changed with differentiation into mTGCs. Mouse TS cells grown for 6 days in the absence of FGF4, heparin, and fibroblast-conditioned medium differentiated from proliferative mTS into mTGCs. Mouse TS cell differentiation was characterized by typical morphological transformation from compact proliferative mTS cells into expanded endoreduplicating mTGCs (Fig. 1, A and B). Day-6 mTGCs had a 13-fold decrease in Lin28a mRNA (P < 0.01) compared to mTS maintained in proliferative culture conditions (Fig. 1C). The decreases observed in Lin28a mRNA data were corroborated by decreases in LIN28A protein, as determined by Western blot of whole-cell lysate and immunohistochemistry (Fig. 1, D–F). The decreased levels of LIN28A mRNA and protein in mTGCs were accompanied by significantly increased levels of all let-7 miRNA family members (P < 0.05) with the exception of let-7b (Fig. 1G). These data suggest that mTS cell differentiation may be a LIN28A-mediated event, and that LIN28A acts to inhibit let-7 miRNA maturation in proliferative mTS cells.
FIG. 1.
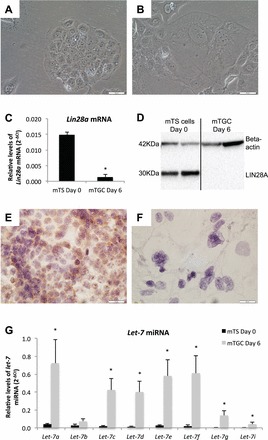
LIN28A and let-7 miRNA levels in proliferative mTS cells and differentiated mTGCs. A and B) Brightfield microscopy (20×) depicts mTS cell morphology at Day 0 under proliferative conditions, and after 6 days under differentiation conditions. C and D) LIN28A mRNA and protein levels are decreased in differentiated mTGCs compared to proliferative mTS cells. Immunohistochemistry demonstrates that LIN28A in proliferating mTS (E) is decreased when cells differentiate into mTGCs (F) after 6 days without FGF4, heparin, or conditioned medium. G) Levels of let-7 miRNA were increased in mTGCs grown under differentiating conditions for 6 days. Error bars represent SEM. Asterisk indicates P < 0.05. Bar = 0.05 mm.
In Vivo Localization of LIN28A in E11.5 Mouse Placenta
Immunohistochemistry, using serial sections of E11.5 mouse placenta, was used to determine in vivo LIN28A protein localization during midgestation. Distinct areas along the chorioallantoic interface, as well as the extraembryonic membranes, stained positive for LIN28A (Fig. 2, A1 and B1). The trophoblast cells of the labyrinth layer had strong, diffuse LIN28A staining throughout (Fig. 2, A2 and B2). Allantois, spongiotrophoblast, and mTGCs in the junctional zone had little or no LIN28A reactivity (Fig. 2, A3 and B3). Maternal decidua also had little to no LIN28A staining. These data demonstrate that LIN28A protein is present in midgestational mouse placenta, and may have a role in the regulation of trophoblast differentiation and/or function.
FIG. 2.
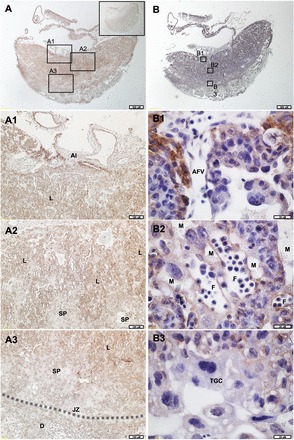
LIN28A in E11.5 mouse placenta. A) E11.5 mouse placenta stained for LIN28A (2×). Bar = 0.5 mm. Boxed areas represent areas of 10× magnification depicted in A1–A3. Inset shows preabsorbed control. B) E11.5 mouse placenta stained for LIN28A and counterstained with hematoxylin (2×). Boxed areas represent areas of 60× magnification depicted in B1–B3. Al, allantois; AFV, allantoic-derived fetal vessel; D, decidua; F, fetal vessel; JZ (and dashed line), junctional zone; L, labyrinth layer; M, maternal vessel; SP, spongiotrophoblast; TGC, trophoblast giant cell.
Loss of Lin28a mRNA in Mouse Trophoblast Cells
To determine the role of LIN28A in mTS cell differentiation and function, a Lin28a mRNA KD mTS cell line was created. Decreased levels of LIN28A protein were confirmed in Lin28a KD mTS cells via Western blot (Fig. 3A). Densitometry analysis of the Western blot data demonstrated an 86% reduction of LIN28A in Lin28a KD mTS cells, compared to a 95% reduction of LIN28A observed in mTGCs after 6 days without FGF4, heparin, and conditioned medium (Fig. 3B). Lin28a KD mTS cells resulted in increased levels of let-7 miRNA similar to mTGC differentiated for 6 days without FGF4, heparin, or conditioned medium (Fig. 3C). These miRNA data suggest that 86% loss of LIN28A is sufficient to induce a comparable increase in the levels of let-7 miRNA to that achieved by removal of FGF4, heparin, and fibroblast-conditioned medium. Notably, when Lin28a KD mTS cells were induced to differentiate by the removal of FGF4, heparin, and conditioned medium, let-7a, let-7d, and let-7g miRNA levels exceeded the levels observed in nontarget control cells (Fig. 3C). Despite losses of LIN28A mRNA and protein, increases in let-7 miRNA, and an apparent increased capacity for let-7 miRNA responsiveness, Lin28a KD mTS cells did not demonstrate increased levels of spontaneous differentiation or loss of proliferative capacity over 15 passages in proliferative culture conditions (Fig. 4).
FIG. 4.
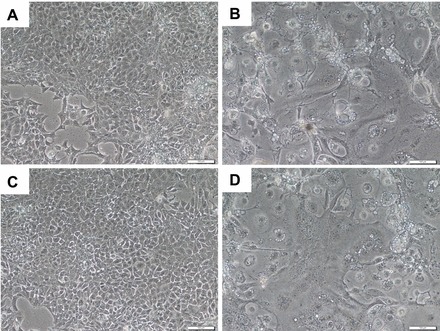
Mouse TS cell morphology. Brightfield microscopy (10×). A) Proliferative nontarget control mTS cells at Day 0. B) Differentiated mTGC after 6 days in differentiation medium. C) Proliferative Lin28a KD mTS cells at Day 0. D) Differentiated mTGC morphology after 6 days in differentiation medium. Bar = 0.1 mm.
LIN28A mRNA and Protein in Cultured Human Trophoblast Cells
The immortalized first-trimester human trophoblast cell line, ACH-3P, was used to determine if LIN28A had a role in regulating human trophoblast differentiation and function. ACH-3P cells were treated with 40 μM forskolin to induce differentiation, and responded with a six-fold increase in soluble hCG-beta in culture medium compared to dimethyl sulfoxide (DMSO) controls, demonstrating that forskolin treatment was effective at inducing differentiation of ACH-3P (Fig. 5A). Forskolin-induced differentiated cells had decreased LIN28A mRNA and protein (24% and 38%, respectively; P < 0.05) compared to DMSO-treated controls (Fig. 5, B–D). To determine if loss of LIN28A was sufficient to induce differentiation in human trophoblast, a LIN28A KD ACH-3P cell line was established. LIN28A mRNA KD resulted in a 96% reduction of LIN28A mRNA (P < 0.01) and a 95% reduction in protein (densitometry not shown) compared to nontarget control ACH-3P cells (Fig. 6, A and B). The resulting LIN28A KD ACH-3P cells had increased levels of miRNAs let-7c, let-7d, and let-7e (P < 0.05; Fig. 6C). ELISA analysis of culture medium collected from LIN28A KD ACH-3P cells had 13-fold (P < 0.01) increased per-cell concentrations of soluble hCG-beta (Fig. 7A), as well as increased hCG-beta immunostaining (Fig. 7, C and D), compared to nontarget control cells. The increased level of hCG protein was accompanied by elevated levels of mRNA for both the alpha (CGA) and beta (CGB) polypeptide subunits (1.8- and 3.5-fold, respectively; P < 0.01) compared to nontarget control cells (Fig. 7B). In addition to increased hCG production, LIN28A KD ACH-3P cells had six-fold higher levels of LGALS13 mRNA (P < 0.01), a syncytiotrophoblast-specific marker (Fig. 8A), 3.5-fold increased levels of ERVW-1 mRNA, which encodes for the fusion protein syncytin-1 (P < 0.01; Fig. 8B), as well as increased morphological differentiation, characterized by an increased spontaneous formation of large, multinucleated syncytial plaques (Fig. 8, C and D). These data suggest that LIN28A may contribute to the regulation of human trophoblast differentiation into the syncytiotrophoblast sublineage. Finally, LIN28A KD ACH-3P cells treated with 40 μM forskolin had additional losses of LIN28A mRNA (P < 0.01; Fig. 9A). While levels of ERVW-1 mRNA did not increase in LIN28A KD ACH-3P cells treated with 40 μM forskolin (Fig. 9B), levels of LGALS13 mRNA increased an additional 15-fold (P < 0.01; Fig. 9C), and soluble hCG in the culture medium rose an additional seven-fold over DMSO controls (P < 0.01; Fig. 9D). These data suggest that, like Lin28a KD mTS cells, LIN28A KD ACH-3P cells have an increased potential for differentiation.
FIG. 5.
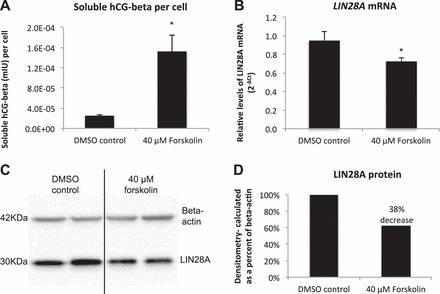
LIN28A response to forskolin-induced differentiation. A) ELISA analysis of soluble hCG (mIU) in ACH-3P cell culture medium. B) LIN28A mRNA in ACH-3P cells treated with forskolin (qPCR). C and D) LIN28A protein and densitometry analysis of ACH-3P cells treated with forskolin. Error bars represent SEM. Asterisks indicate P < 0.05.
FIG. 6.
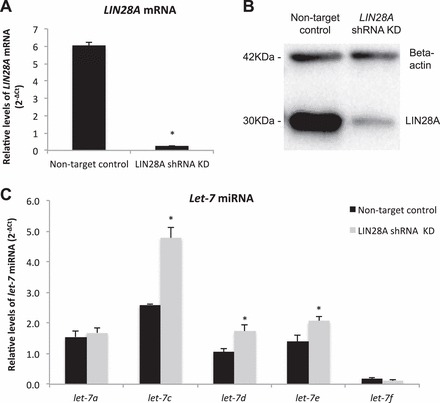
LIN28A and let-7 miRNA in LIN28A KD ACH-3P cells. A and B) LIN28A mRNA (qPCR) and protein in LIN28A KD ACH-3P cells. C) The let-7 miRNA in LIN28A KD ACH-3P cells. Error bars represent SEM. Asterisk indicates P < 0.05.
FIG. 7.
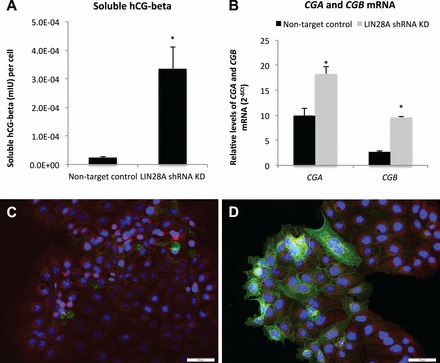
Human chorionic gonadotropin in LIN28A KD ACH-3P cells. A) ELISA analysis of soluble hCG (mIU) in culture medium from LIN28A KD ACH-3P cells. B) Levels of CGA and CGB mRNA (qPCR) in LIN28A KD ACH-3P cells compared to nontarget control ACH-3P cells. Nontarget control ACH-3P cells (C) and LIN28A KD ACH-3P cells (D). Magnification ×20. Immunofluorescence: red, plasma membrane; blue, nuclei; green, hCG-beta. Error bars represent SEM. Asterisks indicate P < 0.01. Bar = 0.05 mm.
FIG. 8.
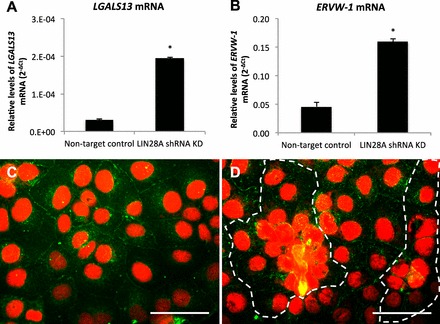
Spontaneous syncytialization in LIN28A KD in ACH3P cells. A and B) LGALS13 and ERVW-1 mRNA (qPCR) in LIN28A KD ACH-3P cells. C and D) Immunofluorescence of LIN28A KD ACH-3P cells: red, nuclei; green, plasma membrane; dashed line signifies boundary of multinucleated cell plaque. Error bars represent SEM. Asterisks indicate P < 0.01. Bar = 0.05 mm.
FIG. 9.
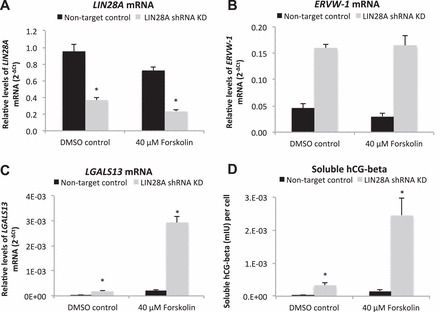
Differentiation markers in LIN28A KD ACH-3P cells treated with forskolin. A–C) LIN28A, ERVW-1, and LGALS13 mRNA (qPCR) in LIN28A KD ACH-3P treated with forskolin. D) ELISA analysis of hCG-beta in cell culture medium of LIN28A KD ACH-3P cells treated with forskolin. Error bars represent SEM. Asterisks indicate P < 0.01.
DISCUSSION
Proper regulation of trophoblast proliferation, differentiation, and function are the fundamental mechanisms supporting proper placenta development and function. However, ethical dictates limit the extent to which human pregnancy is studied, so surrogate models are often necessary to understand trophoblast function. Human ESCs (hESCs) are one such model, and have been used to understand the molecular mechanisms regulating the initial differentiation of trophectoderm and subsequent differentiation events resulting in trophoblast cell lineages [39–41]. To date, LIN28A has been demonstrated to be a potent regulator of pluripotency in hESCs, regulating the initiation of differentiation down embryonic cell lineages [13, 14, 16, 18]. Conservation of LIN28A expression and function in TS progenitors demonstrates that there is a fundamental role for LIN28A in the regulation of trophoblast pluripotency and differentiation, and ultimately in placenta development.
Our data demonstrate that LIN28A is present in mouse placenta tissue, as well as mouse and human trophoblast cells, suggesting that, in addition to being a potent regulator of pluripotency in hESCs, there may be a conserved functional role for LIN28A in trophoblast differentiation. Our qPCR data found Lin28a mRNA to be significantly decreased, and let-7 miRNA to be significantly increased in mTGCs compared to proliferative TS cells (mTS). Western blot assessment of LIN28A in cultured mTS cells confirmed the qPCR data, suggesting that trophoblast differentiation was a LIN28A-regulated event, and that it was possibly acting through let-7 miRNA-mediated pathways similar to those observed in ES cells [16, 26, 27].
Using immunohistochemistry assessment of serial sections of midgestational mouse placenta, we found distinct LIN28A immunostaining in the fetal membranes, along the chorioallantoic interface, and throughout the labyrinth layer, suggesting that these areas may maintain progenitor cell populations. Our data are consistent with observations made by Yang and Moss [28], who noted that early gestational mouse trophoblast and yolk epithelium were positive for LIN28A. In the mouse, labyrinthine differentiation begins at E8.5, when the chorionic and allantoic layers come together to form a trilaminar structure composed of mononuclear TGCs in direct contact with maternal blood, overlaying two layers of multinucleated syncytiotrophoblast layers [42, 43]. Coculture of trophoblast cells with allantois has demonstrated a direct effect on trophoblast morphological differentiation [43], suggesting that these tissues have a direct role in regulating trophoblast differentiation and function. Our observation that LIN28A is present along the chorioallantoic interface and throughout the labyrinth layer suggest that continued expression of pluripotency factors, such as LIN28A, may be necessary for continued trophoblast differentiation and remodeling through midgestation. Additionally, the midgestation chorio-allantoic interface has been reported to be the site of hematopoietic progenitor stem cells [44, 45], suggesting that pluripotency factors, such as LIN28A, may be functioning to maintain stem cell progenitor niches in these tissues. Decreased levels of LIN28A immunostaining in the spongiotrophoblast and the mTGCs along the junctional zone suggest that, by midgestation, these cells have undergone terminal differentiation [46].
Our Lin28a KD mTS cell line demonstrates that LIN28A inhibits let-7 miRNA in trophoblast cells, as demonstrated in human ES cells [47]. However, despite significant decreases in Lin28a mRNA and concomitant increases in let-7 miRNA, mTS cell proliferation or differentiation was not affected. This suggests that, while decreased levels of LIN28A and increased levels of let-7 miRNA may be necessary for differentiation to proceed, it is not sufficient to induce differentiation in mTS. It is important to note that FGF4, heparin, and embryonic fibroblast-derived growth factors present in the proliferative culture medium are potent regulators of the cell cycle, and may act to prevent mTGC differentiation, and their presence in the mTS culture medium may ultimately override any effects resulting from the loss of Lin28a.
While Lin28a KD mTS cells did not exhibit a phenotypic change, when we applied the same strategy for LIN28A mRNA KD in the ACH-3P cells, increased levels of let-7 miRNA were accompanied by increased levels of spontaneous biochemical and morphological differentiation (Fig. 10). Differentiation was characterized by increased levels of hCG, syncytiotrophoblast-specific LGALS13 mRNA, and ERVW-1 mRNA (the transcript encoding for the fusion protein, syncytin-1), and the spontaneous formation of large, multinucleated cell plaques. These data suggested that LIN28A KD was inducing ACH-3P cells to differentiate toward the syncytiotrophoblast trophoblast sublineage.
FIG. 10.
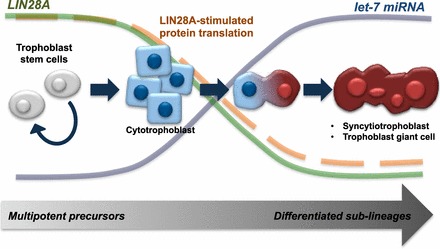
Proposed mechanism for LIN28A regulation of trophoblast cell differentiation. LIN28A functions to maintain pluripotency in proliferative trophoblast progenitors, suppressing let-7 miRNA inhibition of mRNA and supporting translational activation of downstream targets. As LIN28A decreases with differentiation, let-7 miRNA is allowed to mature, suppressing downstream mRNA targets. Additionally, translational activation directly mediated by LIN28A binding target mRNA is lost. Consequently, inhibition to differentiation is removed, and cells progressively differentiate from proliferative precursors into trophoblast sublineages.
Despite the increased levels of spontaneous syncytialization, the LIN28A KD ACH-3P cells continued to passage, suggesting that loss of LIN28A and increased levels of let-7 miRNA were not sufficient to completely suppress proliferation. Additionally, when LIN28A KD ACH-3P cells were treated with forskolin, LIN28A levels decreased further and syncytiotrophoblast markers, hCG, and LGALS13 mRNA increased substantially beyond the levels observed in forskolin-treated nontarget controls. This increased differentiation potential in the LIN28A KD ACH-3P cells subjected to forskolin suggests that, although LIN28A KD induced syncytiotrophoblast formation, it was not complete, and could still be induced further. In mouse TS cells, loss of Lin28a did not result in induced differentiation, suggesting that KD in Lin28a alone does not promote TS cell differentiation. These data are consistent with data presented by Yu et al. [16], which demonstrated that overexpression of Lin28a alone was not sufficient for initiating retrodifferentiation into iPSCs or for maintaining resulting colonies, but improved the frequency of iPSC reprogramming events.
It is important to note that LIN28A has been shown to directly regulate mRNA translation efficiency, stimulating the translation of the growth factor IGF2, pluripotency factor OCT4, and a number of genes important for cell cycle regulation, including histone H2a, cdk4, and cyclins A and B [21, 48–52]. This would suggest that LIN28A likely has regulatory roles independent of let-7 miRNA-mediated pathways, and may act in concert with a number of factors to regulate trophoblast differentiation. Such let-7 miRNA-independent regulatory pathways may explain the differences in effects observed between human and mouse trophoblast cell lines.
Our data demonstrate that LIN28A is present in the mouse placenta; however, the functional role of LIN28A in the regulation of trophoblast differentiation into the sublineages vital for proper placenta development appears to be different between human and mouse. We conclude from these data that LIN28A likely acts in concert with a number of factors to regulate the progression of trophoblast differentiation through let-7 miRNA-dependent and -independent pathways, and that loss of LIN28A results in an increased potential for trophoblast differentiation to proceed, rather than actively initiating differentiation.
ACKNOWLEDGMENT
The authors are grateful to Colin Clay and Jennifer De Luca for their helpful suggestions and editorial support. The authors would like to extend thanks to Lindsey Goetzmann for her help and dedication in the lab.
Footnotes
Supported by Agriculture and Food Research Initiative competitive grant HD 067431-01 from the USDA National Institute of Food and Agriculture, and by National Institutes of Health grant HD067431.
REFERENCES
- Ilekis JV, Reddy UM, Roberts JM. Review article: preeclampsia, a pressing problem: an executive summary of a National Institute of Child Health and Human Development workshop. Reprod Sci 2007; 14: 508–523. [DOI] [PubMed] [Google Scholar]
- Regnault TRH, Galan HL, Parker TA, Anthony RV. Placental development in normal and compromised pregnancies: a review. Placenta 2002; 23 (suppl A): S119–S129. [DOI] [PubMed] [Google Scholar]
- Chiswick ML. Intrauterine growth retardation. Br Med J (Clin Res Ed) 1985; 291: 845–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson ES, Brownbill P, Jones NW, Abrahams VM, Baker PN, Sibley CP, Crocker IP. Utero-placental haemodynamics in the pathogenesis of pre-eclampsia. Placenta 2009; 30: 634–641. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 2005; 308: 1592–1594. [DOI] [PubMed] [Google Scholar]
- Langbein M, Strick R, Strissel PL, Vogt N, Parsch H, Beckmann MW, Schild RL. Impaired cytotrophoblast cell-cell fusion is associated with reduced Syncytin and increased apoptosis in patients with placental dysfunction. Mol Reprod Dev 2008; 75: 175–183. [DOI] [PubMed] [Google Scholar]
- Chaddha V, Viero S, Huppertz B, Kingdom J. Developmental biology of the placenta and the origins of placental insufficiency. Semin Fetal Neonatal Med 2004; 9: 357–369. [DOI] [PubMed] [Google Scholar]
- Krebs C, Macara LM, Leiser R, Bowman AW, Greer IA, Kingdom JCP. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am J Obstet Gynecol 1996; 175: 1534–1542. [DOI] [PubMed] [Google Scholar]
- Bellamy L, Casas J-P, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007; 335: 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons RA. Developmental origins of diabetes: the role of oxidative stress. Best Pract Res Clin Endocrinol Metab 2012; 26: 701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarr O, Yang K, Regnault TRH. In utero programming of later adiposity: the role of fetal growth restriction. J Pregnancy 2012; 2012: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ 1990; 301: 259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darr H, Benvenisty N. Genetic analysis of the role of the reprogramming gene LIN-28 in human embryonic stem cells. Stem Cells 2009; 27: 352–362. [DOI] [PubMed] [Google Scholar]
- Heo I, Joo C, Cho J, Ha M, Han J, Kim VN. Lin28 mediates the terminal uridylation of let-7 precursor microRNA. Mol Cell 2008; 32: 276–284. [DOI] [PubMed] [Google Scholar]
- Viswanathan SR, Daley GQ. Lin28: a microRNA regulator with a macro role. Cell 2010; 140: 445–449. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R. Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007; 318: 1917–1920. [DOI] [PubMed] [Google Scholar]
- Xue D, Peng Y, Wang F, Allan RW, Cao D. RNA-binding protein LIN28 is a sensitive marker of ovarian primitive germ cell tumours. Histopathology 2011; 59: 452–459. [DOI] [PubMed] [Google Scholar]
- Viswanathan SR, Powers JT, Einhorn W, Hoshida Y, Ng TL, Toffanin S, O'Sullivan M, Lu J, Phillips LA, Lockhart VL, Shah SP, Tanwar PS, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet 2009; 41: 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CE, Cuatrecasas M, Castells A, Sepulveda AR, Lee J-S, Rustgi AK. LIN28B promotes colon cancer progression and metastasis. Cancer Res 2011; 71: 4260–4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano R, Miyata H, Yamasaki M, Sugimura K, Tanaka K, Kurokawa Y, Nakajima K, Takiguchi S, Fujiwara Y, Mori M, Doki Y. High expression of Lin28 is associated with tumour aggressiveness and poor prognosis of patients in oesophagus cancer. Br J Cancer 2012; 106: 1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Chen L-L, Lei X-X, Yang L, Lin H, Carmichael GG, Huang Y. Genome-wide studies reveal that Lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells. Stem Cells 2011; 29: 496–504. [DOI] [PubMed] [Google Scholar]
- Thornton JE, Chang H-M, Piskounova E, Gregory RI. Lin28-mediated control of let-7 microRNA expression by alternative TUTases Zcchc11 (TUT4) and Zcchc6 (TUT7). RNA 2012; 18: 1875–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JP, Piskounova E, Gregory RI. Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nat Struct Mol Biol 2009; 16: 1021–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guled M, Lahti L, Lindholm PM, Salmenkivi K, Bagwan I, Nicholson AG, Knuutila S. CDKN2A, NF2, and JUN are dysregulated among other genes by miRNAs in malignant mesothelioma—a miRNA microarray analysis. Genes Chromosomes Cancer 2009; 48: 615–623. [DOI] [PubMed] [Google Scholar]
- Boyerinas B, Park S-M, Hau A, Murmann AE, Peter ME. The role of let-7 in cell differentiation and cancer. Endocr Relat Cancer 2010; 17: F19–F36. [DOI] [PubMed] [Google Scholar]
- Zhong X, Li N, Liang S, Huang Q, Coukos G, Zhang L. Identification of microRNAs regulating reprogramming factor LIN28 in embryonic stem cells and cancer cells. J Biol Chem 2010; 285: 41961–41971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussing I, Slack FJ. Grosshans H. let-7 microRNAs in development, stem cells and cancer. Trends Mol Med 2008; 14: 400–409. [DOI] [PubMed] [Google Scholar]
- Yang D-H, Moss EG. Temporally regulated expression of Lin-28 in diverse tissues of the developing mouse. Gene Expr Patterns 2003; 3: 719–726. [DOI] [PubMed] [Google Scholar]
- Vogt EJ, Meglicki M, Hartung KI, Borsuk E, Behr R. Importance of the pluripotency factor LIN28 in the mammalian nucleolus during early embryonic development. Development 2012; 139: 4514–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger QA, Guttormsen J, Gavin H, Bhushan F. Heat shock protein 1 and the mitogen-activated protein kinase 14 pathway are important for mouse trophoblast stem cell differentiation. Biol Reprod 2007; 76: 884–891. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kunath T, Hadjantonakis A-K, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science 1998; 282: 2072–2075. [DOI] [PubMed] [Google Scholar]
- Hiden U, Wadsack C, Prutsch N, Gauster M, Weiss U, Frank H-G, Schmitz U, Fast-Hirsch C, Hengstschlager M, Potgens A, Ruben A, Knofler M, et al. The first trimester human trophoblast cell line ACH-3P: a novel tool to study autocrine/paracrine regulatory loops of human trophoblast subpopulations—TNF-alpha stimulates MMP15 expression. BMC Dev Biol 2007; 7: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wice B, Menton D, Geuze H, Schwartz AL. Modulators of cyclic AMP metabolism induce syncytiotrophoblast formation in vitro. Exp Cell Res 1990; 186: 306–316. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Kappes SM, Warren WC, Pratt SL, Liang R, Anthony RV. Quantification and cellular localization of ovine placental lactogen messenger ribonucleic acid expression during mid- and late gestation. Endocrinology 1992; 131: 2829–2838. [DOI] [PubMed] [Google Scholar]
- Jeckel KM, Limesand SW, Anthony RV. Specificity protein-1 and −3 trans-activate the ovine placental lactogen gene promoter. Mol Cell Endocrinol 2009; 307: 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Clay CE, Monjazeb A, Thorburn J, Chilton FH, High KP. 15-Deoxy-delta 12,14-prostaglandin J2-induced apoptosis does not require PPARgamma in breast cancer cells. J Lipid Res 2002; 43: 1818–1828. [DOI] [PubMed] [Google Scholar]
- Udayashankar R, Baker D, Tuckerman E, Laird S, Li TC, Moore HD. Characterization of invasive trophoblasts generated from human embryonic stem cells. Hum Reprod 2011; 26: 398–406. [DOI] [PubMed] [Google Scholar]
- Schulz LC, Ezashi T, Das P, Westfall SD, Livingston KA, Roberts RM. Human embryonic stem cells as models for trophoblast differentiation. Placenta 2008; 29: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand M, Horcajadas JA, Esteban FJ, McElroy SL, Fisher SJ, Giudice LC. Transcriptomic signature of trophoblast differentiation in a human embryonic stem cell model. Biol Reprod 2011; 84: 1258–1271. [DOI] [PubMed] [Google Scholar]
- Simmons DG, Natale DRC, Begay V, Hughes M, Leutz A, Cross JC. Early patterning of the chorion leads to the trilaminar trophoblast cell structure in the placental labyrinth. Development 2008; 135: 2083–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross JC, Nakano H, Natale DRC, Simmons DG, Watson ED. Branching morphogenesis during development of placental villi. Differentiation 2006; 74: 393–401. [DOI] [PubMed] [Google Scholar]
- Fernandes RA, Wenceslau CV, Reginato AL, Kerkis I, Miglino MA. Derivation and characterization of progenitor stem cells from canine allantois and amniotic fluids at the third trimester of gestation. Placenta 2012; 33: 640–644. [DOI] [PubMed] [Google Scholar]
- Zeigler BM, Sugiyama D, Chen M, Guo Y, Downs KM, Speck NA. The allantois and chorion, when isolated before circulation or chorio-allantoic fusion, have hematopoietic potential. Development 2006; 133: 4183–4192. [DOI] [PubMed] [Google Scholar]
- Müntener M, Hsu YC. Development of trophoblast and placenta of the mouse. Cells Tissues Organs 1977; 98: 241–252. [PubMed] [Google Scholar]
- Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science 2008; 320: 97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Huang Y. Histone H2a mRNA interacts with Lin28 and contains a Lin28-dependent posttranscriptional regulatory element. Nucleic Acids Res 2009; 37: 4256–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Zhang K, Huang Y. Lin28 modulates cell growth and associates with a subset of cell cycle regulator mRNAs in mouse embryonic stem cells. RNA 2009; 15: 357–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzer E, Moss EG. Localization of the developmental timing regulator Lin28 to mRNP complexes, P-bodies and stress granules. RNA Biol 2007; 4: 16–25. [DOI] [PubMed] [Google Scholar]
- Qiu C, Ma Y, Wang J, Peng S, Huang Y. Lin28-mediated post-transcriptional regulation of Oct4 expression in human embryonic stem cells. Nucleic Acids Res 2010; 38: 1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesskaya A, Cuvellier S, Naguibneva I, Duquet A, Moss EG, Harel-Bellan A. Lin-28 binds IGF-2 mRNA and participates in skeletal myogenesis by increasing translation efficiency. Genes Dev 2007; 21: 1125–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]


