ABSTRACT
Assisted reproductive technologies (ART) have been associated with several adverse perinatal outcomes involving placentation and fetal growth. It is critical to examine each intervention individually in order to assess its relationship to the described adverse perinatal outcomes. One intervention ubiquitously used in ART is superovulation with gonadotropins. Superovulation results in significant changes in the hormonal milieu, which persist during the peri-implantation and early placentation periods. Epidemiologic evidence suggests that the treatment-induced peri-implantation maternal environment plays a critical role in perinatal outcomes. In this study, using the mouse model, we have isolated the exposure to the peri-implantation period, and we examine the effect of superovulation on placentation and fetal growth. We report that the nonphysiologic peri-implantation maternal hormonal environment resulting from gonadotropin stimulation appears to have a direct effect on fetal growth, trophoblast differentiation, and gene expression. This appears to be mediated, at least in part, through trophoblast expansion and invasion. Although the specific molecular and cellular mechanism(s) leading to these observations remain to be elucidated, identifying this modifiable risk factor will not only allow us to improve perinatal outcomes with ART, but help us understand the pathophysiology contributing to these outcomes.
Keywords: ART, epigenetics, estradiol, gonadotropins, implantation
The effect of altering the peri-implantation hormonal milieu on fetal growth, trophoblast differentiation, gene expression, and imprint establishment is examined in the mouse.
INTRODUCTION
More than 200 000 children in the United States are born yearly with the aid of assisted reproductive technologies (ART). Though the majority of the infants are born healthy, recent epidemiological studies suggest that these treatments are associated with an increased risk of adverse perinatal outcomes, including fetal growth restriction, low birth weight, preterm labor, preeclampsia, and some rare genetic and epigenetic diseases [1–7]. In addition, ART studies from both mouse and human have demonstrated long-term metabolic effects on the offspring, including an increase in body fat and impaired glucose tolerance [8–10].
Given that in vitro fertilization (IVF) utilizes multiple clinical and laboratory interventions to generate a cohort of embryos potentially capable of implantation and development to term, it is critical to examine each intervention individually in order to assess its contribution, if any, to the described adverse perinatal outcomes. One intervention ubiquitously used in ART is superovulation with gonadotropins. Gonadotropin administration during ART results in an increase in the number of developing follicles and serum estradiol levels up to ten times greater than that found in natural cycles. A recent study found that an optimal range of serum estradiol correlates with IVF success, with higher levels leading to lower pregnancy and delivery rates [11]. During IVF, superovulation and the associated high estradiol levels on the day of human chorionic gonadotropin administration are associated with disorders of placentation, including intrauterine growth restriction and hypertensive disorders of pregnancy [12, 13]. Epidemiologic studies have demonstrated similar disorders in infants born following superovulation protocols without IVF. Even when restricting the analysis to singleton pregnancies, infants born following ovulation induction with gonadotropins are more likely to be born preterm or small for gestational age [14].
How superovulation affects the resulting oocyte and embryo is unclear. Studies in the mouse have shown that gonadotropin administration can affect gene expression of certain imprinted genes, suggesting that epigenetic changes are responsible for some of the adverse effects seen with ART [15]. This suggestion is consistent with the epidemiologic data that suggests that IVF is associated with an increase in loss-of-imprinting syndromes, such as Beckwith-Wiedemann and Angelman syndromes [1, 2, 7]. Moreover, molecular studies in both mouse and human have demonstrated epigenetic changes following ART [7, 16, 17]. Recently, human studies have found that placentas from in vitro conception differ from control placentas [18, 19]. Placentas from infants conceived by ART have lower mean methylation at CpG sites and are more likely to have aberrant DNA methylation at certain imprinted genes. A recent comparison of human placentas from in vivo or in vitro conceptions revealed differences in the DNA methylation of Grb10, an imprinted gene that regulates fetal and placental growth. These placentas also exhibited altered gene expression of both imprinted and nonimprinted genes [18, 19].
Superovulation may affect the hormonal milieu and the developing embryo at two distinct time points. Initially, during the critical period of oocyte development, the developing gamete is exposed to high doses of gonadotropins that lead to supraphysiological hormone concentrations [20]. Later on, especially in fresh IVF cycles following transfer, the early embryo is exposed to an altered hormonal milieu during peri-implantation development [21]. Thus, it is possible that the postimplantation environment affects the developing fetus. Consistent with this proposal is a recent analysis of the Society for Assisted Reproductive Technology/Centers for Disease Control database indicating that the incidence of low birth weight is greater when embryos are transferred to women following a hormonally stimulated cycle as compared to cycles when the embryos are transferred in a hormonal milieu that is more physiologic, as in the case of frozen embryo transfer [22]. Importantly, there is no difference in low birth weight in singletons born to oocyte donor recipients following fresh versus frozen embryo transfer, demonstrating that it is the hormonal environment, not the freeze-thaw process, that affects the developing embryo [22]. These findings suggest that the treatment-induced maternal environment at the time of embryo transfer may represent an independent mechanism contributing to the adverse effects seen following ART. To test this hypothesis and study the molecular changes occurring in these offspring, we utilized a mouse model to examine the effect of altering the peri-implantation hormonal milieu by superovulation on the fetus. Utilizing in vivo conceived blastocysts, not exposed to gonadotropins, and transferring the embryos to pseudopregnant females following gonadotropin administration or natural mating, we analyzed fetal and placental growth, gene expression, DNA methylation of imprinted genes, and histology in extraembryonic and embryonic tissues to determine the role of the postimplantation environment on the resulting offspring.
MATERIALS AND METHODS
Embryos and Embryo Transfers
All the experiments and procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee Review Board. Female CF-1 (Harlen Sprague Dawley), male C57BL/6J (Jackson Laboratories), and vasectomized C57Bl/6J males (Jackson Laboratories) were obtained and housed in a temperature- and light-controlled environment and fed ad libitum. To obtain blastocysts for transfer, females were mated with males and mating confirmed by the presence of a copulatory plug the morning following mating (0.5 days postcoitum). On Postcoitum Day 3.5, blastocysts were flushed from the uterine horns using standard technique [23], and the embryos cultured in KSOM +AA (Specialty Media; Millipore) under mineral oil at 37°C in an incubator for no more than 1 h prior to transfer. To obtain the experimental group of pseudopregnant females, a group of CF-1 females was superovulated (SO) with an intraperitoneal injection of 5 international units of equine chorionic gonadotropin followed by 5 international units of human chorionic gonadotropin 48 h later and then mated with vasectomized males. Mating was confirmed by the presence of a copulatory plug. Control recipients were obtained by mating CF-1 females with the vasectomized males and mating confirmed by the presence of a copulatory plug. On Postcoital Day 2.5, 10 blastocysts were transferred into a single horn of either a control or SO recipient using the Nonsurgical Embryo Transfer Device (Paratechs) per the manufacturer's protocol.
Fetal Evaluation
On Embryonic Day 18.5 (E18.5), the recipient mice were sacrificed and the gravid uterine horns removed from the abdominal cavity. The number of implantation sites was counted for each recipient. The amniotic membranes were ruptured, and the gestations (fetus and placenta) were then carefully dissected from the uterine horn and, for each gestation, the fetus and placenta was separated, blotted dry, and weighed. Each placenta was bivalved through the midplacental plane, and half were placed in 10% phosphate-buffered formalin for histologic examination. The other half of the placenta was snap frozen in liquid nitrogen and stored at −80°C. Fetal liver, kidney, and brain were snap frozen in liquid nitrogen and stored at −80°C.
DNA/RNA Extraction and cDNA Preparation
Genomic DNA was extracted from frozen placenta and liver using Qiagen QiAmp DNA Micro Kit according to manufacturer's recommendation. RNA extraction and isolation were performed using the Qiagen RNAeasy Micro Kit according to the manufacturer's instructions. RNA concentration and quality was determined with a NanoDrop spectrophotometer (Thermo Fischer Scientific). Synthesis of cDNA was performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) using 2 μg of RNA.
Sex Determination
Sex determination of the fetus was performed using isolated liver DNA. PCR was performed using primers for the Sry gene: Sry forward primer: 5′-TTG TCT AGA GAG CAT GGA GGG CCA TGT CAA, and reverse primer: 5′-CCA CTC CTC TGT GAC ACT TTA GCC CTC CGA. The reactions were performed in 25 μl for 26 cycles (95°C for 30 sec, 60°C for 1 min, 72°C for 30 sec).
Gene Expression
Real-time quantitative PCR (qRT-PCR) was performed with the ABI Prism 7900HT Sequence Detection System (Applied Biosystems). Each reaction was performed in10 μl with 50 ng of cDNA template. Each sample was run in triplicate, and the reactions were carried out for 40 cycles. The following taqman probes were used (Applied Biosystems): Igf2 (Mm00439564_m1), Igf2r (Mm00439576_m1), H19 (Mm01156721_g1), Grb10 (Mm01180443_m1), Rplpo (Mm00725448_s1), Hand1 (Mm00433931_m1), Tpbpa (Mm00493788_g1), Tec (Mm00443230_m1), and Tek (Mm00443243_m1). For each gene of interest, a standard curve ranging from 2.5 to 100 ng was generated with cDNA derived from the placenta of a control recipient. Relative concentration of a given transcript was extrapolated from the standard curve, and the amount of transcript was normalized to the reference gene Rplpo. For each gene of interest, the mean expression level in the control group was set to 1, and the fold-change in gene expression was expressed relative to this reference value.
Placenta Histology
Placentas were fixed overnight at 4°C, dehydrated, and embedded in a paraffin block. Care was taken to orient the bivalved section vertically so that cross-sections of the placenta could be obtained. Serial tissue sections of 4 μm thickness were cut and mounted on glass slides by the Abramson Cancer Center Histology Core (University of Pennsylvania, Philadelphia, PA). Tissues were deparaffinized using citrate buffer, pH 6.0 (lot no. 1994669; Millipore) and stained with hematoxylin and eosin. Images were collected on a Leica DMRBE upright wide-field microscope with 2.5, 5, and 10× objective and a 5.0 MegaPixel color charge-coupled device camera and iVision acquisition software. Micrographs were analyzed and measured using ImageJ v1.4.5 (National Institute of Health). Measurements were made through the midsagittal plane of the placenta and performed as previously described [24]. All the measurements were performed by two independent observers unaware of the treatment group and the measurements averaged. When measurements differed by greater than 10%, a third observer made a measurement, and the two closest measurements were then averaged. The subsequent section was then stained for GRB10 using an antibody to GRB10 (1:500; Santa Cruz-1026) overnight at 4°C. Anti-rabbit secondary antibodies (Vector Laboratories) were used at a 1:10000 dilution for 1 h at 37°C followed by using an ABC Linker Kit (Vector Laboratories) for 30 min at 37°C. Samples were developed using the 4′,6-diamidino-2-phenylindole peroxidase substrate kit (SK-4100; Vector Laboratories). Nuclei were counterstained using hematoxylin. Periodic acid Schiff staining was performed on the following serial section. Slides were deparaffinized, rehydrated, and incubated with periodic acid solution for 5 min. The slides were then rinsed and immersed in the Schiff reagent for 15 min at room temperature. The slides were again rinsed and counterstained in hematoxylin solution.
Western Blot Analysis
The tissues were solubilized for 30 min on ice in modified RIPA buffer (Sigma-Aldrich) supplemented with protease inhibitor (P8340; Sigma-Aldrich). Cleared tissue lysates were resolved on a 4%–15% gradient SDS-PAGE gel and transferred to nitrocellulose membrane. GRB10 was detected using antibodies against GRB10 (AbCAM125583; 1:100 dilution) followed by secondary horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (Transduction Laboratories). Actin was detected using primary monoclonal anti-β actin (A-5441; 1:5000 dilution; Sigma) and secondary horseradish peroxidase- conjugated sheep anti-mouse immunoglobulin G (Amersham). The secondary antibodies were detected using ECL detection reagents (Amersham).
DNA Bisulfite Treatment, Pyrosequencing Assay, and Luminometric Methylation Assay
One microgram of placental DNA was treated with sodium bisulfite using the EpiTect bisulphite kit (Qiagen) according to the manufacturer's protocol. The imprinted control regions from genes of interest were amplified using 50 ng of bisulfite-treated DNA with 10 μM of forward and reverse primers using Qiagen PyroMark PCR Kit per the manufacturer's protocol. Bisulfite pyrosequencing was performed using the PyroMarkQ96 System (Qiagen) and Pyro Gold reagents (Qiagen) to examine the percent methylation at each CpG site analyzed. Primer sequences for amplification and sequencing can be found in Supplemental Table S1 (available online at www.biolreprod.org) [25, 26]. Global methylation was analyzed using the luminometric methylation assay as previously published [27]. Briefly, 200 ng of genomic DNA was digested with HpaII plus EcoRI or Mspi plus EcoRI for 4 h. Each reaction was then combined with 15 μl of annealing buffer and 30 μl of the reaction added to the pyrosequencing plate and analyzed on the PyroMarkQ96 System (Qiagen). The percentage methylation was calculated using the formula: methylation percent = 100 × (1 − HpaII/MspI).
Enzyme-Linked Immunosorbent Assay
A cohort of ten mice were sacrificed on Postcoital Day 1 following either superovulation and mating or following natural mating with mating confirmed by the presence of a vaginal plug. Another 10 samples were isolated on Postcoital Day 4 following either superovulation or natural mating. Blood samples were collected by cardiac puncture, collected into eppendorf tubes, and allowed to clot for 90 min at room temperature. After clotting, the samples were centrifuged at 2000 × g for 15 min at room temperature. After centrifugation, the serum was separated and frozen at −20°C prior to analysis. The estradiol assay was run by the University of Virginia School of Medicine Ligand Assay and Analysis Core (Charlottesville, VA), using an enzyme-linked immunosorbent assay (CalBioTech). Serum VEGF levels were analyzed using R&D Systems Immunoassay VEGF as per the manufacturer's protocol.
Statistical Analysis
The data are expressed as mean ± SEM. All the data sets were analyzed for normality. To determine statistical significance, two-tailed Student t-test was performed for all normally distributed data, and a Mann-Whitney test was performed for nonparametric data. A P < 0.05 was considered significant. Statistical analysis was performed using GraphPad Prism 5.0 software.
RESULTS
Experimental Model
To identify the effects of superovulation on postimplantation development, blastocysts obtained following natural matings were transferred into either control or SO recipients. Pseudopregnant control recipients were generated through natural mating of female mice with vasectomized males. Pseudopregnant SO recipients were generated by administration of gonadotropins to female mice prior to mating with vasectomized males. Ten naturally conceived blastocysts were transferred to both control and SO recipients to determine the effect of the peri-implantation environment on the resulting fetus. There was no difference in age or weight of the recipients in the two groups at the time of transfer.
Fetal and Placental Weights and Placental Histology
We first examined serum estradiol levels in both control and SO mice immediately after mating and at the time of embryo transfer to establish how the hormonal milieu was changed during the peri-implantation period in our model. On the day of mating, SO mice had mean serum estradiol levels over twice that of the control mice. However, by the time of embryo implantation, serum estradiol levels were not different between the two sets of mice. We also examined expression of VEGF, a gene produced by the corpora lutea and known to affect placentation. Following superovulation, and during the period of implantation, serum VEGF levels were approximately 40% higher in the SO mice compared to control mice (Fig. 1).
FIG. 1.
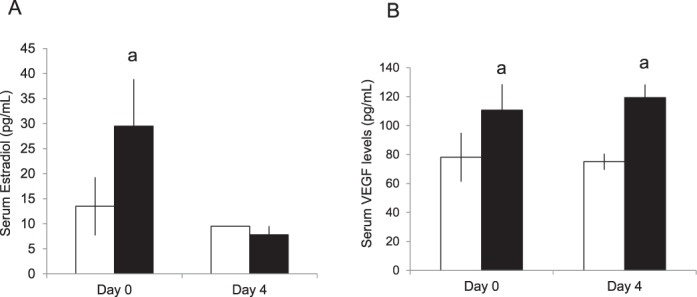
Serum hormone levels in mice following natural mating (white bar) or mating following superovulation with gonadotropins (black bar). A) Serum estradiol levels were significantly elevated in those mice treated with gonadotropins compared to controls on the day of mating. However, by Postcoital Day 4, estradiol levels had returned to baseline. B) VEGF levels were significantly elevated following superovulation when compared to controls, and this elevation persisted into the peri-implantation period. aP < 0.05 versus natural mating. Data are expressed as mean ± SEM.
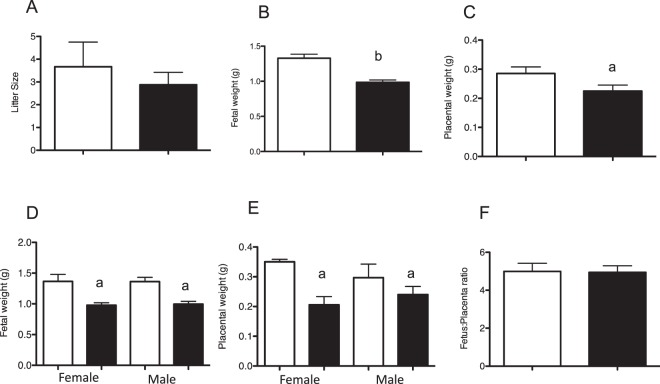
To determine the effect of altering the maternal environment during peri-implantation on fetal and placental growth, pregnant dams were sacrificed on Day 18.5 of gestation. Twenty-three pups from eight SO recipients as well as 16 mice from five control recipients were analyzed. There was no difference in litter size between the control and the SO recipients (Fig. 2A). The fetuses derived from SO dams were significantly smaller than those derived from control recipients, with a greater than 25% difference in mean fetal weight (SO pups: mean ± SEM of 0.99 ± 0.15 g compared to control pups: 1.33 ± 0.23 g; P < 0.0001) (Fig. 2B). Mean placental weight was also significantly smaller in SO mice (SO pups: mean ± SEM of 0.23 ± 0.09 g compared to control pups 0.29 ± 0.09 g; P < 0.05) (Fig. 2C). Both fetuses and placentas were equally affected because there was no difference in the fetus to placenta weight ratio between the two groups. Nevertheless, both male and female pups had lower fetal and placental weight in the SO group (Fig. 2, D–F).
FIG. 2.
Five control (white bars) and eight SO dams (black bars) were sacrificed at E18.5. A) Mean litter size was not different between the groups. Fetal weights (B) and placental weights (C) were significantly different between the two groups, regardless of sex of the offspring (D, E) (control: n = 16, 7 female, 9 male; SO: n = 23, 7 female, 16 male). F) Fetus to placenta weight ratio was not different between the groups. aP < 0.05 versus natural mating (each sex compared to offspring of the same sex). bP < 0.001 versus natural mating. Data are expressed as mean ± SEM.
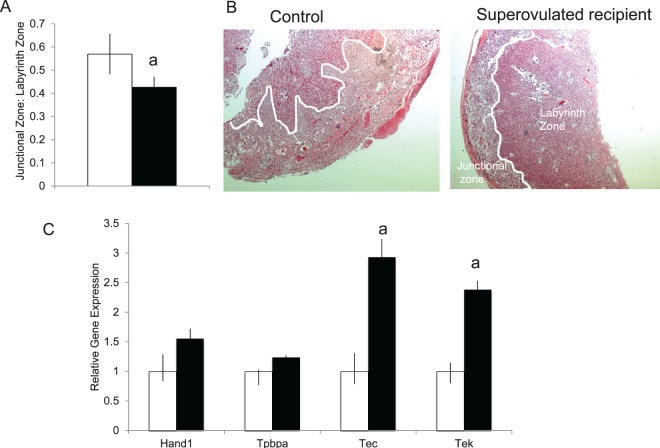
Although fetal weight was most significantly affected in our mouse model, previous studies have suggested that the primary insult during ART is the result of abnormal placentation. Therefore, we examined the histology of the placenta from both control and SO recipients. The gross morphology of the placentas from SO recipients did not differ from that of the control recipients. To determine which placental zones were contributing to the lower placental mass in the SO placentas, histologic, morphometric and immunohistochemical analysis of placentas from both groups were analyzed. The area of the labyrinth zone, the primary area of maternal-fetal nutrient exchange, was examined to determine if changes in the labyrinth could be responsible for both the decreased placental and fetal growth. At E18.5, the placenta from SO recipients revealed a reduced junctional to labyrinth zone ratio compared to placentas from control recipients (SO pups: mean ± SEM of 0.57 ± 0.09 compared to control pups 0.43 ± 0.05; P < 0.05) (Fig. 3A). In addition, placentas from SO recipients showed attenuated branching with limited invasion of the junctional zone by the labyrinth (Fig. 3B). We examined the percentage of vacuolated glycogen cells and the nonvacuolated spongiotrophoblast cells in the junctional layer and found no difference in the distribution of these cell types between our SO and our control placentas. In order to confirm that our model is altering specific regions of the murine placenta and to determine the cell type(s) affected, we performed qRT-PCR using markers for specific cell types. Both Tec, a labyrinth specific marker, and Tek, an endothelial cell marker, were increased in our SO placentas compared to controls (Fig. 3C). There was no difference in Tpbpa, a junctional zone marker, and Hand1, a giant cell-specific marker, between the two groups. These results suggest that the altered maternal environment during implantation resulting from superovulation may affect trophoblast differentiation and ultimately fetal growth.
FIG. 3.
Histopathological examination of placentas from control and SO recipients. A) Placentas from SO recipients (black bar) demonstrated a reduced junctional to labyrinth zone ratio compared to placentas from control recipients (white bar) (n = 10 control, 5 male, 5 female; n = 20 SO, 12 male, 8 female). B) Placentas from SO recipients showed attenuated branching with limited invasion of the junctional zone by the labyrinth. C) Placental expression of selected markers of different placental cell types in SO recipients (black bar) and control recipients (white bar). aP < 0.05 versus natural mating. Data are expressed as mean ± SEM. Original magnification ×2.5.
Expression of Growth-Related Genes in Extraembryonic and Embryonic Tissue
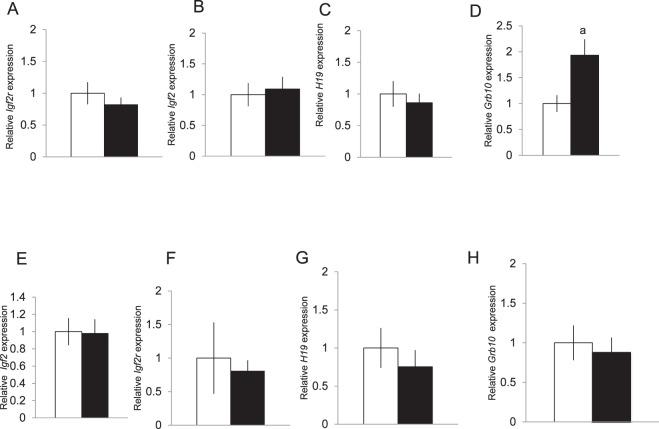
Although serum estradiol levels in the SO group had returned to control values by the time of implantation, the altered environment created by superovulation could alter gene expression in the uterine environment and such changes could lead to changes in gene expression in the developing fetus and/or placenta. Therefore, we analyzed genes that affect fetal and placental growth in placenta from the control and SO recipients; expression of these candidate genes, including Igf2, Igf2r, and H19, is affected by ART. Real-time quantitative PCR revealed no difference in expression of these genes between the placenta and liver of pups derived from control and SO recipients (Fig. 4, A–C). We also analyzed expression of Grb10, a gene that is differentially methylated in the placenta of control and ART children. In the placentas from SO dams, we found a two-fold increase in expression of Grb10 compared to their non-SO counterparts (Fig. 4D). Although Grb10 is also expressed in embryonic tissues such as brain, kidney, and liver, we found no difference in Grb10 expression in embryonic tissues (Fig. 4, E–H).
FIG. 4.
Placental expression of Grb10 was elevated in placentas obtained from SO recipients (black bar) compared to control recipients (white bar) though expression of other growth-related genes did not differ between the two groups (A–D). Gene expression of Grb10, Igf2, Igf2r, and H19 did not differ in the liver (E–H) or other embryonic tissues (data not shown). Gene expression was measured by qRT-PCR at E18.5. Fold change values for each time point are relative to control and normalized to Rplpo. At least eight placentas were examined in each group, four males and four females. aP < 0.05. Data are expressed as mean ± SEM.
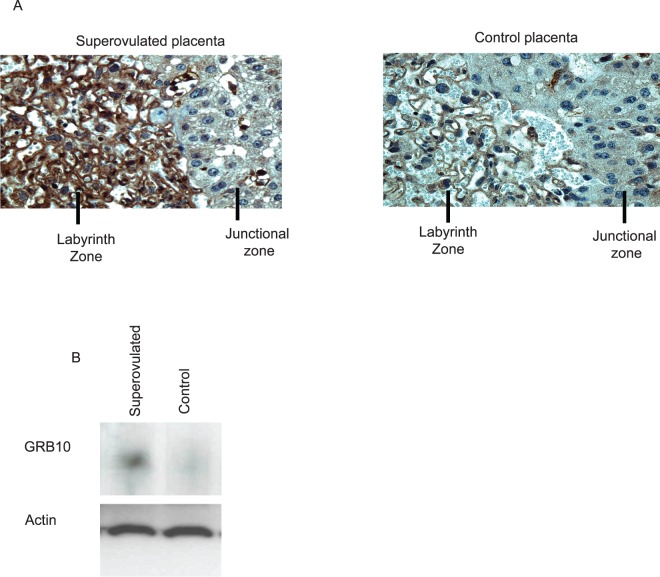
The increase in placental Grb10 expression in the SO group led to an increase in the amount of GRB10 protein in the placenta as determined by both immunohistochemistry and Western blot analysis. In the cross-sections of placenta from SO recipients, we found significantly higher expression of Grb10 in the trophoblast cells of the placental labyrinth zone. Endothelial cell expression did not appear to differ between the two groups (Fig. 5A). We also examined whole protein extracts between placentas from control and SO recipients. By Western blot analyses, SO placentas had an approximately 30% increase in protein expression compared to control placenta when normalized to beta-actin (Fig. 5B).
FIG. 5.
A) Immunohistochemistry using an antibody to GRB10 shows GRB10 expression primarily in the labyrinth zone of the placenta, with increased expression in the SO placenta. B) Western blot analysis of protein lysates from E18.5 placenta from control or SO recipients with an antibody against the C terminus of GRB10. An anti-actin antibody was used as a loading control. (Representative images, eight placenta examined in each group.) Original magnification ×40.
DNA Methylation at Imprinting Control Regions
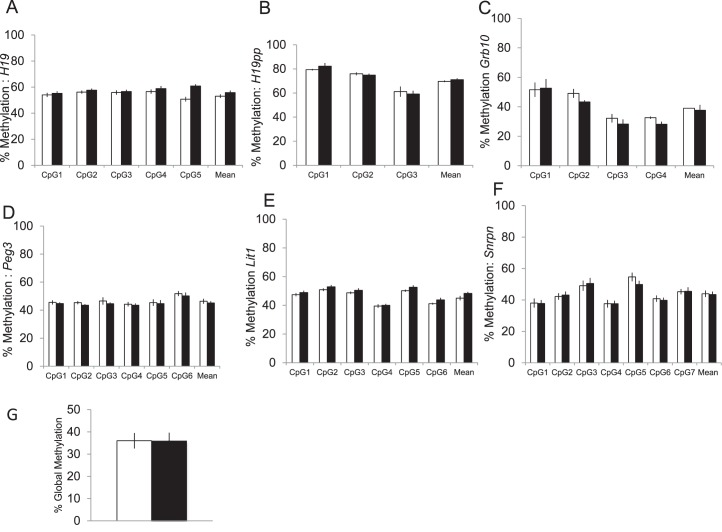
Although the tissue used for the aforementioned analyses was retrieved at E18.5, the primary exposure to the recipients occurred early on during implantation and therefore gene and protein expression may not reflect the early molecular changes occurring as a result of the gonadotropin exposure. Because gonadotropin exposure can affect DNA methylation of imprinted genes and may have long-term sequelae for the offspring [16], we used pyrosequencing to examine DNA methylation in CpG rich regions of five imprinted genes previously known to be affected by ART [17, 18, 28]. We found no difference in mean methylation at any of these sites between the placentas of SO recipients and control recipients (Fig. 6, A–F). We also examined embryonic tissue from these pups and similarly found no difference in methylation in the embryonic tissues of these offspring (data not shown). In addition, we analyzed global methylation in the placenta using a luminometric methylation assay and again found no difference in mean global methylation between placentas from SO recipients and control placentas (Fig. 6G).
FIG. 6.
DNA methylation at the differentially methylated region of six imprinted genes thought to be affected by ART (A–F). Pyrosequencing found no difference in mean DNA methylation at these sites in placental tissue from control (white bars) and SO placentas (black bars). G) There was also no difference in global methylation in the placenta between the two groups. At least eight placentas were examined in each group, four males and four females. Data are expressed as mean ± SEM.
DISCUSSION
Fetal growth depends on proper development and function of the placenta. In this study, we find that altering the hormonal milieu during preimplantation development perturbs placentation by affecting trophoblast differentiation and changing the distribution of cell types in the placenta, which could affect fetal and placental growth. In addition, transfer of naturally conceived embryos into a SO environment results in increased expression of Grb10, a negative growth regulator, in the near-term placenta.
Grb10 codes for an adaptor protein that functions downstream of critical growth-regulating genes, such as the insulin receptor, insulinlike growth factor receptor, and epidermallike growth factor receptor [29]. In both the mouse and human, Grb10 is imprinted with placental expression originating from the hypermethylated maternal allele. Disruption of the maternal allele results in overgrowth of the offspring as well as an enlarged placenta, whereas maternal duplication results in growth retardation, that is, Grb10 likely functions as a negative regulator of fetal growth [30, 31]. In our model, we observe increased expression of Grb10 in our growth-restricted fetuses from the SO recipients, suggesting that such misexpression may be a contributing factor to the phenotype. The increase in Grb10 may also be related to the relative increase in labyrinth zone area seen in the SO placenta. We do not observe any differences in the level of DNA methylation in the differentially methylated region that we examined in Grb10 between the SO and control placentas. It is possible that this failure to detect a difference is attributed to our examining whole placenta and not individual cell types and therefore did not detect changes in DNA methylation in a subset of cells in which loss-of-imprinting occurred. Alternatively, the changes in expression may not be due to aberrant DNA methylation but rather changes in histone modifications, which may be another epigenetic mechanism that regulates Grb10 expression via suppression of the paternal allele [32, 33]. Though previous studies have demonstrated an effect of ART on DNA methylation, it appears that these changes may be a result of an effect on the developing gamete or early embryo preimplantation. In this study, isolating the exposure of the maternal hormonal milieu, we do not detect changes in DNA methylation. This is consistent with previous data that suggests the effect of superovulation may be on the developing gamete through disruption of imprint acquisition and maternal effect gene products [34].
High estradiol levels created through superovulation are implicated to be detrimental to implantation, placentation, and fetal growth [12, 13]. Patients who experience ovarian hyperstimulation syndrome, which results following IVF cycles with extremely high serum estradiol levels, are approximately three times more likely to have adverse perinatal outcomes than patients who do not suffer from ovarian hyperstimulation syndrome [35]. This suggests that the elevated estradiol levels created following superovulation may disrupt proper placentation. However, unlike in humans, in mouse, serum estradiol levels rapidly return to baseline following superovulation, and by the day of blastocyst transfer do not differ from control recipients. Another consequence of gonadotropin stimulation is multiple corpus lutea producing elevated serum VEGF levels [36]. Following controlled ovarian hyperstimulation, in humans, VEGF levels remain elevated for several weeks [37]. We found that in mice, VEGF levels following superovulation are similarly significantly higher than in controls, and this elevation persists during the implantation period. VEGF is implicated in disorders of placentation and as a possible marker for other adverse outcomes associated with IVF such as preeclampsia [38]. VEGF also up-regulates Grb10 in certain cell lines [39]. Thus, elevated VEGF levels in our SO recipients may affect trophoblast differentiation and/or gene expression, including Grb10 expression, in the developing placenta. We are currently examining the role of VEGF in regulation of Grb10 in both human and mouse trophoblasts as well as the vascular cells of the placenta.
ART is associated with several adverse outcomes involving abnormal placentation and fetal growth [4, 9, 40]. These findings have persisted even when examining singleton, term infants born following IVF. In this study, we have removed the confounding effects of superovulation on the oocyte, IVF, and prolonged embryo culture, all which affect the resulting offspring [34, 41, 42]. We have identified one exposure, altered hormonal environment during the peri-implantation period, which alone can lead to adverse outcomes in a mouse model. These data confirm the large clinical studies that have found that the hormonal environment following fresh IVF cycles is detrimental to placentation and fetal growth [22]. Until recently, fresh IVF cycles were preferred to frozen embryo transfers secondary to fear of embryo loss during the freeze/thaw process. However, with the advent of vitrification, the success of frozen embryo transfer is at least equal to that of fresh embryo transfer [43–45]. We suggest, in light of both the animal data presented here and recent human data, that frozen embryo transfers may be preferable to fresh embryo transfers due to the more physiologic environment during implantation, at least for those patients at highest risk for perinatal morbidity. Clearly more research is necessary to identify this group of patients in order to identify those with this modifiable risk factor. In addition, furthering our understanding of factors affecting early implantation will likely lead to a better understanding of the pathophysiology of other conditions of aberrant placentation, such as preeclampsia, that contribute to perinatal morbidity in both natural and assisted conceptions.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Richard Schultz for critically reading the manuscript. We gratefully acknowledge the University of Virginia Center for Research in Reproduction Ligand Core Laboratory for performing the estradiol assays.
Footnotes
Supported by a grant from the Reproductive Scientists Development Program/NIH K12 HD000849-26 (to M.A.M.) and by grant U54-HD-068157 (to C.C., C.S., and M.B.). Presented, in part, at the 46th Annual Meeting of the Society for the Study of Reproduction, July 22–26, 2013, Montreal, Canada.
REFERENCES
- Gosden R, Trasler J, Lucifero D, Faddy M. Rare congenital disorders, imprinted genes, and assisted reproductive technology. Lancet 2003; 361: 1975–1977. [DOI] [PubMed] [Google Scholar]
- Maher ER, Brueton LA, Bowdin SC, Luharia A, Cooper W, Cole TR, Macdonald F, Sampson JR, Barratt CL, Reik W, Hawkins MM. Beckwith-Wiedemann syndrome and assisted reproduction technology (ART). J Med Genet 2003; 40: 62–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med 2002; 346: 731–737. [DOI] [PubMed] [Google Scholar]
- Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol 2004; 103: 551–563. [DOI] [PubMed] [Google Scholar]
- Chen X-K, Wen SW, Bottomley J, Smith GN, Leader A, Walker MC. In vitro fertilization is associated with an increased risk for preeclampsia. Hypertens Pregnancy 2009; 28: 1–12. [DOI] [PubMed] [Google Scholar]
- Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med 2002; 346: 725–730. [DOI] [PubMed] [Google Scholar]
- Cox GF, Burger J, Lip V, Mau UA, Sperling K, Wu B-L, Horsthemke B. Intracytoplasmic sperm injection may increase the risk of imprinting defects. Am J Hum Genet 2002; 71: 162–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Prein J, Smit JJ, Vermeiden JPW, Spreeuwenberg M, van Leeuwen FE, Delemarre-van de Waal HA. Growth during infancy and early childhood in relation to blood pressure and body fat measures at age 8–18 years of IVF children and spontaneously conceived controls born to subfertile parents. Hum Reprod 2009; 24: 2788–2795. [DOI] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Vermeiden JPW, van Leeuwen FE, Delemarre-van de Waal HA. Growth and development of children born after in vitro fertilization. Fertil Steril 2008; 90: 1662–1673. [DOI] [PubMed] [Google Scholar]
- Scott KA, Yamazaki Y, Yamamoto M, Lin Y, Melhorn SJ, Krause EG, Woods SC, Yanagimachi R, Sakai RR, Tamashiro KLK. Glucose parameters are altered in mouse offspring produced by assisted reproductive technologies and somatic cell nuclear transfer. Biol Reprod 2010; 83: 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo BS, Park SH, An BM, Kim KS, Moon SE, Moon HS. Serum estradiol levels during controlled ovarian hyperstimulation influence the pregnancy outcome of in vitro fertilization in a concentration-dependent manner. Fertil Steril 2010; 93: 442–446. [DOI] [PubMed] [Google Scholar]
- Imudia AN, Awonuga AO, Doyle JO, Kaimal AJ, Wright DL, Toth TL, Styer AK. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril 2012; 97: 1374–1379. [DOI] [PubMed] [Google Scholar]
- Farhi J, Ben-Haroush A, Haroush AB, Andrawus N, Pinkas H, Sapir O, Fisch B, Ashkenazi J. High serum oestradiol concentrations in IVF cycles increase the risk of pregnancy complications related to abnormal placentation. Reprod Biomed Online 2010; 21: 331–337. [DOI] [PubMed] [Google Scholar]
- Klemetti R, Sevon T, Gissler M, Hemminki E. Health of children born after ovulation induction. Fertil Steril 2010; 93: 1157–1168. [DOI] [PubMed] [Google Scholar]
- Fortier AL, Lopes FL, Darricarrere N, Martel J, Trasler JM. Superovulation alters the expression of imprinted genes in the midgestation mouse placenta. Hum Mol Genet 2008; 17: 1653–1665. [DOI] [PubMed] [Google Scholar]
- Sato A, Otsu E, Negishi H, Utsunomiya T, Arima T. Aberrant DNA methylation of imprinted loci in superovulated oocytes. Hum Reprod 2007; 22: 26–35. [DOI] [PubMed] [Google Scholar]
- Borghol N, Lornage J, Blachere T. Sophie Garret A, Lefevre A. Epigenetic status of the H19 locus in human oocytes following in vitro maturation. Genomics 2006; 87: 417–426. [DOI] [PubMed] [Google Scholar]
- Katari S, Turan N, Bibikova M, Erinle O, Chalian R, Foster M, Gaughan JP, Coutifaris C, Sapienza C. DNA methylation and gene expression differences in children conceived in vitro or in vivo. Hum Mol Genet 2009; 18: 3769–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan N, Katari S, Gerson LF, Chalian R, Foster MW, Gaughan JP, Coutifaris C, Sapienza C. Inter- and intra-individual variation in allele-specific DNA methylation and gene expression in children conceived using assisted reproductive technology. PLoS Genet 2010; 6: e1001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Ku SY, Jee BC, Suh CS, Kim SH, Choi YM, Kim JG, Moon SY. Dynamics of early estradiol production may be associated with outcomes of in vitro fertilization. Fertil Steril 2010; 94: 2868–2870. [DOI] [PubMed] [Google Scholar]
- Hubayter ZR, Muasher SJ. Luteal supplementation in in vitro fertilization: more questions than answers. Fertil Steril 2008; 89: 749–758. [DOI] [PubMed] [Google Scholar]
- Kalra SK, Ratcliffe SJ, Coutifaris C, Molinaro T, Barnhart KT. Ovarian stimulation and low birth weight in newborns conceived through in vitro fertilization. Obstet Gynecol 2011; 118: 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2003.
- Dokras A, Hoffmann DS, Eastvold JS, Kienzle MF, Gruman LM, Kirby PA, Weiss RM, Davisson RL. Severe feto-placental abnormalities precede the onset of hypertension and proteinuria in a mouse model of preeclampsia. Biol Reprod 2006; 75: 899–907. [DOI] [PubMed] [Google Scholar]
- Susiarjo M, Sasson I, Mesaros C, Bartolomei MS. Bisphenol a exposure disrupts genomic imprinting in the mouse. PLoS Genet 2013; 9: e1003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerschmidt DM, de Vries W, Ito M, Solter D, Ferguson-Smith A, Knowles BB. Trim28 is required for epigenetic stability during mouse oocyte to embryo transition. Science 2012; 335: 1499–1502. [DOI] [PubMed] [Google Scholar]
- Karimi M, Johansson S, Stach D, Corcoran M, Grander D, Schalling M, Bakalkin G, Lyko F, Larsson C, Ekstrom TJ. LUMA. (LUminometric Methylation Assay)—a high throughput method to the analysis of genomic DNA methylation. Exp Cell Res 2006; 312: 1989–1995. [DOI] [PubMed] [Google Scholar]
- Kerjean A, Couvert P, Heams T, Chalas C, Poirier K, Chelly J, Jouannet P, Paldi A, Poirot C. In vitro follicular growth affects oocyte imprinting establishment in mice. Eur J Hum Genet 2003; 11: 493–496. [DOI] [PubMed] [Google Scholar]
- Dufresne AM, Smith RJ. The adapter protein GRB10 is an endogenous negative regulator of insulin-like growth factor signaling. Endocrinology 2005; 146: 4399–4409. [DOI] [PubMed] [Google Scholar]
- Charalambous M, Cowley M, Geoghegan F, Smith FM, Radford EJ, Marlow BP, Graham CF, Hurst LD, Ward A. Maternally-inherited Grb10 reduces placental size and efficiency. Dev Biol 2010; 337: 1–8. [DOI] [PubMed] [Google Scholar]
- Shiura H, Nakamura K, Hikichi T, Hino T, Oda K, Suzuki-Migishima R, Kohda T, Kaneko-ishino T, Ishino F. Paternal deletion of Meg1/Grb10 DMR causes maternalization of the Meg1/Grb10 cluster in mouse proximal chromosome 11 leading to severe pre- and postnatal growth retardation. Hum Mol Genet 2009; 18: 1424–1438. [DOI] [PubMed] [Google Scholar]
- Yamasaki-Ishizaki Y, Kayashima T, Mapendano CK, Soejima H, Ohta T, Masuzaki H, Kinoshita A, Urano T, Yoshiura K, Matsumoto N, Ishimaru T, Mukai T, et al. Role of DNA methylation and histone H3 lysine 27 methylation in tissue-specific imprinting of mouse Grb10. Mol Cell Biol 2007; 27: 732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield AS, Cowley M, Smith FM, Moorwood K, Stewart-Cox JE, Gilroy K, Baker S, Xia J, Dalley JW, Hurst LD, Wilkinson LS, Isles AR, et al. Distinct physiological and behavioural functions for parental alleles of imprinted Grb10. Nature 2011; 469: 534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Market-Velker BA, Zhang L, Magri LS, Bonvissuto AC, Mann MRW. Dual effects of superovulation: loss of maternal and paternal imprinted methylation in a dose-dependent manner. Hum Mol Genet 2010; 19: 36–51. [DOI] [PubMed] [Google Scholar]
- Chung K, Coutifaris C, Chalian R, Lin K, Ratcliffe SJ, Castelbaum AJ, Freedman MF, Barnhart KT. Factors influencing adverse perinatal outcomes in pregnancies achieved through use of in vitro fertilization. Fertil Steril 2006; 86: 1634–1641. [DOI] [PubMed] [Google Scholar]
- Lee A, Christenson LK, Stouffer RL, Burry KA, Patton PE. Vascular endothelial growth factor levels in serum and follicular fluid of patients undergoing in vitro fertilization. Fertil Steril 1997; 68: 305–311. [DOI] [PubMed] [Google Scholar]
- Licht P, Neuwinger J, Fischer O, Siebzehnrubl E, Wildt L. VEGF plasma pattern in ovulation induction: evidence for an episodic secretion and lack of immediate effect of hCG. Exp Clin Endocrinol Diabetes 2002; 110: 130–133. [DOI] [PubMed] [Google Scholar]
- Mutter WP, Karumanchi SA. Molecular mechanisms of preeclampsia. Microvasc Res 2008; 75: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetti-Peraldi S, Murdaca J, Mas JC, Van Obberghen E. The adapter protein, Grb10, is a positive regulator of vascular endothelial growth factor signaling. Oncogene 2001; 20: 3959–3968. [DOI] [PubMed] [Google Scholar]
- Omland AK, Bjercke S, Ertzeid G, Fedorcsak P, Oldereid NB, Storeng R, Abyholm T, Tanbo T. Intracytoplasmic sperm injection (ICSI) in unexplained and stage I endometriosis-associated infertility after fertilization failure with in vitro fertilization (IVF). J Assist Reprod Genet 2006; 23: 351–357. [DOI] [PubMed] [Google Scholar]
- Rinaudo P, Schultz RM. Effects of embryo culture on global pattern of gene expression in preimplantation mouse embryos. Reproduction 2004; 128: 301–311. [DOI] [PubMed] [Google Scholar]
- Doherty AS, Mann MR, Tremblay KD, Bartolomei MS, Schultz RM. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod 2000; 62: 1526–1535. [DOI] [PubMed] [Google Scholar]
- Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril 2011; 96: 344–348. [DOI] [PubMed] [Google Scholar]
- Shapiro BS, Daneshmand ST, Restrepo H, Garner FC, Aguirre M, Hudson C. Matched-cohort comparison of single-embryo transfers in fresh and frozen-thawed embryo transfer cycles. Fertil Steril 2013; 99: 389–392. [DOI] [PubMed] [Google Scholar]
- Ku PY, Lee RK, Lin SY, Lin MH, Hwu YM. Comparison of the clinical outcomes between fresh blastocyst and vitrified-thawed blastocyst transfer. J Assist Reprod Genet 2012; 29: 1353–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.