Abstract
Stress activates the hypothalamo-pituitary-adrenal (HPA) axis, leading to adrenocortical secretion of glucocorticoids. The magnitude and duration of the HPA axis response is mediated in large part by the glucocorticoid receptor (GR). The nucleus of the solitary tract (NTS) abundantly expresses the GR and is a key brain region for processing autonomic and endocrine stress responses. This study tests the hypothesis that GR within the NTS plays an important role in inhibiting stress-induced endocrine and behavioral responses. Cohorts of rats received bilateral micropellet (30 μg) implantations of crystalline corticosterone, mifepristone (a GR antagonist) or cholesterol (control) directed into the region of the NTS, and were subsequently subjected to either acute psychogenic (restraint) stress or chronic variable stress (CVS). We found that NTS GR antagonism increased acute stress-induced corticosterone levels, whereas GR activation within the NTS attenuated this response. Following CVS, basal and 15 min post-restraint plasma corticosterone levels were increased by NTS GR antagonism, which was associated with an increase in Fos immunoreactivity within the PVN. Using the elevated plus maze (EPM) and forced swim test (FST), we assessed the effect of NTS GR inhibition on anxiety- and depressionlike behaviors, respectively. GR inhibition within the NTS decreased open arm exploratory behavior in the EPM and increased immobility in the FST relative to controls. Together, the findings reveal a novel role of NTS GR signaling for inhibiting both endocrine and behavioral responses to stress.
Keywords: Chronic stress, corticosterone, depression, HPA axis, chronic variable stress
1. Introduction
Stress (a real or anticipated threat to homeostasis or well-being) produces endocrine, autonomic, and behavioral responses to promote physiological and cognitive coping (Chrousos and Gold, 1992; Myers et al., 2013b). The primary neuroendocrine responses to stress involve activation of the hypothalamo-pituitary-adrenocortical (HPA) axis, leading to the release of glucocorticoids (Ulrich-Lai and Herman, 2009). The HPA axis response involves a neuroendocrine cascade, initiated by corticotrophin-releasing hormone (CRH) neurons of the paraventricular nucleus (PVN) of the hypothalamus, which act synergistically with arginine vasopressin (AVP) to stimulate pituitary adrenocorticotrophic hormone (ACTH) release and subsequent adrenocortical synthesis and secretion of glucocorticoids. Glucocorticoids then exert actions in the brain and periphery in order to maintain adaptation in the face of potential threats. Glucocorticoid effects are mediated by the mineralocorticoid receptor (MR) and glucocorticoid receptor (GR), which act via genomic as well as non-genomic mechanisms to regulate intracellular signaling (Joels et al., 2012). Due to differential binding characteristics of the two receptors, the MR is thought to mediate effects of low glucocorticoid levels, whereas the GR is responsible for effects of high circulating hormone levels, as seen during stress responses (Reul and De Kloet, 1985). However, newer evidence indicates that membrane-located MR has a much lower affinity and plays a potential role in initial stress responding (Karst et al., 2005).
Chronic stress has been shown to cause long-term physiological and endocrine changes. Several studies have demonstrated that in rats, chronic stress can lead to attenuation of body weight gain, thymic involution due to increased glucocorticoid levels, adrenal hypertrophy/hyperplasia, and changes in HPA axis responsiveness (Kiss and Aguilera, 1993; Herman et al., 1995; Ulrich-Lai et al., 2006). Various animal models have shown that chronic stress also elevates basal corticosterone and/or ACTH secretion, facilitates endocrine responses to novel stressors, and increases PVN activation as evident from increased expression of CRH and AVP in parvocellular neurons (Imaki et al., 1991; Dallman et al., 1992; Herman et al., 1995). Additionally, prolonged stress exposure can impair adaptive mechanisms and impose potential risk for developing numerous stress-related disorders including depression, anxiety and posttraumatic stress disorder (McEwen and Steller, 1993).
Considerable evidence indicates that stress responses are mediated by many overlapping circuits in forebrain limbic regions, hypothalamus, and brainstem (Herman et al., 2003). In particular, the nucleus of the solitary tract (NTS) is a primary brain region involved in brain integration of autonomic and HPA axis stress responses (Ulrich-Lai and Herman, 2009; Ghosal et al., 2013). The NTS sends direct stress-excitatory projections to PVN CRH neurons (Cunningham and Sawchenko, 1988; Ulrich-Lai and Herman, 2009; Rinaman, 2011), and receives descending inputs from forebrain limbic stress-responsive regions, such as the prefrontal cortex and central nucleus of amygdala (CeA) (Schwaber et al., 1982; Hurley et al., 1991). Moreover, Fos induction is observed in NTS neurons following homeostatic challenges, including visceral illness (Rinaman, 1999), immune challenge (Ericsson et al., 1994) and hypoxia (Teppema et al., 1997), as well as psychogenic stressors (e.g., restraint, forced swim, or immobilization) (Pezzone et al., 1993; Cullinan et al., 1995; Sawchenko et al., 2000), indicating possible involvement of the NTS in processing systemic and emotional stress responses. Increased activation of NTS neurons is also observed under conditions of chronic variable stress (as evident by increased ΔFosB immunoreactivity), consistent with a role in long-term stress regulation (Flak et al., 2012).
The NTS abundantly expresses MR and GR (Herman, 1993), and is positioned to modulate glucocorticoid actions. Moreover, colocalization studies indicate that both stress excitatory catecholaminergic and GLP-1 containing neurons contain GR (but not MR) (Harfstrand et al., 1986; Rinaman, 2011), suggesting that glucocorticoids may affect output of these neurons via the GR. Consistent with a functional role for the GR at the level of NTS, it has been shown that chronic stimulation of corticosteroid receptors within the hindbrain increases arterial pressure to acute restraint stress (Scheuer et al., 2007). A previous study also demonstrated that endogenous glucocorticoids act via hindbrain GR perhaps at the level of NTS to provide adaptation of the arterial pressure response to stress in normotensive rats (Bechtold et al., 2009). In addition, local infusions of glucocorticoids into the NTS facilitate memory formation in the basolateral amygdala (Roozendaal et al., 1999), consistent with an important role in forebrain function. Despite evidence showing various stress-regulatory effects of NTS, the role of NTS GR signaling in stress regulation has yet to be determined. Here, we have implanted micropellets (30 μg) of crystalline corticosterone (CORT), mifepristone (a GR antagonist) or cholesterol (CHOL) (control) targeting the caudal NTS, which contains the majority of PVN-projecting neurons, to test the hypothesis that NTS GR signaling plays an important role in inhibiting neuroendocrine and behavioral responses to stress. Our results indicate that GR signaling in the NTS attenuates endocrine stress responses and decreases anxiety- and depression-like behaviors, consistent with a role in limiting the impact of stress on neural and somatic endpoints.
2. Materials and Methods
2.1. Animals
Adult male Sprague Dawley rats (Harlan, Indianapolis, IN; 250-300 g) were housed singly at the Metabolic Diseases Institute of the University of Cincinnati under standard conditions in a temperature and humidity-controlled room on a 12/12 h light/dark cycle (lights on at 0600 h and lights off at 1800 h) with food (Teklad, Harlan) and water available ad libitium. All animal procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee. All rats were habituated to the animal facility for at least one week before the beginning of an experiment.
2.2. Stereotaxic pellet implantation
2.2.1. Experiment 1
A cohort of 35 rats was matched for body weight and divided into three groups. Each rat received bilateral 30 μg pellet of crystalline CORT (n=12) (Sigma-Aldrich, St. Louis, MO), mifepristone (n=13) (Sigma-Aldrich, St. Louis, MO) or CHOL (n=10) (Sigma-Aldrich, St. Louis, MO). These micropellet implants were targeted to the caudal NTS (Paxinos and Watson coordinate system (Paxinos and Watson, 1998)), which encompasses the majority of NTS neurons projecting to the PVN (Rinaman, 2011). Micropellets were made as described previously (Myers and Greenwood-Van Meerveld, 2007). Before surgery, rats were anaesthetized by intraperitoneal injection of a mixture of ketamine (87 mg/kg) and xylazine (13 mg/kg) and were administered an analgesic (butorphanol) and an antibiotic (gentamicin). Each rat was mounted in a stereotaxic surgical frame (Kopf, Tujunga, CA). Following a midline incision, we verified that the skull was leveled. Small holes were drilled in the skull at the calculated coordinates to target the caudal NTS (-14.3 mm posterior to bregma, ±0.6 mm lateral to midline, and -7.8 mm ventral from skull). A 25-gauge stainless steel cannula (Plastic One, Roanoke, VA) containing a micropellet of CORT (30 μg), CHOL (30 μg), or mifepristone (30 μg) was lowered dorsally from the skull such that the micropellet was implanted at the dorsal margin of the targeted NTS region as described previously in other brain regions (Shepherd et al., 2003; Myers and Greenwood-Van Meerveld, 2007). Briefly, CORT (30 μg), CHOL (30 μg), or mifepristone (30 μg) was tamped into the cannula. The cannula was then stereotaxically positioned at the targeted NTS region and the micropellet was extruded by inserting a stylet into the cannula, after which the cannula and stylet were removed. After surgery, rats were allowed to recover for 5-7 days, with body weight, food intake, and behavior monitored to ensure that they were not in post-surgical discomfort. Following recovery, rats were subjected to acute restraint stress challenge (see below). At the end of the study, rats were perfused and brain tissue from each rat was processed for Nissl stain to confirm micropellet placement.
2.2.2. Experiment 2
Forty eight rats were matched for body weight and received bilateral micropellets of 30 μg of mifepristone (n=24) or CHOL (n=24) at the dorsal margin of NTS as described in experiment 1. After recovery for 5 days, rats were subjected to chronic variable stress (CVS) (n=12 for mifepristone; n=12 for cholesterol) as described below or served as unstressed controls. At the end of the study, rats were perfused and placement of micropellets was verified by Nissl staining.
2.3. Stress Protocols
2.3.1. Acute restraint stress testing (experiment 1)
Seven days post-implantation, rats were subjected to an acute restraint stress (serving as a psychogenic stress) at 0900h, during the circadian trough of corticosterone secretion. In this test, rats were placed in well-ventilated Plexiglas restraint tubes for a 30 min period. Blood samples (approximately 250 μl) were collected by tail clip as described previously (Vahl et al., 2005) for the measurement of hormone (corticosterone) levels. A basal blood sample was collected at the beginning of the restraint (0 min). At the end of the 30 min restraint, another blood sample was collected from each rat, and the rats were released from the restrainers back into their home cage to recover. Additional tail blood samples were collected at 60 and 120 min after the initiation of restraint from unrestrained, freely-moving rats by gently removing the clot. Each blood sample was collected in less than 3 min to prevent any increase in ACTH or corticosterone levels due to sampling (Vahl et al., 2005).
2.3.2. Chronic variable stress (experiment 2)
After recovery from surgery, rats were subjected to chronic variable stress (CVS). The CVS protocol lasted for 7 consecutive days to ensure that pellets were delivering steroid throughout the entire experiment (Myers and Greenwood-Van Meerveld, 2012). Previous studies from our laboratory have shown that one week of CVS exposure causes attenuation in body weight gain, adrenal hypertrophy, thymic atrophy, and increased HPA axis activity (Tauchi et al., 2008; Flak et al., 2009). All CVS animals received twice daily exposure to one of several different stressors given in unpredictable order with occasional overnight stressors for one week. Morning stressors were conducted between 0900h and 1130h, while afternoon stressors were conducted between 1300 h and 1600 h. Overnight stressors started immediately after the end of the afternoon stressors and ended before the initiation of the next morning's stressor. Stressors consisted of 5 min of elevated plus maze (first stressor of the CVS regimen), 1 h at 100 rpm on an orbital shaker, 5 min of open field test, 1 h in a cold room (4° C), 30 min of hypoxia (8% Oxygen and 92% nitrogen), overnight crowding, and a 10 min forced swim test (at 23-25° C) on the morning of the last day (day 7) (between 9:00-11:30 AM). The morning after the forced swim test all rats received a 30 min restraint exposure, which was a novel stressor for the CVS group. Blood samples (approximately 250 μl) were collected by tail clip at 0 min (basal) before and 15, 30, 60, and 120 min immediately after onset of the novel stress. Blood samples were collected in less than 3 min as described previously (Vahl et al., 2005).
2.4. Behavioral testing
2.4.1. Elevated plus maze (EPM)
5 min exposure to EPM was used as the first stressor of the CVS protocol (Experiment 2) to assess locomotor activity and anxiety-related behaviors. In this test, rats (n= 8-12/group) were placed in the center of the plus maze facing an open arm and behavior was recorded for the entire 5 min using an overhead mounted camera. Recorded parameters including open and closed arm time, total locomotor activity (total distance travelled in the maze) and entries to the open arm were scored using the Topscan program (Clever System Inc.). We measured percentage of time spent in the open arm (time in open arm/total time on the maze X 100%), and frequency and duration of stretch-attend postures (exploring the open arm from the closed arm), as described previously (Carobez and Bertoglio, 2005). Anxiety-related behavior is associated with less exploration of the open arm relative to overall exploration of all arms (Carobez and Bertoglio, 2005).
2.4.2. Forced swim test (FST)
The FST was selected to assess the impact of NTS GR antagonism on depression-like behavior with and without a prior history of chronic stress. The modified FST was conducted as described previously (Cryan et. al., 2005; Wulsin et al., 2010). Rats (n= 8-12/ group) were exposed to the FST tank (Plexiglas cylinder with a height of 45 cm and a diameter of 20 cm) filled with 31 cm of water (23-25° C) only once for 10 min. The entire behavioral session was videotaped. Measured parameters included immobility, swimming, diving, and climbing, and were scored using Hindsight version 1.5 (Hindsight, MS-dos, version 1.5, Scott Weiss). Active behaviors (swimming, climbing, and diving) versus immobility (not making any active movement or floating in the water without struggling) were defined as described previously (Cryan et. al., 2005; Wulsin et al., 2010).
2.4.3. Homecage locomotor activity
General homecage locomotor activity was determined as described previously (Thompson et al., 2009). Briefly, each rat cage was placed inside a smart frame stainless steel cage rack frame (Hamilton-Kinder Scientific Company, Poway, CA). Infrared photobeam interruptions sensors (7X and 15Y) mounted in the frame detected vertical and horizontal movement which was recorded for 24 h and analyzed using the HMM100 Motor Monitor software. Data were analyzed as the mean number of beam interruptions per group.
2.5. Blood collection and radioimmunoassay
Blood was collected by the tail clip procedure that involves clipping the tail with a sterile scalpel blade and collecting blood (250 μl) in chilled tubes containing 10 μl of 100 mM EDTA as described previously (Vahl et al., 2005). Blood samples were centrifuged at 3000X g for 15 min at 4° C and plasma was stored at -20° C for subsequent hormone analysis. Plasma corticosterone levels were measured using a 125I RIA kit (MP Biomedical, Solon, OH) as described previously (Ulrich-Lai et al., 2006). Plasma ACTH levels were measured using an antiserum donated by Dr. William Engeland (University of Minnesota, MN) and 125I-labeled ACTH (Amersham Biosciences, Piscataway, NJ) as tracer, as described previously (Ulrich-Lai et al., 2006). All samples were run in duplicate in the same assay.
2.6. Brain and organ collection
Ninety minutes after the termination of novel restraint stress challenge, all rats were administered an overdose of sodium pentobarbital (150mg/kg) and intracardially perfused with 100 ml of 0.9% saline followed by 250-300 ml of 4% paraformaldehyde, pH 7.6. Brains were collected and post fixed in 4% paraformaldehyde overnight and then transferred to 30 % sucrose (4 ° C). In order to determine the effects of NTS GR antagonism on somatic markers of stress, adrenal and thymus glands were removed, cleaned and weighed.
2.7. Fos immunoreactivity
Coronal brain sections (30 μm) were collected in 1:12 series using a freezing microtome and stored at -20°C in cryoprotectant (0.1 phosphate buffer, 30% sucrose, 1% polyvinylpyrrolidone, and 30% ethylene glycol). For Fos immunoreactivity, a one in 12 series of brain sections were rinsed (5 min X 5) in 50 mM potassium phosphate-buffered saline (KPBS) (pH 7.2), followed by incubation in 1% hydrogen peroxide solution for 10 min to quench endogenous peroxides and subsequently rinsed in KPBS. Tissue was then incubated in blocking buffer (KPBS) plus 0.25% bovine serum albumin and 0.4% Triton X-100) for 1 h at room temperature followed by incubation with a c-Fos-specific antibody (1:2500; Santa Cruz Biotechnology, Santa Cruz, CA, no. sc-52) in blocking buffer overnight at 4° C. The next day, sections were rinsed in KPBS (5 min X 5 times) followed by 1 h incubation in biotinylated donkey anti-rabbit antibody (1:500; Vector Laboratories, Burlingame, CA; no. BA1000). Sections were washed in KPBS and reacted with avidin-biotin solution (1:1000; Vector Laboratories, Burlingame) for 1 h at room temperature. Sections were washed once more and c-Fos immunoreactivity was visualized with 0.02% 3, 3′-diaminobenzidine (DAB) (Sigma)/hydrogen peroxide in KPBS solution.
2.8. Quantification of Fos immunoreactivity
2-3 brain sections per rat containing the mid-level of PVN (approximately -1.8 mm caudal to the bregma) were identified using a rat brain atlas (Paxinos and Watson, 1998) and analyzed for Fos immunoreactivity. The targeted region contains the richest populations of hypophysiotrophic parvocellular neurons, which are preferentially targeted by NTS afferents (Cunningham and Sawchenko, 1988). Adjacent images were captured at 5x objective using a Carl Zeiss Imager Z.1 (Carl Zeiss Microimaging, Thornwood, NY). For analysis, images were imported into Scion Image (version 4 0.3.2), and a uniform threshold was applied to all images. The number of Fos-positive cells was counted bilaterally for each section and expressed as cells per unit area (mm2). Cell counting was analyzed by an observer blinded to the treatment conditions.
2.9. Statistical analysis
Data are shown as mean ± standard error of the mean. To assess CVS-induced changes, organ weights were expressed as organ weight per 100 g of body weight. Body weight data from experiment 1 were analyzed with a two-way repeated measure analysis of variance (ANOVA) (Drug X time), time being the repeated measure using Sigma Stat (SYSTAT, San Jose CA) software. Final body weight after CVS, organ weights, behavioral data from FST, and overall locomotor activity were analyzed using two-way ANOVA (time X drug) or (stress X drug) as appropriate. Percent change in body weight through CVS was analyzed with three-way repeated measures ANOVA (Stress X drug X time), with time being the repeated measure using Statistica (Statsoft, Tulsa, OK). Baseline plasma hormone levels, integrated (AUC) corticosterone response to stress, immunoreactive counts and behavioral data from EPM were analyzed with one-way ANOVA or t-test as appropriate. Time course hormonal data were analyzed with two-way repeated measure ANOVA (Drug X time), time being the repeated measure. In all ANOVA designs, a priori planned comparisons were included to compare stress differences within drug group or vice versa. Main effects and interactions were analyzed using Fisher's LSD post hoc tests. Effects were considered significant with a critical value α set at p <0.05. Outliers were set by standardized scores >1.96 x the standard deviation from mean and, when necessary, data were reanalyzed following removal.
3. Results
3.1. Verification of pellets placement in the NTS
Micropellets containing CHOL, CORT, or mifepristone were targeted to the caudal NTS. Following each of the experiments, micropellet placement was verified using Nissl stain (Figure.1 A). Previous studies have demonstrated that the diffusion range of these micropellets is approximately 750 μm (Shepherd et al., 2003). Therefore, only rats with bilateral micropellets on the dorsal margin of the NTS between 13.30 mm and 14.30 mm posterior to bregma and placed within the diffusion range of NTS were included in the final data analysis in these studies (Figure.1 B, C). These rats were classified as “hits”, whereas rats having CORT or mifepristone pellets implanted outside the targeted regions (e.g. pellets in the cerebellum and/or in ventrolateral medulla) were considered “misses”.
Figure 1.
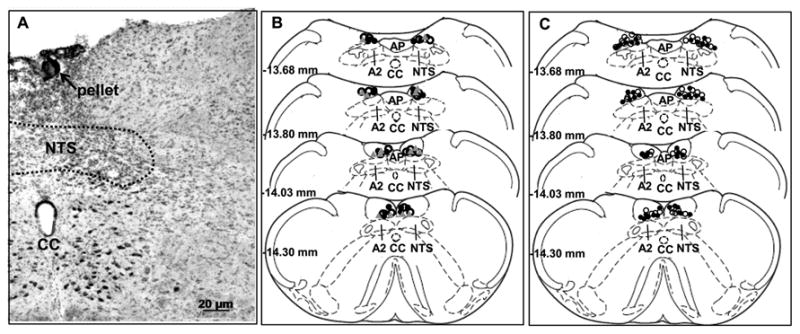
Location of NTS micropellets. (A) Example of micropellet placement directed to targeted NTS region as indicated by the black arrow. (B) Micropellet placement of animals subjected to acute restraint (experiment 1). (C) Location of micropellets of animals exposed to CVS (experiment 2). CHOL (30 μg): open circle; CORT (30 μg): grey triangle; mifepristone (30 μg): closed black circle. For experiment 1: n=8 (CORT implanted animal), n= 8 (mifepristone implanted animal), n=10 (CHOL implanted animals). For experiment 2: n= 8 (mifepristone implanted CVS rats), n=12 (CHOL implanted CVS rats), n=8 (mifepristone implanted no CVS rats), n=12 (CHOL implanted no CVS rats). Schematic coronal sections adapted from Paxinos and Watson (Paxinos and Watson, 1998). CC= Central canal, AP= area postrema, A2= adrenergic cell group, NTS= nucleus of solitary tract.
3.2. Body weight and organ weights
Body weight data for both experiments are presented in Figure 2. While surgery led to temporary decrease in body weight, there were no significant effects based on treatment (Figure. 2 A), suggesting that content of the pellets (CHOL or CORT or mifepristone) did not alter the body weight gain. In Experiment 2, rats with CHOL or mifepristone micropellets in the NTS subjected to CVS had reduced final body weight (Supplementary table 1) (F 1, 39 = 9.84; p < 0.05) compared to control rats, suggesting that the CVS regimen was sufficient to attenuate body weight gain as demonstrated previously (Tauchi et al., 2008). However, there was no drug X CVS interaction (p > 0.05) on the final body weight. Importantly, analyses of percent body weight gain over pre-CVS body weight showed a main effect of CVS (F 1, 39 = 18.18; p < 0.05), day (F 2, 74 = 198.18; p < 0.05) to decrease body weight gain (Figure. 2 B). Importantly, this analysis also revealed a main effect of mifepristone to decrease body weight gain (F 1, 39 = 8.84; p < 0.05). Specifically, post hoc analyses revealed that after chronic stress, CVS groups gained less weight than respective no CVS on day 4 (p < 0.05), and day 7 (p < 0.05) (Figure. 2 B). Moreover, body weight was significantly lower in CVS-mifepristone group than all other groups on day 4 (p < 0.05) and day 7 (p < 0.05) (Figure. 2 B), suggesting that both NTS GR blockade and chronic stress decreased body weight gain. Overall the data suggest a selective effect of GR manipulation on body weight under chronic stress conditions and does not under basal (non-stress) conditions.
Figure 2.
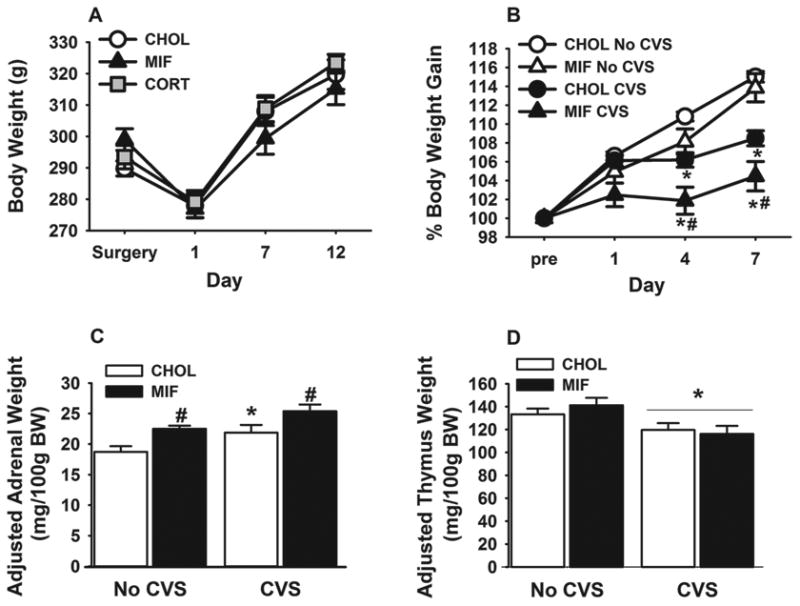
Body weight, adrenal weight, and thymus weight of rats with bilateral NTS micropellets. (A) 30 μg of CHOL, CORT or mifepristone micropellets implantation on the NTS does not alter body weight in the absence of stress (experiment 1, n=8-10/group). (B) NTS mifepristone micropellet (30 μg) and CVS-induced percent change in body weight. (C) Adjusted adrenal weight is increased by both CVS exposure and NTS mifepristone micropellet implants. (D) CVS exposure reduced adjusted thymi weight relative to no CVS control. Data are shown as mean ± SEM (n=8-12 per group). *p <0.05 vs. no CVS. #p <0.05 vs. CHOL.
Mifepristone micropellets in the NTS increased both absolute (F 1, 39 = 11.03; p < 0.05) and adjusted adrenal weight (adrenal weight/100g body weight) (F 1, 39 = 17.70; p < 0.05), suggesting that inhibition of GR in the NTS can cause modest adrenal hypertrophy (Figure. 2 C, Supplementary Table 1). In addition, we observed a main effect of CVS on adjusted adrenal weight (F 1, 39 = 17.44; p < 0.05), but no drug X CVS interaction (p > 0.05). Multiple comparisons indicated that all CVS rats had greater adjusted adrenal weights than their respective non-CVS counterparts (p < 0.05) (Figure. 2 C), consistent with an increased cumulative effect of CVS paradigm on adrenal growth. In addition, CVS decreased both absolute (F 1, 39 = 14.28; p < 0.05) and adjusted thymus weights (actual thymi weight/100g body weight) (main effect of CVS; F 1, 39 = 10.26; p < 0.05) (Figure. 2 D, Supplementary Table 1). There was no drug X CVS interaction (p > 0.05), suggesting that CVS decreased thymic weights regardless of drug implant. Collectively, the CVS-induced adrenal hypertrophy/hyperplasia and thymic atrophy are consistent with our previous findings (Tauchi et al., 2008; Flak et al., 2009), indicative of increased ACTH exposure and elevated corticosterone response. Moreover, NTS GR antagonism alone increased adjusted adrenal weights with and without a history of chronic stress, suggesting elevated cumulative ACTH secretion.
3.3. Basal and acute restraint-induced glucocorticoid levels
CHOL, CORT or mifepristone micropellet-implanted rats showed no significant differences in resting morning plasma corticosterone concentration (Figure. 3 A), consistent with previous reports (Diorio et al., 1993; Sheperd et al., 2003; Myers and Greenwood-Van Meerveld, 2012), showing that there is no effect of site specific steroid implants on normal basal corticosterone levels. Next, we tested the necessity of NTS GR for glucocorticoid responses to acute stress. The time course analysis of plasma corticosterone to acute restraint challenge revealed a main effect of drug (F 2, 69 = 7.18; p < 0.05) and time (F 3, 69 = 61.53; p < 0.05), and a significant drug X time interaction (F 6, 69 = 5.94; p < 0.05). Mifepristone micropellet implantation in the NTS caused a significant increase in the plasma corticosterone response 60 min after onset of the restraint stress (p < 0.05) (Figure. 3 B), whereas CORT micropellet-implanted animals showed a significant reduction in acute restraint-induced plasma corticosterone levels at this time point (p < 0.05) (Figure. 3 B). The data indicate that GR activation by CORT in the NTS attenuates acute psychogenic stress-induced corticosterone response, whereas GR inhibition by mifepristone facilitates this response. Additionally, mifepristone micropellet implanted rats showed a significant increase in integrated post-restraint stress corticosterone release as compared to both CHOL and CORT micropellet-implanted animals (p < 0.05) (Figure. 3 C), suggesting that NTS GR signaling inhibits the glucocorticoid response to acute psychogenic stress. Notably, no significant differences in the restraint-induced plasma corticosterone levels were found among the animals with mifepristone, CORT or CHOL pellets localized to adjacent off target areas (misses) (Figure. 3E, F), suggesting that these effects are specific to NTS GR signaling.
Figure 3.
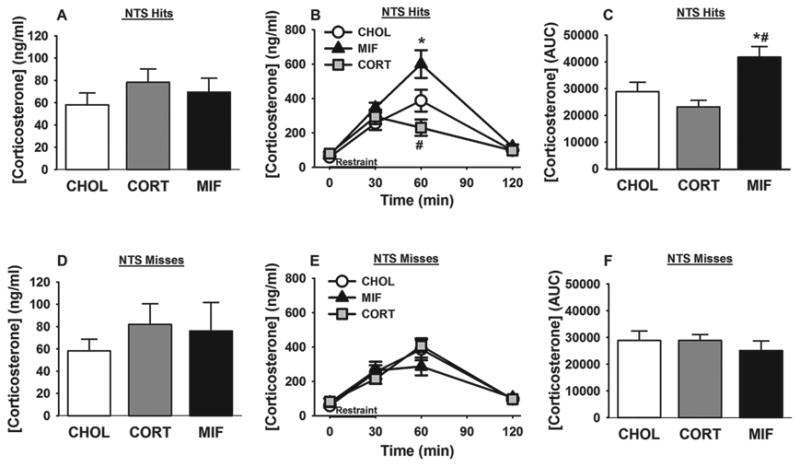
NTS GR signaling inhibits corticosterone response to acute restraint. (A) NTS CHOL, CORT or mifepristone micropellets does not alter baseline AM corticosterone levels. (B) At 60 min after initiation of restraint, mifepristone micropellet-implanted rats have increased plasma corticosterone levels, whereas CORT micropellet-implanted rats exhibited decreased corticosterone levels. (C) NTS mifepristone micropellets increased integrated corticosterone response to acute stress. (D-F) Animals with CHOL, CORT or mifepristone micropellet-implanted to adjacent off target areas showed no difference in their baseline AM plasma corticosterone levels, post-restraint time course corticosterone levels, or total corticosterone response to acute restraint. Data are presented as mean ± SEM. For NTS hits: n=8 (CORT implanted), n= 8 (mifepristone implanted), n=10 (CHOL implanted animals). For NTS misses: n= 4 (CORT implanted) n=5 (mifepristone implanted). *p <0.05 vs. CHOL and CORT. #p <0.05 vs. CHOL and mifepristone. *#p <0.05 vs CHOL and CORT. AUC= area under curve.
3.4. Basal and novel stress-induced HPA axis response in CVS animals
Next, we tested the necessity of NTS GR for inhibiting CVS-induced HPA axis activity. We measured the resting morning plasma ACTH and corticosterone levels of the pellet implanted rats after the termination of the CVS regimen. Exposure to CVS significantly elevated both the resting AM plasma ACTH (t (18) = 2.06; p < 0.05)) (Figure. 4 A) and corticosterone levels in animals with mifepristone micropellets in the NTS as compared to CHOL implanted rats (t (18) = 2.39; p < 0.05)) (Figure. 4 B). These results suggest that GR in the NTS is necessary for basal inhibition of the HPA axis after CVS. In addition, there were significant effects of drug (F 1, 99 = 8.22; p < 0.05) and time (F 4, 99 = 132.53; p < 0.05) on corticosterone responses to a 30 min novel restraint, as well as a significant drug X time interaction (F 4, 99 = 10.75; p < 0.05). Mifepristone micropellet in the NTS potentiated plasma corticosterone levels at the 15 min time point compared to animals with control micropellets (p < 0.05) (Figure. 4 C). However, the integrated post-restraint stress corticosterone response did not differ between groups (CHOL=39408.02±2007; mifepristone= 39193.36±3526). As in experiment 1, NTS “misses” did not differ significantly in plasma corticosterone responses (data not shown), thus providing additional verification that these responses are specific to NTS GR signaling. Collectively, these data support the hypothesis that GR in the NTS plays a role in inhibiting stress-induced glucocorticoid release.
Figure 4.
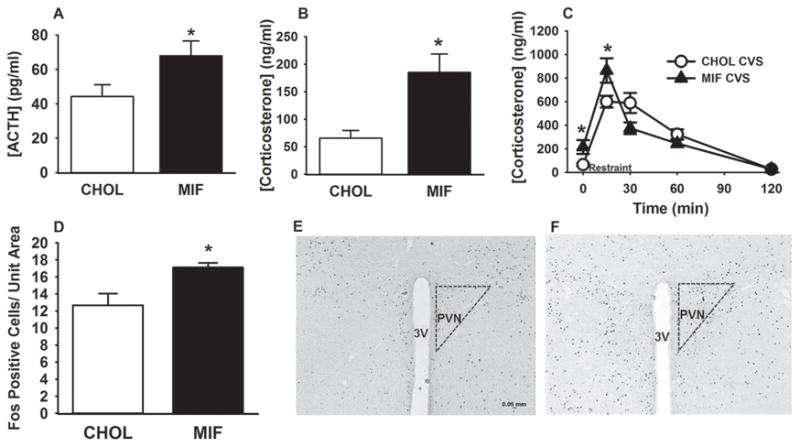
Increased HPA axis activity and PVN activation in the mifepristone-implanted rats after CVS. (A) After CVS, NTS mifepristone-implanted rats showed increased basal AM ACTH levels. (B) GR inhibition in the NTS increased basal corticosterone secretion in CVS exposed rats. (C) Exposure to CVS elevated plasma corticosterone response at 15min after onset of restraint in mifepristone micropellets-implanted animals. (D-F) NTS GR inhibition significantly increased novel stress-induced FOS immunoreactivity within the PVN. (E-F) Example image of Fos immunoreactivity in CHOL (E) and mifepristone (F) animals. Data are presented as mean ± SEM. n= 8 (mifepristone implanted CVS rats), n=12 (CHOL implanted CVS rats). *p <0.05 vs. CHOL CVS rats. 3V= third ventricle, Scale bar in figure 4 E also applies to figure 4 F.
3.5. PVN Fos immunoreactivity
To determine whether changes in the HPA axis response in the CVS-exposed rats are associated with increased activation of PVN neurons, Fos positive cells were counted within the PVN as an indicator of PVN neuronal activation following a novel restraint stress (Figure. 4 D, E, F). We found a significant increase in the average number of Fos positive nuclei per unit area in the PVN in response to acute restraint challenge in the mifepristone pellet-implanted rats relative to CHOL rats (t(12)=3.43; p<0.05) (Figure 4. D, E, F), suggesting that NTS GR inhibition increases acute stress-induced activation of PVN neurons following CVS.
3.6. Elevated plus maze
We used the elevated plus maze (EPM) to assess anxiety-like behavior. We found that there was no significant difference in the amount of time spent in open arm between CHOL (30.0 ±6.15 seconds) and mifepristone (26.3 ±4.98 seconds) micropellet-implanted rats or their total locomotor activity (CHOL=2016.78±254.0 mm; mifepristone=2213.83±168.2 mm). However, there was a significant reduction in the total number of entries into the open arm (t (18) = 3.08; p < 0.05)) (Figure. 5 A) and time spent in stretch attend postures (t (18) = 2.8; p < 0.05)) (Figure. 5 B) in the mifepristone micropellet-implanted rats. These behavioral data suggest reduced exploratory responses in response to the novel context (Carobez and Bertoglio, 2005). Thus, the NTS GR appears to play a role in anxiety-like behavior in the EPM.
Figure 5.
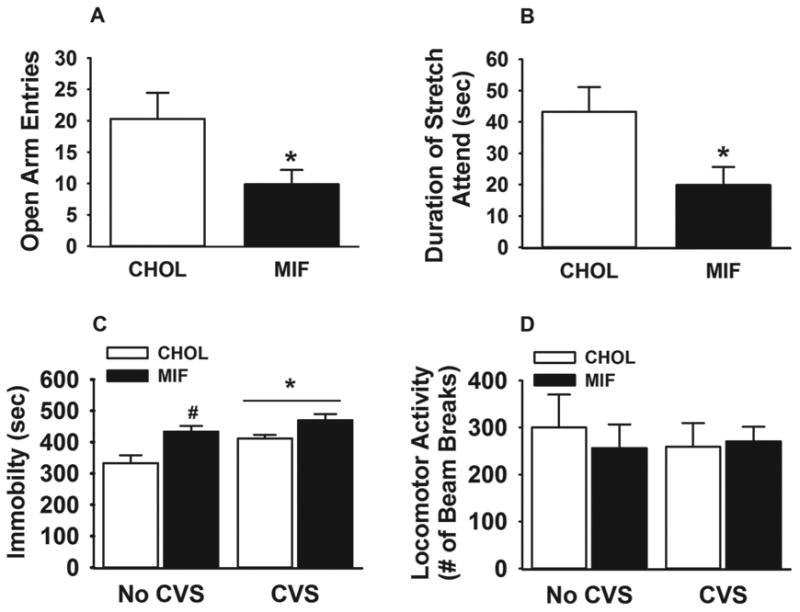
NTS GR inhibition decreases open arm exploration in the EPM and increases helplessness behavior in FST. (A-B) Animals with bilateral mifepristone micropellets in NTS exhibited fewer entries into the open arm of the EPM, and spent less time stretch attending from closed to open arms from closed arm. (C) Both chronic stress exposure and NTS GR inhibition alone increased immobility in the FST. (D) Overall locomotor activity was not affected by bilateral mifepristone implants in the NTS or chronic stress exposure. Data are presented as mean ± SEM. n= 8 (mifepristone implanted CVS rats), n=12 (CHOL implanted CVS rats), n=8 (mifepristone implanted no CVS rats), n=12 (CHOL implanted no CVS rats). *p <0.05 vs. CHOL-implanted rats in figure 5 A and B. #p <0.05 vs. no CVS cholesterol in figure 5 C with a main effect of stress.
3.7. Forced swim test
We also assessed the role of GR within the NTS in regulating depression-like behavior using forced swim test. As expected, CVS significantly increased immobility time (F 1, 39 = 10.47; p < 0.05) (Figure. 5 C), and decreased time spent in active behaviors (F 1, 39 = 14.45; P < 0.05) (Supplementary Figure. 1) in the FST. Moreover, there was a significant main effect of drug (F 1, 39 = 12.02; p < 0.05). Post hoc analyses of immobility duration revealed a differential effect of NTS mifepristone on immobility in control versus CVS-exposed rats. In the case of control (no CVS) rats, NTS mifepristone micropellets significantly elevated immobility duration and decreased active behaviors, suggesting that in animals with no history of stress NTS GR inhibition facilitates depression-like behavior (p < 0.05) (Figure. 5 C). In contrast, there was no significant effect of mifepristone in rats with a history of CVS in further increasing immobility behavior beyond CVS-exposed cholesterol-implanted rats (Figure. 5 C). Importantly, there was no difference in general homecage locomotor activity as measured by the number of beam breaks between treatment or stress group (Figure. 5 D), suggesting that changes in FST mobility are not a product of motor deficits. These behavioral data indicate that GR in the NTS limits depressionlike responses to the FST.
4. Discussion
The results of this study demonstrate that GR signaling within the NTS region encompassing PVN-projecting neurons is involved in inhibitory regulation of stress-induced HPA axis activity and attenuates anxiety- and depression-like behavior. Specifically, we show that inhibition of GR in the NTS enhanced both peak and integrated corticosterone responses after acute restraint stress, whereas activation of GR in the NTS decreased peak corticosterone response to acute restraint stress. Following chronic stress exposure, rats with mifepristone pellets implanted in the NTS had increased basal HPA axis activity and enhanced responsiveness to a novel stressor, which was accompanied by increased Fos expression in the PVN. Moreover, inhibition of NTS GR increased immobility in the FST and decreased open arm exploratory behavior in the EPM. Collectively, our data support the hypothesis that NTS GR plays an inhibitory role in acute endocrine stress responses and reduces anxiety-like behavior to a novel stressor. Moreover, our data provide new evidence that the hindbrain plays a critical role in coordination of endocrine as well as anxiety- and depression-like behavioral responses, and may be involved in mitigating the impact of prolonged stress on the organism.
The role of NTS GR in the neuroendocrine and behavioral stress responses was assessed using local application of CORT or selective GR antagonist, mifepristone, in the NTS. This method has been used in several prior studies targeting medial prefrontal cortex, the central amygdala, or bed nucleus of stria terminalis (Diorio et al., 1993; Sheperd et al., 2003; Sheperd et al., 2009; Myers and Greenwood-Van Meerveld, 2012), to investigate GR-mediated effects on endocrine and behavioral responses. Previous studies have also shown that this dose (30 μg) of CORT micropellet implant in brain regions does not alter the normal range of plasma corticosterone levels in unstressed rats (Sheperd et al., 2003). Micropellets, at a concentration of 30 μg have a diffusion radius of approximately 750 μm (Sheperd et al., 2003), thus defining the spread of steroid from the injection site. Additionally, mifepristone (30 μg) micropellets is effective in blocking behavioral effects of corticosterone (Myers and Greenwood-Van Meerveld, 2007), thus confirming the efficacy of the method for generating physiological/behavioral effects. Our results also indicate no significant differences between ‘NTS misses’ and CHOL implanted rats on HPA axis activity, providing support that effects of CORT and mifepristone micropellet implants observed in this study are specific to NTS GR signaling, and not due to diffusion into the adjacent areas or cerebrospinal circulation. Moreover, both CORT and mifepristone micropellet implants in the NTS did not alter the morning basal plasma corticosterone, as would have been expected if CORT or mifepristone were diffusing into peripheral circulation (Akana et al., 1992; Scheuer et al., 2004). While mifepristone also has anti-progestin actions (Philibert et al., 1985), prior studies suggest that NTS effects are mediated primarily by the GR (Scheuer and Bechtold, 2002). Moreover, our data indicate that NTS CORT micropellets oppose the effects observed following mifepristone administration, strengthening our conclusion that mifepristone is selectively inhibiting glucocorticoid-induced effects. Note that the NTS also expresses MR (Herman, 1993), and thus our results do not address a possible role for MR in stress responses.
Previous studies from our group demonstrated that CVS causes numerous physiological changes, including decreases in body weight gain (Herman et al., 1995), adrenal hypertrophy/hyperplasia due to increased ACTH exposure (Ulrich-Lai et al., 2006; Tauchi et al., 2008; Flak et al., 2009), and thymic atrophy due to increased glucocorticoid levels (Tauchi et al., 2008; Flak et al., 2009). In agreement with the previous reports, the week long CVS protocol used in this study also generated attenuated body weight gain, adrenal hypertrophy/hyperplasia and thymic involution. Moreover, the CVS rats implanted with mifepristone showed a further decrease in percent body weight gain and an increase in adrenal weights relative to the CVS cholesterol-implanted rats, suggesting that GR inhibition in the NTS exacerbates impact of stress on these physiological endpoints. Our finding is in agreement with a previous study reporting that blockade of hindbrain GR using mifepristone increased adrenal weights and attenuated body weight gain in rats following repeated restraint (Bechtold et al., 2009). Also as reported earlier (Bechtold et al., 2009), an increase in normalized adrenal weights is observed in the mifepristone- implanted rats with no prior history of chronic stress, suggesting that NTS GR inhibition can cause modest adrenal hypertrophy/hyperplasia. Together, these data are consistent with our hypothesis that inhibition of NTS GR alters major physiological indices of stress.
Ascending NTS projections are critical for relaying cardiovascular, inflammatory and nociceptive stimuli to the PVN. Prior studies suggest that NTS projections are not required for regulation of psychogenic stress responses (e.g., footshock, swim); however, more recent studies have cast some doubts on this conclusion, given that 1) DSAP lesion of the NTS reduces the corticosterone response to acute restraint (Daubert et al., 2012) and 2) restraint-induced Fos immunoreactivity in the PVN neurons is decreased after ibotenate lesion of NTS (Dayas et al., 2001). Our data further suggest that ascending activation of the HPA axis by the NTS is subject to regulation by glucocorticoids, presumably as a feedback mechanism to decrease excitatory drive to the PVN.
Pharmacological blockade of NTS GR signaling decreases open arm entries and time spent in stretch-attend posture towards open arms without altering the total locomotor activity in the maze. Previous studies report that increased open arm avoidance is an indication of anxiety-related behaviors in rats (Carobez and Bertoglio, 2005). Thus, the present data suggest that local blockade of the NTS GR increases the magnitude of the anxiety-like behavior as indicated by decreased exploration to the open arm in the EPM. Previous studies link HPA axis dysregulation with the development of depression-like behavior (Chrousos and Gold, 1992). Consistent with previous reports, our results demonstrate that a history of prior stress significantly increased immobility duration and decreased active behaviors in cholesterol pellet-implanted rats as compared to the no CVS cholesterol group. In addition, previous reports from our laboratory and others suggest a differential effect of GR signaling on depression-like behavior depending upon the routes of administration and targeted brain regions. For example, disruption of GR signaling in the forebrain or infralimbic prefrontal cortex increases immobility in the forced swim test, indicative of increased depression-like behavior in rodents (Solomon et al., 2012; McKlveen et al., 2013), whereas administration of mifepristone in the dentate gyrus of hippocampus or peripherally decreases immobility (de Kloet et al., 1988; Wulsin et al., 2010). Interestingly, our study demonstrates that inhibition of GR in the NTS by mifepristone increases immobility behavior in the forced swim test, suggesting that GR signaling in the NTS attenuates depressionlike behavior. Notably, within the CVS group, mifepristone did not further increase immobility duration. This may be due to the fact that prolonged stress exposure robustly increases immobility in the FST, and thus GR blockade is not sufficient to further increase the depressionlike behavior. Together, our results are in agreement with previous reports of increased depression-like behavior following chronic stress, and provide new data linking ascending NTS pathways with behavioral despair.
In conclusion, we have provided evidence that GR signaling in NTS is involved in the inhibition of HPA axis response to stress and attenuates anxiety- and depression-related behaviors. These results identify the NTS as a site for glucocorticoid inhibition of stress responses, and lend support to the notion that glucocorticoid feedback inhibition of the HPA axis is a distributed process involving multiple brain regions. Moreover, our data suggest that the NTS plays a prominent role in regulating endocrine and behavioral stress reactivity, and may contribute to pathologies associated with dysfunctional stress regulation. While the circuits controlling engagement of the NTS remain to be established, it is important to note that this region receives input from the infralimbic cortex and central amygdaloid nucleus (Schwaber et al., 1982; Hurley et al., 1991), which are involved in coordinating emotional responses to stressors. Moreover, NTS neurons project heavily to key stress-responding regions such as basolateral amygdala (Roozendaal et al., 1999) and bed nucleus of the stria terminalis (Ricardo and Koh, 1978), which in turn are also interconnected (Myers et al., 2013a) (Figure. 6). Thus, based on this diversity of output and input connections of the NTS, it is likely that the NTS may be part of a distributed circuit that drives general emotional reactivity, including both endocrine and behavioral responses.
Figure 6.
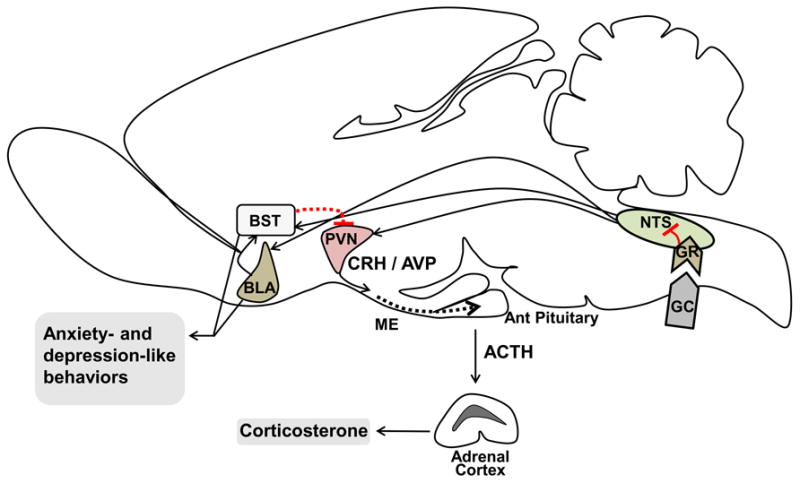
Potential neural circuitry recruited for NTS GR-mediated endocrine and behavioral effects. Our study shows that GR within the NTS is necessary to inhibit endocrine and behavioral responses to stress. Previous work has shown that glucocorticoid infusion in NTS modulates basolateral amygdala (BLA) mediated functions (Roozendaal et al., 1999). In addition, the NTS sends direct projections to the PVN and the bed nucleus of the stria terminalis (BST) (Ricardo and Koh, 1978). The BST also receives projection from BLA (Myers et al., 2013a), and in turn innervates the PVN. Thus, it is possible that the stress-induced effects of NTS GR are mediated predominantly by the PVN, along with other forebrain stress regulatory regions including the BLA and BST. However, future studies are needed to test this potentially recruited pathway.
ME= Median Eminence, Ant Pituitary= Anterior Pituitary, CRH= Corticotropin-Releasing Hormone, AVP= Arginine Vasopressin, GC= Glucocorticoid, GR= Glucocorticoid receptor
Supplementary Material
Acknowledgments
This research work is supported by US National Institute of Health Grant MH069860 to James P. Herman. Brent Myers is supported by American Heart Association Grant 13POST17070152. Sriparna Ghosal is supported by American Heart Association Predoctoral Fellowship # 13PRE17100141. The authors thank Jessica McKlveen and Dr. Matia Solomon for their helpful comments with the preparation of the manuscript, Benjamin Packard, Brittany Kopp, Dayna Wick for their help with blood and tissue collection, and Brad Chambers for help with behavioral testing.
Funding Sources: This research work is supported by US National Institute of Health Grant MH069860 to James P. Herman. Brent Myers is supported by American Heart Association Grant 13POST17070152. Sriparna Ghosal is supported by Ryan Foundation award.
Footnotes
The authors declare no financial or personal conflicts of interest.
Author's contribution: SG designed and performed experiments, interpreted data, and wrote and edited the manuscript.
JB performed experiments and edited the manuscript.
CMD performed experiments.
BM designed experiments, interpreted data, wrote and edited the manuscript.
JPH designed experiments, interpreted data, wrote and edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akana SF, Scribner KA, Bradbury MJ, Strack AM, Walker CD, Dallman MF. Feedback sensitivity of the rat hypothalamo-pituitary-adrenal axis and its capacity to adjust to exogenous corticosterone. Endocrinology. 1992;131:585–594. doi: 10.1210/endo.131.2.1322275. [DOI] [PubMed] [Google Scholar]
- Bechtold AG, Patel G, Hochhaus G, Scheuer DA. Chronic blockade of hindbrain glucocorticoid receptors reduces blood pressure responses to novel stress and attenuates adaptation to repeated stress. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1445–54. doi: 10.1152/ajpregu.00095.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Scribner KA, Bradbury MJ, Walker CD, Strack AM, Cascio CS. Stress, feedback and facilitation in the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol. 1992;4:517–526. doi: 10.1111/j.1365-2826.1992.tb00200.x. [DOI] [PubMed] [Google Scholar]
- Daubert DL, McCowan M, Erdos B, Scheuer DA. Nucleus of the solitary tract catecholaminergic neurons modulate the cardiovascular response to psychological stress in rats. J Physiol. 2012;590:4881–4895. doi: 10.1113/jphysiol.2012.232314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Medullary neurones regulate hypothalamic corticotropin-releasing factor cell responses to an emotional stressor. Neuroscience. 2001;105:707–719. doi: 10.1016/s0306-4522(01)00213-5. [DOI] [PubMed] [Google Scholar]
- Dekloet ER, Dekock S, Schild V, Veldhuis HD. Antiglucocorticoid Ru-38486 Attenuates Retention of a Behavior and Disinhibits the Hypothalamic-Pituitary Adrenal Axis at Different Brain Sites. Neuroendocrinology. 1988;47:109–115. doi: 10.1159/000124900. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak JN, Ostrander MM, Tasker JG, Herman JP. Chronic stress-induced neurotransmitter plasticity in the PVN. J Comp Neurol. 2009;517:156–165. doi: 10.1002/cne.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak JN, Solomon MB, Jankord R, Krause EG, Herman JP. Identification of chronic stress-activated regions reveals a potential recruited circuit in rat brain. Eur J Neurosci. 2012;36:2547–2555. doi: 10.1111/j.1460-9568.2012.08161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S, Myers B, Herman JP. Role of central glucagon-like peptide-1 in stress regulation. Physiol Behav. 2013;122:201–207. doi: 10.1016/j.physbeh.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikström AC, Okret S, Yu ZY, Goldstein M, Steinbusch H, Verhofstad A, Gustafsson JA. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad Sci USA. 1986;83:9779–9783. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP. Regulation of adrenocorticosteroid receptor mRNA expression in the central nervous system. Cell Mol Neurobiol. 1993;13:349–372. doi: 10.1007/BF00711577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991;11:585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, Sarabdjitsingh RA, Karst H. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacol Rev. 2012;64:901–938. doi: 10.1124/pr.112.005892. [DOI] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schütz G, Joëls M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal; glutamate transmission by corticosterone. Proc Natl Acad Sci USA. 2005;102:19204–7. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A, Aguilera G. Regulation of the hypothalamic pituitary adrenal axis during chronic stress: responses to repeated intraperitoneal hypertonic saline injection. Brain Res. 1993;630:262–270. doi: 10.1016/0006-8993(93)90665-a. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- McKlveen JM, Myers B, Flak JN, Bundzikova J, Solomon MB, Seroogy KB, Herman JP. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol Psychiatry. 2013;74:672–679. doi: 10.1016/j.biopsych.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B, Greenwood-Van Meerveld B. Corticosteroid receptor-mediated mechanisms in the amygdala regulate anxiety and colonic sensitivity. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1622–1629. doi: 10.1152/ajpgi.00080.2007. [DOI] [PubMed] [Google Scholar]
- Myers B, Greenwood-Van Meerveld B. Differential involvement of amygdale corticosteroid receptors in visceral hyperalgesia following acute or repeated stress. Am J Physiol Gastrointest Liver Physiol. 2012;302:G260–266. doi: 10.1152/ajpgi.00353.2011. [DOI] [PubMed] [Google Scholar]
- Myers B, Mark Dolgas C, Kasckow J, Cullinan WE, Herman JP. Central stress-integrative circuits: forebrain glutamatergic and GABAergic projections to the dorsomedial hypothalamus, medial preoptic area, and bed nucleus of the stria terminalis. Brain Struct Funct. 2013a:1–17. doi: 10.1007/s00429-013-0566-y. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B, McKlveen JM, Herman JP. Glucocorticoid actions on synapses, circuits, and behavior: Implications for the energetics of stress. Front Neuroendocrinol. 2013b doi: 10.1016/j.yfrne.2013.12.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th Academic Press; San Diego, California: 1998. [Google Scholar]
- Pezzone MA, Lee WS, Hoffman GE, Pezzone KM, Rabin BS. Activation of brainstem catecholaminergic neurons by conditioned and unconditioned aversive stimuli as revealed by c-Fos immunoreactivity. Brain Res. 1993;608:310–318. doi: 10.1016/0006-8993(93)91472-5. [DOI] [PubMed] [Google Scholar]
- Philibert D, Moguilewsky M, Mary L, Lecaue D, Tournemine C, Secchi J, Deraedt R. Pharmacological profile of RU 486 in animals. In: Baulieu E, Segal SJ, editors. The Antiprogestin Steroid RU 486 and Human Fertility Control. New York: Plenum; 1985. pp. 49–68. [Google Scholar]
- Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–11. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol. 1999;277:R582–590. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol. 2011;300:R222–235. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Williams CL, McGaugh JL. Glucocorticoid receptor activation in the rat nucleus of the solitary tract facilitates memory consolidation: involvement of the basolateral amygdala. Eur J Neurosci. 1999;11:1317–1323. doi: 10.1046/j.1460-9568.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- Scheuer DA, Bechtold AG. Glucocorticoids modulate baroreflex control of heart rate in conscious normotensive rats. Am J Physiol Regul Integr Comp Physiol. 2002;282:R475–483. doi: 10.1152/ajpregu.00300.2001. [DOI] [PubMed] [Google Scholar]
- Scheuer DA, Bechtold AG, Shank SS, Akana SF. Glucocorticoids act in the dorsal hindbrain to increase arterial pressure. Am J Physiol Heart Circ Physiol. 2004;286:H458–467. doi: 10.1152/ajpheart.00824.2003. [DOI] [PubMed] [Google Scholar]
- Scheuer DA, Bechtold AG, Vernon KA. Chronic activation of dorsal hindbrain corticosteroid receptors augments the arterial pressure response to acute stress. Hypertension. 2007;49:127–133. doi: 10.1161/01.HYP.0000250088.15021.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaber JS, Kapp BS, Higgins GA, Rapp PR. Amygdaloid and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. J Neurosci. 1982;2:1424–1438. doi: 10.1523/JNEUROSCI.02-10-01424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA. Stereotaxic localization of corticosterone to the amygdala enhances hypothalamo-pituitary-adrenal responses to behavioral stress. Brain Res. 2003;963:203–213. doi: 10.1016/s0006-8993(02)03978-1. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Chambers CO, Busch C, Mount A, Schulkin J. Chronically elevated corticosterone in the dorsolateral bed nuclei of stria terminalis increases anxiety-like behavior. Behav Brain Res. 2009;203:146–149. doi: 10.1016/j.bbr.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Furay AR, Jones K, Packard AEB, Packard BA, Wulsin AC, Herman JP. Deletion of Forebrain Glucocorticoid Receptors Impairs Neuroendocrine Stress Responses and Induces Depression-Like Behavior in Males but. Not Females Neuroscience. 2012;203:135–143. doi: 10.1016/j.neuroscience.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchi M, Zhang R, D'Alessio DA, Seeley RJ, Herman JP. Role of central glucagon-like peptide-1 in hypothalamo-pituitary-adrenocortical facilitation following chronic stress. Exp Neurol. 2008;210:458–466. doi: 10.1016/j.expneurol.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Thompson VB, Heiman J, Chambers JB, Benoit SC, Buesing WR, Norman MK, Norman AB, Lipton JW. Long-term behavioral consequences of prenatal MDMA exposure. Physiol Behav. 2009;96:593–601. doi: 10.1016/j.physbeh.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006;291:E965–973. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D'Alessio DA, Herman JP. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 2005;289:E823–828. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- Wulsin AC, Herman JP, Solomon MB. Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology. 2010;35:1100–1112. doi: 10.1016/j.psyneuen.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


