Abstract
As a social species, humans are acutely aware of cues that signal inclusionary status. The present study characterizes behavioral and neural responses when individuals anticipate social feedback. Across two functional magnetic resonance imaging studies, participants (N = 42) made social judgments about supposed peers and then received feedback from those individuals. Of particular interest was the neural activity occurring when participants were awaiting social feedback. During this anticipatory period, increased neural activity was observed in the ventral striatum (VS), a central component of the brain’s reward circuitry, and dorsomedial prefrontal cortex (dmPFC), a brain region implicated in mentalizing about others. Individuals high in rejection sensitivity exhibited greater responses in both the VS and dmPFC when anticipating positive feedback. These findings provide initial insight into the neural mechanisms involved in anticipating social evaluations, as well as the cognitive processes that underlie rejection sensitivity.
Keywords: fMRI, social feedback, anticipation, dorsomedial prefrontal cortex, ventral striatum, rejection sensitivity, reward, mentalizing
To effectively navigate our complex social worlds, people must be attuned to social context and aware of social cues related to their status as group members (Heatherton, 2011). Evaluative information provided by others signals group inclusion status (Baumeister & Leary, 1995). Such cues are salient, emotionally evocative, and elicit a suite of neural responses distinct from nonsocial forms of feedback.
Importantly, feedback of this kind typically occurs within specific social contexts; being called into a colleague’s office after giving a presentation, hearing your phone beep with a text response from a potential date, or hearing the words “We need to talk” from a partner are a few examples in which evaluative feedback is imminent, but outcome information is not yet known. As such, humans often possess the capacity to predict the circumstances under which the receipt of social feedback is likely. How humans anticipate social encounters is a critical yet poorly understood aspect of our tendency to be attuned to social status. Moreover, there are likely to be individual differences in the extent to which people anticipate positive or negative evaluations from others. For instance, those high in rejection sensitivity anxiously expect negative social evaluations (Downey & Feldman, 1996) and those with high self-esteem show a positivity bias in expected interpersonal evaluations (Brockner & Lloyd, 1986). Here, we sought to characterize behavioral and neural responses when individuals anticipate social evaluation. We also examined how individual differences in rejection sensitivity modulated these responses.
The capacity to anticipate future positive and negative outcomes is a key mechanism underlying reinforcement learning. The ventral striatum (VS) has a primary role in associative learning between a cue and subsequent reward, and dopaminergic neurons in the mesolimbic dopamine circuit (including the VS) fire robustly when anticipating a predicted reward (Schultz, Dayan & Montague, 1997). This response is domain independent, as anticipating different types of rewards elicits the same pattern of activity (Schultz, 1998). Recently, it has been demonstrated that learning to associate peers with different probabilities of social acceptance through implicit learning drew on the same striatal mechanisms as those that link nonsocial cues with monetary reward (Jones et al., 2011; Korn et al., 2012; Lin, Adolphs & Rangel 2012). Thus, the VS plays a critical role in the prediction of rewarding social events, such as being liked by others (Davey, Allen, Harrison, Dwyer & Yucel, 2010). To the extent that anticipatory activity in the VS is a signal of reward expectation, we hypothesized that activity in this region should be heightened when anticipating the receipt of positive social feedback.
Effective social behavior requires the ability to understand the intentions and thoughts of those in our surrounding social worlds. This capacity to mentalize allows people to understand that they are the targets of social evaluation (Heatherton, 2011). Recently, we provided evidence (Powers, Wagner, Norris & Heatherton, 2013) that experiencing social evaluation, such as social exclusion, differentially engages dorsomedial prefrontal cortex (dmPFC), a central component of the neural systems that support mentalizing about social knowledge (Frith & Frith, 2001; Mitchell et al., 2002; Gallagher & Frith, 2003; Mitchell et al., 2006; Gobbini et al., 2007; Wagner et al., 2011). Specifically, social exclusion alters the processing of social cues such that dmPFC exhibits more activity when viewing positive compared to negative social information. Thus, dmPFC activity is influenced by momentary assessments of social relationship status, and reflects differential motivations for future social interactions. We therefore hypothesized that dmPFC should be similarly engaged when anticipating social evaluation, and activity in this region should be greater when positive feedback is expected.
To test these hypotheses, participants underwent fMRI scanning while making social judgments about and receiving feedback from supposed peers. Of particular interest was the neural activity that occurred when participants were awaiting social feedback, and whether this anticipatory activity differed as a function of whether the participants were expecting to receive positive or negative social feedback. In Study 1, participants rated images of prospective peers based on whether they thought they would like the peer; in Study 2, participants attempted to predict whether each peer would like the participant. Participants then received feedback indicating whether the prospective peer liked or disliked the participant. Importantly, both studies contained an anticipatory period between the participant’s judgments and the peers’ feedback (see Figure 1). To the extent that we expect the people we like to like us back (Condon & Crano, 1988; Newcomb, 1961), positive social evaluations (i.e., “I think I would like this person” [Study 1] and “I think this person would like me” [Study 2]) represented situations in which positive feedback was expected in return. We hypothesized that the VS and dmPFC would be selectively engaged when anticipating positive versus negative feedback.
Figure 1.
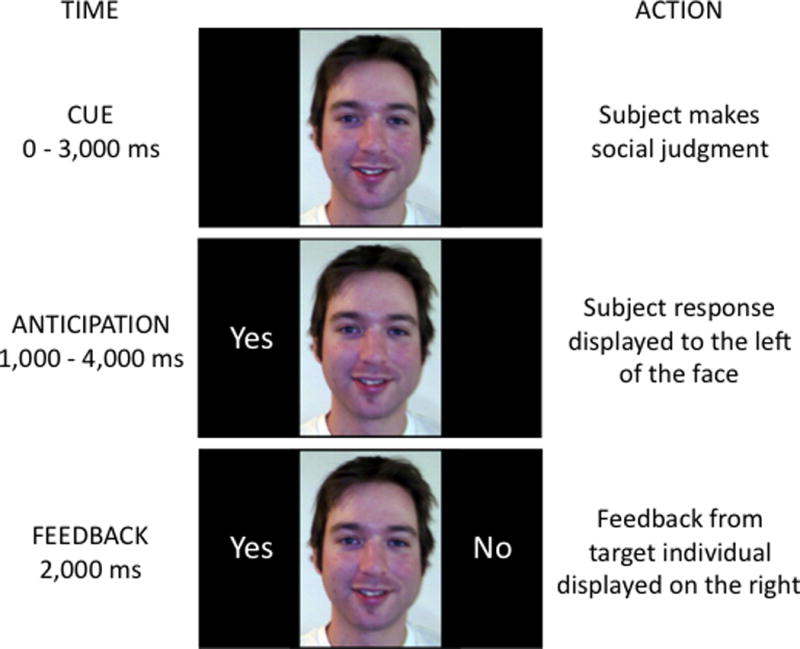
Representation of a complete experimental trial. Time indicates the duration of each subcomponent. The current findings focus on responses during the ANTICIPATION period, after participants have rendered social judgments about the pictured individuals while they are anticipating the receipt of social feedback.
Additionally, we examined how individual differences in rejection sensitivity (RS) modulate neural responses to the anticipation of positive and negative social feedback. Individuals high in RS are characterized by particularly anxious expectations of social evaluation, and these expectations ultimately undermine their interpersonal behaviors and relationships (Downey & Feldman, 1996; Ayduk, Mendoza-Denton, Mischel & Downey, 2000). Although the lack of involvement of prefrontal cognitive control regions is thought to contribute to the especially intense reactions characteristic of high RS individuals (Kross et al., 2007), how RS affects anticipatory neural responses is unknown. Thus, we further hypothesized that neural activity in VS and dmPFC when anticipating social evaluation would be modulated by individual differences in RS. Finally, we collected behavioral measures examining retrospective accounts of social evaluation experiences to test potential linkages between anticipatory processes and retrospective accounts of social evaluative experiences.
Method
Participants
Forty-eight participants (N = 42 final sample; 21 female; age range 18–24) were recruited from the local Dartmouth community. All participants were right-handed, native English speakers, had no history of neurological problems, and had normal or corrected-to-normal vision. They received course credit or were paid for their participation, and gave informed consent in accordance with the guidelines set by the Committee for the Protection of Human Participants at Dartmouth College.
Stimuli
Photographs of college-aged individuals were collected prior to the experiment from the mass media and Internet. A total of 400 photographs (200 male, 200 female) were selected. All photographs were closely cropped to the head and shoulders of the target face and did not contain any other faces in the background of the picture.
Pre-scan procedure
Approximately two weeks prior to fMRI scanning, participants came to the lab for a brief informational session, in which they were given a detailed cover story outlining the purpose of the study. Participants were told that they were participating in a multi-university study on how individuals make first impressions. They were told that participants’ photographs would be traded between participating universities and rated by the other participants from these schools. In this way, individuals from other universities had supposedly rated the faces of the current participants, and in turn, the current participants would rate these same individuals during fMRI scanning. To ensure believability, we took a photograph of each participant, which they believed would be sent to the other universities, and rated by the other participants prior to their fMRI scanning session. Following this session, their photographs were deleted and all ratings and face stimuli were created and compiled randomly by experimenters.
fMRI design and procedure
During fMRI scanning, complex trials were employed to determine unique neural responses to different processes within the reciprocal social evaluation trials (Ollinger, 2001; see Figure 1). During the cue period, participants viewed an individual face and answered the question, “Would I like this person?” (Study 1; 20 participants) or “Would this person like me?” (Study 2; 22 participants) via two possible button responses. Following the button press, the trial shifted to the anticipation period (1000–4000 msec; mean duration 2686.5 msec). During the anticipation period, the participant’s response was displayed on the left side of the screen, while the participant passively awaited feedback indicating whether the pictured individual supposedly liked or disliked the participant’s photograph. The trial then shifted to the feedback portion (2000 msec), in which social feedback, indicating the pictured individual’s impression of the participant, was presented to the right of the face.
Catch trials (partial trials that did not run to completion) were included so that unique estimates of the hemodynamic response function could be computed for each subcomponent of the trial (Ollinger, Corbetta & Shulman, 2001). Specifically, 20% of trials terminated after the cue period, and 20% of trials terminated after the anticipation period. The remaining 60% of trials ran to completion. In total, each participant completed 160 partial and 240 complete trials. To avoid confusion on the participant’s part, participants were told that if the experimenter had not yet received an evaluation from the potential peer, the trial would simply end. All trials were randomly intermixed with periods of fixation during which participants viewed a white cross-hair at the center of the screen (2000–6000 msec).
Faces were counterbalanced across participants to provide positive feedback to half of the participants and negative feedback to the other half. To accomplish this, five different counterbalanced orderings were used across participants, each with equal numbers of male and female faces in each condition. Feedback type was assigned by experimenters and occurred in a pseudorandomized order across runs.
Post-scan assessments
Following the fMRI scanning session, participants completed several personality questionnaires, including the Rejection Sensitivity Questionnaire (RSQ; Downey & Feldman, 1996). The RSQ contains 18 items that describe hypothetical situations (e.g., “You ask your boyfriend/girlfriend to move in with you”) in which participants are instructed to imagine themselves and subsequently report on their anxiety and expectations.
Participants were then given an exit questionnaire, where no participant reported that they did not believe the cover story. Moreover, when debriefed, all participants expressed surprise when told the cover story was a ruse. Also included on this questionnaire was an item that asked participants to retrospectively estimate the percentage of positive feedback they expected to receive (“Of the faces you rated, what percentage did you think would like you?”).
fMRI parameters and analysis
Structural and functional whole-brain imaging was performed on a 1.5 Tesla General Electric Signa scanner at Dartmouth College (General Electric Medical Systems, Milwaukee, WI). Visual stimuli were generated using PsyScope software (Cohen, MacWhinney, Flatt, & Provost, 1993) and presented using an LCD projector (Epson model ELP-7000), viewable by an angled mirror mounted on top of the head coil.
A T-1 weighted high-resolution anatomical image was acquired using a 3-D spoiled gradient sequence (SPGR; 124 sagittal slices, TE = 6 ms, TR = 25 ms, flip angle = 25°, 1 × 1 × 1.2 mm voxels). Functional images were collected in four functional runs of 338 time points each, using a gradient spin-echo, echo-planar sequence sensitive to blood-oxygen level-dependent contrast (T2*) (20 axial slices per whole-brain volume, 3.75 mm in-plane resolution, 5 mm thickness, 1 mm skip, TR = 2000 ms, TE = 35 ms, flip angle = 90°).
Neuroimaging data were preprocessed and analyzed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK). First, functional data were preprocessed using a standard routine that corrected for differences in slice acquisition time, realigned data within and across functional runs to correct for head movement, and unwarped to reduce residual movement-related image distortions not corrected by realignment. Functional data were then normalized into standard space (3mm isotropic voxels) based on the SPM8 EPI template that conforms to the ICBM 152 brain template (Montreal Neurological Institute, MNI). Finally, normalized data were spatially smoothed using a 6mm full-width-at-half-maximum Gaussian kernel. Participants with more than 2 mm total movement in any plane (n = 6) were excluded (final N = 42).
A general linear model (GLM) incorporating seven task regressors (cue, anticipation-after like, anticipation-after dislike, and four types of feedback – congruent-like, congruent-dislike, incongruent-like, incongruent-dislike), and covariates of non-interest (a session mean, a linear trend, and six movement parameters derived from realignment corrections) was specified for each participant. Catch trials were fully incorporated into the cue, anticipation-after like, and anticipation-after dislike regressors. Each trial onset was modeled as a stick function convolved with a canonical hemodynamic response function (HRF). For participants who had more than twelve trials to which they did not register a button response during the cue period (Study 1: n = 5, Study 2: n = 5), no-response trials were modeled as an additional task regressor that was not further analyzed.
Although the neural response was estimated separately for the cue, anticipation, and feedback periods, here we focus on activity during the anticipation period, when participants were awaiting evaluative social feedback after rendering their own social judgments. Specifically, we compared neural activity during the anticipation period as a function of the social judgments participants made (like or dislike) about their supposed peers in the preceding cue period. To this end, parameter estimates from first-level models were used to generate contrast images (weighted parameter estimates) for each participant comparing activity in the anticipation period following like judgments compared to dislike judgments. We then conducted a region of interest (ROI) analysis by extracting parameter estimates (β) for each participant from our a priori defined brain regions of interest (VS, defined anatomically, and dmPFC, defined functionally) and submitting them to offline statistical analyses. To ensure independence in ROI identification, the VS ROI was anatomically defined (anatomical mask from the Harvard-Oxford structural atlas, as implemented in FSL; http://www.fmrib.ox.ac.uk) and the dmPFC ROI was functionally defined from an independent data set (from Powers et al., 2013; MNI coordinates 6, 54, 21; 6mm sphere).
To supplement the targeted analyses, Table 1 summarizes the results of a whole brain, random effects analysis comparing regions displaying greater activity in the anticipation period following like judgments compared to dislike judgments (FWE corrected, p < .05, k > 20). Feedback-related neural activity has been reported elsewhere (Somerville et al., 2006; 2010) and will not be presented here.
Table 1.
Brain regions demonstrating greater activation following like judgments compared to dislike judgments
| Region | Coordinates | t-value | voxels | ||
|---|---|---|---|---|---|
| x | y | z | |||
| vACC | −6 | 42 | −6 | 6.9 | 33 |
| ventral striatum | 6 | 15 | −3 | 7.78 | 51 |
| medial prefrontal cortex | 6 | 42 | −3 | 7.78 | 132 |
| thalamus | 15 | −18 | 9 | 10.72 | 170 |
| middle frontal gyrus | −24 | 21 | 60 | 6.83 | 51 |
| motor/sensory cortex | 42 | −21 | 57 | 15.94 | 2298 |
| cerebellum | −18 | −54 | −24 | 16.96 | 558 |
| cuneus | 30 | −87 | 24 | 6.82 | 33 |
| visual association cortex | −51 | −84 | −3 | 9.49 | 168 |
Notes: Coordinates are reported in Montreal Neurological Institute (MNI) stereotaxic space. FWE corrected, p < .05, k > 20. vACC = ventral anterior cingulate cortex
Results
Behavioral results
RSQ
Scores on the Rejection Sensitivity Questionnaire ranged from 2.83 to 15.5 (possible range: 1 to 36). A median split (median = 8.39) was performed to identify individuals high and low in RS. The mean RSQ score for low RS individuals (n = 21) was 6.1 (SD = 1.7) and the mean score for the high RS individuals (n = 21) was 11.1 (SD = 2.5).
Reaction times
When making social judgments about the pictured individuals during the cue period, participants were faster to make like judgments (M = 1302.8 ms, SD = 221.7) than dislike judgments (M = 1343.1 ms, SD = 219.0), F(1,40) = 7.9, p = .008. There was also a judgment by RS interaction, F(1,40) = 6.2, p = .017, such that low RS individuals were quicker to make like judgments (p = .001), but this difference was not evident for high RS individuals (p = .82).
Expectations of being liked
To examine how RS influenced expectations of being liked by others, an independent samples t-test was performed on the participant generated estimates of liking expectations collected from the post-scan exit questionnaire (i.e., responses to “Of the faces you rated, what percentage did you think would like you?”). High RS individuals reported that they had expected to be liked less often (M = 51.9 percent, SD = 18.6) than low RS individuals (M = 64.8 percent, SD = 12.8), t(35.52) = 2.6, p = .012. Levene’s test indicated unequal variances (F = 7.5, p = .009), so degrees of freedom were adjusted from 40 to 35.52. When compared to the actual percentage of positive feedback provided to participants (50%), the percentage of positive feedback expected by high RS individuals did not differ from the actual proportion (p = .7), while low RS individuals significantly overestimated how often they would be liked by others (p < .001).
fMRI results
There were no statistical differences in neural activity during the anticipatory period in VS or dmPFC as a function of which question participants had responded to during the cue period (“Would I like this person?” [Study 1] or “Would this person like me?” [Study 2]), evidenced by a lack of main effects of Study (all ps > .2) or interactions involving Study (all ps > .7). Therefore, all data presented here represent statistical contrasts including data from all 42 participants. Moreover, pooling data across studies importantly improved the statistical power to detect effects, particularly in the individual difference analyses.
BOLD response in the VS
A mixed-model ANOVA was performed on parameter estimates extracted from the VS with RS (high, low) as a between-subjects factor and anticipation condition (expecting positive feedback versus negative feedback) as a within-subjects factor. There was a main effect of anticipation condition, such that the VS was preferentially engaged when participants anticipated positive social feedback, F(1,40) = 25.1, p < .001 (left VS); F(1,40) = 44.9, p < .001 (right VS). Moreover, the left VS showed a RS by anticipation interaction, F(1,40) = 6.2, p = .017, such that the VS response to anticipating positive versus negative social feedback was more pronounced in high RS individuals. Indeed, when considered within each group, VS activity during the anticipation of positive versus negative social feedback was significantly different only for the high RS individuals (p < .001) (low RS individuals: p = .08) (see Figure 2A). The interaction between RS and anticipation condition was not significant in the right VS, F(1,40) = 2.8, p = .103, although a similar overall pattern of activity was evident.
Figure 2.

A). Results from ROI analysis revealing a RS by anticipation condition interaction in the VS (p = .017). The VS was more engaged when high RS individuals anticipated positive than negative social feedback, whereas this distinction was less pronounced for low RS individuals. Inset displays location of left VS ROI (anatomical mask from the Harvard-Oxford structural atlas). B). Results from ROI analysis revealing a RS by anticipation condition interaction in dmPFC (p = .011). Again, high RS individuals revealed a more polarized response when anticipating positive versus negative social feedback. Inset displays location of dmPFC ROI (defined from Powers et al., 2013; MNI coordinates 6, 54, 21). Bars indicate standard error of the mean. RS = rejection sensitivity.
Next, we separately tested whether the VS was engaged relative to baseline for each anticipation condition. One-sample t-tests revealed that activity in both the left and right VS was significantly greater than baseline when anticipating positive social feedback (both ps < .001) but not when anticipating negative social feedback (both ps > .5).
Past research has linked physical attractiveness to changes in VS activity (Cloutier, Heatherton, Whalen & Kelley, 2008). To ensure that observed differences in VS activity were not confounded with differences in attractiveness, photographs were rated for attractiveness using a 1 (very unattractive) to 7 (very attractive) scale by a separate group of participants (N=45; 22 female). For each participant, we separately calculated the average attractiveness of faces participants were expecting positive feedback from and those they were expecting negative feedback from, and included this difference score (positive – negative) as a covariate for each participant. This did not alter the significance of any of the reported VS effects.
BOLD response in dmPFC
A similar mixed-model ANOVA was performed on signal change in dmPFC with RS as a between-subjects factor and anticipation condition as a within-subjects factor. Like the VS, a main effect of anticipation was evident, such that dmPFC was preferentially engaged when participants anticipated positive social feedback, F(1,40) = 7.4, p = .01. Moreover, paralleling the pattern of activity observed in the VS, results revealed a RS by anticipation condition interaction, F(1,40) = 7.1, p = .011. Pairwise comparisons revealed that high RS individuals showed a greater response when anticipating positive versus negative social feedback (p < .001); low RS individuals did not (p = .97) (see Figure 2B).
We separately determined whether dmPFC was engaged relative to baseline for each anticipation condition. One-sample t-tests revealed that dmPFC was active when anticipating both positive (p < .001) and negative (p < .001) social feedback.
Specificity of RS effects
To determine whether these effects are specific to the anticipation condition, we conducted similar analyses to examine the influence of RS on neural responses to receiving positive and negative feedback (i.e., the feedback period). Although the VS was engaged bilaterally when receiving positive versus negative feedback (left VS: F(1,40) = 28.8, p < .001; right VS: F(1,40) = 28.04, p < .001), there was no significant RS by feedback interaction in either the left VS (p = .2) or right VS (p = .09). The dmPFC was also preferentially engaged when receiving positive feedback, F(1,40) = 4.0, p = .05, but this effect was not modulated by RS (p = .7). That RS did not modulate neural responses when receiving social feedback provides evidence of the specificity of these effects to the neural processes involved during anticipation.
Supplementary whole-brain analysis
To supplement analyses of our a priori ROIs, we have included results of a whole brain, random effects analysis comparing regions displaying greater activity in the anticipation period following like judgments compared to dislike judgments (see Table 1).
Discussion
In the current study, we examined neural and behavioral responses when individuals were anticipating social feedback from supposed peers. We observed increased neural activity in VS and dmPFC when feedback was expected to be positive. Individual differences in RS produced an increased neural sensitivity when anticipating positive feedback, as well as biased retrospective accounts of social feedback experiences.
RS biased retrospective estimates of receiving positive feedback, such that high RS individuals reported expecting to be liked by other people less often. Anxious expectations of social evaluation, which are characteristic of high RS individuals (Downey & Feldman, 1996), contribute to a predisposition to readily perceive rejection in a variety of social cues and situations, even if ambiguous or relatively benign (Downey & Feldman, 1996; Ayduk et al., 2000). Such a persistent expectation of negative social evaluation likely contributes to the general state of increased awareness and reactivity exhibited by high RS individuals within evaluative situations. We note the possibility that this retrospective estimate is not a difference in expectation, but rather reflects a more general memory bias for past social encounters.
High RS individuals were also more accurate in their expectations of being liked. This pattern is broadly consistent with prior work demonstrating that individuals with low self-esteem more accurately estimate how often other people will like them (Somerville et al., 2010), and that depressed patients are more realistic in self-perceptions than non-psychiatric controls (Lewinsohn, Mischel, Chaplin & Barton, 1980). Our findings contribute to this collective literature by providing another example in which individuals especially sensitive to social evaluation demonstrate a more accurate understanding of their own social reality.
Our fMRI results show that the VS was engaged when anticipating positive social feedback. Animal and human studies have identified the VS as a critical component of the neural circuitry underlying the anticipation of rewards across various domains (Schultz 1998; 2002, Knutson, Adams, Fong & Hommer, 2001; O’Doherty, Deichmann, Critchley, & Dolan, 2002). The ability to predict the occurrence of rewards allows for behaviors to be modified in the interest of ultimately obtaining these rewards (Schultz et al., 1997). Our findings extend the function of the VS by demonstrating that the VS signals the occurrence of situations in which social rewards may be obtained. Involvement of the same neural mechanisms that underlie motivational behaviors directed at obtaining primary rewards such as food and sex suggests that existing neural systems that enable basic contingency learning and reward detection subserve reward seeking behaviors in other (i.e., social) domains.
We also observed engagement of dmPFC when anticipating positive social feedback. dmPFC was also active when anticipating negative feedback relative to baseline, albeit to a lesser degree than positive feedback. That anticipation of both positive and negative feedback recruits dmPFC is suggestive of a broader role for dmPFC in anticipating social evaluation. The capacity subserved by this brain region to understand the thoughts and intentions of others (Frith & Frith, 2001; Mitchell et al., 2002; Gallagher & Frith, 2003; Hooker et al., 2008; Rameson et al., 2012) is critical for effectively navigating social encounters (Heatherton, 2011). Here, we show that dmPFC is recruited in circumstances when evaluative feedback is forthcoming, especially when that feedback is expected to be positive. One speculation is that people engage attributional processes during anticipation in an effort to understand the intentions of others in their surrounding social world. These findings are consistent with prior work investigating anticipation of social interactions more generally. Specifically, Walter and colleagues (2004) demonstrated involvement of dmPFC when participants were imagining others in upcoming social interactions. Such increased mentalizing efforts during anticipation may function to improve understanding of complex social situations and aid in generating appropriate social behaviors.
Individuals high in RS demonstrated a particular neural sensitivity when anticipating positive social feedback. Specifically, in both the VS and dmPFC, we observed a more polarized response when anticipating positive feedback relative to negative feedback for the high RS individuals. High RS people tend to expect rejection from known others, and these expectations are derived from associations learned over time (Downey & Feldman, 1996). Consistent with this theoretical framework, prior work has primarily focused on the role of RS in relationships with significant others (e.g., romantic partners, family members, close friends). In contrast, the current study involves discrete social encounters with unknown peers. In novel social situations with unknown others, relational associations are not established, thus the potential for inclusion and acceptance may remain intact. That RS is rooted in consistent experiences of interpersonal rejection over time (Downey & Feldman, 1996) suggests that situations in which social acceptance is expected are uncommon for high RS individuals. Prior work has demonstrated engagement of the VS in novel situations in which rewarding stimuli may be present (Horvitz, 2000; Schultz, 2002). From this perspective, expecting social acceptance represents a novel context containing infrequent social rewards for high RS individuals, and this novelty is reflected in heightened VS activity. It may be important for future work on RS to clarify whether the patterns of neural activity observed here persists across multiple social contexts, including anticipating feedback from a known other.
The pattern of neural activity evident when anticipating social feedback mirrors the neural responses observed during other instances of social cognition, notably cooperation and social reciprocity. Prior work has shown that the VS is recruited when individuals experience instances of mutual cooperation (Rilling et al., 2002), engage in interactions with trustworthy partners (Phan, Sripada, Angstadt & McCabe, 2010), and receive fair treatment from others (Tabibnia & Lieberman, 2007). dmPFC activity has also emerged as a reliable predictor of prosocial behaviors, such as making donations to family members (Telzer et al., 2011) and acts of altruism (Waytz, Zaki & Mitchell, 2012). That we have demonstrated involvement of these same neural regions in the current study is perhaps not surprising, as the reciprocal nature of a social exchange closely resembles instances of cooperative behavior. In the current study, participants rendered social judgments of supposed peers, and then awaited feedback indicating the social impressions from those peers. Similarly, when an individual makes a decision to cooperate, she must then wait to see whether or not her partner will cooperate or not. Prosocial behaviors, such as cooperation and reciprocity, have proved advantageous in sustaining social relationships (Rilling et al., 2002). The involvement of the same brain regions when anticipating social evaluation suggests that similar neural mechanisms exist to facilitate effective navigation of upcoming social encounters. To the extent that expectancies direct attention (Olson et al., 1996) and facilitate learning, neural activity across these domains may broadly reflect an allocation of resources to where they might be the most socially profitable in upcoming social situations.
The current study also highlights the important interplay of reward and mentalizing systems in social behavior. Recently, Korn and colleagues (2012) demonstrated that both VS and dmPFC are involved in receiving social feedback, and specifically, in positively biasing self-relevant feedback. That is, the combination of both reward and mentalizing neural signals mediate how social feedback is processed and integrated into one’s self-concept. By demonstrating that both of these neural systems are likewise involved in anticipating self-relevant positive social feedback, we further underscore the importance of considering both neural signals related to reward and mentalizing in understanding dynamic interpersonal behavior.
Across two experiments, we have demonstrated that anticipating evaluative feedback is characterized by contributions from neural systems involved in reward and mentalizing, and that high RS individuals have behaviorally and neurally distinct expectations of positive and negative social evaluation. As a social species, humans are acutely aware of social cues that signal group inclusion, and how we anticipate evaluative social encounters is an important aspect of our tendency to be attuned to social status. Our beliefs about whether we are valued and liked by others are vital aspects of the human experience and have profound effects on our social relations. Individual differences in these beliefs are associated with a range of social impairments (Downey & Feldman, 1996). These findings provide insight into the neural mechanisms involved in anticipating social evaluations as well as the cognitive processes that underlie rejection sensitivity.
Acknowledgments
Funding
This research was supported by grants from the National Institute of Mental Health R01MH059282 (T.F.H) and R00MH087813 (L.H.S).
References
- Ayduk O, Mendoza-Denton R, Mischel W, Downey G. Regulating the interpersonal self: Strategic self-regulation for coping with rejection sensitivity. Journal of Personality and Social Psychology. 2000;79:776–792. doi: 10.1037//0022-3514.79.5.776. [DOI] [PubMed] [Google Scholar]
- Berenson KR, Gyurak A, Ayduk O, Downey G, Garner MJ, Mogg K, Bradley BP, Pine DS. Rejection sensitivity and disruption of attention by social threat cues. Journal of Research in Personality. 2009;43:1064–1072. doi: 10.1016/j.jrp.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockner J, Lloyd K. Self-esteem and likeability: Separating fact from fantasy. J Res Pers. 1986;20:496–508. [Google Scholar]
- Cloutier J, Heatherton TF, Whalen PJ, Kelley WM. Are attractive people rewarding? Sex differences in the neural substrates of facial attractiveness. Journal of Cognitive Neuroscience. 2008;20(6):941–951. doi: 10.1162/jocn.2008.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. Psyscope: A new graphic interactive environment for designing psychology experiments. Behavioral Research Methods, Instruments, and Computers. 1993;25:257–271. [Google Scholar]
- Condon JW, Crano WD. Inferred evaluation and the relation between attitude similarity and interpersonal attraction. Journal of Personality and Social Psychology. 1988;54(5):789. [Google Scholar]
- Davey CG, Allen NB, Harrison BJ, Dwyer DB, Yücel M. Being liked activates primary reward and midline self-related brain regions. Human Brain Mapping. 2009;31(4):660–668. doi: 10.1002/hbm.20895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey G, Feldman SI. Implications of rejection sensitivity for intimate relationships. Journal of Personality and Social Psychology. 1996;70:1327–1343. doi: 10.1037//0022-3514.70.6.1327. [DOI] [PubMed] [Google Scholar]
- Downey G, Mugious V, Ayduk O, London B, Shoda Y. Rejection sensitivity and the defensive motivational system: Insights from the startle response to rejection cues. Psychological Science. 2004;15:668–673. doi: 10.1111/j.0956-7976.2004.00738.x. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith C. The biological basis of social interaction. Current Directions in Psychological Science. 2001;10(5):151–155. [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends in Cognitive Psychology. 2003;7(2):77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV. Two takes on the social brain: A comparison of theory of mind tasks. Journal of Cognitive Neuroscience. 2007;19(11):1803–1814. doi: 10.1162/jocn.2007.19.11.1803. [DOI] [PubMed] [Google Scholar]
- Heatherton TF. Neuroscience of self and self-regulation. Annual Review of Psychology. 2011;62:363–390. doi: 10.1146/annurev.psych.121208.131616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Germine LT, Knight RT, D’Esposito M. Mentalizing about emotion and its relationship to empathy. Social Cognitive and Affective Neuroscience. 2008;3(3):204–217. doi: 10.1093/scan/nsn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96(4):651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Jones RM, Somerville LH, Li J, Ruberry EJ, Libby V, Glover G, Casey BJ. Behavioral and neural properties of social reinforcement learning. The Journal of Neuroscience. 2011;31(37):13039–13045. doi: 10.1523/JNEUROSCI.2972-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Mischel W, Chaplin W, Barton R. Social competence and depression: the role of illusory self-perceptions. Journal of Abnormal Psychology. 1980;89(2):203–212. doi: 10.1037//0021-843x.89.2.203. [DOI] [PubMed] [Google Scholar]
- Lin A, Adolphs R, Rangel A. Social and monetary reward learning engage overlapping neural substrates. Social Cognitive and Affective Neuroscience. 2012;7(3):274–281. doi: 10.1093/scan/nsr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21(16):1–5. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn CW, Prehn K, Park SQ, Walter H, Heekeren HR. Positively Biased Processing of Self-Relevant Social Feedback. The Journal of Neuroscience. 2012;32(47):16832–16844. doi: 10.1523/JNEUROSCI.3016-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Egner T, Ochsner K, Hirsch J, Downey G. Neural dynamics of rejection sensitivity. Journal of Cognitive Neuroscience. 2007;19(6):945–956. doi: 10.1162/jocn.2007.19.6.945. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Heatherton TF, Macrae CN. Distinct neural systems subserve person and object knowledge. Proceedings of the National Academy of Sciences. 2002;99(23):15238–15243. doi: 10.1073/pnas.232395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50(4):655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Newcomb TM. The acquaintance process. New York, NY: Holt, Rinehart & Winston; 1961. [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Olson JM, Roese NJ, Zanna MP. Expectancies. In: Higgins ET, Kruglanski AW, editors. Social psychology: Handbook of basic principles. New York, NY: The Guilford Press; 1996. pp. 211–238. [Google Scholar]
- Phan KL, Sripada CS, Angstadt M, McCabe K. Reputation for reciprocity engages the brain reward center. Proceedings of the National Academy of Sciences. 2010;107(29):13099–13104. doi: 10.1073/pnas.1008137107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak J, Downey G, Ayduk O. Rejection sensitivity as an interpersonal vulnerability. In: Baldwin M, editor. Interpersonal cognition. 2005. pp. 62–84. [Google Scholar]
- Powers KE, Wagner DD, Norris CJ, Heatherton TF. Socially excluded individuals fail to recruit medial prefrontal cortex for negative social scenes. Social, Cognitive and Affective Neuroscience. 2013;8(2):151–157. doi: 10.1093/scan/nsr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameson LT, Morelli SA, Lieberman MD. The neural correlates of empathy: Experience, automaticity, and prosocial behavior. Journal of Cognitive Neuroscience. 2012;24:235–245. doi: 10.1162/jocn_a_00130. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysio. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9(8):1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Kelley WM, Heatherton TF. Self-esteem modulates medial prefrontal cortical responses to evaluative social feedback. Cerebral Cortex. 2010;20:3005–3013. doi: 10.1093/cercor/bhq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand DG, Greene JD, Nowack MA. Spontaneous giving and calculated greed. Nature. 2012;489(7416):427–430. doi: 10.1038/nature11467. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Gutman DA, Zeh TR, Pagnoni G, Berns GS, Kilts CD. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Sanfey AG, Aronson JA, Nystrom LE, Cohen JD. Opposing BOLD responses to reciprocated and unreciprocated altruism in putative reward pathways. Neuroreport. 2004;15:2539–2543. doi: 10.1097/00001756-200411150-00022. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, Lieberman MD. Fairness and cooperation are rewarding: Evidence from social cognitive neuroscience. New York Academy of Sciences. 2007;1118:90–101. doi: 10.1196/annals.1412.001. [DOI] [PubMed] [Google Scholar]
- Telzer EH, Masten CL, Berkman ET, Lieberman MD, Fuligni AJ. Neural regions associated with self control and mentalizing are recruited during prosocial behaviors towards the family. Neuroimage. 2011;58:242–249. doi: 10.1016/j.neuroimage.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi E, Rangel A, Camerer CF, O’Doherty JP. Neural evidence for inequality-averse social preferences. Nature. 2010;463:1089–1091. doi: 10.1038/nature08785. [DOI] [PubMed] [Google Scholar]
- Wagner DD, Kelley WM, Heatherton TF. Individual differences in the spontaneous recruitment of brain regions supporting mental state understanding when viewing natural social scenes. Cerebral Cortex. 2011;21:2788–2796. doi: 10.1093/cercor/bhr074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, Adenzato M, Ciaramidaro A, Enrici I, Pia L, Bara BG. Understanding intentions in social interaction: the role of the anterior paracingulate cortex. Journal of Cognitive Neuroscience. 2004;16(10):1854–1863. doi: 10.1162/0898929042947838. [DOI] [PubMed] [Google Scholar]
- Waytz A, Zaki J, Mitchell JP. Response of dorsomedial prefrontal cortex predicts altruistic behavior. Journal of Neuroscience. 2012;32(22):7646–7650. doi: 10.1523/JNEUROSCI.6193-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Mitchell J. Equitable decision making is associated with neural markers of intrinsic value. PNAS. 2011;108:19761–19766. doi: 10.1073/pnas.1112324108. [DOI] [PMC free article] [PubMed] [Google Scholar]


