Abstract
Calcyon regulates activity dependent internalization of AMPA glutamate receptors and long term depression of excitatory synapses. Elevated levels of calcyon are consistently observed in brains from schizophrenic patients, and the calcyon gene is associated with attention deficit hyperactivity disorder. Executive function deficits are common to both disorders, and at least for schizophrenia, the etiology appears to involve both heritable and neurodevelopmental factors. Here, we show with calcyon overexpressing CalOE transgenic mice that lifelong calcyon upregulation impairs executive functions including response inhibition and working memory, without producing learning and memory deficits in general. As response inhibition and working memory, as well as the underlying neural circuitry continue to mature into early adulthood, we functionally silenced the transgene during postnatal days 28–49, a period corresponding to adolescence. Remarkably, the response inhibition and working memory deficits including perseverative behavior were absent in adult CalOE mice with the transgene silenced in adolescence. Suppressing the calcyon transgene in adulthood only partially rescued the deficits, suggesting calcyon upregulation in adolescence irreversibly alters development of neural circuits supporting mature response inhibition and working memory. Brain regional immunoblots revealed a prominent down-regulation of AMPA GluR1 subunits in hippocampus, and GluR2/3 subunits in hippocampus and prefrontal cortex of the CalOE mice. Silencing the transgene in adolescence prevented the decrease in hippocampal GluR1, further implicating altered frontohippocampal connectivity in the executive function deficits observed in the CalOE mice. Treatments that mitigate the effects of high levels of calcyon during adolescence could preempt adult deficits in executive functions in individuals at-risk for serious mental illness.
Keywords: schizophrenia, ADHD, mice, fear conditioning, watermaze, working memory, executive functions
Introduction
The exertion of voluntary cognitive control via response inhibition and working memory facilitates goal-directed behavior, and is key to mature decision making. Working memory entails the ability to retain task relevant information 'on-line,' in order to make a planned, goal-directed response, whereas response inhibition involves suppressing a conditioned tendency or habit in order to make a task appropriate response1. Peak performance in both types of cognitive control is attained much later in life than for declarative or reference memory2, 3. For example, while infants older than six months can suppress attention to a 'distractor' stimulus in order to produce a task-appropriate response, their rate of correct inhibitory responses continues to improve until mid- to late adolescence, at which point performance approximates adult levels4. Performance on spatial working memory tasks demanding cognitive flexibility similarly increases into adulthood5, 3. Brain imaging studies indicate that agerelated improvement in working memory and response inhibition correlates with increased activity in a highly distributed neural circuitry including both cortical and subcortical regions1, 6. However, little is known about the molecular or genetic mechanisms involved, although it is well established that adolescence is characterized by massive elimination of excitatory synapses in prefrontal cortex (PFC)7, 8, 9.
Deficits in response inhibition and working memory manifest as a variety of abnormal behaviors including reduced flexibility in decision making, incessant rumination, impulsiveness, hyperactivity, as well as the inability to suppress emotionally disturbing memories. Such maladaptive behaviors are observed in a number of psychiatric illnesses including bipolar disorder, depression, attention deficit hyperactivity disorder (ADHD), schizophrenia, and post-traumatic stress disorder (PTSD), even when general cognitive deficits are absent. Given the late maturation of executive functions, it is perhaps not coincidental that these psychopathologies often emerge or intensify during adolescence10. Although these illnesses exhibit heritability, substantive evidence has yet to emerge to support a role for any gene or genetic pathway as a causal factor in executive function maturation during adolescence.
Calcyon is a particularly promising candidate in this regard for a number of reasons including studies implicating the calcyon gene in schizophrenia11–15 and bipolar disorder16, 17. Additionally, calcyon is required for activity-dependent internalization of α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) glutamate receptors and long term depression (LTD)18, a form of synaptic plasticity associated with synapse elimination and circuit remodeling19–21. The elevated calcyon levels associated with schizophrenia22–25 would be expected to decrease excitatory transmission, consistent with findings suggestive of glutamatergic hypofunction in the etiology or pathophysiology of schizophrenia26, 27. Expression of calcyon is also significantly upregulated in the spontaneous hypertensive rat (SHR), currently the best studied rodent model of ADHD28, 29. Indeed, genome-wide scans, association studies and case studies point to q26, where the calcyon locus resides on chromosome 10 as the site of a susceptibility gene for ADHD30, 31.
We tested the hypothesis that calcyon upreglation impairs response inhibition and working memory by assessing these executive functions in CalOE transgenic mice, a murine model of elevated calcyon expression in brain as observed in schizophrenia. Response inhibition was measured using the Pavlovian paradigm of fear extinction after rapid aversive learning via contextual fear conditioning (CFC)32, and spatial working memory with the Morris water maze33. Expression of the human calcyon transgene in the CalOE mice is dependent on CaMKIIα-tTA driver expression34. CaMKIIα, like endogenous calcyon is primarily expressed in the principle cells of forebrain nuclei35–38. Transgenic calcyon shows a similar expression pattern39.
Fear extinction behavior and working memory are developmentally regulated in rodents, as they are in humans40, 2. Therefore, we also asked whether calcyon plays a neurodevelopmental role by silencing the transgene during adolescence or in adulthood with the tTA protein inhibitor, doxycline (Dox).
Similarly, as excess synaptic pruning and/or abnormal synaptic development have been implicated in the etiology of diseases involving executive function deficits41, we also assessed the effects of calcyon upregulation on AMPA receptors in well-established nodes of the fear extinction/working memory neural circuitry.
Materials and Methods
Subjects
All animal procedures were approved by the institutional IACUC committee. The subjects were αCamKII-tTA (tTA)34 (gift of Dr. T. Abel, U. Penn) single transgenic and TRE-Cal/tTA (CalOE) double transgenic mice bred from matings of tTA and TRE-Cal single transgenic parents, and identified by PCR of genomic DNA isolated from mouse tails39. Prior to carrying out the present studies, the TRE-Cal and tTA mice were back-crossed to C57Bl/6 for eight generations. Mice were housed 2–3 per cage with food and water freely available on a 12:12 hr light-dark cycle, lights on at 7:00 am. All mice were kept with mothers for at least 3 weeks after birth, and the dams provided with nesting material (cotton batting). Doxycycline (20 mg/ml) was administered ad libitum via tinfoil-wrapped standard cage bottles filled with a 5% sucrose solution in water, renewed 3 times a week42.
Behavioral Procedures
Mice were handled for a week before the behavioral training. All training was done between 11:00 am and 5:00 pm. All behavior was videotaped and analyzed by an experimenter blinded to the animal’s genotype.
Contextual Fear Conditioning (CFC)
The apparatus was 260-mm long, 30 mm at the floor and 85 mm at the top. The floor metal plates were separated by 5 mm. Each mouse was allowed to explore the apparatus for 5 min. On the following day, after a 45-second habituation period in the apparatus, each mouse received 3 footshocks, 0.75 mA (Fig. 1&3) and 0.5 mA (Fig. 4), 45 seconds apart, and were removed 45 seconds after the last footshock. Freezing behavior, identified as immobility except that needed for breathing, was recorded for each 45-second period. Extinction was started 24 hrs after CFC training, by placing mice in the apparatus for 5 min/day for 4 days; no footshocks were delivered. The first day of extinction training (Day 1) was also used as a measure of CFC memory.
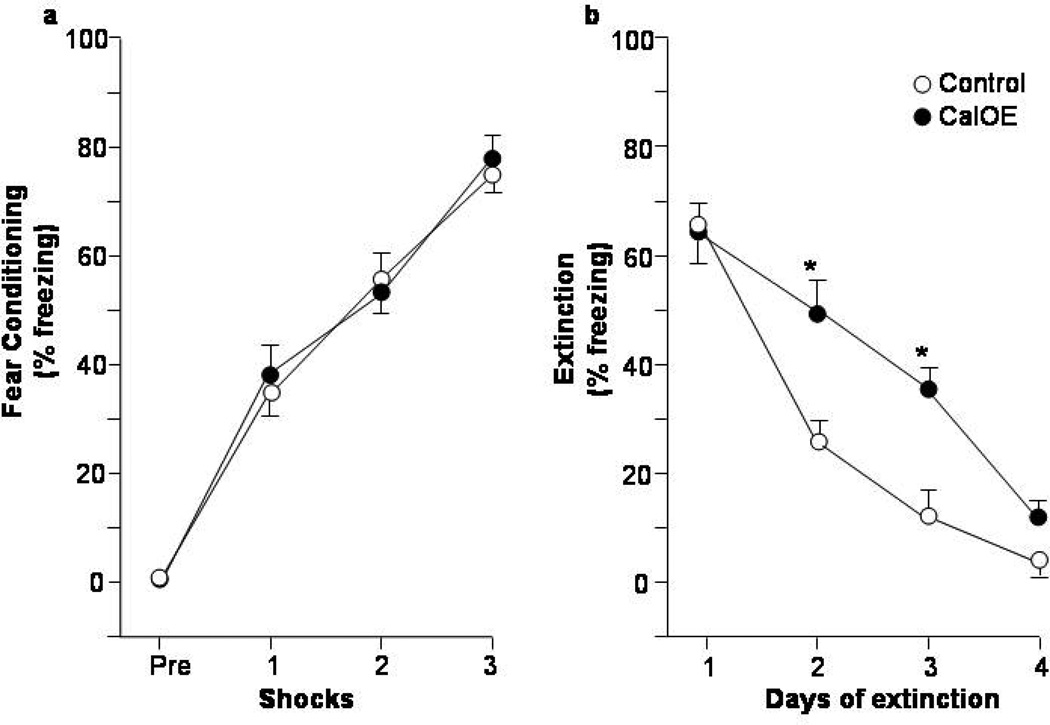
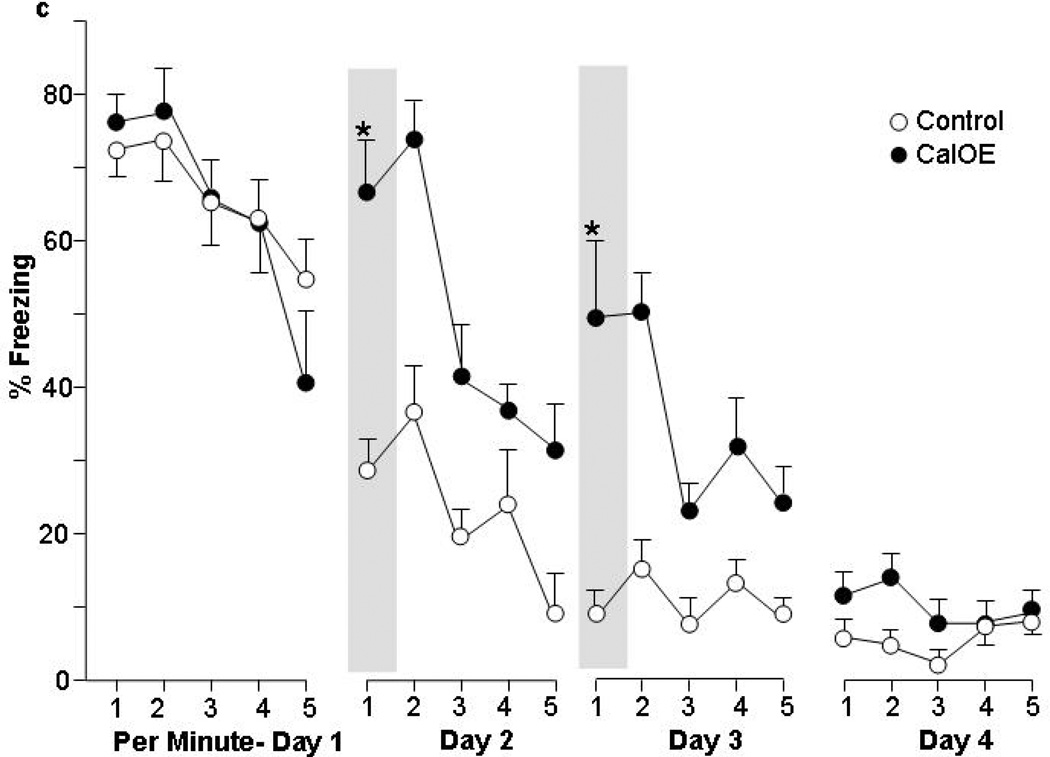
Figure 1. CalOE mice are impaired in fear extinction after contextual fear conditioning.
Percent of time exhibiting freezing behavior during: (a) acquisition of contextual fear conditioning (CFC), and daily CFC extinction training (b) as a whole, as well as (c) on a per minute basis. * p<0.01 compared to control mice.
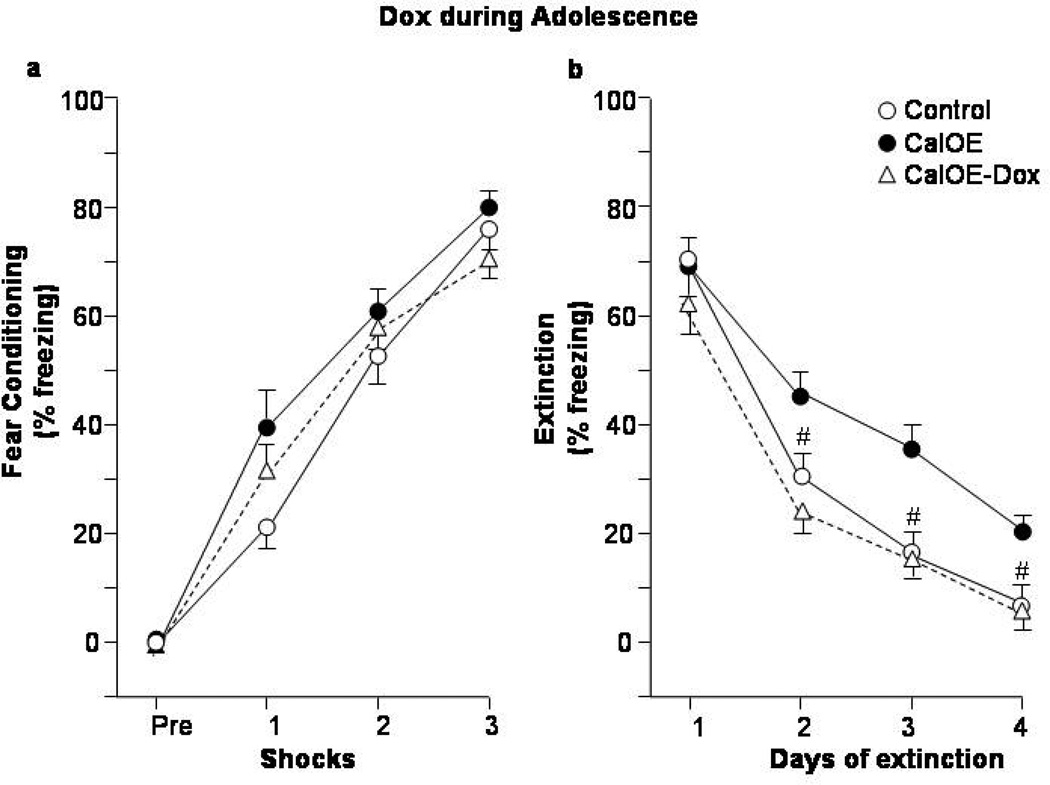
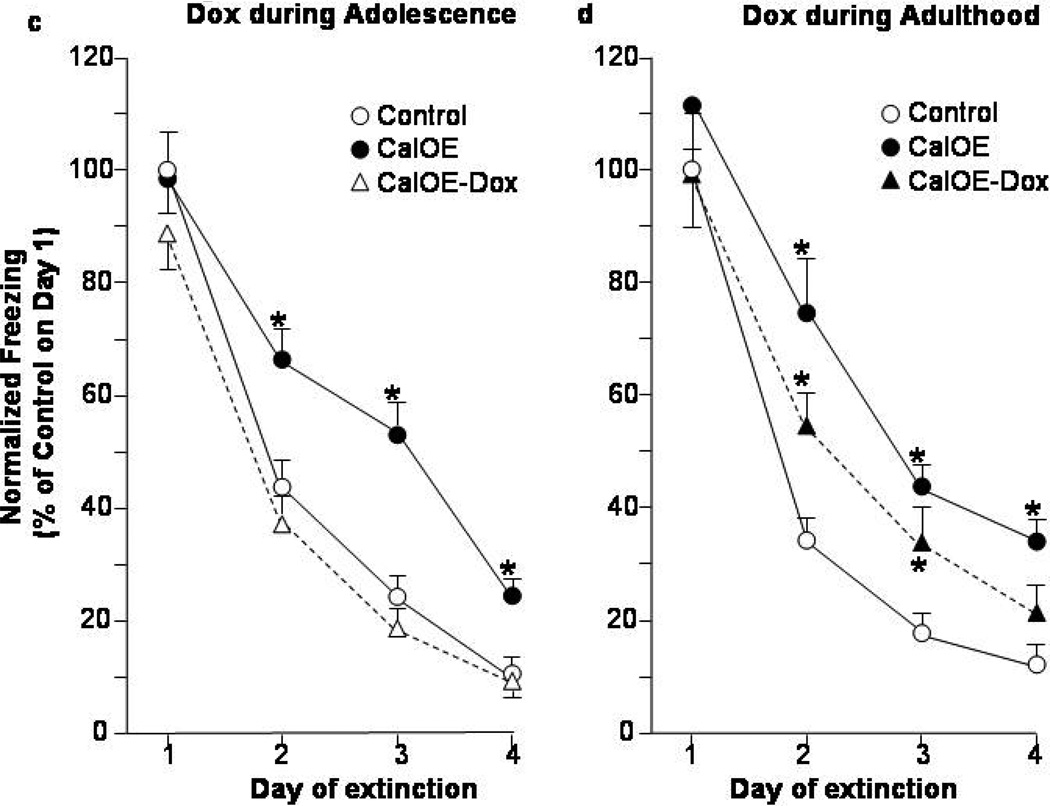
Figure 3. Dox treatment during adolescence preempts fear extinction deficits in adult CalOE mice.
Percent of time spent exhibiting freezing behavior during (a) CFC acquisition; and (b) daily CFC extinction training. CalOE -Dox mice, are CalOE mice that received Dox from postnatal days 28–49. # p< 0.01 compared to the CalOE groups.
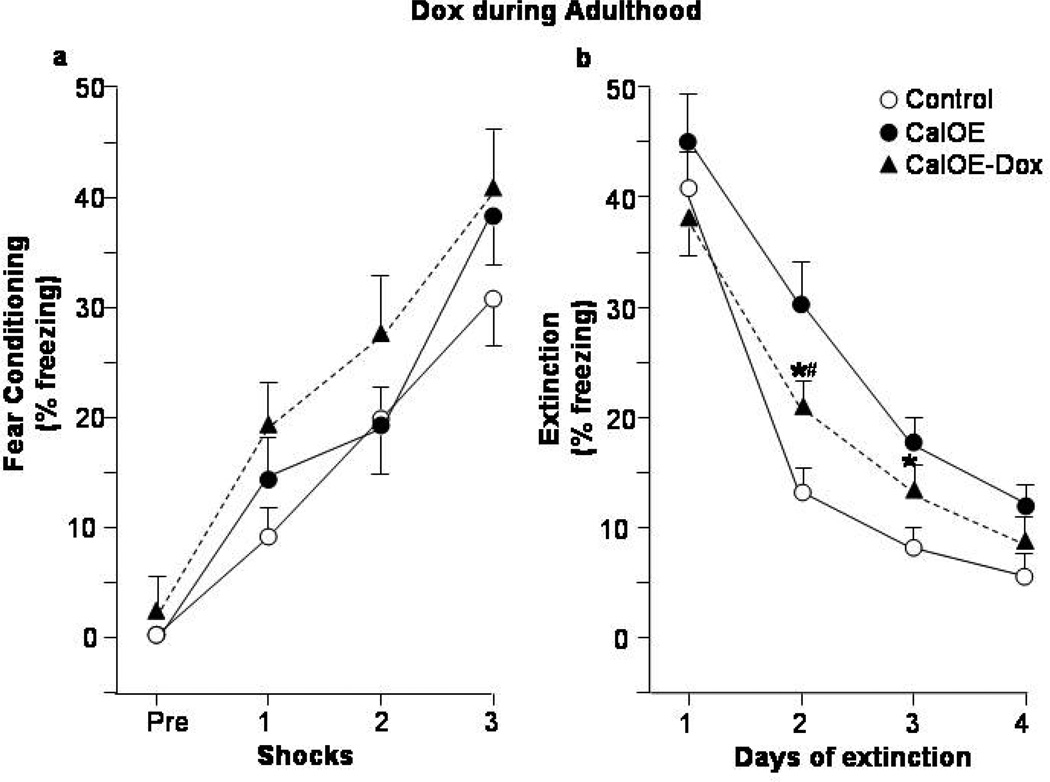
Figure 4. Dox treatment in adulthood partially rescues fear extinction deficits in adult CalOE mice.
Freezing behavior during: (a) CFC acquisition, and (b) daily CFC extinction learning and expression. CalOE -Dox mice are CalOE mice treated with Dox for 3 weeks in adulthood as shown in Fig. 2a. Daily CFC extinction training normalized to that of the controls on Day 1: (c) Dox in adolescence and (d) adulthood studies. * p< 0.01 compared to the control group; # p= 0.01 for CalOE vs. CalOE.
Watermaze task
The apparatus was a tank, 120 cm in diameter. It was surrounded with walls on three sides on which salient extramaze cues were mounted. Titanium dioxide in the water made the submerged square platform (12 cm) not visible from the surface. The water temperature was 23 °C and the platform was submerged at 11 cm below the surface in one of 4 fixed locations. The center of each location was 23 cm off the wall and equidistant from the two closest starting points. The initial training consisted of 5 daily sessions of 3 trials per day. At the beginning of the training, each mouse was placed on the platform for 30 seconds and the trials were initiated 30 seconds later. Each trial consisted of releasing a mouse into the watermaze from one of 4 starting locations in a pseudorandom order allowing it to swim to Location 1 until it mounted the platform and remained on it for 10 sec. Latency to reach the platform was scored with a maximum of 60 seconds. The inter-trial interval was 30 seconds. The reversal training consisted of 4 daily sessions (3 trials/day). All parameters were kept the same, except that the platform was moved to Location 2 which was diametrically opposite to Location 1.
Spatial memory test
Assessing spatial memory was done with probe tests conducted 2 days after the end of initial training (probe test 1) and reversal training (probe tests 2&3, Supplemental figure 2). During a probe test, all procedures were the same as during training, except that the platform was removed. Performance was evaluated by measuring time spent in the target and opposite zones, proximity to the target location, initial latency to the target and the opposite platform locations, and number of crossings of these locations. Target and opposite zones were defined as circles (17 cm in radius) centered on the center of the respective platform locations. These zones covered 1/12th (8%) of the watermaze surface. Analysis was done with EthoVision XT 7 (Noldus, Leesburg, VA).
Working memory training
A week after the second probe test, the mice were subjected to three days of delayed match-to-sample training (4 trials/day) according to the following protocol: 1) on each trial the submerged platform was placed in one of three fixed locations none of which was Location 2; 2) a platform of the same size was placed over the submerged platform, such that it was just above the water; 3) a mouse was placed on the above-water platform for 10 seconds; 4) the mouse was picked up from the above-water platform and that platform was removed (the submerged platform stayed in place); 5) after a 10-second delay period the mouse was released into the pool from a constant starting point and allowed 30 seconds to locate the platform. The performance of each mouse was evaluated by scoring the number of errors before reaching the current trial target area. An error was scored when the mouse swam through an area that was not a target for the current trial. Each area was defined as a circle with a 17 cm radius centered on the center of the respective platform position. As the area around location Location 2 was never a target it was never reinforced. The mice tested for spatial reference memory and working memory had completed fear extinction testing two weeks earlier.
Immunohistochemistry
Mice were anesthetized with isoflurane and the brains were quickly flash-frozen in 2-methyl butane (Sigma). Twenty mm-thick sections containing the dorsal hippocampus (HPC) and prefrontal cortex (PFC) were captured on slides and used for immunohistochemistry as described previously37. The anti-human calcyon antibody (Ab) was affinity purified polyclonal rabbit Ab (1:100)38. This antibody does not detect endogenous mouse calcyon39. Rabbit polyclonal or murine monoclonal antibodies specific for GluR1 and GluR2/3 were used at 1:50. The secondary antibodies were biotinylated anti-rabbit and anti-mouse Abs (1:600, Vector Laboratories, Burlingame, CA). The signal from the secondary Abs was amplified with an ABC kit and visualized with cyanine 3 (CY3) TSA fluorescence system (PerkinElmer Life Sciences). Nuclei were counterstained with SYTOX Green (Invitrogen) or DAPI (Invitrogen) nucleic acid stains. Specificity of the staining was established by incubating control slides without either the primary or secondary antibodies.
Imaging and quantification
Mosaics of image stacks (z-stacks) from the HPC were collected with a 25x objective on a Zeiss AxioImager/ Apotome system. Excitation source intensity and exposure settings were first optimized and then kept constant for all brains. GluR1 and GluR2/3 labeling in the dendritic zone stratum radiatum of CA1 was quantified by measuring the ‘per pixel’ intensity from ~40,000 pixels per image, representing a dendritic volume of ~130,000 µm3 in 2–3 images per animal. The values were expressed as percent of the average of the control mice.
Immunoblots
Forebrains of CalOE mice were homogenized with 8 times volume using lysis buffer (150mM NaCl, 2mM EDTA, and 1% Triton-X-100 in 50mM Tris-Cl, pH 7.4) containing protease inhibitors (Roche) and nutated for an hour at 4°C. Post-nuclear supernatant (PNS) fractions were obtained by centrifugation at 1000x g for 5 minutes at 4°C. Proteins were resolved by SDS-gel electrophoresis on step gradient gels containing 8, 10 and 12 percent polyacrylamide, and transferred to PVDF membranes. Blots were blocked with 5% non-fat dry milk (NFDM) in 1x PBST, probed with anti-FLAG HRP (sigma) at 1:1000 dilution, and the signal developed using Amersham ECL Plus (GE). The blots were stripped and re-probed with anti-Hsp90 antibody (BD Biosciences) at 1:1000 dilution, followed by anti-mouse HRP (Jackson ImmunoResearch) at 1:20,000.
Tissue for brain regional immunoblot studies was obtained from behaviorally naïve adult male CalOE (n=4) and tTA (n=4) mice. Samples of infralimbic/prelimbic prefrontal cortex (IL/PrL), and al HPC were taken using a tissue puncher (0.3µm diameter) (Stoetling Instruments, Wood Dale, IL) from 1 mm thick sections of flash-fozen brains. Tissue was homogenized on ice in 0.1% SDS containing protease and phosphatase inhibitors (Roche) by brief (10 s) sonication. The blots were probed with rabbit polyclonal or murine monoclonal antibodies specific for GluR1 (CalBiochem, 1:50), GluR2/3 (Chemicon, 1:200), and GAPDH (company, 1:200,000). Immunoreactive bands were quantified using Image J (NIH), and the GluR band intensity normalized to that of GAPDH immunoreactive bands in the same lane.
GST pull-down and immunoprecipitation studies
Equivalent amounts of glutathione-S-transferase (GST) or GST-GluR1 or GST-GluR2 or GST-GluR3 ‘C’ terminus43 were bound to glutathione resin (Amersham Biosciences) and blocked using 1% bovine serum albumin (BSA) in binding buffer (150mM NaCl and 1% Triton-X-100 dissolved in 20mM Tris-Cl, pH 7.4) containing protease inhibitors (Roche). An equivalent amount of purified S-tagged human calcyon (residues 93–217)18 was added to each sample, and nutated for an hour at room temperature (RT) in the binding buffer containing 1% BSA. The resin was transferred to spin filters, washed five times with binding buffer, and protein complexes eluted in gel loading buffer as above. Blots were probed with S-protein HRP (Novagen) at 1:5000 dilution.
For the immunoprecipitation studies, CalOE forebrain PNS fractions (pre-cleared with non-immune IgG resin) were added to equivalent amounts of either IgG resin or anti-FLAG resin, and nutated overnight at 4°C. After washing the resin in spin filters, proteins were eluted by boiling for 5 minutes in 2x SDS gel loading buffer with 5% β-ME. Blots were probed with anti-GluR1 antibodies (Calbiochem) at 1:50 dilution, and re-probed with anti-GluR2/3 antibody (Chemicon International) at 1:500, anti-FLAG HRP (Sigma) at 1:1000, or anti-Hsp90 antibody (BD Biosciences) at 1:1000 dilution.
Statistical Analyses
Group differences were evaluated with factorial and factorial mixed-design ANOVA tests with between group factors genotype and Dox treatment and within-group factors training or extinction, as appropriate. For the experiments where more than two groups were evaluated, a priori hypotheses were evaluated with Bonferonni/Dunn post-hoc tests. Evaluating above chance levels for each group was done with a one sample t-test where the hypothesized mean was the ‘at chance’ level. The level of significance was set at 0.05.
Results
Fear Extinction is Impaired in the CalOE mice
In the first set of experiments, we assessed response inhibition in the CalOE and tTA mice with CFC fear extinction which involves the ability to suppress the fear response during subsequent exposure to the conditioning context in the absence of the aversive stimulus. The tTA (n=7) and CalOE (n=6) mice exhibited no differences in freezing behavior or number of crossings during exploration of the CFC apparatus either on the pre-training day (data not shown), or during the habituation period prior to the first footshock (Fig. 1a). Additionally, both groups acquired fear of the CFC apparatus as revealed by a significant training effect (ANOVA-RM: F(3,33)= 123.06, p< 0.0001). There was neither a genotype effect, nor an interaction of training×genotype. The tTA and CalOE mice also did not differ in their ability to recall or express memory for the CFC training (Fig. 1b), as indicated by comparably high levels of freezing during Day 1 of extinction. Altogether, these findings indicate that abnormally high levels of calcyon do not disrupt learning, consolidation or retrieval of fear-motivated learning.
The CalOE mice showed impaired CFC extinction, although like controls, they did eventually learn to suppress fear of the CFC context once it was no longer paired with foot shock, as evidenced by a significant effect of extinction training (F(3,33)= 127.16, p<0.0001) (Fig. 1b). There were significant group differences in the rate of extinction based on genotype (F(1,11)=9.15, p=0.011). The CalOE mice showed higher freezing behavior on Day 2 of extinction compared to the control mice (post-hoc t-test: p< 0.001), and they also froze more on Day 3 (p< 0.0001). These differences were not due to performance of Day 1 of extinction (p=0.804). These data suggest that high levels of calcyon throughout life impair fear extinction in adulthood. We address this point from a developmental perspective below.
Extinction behavior involves distinct phases of memory acquisition, consolidation, retrieval and expression32. Therefore, we analyzed the five minute extinction sessions on a per minute basis to assess whether the deficits observed in CalOE mice involved impaired acquisition or consolidation/retrieval (Fig. 1c). This analysis showed that like the tTA mice, CalOE mice eventually suppressed fear behavior within each one of the first 3 daily extinction sessions. (Extinction training effect for Days 1–3: F(4,44)= 6.71; 10.43 and 3.37, p<0.05, and no significant interaction between extinction training and genotype (F(4,44)= 1.04; 2.41 and 2.34, ns. Freezing levels on Day 4 were too low (<10%) to yield meaningful results by this analysis.) Such behavior is indicative of intact acquisition of extinction learning. However, during the first 2 minutes of the extinction sessions on Days 2 and 3, the CalOE mice showed significantly more freezing than the tTA control mice (Fig. 1c, p<0.01 for minute 1 is shown). This difference in the early phase of each session is consistent with deficits in inhibiting the freezing response due to impaired consolidation and/or retrieval of fear extinction learning.
Silencing the calcyon transgene in adolescence averts impairment in fear extinction
A powerful feature of the tTA/TRE transgenic system is that it is possible to turn TRE transgene expression 'off' as well as back 'on' again due to the ability of a low concentration of doxycycline (Dox) to inhibit the tTA protein in a reversible fashion42. The utility of Dox (20 mg/ml) in silencing transgene expression was confirmed by human calcyon (hCal) antibody staining of HPC and infralimbic/prelimbic (IL/PrL) area of PFC (Supplementary Fig. 1a). Critically, these studies showed that Dox silencing of the calcyon transgene appears to be fully reversible within a one week drug wash-out period (Supplementary Fig. 1a). Immunoblotting studies confirmed that suppression occurs within one week of Dox treatment (Supplementary Fig. 1b).
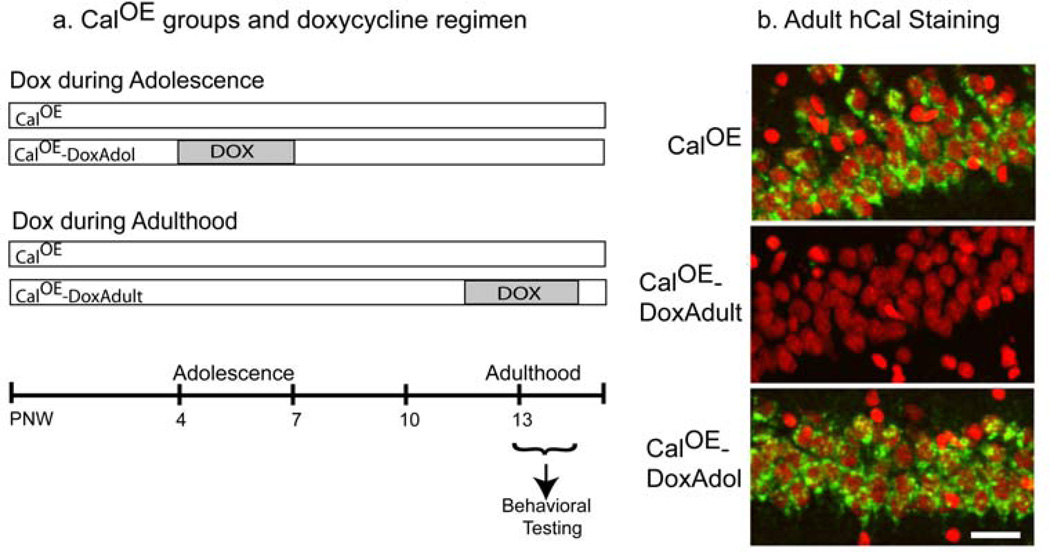
We exploited the 'suppressible/reversible' feature of the tTA/TRE system to probe causality in the CalOE fear extinction deficits. Adolescence is considered a 'critical period' for maturation of the neural circuitry involved in gating response inhibition and goal-directed behavior10. Therefore, we hypothesized that normalizing levels of calcyon with Dox during this period such as shown in Fig. 2a would alleviate the behavioral deficits observed in the adult CalOE mice. The 'adolescence Dox' study involved four groups: untreated CalOE (n=9) and tTA (n= 4); and tTA-DoxAdol (n= 6) and CalOE - DoxAdol (n= 9). The DoxAdol mice were treated with Dox from postnatal day (PND) 28 to PND 49, a period thought to represent adolescence in rodents 44. For comparison, we conducted a separate study with mice treated with Dox for a similar length of time in adulthood. The 'adult Dox' study involved an additional four groups: tTA (n=7), CalOE (n=8), tTA-DoxAdult (n=8) and CalOE -DoxAdult (n=9) (Fig. 2a). Immunohistochemical analysis after testing revealed hCal transgene expression in the forebrains of the CalOE and CalOE -DoxAdol, but not the tTA mice or CalOE -Dox Adult (Fig. 2b).
Figure 2. Scheme for Dox administration in adolescence or adulthood.
(a) Diagram of Dox treatment regimen. In both studies, mice were treated with Dox for 3 weeks. PNW, postnatal week. (b) Immunohistochemistry was performed with the hCalcyon antibody after behavioral testing. As shown here for the CA1 region of dorsal HPC, transgene expression can be detected in untreated and DoxAdol treated, but not DoxAdult treated CalOE mouse brain. hCalcyon is in green and nuclei are in red. Scale bar, 20 µm.
Remarkably, Dox treatment during adolescence prevented the extinction deficits in the CalOE mice as evidenced by the lower levels of freezing during Days 2–4 in the CalOE -DoxAdol mice compared to the CalOE mice (p< 0.01 for all days) (Fig. 3b). In both studies, there were no between-group differences for the tTA and tTA-Dox treated mice, so we assessed the CalOE, and CalOE -Dox groups relative to a 'control' group comprised of both tTA and tTA-Dox mice. As in the initial study (Fig. 1b), there were no differences between groups in both studies during exploration of the CFC apparatus on the pre-training day (data not shown), or prior to the first foot shock (Figs. 3a, 4a). The effects of CFC training were significant in both studies (ANOVA-RM: F(3,75)= 190.98, p<0.0001 and F(3,87)= 31.37, p< 0.0001 for the Dox-Adol and Dox-Adult studies, respectively), as were those for extinction training (F(3,75)=142.60 and F(3,87)= 92.32, p<0.0001 for both) and genotype (F(2,25)=6.69 and F(2,29)=6.45, p<0.01 for both). The control and CalOE mice in both the Dox in adolescence and adulthood studies also did not differ in their ability to acquire, recall or express memory for the CFC training as indicated by comparably high levels of freezing during Day 1 of extinction (Figs. 3b,4b).
The extinction training and genotype effects were due to higher levels of freezing of the untreated CalOE groups on Days 2–4 of extinction compared to the tTA controls (p< 0.01 for all days) (Fig. 3b, 4b). While the CalOE -DoxAdol mice froze less than untreated CalOE mice on Day 2 to 4 of extinction, the CalOE -DoxAdult mice froze less than untreated CalOE mice on Days 2 and 3, but greater than control mice on Day 2 (Fig. 4b). They froze significantly less than the CalOE mice on Day 2 of extinction (p<0.05), but comparably to them on Days 3 and 4. When the data from the two Dox studies were normalized to the freezing behavior of control groups on Day 1 of extinction, it was evident that the CalOE -DoxAdult mice extinguish at a rate intermediate to both control and untreated CalOE groups (fig. 4d), whereas the extinction curve of the CalOE -DoxAdol group closely paralleled that of the controls (fig. 4c). Altogether, the results of the initial study (Fig. 1) and the two Dox studies (Figs. 3,4) suggest that 1) high levels of calcyon throughout life impair fear extinction in adulthood, 2) the deficit can be only partially overcome by suppressing the calcyon transgene during training and testing in adulthood, and 3) silencing the gene during rodent 'adolescence' averts the deficits.
Perseverative behavior of CalOE mice is avoided by silencing the transgene in adolescence
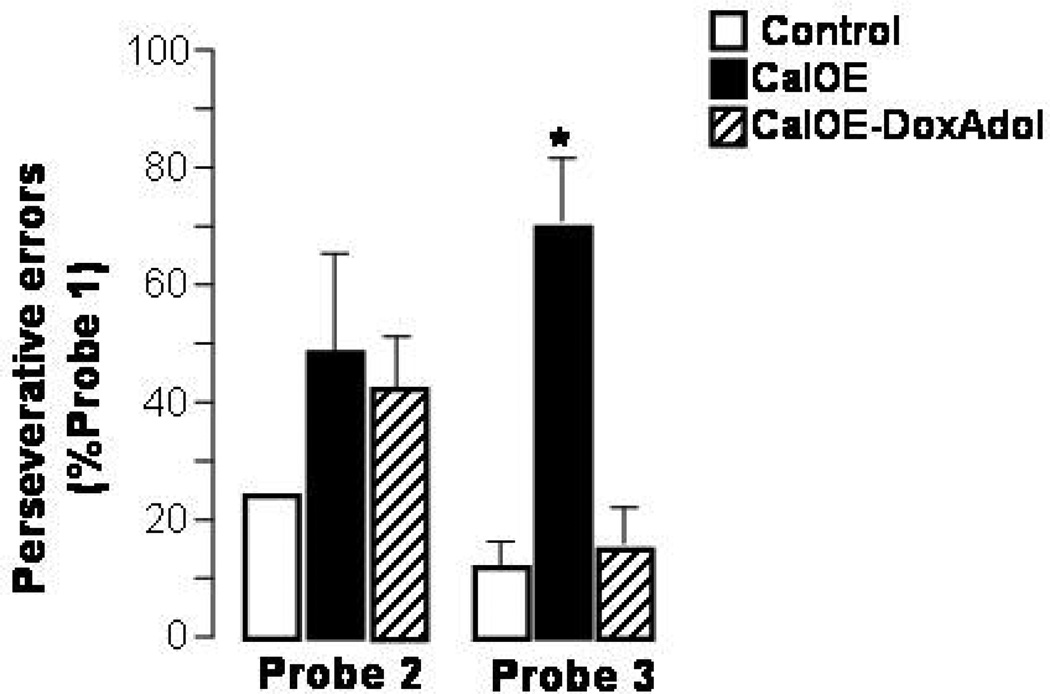
To assess whether the extinction deficit reflects a broader impairment in executive functions, we tested the mice for perseverative behavior and working memory deficits using the Morris water maze. This permitted assessment of spatial reference memory and spatial working memory in the same apparatus used for reference memory, with the mice performing the same behavior, with presumably the same motivation. Untreated CalOE, CalOE -DoxAdol and control mice were trained to locate, based on spatial cues, a submerged platform in the Morris water maze. Both groups of CalOE mice performed on par with the control animals during training and on the initial reference memory probe test when the platform was removed and the mice were allowed to swim in the maze for 60 seconds (Supplementary Fig. 2). CalOE mice could also learn, over the course of four days, a new location of the escape platform (Supplementary Fig. 2). However, during probe tests 2 and 3 conducted two and seven days after the end of the reversal training, respectively, CalOE mice showed evidence of perseverative behavior by visiting the initial target location more often than did control mice. While this perseverative behavior was only a tendency during probe test 2, it was unmistakable during probe test 3 (Fig. 5). Thus, as observed in the CFC and extinction studies, untreated CalOE mice fell short on a task requiring suppression of a previously learned behavior, although basic associative learning and reference memory were not impaired. In contrast, the performance of the CalOE -DoxAdol animals was comparable to that of controls as it had been in the CFC and extinction studies, although the two tasks required completely different behavioral responses (freezing vs. swimming) (Fig. 5).
Figure 5. Perseverative behavior in CalOE mice can be prevented by turning-off calcyon overexpression during adolescence.
CalOE mice exhibit a higher number of perseverative errors than both control and CalOE -Dox-Adol mice (p<0.01). A perseverative error is a target 1 crossing during probe test 2 or 3. Target 1 was an escape location only during the initial reference memory training (Supplementary Fig. 2), but never during the reversal learning task. Swim speeds did not differ between groups.
CalOE, but not CalOE -DoxAdol mice perseverate on a spatial working memory task
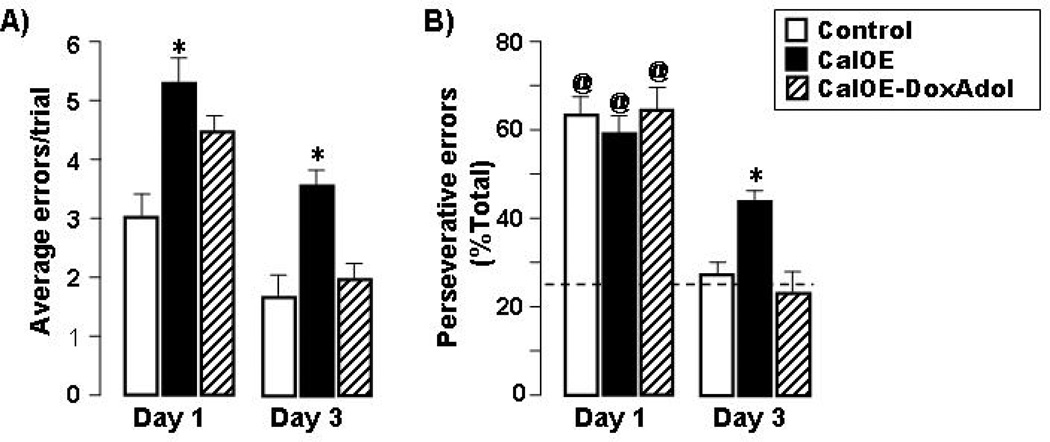
We assessed executive functions in CalOE mice further by testing working memory in the water maze. After exposure to the escape platform for 10 seconds, the mice were tested for recall of the current trial location of the escape platform following a delay of 10 seconds. Compared to controls, CalOE mice committed more errors per trial during both early (day 1) and late (day 3) stages of this spatial working memory training (Fig. 6a). Suppressing calcyon overexpression during adolescence reversed this deficit, as reflected in the performance of CalOE -DoxAdol mice as it was on par with that of controls. Similarly, the CalOE mice committed more perseverative errors, defined as crossings of the platform location used during the second reference memory training. This location was never reinforced during the working memory training. Nevertheless, CalOE mice continued to visit the platform location from the previous reference memory training even in the late stages of the working memory task, whereas the control and CalOE -DoxAdol mice visited this location at near chance levels (27% and 23%, respectively, vs. 25%=chance, Fig. 6b). Hence, suppressing calcyon over-expression throughout adolescence is sufficient to preempt perseverative behavior on working memory tasks in adulthood.
Figure 6. Dox treatment during adolescence prevents perseverative behavior and working memory deficits in adult CalOE mice.
Performance during early (Day 1) and later (Day 3) training on a spatial working memory task (10 s delay). (a) Average errors per trial. (b) Perseverative behavior indicated by errors to target 2 expressed as percent of total errors. Target 2 was an escape location only during reversal training, but never during this task. Dashed line is chance level. Mean and SEM of the data for control, CalOE , and CalOE -DoxAdol mice are shown in the open, black, and gray bars, respectively, @ p< 0.001 compared to performance of the respective group on Day3, * p< 0.01 compared to the control group.
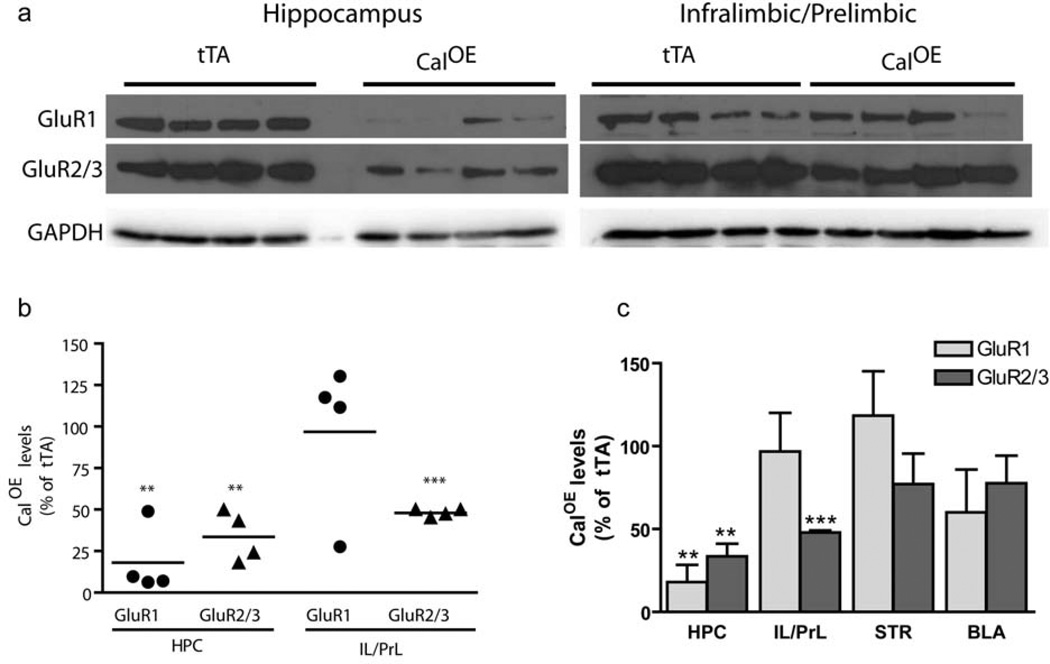
Decreased AMPA subunits in the CalOE IL/PrL cortex and HPC
Calcyon regulates activity dependent internalization endocytosis of AMPA receptors and AMPA-mediated LTD, a synaptic mechanism implicated in the activity-dependent remodeling of circuits during postnatal brain development18, 19, 21. Accordingly, we investigated the impact of calcyon upregulation on GluR1 and GluR2/3 subunit expression in key nodes of the fear extinction/working memory circuit by immunoblotting. The robust differences between GluR1 and GluR2/3 levels in the CalOE and tTA mice suggested that the neural circuitry important for fear extinction and working memory is acutely sensitive to calcyon expression levels. Specifically, levels of GluR1 in HPC were reduced by more than 80% in the CalOE samples (n=4) compared to those from tTA mice (n=4) (Fig. 7) (p=0.001). GluR2/3 subunits were similarly down-regulated in HPC, and infralimbic/prelimbic (IL/PrL) areas of PFC of the CalOE mice (p<0.001). In contrast, GluR1 subunit levels in the CalOE PFC did not differ from that of controls. Genotype specific differences were not observed in either the BLA or striatal samples (Fig. 7c, Supplementary Fig. 3).
Figure 7. Down-regulation of AMPA subunits in CalOE mice.
(a) Immunoblots of HPC and IL/PrL cortex from CalOE (n=4) and tTA (n=4) mice probed with GluR1, GluR2/3 or GAPDH antibodies as indicated. (b) Scatter plot of GluR1 (circles) and GluR2/3 (triangles) levels in HPC (HPC) and infralimbic and prelimbic (IL/PrL) area in each CalOE mouse normalized to the average value in the tTA group. Line shows the mean; ** and *** , p = and <0.001, respectively. (c) Histogram summarizing the results of all brain areas tested, dorsal striatum (STR) basal lateral amygdala (AMY). Bars and error bars show the mean and SEM of the group data.
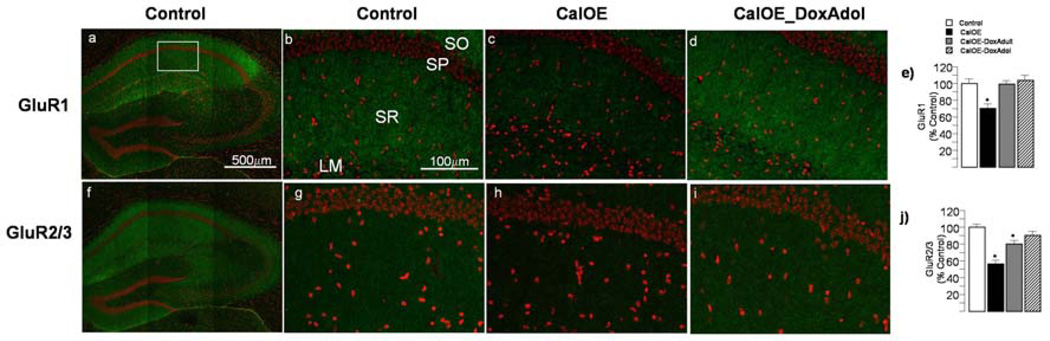
We also compared GluR1 (Fig. 8a–c) and GluR2/3 (Fig. 8e–g) antibody staining in tissue sections of untreated adult CalOE mice with those treated with Dox in adolescence (Fig. 8). Consistent with the immunoblot data, antibody staining of both AMPA subunits in the CA1 region of dorsal HPC of untreated CalOE mice was significantly weaker than that of the tTA controls (Fig. 8b–d;f–h). Whereas GluR1 staining in CA1 of the Dox_Adol and Dox_Adult CalOE mice did not differ from controls (Fig. 8d), GluR2/3 staining in the CalOE mice was not corrected by Dox treatment in adulthood (Fig. 8h).
Figure 8. Dox in adolescence normalizes GluR1 and GluR2/3 levels in adult CalOE mice, whereas Dox in adulthood normalizes GluR1, and only partially normalizes GluR2/3 levels.
Lower magnification mosaic images of the hippocampus showing the general pattern of GluR1 (a) and GluR2/3 (f) antibody staining (in green). Nuclei are in red. Box indicates the size and approximate location of images in the CA1 region of dorsal hippocampus shown in panels b-d and g-i. GluR1 (b-d) and GluR2/3 (g-i) antibody staining (green) of adult control (b,g), CalOE (c,h), and CalOE mice treated with Dox during adolescence (d,i). GluR1 (e) and GluR2/3 (j) staining intensity in the stratum radiatum (SR) in the CA1 of CalOE, CalOE_DoxAdult and CalOE_DoxAdol mice normalized to control. Bars and error bars show the mean and SEM of group data. SR, stratum radiatum, SP, stratum pyramidale, SO, stratum oriens, LM, lacunosum moleculare.
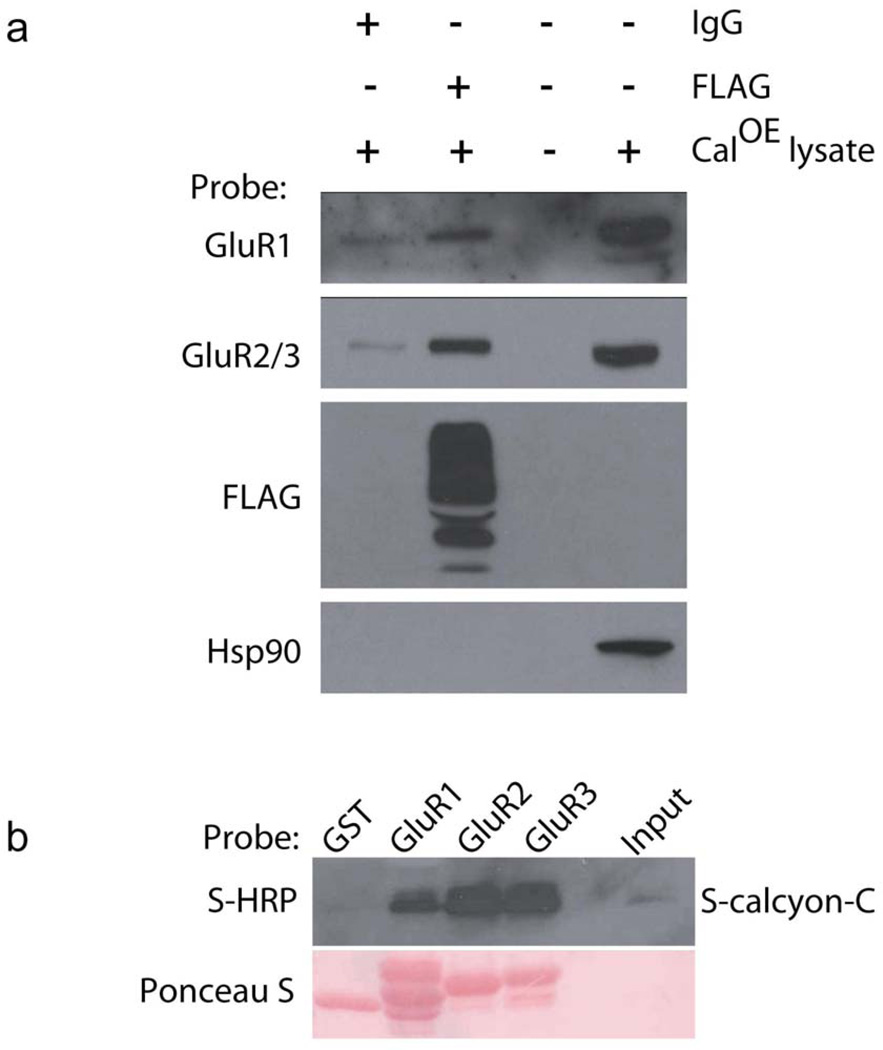
To obtain further insight into potential mechanism(s) underlying this relationship, we conducted protein co-precipitation studies to assess whether calcyon and AMPA receptors physically interact. Consistent with this possibility, antibodies to transgenic calcyon, but not non-immune IgG, immunoprecipitated GluR1 and Glur2/3 subunits from CalOE brain homogenates (Fig. 9a). Pull-down studies further indicated that the proteins interact directly via cytoplasmic sequences. GST fusion proteins including the C-terminal tails of GluR1, GluR2 and GluR3 effectively retained S-protein fused to the C-terminus of calcyon, whereas GST only did not (Fig. 9b).
Figure 9. Direct physical association of calcyon and AMPA receptors.
(a) Immunoblot showing co-immunoprecipitation of endogenous GluR1 and GluR2/3 subunits, but not HSP90 with transgenic FLAG-calcyon from CalOE brain homogenates. Similar results were obtained in two independent experiments. (b) Upper panel, immunoblot of material eluted from glutathione resin bound with GST, GST-GluR1, GluR2 or GluR3 fusion protein as indicated, following incubation with purified S-calcyon-C probed with the HRP-conjugated S protein antibody. Lower panel is same blot later stained with ponceau S indicating that the GST fusion proteins were present in equivalent amounts. Similar results were obtained in three independent experiments.
Discussion
There are four main observations: (1) compared to litter-mate controls, CalOE mice exhibited deficits in suppressing a learned response when it became no longer appropriate, and exhibited perservative behavior when performing tasks that required inhibition of a previously learned response; (2) despite such deficits, CalOE mice exhibited normal aversion-cued as well as reference learning and memory; (3) the response inhibition deficits and perseverative behavior were absent in adult CalOE mice in which calcyon levels had been normalized during adolescence, but only partially attenuated in CalOE mice in which calcyon overexpression was suppressed in adulthood; and (4) there were striking reductions in AMPA receptor GluR1 and/or GluR2/3 subunits in HPC and the IL/PrL areas of PFC that were absent in CalOE mice with the transgene silenced in adolescence.
Executive function deficits
CalOE mice, compared to controls, showed an inability to suppress behavior that is no longer required by the current task in that they demonstrated greater fear behavior during repeated exposure to a shock-associated context during extinction training. Their extinction deficits could not be attributed to difficulty updating the emotional valence of the contextual stimuli, or suppressing fear behavior because within each daily extinction session, freezing decreased as a function of time. Instead, the much higher levels of freezing observed upon re-introduction to the extinction context, suggest the CalOE deficits arise from impaired consolidation and/or retrieval of memory for fear extinction. PFC participates in the consolidation and expression of fear extinction along with other forebrain regions, including the HPC, BLA and nucleus accumbens32. Considerable evidence suggests connections between HPC and PFC play an important role in retention of fear extinction45,46. Based on reductions in AMPA receptors in the respective brain areas, our data indicate altered connectivity between HPC and the IL/PrL areas of PFC could contribute to the extinction deficits of the CalOE mice. Similarly, the lack of alterations in GluR levels in BLA is consistent with unimpaired BLA function as reflected in the ability of the CalOE mice to acquire and recall the context-footshock association as well as controls47.
Difficulty inhibiting adaptive responses was also evident in the spatial working memory task by the tendency of the CalOE mice to visit a location previously associated with escape. These were working memory deficits as the same mice performed as well as controls during tests of reference memory in the same task. However, their tendency to perseverate was also evident in the reversal learning probe tests. The errors in fear extinction, reversal learning, and working memory point to a general deficit in cognitive flexibility. Additionally, the perseverative behavior implicates IL/PrL and/or hippocampal dysfunction as both brain regions are required for spatial working memory tasks involving delays of 10 seconds as in our experiments48–51 whereas working memory tasks with short (~2 sec) delays do not tap hippocampal functions49. During long delays, the HPC is proposed to assist the PFC in processing and remembering minimal cue information for relatively long periods, possibly by entraining theta (4 to 12 Hz) rhythmic firing in PFC50, 52, 53.
In light of the previously observed reduced anxiety-like behavior in the open-field, light-dark and elevated plus maze tasks39, the current results indicates that CalOE mice exhibit complex deficits in control over behavior. Thus, multiple lines of evidence suggest that high levels of calcyon in forebrain disrupt executive functions54. As impaired executive functions are a hallmark of disorders such as ADHD and schizophrenia, this study, although conducted in rodents, provides strong evidence that calcyon overexpression contributes to the phenotype observed in these disorders. Intriguingly, recent work indicates that schizophrenic subjects, like the CalOE mice, also show impaired expression of extinction memory but normal fear extinction learning55. Similarly, adults with ADHD show impairment in working memory and response inhibition tasks56.
Altered AMPA expression in the fear extinction and working memory circuitry
The alterations in AMPA receptor GluR1 and GluR2/3 subunits detected in CalOE brain correspond well with the pattern of calcyon transgene expression. For example, GluR1 and GluR2/3 levels are significantly down-regulated in HPC where they and the calcyon transgene are strongly expressed in pyramidal neurons in all CA regions and the dentate gyrus due to the expression of the CaMKII-tTA driver57. In cortex, transgene expression is limited to pyramidal neurons. Given this, the selective down-regulation of GluR2/3 observed in IL/PrL presumably reflects the more prominent expression of these subunits in the same cells. Down-regulation of GluR1 in cortex in pyramidal cells could be masked by the more dense expression of GluR1 in non-pyramidal in cortex57–60. Indeed, the apparent lack of effect on AMPA subunit expression in BLA or striatum may reflect lower levels of transgene expression in these brain regions or greater discordance in the respective cellular expression patterns. Previous studies suggest calcyon associates with molecular machinery involved in clathrin mediated endocytosis61, and that calcyon facilitates activity dependent, clathrin-mediated internalization of AMPA receptors18. Therefore, GluR1 and GluR2/3 down-regulation could be attributed to inappropriately high levels of AMPA receptor endocytosis, and subsequent degradation of these subunits. Further, the protein co-precipitation and pull-down data suggest that the down-regulation of GluR1 and GluR2/3 is in part mediated by direct association with calcyon. However, as silencing the transgene in adolescence is sufficient to reverse the alterations, a major finding reported here is that altered neurodevelopment plays a role in the adult AMPA receptor deficits. Similarly, stable alterations in the circuitry or molecules regulating GluR2/3 expression could explain the failure of silencing the calcyon transgene in adulthood to completely reverse the reduction in GluR2/3 levels.
The possibility that down-regulation of hippocampal and IL/PrL AMPA receptors contributes to the behavioral deficits is consistent with numerous studies indicating that GluRs play a role in fear extinction62–65 and working memory66. For example, GluR1 knockout, mice much like the untreated CalOE mice which express only about ~ 15% of normal GluR1 in HPC, exhibit deficits in spatial working memory, but perform normally in tests of spatial reference memory67–69. Restoration of hippocampal GluR1 partially rescues the deficits in GluR1 knockout mice70. Similarly, we found that GluR1 levels in HPC of CalOE-DoxAdol mice are comparable to those of controls, consistent with their 'normal' performance in reversal learning and spatial working memory. On the other hand, low GluR2/3 levels in IL/PrL could underlie the fear extinction deficits of the CalOE mice as contextual fear extinction performance correlates well with GluR2/3 subunit expression in PFC64,65. Further, the failure of Dox in adulthood to fully reverse the Glur2/3 deficits correlates with the partial rescue of the extinction deficits observed in the CalOE -DoxAdult mice.
Altered cortical excitability could also explain the inability of silencing the transgene in adulthood to fully rescue the CalOE behavioral deficits. The general down-regulation of hippocampal excitatory output in the CalOE mice, a potential consequence of reduced GluR1 and GluR2/3 levels, would be expected to lessen the ability of HPC to entrain the PFC during spatial working memory tasks50, 52. Either a selective decrease in GluR2/3 subunits71 or a general down-regulation of AMPA receptors in pyramidal neurons in cortex could produce alterations in excitability, and render the PFC less capable of integrating signals from other cortical and subcortical areas such as HPC45.
Anatomical and brain imaging data suggests that forebrain regions undergo extensive remodeling at the circuit level during adolescence. Calcyon regulates NMDA and activity-dependent synaptic plasticity, a mechanism proposed to accomplish both the loss of some connections and the strengthening of others during development18,19. Therefore, transgene upregulation during adolescence, especially, could exert a long term impact on adult behavior by disrupting activity-dependent developmental mechanisms involved in maturation of circuits important for working memory and response inhibition. Alternatively, the partial rescue in adulthood can be explained by proposing that there is a 'sensitive period' for establishing optimal functional connectivity in the HPC-PFC system. Our data suggest that this period includes adolescence, consistent with the reported peak in synaptic proteins, including glutamatergic NMDA and AMPA receptors associated with plasticity in the HPC and cortex shortly before and during early adolescence72, 73.
Conclusion
Adolescence is considered a 'critical period' for maturation of PFC and a network of limbic structures involved in gating response inhibition and goal-directed behavior74. Further, the maturation of working memory is influenced to neurodevelopmental events intrinsic as well as extrinsic to the PFC 2,75,76. Taking advantage of the inducible and reversible feature of the tetracycline transactivator (tTA) /TRE transgenic system, we show that Dox treatment during rodent adolescence effectively preempts the working memory and extinction deficits typically observed in adult CalOE animals. While several animal studies have investigated the influence of early untoward experiences and environmental factors (e.g., stress) on adult executive functions77,78 to our knowledge, this is the first to address the impact of alterations in a single gene during early postnatal development. Similar approaches could be fruitful in identifying other genes involved in the maturation of executive functions, or in assessing the contribution of other developmental periods.
Based on the performance of CalOE mice in three different behavioral paradigms, this work supports the hypothesis that environmental conditions and/or genetic alterations resulting in supranormal calcyon levels during adolescence undermines neural processes involved in the maturation of adult working memory and response inhibition. These findings have clinical as well as neurodevelopmental implications by linking two observations of schizophrenia post-mortem analyses: elevated levels of calcyon22–25 and GluR2 in prefrontal cortex79. Additionally, hippocampal deficits and/or disrupted fronto-temporal lobe80 or fronto-hippocampal connectivity are frequently reported in subjects with schizophrenia, their first degree relatives and off-spring81–84. Importantly, longitudinal studies of the non-psychotic relatives and off-spring of schizophrenics indicate that executive function deficits can be detected even in adolescence, and deteriorate with age85,86. In light of the degenerative nature of these deficits and the marginal efficacy of the currently available anti-psychotic medications when used in adults87, 88 the present findings make a strong case for further investigating the therapeutic merits of early interventions to avert or mitigate later losses in executive functions, and specifically highlight adolescence as a 'window of therapeutic opportunity.'
Supplementary Material
Acknowledgements
We thank Dr. Sang Lee, Medical College of Wisconsin for the GluR plasmids, Drs. Lynn Selemon, David Blake, Philip Wang and Jay Hegde for their comments on the manuscript, Kyle Layman, Nita Vakil and Jonathan Bean for technical support, and Dr. Lin Mei for use of the EthoVision XT software. This work was supported by DoD Concept Award PT0713 and MCG PSRP grant (CB) and MCG start-up funds and NIH grant 1R21MH083188 (AV).
Footnotes
Author Contributions: NM performed protein interaction studies, KB performed and quantified immunohistochemistical studies. AV: performed and analyzed behavioral experiments; CB: performed immunoblots, and wrote the manuscript. AV and CB co-conceived the study.
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Luna B, Padmanabhan A, O'Hearn K. What has fMRI told us about the Development of Cognitive Control through Adolescence? Brain and Cognition. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green RJ, Stanton ME. Differential ontogeny of working memory and reference memory in the rat. Behav. Neurosci. 1989;103:98–105. doi: 10.1037//0735-7044.103.1.98. [DOI] [PubMed] [Google Scholar]
- 3.Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond A, Goldman-Rakic PS. Comparison of human infants and rhesus monkeys on Piaget's AB task: evidence for dependence on dorsolateral prefrontal cortex. Exp Brain Res. 1989;74:24–40. doi: 10.1007/BF00248277. [DOI] [PubMed] [Google Scholar]
- 5.Diamond A. Developmental time course in human infants and infant monkeys, and the neural bases of inhibitory control in reaching. Ann. N. Y. Acad. Sci. 1990;608:637–669. doi: 10.1111/j.1749-6632.1990.tb48913.x. discussion 669–676. [DOI] [PubMed] [Google Scholar]
- 6.Hare T, Tottenham N, Galvan A, Voss H, Glover G, Casey B. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol.Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huttenlocher P. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 8.Bourgeois J, Goldman-Rakic P, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb.Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- 9.Woo T, Pucak M, Kye C, Matus C, Lewis D. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997;80:1149–1158. doi: 10.1016/s0306-4522(97)00059-6. [DOI] [PubMed] [Google Scholar]
- 10.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams N, Norton N, Williams H, Ekholm B, Hamshere M, Lindblom Y, et al. A systematic genomewide linkage study in 353 sib pairs with schizophrenia. Am.J.Hum.Genet. 2003;73:1355–1367. doi: 10.1086/380206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holliday EG, McLean DE, Nyholt DR, Mowry BJ. Susceptibility locus on chromosome 1q23-25 for a schizophrenia subtype resembling deficit schizophrenia identified by latent class analysis. Arch. Gen. Psychiatry. 2009;66:1058–1067. doi: 10.1001/archgenpsychiatry.2009.136. [DOI] [PubMed] [Google Scholar]
- 13.Mowry BJ, Ewen KR, Nancarrow DJ, Lennon DP, Nertney DA, Jones HL, et al. Second stage of a genome scan of schizophrenia: study of five positive regions in an expanded sample. Am. J. Med. Genet. 2000;96:864–869. [PubMed] [Google Scholar]
- 14.Fallin MD, Lasseter VK, Wolyniec PS, McGrath JA, Nestadt G, Valle D, et al. Genomewide linkage scan for schizophrenia susceptibility loci among Ashkenazi Jewish families shows evidence of linkage on chromosome 10q22. Am. J. Hum. Genet. 2003;73:601–611. doi: 10.1086/378158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon HJ, Yim S, Lee WK, Jeon Y, Kim YH, Ko YJ, et al. Identification of DNA copynumber aberrations by array-comparative genomic hybridization in patients with schizophrenia. Biochem. Biophys. Res. Commun. 2006;344:531–539. doi: 10.1016/j.bbrc.2006.03.156. [DOI] [PubMed] [Google Scholar]
- 16.Ewald H, Flint T, Jorgensen T, Wang A, Jensen P, Vang M, et al. Search for a shared segment on chromosome 10q26 in patients with bipolar affective disorder or schizophrenia from the Faroe Islands. Am.J.Med.Genet. 2002;114:196–204. [PubMed] [Google Scholar]
- 17.Venken T, Alaerts M, Souery D, Goossens D, Sluijs S, Navon R, et al. Chromosome 10q harbors a susceptibility locus for bipolar disorder in Ashkenazi Jewish families. Mol. Psychiatry. 2008;13:442–450. doi: 10.1038/sj.mp.4002039. [DOI] [PubMed] [Google Scholar]
- 18.Davidson H, Xiao J, Dai R, Bergson C. Calcyon is necessary for activity-dependent AMPA receptor internalization and LTD in CA1 neurons of hippocampus. Eur.J.Neurosci. 2009;29:42–54. doi: 10.1111/j.1460-9568.2008.06563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tessier CR, Broadie K. Activity-dependent modulation of neural circuit synaptic connectivity. Front Mol Neurosci. 2009;2:8. doi: 10.3389/neuro.02.008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch GS, Dunwiddie T, Gribkoff V. Heterosynaptic depression: a postsynaptic correlate of long-term potentiation. Nature. 1977;266:737–739. doi: 10.1038/266737a0. [DOI] [PubMed] [Google Scholar]
- 21.Bastrikova N, Gardner G, Reece J, Jeromin A, Dudek S. Synapse elimination accompanies functional plasticity in hippocampal neurons. Proc.Natl.Acad.Sci.U.S.A. 2008;105:3123–3127. doi: 10.1073/pnas.0800027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koh P, Bergson C, Undie A, Goldman-Rakic P, Lidow M. Up-regulation of the D1 dopamine receptor-interacting protein, calcyon, in patients with schizophrenia. Arch.Gen.Psychiatry. 2003;60:311–319. doi: 10.1001/archpsyc.60.3.311. [DOI] [PubMed] [Google Scholar]
- 23.Bai J, He F, Novikova S, Undie A, Dracheva S, Haroutunian V, et al. Abnormalities in the dopamine system in schizophrenia may lie in altered levels of dopamine receptor-interacting proteins. Biol.Psychiatry. 2004;56:427–440. doi: 10.1016/j.biopsych.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Clinton S, Ibrahim H, Frey K, Davis K, Haroutunian V, Meador-Woodruff J. Dopaminergic abnormalities in select thalamic nuclei in schizophrenia: involvement of the intracellular signal integrating proteins calcyon and spinophilin. Am.J.Psychiatry. 2005;162:1859–1871. doi: 10.1176/appi.ajp.162.10.1859. [DOI] [PubMed] [Google Scholar]
- 25.Baracskay K, Haroutunian V, Meador-Woodruff J. Dopamine receptor signaling molecules are altered in elderly schizophrenic cortex. Synapse. 2006;60:271–279. doi: 10.1002/syn.20292. [DOI] [PubMed] [Google Scholar]
- 26.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell. Mol. Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- 27.Sodhi M, Wood KH, Meador-Woodruff J. Role of glutamate in schizophrenia: integrating excitatory avenues of research. Expert Rev Neurother. 2008;8:1389–1406. doi: 10.1586/14737175.8.9.1389. [DOI] [PubMed] [Google Scholar]
- 28.Heijtz R, Kolb B, Forssberg H. Motor inhibitory role of dopamine D1 receptors: implications for ADHD. Physiol Behav. 2007;92:155–160. doi: 10.1016/j.physbeh.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 29.Dasbanerjee T, Middleton F, Berger D, Lombardo J, Sagvolden T, Faraone S. A comparison of molecular alterations in environmental and genetic rat models of ADHD: a pilot study. Am.J.Med.Genet.B Neuropsychiatr.Genet. 2008;147B:1554–1563. doi: 10.1002/ajmg.b.30877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loo S, Fisher S, Francks C, Ogdie M, MacPhie I, Yang M, et al. Genome-wide scan of reading ability in affected sibling pairs with attention-deficit/hyperactivity disorder: unique and shared genetic effects. Mol.Psychiatry. 2004;9:485–493. doi: 10.1038/sj.mp.4001450. [DOI] [PubMed] [Google Scholar]
- 31.Fisher S, Francks C, McCracken J, McGough J, Marlow A, MacPhie I, et al. A genomewide scan for loci involved in attention-deficit/hyperactivity disorder. Am.J.Hum.Genet. 2002;70:1183–1196. doi: 10.1086/340112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quirk G, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spooner RI, Thomson A, Hall J, Morris RG, Salter SH. The Atlantis platform: a new design and further developments of Buresova's on-demand platform for the water maze. Learn. Mem. 1994;1:203–211. [PubMed] [Google Scholar]
- 34.Mayford M, Abel T, Kandel E. Transgenic approaches to cognition. Curr.Opin.Neurobiol. 1995;5:141–148. doi: 10.1016/0959-4388(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 35.Zelenin S, Aperia A, Diaz HR. Calcyon in the rat brain: cloning of cDNA and expression of mRNA. J.Comp Neurol. 2002;446:37–45. doi: 10.1002/cne.10198. [DOI] [PubMed] [Google Scholar]
- 36.Oakman S, Meador-Woodruff J. Calcyon transcript expression in macaque brain. J.Comp Neurol. 2004;468:264–276. doi: 10.1002/cne.10993. [DOI] [PubMed] [Google Scholar]
- 37.Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley P, Barnes C. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J.Neurosci. 2005;25:1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Negyessy L, Bergson C, Garab S, Simon L, Goldman-Rakic P. Ultrastructural localization of calcyon in the primate cortico-basal ganglia-thalamocortical loop. Neurosci.Lett. 2008;440:59–62. doi: 10.1016/j.neulet.2008.05.047. [DOI] [PubMed] [Google Scholar]
- 39.Trantham-Davidson H, Vazdarjanova A, Dai R, Terry A, Bergson C. Up-regulation of calcyon results in locomotor hyperactivity and reduced anxiety in mice. Behav.Brain Res. 2008;189:244–249. doi: 10.1016/j.bbr.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JH, Hamlin AS, Richardson R. Fear extinction across development: the involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J. Neurosci. 2009;29:10802–10808. doi: 10.1523/JNEUROSCI.0596-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frankle WG, Lerma J, Laruelle M. The synaptic hypothesis of schizophrenia. Neuron. 2003;39:205–216. doi: 10.1016/s0896-6273(03)00423-9. [DOI] [PubMed] [Google Scholar]
- 42.Chen J, Kelz M, Zeng G, Sakai N, Steffen C, Shockett P, et al. Transgenic animals with inducible, targeted gene expression in brain. Mol.Pharmacol. 1998;54:495–503. doi: 10.1124/mol.54.3.495. [DOI] [PubMed] [Google Scholar]
- 43.Lee S, Liu L, Wang Y, Sheng M. Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron. 2002;36:661–674. doi: 10.1016/s0896-6273(02)01024-3. [DOI] [PubMed] [Google Scholar]
- 44.Spear LP. Adolescent Brain Development and Animal Models. Annals of the New York Academy of Sciences. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- 45.Farinelli M, Deschaux O, Hugues S, Thevenet A, Garcia R. Hippocampal train stimulation modulates recallof fear extinction independently of prefrontalcortex synaptic plasticity and lesions. Learning & Memory. 2006;13:329–334. doi: 10.1101/lm.204806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia R, Spennato G, Nilsson-Todd L, Moreau J, Deschaux O. Hippocampal lowfrequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiol.Learn.Mem. 2008;89:560–566. doi: 10.1016/j.nlm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Vazdarjanova A, McGaugh J. Basolateral amygdala is not critical for cognitive memory of contextual fear conditioning. Proc.Natl.Acad.Sci.U.S.A. 1998;95:15003–15007. doi: 10.1073/pnas.95.25.15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Izaki Y, Takita M, Akema T. Specific role of the posterior dorsal hippocampus-prefrontal cortex in short-term working memory. Eur. J. Neurosci. 2008;27:3029–3034. doi: 10.1111/j.1460-9568.2008.06284.x. [DOI] [PubMed] [Google Scholar]
- 49.Lee I, Kesner RP. Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. J. Neurosci. 2003;23:1517–1523. doi: 10.1523/JNEUROSCI.23-04-01517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J. Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 53.Paz R, Bauer EP, Paré D. Theta synchronizes the activity of medial prefrontal neurons during learning. Learn. Mem. 2008;15:524–531. doi: 10.1101/lm.932408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duncan J, Owen A. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 55.Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, et al. Extinction memory is impaired in schizophrenia. Biol. Psychiatry. 2009;65:455–463. doi: 10.1016/j.biopsych.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clark L, Blackwell A, Aron A, Turner D, Dowson J, Robbins T, et al. Association Between Response Inhibition and Working Memory in Adult ADHD: A Link to Right Frontal Cortex Pathology? Biological Psychiatry. 2007;61:1395–1401. doi: 10.1016/j.biopsych.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 57.Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPAselective glutamate receptors in the rat brain. J. Comp. Neurol. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- 58.Vickers JC, Huntley GW, Edwards AM, Moran T, Rogers SW, Heinemann SF, et al. Quantitative localization of AMPA/kainate and kainate glutamate receptor subunit immunoreactivity in neurochemically identified subpopulations of neurons in the prefrontal cortex of the macaque monkey. J. Neurosci. 1993;13:2982–2992. doi: 10.1523/JNEUROSCI.13-07-02982.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin LJ, Blackstone CD, Levey AI, Huganir RL, Price DL. AMPA glutamate receptor subunits are differentially distributed in rat brain. Neuroscience. 1993;53:327–358. doi: 10.1016/0306-4522(93)90199-p. [DOI] [PubMed] [Google Scholar]
- 60.Ye E, Kim T, Choi J, Jin M, Jeon Y, Kim M, et al. Ionotropic glutamate receptor GluR1 in the visual cortex of hamster: distribution and co-localization with calcium-binding proteins and GABA. Acta Histochem Cytochem. 2006;39:47–54. doi: 10.1267/ahc.05058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiao J, Dai R, Negyessy L, Bergson C. Calcyon, a novel partner of clathrin light chain, stimulates clathrin-mediated endocytosis. J.Biol.Chem. 2006;281:15182–15193. doi: 10.1074/jbc.M600265200. [DOI] [PubMed] [Google Scholar]
- 62.Dalton G, Wang Y, Floresco S, Phillips A. Disruption of AMPA receptor endocytosis impairs the extinction, but not acquisition of learned fear. Neuropsychopharmacology. 2008;33:2416–2426. doi: 10.1038/sj.npp.1301642. [DOI] [PubMed] [Google Scholar]
- 63.Matsuo N, Reijmers L, Mayford M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science. 2008;319:1104–1107. doi: 10.1126/science.1149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gourley SL, Kedves AT, Olausson P, Taylor JR. A History of Corticosterone Exposure Regulates Fear Extinction and Cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2008;34:707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zushida K, Sakurai M, Wada K, Sekiguchi M. Facilitation of extinction learning for contextual fear memory by PEPA: a potentiator of AMPA receptors. J.Neurosci. 2007;27:158–166. doi: 10.1523/JNEUROSCI.3842-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bannerman DM, Deacon RMJ, Seeburg PH, Rawlins JNP. GluR-A-Deficient mice display normal acquisition of a hippocampus-dependent spatial reference memory task but are impaired during spatial reversal. Behav. Neurosci. 2003;117:866–870. doi: 10.1037/0735-7044.117.4.866. [DOI] [PubMed] [Google Scholar]
- 67.Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- 68.Reisel, D.,Bannerman DM, Schmitt WB, Deacon RMJ, Flint J, Borchardt T, et al. Spatial memory dissociations in mice lacking GluR1. Nat. Neurosci. 2002;5:868–873. doi: 10.1038/nn910. [DOI] [PubMed] [Google Scholar]
- 69.Bannerman DM. Fractionating spatial memory with glutamate receptor subunit-knockout mice. Biochem. Soc. Trans. 2009;37:1323–1327. doi: 10.1042/BST0371323. [DOI] [PubMed] [Google Scholar]
- 70.Schmitt WB, Sprengel R, Mack V, Draft RW, Seeburg PH, Deacon RMJ, et al. Restoration of spatial working memory by genetic rescue of GluR-A-deficient mice. Nat. Neurosci. 2005;8:270–272. doi: 10.1038/nn1412. [DOI] [PubMed] [Google Scholar]
- 71.Pellegrini-Giampietro D, Gorter J, Bennett M, Zukin R. The GluR2 (GluR-B) hypothesis: Ca(2+)-permeable AMPA receptors in neurological disorders. Trends Neurosci. 1997;20:464–470. doi: 10.1016/s0166-2236(97)01100-4. [DOI] [PubMed] [Google Scholar]
- 72.Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain--I. N-methyl-D-aspartate and quisqualate receptors. Neuroscience. 1990;35:31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- 73.Swulius MT, Kubota Y, Forest A, Waxham MN. Structure and composition of the postsynaptic density during development. J. Comp. Neurol. 2010;518:4243–4260. doi: 10.1002/cne.22451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spear L. The adolescent brain and age-related behavioral manifestations. Neurosci.Biobehav.Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 75.Freeman JH, Stanton ME. Fimbria-fornix transections disrupt the ontogeny of delayed alternation but not position discrimination in the rat. Behav. Neurosci. 1991;105:386–395. doi: 10.1037//0735-7044.105.3.386. [DOI] [PubMed] [Google Scholar]
- 76.Lipska B, Aultman J, Verma A, Weinberger D, Moghaddam B. Neonatal damage of the ventral hippocampus impairs working memory in the rat. Neuropsychopharmacology. 2002;27:47–54. doi: 10.1016/S0893-133X(02)00282-8. [DOI] [PubMed] [Google Scholar]
- 77.Andersen S, Teicher M. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 78.Akers K, Nakazawa M, Romeo R, Connor J, McEwen B, Tang A. Early life modulators and predictors of adult synaptic plasticity. Eur.J.Neurosci. 2006;24:547–554. doi: 10.1111/j.1460-9568.2006.04921.x. [DOI] [PubMed] [Google Scholar]
- 79.Vawter MP, Crook JM, Hyde TM, Kleinman JE, Weinberger DR, Becker KG, et al. Microarray analysis of gene expression in the prefrontal cortex in schizophrenia: a preliminary study. Schizophr. Res. 2002;58:11–20. doi: 10.1016/s0920-9964(01)00377-2. [DOI] [PubMed] [Google Scholar]
- 80.Folley BS, Astur R, Jagannathan K, Calhoun VD, Pearlson GD. Anomalous neural circuit function in schizophrenia during a virtual Morris water task. Neuroimage. 2010;49:3373–3384. doi: 10.1016/j.neuroimage.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boos HBM, Aleman A, Cahn W, Pol HH, Kahn RS. Brain Volumes in Relatives of Patients With Schizophrenia: A Meta-analysis. Arch Gen Psychiatry. 2007;64:297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- 82.Sişmanlar SG, Anik Y, Coşkun A, Ağaoğlu B, Karakaya I, Yavuz CI. The volumetric differences of the fronto-temporal region in young offspring of schizophrenic patients. Eur Child Adolesc Psychiatry. 2010;19:151–157. doi: 10.1007/s00787-009-0052-5. [DOI] [PubMed] [Google Scholar]
- 83.Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch. Gen. Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- 84.Wolf RC, Vasic N, Sambataro F, Höse A, Frasch K, Schmid M, et al. Temporally anticorrelated brain networks during working memory performance reveal aberrant prefrontal and hippocampal connectivity in patients with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:1464–1473. doi: 10.1016/j.pnpbp.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 85.Bhojraj TS, Diwadkar VA, Sweeney JA, Prasad KM, Eack SM, Montrose DM, et al. Longitudinal alterations of executive function in non-psychotic adolescents at familial risk for schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:469–474. doi: 10.1016/j.pnpbp.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Keshavan MS, Kulkarni S, Bhojraj T, Francis A, Diwadkar V, Montrose DM, et al. Premorbid cognitive deficits in young relatives of schizophrenia patients. Front Hum Neurosci. 2010;3:62. doi: 10.3389/neuro.09.062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Keefe RSE, Sweeney JA, Gu H, Hamer RM, Perkins DO, McEvoy JP, et al. Effects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164:1061–1071. doi: 10.1176/ajp.2007.164.7.1061. [DOI] [PubMed] [Google Scholar]
- 88.Lewis D. Atypical antipsychotic medications and the treatment of schizophrenia. Am.J.Psychiatry. 2002;159:177–179. doi: 10.1176/appi.ajp.159.2.177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.