Abstract
Dementia is one of the most important problems nowadays. Aging is associated with learning and memory impairments. Diet rich in cholesterol has been shown to be detrimental to cognitive performance. This work was carried out to compare the effect of high cholesterol diet on the hippocampus of adult and aged male albino rats. Twenty adult and twenty aged male rats were used in this study. According to age, the rats were randomly subdivided into balanced and high cholesterol diet fed groups. The diet was 15 g/rat/day for adult rats and 20 g/rat/day for aged rats for eight weeks. Serial coronal sections of hippocampus and blood samples were taken from each rat. For diet effect evaluation, Clinical, biochemical, histological, immunohistochemical, and morphometric assessments were done. In compare to a balanced diet fed rat, examination of Cornu Ammonis 1 (CA 1) area in the hippocampus of the high cholesterol diet adult rats showed degeneration, a significant decrease of the pyramidal cells, attenuation and/or thickening of small blood vessels, apparent increase of astrocytes and apparent decrease of Nissl's granules content. Moreover, the high cholesterol diet aged rats showed aggravation of senility changes of the hippocampus together with Alzheimer like pathological changes. In conclusion, the high cholesterol diet has a significant detrimental effect on the hippocampus and aging might pronounce this effect. So, we should direct our attention to limit cholesterol intake in our food to maintain a healthy life style for a successful aging.
Keywords: Cholesterol, Aging, Cornu Ammonis, Hippocampus, Pyramidal cells
Introduction
Progressive disorders affecting behavior, thinking, memory and daily activities is known as dementia syndrome. The most common type of dementia is Alzheimer's disease [1]. A person who has dementia is always developing agitation and other behavioral symptoms, making it much harder to care for him [2]. In many vertebrates hippocampus forms a major component of the brains. The consolidation of information from short-term memory to long-term memory is a role of hippocampus [3]. Cornu Ammonis 1 (CA1) is the zone that is most sensitive to various insults [4]. Aging is associated with impairment in certain aspects of cognition, especially learning and memory. The hippocampus formation is one of the most brain areas affected by aging in both humans and other mammalian species [5]. Several studies demonstrated that greater intake of cholesterol increased atherosclerotic picture in target organs [6]. Thus, the present study was performed to investigate the effect of high cholesterol diet on the structure of hippocampus in the adult and aged rats.
Materials and Methods
The animals
Twenty adult (three-month-old) and twenty elderly aged (18-24 month-old) male albino rats (Wistar strain) obtained from the National Center of Researches (Cairo, Egypt) were used in this study. The rats were kept in the laboratory under constant conditions of temperature (24±2℃) for, at least, one week before and through the experimental work.
Diet
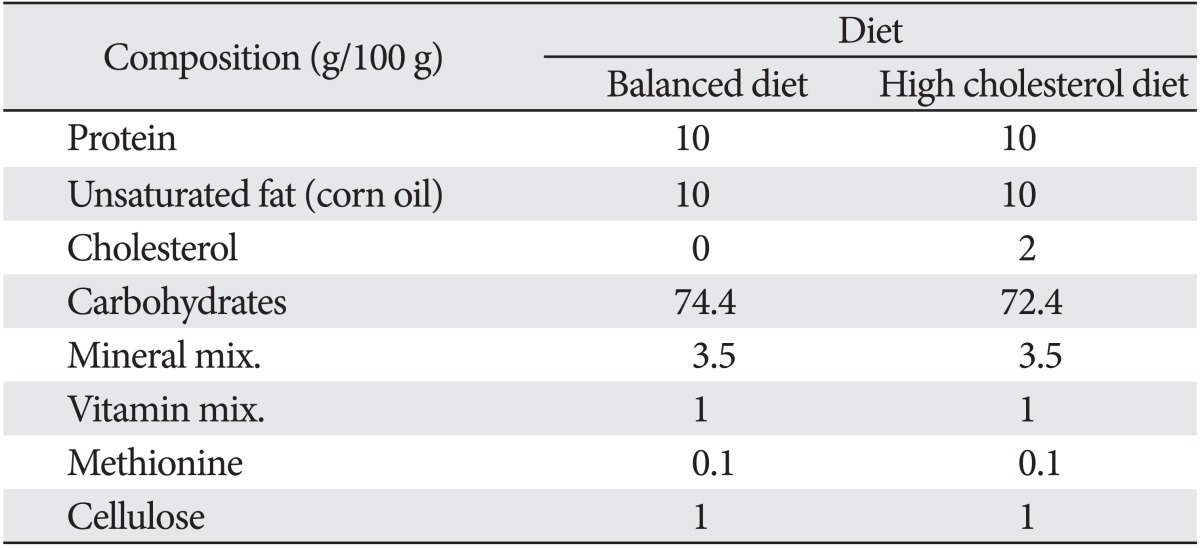
Two different types of diet (balanced and high cholesterol diets) were used in this study. They were prepared in the National Center of Researches, Nutrition Department (Table 1) [8, 9]. Cholic acid (0.25 g/100 g) was added to high cholesterol diet to increase the absorption of cholesterol [7]. Diet was given to rats as 15 g/rat/day for adult rats and 20 g/rat/day for aged rats for eight weeks [8]. The rats were put individually in separate cages to allow for the careful control of food intake for each animal. The cages were provided with white paper bedding to allow complete collection of any remaining food. The rats received food in heavy pot containers and received water ad-libitum. The food was introduced daily and any remaining food was removed, weighed and recorded.
Table 1.
The experimental protocol
According to age and type of diet the animals were classified into adult balanced group; adult high cholesterol diet group; aged balanced group and aged high cholesterol diet group. At the end of the experiment, all rats were fasted for 12 hours, anaesthetized and blood samples were collected from retro orbital venous plexus. The specimens of the brain were obtained after an injection of the heart by 10% formalin.
Clinical assessment
All rats were observed for activity, bowel habits, food intake and changes in body weight that were calculated by using the following formula:
[10]
W1=Weight at the beginning of the experiment.
W2=Weight at the end of the experiment.
Biochemical assessment
The collected blood samples were used to detect serum levels of low density lipoprotein (LDL) [11], high density lipoprotein (HDL), cholesterol and triglycerides [12].
Histological assessment
Each brain was cut into two halves, one was fixed in 10% formalin and the other was fixed in Golgi Cox fixative and staining solution for two months. Each cerebral hemisphere was cut coronally into two halves to reach the site of hippocampus and then the specimens were dehydrated, cleared, and embedded in paraffin blocks. Serial coronal sections were cut into 5-7 µm thick and stained with haematoxylin and eosin (H&E), toluidine blue [13], and Golgi Cox [14] stains.
Immunohistochemical assessment
The paraffin embedded tissues were used for immunohistochemical localization of glial fibrillary acidic protein (GFAP), a marker of astrocytes (1:50-1:100, no: M076101, Dako, Glostrup, Denmark). Formalin fixed paraffin embedded tissue sections were deparaffinized and endogenous peroxidase was blocked with H2O in methanol. The sections were heated in 0.01 mol/l citrate buffer in a microwave pressure cooker for 20 minutes. The slides were allowed to cool to the room temperature, and nonspecific binding was blocked with normal horse serum for 20 minutes at the room temperature. The MIB-1 monoclonal antibody was used for detection of nuclear GFAP. Counterstaining was done using Mayer's hematoxylin (Cat. No.94585, BioGenex, Antony, France) [15].
Morphometric assessment
Five different H&E-stained sections from five different rats were examined in each group to 1) count the number of pyramidal cells in H&E sections and the number of astrocytes in GFAP-immunostained sections [8]. 2) Measure the thickness of hippocampus [16]. The data were obtained by using computerized image analyzer (Lecia Imaging System Ltd., Cambridge, England). Hippocampus sections were randomly selected for morphometric measurements. Ten readings were obtained in each specimen and the mean values were obtained.
The scoring system for toluidine blue was tested according to the following semi-quantitative scale: (-), negative; (+), very weakly positive; (++), weakly positive; (+++), moderately positive; (++++), strongly positive; (+++++), very strongly positive.
Statistical analysis
All statistical analyses were performed with SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). Measurement data were expressed as mean±SD. Mann-Whitney and Wilcoxon analysis of variance were used to compare continuous variables among groups. P<0.05 was considered statistically significant.
Results
General appearance and body weight of the animals
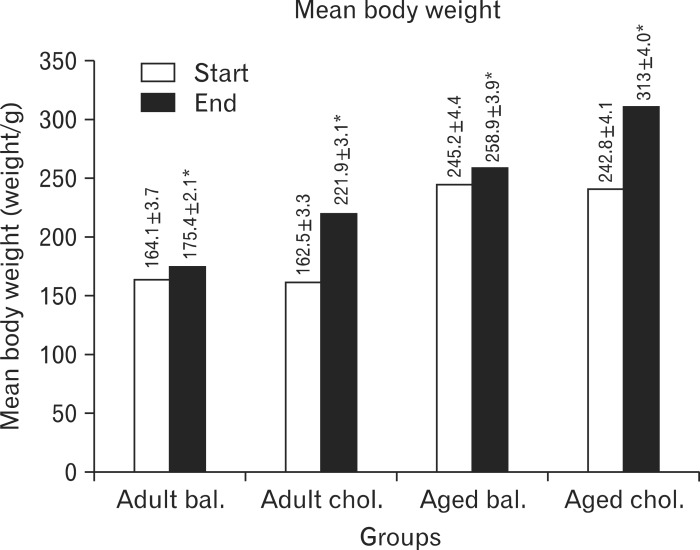
All the rats in the adult and aged groups showed no change in their physical activity or in the bowel habits all over the period of the experiment. However, there was an increase in food intake observed from the second week till the end of the experiment in high cholesterol rats as compared to balanced rats. All the animals showed a significant increase in the body weight at the end of the experiment as compared to the beginning of the experiment (P<0.05). However, the percentage (%) of increase in the body weight was higher in the rats that received high cholesterol diet (adult 36.6% and aged 28.9%) than in those that received balanced diet (adult 6.9% and aged 5.6%) (Fig. 1).
Fig. 1.
Mean body weight of the experimental groups (in grams) at the start and the end of the experiment. bal., balanced; chol., cholesterol. *Significant difference (P<0.05).
Biochemical results
In both adult and aged groups, the serum of cholesterol fed animals showed a significant decrease in the level of HDL (P<0.01) and increase in the levels of LDL (P<0.01), total cholesterol (P≤0.001), and triglycerides (P<0.01), when compared with the balanced groups. However, these changes were more significant in the aged than in the adult groups (P≤0.01) (Table 2).
Table 2.
Mean±SD of the serum levels (in mg/dl) lipid profile in experimental groups
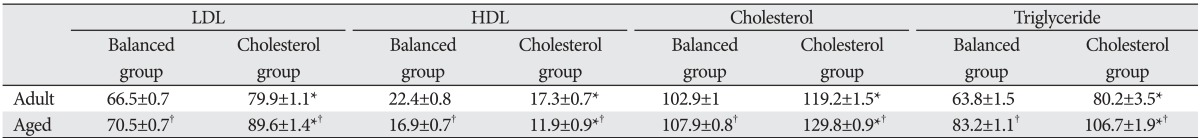
SD, standered deviation; LDL, low density lipoprotein; HDL, high density lipoprotein. *Significant in comparison with balanced group of the same age. †Significant in comparison with the corresponding adult group.
Histological and immunohistochemical results
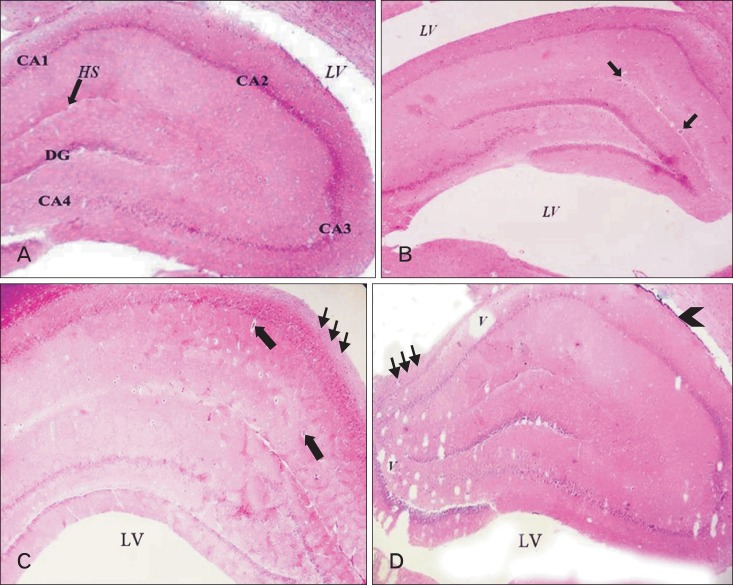
Light microscopic results of H&E-stained sections in adult balanced diet fed rat revealed that the hippocampus is composed of four areas; CA1-CA4 (Fig. 2A). Each area appeared in three layers; polymorphic, pyramidal, and molecular. Capillaries and different glial cells scattered inside molecular and polymorphic layers (Fig. 3A). Microglia, oligodendroglia and astrocytes constituted the glial cell types (Fig. 4A). The pyramidal nerve cells appeared as large triangular cells with large vesicular nuclei and prominent processes (Fig. 5A). Comparing the hippocampus of adult balanced diet fed rats with aged balanced diet fed rats, it could be noted that the aged rats showed a significant (P<0.01) decrease in the hippocampus thickness (Table 3, Fig. 2B), slight disorganization of the pyramidal cell layer (Fig. 3B), few degeneration of pyramidal cells and slight spongiosis (Fig. 4B). Also, there were senile neuritic plaques, intracellular Hirano's bodies, and lipofuschin pigmentation (Fig. 5B, C).
Fig. 2.
A section of hippocampus showing: (A) adult balanced diet fed rats composed of four areas: CA1, CA2, CA3, and CA4. There is a narrow hippocampus sulcus (HS), and dentate gyrus (DG). (B) Aged balanced diet fed rats shows, apparent decrease in the hippocampus thickness compared with adult balanced. Also, there is increased vascularization (arrows) inside the hippocampus sulcus. (C) Adult cholesterol diet fed rats shows, vascularization (arrows) and peripheral degeneration (thin arrows). (D) Aged cholesterol diet fed rats shows, multiple vacuolations (V) especially in CA1 area, fibrotic meninges (arrowhead) and peripheral degeneration (thin arrows). LV, lateral ventricle (H&E, ×40).
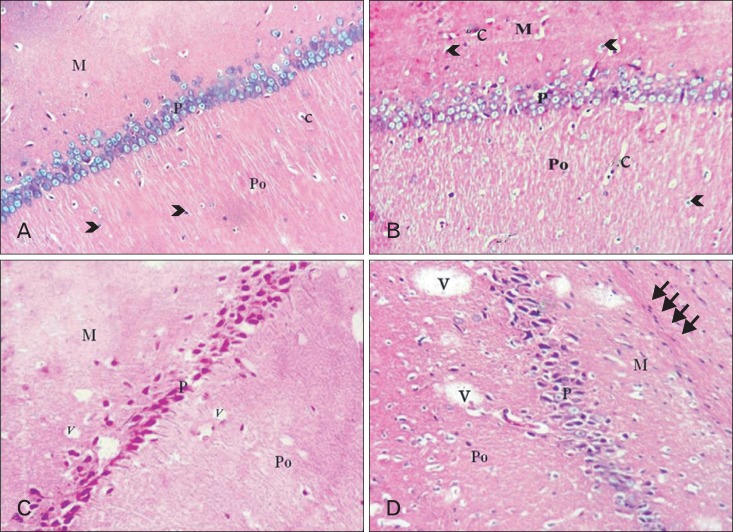
Fig. 3.
CA1 area of hippocampus. (A) Adult balanced diet fed rats shows, molecular (M), pyramidal (P), and polymorphic (Po) layers. The glial cells (arrowheads) and capillaries (c) are scattered inside molecular and polymorphic layers. (B) Aged balanced diet fed rats shows, slight disorganization of the pyramidal cell layer (P) with presence of many capillaries (c) and neuralgia cells (arrowheads). (C) Adult cholesterol diet fed rats shows, disorganization, degeneration and shrinkage of all pyramidal cells and presence of dispersed small vacuolations (V). (D) Aged cholesterol diet fed rats shows, shrinkage and darkness of all pyramidal cells, vacuolations (V) and massive neurogliosis and degeneration (thin arrows) in the molecular layer (M) (A-D, H&E, ×100).
Fig. 4.
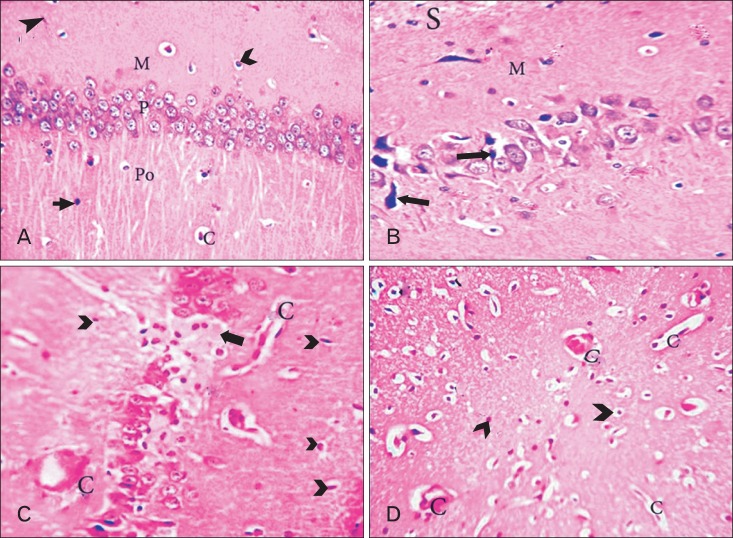
CA1 area of hippocampus. (A) Adult balanced diet fed rats shows, well organized pyramidal cells. There are different types of the neuralgia cells are scattered inside the neuropil matrix such as astrocytes (tailed arrowhead) with small vesicular nuclei; oligodendroglia (hollow arrowhead) with small dark nuclei and perinuclear halos and microglia with rod-shaped nuclei (triangle arrowhead). P, pyramidal layer; Po, polymorphic layer. (B) Aged balanced diet fed rats shows, apparent decrease in the number of pyramidal cells with presence of some shrunken degenerated cells (black arrows). There is slight vacuolations (spongiosis) (S) in the neuropil of molecular layer (M). (C) Adult cholesterol diet fed rats shows, hyalinized pyramidal cells with eccentric nuclei (black arrow), many glial cells (arrowheads) and presence of both thickened and attenuated capillaries (c). (D) Aged cholesterol diet fed rats shows, increased number of thickened and attenuated capillaries (c) with gliosis (arrowheads) (A-D, H&E, ×400).
Fig. 5.
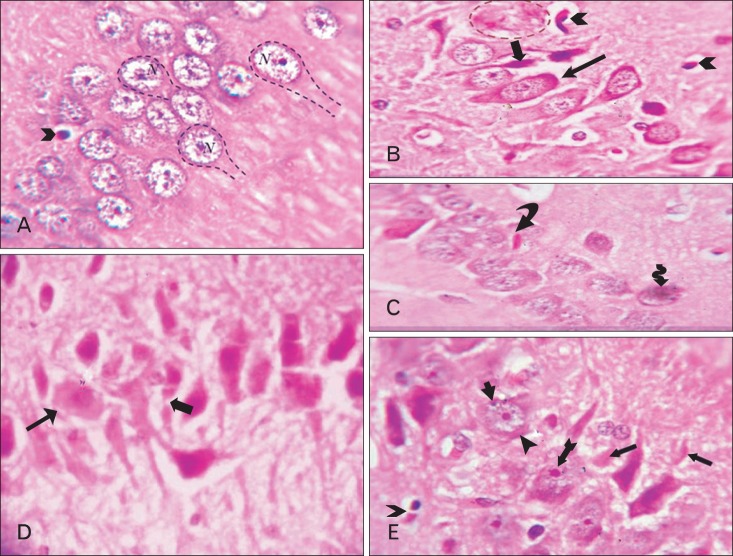
CA1 area of hippocampus. (A) Adult balanced diet fed rats shows, typical pyramidal cells (dashed lines) with triangular bodies, basophilic rim of cytoplasm, large vesicular nuclei (N) and prominent nucleoli. The oligodendroglia cell (arrowhead) lies adjacent to the pyramidal cell membrane. (B, C) Aged balanced diet fed rats shows, a senile neuritic plaque (dashed circle), apoptotic oligodendroglia (arrowheads) and the pyramidal cells either sclerotic (thin arrow) or dark shrunken (thick arrow). (D) Adult cholesterol diet fed rats shows, eosinophilic degenerated pyramidal cell with disintegrated axon (thick arrow), other pyramidal cells become swelling (thin arrow). Also, there are eosinophilic Hirano body (curved arrow) and Lipofuscin pigmentation (corrugated arrow). (E) Aged cholesterol diet fed rats shows, extra neural Hirano bodies (arrows), both intracellular tangles (triangle arrowhead) and granulovacuolar degeneration (tailed arrowhead), Lewy's body (crossed arrow) and apoptotic oligodendroglia (hollow arrowhead) (A-E, H&E, ×1,000).
Table 3.
Mean±SD of both thickness of hippocampus and pyramidal cells number

SD, standered deviation. *Significant in comparison with balanced group of the same age. †Significant difference from corresponding adult group.
Comparing the adult balanced diet fed rats with the adult cholesterol diet fed rats, hippocampus showed, no significant change in thickness (P≥0.05), numerous blood capillaries and peripheral degeneration (Table 3, Fig. 2C). Dispersed small vacuolations, disorganization and shrinkage of the pyramidal cells (Fig. 3C). Also, there was hyalinized pyramidal cells with eccentric nuclei, presence of either thickened and attenuated capillaries (Fig. 4C) and many glial cells. Some pyramidal cell showed disintegrated axon (Fig. 5D).
Comparing the aged balanced diet fed rats with the aged cholesterol diet fed, the hippocampus of the latter showed multiple vacuolations specially in CA1 area, meningeal fibrosis and peripheral degeneration with no significant change in hippocampus thickness (P≥0.05) (Fig. 2D). Also, there was many degenerated pyramidal cells, vacuolations and degeneration in the molecular layer (Fig. 3D) with massive neurogliosis (Figs. 3, 4D) and many thickened and attenuated capillaries (Fig. 4D). In addition, extra neural Hirano bodies, intracellular tangles and granulovacuolar degeneration, Lewy bodies and apoptotic oligodendroglia appeared (Fig. 5E).
Comparison between adult and aged groups showed that there was a significant decrease in the mean number of pyramidal cells (P<0.001) in all aged groups than in all adult groups. In both adult and aged animals, the number of pyramidal cells showed a significant decrease in the cholesterol groups, when compared with the balanced groups. This decrease was more significant in the aged (P<0.001) than in the adult rats (P<0.01) (Table 3).
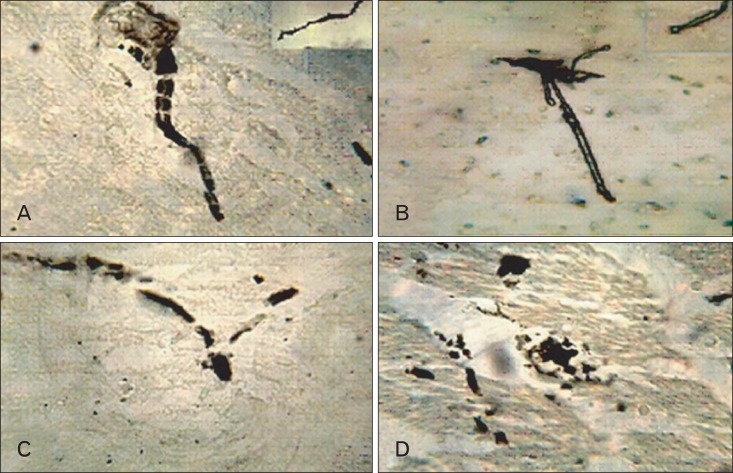
Golgi Cox-stained sections recorded the shape of pyramidal cells in adult balanced group (Fig. 6A) in comparison to others groups. They revealed that, the pyramidal cell in aged group showed sclerotic appearance, body deformity, short thickened hollow axon and few non-branched spineless hollow dendrites (Fig. 6B); adult cholesterol cell showed ballooned non-dendritic body with disintegrated axon (Fig. 6C) and lastly, aged cholesterol cells showed; non-dendritic clumping body with severely disintegrated axon (Fig. 6D).
Fig. 6.
CA1 area pyramidal cells. (A) Adult balanced diet fed rats shows with triangle cell body with nodded axon and multiple branched dendrites with spines (inset) (B). (B) Aged balanced diet fed rats with sclerotic cell membrane, short hollow axon and few non branched, hollow, spineless dendrites (inset). (C) Adult cholesterol diet fed rats shows, with ballooned non-dendritic body and disintegrated axon. (D) Aged cholesterol diet fed rats shows, with either clumped or ballooned non-dendritic body and severely disintegrated axon (A-D, Golgi Cox, ×1,000).
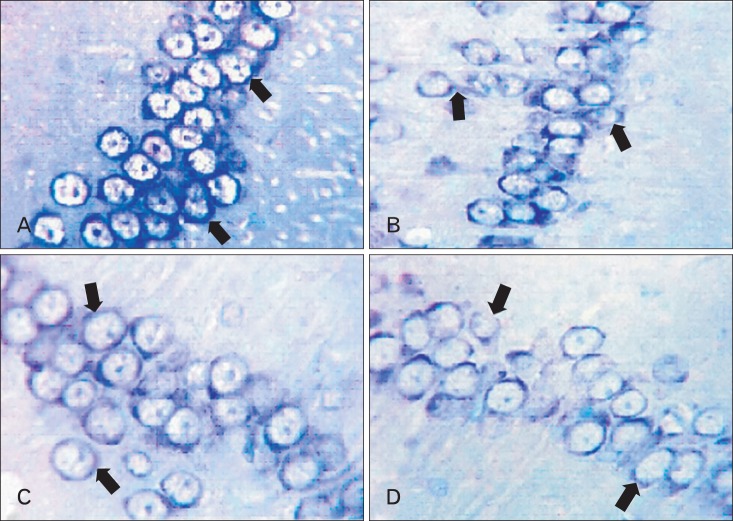
Light microscopic results of toluidine blue stained sections recorded the strongest pyramidal cell activity (Nissl's granules density) in the adult balanced group (++++) (Fig. 7A) in comparison with other experimental groups. The activity became decreased in aged balanced group (Fig. 7B) and adult cholesterol group (++) (Fig. 7C) and very decreased in aged cholesterol group (+) (Fig. 7D).
Fig. 7.
CA1 area of hippocampus. (A) Adult balanced diet fed rats shows, strong (++++) positive touildine blue expression (arrows). (B, C) Aged balanced diet fed rats and adult cholesterol diet fed rats show, week positive (++) toluidine blue expression (arrows). (D) Aged cholesterol diet fed rats shows, very week (+) positive touildine blue expression in aged cholesterol (arrows) (A-D, toluidine blue, ×1,000).
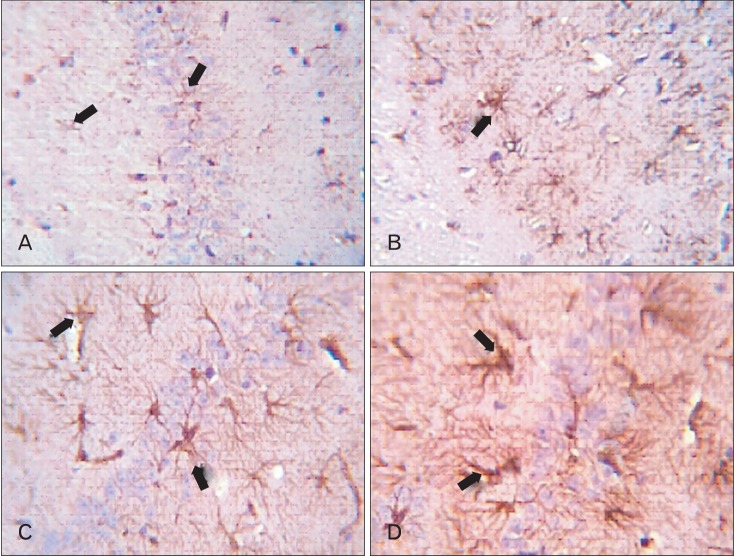
Immunohistochemical results for GFAP-stained sections revealed that CA1 area had a cytoplasmic brown reaction in few small dispersed non-branched astrocytes in adult balanced diet fed rats (Fig. 8A), small branched astrocytes in aged balanced diet fed rats (Fig. 8B), and large branched astrocytes in both adult and aged cholesterol rats (Fig. 8C, D). The reaction became very strong in the latter. Comparison between adult and aged groups showed that there was a significant increase in the number of astrocytes (P<0.0001) in all aged groups than in all adult groups. In both adult and aged animals, the number of astrocytes showed a significant increase in the cholesterol groups, when compared with the balanced groups (P<0.0001) (Table 3).
Fig. 8.
CA1 area of hippocampus. (A) Adult balanced diet fed rats shows, slight positive brown glial Fibrillary acidic protein (GFAP) reaction small non branched dispersed astrocytes (arrows). (B) Aged balanced diet fed rats shows, positive brown expression of GFAP in small branched astrocytes (arrow). (C) Adult cholesterol diet fed rats shows, positive brown expression of GFAP in large branched astrocytes (arrows). (D) Aged cholesterol diet fed rats shows, dense positive brown expression of GFAP in large branched astrocytes (arrows) (A-D, GFAP, ×1,000).
Discussion
In the present study, the possibility that aging process decreases the memory function might be proved through the decrease in the amount of Nissl's granules and the increase in number and size of astrocytes [8]. Moreover, through the disarrangment and shrinkage of pyramidal cells [17], the decrease in dendritic arborisation and spine numbers, the latter was assured by a notable correlation between age-related reductions in spine densities and age-dependent declines in learning and memory [18]. Also, the synchronization of lipofuscin pigment neuritic plaques, granulovacuolar degeneration, and Hirano bodies recorded in this study might be associated with memory decline, as they had been observed in the hippocampus of elderly as well as in Alzheimer's disease [4]. The pathogenesis of hippocampus aging was proven to occur through several mechanisms; the blood vessel pathology detected in the present study supported the reduction in plasticity of the brain microvasculature as a causing mechanism [19]. In the present study, the histological and morphometric results of high cholesterol diet fed adult rats were observed by other researchers [20, 21, 22]. In the present study, high cholesterol diet fed to aged rats resulted in aggravation of senility changes of the hippocampus together with marked pathological changes that accompanied cholesterol administration. Several studies have been made to know how high cholesterol diet can produce these changes in the hippocampus. The exact mechanism may be due to neurodegeneration that could be attributed to the increase in the expression of inflammatory mediators [23] or to the atherosclerosis that accompanies hypercholesterolemia [24]. The present study doubt on the possibility of occurrence of Alzheimer's disease in old age when high cholesterol diet is taken for a long time. This could be proven through; the pathological changes observed in the microvessels of the hippocampus as [20] who found this similar to the vascular pathology seen in Alzheimer's disease. Also, through the increase in numbers of neuritic plaques when associated with the increase in the numbers of tangles and both triggering the degree of cognitive decline [25]. Also, Lewy's bodies noticed in this work might trigger dementia through blocking the effects of dopamine and acetylcholine [26]. Moreover, Lewy's bodies often co-occur with Alzheimer's disease if accompanied with senile plaques and granulovacuolar degeneration [27]. The free extracellular Hirano bodies as well as neurofibrillary tangles (NFTs) or 'ghost tangles' might be the remnant of the dead neurons [28]. The increase in tangles' number correlated with the incidence of Alzheimer's disease [25].
NFT and senile plaques are the main pathological features of Alzheimer's disease. They are formed by accumulation of abnormally hyperphosphorylated tau and proteolytic processing of amyloid precursor protein (APP) into β-amyloid peptide (Aβ) respectively [29]. Hypercholesterolemia strongly accelerated Aβ accumulation and tau pathology that accompanied by microglia activation and aggravation of memory impairment in mouse models [30] and rabbits [31]. A diet-induced hypercholesterolemia increased Aβ levels and extracellular deposition in the brain in transgenic mouse model of Alzheimer's disease [32, 33, 34]. A disturbed cholesterol homeostasis within lipid rafts might influence APP processing [35, 36]. Also, cholesterol disturbance in diabetic rat increases tau phosphorylation that might be due to either increase JNK activity or activation of the tau kinase, glycogen synthase kinase 3β [37, 38].
It could be concluded that, aging process has degenerative changes in the hippocampus. High cholesterol diet is a risk factor for the hippocampus of both adult and aged but it is more serious for the latter as it can produce Alzheimer's disease like pathological changes. So, it is recommended that a life style depending on a low cholesterol diet should be maintained.
Acknowledgements
We acknowledge the contribution of Menoufia University in funding this study.
References
- 1.Wimo A, Prince M. The global economic impact of dementia. A conceptual framework. Alzheimer's disease international [Internet] NHS Choices; 2010. [cited 2013 May 29]. Available from: http://www.nhs.uk/Conditions/Dementia/Pages/Causes.aspx. [Google Scholar]
- 2.Kahn DA, Gwyther LP, Frances A. Agitation in older persons with dementia, expert consensus guideline series. A guide for families and caregivers [Internet] Alzheimer's Outreach; [cited 2014 May 16]. Available from: http://www.zarcrom.com/users/alzheimers/ [Google Scholar]
- 3.Pearce JM. Ammon's horn and the hippocampus. J Neurol Neurosurg Psychiatry. 2001;71:351. doi: 10.1136/jnnp.71.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mills SE. Histology for pathologists. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 274–319. [Google Scholar]
- 5.Driscoll I, Sutherland RJ. The aging hippocampus: navigating between rat and human experiments. Rev Neurosci. 2005;16:87–121. doi: 10.1515/revneuro.2005.16.2.87. [DOI] [PubMed] [Google Scholar]
- 6.Elias PK, Elias MF, D'Agostino RB, Sullivan LM, Wolf PA. Serum cholesterol and cognitive performance in the Framingham Heart Study. Psychosom Med. 2005;67:24–30. doi: 10.1097/01.psy.0000151745.67285.c2. [DOI] [PubMed] [Google Scholar]
- 7.Ando K, Higami Y, Tsuchiya T, Kanematsu T, Shimokawa I. Impact of aging and life-long calorie restriction on expression of apoptosis-related genes in male F344 rat liver. Microsc Res Tech. 2002;59:293–300. doi: 10.1002/jemt.10207. [DOI] [PubMed] [Google Scholar]
- 8.Abdel Tawab SM, Nada HF, Mohammed SA, Bahaa ES. Histological and immunohistochemical study on the effect of hypo and hypercaloric diet on the structure of hippocampus of male albino rat. Egypt J Histol. 2008;31:103–114. [Google Scholar]
- 9.Granholm AC, Bimonte-Nelson HA, Moore AB, Nelson ME, Freeman LR, Sambamurti K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J Alzheimers Dis. 2008;14:133–145. doi: 10.3233/jad-2008-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carol JV. Calculating percent increase and decrease. A conceptual framework [Internet] Honolulu: Atlantic International University; c2004-2013. [cited 2013 May 30]. Available from: http://www.onemathematicalcat.org/algebra_book/online_problems/calc_percent_inc_dec.htm. [Google Scholar]
- 11.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 12.Rifai N, Bachori KP, Alber S. Lipids, lipoprotein and apolipoprotein. In: Aldrich JE, editor. Tiez Fundamental of Clinical Chemistry. 5th ed. Pennsylvania: W.B. Saunder Co.; 2001. pp. 484–489. [Google Scholar]
- 13.Kiernan JA. Histological and histochemical methods: theory and practice. 3rd ed. Oxford: Linacre House, Jordan Hill; 1999. [Google Scholar]
- 14.Drury RA. Wallington EA. Carleton's histological techniques. 5th ed. New York: Oxford University Press; 1980. [Google Scholar]
- 15.Bancroft JD, Gamble M. Theory and practice of histological techniques. 5th ed. London: Churchill Livingstone; 2002. [Google Scholar]
- 16.Mostafa H, Anwar M, Ahmed S, Yahia N. Effect of vincamine on age related structural changes in the frontal cortex and the hippocampus of male albino rat [dissertation] Cairo: Ain Shams University; 2006. p. 188. [Google Scholar]
- 17.Hashem HE, Elmasry SM, Eladl MA. Dentate gyrus in aged male albino rats (histological and Tau-immunohistochemical study) Egypt J Histol. 2010;33:659–670. [Google Scholar]
- 18.von Bohlen und Halbach O, Zacher C, Gass P, Unsicker K. Age-related alterations in hippocampal spines and deficiencies in spatial memory in mice. J Neurosci Res. 2006;83:525–531. doi: 10.1002/jnr.20759. [DOI] [PubMed] [Google Scholar]
- 19.Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res Rev. 2003;2:149–168. doi: 10.1016/s1568-1637(02)00064-8. [DOI] [PubMed] [Google Scholar]
- 20.Franciosi S, Gama Sosa MA, English DF, Oler E, Oung T, Janssen WG, De Gasperi R, Schmeidler J, Dickstein DL, Schmitz C, Gandy S, Hof PR, Buxbaum JD, Elder GA. Novel cerebrovascular pathology in mice fed a high cholesterol diet. Mol Neurodegener. 2009;4:42. doi: 10.1186/1750-1326-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohamed GF, Ahmed MA, Omar SM. Light and electron microscopic study on the effect of caffeine on blood brain barrier integrity in a rabbit model of Alzheimer's disease. Egypt J Histol. 2010;33:467–478. [Google Scholar]
- 22.Dickson DW, Weller RO. Neurodegeneration: the molecular pathology of dementia and movement disorders. 2nd ed. New Jersey: Wiley-Blackwell; 2011. pp. 62–91. [Google Scholar]
- 23.Rahman SM, Van Dam AM, Schultzberg M, Crisby M. High cholesterol diet results in increased expression of interleukin-6 and caspase-1 in the brain of apolipoprotein E knockout and wild type mice. J Neuroimmunol. 2005;169:59–67. doi: 10.1016/j.jneuroim.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Cao D, Garber DW, Kim H, Fukuchi K. Association of aortic atherosclerosis with cerebral beta-amyloidosis and learning deficits in a mouse model of Alzheimer's disease. Am J Pathol. 2003;163:2155–2164. doi: 10.1016/s0002-9440(10)63572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenblum WI. Neuropathology mini-course for residents. Dementias [Internet] Richmond: VCU Department of Pathology; 2007. [cited 2013 May 25]. Available from: http://www.pathology.vcu.edu/education/WirSelfInst/dementias.html. [Google Scholar]
- 26.Causes of dementia: a conceptual framework [Internet] NHS Choices; 2012. [cited 2013 May 29]. Available from: http://www.nhs.uk/Conditions/Dementia/Pages/Causes.aspx. [Google Scholar]
- 27.Iseki E, Togo T, Suzuki K, Katsuse O, Marui W, de Silva R, Lees A, Yamamoto T, Kosaka K. Dementia with Lewy bodies from the perspective of tauopathy. Acta Neuropathol. 2003;105:265–270. doi: 10.1007/s00401-002-0644-3. [DOI] [PubMed] [Google Scholar]
- 28.Ha S, Furukawa R, Stramiello M, Wagner JJ, Fechheimer M. Transgenic mouse model for the formation of Hirano bodies. BMC Neurosci. 2011;12:97. doi: 10.1186/1471-2202-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gómez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- 30.Park SH, Kim JH, Choi KH, Jang YJ, Bae SS, Choi BT, Shin HK. Hypercholesterolemia accelerates amyloid beta-induced cognitive deficits. Int J Mol Med. 2013;31:577–582. doi: 10.3892/ijmm.2013.1233. [DOI] [PubMed] [Google Scholar]
- 31.Sparks DL, Scheff SW, Hunsaker JC, 3rd, Liu H, Landers T, Gross DR. Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol. 1994;126:88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- 32.Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 33.Levin-Allerhand JA, Lominska CE, Smith JD. Increased amyloid-levels in APPSWE transgenic mice treated chronically with a physiological high-fat high-cholesterol diet. J Nutr Health Aging. 2002;6:315–319. [PubMed] [Google Scholar]
- 34.Shie FS, Jin LW, Cook DG, Leverenz JB, LeBoeuf RC. Diet-induced hypercholesterolemia enhances brain A beta accumulation in transgenic mice. Neuroreport. 2002;13:455–459. doi: 10.1097/00001756-200203250-00019. [DOI] [PubMed] [Google Scholar]
- 35.Ghribi O. Potential mechanisms linking cholesterol to Alzheimer's disease-like pathology in rabbit brain, hippocampal organotypic slices, and skeletal muscle. J Alzheimers Dis. 2008;15:673–684. doi: 10.3233/jad-2008-15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simons M, Keller P, Dichgans J, Schulz JB. Cholesterol and Alzheimer's disease: is there a link. Neurology. 2001;57:1089–1093. doi: 10.1212/wnl.57.6.1089. [DOI] [PubMed] [Google Scholar]
- 37.Yoon SY, Park JS, Choi JE, Choi JM, Lee WJ, Kim SW, Kim DH. Rosiglitazone reduces tau phosphorylation via JNK inhibition in the hippocampus of rats with type 2 diabetes and tau transfected SH-SY5Y cells. Neurobiol Dis. 2010;40:449–455. doi: 10.1016/j.nbd.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Kim DH, Huh JW, Jang M, Suh JH, Kim TW, Park JS, Yoon SY. Sitagliptin increases tau phosphorylation in the hippocampus of rats with type 2 diabetes and in primary neuron cultures. Neurobiol Dis. 2012;46:52–58. doi: 10.1016/j.nbd.2011.12.043. [DOI] [PubMed] [Google Scholar]