Abstract
Excessive immune responses induced by ischemia-reperfusion injury (IRI) are known to lead to necrotic and apoptotic cell death, and calcineurin plays a major role in this process. Calcineurin dephosphorylates the nuclear factor of activated T-cells (NFAT), permitting its translocation into the nucleus. As a result, calcineurin promotes the release of pro-inflammatory cytokines, such as tumor necrosis factor-α. The overproduction of pro-inflammatory cytokines causes renal cell death. Calcineurin activity is regulated by calpain, a cysteine protease present in the nucleus. Calpain-mediated proteolysis increases the phosphatase activity of calcineurin, resulting in NFAT dephosphorylation. This process has been studied in cardiomyocytes but its role in renal IRI is unknown. Thus, we examined whether calpain regulates calcineurin in renal tubule nuclei. We established an in vivo renal IRI model in mice and identified the protective role of a calcineurin inhibitor, FK506, in this process. Calcineurin is expressed in the nucleus, where it is present in its calpain-cleaved form. FK506 reduced nuclear expression of calcineurin and prevented calcineurin-mediated NFAT activation. Our study shows clearly that FK506 reduces calpain-mediated calcineurin activity. Consequently, calcineurin could not maintain NFAT activation. FK506 reduced renal cell death by suppressing the transcription of pro-inflammatory cytokine genes. This study provides evidence that FK506 protects against inflammation in a renal IRI mouse model. We also provided a mechanism of calcineurin action in the nucleus. Therefore, FK506 could improve renal function by decreasing calcineurin activity in both the cytoplasm and the nucleus of renal tubule cells.
Keywords: Calcineurin, Calpain, FK506, Ischemic kidney
Introduction
Ischemia-reperfusion injury (IRI) is an inevitable consequence of renal transplantation and appears in early kidney transplant dysfunction [1, 2]. IRI results in acute kidney failure, which is a major cause of delayed graft function and leads to allograft rejection [3].
The pathogenesis of IRI is not completely understood but has been demonstrated to be related to inflammatory responses and consequent renal cell death. Infiltrating leukocytes damage the renal membrane and produce various pro-inflammatory cytokines. Thus, excessive immune responses result in renal cell death and cause allograft failure. In order to suppress inflammatory responses and increase the rate of transplant success, calcineurin inhibitors (CNIs) are widely used as immunosuppressant [2, 4].
Calcineurin is a serine/threonine phosphatase whose activity is required to mediate various intracellular calcium signaling pathways [5]. In response to various stimuli, calcineurin dephosphorylates transcription factors such as the nuclear factor of activated T-cells (NFAT) [6]. Dephosphorylated NFAT induces the release of interleukin-2, -3 and -4, interferon-γ, and tumor necrosis factor-α (TNF-α) and results in various immune responses [6]. Calcineurin, in particular, plays an essential role in T-cell activation [6] as a key component regulating T lymphocyte signal transduction [7, 8, 9, 10]. In myocardial ischemia, calcineurin has been shown to regulate both pro- and anti-apoptotic factors [7, 11]. Because of the properties of calcineurin, CNIs are widely used to suppress immune responses. FK506 is a well-known CNI [5, 11] that inhibits calcineurin activity though binding to the FK binding protein (FKBP) domain [11, 12, 13, 14]. However, FK506 has shown nephrotoxicity when used in excess, inducing renal cell death [10, 12].
In this study we examined the effects of FK506 in a renal IRI mouse model. First, we showed calcineurin expression induced by IRI and determined a minimum concentration of FK506 that is effective in reducing calcineurin activity without kidney damage. Calcineurin is composed of calcineurin A and calcineurin B subunits. FK506 has been shown to bind to FKBP12, which is a protein distinct from calcineurin A. The FK506-FKBP12 complex inhibits calcineurin activity [12, 15, 16]. Further, we suggest that the role of FK506 in the nucleus is different from its role in the cytoplasm. Nuclear calcineurin has been reported in myocardial hypertrophy [8, 17, 18, 19, 20]. According to these studies, calcineurin senses intracellular Ca2+ by undergoing a conformational change in its C-terminal autoinhibitory (AI) domain upon binding to Ca2+. Targeted proteolysis of the calcineurin AI domain by calpain increases calcineurin activity, which contributes to the translocation of NFAT to the nucleus [17]. In cardiomyocytes, calpain stimulation by angiotensin II causes the proteolytic cleavage of calcineurin [21]. The cleaved calcineurin functions in the nucleus with increased phosphatase activity. Once activated, calcineurin dephosphorylates NFAT, translocates to the nucleus, and activates NFAT-mediated transcription-inducing genes related to pro-inflammatory cytokines. Thus, we assume that FK506, a typical inhibitor of calcineurin, attenuates the cleavage of calcineurin and consequent inflammatory responses. In addition, we clearly demonstrated the expression of calcineurin and related inflammatory molecules through immunohistochemistry, which has been difficult to show directly in the kidney in IRI models in previous studies. Here, we re-evaluated FK506 as a new therapeutic agent as an anti-inflammatory factor for treating IRI as well as other, related diseases.
Materials and Methods
Animals
Male C57BL/6 mice were purchased from Central Laboratory Animal, Inc. (Seoul, Korea). Mice used in all experiments were 12 weeks old. These mice were housed in a specific pathogen-free facility with appropriate temperature and humidity and allowed free access to food and water. The mice for this study (GNU-121108-M0046) were approved by the Institutional Animal Care and Use Committee at Gyeongsang National University.
IRI mouse model
The mice were anesthetized by intramuscular injection of Zoletil (30 mg/kg, Virbac Laboratories, Carros, France) and Rompun (10 mg/kg, Bayer Korea, Seoul, Korea). The temperature of the anesthetized mice was adjusted to near 37℃ using a warming light. Animals were allowed to self-breathe room air. Both kidneys were exposed by a longitudinal incision, and the renal pedicle was occluded by atraumatic microaneurysm clamps for 25 minutes to induce ischemia. After 25 minutes, the clamp was removed for reperfusion. The sham group was performed by the same surgical method except that the clamping was not applied. FK506 was injected intraperitoneally 30 minutes before surgery. The concentrations were 0.01, 0.1, 1, 10, and 100 µg/kg. Twenty-four hours after surgery, the mice were anesthetized and perfused transcardially with heparinized saline. One kidney was removed and frozen in liquid nitrogen immediately and the other kidney was fixed in 4% neutral buffered formalin. Blood samples were collected to measure creatinine levels from the heart before perfusion.
Measurement of creatinine levels
Blood samples from the heart were collected in glass evacuated blood collection tubes (Greiner Bio-one, Frickenhausen, Germany) and centrifuged at 4℃. Blood serum analysis was requested for measurement of creatinine level at the Korea Green Cross Corp. (Yongin, Korea).
Immunohistochemistry
After fixation, kidney tissues were paraffin-embedded and cut into 5-µm-thick longitudinal sections. After deparaffinization, the slide samples were incubated with appropriate primary antibodies as follows. Antibodies against calcineurin (BD Bioscience, San Jose, CA, USA), neutrophil infiltration protein Ly6B.2 (clone 7/4, AbD Serotec, San Diego, CA, USA), TNF-α (Abcam, Cambridge, UK), caspase-3 (Asp175, Cell Signaling Technology, Danvers, MA, USA), NFATc1 (NFAT2, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and intercellular adhesion molecule-1 (ICAM-1; Sino Biological Inc., Beijing, China) were used. Sections were incubated with primary antibody at 4℃ overnight. After washing three times with 0.1 M phosphate buffered saline (PBS), sections were incubated with the biotin tagged an appropriate secondary antibody at room temperature (RT) for 1 hour. After washing three times with 0.1 M PBS, sections were incubated in avidin-biotin-peroxidase complex solution (Vector Laboratories, Inc., Burlingame, CA, USA) and developed with diaminobenzidine substrate kit (Vector Laboratories, Inc.). After dehydration, sections were mounted and visualized with a BX50 microscope (Olympus, Tokyo, Japan), and digital images were captured. Captures were randomly distributed on 5 areas of all field and represented as high power field using Image J.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
After fixation, kidney tissues were paraffin-embedded and cut into 5-µm-thick longitudinal sections. After deparaffinization, sections were treated with 3% H2O2 in methanol for peroxidase quenching, permeabilized with 0.1% Triton X-100, and labeled using the In Situ Cell Death Detection Kit (Roche Applied Science, Mannheim, Germany). The fluorescence images were captured with a BX50 microscope (Olympus). Statistical analysis was performed using the Image J.
Tissue fractionation and western blot
Frozen kidney tissues were fractionated using the Nuclear/Cytosol Fractionation Kit (BioVision Inc., Milpitas, CA, USA) according to the manufacturer's protocol. Protein concentration was determined using a bicinchoninic acid kit (Pierce, Rockford, IL, USA). Isolated nuclear and cytoplasmic extracts were separated by 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were incubated in blocking solution (3% bovine serum albumin in TBS containing 0.1% Tween-20) at RT for 1 hour and then with the following primary antibodies. Antibodies against calcineurin (BD Biosciences), calpain 1 (H-65), the cytosol marker PRX II (Santa Cruz Biotechnology), and the nuclear protein p84 (Abcam) were used. The protein bands were visualized by an enhanced chemiluminescence western blot detection system (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Digital images were capture with an ImageQuant LAS-4000 Imager (GE Healthcare Life Sciences, Buckinghamshire, UK) and analyzed by Image J.
Statistical analysis
Statistical analysis was performed using SPSS version 21 (IBM Co., Armonk, NY, USA). Results are represented as mean±SD. Analysis was based on three independent experiments, each of which included 4 mice per group. P-values were determined through one-way ANOVA, and less than 0.01 were considered statistically significant.
Results
Renal IRI mouse model and changes in creatinine levels
FK506 is a widely used immunosuppressant in most organ transplantation procedures [1, 22]. We examined the effects of FK506, which is known to inhibit calcineurin activity, and consequent renal cell death in renal ischemic injury. To address this, we established an in vivo renal IRI mouse model. FK506 pre-conditioning was performed by injection 30 minutes before surgery. By clamping renal arteries, we confirmed the color of kidney became darkened, and reperfused by unclamping. After 24 hours, the kidneys were surgically removed, and we found that the injured kidney displayed an infarct in the outer medulla compared to the control kidney, as shown in Fig. 1A.
Fig. 1.
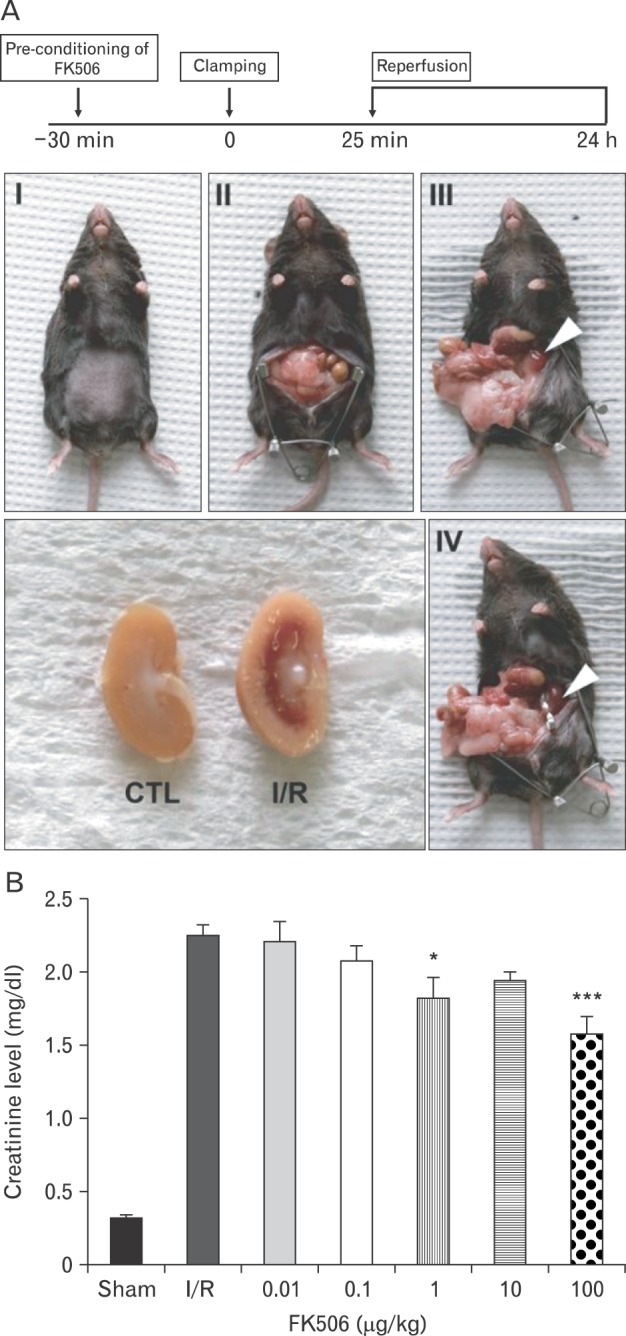
Establishment of in vivo model of renal ischemia-reperfusion injury (IRI). (A) FK506 pre-conditioning was performed 30 minutes before surgery. Renal pedicles were occluded by clamping for 25 minutes, and reperfusion followed by removing these clips. Arrowheads indicate the color change in the kidney from renal ischemia/reperfusion (I/R) mice. One day later, the cross section of the IRI kidney revealed hemorrhagic infarcts in the outer medulla. (B) Creatinine levels were measured in serum from I/R or FK506-preconditioned I/R mice. Creatinine levels in I/R mice increased to more than 5-fold those in the sham control (CTL) mice. FK506 reduced these levels, which was statistically significant at 1 µg/kg. All experiments were performed in triplicate and repeated twice. *P<0.01, ***P<0.0001.
We first examined the effect of FK506 on renal function in our IRI model. To determine the dose-dependent effect of FK506, the mice were treated with FK506 at various concentrations, as indicated. To assess renal function, we measured serum creatinine levels, elevations of which are indicative of renal filtration dysfunction. We confirmed that creatinine levels were significantly increased more than 5-fold in ischemia/reperfusion (I/R) mice compared to the sham control mice. FK506 caused reductions in creatinine levels at concentrations of 1 µg/kg or greater (Fig. 1B), showing that FK506 can improve renal function. In addition, FK506 dose-dependently reduced infarction of outer medulla (data not shown).
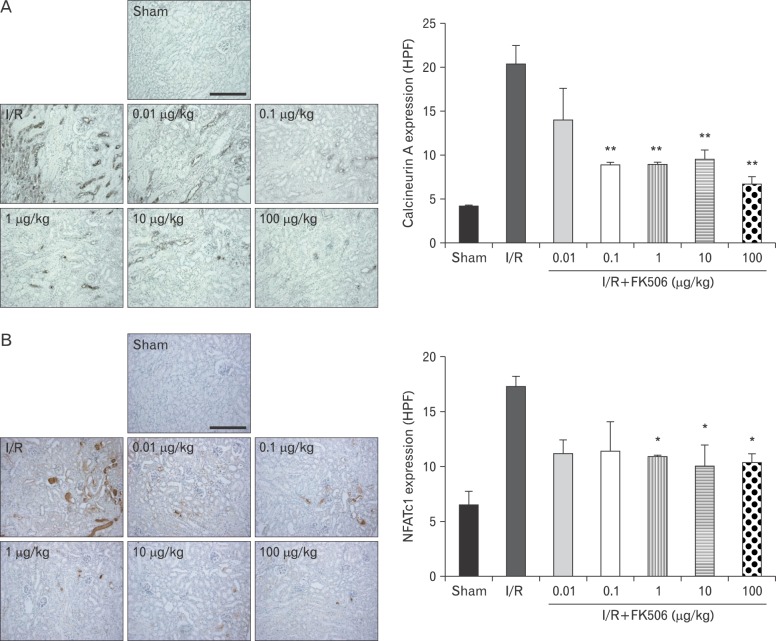
FK506 reduced the expression of calcineurin and NFAT2 in renal IRI mice
Because FK506 is a well-known inhibitor of calcineurin [5], we examined changes of calcineurin expression after FK506 treatment by performing immunohistochemistry (Fig. 2A). As previously reported [13, 23], calcineurin expression was observed in the proximal and distal tubules and was found to be highly expressed in the outer medulla after IRI. We confirmed that calcineurin expression was significantly increased by I/R and decreased by FK506 treatment at concentrations of 0.1 µg/kg or higher. The sham control kidneys had low levels of calcineurin. Therefore, these results demonstrate that FK506 is effective in reducing calcineurin expression.
Fig. 2.
FK506 reduced the expression of calcineurin and NFAT2. (A) Calcineurin expression was observed in the distal tubules near glomeruli and the tubules in the outer medulla. FK506 treatment gradually decreased the levels of calcineurin. I/R, ischemia/reperfusion; HPF, high power field. (B) NFAT2 expression was observed in the damaged tubules around glomeruli and in the outer medulla. In the FK506-treated group, NFAT2 expression was noticeably weaker or confined to the distal tubules. All experiments were performed in triplicate and repeated twice. *P<0.01, **P<0.001. Densitometry was measured from more than 10 random images per each sample. Scale bars=100 µm (A, B).
NFAT is an important transcription factor that directly regulates the expression of pro-inflammatory cytokines in T and B lymphocytes during adaptive immune responses [24, 25, 26, 27, 28]. Calcineurin dephosphorylates NFAT2 in the cytoplasm, and the dephosphorylated NFAT2 translocates to the nucleus. Nuclear NFAT regulates transcriptional activity of target genes. Thus, by immunohistochemistry we measured changes in NFAT2 expression following FK506 treatment (Fig. 2B). As expected, NFAT2 was dramatically induced in I/R mice about 3-fold compared to the sham control mice and reduced by FK506. Here, we showed that reduced expression by FK506 could negatively regulate the calcineurin-NFAT2 signaling. Eventually, FK506 mitigate the inflammation of kidney outer medulla by excessive expression of calcineurin-NFAT2.
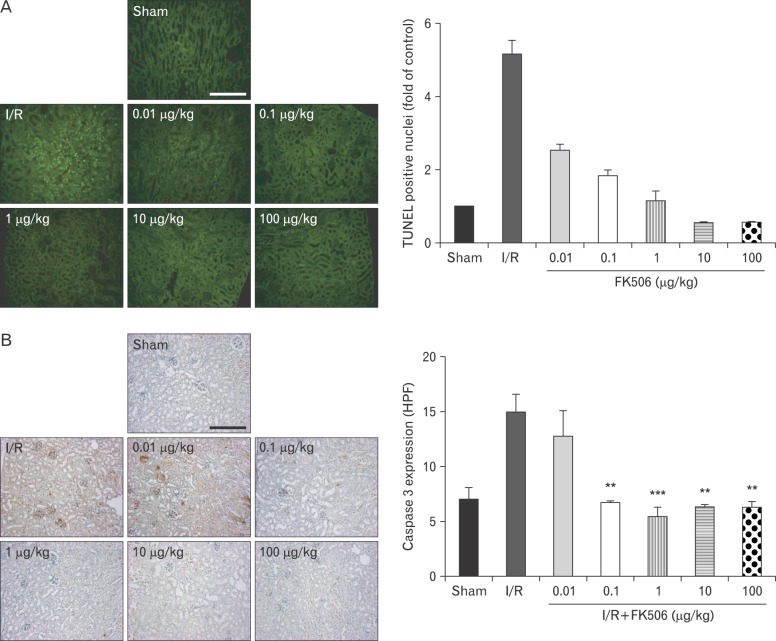
FK506 reduced apoptotic cell death, pro-inflammatory cytokine expression, and neutrophil infiltration protein in renal IRI mice
To investigate whether FK506 has a protective role in renal cell death, we performed the TUNEL assay. Apoptotic nuclear staining was evident in renal IRI mice and dramatically reduced by FK506 treatment to a level similar to that in sham control mice (Fig. 3A). Then, we examined levels of cleaved caspase-3 as an apoptotic marker by immunohistochemistry (Fig. 3B). In particular, a FK506 dose of 0.1 µg/kg, which is 1,000-fold lower than the widely used dose, was sufficient to clearly reduce caspase-3 expression.
Fig. 3.
FK506 reduced apoptotic cell death induced by renal ischemia-reperfusion injury. (A) The extent of apoptosis was determined by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. Noticeable reductions in renal cell death were observed in groups treated with FK506. I/R, ischemia/reperfusion. (B) Cleaved caspase 3 was measured as an apoptotic marker and was found to decrease significantly after FK506 treatment at doses of 0.1 µg/kg or higher. All experiments were performed in triplicate and repeated twice. HPF, high power field. **P<0.001, ***P<0.0001. Scale bars=100 µm (A, B).
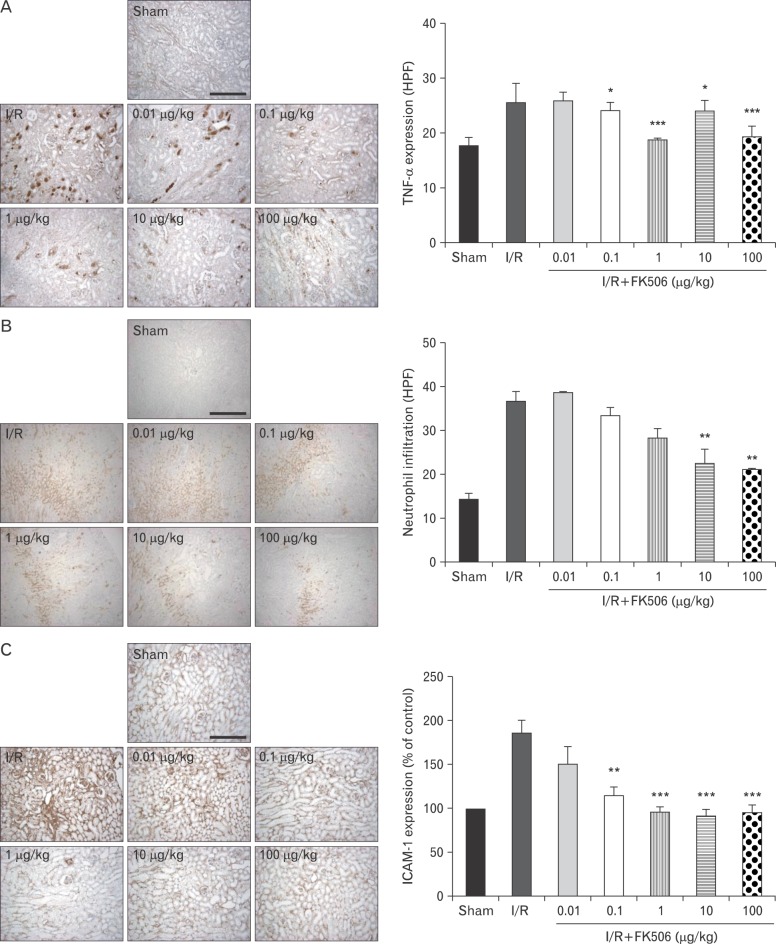
Then, we examined the inflammatory signaling pathways that could be affected by FK506 and measured the expression of TNF-α, a pro-inflammatory cytokine, by immunohistochemistry (Fig. 4A). The number of TNF-α-positive cells was significantly increased, and the immunoreactivity was intense in renal IRI mice compared to the sham control mice. TNF-α expression was strong in the proximal and distal tubules around the glomerulus, and FK506 particularly reduced its expression in distal tubules of the cortex and tubules in the outer medulla. We also found that calcineurin and TNF-α were expressed in identical regions.
Fig. 4.
FK506 reduced pro-inflammatory cytokine expression and neutrophil infiltration. Tumor necrosis factor-α (TNF-α) expression was strong in the tubules around glomeruli, where calcineurin expression was high, as shown in Fig. 2A. FK506 reduced TNF-α expression in the distal tubules in the cortex and the tubules in the outer medulla. I/R, ischemia/reperfusion; HPF, high power field. (B) Expression of Ly6B.2, a neutrophil infiltration marker, was observed at high levels in the outer medulla and particularly in the interstitium. Levels of Ly6B.2 were significantly reduced by FK506 treatment. (C) Expression of intercellular adhesion molecule-1 (ICAM-1) was observed mostly in the tubules and interstitium. FK506 decreased ICAM-1 to sham control levels. All experiments were performed in triplicate and repeated twice. *P<0.01, **P<0.001, ***P<0.0001. Scale bars=100 µm (A-C).
Stimuli such as neutrophil infiltration evoke excessive expression of pro-inflammatory cytokines and are also known to mediate IRI [29]. Thus, we investigated whether FK506 affects neutrophil infiltration by immunohistochemistry (Fig. 4B). Staining of neutrophil infiltration protein Ly6B.2 was apparent in the outer medulla of IRI mice and increased about 3-fold compared to the sham control mice. In addition, neutrophil infiltration was apparent in the interstitium, where blood vessels are mostly distributed, of the I/R mice. However, the level of neutrophil infiltration in the interstitial tubules was greatly reduced after FK506 treatment. These results suggest that FK506 exerts its anti-apoptotic function in IRI through reducing inflammatory cytokines and neutrophil infiltration.
FK506 reduced extracellular matrix accumulation in renal IRI mice
Excessive pro-inflammatory cytokines can induce accumulation of the extracellular matrix (ECM), which is known to cause renal diseases such as fibrosis [30] and diabetic nephropathy [7, 23, 30]. We observed that FK506 treatment decreased TNF-α, expression and therefore would suppress ECM accumulation. Thus, we examined the expression of ICAM-1. ICAM-1 is a well-known ligand for integrin LFA-1 and regulates the recruitment of inflammatory leukocytes. As shown in Fig. 4C, we found that ICAM-1 expression was dramatically increased in I/R mice and FK506 treatment suppressed ICAM-1 expression to a level similar to that in the sham control mice. ICAM-1 was normally expressed in the interstitium of the kidney, where blood vessels are distributed, as shown in the sham mice. ICAM-1 expression was further induced in the glomerulus, where capillaries are distributed, in the I/R mice. Thus, we found strong ICAM-1 staining in the thickened interstitium. FK506 at a dose of 1 µg/kg was sufficient to reduce the expression of ICAM-1.
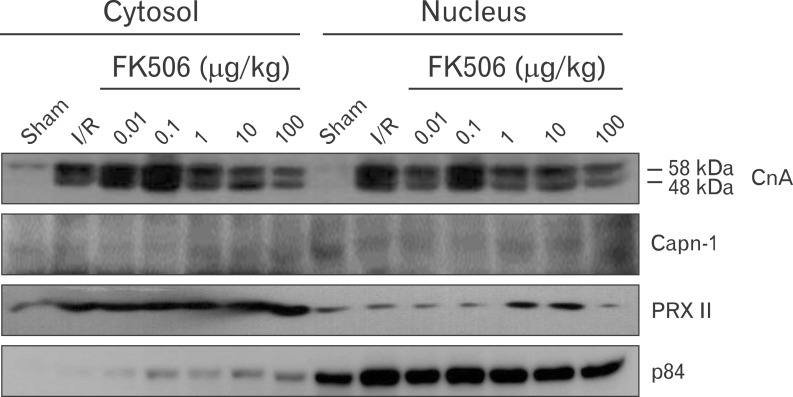
FK506 reduced calpain-mediated calcineurin activity
We next investigated the activity of calcineurin in the nucleus after renal IRI. In cardiomyocytes, calcineurin is regulated by calpain-mediated proteolysis [8, 17]. We assumed that calcineurin activity might be regulated in a similar way in the kidney and play a crucial role during renal IRI. Thus, we prepared cytosolic and nuclear extracts from kidney of IRI or sham control mice and examined calcineurin expression patterns (Fig. 5). We detected the 48-kDa cleaved form of calcineurin along with the 58-kDa full-length form of calcineurin. The cleaved form of calcineurin was found only in IRI mice and was presumably processed by calpain. This result showed that FK506 decreased calcineurin expression and further reduced levels of the cleaved form of calcineurin. FK506 did not appear to affect calpain activity directly; no substantial changes were observed in nuclear calpain levels. This result was confirmed by immunohistochemistry using calpain-1 antibody (data not shown). We suggest that FK506 reduces the activity of calcineurin in both the nucleus and the cytoplasm and that particularly in the nucleus FK506 has a cytoprotective effect by inhibiting NFAT2-mediated transcriptional activity.
Fig. 5.
FK506 inhibits the calpain-mediated proteolytic cleavage of calcineurin. The nucleus and cytoplasm were separated by tissue fractionation, and specific protein expression was analyzed by western blot. Putative cleaved calcineurin (CnA) was detected in both the cytosolic and nuclear fractions. FK506 decreased the expression of calcineurin in a dose-dependent manner. Calpain (Capn-1) expression appeared slightly higher in the nucleus but was not altered by FK506 treatment. The cytosolic PRX II and nuclear p84 proteins were used as markers of the cytosolic and nuclear fractions, respectively. All experiments were performed in triplicate and repeated twice. I/R, ischemia/reperfusion.
Discussion
Renal IRI involves a complex pathophysiologic mechanism, and calcineurin has been proposed as a major molecular mediator of IRI [13]. It is a Ca2+/CaM-dependent protein phosphatase and is an important Ca2+ effector promoting necrosis and apoptosis [23, 31, 32, 33, 34, 35]. Calcineurin activity is predominant in the cytoplasm. However, we suggest here that calcineurin has activity in the nucleus, particularly in a renal I/R model. Previously, its role in the nucleus has been discussed during ischemia in other tissues. In this study, we reinvestigate FK506, a well-known CNI, and confirmed its role in reducing expression of the pro-inflammatory cytokine TNF-α and decreasing neutrophil infiltration. Despite its beneficial roles in reducing inflammatory responses, FK506 exhibits adverse effects when administered in high doses [10, 22, 36]. Therefore, we sought the lowest effective dose of FK506 and demonstrated that 1 µg/kg is sufficient, which is 1,000 times less than the normal dose (1 mg/kg).
We thought that FK506 could improve renal function. Thus, we measured serum creatinine level. However, serum creatinine level is not sufficiently explained renal function. To assess renal function, glomerulus filtration rate is required. Therefore, we could be restricted to only anti-inflammatory effects of FK506. Fig. 1B showed serum creatinine level decreasing by FK506 treatment. The creatinine level was clearly reduced, but not significant than pro-inflammatory cytokine level. The creatinine level is closely linked to the renal failure and impairment. However, creatinine level is influenced by various factors such as status of muscle, protein uptake and high dietary of meat [37, 38]. Thus, serum creatinine level does not need to be exactly same with the reduction of the inflammatory cytokine.
Then, we investigated the role of calcineurin in the nucleus. To our knowledge, this is the first study of nuclear calcineurin in renal IRI. We separated the cortex and medulla of the kidney and performed western blot analysis. We clearly showed a cleaved form of calcineurin in addition to full-length calcineurin. Calpain is known to catalyze the cleavage of calcineurin in the nucleus, which promotes NFAT2 transcriptional activity [17]. We speculated that the inhibitory effect of FK506 is not confined to the cytoplasm as previously shown, but may have a critical role in the nucleus as our results suggest. FK506 may block calpain-mediated calcineurin activity in the nucleus (Fig. 6). FK506 did not directly affect calpain activity, but diminished immune responses presumably by inhibiting the action of cleaved calcineurin. We suggest here that FK506 decreases calcineurin activity not only in the cytoplasm, but also in the nucleus. Furthermore, this dual effect may suppress overall inflammatory responses induced by renal IRI.
Fig. 6.
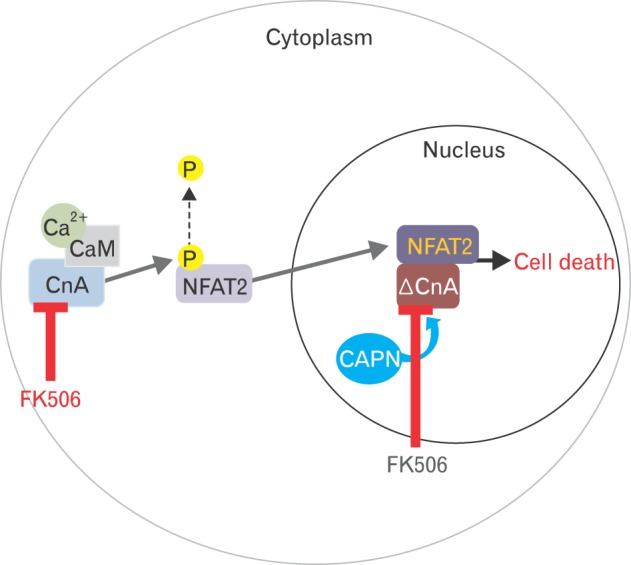
FK506 reduces calcineurin activity in both the cytoplasm and nucleus. In the cytoplasm, FK506 reduces renal tubular cell death by inhibiting calcineurin expression. Inhibition of calcineurin decreases the levels of dephosphorylated NFAT2. Thus, FK506 reduces the neutrophil infiltration and production of pro-inflammatory cytokines induced by renal injury, as shown in this study. FK506 decreases calcineurin activity by inhibiting its calpain-catalyzed cleavage. Cleaved calcineurin is known to be cytotoxic by enhancing NFAT2 transcriptional activity following ischemia/reperfusion injury.
Although the FK506 effect on the medulla was not investigated in this study, we detected the cleaved form of calcineurin in the cytoplasm of cells of the cortex (Fig. 5) and medulla (data not shown). However, the cleaved form of calcineurin in the nucleus has previously been shown not to translocate back to the cytoplasm of cardiomyocytes [8]. Thus, the role of the cleaved form of calcineurin in the cytoplasm of cells of the renal cortex and medulla is particularly interesting. We performed immunohistochemistry to detect calcineurin, which revealed that calcineurin was expressed in the tubules of the medulla. We speculated that this is related to the presence of the cleaved form of calcineurin in the cytoplasm. Therefore, the cleaved form of calcineurin is worth studying further. FK506 is a well-known immunosuppressant that is commonly used during kidney transplantation to reduce renal IRI because it is known to have anti-inflammatory activity. It has therapeutic potential in other diseases associated with inflammatory responses. We propose that it can be applied to auto-immune diseases such as atopic dermatitis and rheumatoid arthritis and even as an anti-cancer drug [26, 39] because these diseases are closely related to excessive inflammation, including leukocyte infiltration and ECM accumulation [26, 40, 41, 42]. Recently, several studies on the anti-cancer effects of FK506 have been reported [39]. In conclusion, we revisited the effect of FK506 as an anti-inflammatory factor in renal IRI and suggest it inhibits calcineurin activity in both the cytoplasm and the nucleus by inhibiting NFAT2-mediated transcriptional activity.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Science, ICT, and Future Planning (No. 2005-0049415).
References
- 1.Neudoerfl C, Mueller BJ, Blume C, Daemen K, Stevanovic-Meyer M, Keil J, Lehner F, Haller H, Falk CS. The peripheral NK cell repertoire after kidney transplantation is modulated by different immunosuppressive drugs. Front Immunol. 2013;4:46. doi: 10.3389/fimmu.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou W, Guan Q, Kwan CC, Chen H, Gleave ME, Nguan CY, Du C. Loss of clusterin expression worsens renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2010;298:F568–F578. doi: 10.1152/ajprenal.00399.2009. [DOI] [PubMed] [Google Scholar]
- 3.Shihab FS, Bennett WM, Andoh TF. Donor preconditioning with a calcineurin inhibitor improves outcome in rat syngeneic kidney transplantation. Transplantation. 2009;87:326–329. doi: 10.1097/TP.0b013e3181945332. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy SE, Erlich JH. Murine renal ischaemia-reperfusion injury. Nephrology (Carlton) 2008;13:390–396. doi: 10.1111/j.1440-1797.2008.00979.x. [DOI] [PubMed] [Google Scholar]
- 5.Akool el-S, Doller A, Babelova A, Tsalastra W, Moreth K, Schaefer L, Pfeilschifter J, Eberhardt W. Molecular mechanisms of TGF beta receptor-triggered signaling cascades rapidly induced by the calcineurin inhibitors cyclosporin A and FK506. J Immunol. 2008;181:2831–2845. doi: 10.4049/jimmunol.181.4.2831. [DOI] [PubMed] [Google Scholar]
- 6.Gooch JL, Barnes JL, Garcia S, Abboud HE. Calcineurin is activated in diabetes and is required for glomerular hypertrophy and ECM accumulation. Am J Physiol Renal Physiol. 2003;284:F144–F154. doi: 10.1152/ajprenal.00158.2002. [DOI] [PubMed] [Google Scholar]
- 7.Gooch JL, Pèrgola PE, Guler RL, Abboud HE, Barnes JL. Differential expression of calcineurin A isoforms in the diabetic kidney. J Am Soc Nephrol. 2004;15:1421–1429. doi: 10.1097/01.asn.0000128076.91545.bb. [DOI] [PubMed] [Google Scholar]
- 8.Heineke J, Ritter O. Cardiomyocyte calcineurin signaling in subcellular domains: from the sarcolemma to the nucleus and beyond. J Mol Cell Cardiol. 2012;52:62–73. doi: 10.1016/j.yjmcc.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Kilka S, Erdmann F, Migdoll A, Fischer G, Weiwad M. The proline-rich N-terminal sequence of calcineurin Abeta determines substrate binding. Biochemistry. 2009;48:1900–1910. doi: 10.1021/bi8019355. [DOI] [PubMed] [Google Scholar]
- 10.Siepert A, Brosel S, Vogt K, Ahrlich S, Schmitt-Knosalla I, Loddenkemper C, Kühl A, Baumgrass R, Gerstmayer B, Tomiuk S, Tiedge M, Viklický O, Brabcova I, Nizze H, Lehmann M, Volk HD, Sawitzki B. Mechanisms and rescue strategies of calcineurin inhibitor mediated tolerance abrogation induced by anti-CD4 mAb treatment. Am J Transplant. 2013;13:2308–2321. doi: 10.1111/ajt.12352. [DOI] [PubMed] [Google Scholar]
- 11.Gorentla BK, Zhong XP. T cell receptor signal transduction in T lymphocytes. J Clin Cell Immunol. 2012;2012(Suppl 12):5. doi: 10.4172/2155-9899.S12-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grigoriu S, Bond R, Cossio P, Chen JA, Ly N, Hummer G, Page R, Cyert MS, Peti W. The molecular mechanism of substrate engagement and immunosuppressant inhibition of calcineurin. PLoS Biol. 2013;11:e1001492. doi: 10.1371/journal.pbio.1001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurji K, Sharma RK. Potential role of calcineurin in pathogenic conditions. Mol Cell Biochem. 2010;338:133–141. doi: 10.1007/s11010-009-0346-4. [DOI] [PubMed] [Google Scholar]
- 14.Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- 15.Cook LG, Chiasson VL, Long C, Wu GY, Mitchell BM. Tacrolimus reduces nitric oxide synthase function by binding to FKBP rather than by its calcineurin effect. Kidney Int. 2009;75:719–726. doi: 10.1038/ki.2008.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacMillan D. FK506 binding proteins: cellular regulators of intracellular Ca2+ signalling. Eur J Pharmacol. 2013;700:181–193. doi: 10.1016/j.ejphar.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 17.Burkard N, Becher J, Heindl C, Neyses L, Schuh K, Ritter O. Targeted proteolysis sustains calcineurin activation. Circulation. 2005;111:1045–1053. doi: 10.1161/01.CIR.0000156458.80515.F7. [DOI] [PubMed] [Google Scholar]
- 18.Hisamitsu T, Nakamura TY, Wakabayashi S. Na(+)/H(+) exchanger 1 directly binds to calcineurin A and activates downstream NFAT signaling, leading to cardiomyocyte hypertrophy. Mol Cell Biol. 2012;32:3265–3280. doi: 10.1128/MCB.00145-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu G, Li M, Daneshgari F. Calcineurin and Akt expression in hypertrophied bladder in STZ-induced diabetic rat. Exp Mol Pathol. 2012;92:210–216. doi: 10.1016/j.yexmp.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkins BJ, Molkentin JD. Calcineurin and cardiac hypertrophy: where have we been? Where are we going? J Physiol. 2002;541(Pt 1):1–8. doi: 10.1113/jphysiol.2002.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu HY, Tomizawa K, Matsui H. Calpain-calcineurin signaling in the pathogenesis of calcium-dependent disorder. Acta Med Okayama. 2007;61:123–137. doi: 10.18926/AMO/32905. [DOI] [PubMed] [Google Scholar]
- 22.Williams CR, Gooch JL. Calcineurin inhibitors and immunosuppression: a tale of two isoforms. Expert Rev Mol Med. 2012;14:e14. doi: 10.1017/erm.2012.8. [DOI] [PubMed] [Google Scholar]
- 23.Gooch JL. An emerging role for calcineurin Aalpha in the development and function of the kidney. Am J Physiol Renal Physiol. 2006;290:F769–F776. doi: 10.1152/ajprenal.00281.2005. [DOI] [PubMed] [Google Scholar]
- 24.Abdin AA. Targeting sphingosine kinase 1 (SphK1) and apoptosis by colon-specific delivery formula of resveratrol in treatment of experimental ulcerative colitis in rats. Eur J Pharmacol. 2013;718:145–153. doi: 10.1016/j.ejphar.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 25.Feliers D, Gorin Y, Ghosh-Choudhury G, Abboud HE, Kasinath BS. Angiotensin II stimulation of VEGF mRNA translation requires production of reactive oxygen species. Am J Physiol Renal Physiol. 2006;290:F927–F936. doi: 10.1152/ajprenal.00331.2005. [DOI] [PubMed] [Google Scholar]
- 26.Minami T, Jiang S, Schadler K, Suehiro J, Osawa T, Oike Y, Miura M, Naito M, Kodama T, Ryeom S. The calcineurin-NFAT-angiopoietin-2 signaling axis in lung endothelium is critical for the establishment of lung metastases. Cell Rep. 2013;4:709–723. doi: 10.1016/j.celrep.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 28.Xiao HB, Liu RH, Ling GH, Xiao L, Xia YC, Liu FY, Li J, Liu YH, Chen QK, Lv JL, Zhan M, Yang SK, Kanwar YS, Sun L. HSP47 regulates ECM accumulation in renal proximal tubular cells induced by TGF-beta1 through ERK1/2 and JNK MAPK pathways. Am J Physiol Renal Physiol. 2012;303:F757–F765. doi: 10.1152/ajprenal.00470.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schofield ZV, Woodruff TM, Halai R, Wu MC, Cooper MA. Neutrophils: a key component of ischemia-reperfusion injury. Shock. 2013;40:463–470. doi: 10.1097/SHK.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 30.Qi XM, Wu YG, Liang C, Zhang P, Dong J, Ren KJ, Zhang W, Fang F, Shen JJ. FK506 ameliorates renal injury in early experimental diabetic rats induced by streptozotocin. Int Immunopharmacol. 2011;11:1613–1619. doi: 10.1016/j.intimp.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 31.Abdulkader RC, Liborio AB, Malheiros DM. Histological features of acute tubular necrosis in native kidneys and long-term renal function. Ren Fail. 2008;30:667–673. doi: 10.1080/08860220802212460. [DOI] [PubMed] [Google Scholar]
- 32.Bueno OF, Lips DJ, Kaiser RA, Wilkins BJ, Dai YS, Glascock BJ, Klevitsky R, Hewett TE, Kimball TR, Aronow BJ, Doevendans PA, Molkentin JD. Calcineurin Abeta gene targeting predisposes the myocardium to acute ischemia-induced apoptosis and dysfunction. Circ Res. 2004;94:91–99. doi: 10.1161/01.RES.0000107197.99679.77. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto Y, Soderling TR. Regulation of calcineurin by phosphorylation. Identification of the regulatory site phosphorylated by Ca2+/calmodulin-dependent protein kinase II and protein kinase C. J Biol Chem. 1989;264:16524–16529. [PubMed] [Google Scholar]
- 34.Park CH, Kim YS, Kim YH, Choi MY, Yoo JM, Kang SS, Choi WS, Cho GJ. Calcineurin mediates AKT dephosphorylation in the ischemic rat retina. Brain Res. 2008;1234:148–157. doi: 10.1016/j.brainres.2008.07.082. [DOI] [PubMed] [Google Scholar]
- 35.Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- 36.Yousuf S, Atif F, Kesherwani V, Agrawal SK. Neuroprotective effects of Tacrolimus (FK-506) and Cyclosporin (CsA) in oxidative injury. Brain Behav. 2011;1:87–94. doi: 10.1002/brb3.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function: measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 38.Stevens LA, Levey AS. Measurement of kidney function. Med Clin North Am. 2005;89:457–473. doi: 10.1016/j.mcna.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Kashyap T, Rabinovitz I. The calcium/calcineurin pathway promotes hemidesmosome stability through inhibition of beta4 integrin phosphorylation. J Biol Chem. 2012;287:32440–32449. doi: 10.1074/jbc.M112.385245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gooch JL, Gorin Y, Zhang BX, Abboud HE. Involvement of calcineurin in transforming growth factor-beta-mediated regulation of extracellular matrix accumulation. J Biol Chem. 2004;279:15561–15570. doi: 10.1074/jbc.M308759200. [DOI] [PubMed] [Google Scholar]
- 41.Stefater JA, 3rd, Rao S, Bezold K, Aplin AC, Nicosia RF, Pollard JW, Ferrara N, Lang RA. Macrophage Wnt-Calcineurin-Flt1 signaling regulates mouse wound angiogenesis and repair. Blood. 2013;121:2574–2578. doi: 10.1182/blood-2012-06-434621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang B, Shi W, Ma J, Sloan A, Faul C, Wei C, Reiser J, Yang Y, Liu S, Wang W. The calcineurin-NFAT pathway allows for urokinase receptor-mediated beta3 integrin signaling to cause podocyte injury. J Mol Med (Berl) 2012;90:1407–1420. doi: 10.1007/s00109-012-0960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]