Abstract
Objective
To investigate prognostic predictors of long-term survival of patients with cardiac amyloidosis (CA), and to determine predictive value of high-sensitivity cardiac troponin T (hs-cTnT) in CA patients.
Methods
We recruited 102 consecutive CA cases and followed these patients for 5 years. We described their clinical characteristics at presentation and used a new, high-sensitivity assay to determine the concentration of cTnT in plasma samples from these patients.
Results
The patients with poor prognosis showed older age (56 ± 12 years vs. 50 ± 15 years, P = 0.022), higher incidences of heart failure (36.92% vs. 16.22%, P = 0.041), pericardial effusion (60.00% vs. 35.14%, P = 0.023), greater thickness of interventricular septum (IVS) (15 ± 4 mm vs. 13 ± 4 mm, P = 0.034), higher level of hs-cTnT (0.186 ± 0.249 ng/mL vs. 0.044 ± 0.055 ng/mL, P = 0.001) and higher NT-proBNP (N-terminal pro-B-type natriuretic peptide) levels (11,742 ± 10,464 pg/mL vs. 6,031 ± 7,458 pg/mL, P = 0.006). At multivariate Cox regression analysis, heart failure (HR: 1.78, 95%CI: 1.09–2.92, P = 0.021), greater wall thickness of IVS (HR: 1.44, 95%CI: 1.04–3.01, P = 0.0375) and higher hs-cTnT level (HR: 6.16, 95%CI: 2.20–17.24, P = 0.001) at enrollment emerged as independent predictors of all-cause mortality.
Conclusions
We showed that hs-cTnT is associated with a very ominous prognosis, and it is also the strongest predictor of all-cause mortality in multivariate analysis. Examination of hs-cTnT concentrations provides valuable prognostic information concerning long-term outcomes.
Keywords: Cardiac amyloidosis, Long-term survival, Troponin T
1. Introduction
Amyloidosis is a rare disease with alterations in protein conformation that result in systemic deposition of abnormal fibrils. Cardiac involvement was reported in 50% of amyloidosis cases and was associated with the worst prognosis with reported median survival of 6 to 24 months, with 4 months in the setting of advanced heart failure (HF).[1]–[3] The outcome of patients with cardiac amyloidosis (CA) is heterogeneous, so it is crucial to find non-invasive evaluation tools in order to obtain an early recognition of the disease progression. A new generation of sensitive assays for cardiac troponins has been introduced recently, and is significantly associated with the incidences of cardiovascular death and HF.[4],[5] There are few data from studies assessing the use of such assays for the evaluation of prognosis of CA patients. The aim of this study was the identification of independent predictors of all-cause mortality for CA patients.
2. Methods
2.1. Diagnosis of CA
We enrolled 102 patients with CA at our institute from January 2006 to January 2008. Endomyocardial biopsy (EMB) is the gold-standard for diagnosis of CA. Most patients with CA were confirmed by invasive EMB: routine paraffin processed sections were stained with hematoxylin eosin for cell morphology, and amyloid apple-green birefringence of Congo red stained under polarized light is indicative for the presence of amyloid.
2.2. Baseline clinical features
Baseline clinical data included clinical manifestation, laboratory profile and the treatments during their stay in hospital. The clinical manifestations were focused on symptoms and signs of HF, and the New York Heart Association (NYHA) functional class. HF was defined as the presence of symptoms (breathlessness at rest or on exercise, tiredness, and ankle swelling) and/or classical physical signs (tachycardia, pulmonary rales, jugular venous distention, peripheral edema, and hepatomegaly). Renal failure was defined as estimated glomerular filtration rate (eGFR) < 30 mL/min.
Laboratory profile included the results of electrocardiogram (ECG) and transthoracic echocardiography (TTE) for every patient. Low voltage of ECG was defined as peak-to-peak QRS amplitude being < 5 mm in limb leads and < 10 mm in precordial leads. We measured left ventricular (LV) end-diastolic diameter, end-systolic diameter, left atrial diameter, thickness of interventricular septum (IVS), pericardial effusion and ejection fraction (EF) by TTE. Restrictive left ventricular filling pattern was defined as early diastole deceleration time < 130 ms and the peak early velocity and peak atrial velocity (E/A) ratio ≥ 2. We used a new, high-sensitivity assay to determine the concentration of cardiac troponin T (cTnT) in plasma samples from CA patients. The lower detection limit of the highly sensitive cTnT (hs-cTnT) assay was 0.001 ng/mL. Electrochemiluminescence (ECL) technology was used to measure troponin T (Elecsys®, fifth-generation Roche Troponin T) for risk assessment in patients with CA. The 99th percentile value of a healthy population is 0.014 ng/mL. Plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) level (pg/mL) was measured by ECL technology (BNP test kit of Bayer).
2.3. Follow-up
This research was an observational study, and it was approved by the local institutional review board. These patients signed informed consent as part of the 5-years follow-up. Data were obtained by regular doctor visits or telephone calls to patients or their relatives. Clinical interviews were performed once a month. To avoid misclassification of the cause of death, all-cause mortality was selected as the endpoint. Patients with HF were mainly treated with β-blockers, angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor blocker (ARB) based on guidelines, unless there were contraindications to these drugs. We try to explore predictors of long-term survival of patients with CA through a 5-year follow-up observation.
2.4. Statistical analysis
Clinical characteristics and laboratory features of our study population were analyzed. Data were expressed as mean ± SD for continuous variables and as percentages for discrete variables. Continuous variables were compared between groups using the Student t test (for normal distribution) or Mann–Whitney rank sum test (for non-normal distribution). Comparisons between continuous variables among groups were performed with the analysis of variance (ANOVA) using the LSD statistic, while the proportions were compared by means of the Chi-square test, using Fisher exact test. All analyses were performed with the software SPSS 19.0 Statistical Package. Predictions of long-term survival were assessed with logistic regression using Kaplan–Meier survival curves, and a significant P value was set at 0.05.
3. Results
3.1. Study population
Positive biopsies for amyloidosis were obtained from EMB in 102 patients, which are presented in Figure 1. The patients were predominantly men (63.72%). Mean age at presentation was 53 years. The majority (60/102, 58.82%) presented with HF. Twelve patients (11.76%) had renal failure. Seventy patients (68.63%) were classified as primary amyloidosis, 22 patients (21.57%) were classified as chronic inflammatory disease associated amyloidosis and the remaining 10 patients (9.80%) were classified as multiple myeloma associated amyloidosis. The results of ECG showed that 48 of 102 patients (47.06%) had low QRS voltages, 74 of 102 patients (72.55%) had atrial fibrillations. The results of TTE showed that the mean thickness of IVS was 14.6 mm; restrictive left ventricular filling patterns were present in 41% of cases. Medical treatments generally included ACEI (35%), beta-blockers (21%), calcium channel blockers (5%), digitalis (14%) and melphalan (8%). No patients had undergone bone marrow transplantations or heart transplantations.
Figure 1. Myocardial tissue stained with hematoxylin–eosin (A) and Congo red (B), myocardial tissue stained with Congo red showed apple-green birefringence under polarized light (C).
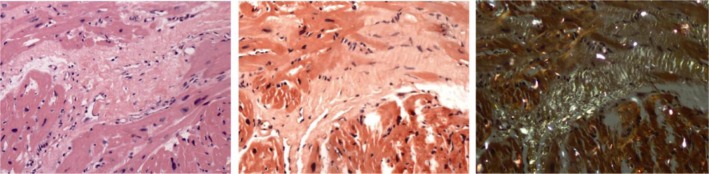
Amyloid apple-green birefringence of Congo red stained under polarized light is indicative for the presence of amyloid in myocardial tissue.
3.2. Natural history
Ninety-one of 102 patients died during the follow-up of 60 months, while 11 patients survived over a 5-year period. Seventy-nine of the 91 patients (87%) died from a cardiac cause (cardiac arrhythmia, cardiac failure or heart block), and the other 12 patients (13%) died from non-cardiac causes. Necropsy confirmed CA in 23 patients of them. Survival from all-cause death was 58%, 35% and 11% at 12, 24, and 60 months. The patients were divided into two groups based on survival of < or > two years. Baseline clinical/laboratory features and therapies of the two subgroups were described in Table 1. Patients with a poor prognosis were characterized by older age (56.29 ± 12.15 years vs. 49.92 ± 15.20 years, P = 0.022), NYHA III–IV (69.23% vs. 43.24 %, P = 0.012), higher levels of hs-cTnT (0.186 ± 0.249 ng/mL vs. 0.044 ± 0.055 ng/mL, P = 0.001) and BNP (11742 ± 10464 pg/mL vs. 6031 ± 7458 pg/mL, P = 0.006). The results of TTE showed that patients with a poor prognosis more frequently presented with pericardial effusion (60.00% vs. 35.14%, P = 0.023) and had greater thickness of IVS (15 ± 4 mm vs. 13 ± 4 mm, P = 0.034). The results of ECG showed that patients with poor prognosis more frequently presented with low QRS voltages (56.92% vs. 29.73%, P = 0.007). Moreover, patients with poor prognosis seemed to be more intolerant to ACEI/ARBs and beta-blockers, although there was no significant difference.
Table 1. Comparisons of baseline clinical/laboratory features and therapies between CA patients divided by survival time of two years.
| Short survive group, n = 65 | Long survive group, n = 37 | P | |
| Gender (Male/ Female) | 46/19 | 19/18 | 0.057 |
| Age, yrs | 56.29 ± 12.15* | 49.92 ± 15.20 | 0.022 |
| SBP, mmHg | 122 ± 26 | 129 ± 30 | 0.208 |
| DBP, mmHg | 77 ± 17 | 78 ± 20 | 0.969 |
| HR, beats/min | 81 ± 16 | 82 ± 18 | 0.812 |
| HF at enrolment | 24 (36.92)* | 6 (16.22) | 0.041 |
| NYHA III–IV | 45 (69.23%)* | 16 (43.24%) | 0.012 |
| Low QRS voltages | 37 (56.92%) | 11 (29.73%) | 0.007 |
| IVS, mm | 15 ± 4 | 13 ± 4 | 0.034 |
| Pericardial effusion | 39 (60.00%)* | 13 (35.14%) | 0.023 |
| Left ventricular ejection fraction (%) | 45.05 ± 10.41* | 50.41 ± 10.75 | 0.015 |
| Renal failure | 8 (12.30%) | 5 (13.51%) | 1.000 |
| Proteinuria > 1 g in 24 h | 26 (40.00%) | 10 (27.03%) | 0.204 |
| BNP, pg/mL | 11742 ± 10464* | 6031 ± 7458 | 0.006 |
| Hs-cTnT, ng/mL | 0.186 ± 0.249* | 0.044 ± 0.055 | 0.001 |
| ACEI/ARBs | 27 (41.54%) | 18 (48.65%) | 0.537 |
| β-Blockers | 38 (58.46%) | 24 (64.86%) | 0.674 |
Data are expressed as mean ± SD or n (%) unless otherwise indicated. ACEI: angiotensin converting enzyme inhibitor; ARBs: angiotensin receptor blocker; BNP: brain natriuretic peptide; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; HR: heart rate; Hs-cTnT: high sensitive cardiac troponin T; IVS: interventricular septum; NYHA: New York Heart Association; SBP: systolic blood pressure.*Different from group with long survive at P < 0.05.
3.3. The relationship between level of hs-cTnT and prognosis of CA
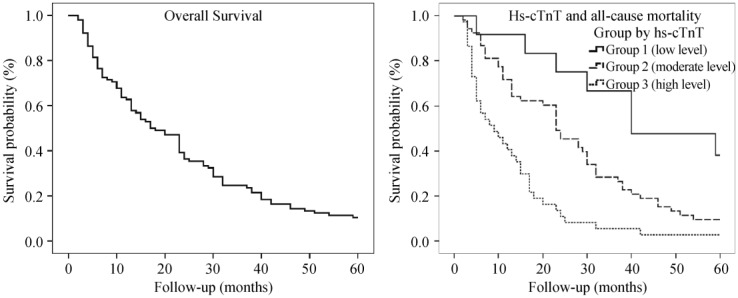
Among the entire population of 102 patients, 90 (88%) patients showed positive hs-cTnT (> 0.014 ng/mL). We divided all patients into 3 groups according to hs-cTnT level. Twelve subjects (12%) belonged to negative hs-cTnT group (Group 1, < 0.014 ng/mL); 53 subjects (52%) belonged to Group 2 (0.014 ng/mL < hs-cTnT < 0.100 ng/mL); and the remaining 37 subjects (36%) assigned to the group with high hs-cTnT levels (Group 3, > 0.100 ng/mL). We described clinical features and outcomes for each group in Table 2 and Figure 2. Compared with patients in Group 1, those in Group 3 were older, inclined to present with HF (51.35 % vs. 25.00%, P = 0.02), showing higher BNP levels (15,428 pg/mL vs. 3,448 pg/mL, P = 0.01), having greater thickness of IVS (16 ± 4 mm vs. 13 ± 5 mm, P = 0.02) and inclined to present with a restrictive left ventricular (LV) filling pattern (64.86% vs. 8.33%, P = 0.01). The level of hs-cTnT was closely correlated with age (P = 0.004), heart function classification (P = 0.043), restrictive LV filling pattern (P = 0.032) or level of BNP (P = 0.001). Median survival times were significantly shorter along with the elevation of hs-cTnT level (39 months vs. 23 months vs. 8 months, P < 0.01). Figure 2 presents Kaplan-Meier curves for survival time in different groups.
Table 2. Comparisons of clinical features of CA patients divided by hs-cTnT level.
| Group 1 (n = 12) | Group 2 (n = 53) | Group 3 (n = 37) | |
| Age, yrs | 49.33 ± 13.07 | 49.83 ± 14.72 | 61.43 ± 8.07* |
| Serum creatinine, µmol/L | 73.8 ± 14.0 | 114.7 ± 110.3 | 121.1 ± 133.1 |
| HF | 3 (25.00%) | 8 (15.09%) | 19(51.35%)* |
| BNP, pg/mL | 3,448 ± 4584 | 7,060 ± 8263* | 15,428 ± 10,469* |
| Restrictive LV filling pattern | 1 (8.33%) | 17 (32.07%)* | 24 (64.86)*,# |
| IVS, mm | 13 ± 5 | 15 ± 5 | 16 ± 4* |
| Median survival time, months | 39 (n = 8) | 23 (n = 48) * | 8 (n = 36)* # |
*Different from group with low hs-cTnT level at P < 0.05; #Different from group with moderate hs-cTnT level at P < 0.05. Group 1: patients with low hs-cTnT level (< 0.014 ng/mL); Group 2: patients with moderate hs-cTnT level (0.014 ng/mL < hs-cTnT < 0.100 ng/mL); Group 3: patients with high hs-cTnT level (> 0.100 ng/mL). Data were expressed as mean ± SD or n (%) unless other indicated. BNP: brain natriuretic peptide; HF: heart failure; Hs-cTnT: high sensitive cardiac troponin T; IVS: interventricular septum; LV: left ventricular.
Figure 2. Kaplan-Meier curves of 5-year follow-up for all CA patients (A) and in different groups divided by Hs-cTnT level (B).
CA: cardiac amyloidosis; Hs-cTnT: high sensitive cardiac troponin T.
3.4. Identification of CA prognostic predictors
By multivariate Cox survival analysis, HF (hazard ratio (HR) = 1.78, P = 0.021), greater thickness of IVS (HR = 1.44, P = 0.0375) and higher hs-cTnT levels (HR = 6.16, P = 0.001) at diagnosis were independently predictors for all-cause mortality in our five-year follow-up observations. The HR associated with relevant variables in multivariate analyses are listed in Table 3. Among baseline variables, strong association between mortality and the level of hs-cTnT was observed, even after adjustment for other factors. Even mild elevations of troponin level could affect the prognoses of CA patients.
Table 3. Multivariate Cox survival analyses of predictors for prognosis in CA patients.
| Variable | Hazard ratio (95% CI) | P value |
| Ages | 1.00 (0.99–1.01) | 0.515 |
| HF | 1.78 (1.09–2.92) | 0.021 |
| Low voltage of lead | 1.44 (0.92–2.25) | 0.113 |
| IVST > 12 mm | 1.76 (1.04–3.01) | 0.037 |
| LAD > 40 cm | 1.59 (0.97–2.63) | 0.067 |
| BNP | 1.01 (1.01–1.02) | 0.117 |
| Hs-cTnT | 6.16 (2.20–17.24) | 0.001 |
BNP: brain natriuretic peptide; CI: confidence interval; HF: heart failure; HR: hazard ratio (increase in mortality risk per 1-unit increase in the variable; for categorical variables, the HR represents the risk of mortality in the presence of the variables); Hs-cTnT: high sensitive cardiac troponin T; IVST: interventricular septal thickness; LAD: left atrium diameter.
4. Discussion
Despite the advances in treatment, the prognosis of CA remains poor, particularly in the presence of HF. The median survival time from diagnosis among all patients is approximately one year, especially in untreated patients.[6] The main causes of death in our study were congestive HF, ventricular tachyarrhythmia, bradyarrhythmia and severe hypotension.
It is necessary to estimate the prognosis through non-invasive clinical examinations for CA patients. Our observational study showed that older age, higher level of BNP, pleural effusion and HF were independently associated with poor prognosis, which was consistent with the reported literature.[7],[8] Other prognostic factors included male gender, recent syncope, restrictive left ventricular filling pattern, LA enlargement, and left ventricular ejection time as reported.[7]–[11] The previous observations showed traditional troponin is a powerful tool in clinical and prognostic assessments of patients with CA. Increased proponing level was associated with poor survival in patients with amyloid light-chain (AL) amyloidosis, and was a significant predictor for all-cause mortality.[7],[12] The predictive value of the determination of hs-cTnT levels in this study was even superior to the traditional troponin assay in the evaluation of all-cause mortality (HR = 6.16).
Our results emphasized the role of hs-cTnT for accurate risk stratification in evaluating patients who were potential candidates for cardiac death. We have improved the previous model of discriminating patients using biomarkers of hs-cTnT instead of serum cTnT. In our study, most of patients are hs-cTnT positive (88.23%, 90/102). Because hs-cTnT is more sensitive than the traditional test for troponin, we could discriminate among patients with different outcomes through the measurement of hs-cTnT levels.
The mechanisms responsible for the release of very low levels of cTnT in patients could include: (1) cardiomyocyte damage due to extracellular deposition of insoluble beta-febrile proteins in the heart; (2) inflammatory processes; (3) cardiomyocyte apoptosis and increased myocardial strain due to pressure or volume overload.[13] The level of hs-cTnT depends on the velocity of amyloid substance deposition in the myocardium, and is highly positively correlated to the progress of the disease.[14] In our study, the thickness of IVS and HF are both associated with hs-cTnT level. Even a minimally increased cTnT level may represent subclinical cardiac injury and has important clinical implications.[15]–[17] Examination of the hs-cTnT level, as an important tool, plays an important role in the prognostic classification of patients affected by amyloidosis.
4.1. Study limitation
A major limitation of the study was the small sample size from a single center. However, CA represents a rare cardiac disease and there is a lack of large number of studies in the literatures. The long follow-up period of the study was mitigated by the relatively small sample size. The sample size limited the ability to add more clinical variables to be tested in a Cox model and led to the lack of statistical power. We were not able to establish the cause of death for all patients, so the endpoint event was not cardiovascular mortality, but the all-cause mortality. We did not compare hs-cTnT levels with traditional troponin T levels, and did not analyze the differences between the two examinations.
4.2. Conclusion
We showed that the high level of hs-cTnT was associated with a very ominous prognosis, and it was the strong predictor of all-cause mortality in multivariate analysis. Examination of the hs-cTnT level provided valuable prognostic information concerning long-term outcome.
References
- 1.Kyle RA, Gertz MA, Greipp PR, et al. A trial of three regimens for primary amyloidosis: colchicine alone, melphalan and prednisone, and melphalan, prednisone, and colchicine. N Engl J Med. 1997;336:1202–1207. doi: 10.1056/NEJM199704243361702. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45–59. [PubMed] [Google Scholar]
- 3.Dispenzieri A, Gertz MA, Kyle RA, et al. Prognostication of survival using cardiac troponins and N-terminal pro-brain natriuretic peptide in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood. 2004;104:1881–1887. doi: 10.1182/blood-2004-01-0390. [DOI] [PubMed] [Google Scholar]
- 4.Melanson SE, Morrow DA, Jarolim P. Earlier detection of myocardial injury in a preliminary evaluation using a new troponin I assay with improved sensitivity. Am J Clin Pathol. 2007;128:282–286. doi: 10.1309/Q9W5HJTT24GQCXXX. [DOI] [PubMed] [Google Scholar]
- 5.Omland T, de Lemos JA, Sabatine MS, et al. Sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyle RA, Gertz MA, Greipp PR, et al. Amyloidosis long-term survival (10 years or more) in 30 patients with primary amyloidosis. Blood. 1999;93:1062–1066. [PubMed] [Google Scholar]
- 7.Bellavia D, Pellikka PA, Dispenzieri A, et al. Comparison of right ventricular longitudinal strain imaging, tricuspid annular plane systolic excursion, and cardiac biomarkers for early diagnosis of cardiac involvement and risk stratification in primary systematic (AL) amyloidosis: a 5-year cohort study. Eur Heart J Cardiovasc Imaging. 2012;13:680–689. doi: 10.1093/ehjci/jes009. [DOI] [PubMed] [Google Scholar]
- 8.Finocchiaro G, Merlo M, Pinamonti B, et al. Long term survival in patients with cardiac amyloidosis. Prevalence and characterisation during follow-up. Heart Lung Circ. 2013;22:647–654. doi: 10.1016/j.hlc.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Finocchiaro G, Pinamonti B, Merlo M, et al. Focus on cardiac amyloidosis: a single-center experience with a long-term follow-up. J Cardiovasc Med (Hagerstown) 2013;14:281–288. doi: 10.2459/JCM.0b013e3283536534. [DOI] [PubMed] [Google Scholar]
- 10.Mohty D, Pibarot P, Dumesnil JG, et al. Left atrial size is an independent predictor of overall survival in patients with primary systemic amyloidosis. Arch Cardiovasc Dis. 2011;104:611–618. doi: 10.1016/j.acvd.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Migrino RQ, Mareedu RK, Eastwood D, et al. Left ventricular ejection time on echocardiography predicts long-term mortality in light chain amyloidosis. J Am Soc Echocardiogr. 2009;22:1396–1402. doi: 10.1016/j.echo.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apridonidze T, Steingart RM, Comenzo RL, et al. Clinical and echocardiographic correlates of elevated troponin in amyloid light-chain cardiac amyloidosis. Am J Cardiol. 2012;110:1180–1184. doi: 10.1016/j.amjcard.2012.05.061. [DOI] [PubMed] [Google Scholar]
- 13.Wallace TW, Abdullah SM, Drazner MH, et al. Prevalence and determinants of troponin T elevation in the general population. Circulation. 2006;113:1958–1965. doi: 10.1161/CIRCULATIONAHA.105.609974. [DOI] [PubMed] [Google Scholar]
- 14.Dispenzieri A, Gertz MA, Kumar SK, et al. High sensitivity cardiac troponin T in patients with immunoglobulin light chain amyloidosis. Heart. 2014;100:383–388. doi: 10.1136/heartjnl-2013-304957. [DOI] [PubMed] [Google Scholar]
- 15.Sato Y, Fujiwara H, Takatsu Y. Cardiac troponin and heart failure in the era of high-sensitivity assays. J Cardiol. 2012;60:160–167. doi: 10.1016/j.jjcc.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Palladini G, Barassi A, Klersy C, et al. The combination of high-sensitivity cardiac troponin T (hs-cTnT) at presentation and changes in N-terminal natriuretic peptide type B (NT-proBNP) after chemotherapy best predicts survival in AL amyloidosis. Blood. 2010;116:3426–3430. doi: 10.1182/blood-2010-05-286567. [DOI] [PubMed] [Google Scholar]
- 17.Kristen AV, Giannitsis E, Lehrke S, et al. Assessment of disease severity and outcome in patients with systemic light-chain amyloidosis by the high-sensitivity troponin T assay. Blood. 2010;116:2455–2461. doi: 10.1182/blood-2010-02-267708. [DOI] [PubMed] [Google Scholar]