Abstract
Background
Cilostazol is a type 3 phosphodiesterase inhibitor which has been previously demonstrated to prevent the occurrence of tachyarrhythmia and improve defibrillation efficacy. However, the mechanism for this beneficial effect is still unclear. Since cardiac mitochondria have been shown to play a crucial role in fatal cardiac arrhythmias and that oxidative stress is one of the main contributors to arrhythmia generation, we tested the effects of cilostazol on cardiac mitochondria under severe oxidative stress.
Methods
Mitochondria were isolated from rat hearts and treated with H2O2 to induce oxidative stress. Cilostazol, at various concentrations, was used to study its protective effects. Pharmacological interventions, including a mitochondrial permeability transition pore (mPTP) blocker, cyclosporine A (CsA), and an inner membrane anion channel (IMAC) blocker, 4′-chlorodiazepam (CDP), were used to investigate the mechanistic role of cilostazol on cardiac mitochondria. Cardiac mitochondrial reactive oxygen species (ROS) production, mitochondrial membrane potential change and mitochondrial swelling were determined as indicators of cardiac mitochondrial function.
Results
Cilostazol preserved cardiac mitochondrial function when exposed to oxidative stress by preventing mitochondrial depolarization, mitochondrial swelling, and decreasing ROS production.
Conclusions
Our findings suggest that cardioprotective effects of cilostazol reported previously could be due to its prevention of cardiac mitochondrial dysfunction caused by severe oxidative stress.
Keywords: Phosphodiesterase-3 inhibitor, Cilostazol, Mitochondria, Heart, Oxidative stress, Membrane potential, Ischemia
1. Introduction
Cilostazol is a selective type-3 phosphodiesterase inhibitor that has been used to treat claudication. Unlike other type-3 phosphodiesterase inhibitors, such as milrinone and vesnarinone which have been shown to increase cyclic adenosine monophosphate (cAMP) levels, arrythmogenisis, and mortality rates in heart failure patients,[1],[2] growing evidence suggests that cilostazol could be cardioprotective.[3]–[14] This could be due to the fact that cilostazol inhibits not only type 3 phosphodiesterase, but also adenosine uptake, thus reducing the cAMP levels.[15],[16] Previous studies also demonstrated the cardioprotective effects of cilostazol by pre-venting the occurrence of fatal arrhythmias in Brugada's syndrome.[17] Our recent study demonstrated that cilostazol could stabilize the cardiac electrophysiology and prevent fatal arrhythmias by increasing the ventricular fibrillation threshold as well as increasing the defibrillation efficacy.[18] Despite these beneficial effects, the definite mechanism of cilostazol in arrhythmia prevention is still unclear.
Cardiac mitochondrial dysfunction has been shown to play an important role in cardiac arrhythmogenesis.[19] It has been shown that depolarization of cardiac mitochondrial membrane potential occurring during various stress conditions, including ischemia-reperfusion, could initiate fatal arrhythmia in the heart.[19] Oxidative stress due to high levels of reactive oxygen species (ROS) produced in the mitochondria during ischemia-reperfusion has been shown to play an important role in triggering the cardiac mitochondrial membrane potential changes.[19],[20] Although several drugs and chemical substances have been demonstrated to attenuate mitochondrial dysfunction caused by oxidative stress;[19],[21]–[23] the role of cilostazol on cardiac mitochondrial function has never been investigated.
In the present study, we tested the hypothesis that cilostazol can prevent cardiac mitochondrial dysfunction caused by H2O2-induced severe oxidative stress by attenuating mitochondrial swelling, preventing mitochondrial depolarization, and reducing ROS production in cardiac mitochondria.
2. Methods
2.1. Animal preparation
This study was approved by the Institutional Animal Care and Use Committee at the Faculty of Medicine, Chiang Mai University. Wistar rats (300–350 g) obtained from the National Laboratory Animal Center, Mahidol University, Bangkok, Thailand, were housed in a room with temperature 22–25°C and had a constant 12-h light/dark cycle. All animals received standard pelleted rat diet and water ad libitum.
2.2. Isolated cardiac mitochondria preparation
Cardiac mitochondria were isolated based upon the protocol described previously.[20] In brief, each rat was injected intraperitoneally with thiopental (80 mg/kg), and the heart was removed and homogenized in ice-cold buffer containing sucrose 300 mmol/L, N-(tris (hydroxymethyl) methyl)-2-aminoethanesulfonic acid sodium salt (TES) 5 mmol/L, and ethylene glycol tetraacetic acid (EGTA) 0.2 mmol/L, pH 7.2 at 4°C. The homogenate was centrifuged at 800 r/min for 5 min and the supernatant was collected and centrifuged at 8,800 r/min for 5 min. Cardiac mitochondrial pellets were resuspended in an ice-cold buffer and centrifuged again at 8,800 r/min for 5 min. Protein concentration was determined according to the bicinchoninic acid (BCA) assay.[24]
2.3. Experimental protocols
Isolated cardiac mitochondria from rat hearts were used in all study protocols. Identification of cardiac mitochondria was performed using electron microscopy. Application of H2O2 at 2 mmol/L with a 5-min incubation time was used to induce oxidative stress in cardiac mitochondria. This concentration and incubation time were chosen as been shown to effectively cause cardiac mitochondrial dysfunction by inducing mitochondrial swelling, increasing mitochondrial ROS production, and depolarizing mitochondrial membrane potential.[20]
In the first protocol, 20-µmol/L cilostazol was applied to cardiac mitochondria at various incubation times (5–20 min) prior to H2O2 application. The time in which cilostazol could effectively protect cardiac mitochondrial dysfunction caused by H2O2 was chosen for the next protocol. In the second protocol, various concentrations of cilostazol (5–40 µmol/L) were studied with the incubation time obtained from the first protocol. Vehicle-treated mitochondria were used as a control group.
In the third protocol, the protective mechanism of cilostazol on cardiac mitochondria was investigated using a pharmacological intervention approach. Cyclosporine A (CsA) and 4′-chlorodiazepam (CDP) were used for this purpose. CsA is a blocker of the mitochondrial permeability transition pore (mPTP),[20],[25] whereas CDP is known to inhibit the opening of the inner membrane anion channel (IMAC).[20] In this protocol, cardiac mitochondria were randomly assigned into 10 treatment groups (n = 8 per group), consisting of the control (vehicle); cilostazol (effective dose of cilostazol obtained from the second protocol); CsA (5 µmol/L); CDP (100 µmol/L); H2O2; H2O2 with cilostazol; H2O2 with CsA; H2O2 with CDP; H2O2 with cilostazol + CsA; and H2O2 with cilostazol + CDP. In each study protocol, cardiac mitochondria in each group were investigated for mitochondrial swelling, mitochondrial ROS production, and mitochondrial membrane potential changes.
2.4. Determination of cardiac mitochondrial swelling, ROS production and membrane potential changes
Cardiac mitochondrial swelling was determined in all groups by measuring the change in the absorbance of the cardiac mitochondrial suspension at 540 nm (A540) using a microplate reader.[20],[25] Mitochondria (0.4 mg/mL) were incubated in respiration buffer (containing 100 mmol/L KCl, 50 mmol/L sucrose, 10 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and 5 mmol/L KH2PO4, pH 7.4 at 37°C) with the addition of 10-mmol/L pyruvate/malate. Mitochondrial swelling was indicated by a decrease in the absorbance of the suspension.[20]
ROS production in cardiac mitochondria was determined using dichlorohydro-fluorescein diacetate (DCFDA),[26] and measured by a fluorescent microplate reader.[20] Cardiac mitochondria (0.4 mg/mL) were incubated at 25°C with 2-µmol/L of DCFDA for 20 min. The mitochondrial suspension was gently agitated and incubated at room temperature for measurements. DCFDA passed through the mitochondrial membranes where it was oxidized in the presence of H2O2 to DCF (a fluorescent form). Fluorescence was determined at λex 485 nm and λem 530 nm according to the spectral characteristics of DCF. The ROS levels were expressed as arbitrary units of fluorescence intensity of DCF.[20]
Changes in the cardiac mitochondrial membrane potential were determined using the dye 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1).[20],[27],[28] The isolated cardiac mitochondria (2 mg/mL) were stained with JC-1 (310 nmol/L) at 37°C for 30 min. The intensity of fluorescence was determined using a fluorescent microplate reader. JC-1 monomer fluorescence (green) was excited at 485 nm and the emission was detected at 530 nm. JC-1 aggregate fluorescence (red) was excited at 485 nm and the emission fluorescence was recorded at 590 nm. Cardiac mitochondrial depolarization was indicated by a decrease in the red/green fluorescence intensity ratio.[20]
2.5. Statistical analysis
All data were expressed as means ± SE. Comparisons among groups were determined by one-way ANOVA, followed by the Fisher post-hoc test. P < 0.05 was considered statistically significant.
3. Results
3.1. Effects of cilostazol on cardiac mitochondrial swellling, ROS production and membrane potential changes
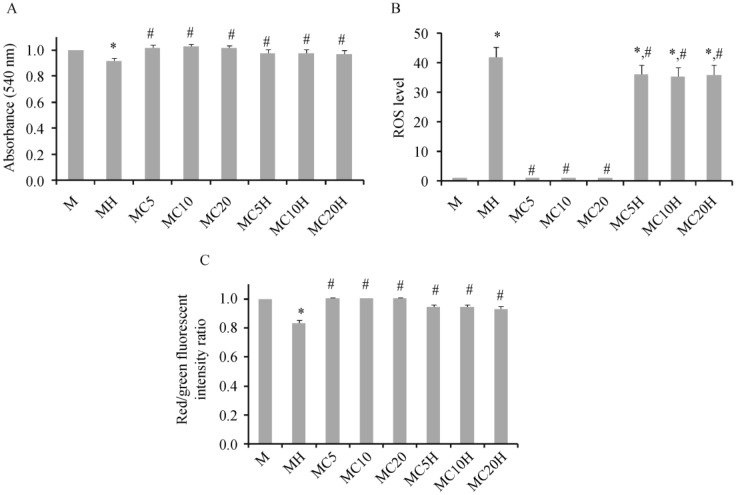
Application of H2O2 at 2 mmol/L caused cardiac mitochondrial dysfunction as represented by decreased absorbance indicating mitochondrial swelling (Figure 1A), increased ROS levels (Figure 1B), and a decreased red/green fluorescent ratio indicating mitochondrial membrane depolarization (Figure 1C), in comparison to the control group. When 20-µmol/L cilostazol was applied to cardiac mitochondria, there was no change in absorbance, ROS levels, or mitochondrial membrane potential in cardiac mitochondria at any of the incubation times. However, the application of cilostazol to cardiac mitochondria for 5, 10, and 20 min prior to H2O2 application could prevent mitochondrial swelling, decrease ROS levels, and prevent mitochondrial depolarization caused by H2O2 (Figure 1). From this protocol, a 10-min incubation time was chosen for cilostazol as the standard period for subsequent protocols in this study.
Figure 1. Effects of incubation times of 20-µmol/L cilostazol on cardiac mitochondrial swelling (A), ROS levels (B), and membrane potential change (C) after 4-min H2O2 application.
Without H2O2, cilostazol applied to cardiac mitochondria for 5, 10, and 20 min did not alter the absorbance, ROS levels, or membrane potential of cardiac mitochondria, in comparison with the control group. Application of H2O2 to cardiac mitochondria caused marked cardiac mitochondrial swelling, increased ROS levels, and a decreased red/green fluorescent intensity ratio (i.e., mitochondrial depolarization). When cilostazol was applied to cardiac mitochondria for 5, 10 and 20 min prior to H2O2 application, it could prevent mitochondrial swelling, decrease ROS levels, and prevent mitochondrial depolarization, in comparison with the H2O2 treated group. M: cardiac mitochondria without any treatment; MH: cardiac mitochondria treated with H2O2; MC5/MC10/MC20: cardiac mitochondria treated with 20 µmol/L cilostazol for 5, 10, and 20 min without H2O2 application, respectively; MC5H/MC10H/MC20H: cardiac mitochondria treated with 20 µmol/L cilostazol for 5, 10, and 20 min prior to H2O2 application, respectively. *P < 0.05 vs. M group, #P < 0.05 vs. H2O2 treated mitochondria. ROS: reactive oxygen species.
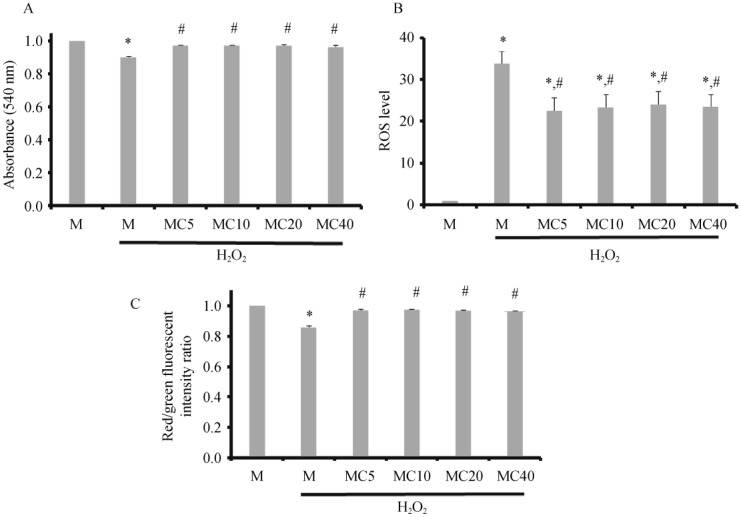
To investigate the dose-dependent effect of the drug, cilostazol at 5, 10, 20, and 40 µmol/L was tested for its protective effect, and the results are shown in Figure 2. All tested concentrations of cilostazol could significantly prevent mitochondrial swelling caused by H2O2 (Figure 2A). Furthermore, all tested concentrations of cilostazol could decrease the ROS levels (Figure 2B) and prevent cardiac mitochondrial depolarization induced by H2O2 application (Figure 2C). From this protocol, 5-µmol/L cilostazol was chosen as the standard concentration for subsequent protocols in this study.
Figure 2. Effects of different concentrations of cilostazol on cardiac mitochondrial swelling (A), ROS levels (B), and membrane potential changes (C) after 4-min H2O2 application.
Cilostazol at 5, 10, and 20 µmol/L with a 10 min incubation period could effectively prevent cardiac mitochondrial swelling, decrease ROS levels, and prevent mitochondrial membrane depolarization, in comparison with the H2O2 treated group. M: cardiac mitochondria without any treatment; MC5/MC10/MC20/MC40: cardiac mitochondria treated with cilostazol at 5, 10, 20, and 40 µmol/L for 5 min, respectively. *P < 0.05 vs. M group; #P < 0.05 vs. H2O2 treated mitochondria. ROS: reactive oxygen species.
3.2. Effects of cilostazol, CsA, and CDP on cardiac mitochondrial swelling, mitochondrial ROS levels and mitochondrial membrane potential changes
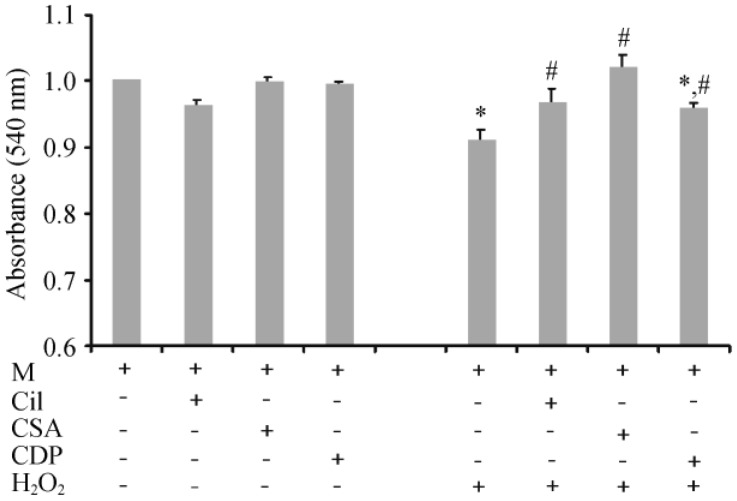
The protective effects of cilostazol, CsA and CDP on cardiac mitochondria were tested, and the results are shown in Figure 3. cilostazol, CsA and CDP did not alter the absorbance in cardiac mitochondria. In H2O2-treated cardiac mitochondria, the groups that were pretreated with Cilostazol and CsA did not show mitochondrial swelling caused by H2O2. Although CDP could attenuate cardiac mitochondrial swelling caused by H2O2, it could not restore the absorbance to the baseline level (Figure 3).
Figure 3. Effects of cilostazol, CsA, and CDP on cardiac mitochondrial swelling.
Cardiac mitochondria were pretreated with cilostazol (5 µmol/L, 10 min incubation), CsA, or CDP prior to the administration of H2O2. Cilostazol and CsA completely protected cardiac mitochondrial swelling caused by H2O2, whereas CDP could only partially protect cardiac mitochondrial swelling. *P < 0.05 vs. M (control group), #P < 0.05 vs. H2O2 treated group. CDP: 4′-chlorodiazepam; Cil: cilostazol; CsA: cyclosporine A; M: cardiac mitochondria without any treatment.
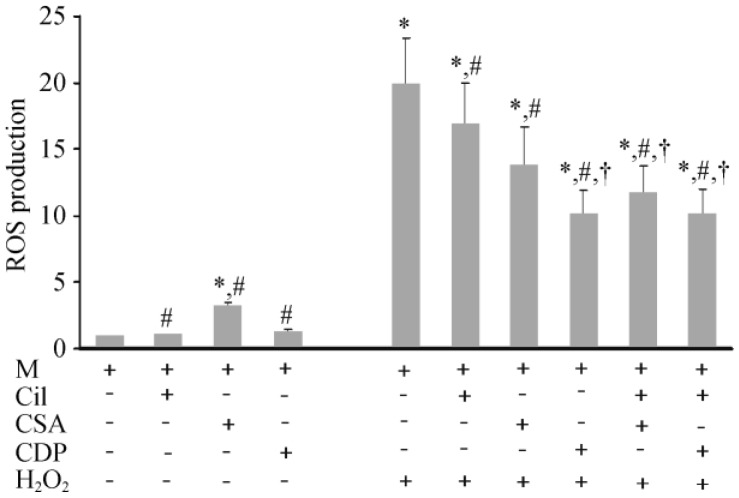
For cardiac mitochondrial ROS production, Cilostazol and CDP did not alter the ROS level, whereas CsA caused a slight increase in ROS levels in cardiac mitochondria without H2O2 application (Figure 4). In H2O2-treated cardiac mitochondria, the groups that were treated with cilostazol, CDP and CsA had decreased ROS levels, compared to the H2O2 treated group. However, the amount of ROS reduction was the greatest in the CsA, cilostazol + CDP, and cilostazol + CsA groups.
Figure 4. Effects of cilostazol, CsA, and CDP on ROS production in cardiac mitochondria.
Without H2O2, cilostazol and CDP did not alter the ROS levels, whereas CsA caused a slight increase in ROS levels in cardiac mitochondria. While H2O2 caused a marked increase in ROS levels, cilostazol, CsA, and CDP could significantly decrease the ROS levels created by H2O2. *P < 0.05 vs. M (control group), #P < 0.05 vs. H2O2 treated group, †P < 0.05 vs. (CsA + H2O2) treated group. CDP: 4′-chlorodiazepam; Cil: cilostazol; CsA: cyclosporine A; M: cardiac mitochondria; ROS: reactive oxygen species.
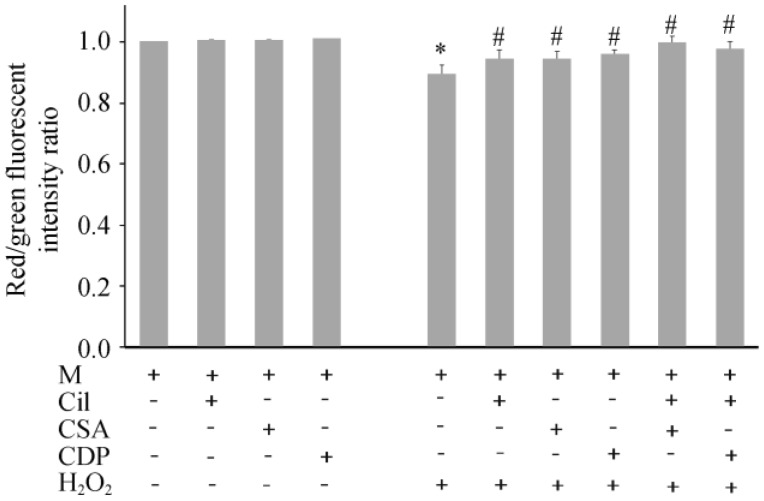
For cardiac mitochondrial membrane potential changes, cilostazol, CsA and CDP did not alter cardiac mitochondrial membrane potential (Figure 5). While H2O2 caused cardiac mitochondrial depolarization, pretreatment with cilostazol, CsA, CDP, cilostazol + CDP, and cilostazol + CsA could prevent cardiac mitochondrial depolarization caused by H2O2.
Figure 5. Effects of cilostazol, CsA, and CDP on cardiac mitochondrial membrane potential changes.
Without H2O2, cilostazol, CsA and CDP did not alter the cardiac mitochondrial membrane potential. While H2O2 caused mitochondrial depolarization, cilostazol, CsA and CDP could completely prevent cardiac mitochondrial membrane depolarization caused by H2O2. *P < 0.05 vs. M (control) group, #P < 0.05 vs. H2O2 treated group. CDP: 4′-chlorodiazepam; Cil: cilostazol; CsA: cyclosporine A; M: cardiac mitochondria.
4. Discussion
The major finding of the present study is that cilostazol prevents cardiac mitochondrial dysfunction caused by severe oxidative stress due to H2O2 application. The protective effects of cilostazol include prevention of cardiac mitochondrial swelling, decreased mitochondrial ROS production, and prevention of cardiac mitochondrial depolarization due to oxidative stress induced by H2O2.
Previous studies demonstrate that cilostazol can have antiarrhythmic effects in both animal models and patients.[18],[29] However, its effects on cardiac mitochondria have never been investigated. Since cardiac mitochondrial dysfunction has been shown to be associated with cardiac arrhythmias,[21] it is possible that the antiarrhythmic effect of cilostazol could, in part, be due to its protective effects regarding cardiac mitochondria. We previously demonstrated that oxidative stress in cardiac mitochondria caused by H2O2 can cause cardiac mitochondrial dysfunction, and that this model can be used to study the efficacy of drugs for their potential cardioprotective effects.[20] As shown in our study, cilostazol demonstrated its effects in preventing cardiac mitochondrial swelling, decreasing mitochondrial ROS production, and preventing cardiac mitochondrial membrane depolarization due to H2O2 application, indicating that this drug can assist in the prevention of cardiac mitochondrial dysfunction caused by severe oxidative stress.
In the present study, the mechanism of cardiac mitochondrial protection by cilostazol was tested using a pharmacological approach. It is known that oxidative stress with increased ROS production can lead to an opening of the mPTP in cardiac mitochondria, resulting in mitochondrial swelling and membrane potential depolarization.[30],[31] In our study, when CsA was applied to cardiac mitochondria prior to H2O2 application, it was possible to prevent cardiac mitochondrial swelling, decrease ROS production, and prevent cardiac mitochondrial membrane depolarization. Although cilostazol offers effects that prevent cardiac mitochondrial dysfunction similar to those found with CsA, the reduction of mitochondrial ROS production in the cilostazol-treated group was less than that of the CsA-treated mitochondria. Nevertheless, cilostazol could still prevent mitochondrial depolarization similar to that of the CsA-treated group, suggesting that the effects of cilostazol on ROS reduction were already sufficient to bring the ROS levels down to a level below the critical threshold that causes mitochondrial depolarization.[20] The prevention of cardiac mitochondrial dysfunction including the restoration of mitochondrial membrane potential by cilostazol could be responsible for its previously reported antiarrhythmic effects.[3],[18] There are several limitations in the present study. It is well known that the roles of PDE-3 involve both cAMP and cyclic guanosine monophosphate (cGMP), however, it has been demonstrated that PDE-3 is functionally a cAMP-hydrolyzing enzyme.[32] In the present study, we did not determine the effects of cilostazol on cAMP and cGMP. Moreover, it has been shown that PDE-3 inhibitor could exert cardioprotective benefits via its activation of cAMP-dependent protein kinase A (PKA) and potentiating the opening of mitochondrial Ca2+-activated K+ channels.[33] In the present study, the roles of cilostazol on the PKA and mitochondrial Ca2+-activated K+ channels were not investigated. Future studies are needed to determine the effects of cilostazol on these signaling cascades.
In conclusion, under oxidative stress, cilostazol prevents cardiac mitochondrial dysfunction by attenuating cardiac mitochondrial swelling, ROS production, and mitochondrial membrane potential changes in cardiac mitochondria. These effects, particularly those regarding the prevention of cardiac mitochondrial depolarization, may explain its previously found antiarrhythmic effect.
Acknowledgments
This study is supported by grants from the Thailand Research Fund BRG (SCC), RTA 5580006 (NC), and Chiang Mai University Excellence Center Award (NC).
References
- 1.Feldman AM, Bristow MR, Parmley WW, et al. Effects of vesnarinone on morbidity and mortality in patients with heart failure. Vesnarinone Study Group. N Engl J Med. 1993;329:149–155. doi: 10.1056/NEJM199307153290301. [DOI] [PubMed] [Google Scholar]
- 2.Packer M, Carver JR, Rodeheffer RJ, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med. 1991;325:1468–1475. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 3.Atarashi H, Endoh Y, Saitoh H, et al. Chronotropic effects of cilostazol, a new antithrombotic agent, in patients with bradyarrhythmias. J Cardiovasc Pharmacol. 1998;31:534–539. doi: 10.1097/00005344-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Cone J, Wang S, Tandon N, et al. Comparison of the effects of cilostazol and milrinone on intracellular cAMP levels and cellular function in platelets and cardiac cells. J Cardiovasc Pharmacol. 1999;34:497–504. doi: 10.1097/00005344-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Feldman AM, McNamara DM. Reevaluating the role of phosphodiesterase inhibitors in the treatment of cardiovascular disease. Clin Cardiol. 2002;25:256–262. doi: 10.1002/clc.4960250603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gamssari F, Mahmood H, Ho JS, et al. Rapid ventricular tachycardias associated with cilostazol use. Tex Heart Inst J. 2002;29:140–142. [PMC free article] [PubMed] [Google Scholar]
- 7.Kambayashi J, Liu Y, Sun B, et al. Cilostazol as a unique antithrombotic agent. Curr Pharm Des. 2003;9:2289–2302. doi: 10.2174/1381612033453910. [DOI] [PubMed] [Google Scholar]
- 8.Kishida M, Watanabe K, Tsuruoka T. [Effects of cilostazol in patients with bradycardiac atrial fibrillation] J Cardiol. 2001;37:27–33. [PubMed] [Google Scholar]
- 9.Kodama-Takahashi K, Kurata A, Ohshima K, et al. Effect of cilostazol on the ventricular escape rate and neurohumoral factors in patients with third-degree atrioventricular block. Chest. 2003;123:1161–1169. doi: 10.1378/chest.123.4.1161. [DOI] [PubMed] [Google Scholar]
- 10.Toyonaga S, Nakatsu T, Murakami T, et al. Effects of cilostazol on heart rate and its variation in patients with atrial fibrillation associated with bradycardia. J Cardiovasc Pharmacol Ther. 2000;5:183–191. doi: 10.1054/JCPT.2000.8696. [DOI] [PubMed] [Google Scholar]
- 11.Tsuchikane E, Fukuhara A, Kobayashi T, et al. Impact of cilostazol on restenosis after percutaneous coronary balloon angioplasty. Circulation. 1999;100:21–26. doi: 10.1161/01.cir.100.1.21. [DOI] [PubMed] [Google Scholar]
- 12.Tsuchiya T. Role of pharmacotherapy in Brugada syndrome. Indian Pacing Electrophysiol J. 2004;4:26–32. [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuji T, Ueda H, Nakatsu T, et al. Effects of cilostazol on heart rate and its variability in patients with sick sinus syndrome. Clin Drug Invest. 2001;21:605–612. [Google Scholar]
- 14.Watanabe K, Ikeda S, Komatsu J, et al. Effect of cilostazol on vasomotor reactivity in patients with vasospastic angina pectoris. Am J Cardiol. 2003;92:21–25. doi: 10.1016/s0002-9149(03)00458-2. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Shakur Y, Yoshitake M, et al. Cilostazol (Pletal): a dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake. Cardiovasc Drug Rev. 2001;19:369–386. doi: 10.1111/j.1527-3466.2001.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang S, Cone J, Fong M, et al. Interplay between inhibition of adenosine uptake and phosphodiesterase type 3 on cardiac function by cilostazol, an agent to treat intermittent claudication. J Cardiovasc Pharmacol. 2001;38:775–783. doi: 10.1097/00005344-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchiya T, Ashikaga K, Honda T, et al. Prevention of ventricular fibrillation by cilostazol, an oral phosphodiesterase inhibitor, in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 2002;13:698–701. doi: 10.1046/j.1540-8167.2002.00698.x. [DOI] [PubMed] [Google Scholar]
- 18.Kanlop N, Shinlapawittayatorn K, Sungnoon R, et al. Cilostazol attenuates ventricular arrhythmia induction and improves defibrillation efficacy in swine. Can J Physiol Pharmacol. 2010;88:422–428. doi: 10.1139/y09-127. [DOI] [PubMed] [Google Scholar]
- 19.Cortassa S, Aon MA, Winslow RL, O'Rourke B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys J. 2004;87:2060–2073. doi: 10.1529/biophysj.104.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thummasorn S, Kumfu S, Chattipakorn S, et al. Granulocyte-colony stimulating factor attenuates mitochondrial dysfunction induced by oxidative stress in cardiac mitochondria. Mitochondrion. 2011;11:457–466. doi: 10.1016/j.mito.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Aon MA, Cortassa S, Marban E, et al. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 22.Brady NR, Hamacher-Brady A, Westerhoff HV, et al. A wave of reactive oxygen species (ROS)-induced ROS release in a sea of excitable mitochondria. Antioxid Redox Signal. 2006;8:1651–1665. doi: 10.1089/ars.2006.8.1651. [DOI] [PubMed] [Google Scholar]
- 23.Chen Q, Moghaddas S, Hoppel CL, et al. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol. 2008;294:C460–C466. doi: 10.1152/ajpcell.00211.2007. [DOI] [PubMed] [Google Scholar]
- 24.Walker JM. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol Biol. 1994;32:5–8. doi: 10.1385/0-89603-268-X:5. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz-Meana M, Garcia-Dorado D, Miro-Casas E, et al. Mitochondrial Ca2+ uptake during simulated ischemia does not affect permeability transition pore opening upon simulated reperfusion. Cardiovasc Res. 2006;71:715–724. doi: 10.1016/j.cardiores.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Novalija E, Kevin LG, Eells JT, et al. Anesthetic preconditioning improves adenosine triphosphate synthesis and reduces reactive oxygen species formation in mitochondria after ischemia by a redox dependent mechanism. Anesthesiology. 2003;98:1155–1163. doi: 10.1097/00000542-200305000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Di Lisa F, Blank PS, Colonna R, et al. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J Physiol. 1995;486(Pt 1):1–13. doi: 10.1113/jphysiol.1995.sp020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong V, Teng XW, Chang TK, et al. Valproic acid II: effects on oxidative stress, mitochondrial membrane potential, and cytotoxicity in glutathione-depleted rat hepatocytes. Toxicol Sci. 2005;86:436–443. doi: 10.1093/toxsci/kfi185. [DOI] [PubMed] [Google Scholar]
- 29.Kanlop N, Chattipakorn S, Chattipakorn N. Effects of cilostazol in the heart. J Cardiovasc Med (Hagerstown) 2011;12:88–95. doi: 10.2459/JCM.0b013e3283439746. [DOI] [PubMed] [Google Scholar]
- 30.Petronilli V, Penzo D, Scorrano L, et al. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J Biol Chem. 2001;276:12030–12034. doi: 10.1074/jbc.M010604200. [DOI] [PubMed] [Google Scholar]
- 31.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 32.Rao YJ, Xi L. Pivotal effects of phosphodiesterase inhibitors on myocyte contractility and viability in normal and ischemic hearts. Acta Pharmacol Sin. 2009;30:1–24. doi: 10.1038/aps.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukasawa M, Nishida H, Sato T, et al. 6-[4-(1-Cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydro-2-(1H)quinolinone (cilostazol), a phosphodiesterase type 3 inhibitor, reduces infarct size via activation of mitochondrial Ca2+-activated K+ channels in rabbit hearts. J Pharmacol Exp Ther. 2008;326:100–104. doi: 10.1124/jpet.108.136218. [DOI] [PubMed] [Google Scholar]