Abstract
Aortic valve stenosis (AS) is common in the elderly. Although surgical replacement of the valve has been the gold standard of management, many patients have been excluded from surgery because they were very old, frail, or had co-morbidities that increased operative risks. In the last decade, transcatheter aortic valve implantation (TAVI) has emerged as a new treatment option suitable for these patients. This article reviews the available literature on the role of TAVI in elderly patients with severe aortic stenosis. Published studies showed that elderly individuals who underwent TAVI experienced better in-hospital recovery, and similar short and mid-term mortality compared to those underwent surgical treatment of AS. However, long-term outcomes of TAVI in elderly patients are still unknown. The available data in the literature on the effect of advanced age on clinical outcomes of TAVI are limited, but the data that are available suggest that TAVI is a beneficial and tolerable procedure in very old patients. Some of the expected complications after TAVI are reported more in the oldest patients such as vascular injures. Other complications were comparable in TAVI patients regardless of their age group. However, very old patients may need closer monitoring to avoid further morbidities and mortality.
Keywords: Aortic stenosis, Transcatheter aortic valve implantation, Surgical aortic valve replacement, Geriatric
1. Introduction
Aortic valve stenosis (AS) is a common valvular disease in the Western world. As the geriatric population has grown, so too has the number of patients presenting with symptomatic aortic valve disease because its prevalence increases with age.
Surgical aortic valve replacement (SAVR) has produced significant results that have been well documented for elderly patients, including improved life expectancy, cardiovascular symptoms, and quality of life.[1],[2] Yet, up to one-third of elderly patients with severe AS have been excluded from surgery.[3] Not surprisingly, advanced age has been a leading barrier to surgical intervention in elderly patients. This is based on the peri-operative mortality rate which is found to increase with age from 1.3% in patients ≤ 70 years old, to about 5% at age 80-85 years, and 10% in patients ≥ 90 years old.[4],[5] It is important, though, to realize that age, per se, is not a predictor of poor outcomes of surgery, and those patients usually enjoy the same survival rates within their age and gender matched population.[6] To help bridge this gap in care, medical innovations such as transcatheter aortic valve implantation (TAVI) have emerged as a new treatment option which improves the clinical course of AS without high surgical risk.
2. TAVI as an alternative therapy for AS in elderly patients
TAVI includes the insertion of a prosthetic valve into the stenotic aortic valve through a vascular access without the need for open heart surgery. Two valves are commonly used for this procedure: the Edwards SAPIEN valve which is a balloon expandable valve and the Core Valve which is a self-expanding one. Both can usually be inserted through a transfemoral retrograde approach. They may also be placed via other arteries such as the axillary artery or directly through the cardiac apex in patients with stenosed iliac and femoral arteries. TAVI is strongly indicated for symptomatic AS patients with multiple comorbidities, or high operative risk [i.e., expected mortality > 20% with the European System for Cardiac Operative Risk Evaluation score (EuroSCORE) or > 10% with the Society of Thoracic Surgeons score (STS)], as long as life expectancy is a year or more.[7]
Pre-procedure multidisciplinary assessment is important for the appropriate selection of patients to undergo TAVI. The team usually includes cardiologists, cardiothoracic surgeons, cardiac anesthesiologists, interventional cardiologists and imaging specialists. Cardiac imaging should assess not only the severity of aortic stenosis, but also morphometric characteristics like the diameter of the aortic root, the conformation of the aortic valve and annulus, and area of valve calcification to ensure that the most appropriate valve is implanted, and that the procedure is likely to be successful. Assessment for atherosclerosis of both coronary and peripheral arteries is also important to identify patients at risk for perioperative complications and to select the optimal site for access.[8]
Following TAVI, an immediate and sustained improvement in aortic valve area, trans-valvular gradient, and left ventricular ejection fraction were reported.[9]–[11] These changes were associated with an enhancement in patients' physical and mental functioning, as well as their quality of life.[12],[13] However, frail elderly patients were found to have smaller gains in functional status after TAVI and they may require multidisciplinary interventions, such as nutrition consultation and exercise-training programs, to achieve desired outcomes.[14]
Due to the mentioned hemodynamic and functional effects of TAVI, the early and midterm survival in patients with AS has significantly improved as shown in Moat, et al.[15] study, who reported life expectancy rates of 92.9%, 78.6% and 73.7% at 30 days, 1 year and 2 years after TAVI, respectively. Studies have shown that transfemoral approach is associated with better survival rates compared to other vascular accesses. This, however, is still controversial because patients who are referred to transapical or transaxillary approaches usually have higher risk profiles which explains the increased mortality after these procedures.[16]
3. TAVI vs. SAVR in elderly patients
As discussed earlier, both TAVI and SAVR have achieved significant improvements in survival rates as well as other outcomes in patients with severe aortic stenosis. Studies have shown that SAVR can be performed in elderly patients with acceptable morbidity and mortality rates. The question then becomes, can TAVI be trusted as a safe and effective alternative to surgical management in elderly patients?
Of the studies that looked at the outcomes of TAVI compared to SAVR, we specifically reviewed the trials where average age of patients who underwent both procedures was ≥ 80 years. Only 8 studies were found (5 observational studies and 3 randomized controlled trials) and summarized in (Table 1).[17]–[24] In these studies, elderly individuals who underwent TAVI experienced better in-hospital recovery, and similar short and mid-term mortality compared to those underwent SAVR (Table 1).
Table 1. Summary of studies compared the outcomes of TAVI vs. SAVR in patients aged ≥ 80 years old.
| References | n | Study design | Age (yrs) (TAVI vs. SAVR) | F/U (mo) | Clinical outcomes that improved with TAVI | Clinical outcomes that worsened with TAVI | Clinical outcomes that were similar in both TAVI and SAVR |
| Zierer, et al.[18] | 51 | OS | 85 vs. 82 | 12 | Operative time, ventilation time, intensive care unit stay, and hospital stay | 30 days and 1 year mortality | |
| De Carlo, et al.[19] | 96 | OS | 83 vs. 82 | 9 | Freedom from major cardiac and cerebrovascular events at 6 months; Cardiac mortality at 6 months | ||
| Smith, et al.[17] | 699 | RCT | 83.6 vs. 84.5 | 12 | Survival at 1 year | ||
| Kodali, et al.[20] | 699 | RCT | 83.6 vs. 84.5 | 24 | Paravalvular regurgitation was more frequent after TAVR and was associated with increased late mortality | Two years mortalitySimilar reduction in symptoms, and similar improvement in valve hemo-dynamics | |
| Conradi, et al.[21] | 164 | OS | 81.9 vs. 82.5 | 6 | Operative time, ventilation time, and intensive care unit stay. The need of blood transfusion | Mortality at 30 days, 90 days, and 180 daysSimilar hospital stay | |
| Nielsen, et al.[22] | 70 | RCT | 80 vs. 82 | 3 | Study terminated early due to the side effects in TAVI group (two deaths, two strokes, and one case of renal failure requiring dialysis) | All-cause mortality at 30 days | |
| Im, et al.[23] | 24 | OS | 82. 5 vs. 83 | 3 | In-hospital (all-cause mortality, major stroke, peri-procedural myocardial infarction, life-threatening bleeding, major vascular complication, and acute kidney injury); The rate of 3-monthall-cause mortality, myocardial infarction, major stroke, and re-hospitalization | ||
| Silberman, et al.[24] | 252 | OS | 84 vs. 80 | 36 | Procedural mortality | One year, two years and three years survival rates |
F/U: duration of follow up; mo: months; OS: observational study; RCT: randomized controlled trial; SAVR: Surgical aortic valve replacement; TAVI: Transcatheter aortic valve implantation.
One of the most important studies is the Placement of AoRtic TraNscathetER Valves (PARTNER) trial which was a multicenter, randomized trial that compared TAVI to surgical management and medical therapy in 1,057 AS patients.[17] In this study, patients were divided into two cohorts: group A which included those with high surgical risk (the mean age of this group was 84 years old), and group B which included those who were not considered to be appropriate candidates for surgery (the mean age was 83 years old). In the high-risk PARTNER trial cohort A, mortality at 30 days was 3.4% in the TAVI group, compared to 6.5% in the SAVR group (P = 0.07). At the 1-year and 2-year follow-up, mortality was 24.2% and 33.9% with TAVI vs. 26.8% and 35.0% in the SAVR groups, respectively (P = 0.44, P = 0.78, respectively).[17],[20] PARTNER trial also showed that the incidences of stroke, myocardial infarction, acute kidney injury (AKI), endocarditis, and pacemaker placement at one and two years after TAVI and SAVR were comparable.[17],[20] However, patients who underwent SAVR experienced more major bleedings, and less major vascular injuries compared to those treated with TAVI.[20] The authors concluded that TAVI is not-inferior to SAVR as a treatment of AS in high risk surgery patients.
Another randomized controlled study was the STACCATO trial which initially planned to assign 200 patients (age > 75 years) to either TAVI vs. SAVR.[22] However, after the inclusion of 70 patients, the trial was prematurely terminated due to unexpectedly poor outcomes in the TAVI cohort. Authors of this trial concluded that current indications for TAVI should remain restricted only to surgically inoperable patients.
Only a few studies have focused on elderly patients with intermediate or low risk for procedural complications. These include the Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI) trial and the OBSERVANT trial. The SURTAVI investigators prospectively enrolled patients with symptomatic severe aortic stenosis and intermediate surgical risk (STS score between 3% and 8%) to either TAVI or SAVR.[25] The study included 255 patients, the mean age was 80.1 years. All-cause mortality at 30 days (7.8% vs. 7.1%, P = 0.74) and 1 year (16.5% vs.16.9%, P = 0.64) was comparable between the two groups. The Italian OBSERVANT study reported similar 30 days mortality for its intermediate risk, propensity-matched subgroups.[26] Differences in rates of blood transfusion (higher in SAVR), vascular damage, permanent atrioventricular block, and residual aortic valve regurgitation (all higher in TAVI) were reported.[26]
Lastly, while the early and mid-term survival rates seem comparable in TAVI and SAVR in most of the outlined studies, not much is known about long-term outcomes of TAVI in elderly patients. Observations by Holzhey, et al.[27] showed that Kaplan-Meier curves diverged between TAVI and SAVR patients after 6 months in favor of conventional surgery. Follow-up studies, therefore, are necessary to assess whether TAVI will remain a comparable approach to SAVR on long-term basis as well.
4. Effect of advanced age on the outcomes of TAVI
Given the growing geriatric population in the Western countries, more and more of nonagenarians are expected to undergo TAVI as the principal treatment for severe AS. Yet, relatively few studies have examined the outcomes of this procedure in very old patients. Jabs, et al.[28] have reported their experience with a 99 year old AS patient who underwent TAVI after presenting with syncope and progressive dyspnea.[28] Following TAVI, aortic valve area improved from 0.6 cm2 to 1.5 cm2 and the patient was discharged to a geriatric rehabilitation unit 2 days after the procedure. For the next four years, the patient remained independent, with mild exertional dyspnea, and without recurrent syncopes. Transesophageal echocardiography at 3.5 years follow-up showed aortic valve area of 1.5 cm2 with persistent optimal positioning of the implanted valve. A recent Swedish study reviewed post-procedure mortality in 29 nonagenarian patients who underwent TAVI between 2008 and 2011.[29] There was no mortality in the first 30 post-procedure days. One-year, two years, and three years mortality were found as 11%, 28%, and 40%, respectively.[29]
The available literature shows only three trials that studied the outcomes of TAVI in very old patients comparing to younger patients. All of these studies were observational and no randomized controlled trials were identified as shown in Table 2.[30]–[32] In a study of 1,386 patients who underwent TAVI at ages ranging between 40–99 years,[30] the researchers divided the patients into four age groups and analyzed the outcome of TAVI in each group. Despite the difference in the groups' characteristics, all groups, including the oldest one, showed immediate and marked improvement of the hemodynamic valve status, functional status, and overall quality of life. Also, each group had similar rates of technical success and 30 day all-cause mortality regardless of age. Havakuk, et al.[31] evaluated 293 patients who underwent TAVI (patients' age ranged between 63 and 98 years and average age was 83 years). The cohort was divided depending on age into 2 groups: younger vs. older than 85 years. Older patients experienced length of in-hospital stay, readmission rates, and 30-day mortality similar to those reported in younger patients. However, overall mortality rate during the entire follow-up (median 480 days, interquartile range 330 to 770 days) was higher in the older age group. In another study, Yamamoto et al.[32] compared the clinical outcomes of TAVI in patients ≤ 90 years old to patients ≥ 90 years old and found similar procedural success in both groups. Although 30-day and 6-month mortality trended to be higher in patients older than 90 years old, the differences in mortality rates were statically insignificant (6% vs. 15%, P = 0.22; and 14% vs. 27%, P = 0.14, respectively). Moreover, the cumulative survival after 13.4 ± 8.0 months of follow-up was comparable between both groups (P = 0.22).
Table 2. Studies that showed outcomes of TAVI in different age groups.
| References | Study design | Age groups (Mean age in each group) | Number of patients in each group | Female in each group (%) | How the oldest patients were different in baseline characteristics | Clinical outcomes that changed with age | Clinical outcomes that did not change with age |
| Buellesfeld, et al.[30] | OS | Group A: 73.4 yrs; Group B: 80.6 yrs; Group C: 84.5 yrs; Group D: 88.9 yrs | Group A: n = 347;Group B: n = 350;Group C: n = 382;Group D: n = 312 | Group A: 44.4%; Group B: 52.3%; Group C: 67.5%; Group D: 67.9% | The prevalence of DM, prior CABG, and COPD was less in the oldest patients; renal insufficiency was more common in this group.Oldest patients had higher surgical risk; they underwent less trans subclavian TAVI, and less large Edwards valves. | Less post-TAVI MI (reported less in both oldest and the youngest groups) | The improvement in functional status and quality of life.The rates of technical success, in-hospital mortality, and 30 day mortality. |
| Havakuk, et al.[31] | OS | ≤ 85 yrs (mean: 80.5 yrs) vs. > 85 yrs (mean: 88.8 yrs) | 200 vs. 93 | 57.5% vs. 70.0%P = 0.043 | The prevalence of DM was less in older patient. They had more HF and prior MIs, but less prior CABG. | Older patient suffered more respiratory failure and minor vascular complicationsOverall mortality rate was higher in the older age group | Length of in-hospital stay, readmission rates, and 30-day mortality. |
| Yamamoto, et al.[32] | OS | < 90 yrs (mean: 82.3 yrs) vs. ≥ 90 yrs (mean: 91.6 yrs) | 110 vs. 26 | 50.0% vs. 81.0%P = 0.004 | Older patients had less BMI, and less Leukocyte count. Smoking and using beta blockers were less common in older patients. The rate of NYHA class IV was greater in the oldest patients | The oldest patients had more major vascular complications | Length of ICU stay and in-hospital stay.Procedural success, 30-day and 6-month mortality. |
BMI: body mass index; CABG: coronary artery bypass graft; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; HF: heart failure; ICU: intensive care unit; MI: myocardial infarction; NYHA: New York Heart Association; OS: observational study; TAVI: transcatheter aortic valve implantation.
In conclusion, the available literature gives an impression that TAVI is a beneficial and tolerable procedure in very old patients with severe AS. This hypothesis, however, still needs further testing in randomized controlled trials in order to be confirmed.
5. Procedure complications and patient's age
Although TAVI has a high success rate with few surgical complications, it still carries risks. Common procedure complications include vascular injuries, bleeding, stroke, atrioventricular conduction system injuries, AKI, and aortic regurgitation. Complication rates differ with operator experience, the size of the device, the site of catheter access, as well as the use of pre-procedural screening.
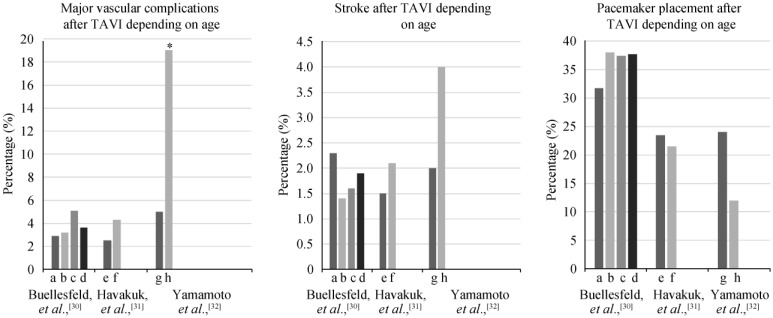
Vascular injuries are the most common complications after TAVI, and range from dissection, to perforation, to acute thrombotic occlusion. The prevalence of these complications seem to increase in very old patients. Yamamoto, et al.[32] reported more frequent major vascular complications in nonagenarians (19% vs. 5%, P = 0.022). Havakuk, et al.[31], though, found that only minor vascular injuries increased in older patients (7.5% vs. 16%, P = 0.02), and reported similar rates of major vascular complications in both age groups of their study (4.3% vs. 2.5%, P = 0.41), as shown in Figure 1.
Figure 1. The rates of post TAVI complications depending on patients' age.[30]–[32].
Y axis represents the percentage of post TAVI complications. X axis includes age groups of TAVI patients as presented in Buellesfeld, et al.,[30] Havakuk, et al.,[31] and Yamamoto, et al.,[32] trials. Asterisk represents any statistically significant difference. a: represents the youngest group of TAVI patients with mean age of 73.4 ± 4.5 years; b: represents the second group of TAVI patients with mean age of 80.6 ± 1.1 years; c: represents the third group of TAVI patients with mean age 84.5 ± 1.1 years; d: represents the second group of TAVI patients with mean age 88.9 ± 2.2 years; e: represents patients who are ≤ 85 years old; f: represents patients who are ≥ 85 years old; g: represents patients who are ≤ 90 years old; h: represent patients who are ≥ 90 years old. TAVI: transcatheter aortic valve implantation.
Patients also are at risk for cerebrovascular accidents (CVA) after TAVI. In the high-risk cohort of the PARTNER trial (cohort A), there was a trend toward more strokes in the TAVI group at 30 days when compared to the SAVR group (4.6% vs. 2.4%, P = 0.12) and at 1-year (6.0% vs. 3.2%, P = 0.08).[17] Up to 10% of TAVI patients experienced CVA, regardless of their age (Figure 1). [30]–[34] The CVA are mostly ischemic, felt to be due to showering of emboli from the aorta or during valve positioning and deployment. To reduce the risk of stroke, aspirin, clopidogrel, or warfarin has been used in TAVI patients. In a small retrospective study that included 171 patients (average age 81.6 years), the use of post-TAVI dual antiplatelet did not protect patients from stroke, but has been associated with worse bleeding compared to the use of single antiplatelet or warfarin.[35] Durand, et al.[36] retrospectively compared the use of mono antiplatelet therapy vs. dual antiplatelet therapy in 292 patients underwent TAVI (average age was 83.6 years), and found that monotherapy reduced life-threatening and major bleedings without increasing the risk of stroke and myocardial infarction compared to dual antiplatelet therapy. Another study has showed that the safest post TAVI regimen was a combination of clopidogrel and Vitamin K antagonists.[37] In this study, advanced age increased risk of early bleeding after TAVI (OR: 5.96, 95% CI: 1.47–24.13, P = 0.01).[37] More studies are needed to evaluate the efficacy and risks of different antithrombotic regimens before recommendations can be made regarding stroke prevention in TAVI patients.
Due to the proximity of the aortic valve to the atrioventricular node and bundle of His, TAVI is associated with a risk of atrioventricular conduction system injuries and permanent pacemaker implantation.[34] The need of a new pacemaker was reported in PARTNER study in similar rates after both TAVI (5% and 6.4%) and SAVR (6.4% and 7.2%), (P = 0.44, P = 0.69, respectively), at one and two years, respectively.[20] However, this complication was observed more commonly with CoreValve implantations presumably because of its longer stent frame, self-expanding nature, or ovoid shape.[38] Advancing age does not increase the risks for atrioventricular nodal block or permanent pacemaker (Figure 1).[30]–[32]
AKI is another potential complication of TAVI and is associated with an increase in post-procedure mortality.[39] Pre-existing hypertension, chronic obstructive pulmonary disease, and blood transfusion predicted AKI in one study.[37] Surprisingly, neither the amount of intravenous contrast agent nor patient's age, nor pre-procedure chronic kidney disease affected the risk of AKI after TAVI.[31],[32],[39],[40]
Paravalvular leak due to incorrect sizing or positioning of the prosthetic valve, or under-expansion of it, can result in aortic regurgitation. This complication was more common in patients undergoing TAVI than SAVR in the PARTNER study (12.0% vs. 0.9%, P < 0.001),[17] but did not increase in very old patients when compared to younger patients.[30]–[32] Studies found that paravalvular leak is more prevalent with transfemoral approach and in patients received self-expandable valves.[38],[41] Valve leak should be followed closely and managed early because severe aortic regurgitation was found to be an independent predictor of mortality after TAVI.[42]
Based on the limited published studies, some of the expected complications after TAVI are reported more in the oldest patients such as vascular injuries. Other complications were comparable in TAVI patients regardless of their age group. However, very old patients may need closer monitoring to avoid further morbidities and mortality.
6. The future of TAVI
TAVI offers a new treatment approach for tens of thousands of elderly patients with severe AS, who otherwise would be treated medically with resultant poor outcomes. As transcatheter technology improves with second generation devices and operator experience increases, indications for TAVI may expand to include patients with moderate surgical risk, especially those who are frail. More studies to identify the indications and contraindications for specific prosthetic valves and methods of vascular access would help physicians tailor treatments to patients.
Outcomes for TAVI might improve with optimization of perioperative management strategies as well. For example, optimal antiplatelet therapy or anticoagulation regimen remains undefined yet. Post-TAVI morbidities and mortality are expected to decrease significantly with determination of the ideal anticoagulation treatment that can reduce the incidence of stroke without rising bleeding risk. Similarly, since paravalvular leak has a bad impact on survival of patients undergoing TAVI, more efforts are made to predict this complication, such as using aortic valve calcium scoring. These efforts may result in better patients' selection for TAVI and decrease the expected mortality.[43]
New indications for transcatheter procedures is on the horizon. Increasingly, valve in valve procedures are of interest. Bioprosthetic valves are preferred for elderly patients undergoing SAVR, yet these tend to fail early, requiring re-operation. Mortality rates for re-operation are high, but theoretically may be lower with valve in valve implantation using the transcatheter technique. Early reports of such procedures have been promising.[44]
7. Conclusions
Many older adults with severe AS are not candidates for SAVR because of high surgical risk, advanced age, frailty, or comorbidity conditions. TAVI seems to be a safe and feasible alternative treatment for these patients. Improvements in devices, technical expertise, and clinical experience, along with perioperative patient management may expand the use of TAVI for elderly patients at lower surgical risk in the future. More studies, however, are needed to explore a multiplicity of aspects of this treatment especially in very old patients.
References
- 1.Varadarajan P, Kapoor N, Bansal RC, et al. Survival in elderly patients with severe aortic stenosis is dramatically improved by aortic valve replacement: results from a cohort of 277 patients aged > or =80 years. Eur J Cardiothorac Surg. 2006;30:722–727. doi: 10.1016/j.ejcts.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 2.Huber CH, Goeber V, Berdat P, et al. Benefits of cardiac surgery in octogenarians—a postoperative quality of life assessment. Eur J Cardiothorac Surg. 2007;31:1099–1105. doi: 10.1016/j.ejcts.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 3.Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J. 2005;26:2714–2720. doi: 10.1093/eurheartj/ehi471. [DOI] [PubMed] [Google Scholar]
- 4.Sawaya F, Stewart J, Babaliaros V. Aortic stenosis: who should undergo surgery, transcatheter valve replacement? Cleve Clin J Med. 2012;79:487–497. doi: 10.3949/ccjm.79a.11043. [DOI] [PubMed] [Google Scholar]
- 5.Assmann A, Minol JP, Mehdiani A, et al. Cardiac surgery in nonagenarians: not only feasible, but also reasonable? Interact CardiovascThorac Surg. 2013;17:340–343. doi: 10.1093/icvts/ivt125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kvidal P, Bergström R, Hörte LG, et al. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol. 2000;35:747–756. doi: 10.1016/s0735-1097(99)00584-7. [DOI] [PubMed] [Google Scholar]
- 7.Vahanian A, Alfieri O, Al-Attar N, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI) EuroIntervention. 2008;4:193–199. doi: 10.4244/eijv4i2a36. [DOI] [PubMed] [Google Scholar]
- 8.O'Sullivan CJ, Stortecky S, Buellesfeld L, et al. Preinterventional screening of the TAVI patient: how to choose the suitable patient and the best procedure. Clin Res Cardiol. 2014;103:259–274. doi: 10.1007/s00392-014-0676-4. [DOI] [PubMed] [Google Scholar]
- 9.Bauer F, Eltchaninoff H, Tron C, et al. Acute improvement in global and regional left ventricular systolic function after percutaneous heart valve implantation in patients with symptomatic aortic stenosis. Circulation. 2004;110:1473–1476. doi: 10.1161/01.CIR.0000134961.36773.D6. [DOI] [PubMed] [Google Scholar]
- 10.Clavel MA, Webb JG, Pibarot P, et al. Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J Am Coll Cardiol. 2009;53:1883–1891. doi: 10.1016/j.jacc.2009.01.060. [DOI] [PubMed] [Google Scholar]
- 11.Webb JG, Pasupati S, Humphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation. 2007;116:755–763. doi: 10.1161/CIRCULATIONAHA.107.698258. [DOI] [PubMed] [Google Scholar]
- 12.Bekeredjian R, Krumsdorf U, Chorianopoulos E, et al. Usefulness of percutaneous aortic valve implantation to improve quality of life in patients >80 years of age. Am J Cardiol. 2010;15; 106:1777–1781. doi: 10.1016/j.amjcard.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Ussia GP, Barbanti M, Cammalleri V, et al. Quality-of-life in elderly patients one year after transcatheter aortic valve implantation for severe aortic stenosis. EuroIntervention. 2011;7:573–579. doi: 10.4244/EIJV7I5A93. [DOI] [PubMed] [Google Scholar]
- 14.Schoenenberger AW, Stortecky S, Neumann S, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI) Eur Heart J. 2013;34:684–692. doi: 10.1093/eurheartj/ehs304. [DOI] [PubMed] [Google Scholar]
- 15.Moat N, Ludman P, de Belder MA, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis. The UK TAVI (United Kingdom Transcatheter Aortic Valve Implantation) registry. J Am Coll Cardiol. 2011;58:2130–2138. doi: 10.1016/j.jacc.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 16.Blackman DJ, Baxter PD, Gale CP, et al. Do outcomes from transcatheter aortic valve implantation vary according to access route and valve type? The UK TAVI Registry. J Interv Cardiol. 2014;27:86–95. doi: 10.1111/joic.12084. [DOI] [PubMed] [Google Scholar]
- 17.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 18.Zierer A, Wimmer-Greinecker G, Martens S, et al. Is transapical aortic valve implantation really less invasive than minimally invasive aortic valve replacement? J Thorac Cardiovasc Surg. 2009;138:1067–1072. doi: 10.1016/j.jtcvs.2009.04.057. [DOI] [PubMed] [Google Scholar]
- 19.De Carlo M, Giannini C, Ettori F, et al. Impact of treatment choice on the outcome of patients proposed for transcatheter aortic valve implantation. EuroIntervention. 2010;6:568–574. doi: 10.4244/EIJV6I5A96. [DOI] [PubMed] [Google Scholar]
- 20.Kodali SK, Williams MR, Smith CR, et al. PARTNER Trial Investigators. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;3; 366:1686–1695. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 21.Conradi L, Seiffert M, Treede H, et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement: a propensity score analysis in patients at high surgical risk. J Thorac Cardiovasc Surg. 2012;143:64–71. doi: 10.1016/j.jtcvs.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen HH, Klaaborg KE, Nissen H, et al. A prospective, randomised trial of transapicaltranscatheter aortic valve implantation vs. surgical aortic valve replacement in operable elderly patients with aortic stenosis: the STACCATO trial. EuroIntervention. 2012;8:383–389. doi: 10.4244/EIJV8I3A58. [DOI] [PubMed] [Google Scholar]
- 23.Im E, Hong MK, Ko YG, et al. Comparison of early clinical outcomes following transcatheter aortic valve implantation versus surgical aortic valve replacement versus optimal medical therapy in patients older than 80 years with symptomatic severe aortic stenosis. Yonsei Med J. 2013;54:596–602. doi: 10.3349/ymj.2013.54.3.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silberman S, Abu Akr F, Bitran D, et al. Comparison between transcatheter and surgical aortic valve replacement: a single-center experience. J Heart Valve Dis. 2013;22:448–454. [PubMed] [Google Scholar]
- 25.Piazza N, Kalesan B, van Mieghem N, et al. A 3-center comparison of 1-year mortality outcomes between transcatheter aortic valve implantation and surgical aortic valve replacement on the basis of propensity score matching among intermediate-risk surgical patients. JACC Cardiovasc Interv. 2013;6:443–451. doi: 10.1016/j.jcin.2013.01.136. [DOI] [PubMed] [Google Scholar]
- 26.D'Errigo P, Barbanti M, Ranucci M, et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis: results from an intermediate risk propensity-matched population of the Italian OBSERVANT study. Int J Cardiol. 2013;67:945–952. doi: 10.1016/j.ijcard.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Holzhey DM, Shi W, Rastan A, et al. Transapical versus conventional aortic valve replacement—a propensity-matched comparison. Heart Surg Forum. 2012;15:E4–E8. doi: 10.1532/HSF98.20111084. [DOI] [PubMed] [Google Scholar]
- 28.Jabs A, Kilic T, Schnelle N, et al. Transcatheter aortic valve implantation and four-year follow up in a 99-year-old patient. J Heart Valve Dis. 2013;22:261–264. [PubMed] [Google Scholar]
- 29.Verouhis D, Yamasaki K, Ivert T, et al. Transcatheter aortic valve implantation is feasible and safe in nonagenarians. J Am Geriatr Soc. 2014;62:189–190. doi: 10.1111/jgs.12620. [DOI] [PubMed] [Google Scholar]
- 30.Buellesfeld L, Gerckens U, Erbel R, et al. Age-stratified baseline and outcome characteristics of patients undergoing transcatheter aortic valve implantation: results from the German multicenter registry. J Invasive Cardiol. 2012;24:531–536. [PubMed] [Google Scholar]
- 31.Havakuk O, Finkelstein A, Steinvil A, et al. Comparison of outcomes in patients ≤ 85 versus > 85 years of age undergoing transcatheter aortic-valve implantation. Am J Cardiol. 2014;113:138–141. doi: 10.1016/j.amjcard.2013.09.044. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto M, Meguro K, Mouillet G, et al. Comparison of effectiveness and safety of transcatheter aortic valve implantation in patients aged ≥ 90 years versus < 90 years. Am J Cardiol. 2012;110:1156–1163. doi: 10.1016/j.amjcard.2012.05.058. [DOI] [PubMed] [Google Scholar]
- 33.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas M, Schymik G, Walther T, et al. Thirty-day results of the SAPIEN aortic bioprosthesis European outcome (SOURCE) registry: a European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2010;122:62–69. doi: 10.1161/CIRCULATIONAHA.109.907402. [DOI] [PubMed] [Google Scholar]
- 35.Poliacikova P, Cockburn J, de Belder A, et al. Antiplatelet and antithrombotic treatment after transcatheter aortic valve implantation - comparison of regimes. J Invasive Cardiol. 2013;25:544–548. [PubMed] [Google Scholar]
- 36.Durand E, Blanchard D, Chassaing S, et al. Comparison of two antiplatelet therapy strategies in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2014;113:355–360. doi: 10.1016/j.amjcard.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 37.Czerwińska-Jelonkiewicz K, Witkowski A, Dąbrowski M, et al. Antithrombotic therapy - predictor of early and long-term bleeding complications after transcatheter aortic valve implantation. Arch Med Sci. 2013;9:1062–1070. doi: 10.5114/aoms.2013.39794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdel-Wahab M, Mehilli J, Frerker C, et al. Comparison of balloon-expandable vs. self-expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. JAMA. 2014;311:1503–1514. doi: 10.1001/jama.2014.3316. [DOI] [PubMed] [Google Scholar]
- 39.Bagur R, Webb JG, Nietlispach F, et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J. 2010;31:865–874. doi: 10.1093/eurheartj/ehp552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goebel N, Baumbach H, Ahad S, et al. Transcatheter aortic valve replacement: does kidney function affect outcome? Ann Thorac Surg. 2013;96:507–512. doi: 10.1016/j.athoracsur.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 41.Gilard M, Eltchaninoff H, Iung B, et al. Registry of transcatheter aortic-valve implantation in high risk patients. N Engl J Med. 2012;366:1705–1715. doi: 10.1056/NEJMoa1114705. [DOI] [PubMed] [Google Scholar]
- 42.Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299–308. doi: 10.1161/CIRCULATIONAHA.110.946533. [DOI] [PubMed] [Google Scholar]
- 43.Colli A, Gallo M, Bernabeu E, et al. Aortic valve calcium scoring is a predictor of paravalvular aortic regurgitation after transcatheter aortic valve implantation. Ann Cardiothorac Surg. 2012;1:156–159. doi: 10.3978/j.issn.2225-319X.2012.07.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khawaja MZ, Haworth P, Ghuran A, et al. Transcatheter aortic valve implantation for stenosed and regurgitant aortic valve bioprostheses CoreValve for failed bioprosthetic aortic valve replacements. J Am Coll Cardiol. 2010;55:97–101. doi: 10.1016/j.jacc.2009.06.060. [DOI] [PubMed] [Google Scholar]