Abstract
Endometriosis is an estrogen-dependent inflammatory disorder characterized by the presence of endometrial tissue outside the uterine cavity. Patients experience chronic pelvic pain and infertility, with the most likely origin of the tissue deposits (lesions) being endometrial fragments shed at menses. Menstruation is an inflammatory process associated with a dramatic increase in inflammatory mediators and tissue-resident immune cells. In the present study, we developed and validated a mouse model of endometriosis using syngeneic menstrual endometrial tissue introduced into the peritoneum of immunocompetent mice. We demonstrate the establishment of endometriotic lesions that exhibit similarities to those recovered from patients undergoing laparoscopy. Specifically, in both cases, lesions had epithelial (cytokeratin+) and stromal (vimentin/CD10+) cell compartments with a well-developed vasculature (CD31+ endothelial cells). Expression of estrogen receptor β was increased in lesions compared with the peritoneum or eutopic endometrium. By performing experiments using mice with green fluorescent protein–labeled macrophages (MacGreen) in reciprocal transfers with wild-type mice, we obtained evidence that macrophages present in the peritoneum and in menses endometrium can contribute to the inflammatory microenvironment of the lesions. In summary, we developed a mouse model of endometriosis that exhibits similarities to human peritoneal lesions with respect to estrogen receptor expression, inflammation, and macrophage infiltration, providing an opportunity for further studies and the possible identification of novel therapies for this perplexing disorder.
Endometriosis occurs in 6% to 10% of women of reproductive age,1 is associated with chronic pelvic pain and infertility, and represents a huge socioeconomic burden.2 The defining feature of the condition is the presence of endometrial tissue outside the uterine cavity, typically on the peritoneal wall3 or the surface of the ovary (endometriomas).4 Spontaneous endometriosis occurs only in humans and some primates,5,6 where the luminal portion of the endometrium sheds at the end of the menstrual cycle. The primary cause of endometriosis is widely accepted to be the introduction of endometrial tissue into the peritoneal cavity via the fallopian tubes, a process known as retrograde menstruation.7 In captive baboons, the incidence of spontaneous endometriosis parallels the number of menstrual cycles experienced, providing a link between shedding of endometrial tissue and the onset of disease.8
Current treatment strategies for peritoneal endometriosis are restricted to surgical excision of the lesions or suppression of ovarian function to mimic premature menopause. In up to 75% of cases, symptoms recur after surgery, with long-term ovarian suppression sometimes ineffective.9 Although it is acknowledged that there is an unmet clinical need for new nonsurgical treatments for endometriosis, their development depends on access to animal models that can recapitulate features of the human disorder.10 Nonhuman primates offer a physiologically relevant model of endometriosis, although their use is limited by cost and ethical concerns. Injecting female baboons with autologous menstrual endometrium into the peritoneal cavity has been shown to result in the development of endometriotic lesions.11 This model has been used in longitudinal studies, allowing new insights into changes in gene expression in lesions as they become established.12 In studies using the same model, expression of aromatase and the β isoform of estrogen receptor (ERβ) was found to be increased in the lesions, a finding in agreement with studies in women.13,14 Rat and mouse models of endometriosis have also been developed, offering the advantage of lower cost and smaller animal size for testing potential therapies. The availability of mice genetically engineered to express fluorescent marker proteins15,16 or targeted deletion of genes implicated in endometrial disease progression17 has made them an attractive alternative to primate studies. To date, studies using mice have usually involved the injection of human endometrial tissue into immunosuppressed mice18 or the introduction of small fragments of mouse uterine tissue that are sutured to the peritoneum or other sites (reviewed in Grummer19). Results obtained using both models have provided new insights into the origins of the disorder and its association with infertility20 and have served as a platform for testing potential therapies, including those targeting angiogenesis in the lesions21; however, both models have limitations. For example, although human tissue injected either under the skin or into the peritoneal cavity can result in the formation of endometriosis-like lesions, these can grow only if the recipients lack the capacity to mount a tissue rejection response, eg, they are homozygous for the PrkdcSCID mutation.18 Models using mice with an intact immune system allow for studies on the role(s) of inflammatory cytokines and immune cells,22,23 but because mice do not exhibit spontaneous decidualization or menstruation, the uterine tissue introduced into the peritoneal cavity does not recapitulate the tissue microenvironment at the time of retrograde menstruation. Furthermore, in most of the autologous/syngeneic mouse models, uterine tissue is secured in the cavity after local abrasion and/or using sutures,16,22 with both procedures likely to induce an artificial inflammatory response.
In the present study, we set out to develop a mouse model of endometriosis that would combine the best features of the current baboon and mouse models. For this we used our recently validated mouse model of menstruation24 as the source of syngeneic mouse menstrual endometrium and introduced this into the peritoneum of immunocompetent mice. To determine whether this approach could mimic the estrogenic and inflammatory features of human peritoneal lesions, we examined gene and protein expression in mice and patients and complemented these studies by undertaking reciprocal transfers between mice in which cells of the granulocyte/macrophage lineage were labeled with green fluorescent protein (GFP).15
Materials and Methods
Animals
Mature (approximately 8-week-old) female C57BL/6 mice were purchased from Harlan Laboratories (Derby, UK) and were allowed to acclimate for 1 week before surgery. Some experiments were performed using transgenic Cfs1r-EGFP mice (MacGreen) that were originally generated as described in detail elsewhere.15 The founder stocks were a gift from Bernadette Dutia and Prof. David Hume (The Roslin Institute, The University of Edinburgh). Two heterozygous enhanced GFP+ males and two heterozygous enhanced GFP+ females were cross-bred with wild-type (WT) C57BL/6 mice to form a breeding colony and were bred under standard conditions. Offspring were genotyped and classified as either MacGreen or WT. Mice were maintained on standard chow and water available ad libitum and were housed in environmentally controlled facilities illuminated between 7:00 am and 7:00 pm. All the animal procedures were performed in accordance with UK legal requirements and under licensed approval from the UK Home Office (London).
Mouse Model of Endometriosis
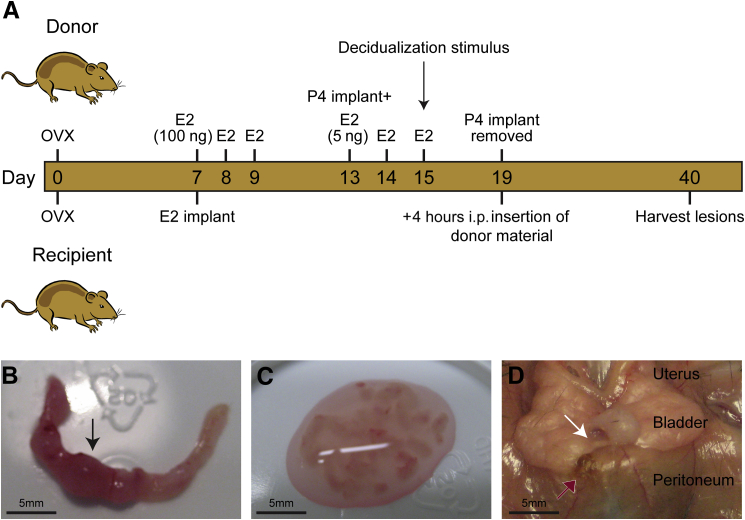
Menstruation was induced in adult donor mice (approximately 8 weeks of age) using a protocol developed in-house24 (Figure 1A). In brief, ovariectomized mice (day 1) were primed with s.c. injections of 100 ng of estradiol-17β (days 7 to 9), treated with progesterone (P4; Sigma-Aldrich, Dorset, UK) delivered via a SILASTIC implant (Dow Corning Corp, Midland, MI) from days 13 to 19, and injected with 5 ng of estradiol-17β in sesame oil on days 13, 14, and 15. Decidualization was induced in one uterine horn using 20 μL of oil (day 15), and endometrial tissue in the process of being shed from the decidualized horn was recovered from mice culled on day 19, 4 hours after P4 withdrawal (removal of pellet) by opening the horn longitudinally in a petri dish and scraping the tissue away from the myometrial layer using a scalpel (Figure 1, B and C). The tissue mass was suspended in 0.2 mL of PBS and passed through a 19-gauge needle before being injected i.p. into anesthetized recipient mice (approximately 8 weeks of age) that had been previously ovariectomized and implanted with an estradiol-17β (Sigma-Aldrich) SILASTIC implant (Figure 1A). Tissue from one decidualized donor horn was used to inoculate each recipient mouse (approximately 40 mg tissue/0.2 mL PBS per mouse). Three weeks after i.p. injection, recipient mice were culled (photographs of the body cavity taken and were taken the lesions carefully dissected from surrounding tissue) and tissues were either fixed in 4% normal buffered formaldehyde for histologic analysis or placed in RNA Save (Geneflow Ltd, Lichfield, UK) for RNA extraction. In total, endometriosis was induced in 18 mice. Of these, 10 mice were used in the MacGreen reciprocal transfers: MacGreen donor and WT recipient (n = 6) and WT donor and MacGreen recipient (n = 4).
Figure 1.
A novel mouse model of endometriosis. A: Schematic showing the timeline of procedures performed on donor and recipient mice. Four hours after P4 withdrawal, mouse menstrual material was collected from donor uteri. The decidualized uterine horn was selected (arrow) and opened longitudinally (B), and the endometrium was removed from the myometrial layer, cut using a scalpel, and resuspended in PBS (C). The tissue was injected into the peritoneum of recipient mice previously primed with an estradiol-17β SILASTIC pellet, and the lesions were allowed to develop for 3 weeks. D: Lesions recovered from recipient mice. The white arrow indicates a neutral lesion associated with the bladder mesentery; red arrow, a peritoneal brown/black lesion.
Human Tissue Collection
Peritoneal endometriotic lesion biopsy samples (n = 19) were obtained from women (aged 18 to 45 years) undergoing laparoscopic surgery for the treatment of endometriosis-associated pain. Endometrial (n = 18) and peritoneal (n = 19) biopsy samples were obtained from women undergoing an explorative laparoscopy for pelvic pain in which no endometriosis was detected (controls). All the samples were collected after informed consent was provided, and ethical approval was obtained from the Lothian Research Ethics Committee. Eighty-two patients were recruited, but samples from those subsequently found to have been taking exogenous hormones up to 3 months before sample collection were not used, and as a result samples from 37 patients were analyzed for the present study. Cycle stage was determined by serum estrogen and progesterone levels and by histologic staining based on Noyes criteria,25 and samples recovered from the proliferative and secretory phases were analyzed. Further details are available in Table 1. Biopsy samples were collected in RNAlater reagent (Applied Biosystems, Warrington, UK) for quantitative RT-PCR and in neutral-buffered formalin for immunohistochemical (IHC) analysis.
Table 1.
Patient Characterization
| Characteristic | Endometriosis | No endometriosis |
|---|---|---|
| Sample size (No.) | 19 | 18 |
| Age [years (means ± SD)] | 33.68 ± 6.903 | 32.38 ± 9.193 |
| Proliferative stage (No.) | 8 | 8 |
| Secretory stage (No.) | 11 | 10 |
Immunodetection
After fixation, human and mouse lesions were stained using H&E; only lesions containing identifiable stromal and epithelial compartments were used for further analysis. Single-color IHC analysis was performed according to standard protocols24,26 with citrate antigen retrieval followed by blocking endogenous peroxidase with 3% H2O2 in methanol. A streptavidin-biotin block (Vector Laboratories, Peterborough, UK) was performed, and any nonspecific staining was reduced using species-specific blocking solution (1:4 serum in Tris-buffered saline + 5% bovine serum albumin). Incubation with the appropriate dilution of primary antibody was performed overnight at 4°C in blocking solution (Table 2). Biotinylated secondary antibodies (dilution 1:500) were diluted in blocking solution and incubated at room temperature for 30 minutes. A streptavidin–horseradish peroxidase conjugate (dilution 1:1000; Sigma-Aldrich) was diluted in Tris-buffered saline and incubated at room temperature for 1 hour, followed by visualization using ImmPACT diaminobenzidine peroxidase substrate (Vector Laboratories). For anti-CD31 IHC analysis, nonspecific epitopes were blocked with Bloxall blocking solution (Dako UK Ltd, Ely, UK), and the secondary antibody was detected using a streptavidin–alkaline phosphatase conjugate and visualized using PermaRed (Dako UK Ltd). Images were captured using AxioVision software version 4.8.2.0 (Carl Zeiss Ltd, Cambridge, UK) and a Provis microscope (Olympus America Inc., Center Valley, PA). Dual immunofluorescence was performed as previously herein with a secondary F (ab) polyclonal antibody to IgG (horseradish peroxidase), and antibody detection was performed using a TSA system kit (PerkinElmer, Waltham, MA) labeled with either Cy3 (red) or fluorescein (green). For detection of the second antigen, sections were microwaved for 2.5 minutes in boiling citrate buffer, and the second primary antibody was applied overnight at 4°C. Secondary antibody was detected as before with an appropriate TSA system, and sections were counterstained with DAPI (dilution 1:500). Slides were mounted in PermaFluor medium (Thermo Fisher Scientific, Waltham, MA). Sections were evaluated using an LSM 710 confocal microscope and ZEN 2009 software (Carl Zeiss Ltd).
Table 2.
List of Antibodies Used in IHC Analysis
| Antibody specificity (supplier) | Raised in | Used on | Dilution |
|---|---|---|---|
| ERβ [AbD Serotec (#MCA1974S)] | Mouse | Human | 1:50 |
| ERβ [Santa Cruz Biotechnology (#SC-8974)] | Rabbit | Mouse | 1:500 |
| ERα (Vector Laboratories) | Mouse | Human and mouse | 1:50 |
| CD31 (Dako) | Rabbit | Mouse | 1:800 |
| Cytokeratin [Sigma-Aldrich (#C2562)] | Mouse | Mouse | 1:2000 |
| Vimentin [Cell Signaling Technology Inc. (#5741)] | Rabbit | Mouse | 1:600 |
| GFP [Invitrogen Molecular Probes (#A11122)] | Rabbit | Mouse | 1:250 |
| Biotinylated anti-mouse (Vector Laboratories) | Goat | 1:500 | |
| Biotinylated anti-rabbit (Vector Laboratories) | Goat | 1:500 | |
| Horseradish peroxidase–conjugated streptavidin (Dako) | NA | 1:200 |
NA, not applicable.
Quantitative RT-PCR
Total RNA was extracted from mouse and human tissues by homogenization in TRI Reagent solution (Life Technologies Ltd, Paisley, UK) using a Qiagen TissueLyser system at 25 Hz for 2.5 minutes, chloroform phase separation was performed, and the lysates were processed using an RNeasy kit (Qiagen Inc., Valencia, CA). Complementary DNA was synthesized using a SuperScript VILO kit (Invitrogen, Paisley, UK), with a starting template concentration of approximately 100 ng of RNA. Real-time PCR reactions were performed using the Roche Universal ProbeLibrary (Roche Applied Science, West Sussex, UK) and express quantitative RT-PCR supermix (Invitrogen). Quantitative RT-PCR was performed using a 7900 fast real-time PCR machine (Life Technologies, Grand Island, NY), with 18S as the endogenous control. Primer sequences are supplied in Table 3.
Table 3.
List of Primer Sequences
| Species | mRNA | Forward | Reverse |
|---|---|---|---|
| Human | ERα | 5′-TTACTGACCAACCTGGCAGA-3′ | 5′-ATCATGGAGGGTCAAATCCA-3′ |
| Human | ERβ | 5′-ATCATGGAGGGTCAAATCCA-3′ | 5′-TGGGCATTCAGCATCTCC-3′ |
| Mouse | ERα | 5′-GCTCCTAACTTGCTCCTGGAC-3′ | 5′-CAGCAACATGTCAAAGATCTCC-3′ |
| Mouse | ERβ | 5′-CCTCAGAAGACCCTCACTGG-3′ | 5′-CACGCACTTCCCCTCATC-3′ |
| Mouse | IL-6 | 5′-GCTACCAAACTGGATATAATCAGGA-3′ | 5′-CCAGGTAGCTATGGTACTCCAGAA-3′ |
| Mouse | CCL2 | 5′-CATCCACGTGTTGGCTCA-3′ | 5′-GATCATCTTGCTGGTGAATGAGT-3′ |
| Mouse | CCL5 | 5′-TGCAGAGGACTCTGAGACAGC-3′ | 5′-GAGTGGTGTCCGAGCCATA-3′ |
| Mouse | TNFα | 5′-CTGTAGCCCACGTCGTAGC-3′ | 5′-TTGAGATCCATGCCGTTG-3′ |
Statistical Analysis
Statistical analysis was performed using a D'Agostino and Pearson omnibus normality test followed by a one-way analysis of variance with a Newman-Keuls post-test or a Student's unpaired t-test. Graphpad Prism 5 (GraphPad Software Inc., San Diego, CA) was used to perform the statistical tests.
Results
Mouse and Human Endometriotic Lesions Share Common Histological Phenotypes
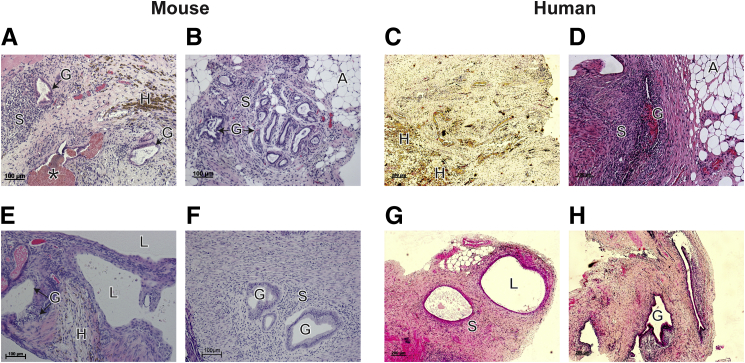
We developed a syngeneic mouse model of endometriosis using mouse menstrual donor tissue to inoculate the peritoneum of recipient mice (Figure 1A). Although the eutopic endometrium of mice exhibits inherent differences to the human counterpart (it does not shed), we confirmed comparable histological features at corresponding cycle stages (Supplemental Figure S1). Specifically, during the estrogen-dominated estrous stage, glands in the mouse uterus were small and round (Supplemental Figure S1A), as were those present in proliferative stage human endometrium (Supplemental Figure S1C). In diestrous, glands were larger and tortuous (Supplemental Figure S1B), comparable with secretory phase human endometrium (Supplemental Figure S1D). After induction of decidualization and 4 hours after P4 withdrawal, mice were culled, the uterus was recovered (Figure 1B), and endometrial tissue was suspended in PBS (Figure 1C). Three weeks after i.p. injection of donor material, lesions were identified on the anterior and posterior walls of the parietal peritoneum (Figure 1D); adipose tissue surrounding the bladder (Figure 1D); visceral peritoneum covering the uterus, gut, and intestines; mesentery associated with the gut and intestines; adipose associated with the kidney; and underneath the kidneys. In agreement with observations made in the patients, lesions in the mice exhibited both macroscopic and microscopic heterogeneity. Phenotypes observed included brown/black lesions (Figure 1D); on histologic examination, these lesions demonstrated evidence of hemosiderin and/or hemorrhage (Figure 2A). Lesions that were neutral in color (Figure 1D) were surrounded by adipose tissue (Figure 2B), and some lesions also had fluid-filled cysts (Figure 2E). The histologic features of these mouse lesions were similar to those recovered from patients: fibrotic lesions containing evidence of hemosiderin (Figure 2C), lesions with associated adipose tissue (Figure 2D), and those with a cystic appearance (Figure 2G). Lesions in mice (Figure 2, A, B, E, and F) and humans (Figure 2, D and H) had a well-developed glandular epithelium. A high proportion of mice (83.3%) developed lesions (Table 4); a mean of 2.06 lesions were recovered from each mouse, and 81% of these contained both glandular and stromal compartments (Table 4). Not all the injected material adhered to the peritoneum, with some floating material detected at harvest, consistent with reports from other researchers.27
Figure 2.
Mouse lesions mimic human histological phenotypes. H&E stain of mouse lesions recovered from the anterior parietal peritoneum (A), the mesentery associated with the intestines (B), the parietal peritoneum–associated fluid-filled cyst (E), and the posterior parietal peritoneum (F). C, D, G, and H: Human lesions recovered from the peritoneal lining. A, adipose; G, glands; H, hemosiderin; L, cyst lumen; S, stroma. Asterisk in A indicates evidence of hemorrhage.
Table 4.
Information about Lesions Recovered from the Mouse Model and Patients
| Variable | Mouse | Human |
|---|---|---|
| Sample size (No.) | 18 | 20 |
| Recovery rate (%) | 83.3 | 100 |
| Number of lesions (mean ± SD) | 2.06 ± 0.32 | ≥1 |
| Lesions with glands and stroma (%) | 81 | 68 |
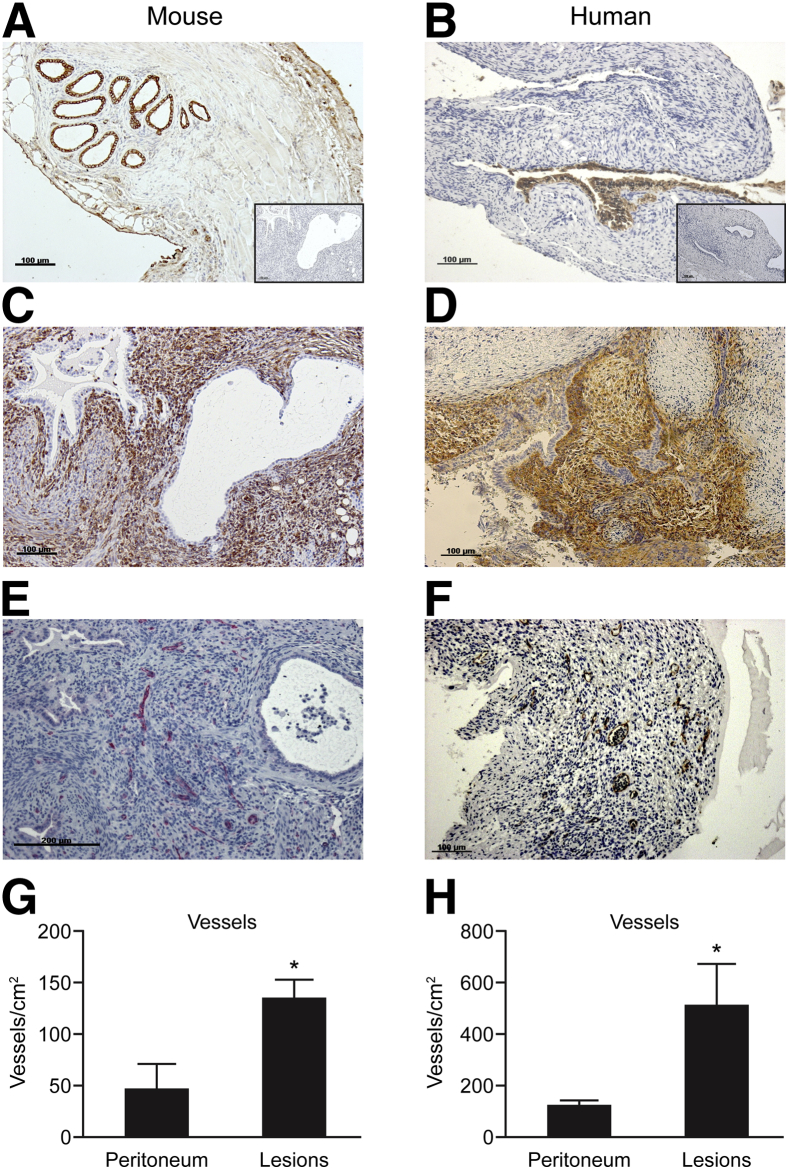
Immunostaining was used to identify cells with an epithelial phenotype (anti-cytokeratin) (Figure 3B), stromal fibroblasts (anti-CD10) (Figure 3D), and endothelial cells in the vasculature (anti-CD31) of human lesions (Figure 3F), with identical cell types confirmed in the lesions from mice (Figure 3, A, C, and E). The recovered lesions were significantly more vascularized compared with the peritoneum in both mice (means ± SEM: 133.1 ± 19.62 and 44.55 ± 26.54, respectively; P < 0.05) (Figure 3G) and humans (511.6 ± 159.1 and 116 ± 25.7, respectively; P < 0.05) (Figure 3H).
Figure 3.
Mouse and human lesions possess glandular, stromal, and vascular components. In lesions recovered from mice (A, C, and E) or women (B, D, and F), IHC analysis identified cytokeratin-positive epithelial cells (brown positive) (A and B) and stromal fibroblasts (C, vimentin; D, CD10, brown positive in both cases). Endothelial cells were immunopositive for CD31 in mice (E, PermaRed) and humans (F, diaminobenzidine). Insets in A and B show negative controls with omission of primary antibody. G and H: When blood vessels were quantified in peritoneum and lesions (n = 5 mice; n = 5 humans for each sample type), a significantly higher density was detected in the lesions. Data are expressed as means ± SD. ∗P < 0.05 by Student's t-test.
Changes in ER Subtype Expression in Human Peritoneal Lesions Is Mirrored by Expression in the Endometriotic Lesions Recovered from Mice
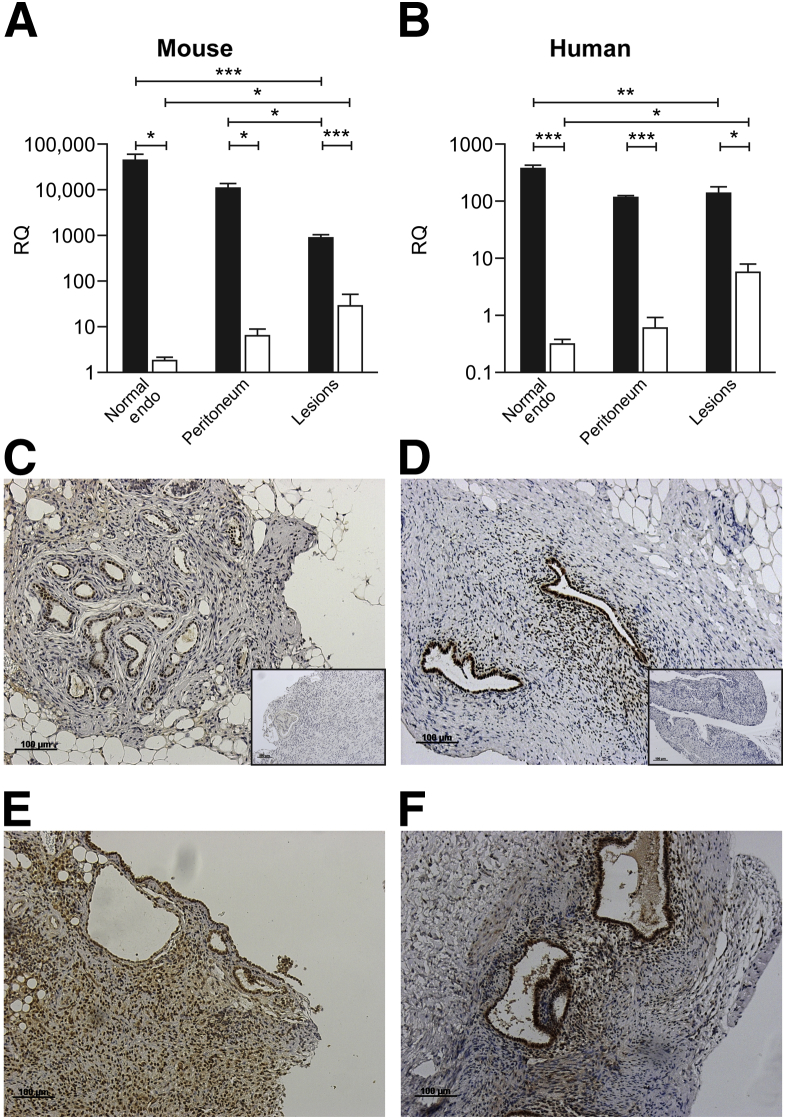
We previously reported patterns of expression of ERα and ERβ during the normal cycle in human28 and mouse29 endometrium. Concentrations of ERα mRNAs were significantly lower in lesions compared with the endometrium in mice (Figure 4A) and humans (Figure 4B) (P < 0.001 and P < 0.01, respectively). In contrast, concentrations of ERβ mRNA in tissue homogenates of lesions were significantly higher than in the endometrium in mice (Figure 4A) and humans (Figure 4B) (P < 0.05 for both). Additional data for patient samples comparing the proliferative and secretory phases are given in Supplemental Figure S2; the only significant difference between cycle stages was that the expression of ERα in lesions in the secretory phase was higher than in the proliferative phase.
Figure 4.
ER expression is comparable in mouse and human lesions. A: Quantitative RT-PCR analysis of ERα (black bars) and ERβ (white bars) in the mouse uterus (n = 6), peritoneum (n = 6), and endometriotic (Endo) lesions (n = 6). B: Quantitative RT-PCR analysis of ERα and ERβ in human endometrium (Endo) (n = 18), peritoneum (n = 19), and peritoneal lesions (n = 19). Quantitative RT-PCR data were analyzed using a one-way analysis of variance and the Newman-Keuls postcomparison test. C: Diaminobenzidine (DAB) IHC analysis using an anti-ERα antibody on mouse lesions revealed moderate immunoreactivity in glandular epithelium. D: DAB IHC analysis using an anti-ERα antibody on human lesions also revealed moderate immunoreactivity in epithelium and stromal areas. Insets in C and D show negative controls with omission of primary antibody. E: DAB IHC analysis using an anti-ERβ antibody on mouse lesions reveals prominent ERβ immunoreactivity in glandular epithelium and stromal regions. F: DAB IHC analysis using an anti-ERβ antibody on human lesions also reveals strong immunoreactivity in glandular and stromal regions. IHC analysis of human lesions was performed on samples dissected during the proliferative (estrogen-dominated) stage of the menstrual cycle. Data are expressed as means ± SD. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. RQ, relative quantification.
ERα protein was localized to the glandular epithelium of mouse lesions (Figure 4C), but rarely in the stromal compartment. In contrast, ERβ IHC analysis revealed intense immunoreactivity in lesions in multiple cell types, including glandular epithelial, stromal fibroblast, and endothelial cells (Figure 4E). Analysis of human lesions detected staining for ERα in stromal and epithelial cells (Figure 4D), with intense ERβ immunopositive staining in multiple cell types (Figure 4F).
Peritoneal Lesions Formed in Mice Have an Altered Cytokine Microenvironment
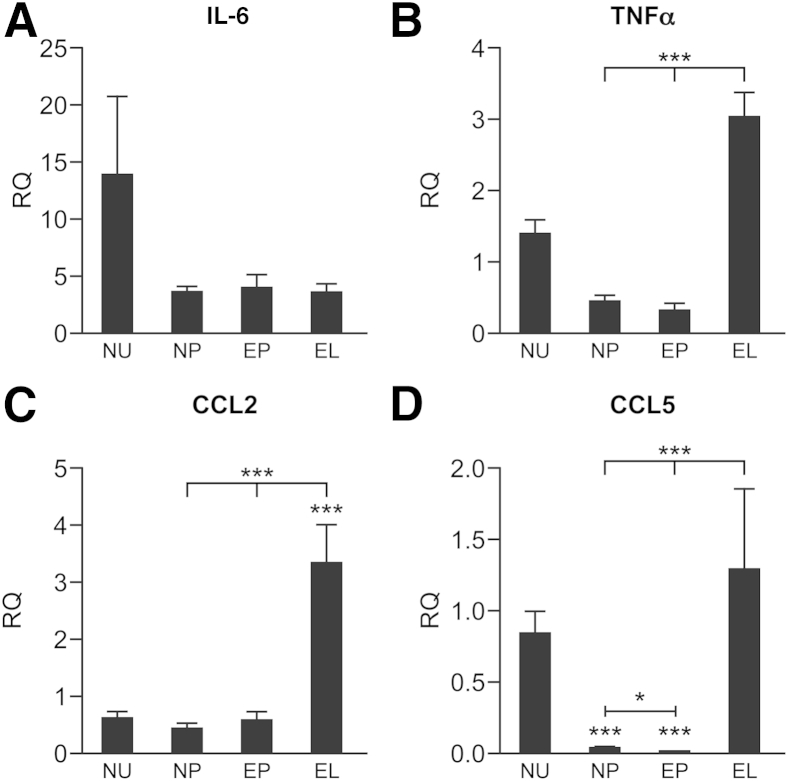
Concentrations of mRNAs encoding the proinflammatory cytokine tumor necrosis factor α (TNFα) and the monocyte chemoattractant proteins chemokine ligands 2 and 5 (CCL2 and CCL5) were significantly higher in endometriotic lesions compared with healthy peritoneum and the peritoneum of endometriotic mice (P < 0.001) (Figure 5, B–D). Concentrations of IL-6 (Figure 5A) and IL-4 (not shown) were not significantly different between samples.
Figure 5.
Analysis of expression of cytokines in mouse tissues revealed altered expression in lesions. Quantitative RT-PCR analysis of inflammatory cytokines in the uterus (NU) and peritoneum (NP) of healthy cycling mice and the peritoneum (EP) and lesions (EL) of mice with endometriosis: IL-6 (A), TNFα (B), CCL2/macrophage chemotactic protein 1 (C), and CCL5/RANTES (D). Quantitative RT-PCR data were analyzed using a one-way analysis of variance and a Newman-Keuls post-comparison test. Data are expressed as means ± SD. ∗P < 0.05, ∗∗∗P < 0.001.
Macrophages Present in the Shed Endometrium Contribute to Endometriotic Lesions
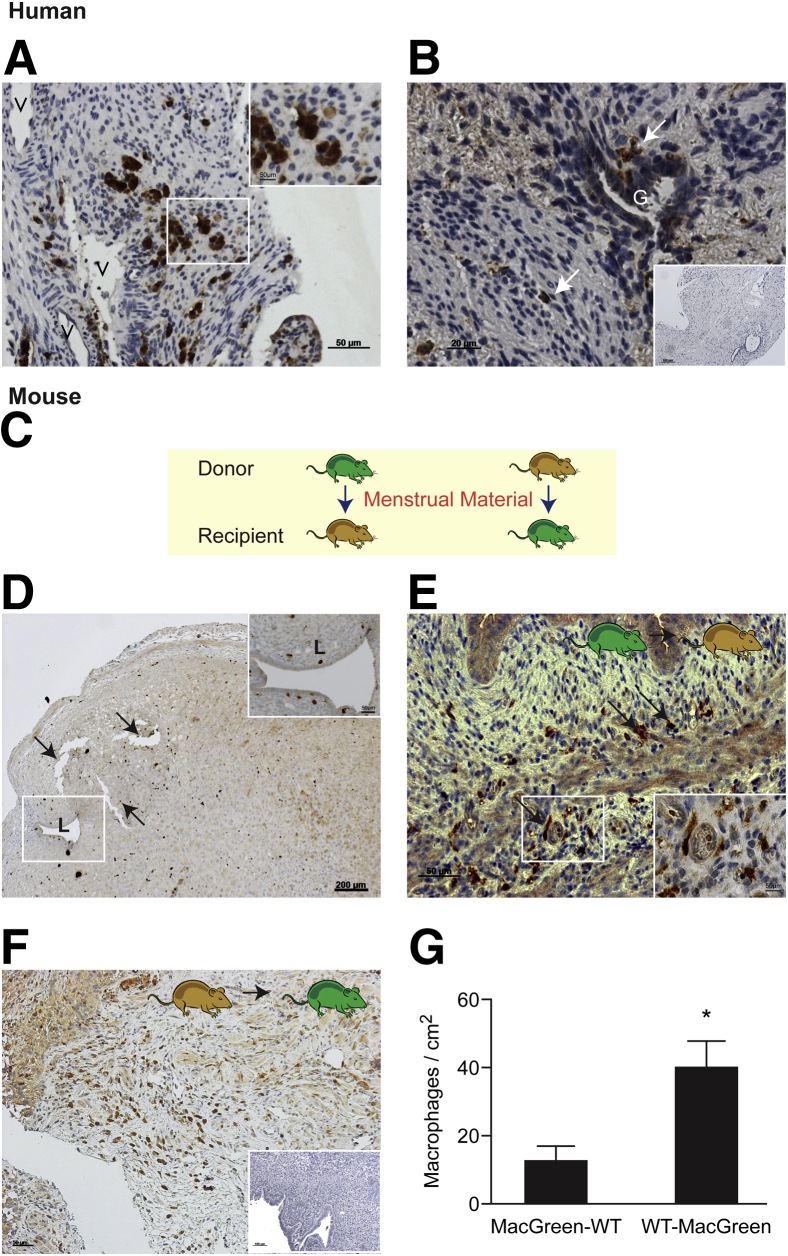
We detected CD68+ macrophages in the stroma of human peritoneal lesions in close proximity to blood vessels (Figure 6A) and the glandular epithelium (Figure 6B). In MacGreen mice, GFP+ cells were readily identified in the uterus after induction of menses-like tissue shedding (Figure 6D and Supplemental Figure S3). The GFP+ cells were present in the endometrial/decidual tissue 4 hours after P4 withdrawal, when tissue was recovered for transfer (Figure 6D). The influx of GFP+ cells was also confirmed in samples recovered at 12 hours, when blood could be detected in the vagina of 100% of mice24 (Supplemental Figure S3). After reciprocal transfers between MacGreen and WT littermates (Figure 6C), GFP+ cells were detected in endometriotic lesions (Figure 6, E and F). Fluorescent IHC analysis was used to show that GFP+ cells present in lesions were all GR-1– (GR-1 antibody directed against Ly-6C+ and Ly-6G+ granulocytes) (Supplemental Figure S4, B–D), although some GR-1/GFP+ cells were present in the mouse uterus 4 hours after P4 withdrawal (Supplemental Figure S4A). Also, in lesions containing GFP+ cells, staining of adjacent sections confirmed that cells were F4/80+ (macrophage marker) (Supplemental Figure S4, E–H), thus validating that GFP+ cells represented a population of tissue-resident macrophages. GFP+ macrophages were present in lesions harvested from WT recipients after peritoneal transfer of menstrual tissue from MacGreen mice (Figure 6E), suggesting that macrophages present in shed endometrium survive and contribute to the inflammatory microenvironment of the lesions. The numbers of GFP+ macrophages were higher in lesions isolated from WT (donor) to MacGreen (recipient) transfers (Figure 6F); this was significant when macrophages were counted and normalized to lesion area (means ± SEM: 12.08 ± 4.69 and 39.64 ± 8.034; P = 0.013) (Figure 5G). We found that GFP+ macrophages were present close to the glandular compartments of lesions (Supplemental Figure 5A) and around the edges of lesions, close to blood vessels (Supplemental Figure S5B). In some lesions, GFP+ macrophages also seemed to be migrating toward the lesion from adjacent blood vessels (Supplemental Figure S5C) and were present in fibrotic areas of lesions (Supplemental Figure S5D).
Figure 6.
Macrophages from the shed endometrium contribute to endometriotic lesions. A and B: In human peritoneal lesions, immunostaining for CD68 identified macrophages in the stroma, some of which were in close proximity to glandular structures in human lesions. The inset in A shows an enlarged image of stromal macrophages; in B, the inset shows negative control with omission of primary antibody. White arrows indicate macrophages (B). C: Schematic indicating reciprocal transfers of menstrual material between MacGreen mice (green) and WT mice (brown). D: Anti-GFP IHC analysis on MacGreen donor uteri 4 hours after P4 withdrawal revealed macrophages present in the decidua and associated with the uterine lumen (inset). Black arrows indicate an area of the decidua beginning to shed from the basal layer of the endometrium. E: GFP+ macrophages were detected using an anti-GFP antibody in lesions isolated from MacGreen (donor) to WT (recipient) mice, demonstrating the presence of macrophages from the shed endometrium in lesions. Black arrows indicate macrophages. F: GFP+ macrophages were also detected in lesions isolated from WT (donor) to MacGreen (recipient) mice, demonstrating infiltration of host macrophages into lesions. The inset shows negative control with omission of primary antibody. G: Graph showing the respective contribution of donor/recipient to the macrophage composition of mouse endometriotic lesions. Statistical analysis was performed using a Student's t-test. Data are expressed as means ± SD. n = 6 (MacGreen-WT); n = 3 (WT-MacGreen). ∗P < 0.05. G, glands; V, vessels. Scale bars: 50 μmol/L (A, A, inset, and E); 20 μmol/L (B); 100 μmol/L (B, inset); 200 μmol/L (D).
Discussion
In this study, we describe a new mouse model of endometriosis that uses syngeneic mouse menstrual donor tissue introduced into the peritoneum of immunocompetent recipient mice. This model mimics the primate baboon model, in which lesions are artificially induced by the injection of menstrual endometrium into the peritoneum,11 but is distinct from previously described mouse models, in which uterine tissue fragments are sutured onto the peritoneum16 or intestinal mesentery.30 To determine whether the use of menstrual material could recapitulate key features of the human disorder in mice, we examined lesions for evidence of endometrial histology, ER expression, inflammatory signaling molecules, and tissue-resident macrophages.
In the present study, lesions recovered from a variety of sites in the peritoneum of the mice shared histological similarities with human lesions, including the presence of hemosiderin, cytokeratin-positive epithelial cells, and close association with adipose cells. Lesions recovered from other mouse models frequently possess a cystic phenotype,31 and although we did observe this histological feature, most lesions had evidence of well-organized stromal and glandular structures. In the mice, total concentrations of ERβ mRNAs were significantly higher in lesions compared with the endometrium and peritoneum, and intense immunostaining was detected in all cell types. In contrast, expression of ERα in the mouse lesions was lower than in the uterus. The findings in this new mouse model seem to phenocopy results obtained in lesions recovered from the patients and are in agreement with reports that alterations in the DNA methylation status of the ESR2 gene promoter is a mechanism responsible for the increase in ERβ mRNAs in endometriotic cells.32 In the healthy endometrium, expression of the P4 receptor is coupled with that of ERα,33 and altered ratios of ERα:ERβ in endometriosis lesions have been implicated in the suppression of P4 receptor expression.34 Because we detected altered expression of ERα and ERβ in lesions in the present mice, we believe it may provide a model for further studies on the mechanisms responsible for the PR-resistant phenotype reported in patients with endometriosis.34
Menstruation is an inflammatory process, the culmination of a regulatory cascade of changes precipitated by the acute withdrawal of P4 and characterized by an increase in a variety of tissue-resident immune cells, with 6% to 10% of cells in the menstrual endometrium reported to be macrophages.6,35 A complex interaction between resident immune cells and uterine stromal cells modulates the biosynthesis and release of proinflammatory cytokines, chemokines, and prostaglandins, resulting in local vasoconstriction, release of enzymes that degrade the extracellular matrix, and shedding of the upper layer of the endometrium.36,37 Thus, menstrual material, including that arriving in the peritoneal cavity by retrograde flow, will contain tissue fragments, including their resident immune cells and a range of inflammatory mediators. The importance of the inflammatory microenvironment of the shed endometrium has been highlighted by reports of an altered inflammatory phenotype in both the peritoneal milieu and eutopic endometrium of women with endometriosis.38 Previous studies performed in the baboon reported a higher number of lesions in animals injected i.p. with menstrual endometrium compared with luteal endometrium, indicating that menstrual material contains factors that enhance its ability to attach to the peritoneal lining.39
Herein, we confirmed that induction of a menses-like event in mice was associated with the presence of macrophages in the uterus and that lesions formed in mice contained tissue-resident macrophages and a proinflammatory microenvironment. These results would be consistent with evidence that macrophages are key players in the pathogenesis of endometriosis. For example, Chuang et al40 suggested that prostaglandin E2–mediated down-regulation of the scavenger receptor CD36 may inhibit the phagocytic ability of peritoneal macrophages, encouraging the establishment of lesions. The macrophage inhibitor factor IL-13 is decreased in women with endometriosis,41 suggesting a mechanism by which endometriosis-associated macrophages are unrestrained. Transforming growth factor β is overexpressed in patients with endometriosis; in a mouse model based on xenotransplantation of human eutopic endometrial tissue, the weight of endometriosis-like lesions was reduced 11-fold when tissue was engrafted into TGFβ null mice, and numbers of macrophages detected in the lesions were significantly reduced, supporting suggestions that macrophages are involved in lesion survival.18
By performing reciprocal transfers between MacGreen and WT (unlabeled) mice, we could determine the source of the macrophages in endometriosis lesions. When lesions were recovered from MacGreen recipients, we readily detected GFP+ macrophages in the lesions, consistent with reports that macrophages in endometriotic lesions are recruited from the circulation42 or from populations present in peritoneal fluid.43 In contrast, the contribution of macrophages present in shed endometrium to the etiology of endometriotic lesions has not been studied in previous mouse models, and, therefore, we were excited to detect GFP+ macrophages in lesions 3 weeks after the transfer of tissue from MacGreen mice into the peritoneum of WT recipients. These data suggest that the macrophages present in shed endometrium persist in the peritoneal microenvironment and contribute to the endometriotic lesions. Macrophages and neutrophils are thought to promote angiogenesis in the early stages of lesion formation, and this has been reported using an autologous mouse model using estrous uterine tissue sutured to the peritoneum.44 We speculate that in the present menses endometriosis model, formation of lesions is promoted by the presence of immune cells, including macrophages, without the need for induced inflammation caused by suturing.
A variety of studies have analyzed the polarization of macrophages present in lesions45; it has been demonstrated that lesion-resident macrophages are alternatively activated (M2 phenotype), exhibiting a repair/resolution phenotype thought to enhance ectopic cell survival. Conversely, the injection of M1-like (proinflammatory) macrophages may suppress lesion development.46 The endometrial macrophage is thought to possess a unique phenotype that alters during the menstrual cycle.47 This study suggests that the population of macrophages in the lesions may be heterogeneous and that macrophages in shed endometrium might survive for long periods and become reprogrammed while resident in the tissue. Further studies are required before we can assess the relative contributions of peritoneum, circulation, and menses material to the complex immune cell complement of the lesions.
To investigate how the presence of immune cells, such as macrophages, affect the lesion microenvironment, we analyzed the expression of macrophage chemotactic factors (CCL2 and CCL5) and macrophage-derived cytokines (IL-6 and TNFα). We found that CCL2 (also known as macrophage chemotactic protein 1) and CCL5 (also known as RANTES) were up-regulated in the lesions of mice with endometriosis, consistent with reports that lesions recruit macrophages. We also found that in lesions, TNFα was overexpressed compared with the peritoneum, indicating that the inflammatory microenvironment of lesions is altered by the presence of macrophages. These results would be consistent with reports that peritoneal macrophages isolated from mice with endometriosis secrete TNFα and IL-6, which up-regulates vascular endothelial growth factor, leading to increased angiogenesis.44
In summary, we describe a new mouse model of endometriosis that offers the chance to fully explore the pathobiology of this complex disorder. The model closely mirrors the human condition as an estrogen-dependent inflammatory disorder, exhibiting similar hallmarks, and has revealed that the immune complement of the menstrual endometrium contributes to the pathologic appearance of peritoneal lesions. We hope that the use of this model will inform future studies investigating the role of immune cells and menstrual tissue on the development of endometriosis, advance our understanding of disease mechanisms of endometriosis, and allow the identification and study of novel targets for endometriosis therapy.
Acknowledgments
We thank Ronnie Grant for preparation of the figures, research nurses Ann Doust and Helen Dewart, and the women who gave informed consent for the use of their material in this study.
Footnotes
Supported by MRC Programme grant G1100356/1 (P.T.K.S.), Wellbeing of Women grant R42533 (A.W.H.), and a Society for Endocrinology early career grant (E.G).
Disclosures: None declared.
Supplemental Data
H&E stain of mouse eutopic endometrium (estrous stage) (A) and diestrous stage (B). H&E stain of the proliferative (C) and secretory (D) stages of human eutopic endometrium.
Quantitative RT-PCR analysis of ERα (A) and ERβ (B) in human endometrium (Endo) [n = 5 proliferative phase (P) and n = 5 secretory phase (S)], peritoneum (n = 10 proliferative and n = 10 secretory), and peritoneal lesions (n = 5 proliferative and n = 5 secretory). Quantitative RT-PCR data were analyzed using a one-way analysis of variance and the Newman-Keuls post-comparison test. Data are expressed as means ± SD. ∗∗P < 0.01. RQ, relative quantification.
Mouse uterus recovered from a MacGreen mouse 12 hours after P4 withdrawal after hormonal stimulation and oil-induced decidualization. Note the influx of macrophages (GFP+ tissue-resident cells) at the margins of the decidual mass (A, with higher magnifications in C and D). Macrophages were also present adjacent to the region of uterus in which epithelial integrity was restored (A and higher magnification in B). Scale bars: 200 μmol/L (A); 100 μmol/L (B and C); 20 μmol/L (D).
IHC analysis for GFP in mouse lesions taken from MacGreen to WT transfers (A and B) and from WT to MacGreen transfers (C and D). E: Negative control (omission of primary antibody). Arrows indicate macrophages. G, glands; L, lumen.
Dual immunofluorescence for GR-1 (red) and GFP (green) on sections of donor mouse endometrium 4 hours after P4 withdrawal (A) and lesions (B–D). Insets in A–C are enlarged areas of interest. The inset in D is a negative control (primary antibody omitted). Red arrows indicate GR-1+ cells; green arrows, GFP+ cells; and white arrows, colabeling. Single immunofluorescence on serial sections of lesions for GFP (E and G; green) and F4/80 (F and H; red). Scale bars: 50 μmol/L.
References
- 1.Giudice L.C., Kao L.C. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 2.Simoens S., Dunselman G., Dirksen C., Hummelshoj L., Bokor A., Brandes I., Brodszky V., Canis M., Colombo G.L., DeLeire T., Falcone T., Graham B., Halis G., Horne A., Kanj O., Kjer J.J., Kristensen J., Lebovic D., Mueller M., Vigano P., Wullschleger M., D'Hooghe T. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27:1292–1299. doi: 10.1093/humrep/des073. [DOI] [PubMed] [Google Scholar]
- 3.Young V.J., Brown J.K., Saunders P.T., Horne A.W. The role of the peritoneum in the pathogenesis of endometriosis. Hum Reprod Update. 2013;19:558–569. doi: 10.1093/humupd/dmt024. [DOI] [PubMed] [Google Scholar]
- 4.Trukhacheva E., Lin Z., Reierstad S., Cheng Y.H., Milad M., Bulun S.E. Estrogen receptor (ER) β regulates ERα expression in stromal cells derived from ovarian endometriosis. J Clin Endocrinol Metab. 2009;94:615–622. doi: 10.1210/jc.2008-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emera D., Romero R., Wagner G. The evolution of menstruation: a new model for genetic assimilation: explaining molecular origins of maternal responses to fetal invasiveness. Bioessays. 2012;34:26–35. doi: 10.1002/bies.201100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabbour H.N., Kelly R.W., Fraser H.M., Critchley H.O. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- 7.Sampson J.A. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3:93–110.43. [PMC free article] [PubMed] [Google Scholar]
- 8.D'Hooghe T.M., Bambra C.S., De Jonge I., Lauweryns J.M., Koninckx P.R. The prevalence of spontaneous endometriosis in the baboon (Papio anubis, Papio cynocephalus) increases with the duration of captivity. Acta Obstet Gynecol Scand. 1996;75:98–101. doi: 10.3109/00016349609033298. [DOI] [PubMed] [Google Scholar]
- 9.Giudice L.C. Clinical practice: endometriosis. N Engl J Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers P.A., D'Hooghe T.M., Fazleabas A., Giudice L.C., Montgomery G.W., Petraglia F., Taylor R.N. Defining future directions for endometriosis research: workshop report from the 2011 World Congress of Endometriosis in Montpellier, France. Reprod Sci. 2013;20:483–499. doi: 10.1177/1933719113477495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Hooghe T.M., Kyama C.M., Chai D., Fassbender A., Vodolazkaia A., Bokor A., Mwenda J.M. Nonhuman primate models for translational research in endometriosis. Reprod Sci. 2009;16:152–161. doi: 10.1177/1933719108322430. [DOI] [PubMed] [Google Scholar]
- 12.Afshar Y., Hastings J., Roqueiro D., Jeong J.W., Giudice L.C., Fazleabas A.T. Changes in eutopic endometrial gene expression during the progression of experimental endometriosis in the baboon, Papio anubis. Biol Reprod. 2013;88:44. doi: 10.1095/biolreprod.112.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bukulmez O., Hardy D.B., Carr B.R., Word R.A., Mendelson C.R. Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology. 2008;149:1190–1204. doi: 10.1210/en.2007-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fazleabas A.T., Brudney A., Chai D., Langoi D., Bulun S.E. Steroid receptor and aromatase expression in baboon endometriotic lesions. Fertil Steril. 2003;80(Suppl 2):820–827. doi: 10.1016/s0015-0282(03)00982-8. [DOI] [PubMed] [Google Scholar]
- 15.Sasmono R.T., Oceandy D., Pollard J.W., Tong W., Pavli P., Wainwright B.J., Ostrowski M.C., Himes S.R., Hume D.A. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101:1155–1163. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- 16.Wilkosz S., Pullen N., de-Giorgio-Miller A., Ireland G., Herrick S. Cellular exchange in an endometriosis-adhesion model using GFP transgenic mice. Gynecol Obstet Invest. 2011;72:90–97. doi: 10.1159/000325826. [DOI] [PubMed] [Google Scholar]
- 17.Fang Z., Yang S., Lydon J.P., DeMayo F., Tamura M., Gurates B., Bulun S.E. Intact progesterone receptors are essential to counteract the proliferative effect of estradiol in a genetically engineered mouse model of endometriosis. Fertil Steril. 2004;82:673–678. doi: 10.1016/j.fertnstert.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 18.Hull M.L., Johan M.Z., Hodge W.L., Robertson S.A., Ingman W.V. Host-derived TGFB1 deficiency suppresses lesion development in a mouse model of endometriosis. Am J Pathol. 2012;180:880–887. doi: 10.1016/j.ajpath.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Grummer R. Animal models in endometriosis research. Hum Reprod Update. 2006;12:641–649. doi: 10.1093/humupd/dml026. [DOI] [PubMed] [Google Scholar]
- 20.Grummer R. Translational animal models to study endometriosis-associated infertility. Semin Reprod Med. 2013;31:125–132. doi: 10.1055/s-0032-1333477. [DOI] [PubMed] [Google Scholar]
- 21.Hull M.L., Charnock-Jones D.S., Chan C.L., Bruner-Tran K.L., Osteen K.G., Tom B.D., Fan T.P., Smith S.K. Antiangiogenic agents are effective inhibitors of endometriosis. J Clin Endocrinol Metab. 2003;88:2889–2899. doi: 10.1210/jc.2002-021912. [DOI] [PubMed] [Google Scholar]
- 22.Lin L., Isacson O. Axonal growth regulation of fetal and embryonic stem cell-derived dopaminergic neurons by Netrin-1 and Slits. Stem Cells. 2006;24:2504–2513. doi: 10.1634/stemcells.2006-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somigliana E., Vigano P., Zingrillo B., Ranieri S., Filardo P., Candiani M., Vignali M. Induction of endometriosis in the mouse inhibits spleen leukocyte function. Acta Obstet Gynecol Scand. 2001;80:200–205. doi: 10.1034/j.1600-0412.2001.080003200.x. [DOI] [PubMed] [Google Scholar]
- 24.Cousins F.L., Murray A., Esnal A., Gibson D.A., Critchley H.O., Saunders P.T. Evidence from a mouse model that epithelial cell migration and mesenchymal-epithelial transition contribute to rapid restoration of uterine tissue integrity during menstruation. PLoS One. 2014;9:e86378. doi: 10.1371/journal.pone.0086378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noyes R.W., Hertig A.T., Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 26.Collins F., MacPherson S., Brown P., Bombail V., Williams A.R., Anderson R.A., Jabbour H.N., Saunders P.T. Expression of oestrogen receptors, ERα, ERβ, and ERβ variants, in endometrial cancers and evidence that prostaglandin F may play a role in regulating expression of ERα. BMC Cancer. 2009;9:330. doi: 10.1186/1471-2407-9-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burns K.A., Rodriguez K.F., Hewitt S.C., Janardhan K.S., Young S.L., Korach K.S. Role of estrogen receptor signaling required for endometriosis-like lesion establishment in a mouse model. Endocrinology. 2012;153:3960–3971. doi: 10.1210/en.2012-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Critchley H.O., Brenner R.M., Henderson T.A., Williams K., Nayak N.R., Slayden O.D., Millar M.R., Saunders P.T. Estrogen receptor β, but not estrogen receptor α, is present in the vascular endothelium of the human and nonhuman primate endometrium. J Clin Endocrinol Metab. 2001;86:1370–1378. doi: 10.1210/jcem.86.3.7317. [DOI] [PubMed] [Google Scholar]
- 29.McNeilly J.R., Saunders P.T., Taggart M., Cranfield M., Cooke H.J., McNeilly A.S. Loss of oocytes in Dazl knockout mice results in maintained ovarian steroidogenic function but altered gonadotropin secretion in adult animals. Endocrinology. 2000;141:4284–4294. doi: 10.1210/endo.141.11.7764. [DOI] [PubMed] [Google Scholar]
- 30.Pelch K.E., Sharpe-Timms K.L., Nagel S.C. Mouse model of surgically-induced endometriosis by auto-transplantation of uterine tissue. J Vis Exp. 2012:e3396. doi: 10.3791/3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laschke M.W., Giebels C., Nickels R.M., Scheuer C., Menger M.D. Endothelial progenitor cells contribute to the vascularization of endometriotic lesions. Am J Pathol. 2011;178:442–450. doi: 10.1016/j.ajpath.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue Q., Lin Z., Cheng Y.H., Huang C.C., Marsh E., Yin P., Milad M.P., Confino E., Reierstad S., Innes J., Bulun S.E. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod. 2007;77:681–687. doi: 10.1095/biolreprod.107.061804. [DOI] [PubMed] [Google Scholar]
- 33.Critchley H.O., Saunders P.T. Hormone receptor dynamics in a receptive human endometrium. Reprod Sci. 2009;16:191–199. doi: 10.1177/1933719108331121. [DOI] [PubMed] [Google Scholar]
- 34.Bulun S.E., Monsavais D., Pavone M.E., Dyson M., Xue Q., Attar E., Tokunaga H., Su E.J. Role of estrogen receptor-β in endometriosis. Semin Reprod Med. 2012;30:39–45. doi: 10.1055/s-0031-1299596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salamonsen L.A., Zhang J., Brasted M. Leukocyte networks and human endometrial remodelling. J Reprod Immunol. 2002;57:95–108. doi: 10.1016/s0165-0378(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 36.Evans J., Salamonsen L.A. Inflammation, leukocytes and menstruation. Rev Endocr Metab Disord. 2012;13:277–288. doi: 10.1007/s11154-012-9223-7. [DOI] [PubMed] [Google Scholar]
- 37.Maybin J.A., Critchley H.O., Jabbour H.N. Inflammatory pathways in endometrial disorders. Mol Cell Endocrinol. 2011;335:42–51. doi: 10.1016/j.mce.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Burney R.O., Giudice L.C. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511–519. doi: 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D'Hooghe T.M., Bambra C.S., Raeymaekers B.M., De Jonge I., Lauweryns J.M., Koninckx P.R. Intrapelvic injection of menstrual endometrium causes endometriosis in baboons (Papio cynocephalus and Papio anubis) Am J Obstet Gynecol. 1995;173:125–134. doi: 10.1016/0002-9378(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 40.Chuang P.C., Lin Y.J., Wu M.H., Wing L.Y., Shoji Y., Tsai S.J. Inhibition of CD36-dependent phagocytosis by prostaglandin E2 contributes to the development of endometriosis. Am J Pathol. 2010;176:850–860. doi: 10.2353/ajpath.2010.090551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLaren J., Dealtry G., Prentice A., Charnock-Jones D.S., Smith S.K. Decreased levels of the potent regulator of monocyte/macrophage activation, interleukin-13, in the peritoneal fluid of patients with endometriosis. Hum Reprod. 1997;12:1307–1310. doi: 10.1093/humrep/12.6.1307. [DOI] [PubMed] [Google Scholar]
- 42.Halme J., Becker S., Wing R. Accentuated cyclic activation of peritoneal macrophages in patients with endometriosis. Am J Obstet Gynecol. 1984;148:85–90. doi: 10.1016/s0002-9378(84)80037-x. [DOI] [PubMed] [Google Scholar]
- 43.McLaren J., Prentice A., Charnock-Jones D.S., Millican S.A., Muller K.H., Sharkey A.M., Smith S.K. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Invest. 1996;98:482–489. doi: 10.1172/JCI118815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin Y.J., Lai M.D., Lei H.Y., Wing L.Y. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology. 2006;147:1278–1286. doi: 10.1210/en.2005-0790. [DOI] [PubMed] [Google Scholar]
- 45.Capobianco A., Rovere-Querini P. Endometriosis, a disease of the macrophage. Front Immunol. 2013;4:9. doi: 10.3389/fimmu.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bacci M., Capobianco A., Monno A., Cottone L., Di Puppo F., Camisa B., Mariani M., Brignole C., Ponzoni M., Ferrari S., Panina-Bordignon P., Manfredi A.A., Rovere-Querini P. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol. 2009;175:547–556. doi: 10.2353/ajpath.2009.081011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thiruchelvam U., Dransfield I., Saunders P.T., Critchley H.O. The importance of the macrophage within the human endometrium. J Leukoc Biol. 2013;93:217–225. doi: 10.1189/jlb.0712327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
H&E stain of mouse eutopic endometrium (estrous stage) (A) and diestrous stage (B). H&E stain of the proliferative (C) and secretory (D) stages of human eutopic endometrium.
Quantitative RT-PCR analysis of ERα (A) and ERβ (B) in human endometrium (Endo) [n = 5 proliferative phase (P) and n = 5 secretory phase (S)], peritoneum (n = 10 proliferative and n = 10 secretory), and peritoneal lesions (n = 5 proliferative and n = 5 secretory). Quantitative RT-PCR data were analyzed using a one-way analysis of variance and the Newman-Keuls post-comparison test. Data are expressed as means ± SD. ∗∗P < 0.01. RQ, relative quantification.
Mouse uterus recovered from a MacGreen mouse 12 hours after P4 withdrawal after hormonal stimulation and oil-induced decidualization. Note the influx of macrophages (GFP+ tissue-resident cells) at the margins of the decidual mass (A, with higher magnifications in C and D). Macrophages were also present adjacent to the region of uterus in which epithelial integrity was restored (A and higher magnification in B). Scale bars: 200 μmol/L (A); 100 μmol/L (B and C); 20 μmol/L (D).
IHC analysis for GFP in mouse lesions taken from MacGreen to WT transfers (A and B) and from WT to MacGreen transfers (C and D). E: Negative control (omission of primary antibody). Arrows indicate macrophages. G, glands; L, lumen.
Dual immunofluorescence for GR-1 (red) and GFP (green) on sections of donor mouse endometrium 4 hours after P4 withdrawal (A) and lesions (B–D). Insets in A–C are enlarged areas of interest. The inset in D is a negative control (primary antibody omitted). Red arrows indicate GR-1+ cells; green arrows, GFP+ cells; and white arrows, colabeling. Single immunofluorescence on serial sections of lesions for GFP (E and G; green) and F4/80 (F and H; red). Scale bars: 50 μmol/L.