Abstract
During embryonic development, amniotes typically form outgrowths from the medial sides of the maxillary prominences called palatal shelves or palatine processes. In mammals the shelves fuse in the midline and form a bony hard palate that completely separates the nasal and oral cavities. In birds and lizards, palatine processes develop but remain unfused, leaving a natural cleft. Adult turtles do not possess palatine processes and unlike other amniotes, the internal nares open into the oral cavity. Here we investigate craniofacial ontogeny in the turtle, Emydura subglobosa to determine whether vestigial palatine processes develop and subsequently regress, or whether development fails entirely. We found that the primary palate in turtles develops similarly to other amniotes, but secondary palate ontogeny diverges. Using histology, cellular dynamics and in situ hybridization we found no evidence of palatine process development at any time during ontogeny of the face in the turtle. Furthermore, detailed comparisons with chicken embryos (the model organism most closely related to turtles from a molecular phylogeny perspective), we identified differences in proliferation and gene expression patterns that correlate with the differences in palate morphology. We propose that, in turtles, palatine process outgrowth is never initiated due to a lack of mesenchymal bone morphogenetic protein 2 (BMP2) expression in the maxillary mesenchyme, which in turn fails to induce the relatively higher cellular proliferation required for medial tissue outgrowth. It is likely that these differences between turtles and birds arose after the divergence of the lineage leading to modern turtles.
Turtles (Testudines) have evolved a unique bauplan which has puzzled taxonomists for generations. Early morphological studies consigned turtles to a basal position within the class Reptilia due to their seemingly primitive cranial structure and unique body shape as reviewed by Zardoya and Meyer (’98). However, several recent molecular studies found that they are actually a sister clade to archosaurs, the ancient reptilian clade consisting of birds and crocodilians (Crawford et al., 2012; Fong et al., 2012; Shaffer et al., 2013; Wang et al., 2013). Birds, turtles and crocodilians represent extant remnants of a deep and diverse radiation of reptiles (Brusatte et al., 2010). Consequently each taxon exhibits considerable physiological and morphological differences, especially within the cranial region. These variations in morphology are likely due to different functional requirements for respiration, food acquisition, and processing. One striking example of these differences is that while crocodilians use their teeth extensively; turtles and birds have evolved to be completely edentulous and became the only amniotes with a keratinized beak (Lee, ’97; Hieronymus and Witmer, 2010).
Other major differences in craniofacial anatomy across amniotes are seen in the secondary palate. In order to better contrast the unique features of reptiles, we will first review the ontogeny of the better known, mammalian secondary palate. In the adult mammal, the hard palate and posterior soft palate fully separate the nasal and oral cavities (Bush and Jiang, 2012). During embryonic development, palatal shelves (the paired medial outgrowths from each embryonic maxillary prominence) first grow vertically beside the tongue and then reorient horizontally to meet and form a midline epithelial seam. The seam degrades allowing a mesenchymal bridge to form joining the palatal shelves with each other and the premaxilla anteriorly (Tamarin, ’82; Bush and Jiang, 2012). Subsequently, ossification of the palatine processes of the maxillary and palatine bones occurs within the palatal shelves after they fuse (Baek et al., 2011).
In contrast to mammals, birds and squamates have palatine processes which look similar to mammalian embryonic palatal shelves and are never fused (Richman et al., 2006). The palatine bones in the adult are positioned on either side of the midline, contacting the maxillary and premaxillary bones anteriorly and pterygoid bones posteriorly. Interestingly, crocodilians are the only reptiles to develop a complete secondary hard palate similar to mammals (Ferguson, ’81).
In contrast to other amniotes, the majority of turtles have a palate that is more similar to basal vertebrates (e.g., amphibians and fishes). The typical turtle has choanae (internal nares) between the premaxilla and palatine bones that open directly into the oral cavity. Turtles also possess a “hard palate” consisting of expanded palatine and maxillary bones, fusing in the midline just posterior to the choanae and articulating with the cranial base posteriorly (Gaffney, ’79). They utilize their palates as a food-processing and prey handling surface (Natchev et al., 2010, 2011; Parham and Pyenson, 2010). Additionally, ancient turtles such as Kayentachelys, Proterochersis, Proganochelys, and Odontochelys possessed marginal as well as palatine teeth (Li et al., 2008; Davit-Beal et al., 2009), which were lost during evolution, possibly due to changes in diet or habitat.
There are many morphological studies of testudine development due to their unique phenotype. These studies consist of several staging series, although most are focused on external features of the embryonic head and limb, sometimes accompanied by wholemount skeletal staining. Some exemplar species are Chelydra serpentina (Yntema, ’68), Apalone spinifera (Greenbaum and Carr, 2002), Pelodiscus sinensis (Tokita and Kuratani, 2001), and Emydura subglobosa (Werneburg et al., 2009). Other studies have focused on the formation of the chondro and dermatocranium. These studies use wholemount skeletal staining (Sheil, 2003, 2005; Sánchez-Villagra et al., 2009) and histological analyses (Tulenko and Sheil, 2007) in a variety of testudine groups ranging from softshell turtles to snapping turtles. Additional studies have focused on turtle-specific features such as the development of the carapace (Gilbert et al., 2001) and positioning of the shoulder girdle inside rather than outside the rib cage (Hirasawa et al., 2013).
To the best of our knowledge, the only study of turtle at early stages of craniofacial morphogenesis was focused on expression of genes involved in tooth development (Tokita et al., 2012) and more recently, on the genesis of the temporal region (Tokita et al., 2013). These studies were performed on the softshell turtle (P. sinensis) and examined different regions of the head than those studied here. Nonetheless, there is a general scarcity of turtle craniofacial ontogeny studies, especially those that encompass the pre-differentiation stages of development.
We studied the pleurodire species, E. subglobosa since a staging series is available for this species (Werneburg et al., 2009) and the adult palate morphology represents the most common pattern seen in turtles. We hypothesize that the divergent palate observed in adult turtles is due to ontogenetic difference arising from differential palatine process outgrowth from the embryonic maxillary prominences. We investigated two possible mechanisms for the failure of palatine process formation: (1) vestigial palatine outgrowths are initiated, but subsequently regresses (possibly through apoptosis) or (2) outgrowths fails to initiate entirely and therefore, are absent at all developmental stages. These studies use a combination of histology, gene expression and cellular dynamics at the relevant stages of palatine process initiation to understand the palatal morphology differences between turtles and their close relatives, the birds.
MATERIALS AND METHODS
Embryo Acquisition, Staging, and Fixation
All animal work was approved under ethics protocol # A11-0352 and carried out at the University of British Columbia. Fertilized E. subglobosa eggs were donated by the Toronto Zoo. Eggs were rinsed with diluted iodine tincture (1:25,000) to limit fungal growth and incubated in moistened vermiculite at 30°C. Embryos were staged as described by Werneburg et al. (2009). Fertilized chicken eggs were incubated at 38°C and staged according to Hamburger and Hamilton (’51). Embryos were euthanized according to approved methods (Turtles: injection of ~50–100 mL of Tricaine methanesulfonate, pH 7, 5–10 min prior to removal from egg followed by decapitation). Subsequently, embryos were fixed overnight in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) pH 7.4 at 4°C.
Fixed Alligator mississippiensis fetuses were imported from the Rockefeller Wildlife Refuge, Louisiana under Foreign CITES export permit #10US766170/9 and Federal Fish and Wildlife Permit #MA003005-0 issued to Louisiana State University, Museum of Natural Science (Baton Rouge, LA). The fetuses were initially fixed in 4% PFA and stored in 70% ethanol during transport. Once at UBC, heads were rehydrated, fixed in Bouin’s fixative for 24 hr, rinsed in water and then demineralized in Morse’s solution (10%, w/v, sodium citrate and 22.5%, v/v, formic acid) for 1.5 days, with moderate stirring at room temperature.
Histological Sections and Micro-CT Analysis
After fixation, specimens were embedded in paraffin, sectioned, and stained with Picrosirius red and Alcian blue for bone and cartilage, respectively (Buchtová et al., 2007). E. subglobosa heads were fixed in 100% ethanol and scanned using a Scanco micro-CT scanner at high resolution.
BrdU, PCNA, and TUNEL Assays
E. subglobosa eggs were injected with 10 μL of 10 mM 5-bromo-2′-deoxyuridine (BrdU) and incubated for 3 hr at 30°C. Embryos were then fixed and embedded in paraffin. Tissues were dewaxed in xylene and dehydrated through a graded ethanol series. Antigen retrieval was performed using a Diva Decloaker solution (Biocare Medical, Concord, CA) at 100°C. Tissues were then digested with Sau3A1 (Roche, Mannheim, Germany) in Buffer A (Roche) at 37°C for 30 min. Subsequently, incorporated BrdU was detected using an Anti-BrdU Antibody (Amersham/GE Healthcare Biosciences Corp., Piscataway, NJ, USA) overnight at 4°C. Stage 5 turtle embryos could not be accessed in ovo to adequately label with BrdU, thus Anti-Proliferating Cell Nuclear Antigen (PCNA) antibodies were used to analyze proliferation patterns as described by Buchtová et al. (2007). Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analysis was performed with the ApopTag plus Peroxidase in situ Apoptosis Detection Kit detected with FITC-tagged anti-HRP (Chemicon International, Temecula, CA, USA, S7101). Slides were coverslipped with Prolong Gold containing 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA, USA). Images were captured using a widefield fluorescence microscope (Zeiss, Jena, Germany; Axioplan2) well as a confocal microscope (Leica Canada, Willowdale, ON, Canada, DM6000 CS). Slides analyzed with confocal microscope were stained with TO-PRO3-iodide stain (diluted 1:5,000, Life Technologies, Inc. Burlington, ON, Canada).
The percentage proliferating or BrdU positive cells was quantified using ImageJ (NIH, Bethesda, MD). The maxillary prominences of E. subglobosa embryos of stage 3 (n = 3), stage 4 (n = 3), and stage 5 (n = 3) were analyzed. Additionally, chicken embryos of stage 24 (n = 6), Stage 28 (n = 6), and stage 29 (n = 6) were analyzed. The percentage of proliferating cells was counted in six regions of the maxillary prominence, three cranial and three caudal. This strategy separated the medial from lateral mesenchyme so that differences in the shelf forming medial region would be more obvious. The data for the six regions at each stage were analyzed using 1-way ANOVA and significant differences between groups determined using Tukey’s post hoc testing (Statistica, v. 6.0; StatSoft, Inc., Tulsa, OK, USA).
Cloning of turtle cDNAs
E. subglobosa RNA and cDNA was prepared from stage 4 embryo RNA as described for other reptiles (Handrigan et al., 2010). DNA amplicons were gel extracted using Qiaquick gel extraction kit (Qiagen Canada; Qiagen, Inc., Mississauga, ON, Canada) and run through a second round of PCR using Taq DNA polymerase (New England Biolabs, Inc., Beverly, MA, USA). PCR products were gel extracted and cloned into pGemT-Easy (Promega, Madison, WI). For each gene of interest, a list of orthologous genes from other species of reptiles, mammals, and amphibians was compiled from NCBI GenBank and Ensembl databases. Partially degenerate PCR primers for BMPs, FGFR2, and for PTCH1 were designed from conserved regions using CODEHOP. E. subglobosa sequences have been deposited in Genbank as follows: BMP7—JQ995295, BMP2—KC834016, FGFR2—KC886401, PTCH1—JQ995296. Plasmids containing Trachemys scripta BMP4 and SPRY2 sequences were generously provided by Moustakas (2008).
In Situ Hybridization
Radioactive in situ hybridization (35S-labelled UTP, Perkin Elmer-Cetus, Norwalk, CT, USA) on histological sections of E. subglobosa were performed as described (Rowe et al., ’92). Probe concentrations were kept at 105 cpm/μL and slides were exposed between 2 and 3 weeks.
RESULTS
Palate Morphology and Histology
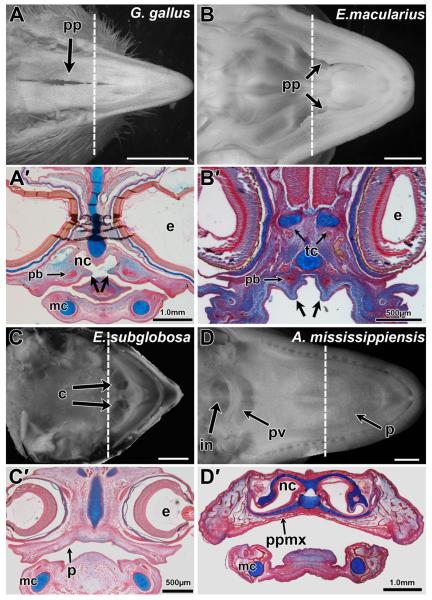
While the skeletal patterns are well described for many vertebrates, the intraoral, soft tissue morphology is not. We began by comparing fetal amniote palates (Fig 1). In birds and squamates palatine processes are visible with a gap in the midline (Fig. 1A,B). Thus there is a communication between the oral and nasal cavities (Fig. 1A,B). In E. subglobosa, the choanae open directly into the oral cavity unobstructed (Fig. 1C). In contrast, crocodilians, like mammals, possess a complete secondary palate that separates the oral and nasal cavities. Furthermore, crocodilians possess a palatal valve which functions in a similar manner to the mammalian soft palate to separate the oral and nasal cavities during respiration (Fig. 1D).
Figure 1.
Palatal phenotypes in reptiles. Palatal views of late stage reptile embryos prior to hatching (A,B) and corresponding histological sections from embryos (A’–D’). Chicken (Gallus gallus) A-stage 41 and A’-stage 34 Leopard gecko (Eublepharis macularius, C,D) B-stage 41 and B’-stage 31 (Wise et al., 2009). Turtle (Emydura subglobosa) C-stage 14 and C’-stage 5 (Werneburg et al., 2009). Alligator (Alligator mississippiensis) D-stage 24 and D’-stage 23 (Ferguson, ’85). Palatine processes and the partially clefted palate can be observed in the chicken and leopard gecko while turtles lack any such processes (A,A’,B,B’,C,C’). Alligators (D,D’) have a complete secondary palate which separate the oral and nasal cavities entirely, with the internal nares opening towards the back of the mouth as in mammals. Frontal sections were stained with alcian blue and picrosirius red, showing the presence of palatine processes in the oral cavity (plane of section is shown by the dashed lines in A–D). Osseous condensations are stained red and cartilage is stained blue. In chicken and gecko the palatine bones ossify adjacent to but not within the palatine processes (arrows) (A’,B’). Key: c, choana; e, eye; mc, in, internal nares; Meckel’s cartilage; nc, nasal cavity; p, palate; pb, palatine bone; pp, palatine process; ppmx, palatine process of maxillary bone; pv, palatal valve; tc, trabeculi cranii. Scale bars: A = 5.0 mm, B,C,F = 2 mm, A’,D’ = 1.0 mm, B’,C’ = 500 μm.
Sections were also prepared to determine the relationship between the palatine bones and the soft tissue of the palatine processes. The appearance is grossly similar in both avian (Fig. 1A’) and squamates embryos (Fig. 1B’) where the palatine processes are small mesenchymal outgrowths of the medial sides of the maxillary prominences. The ossification centers for the palatine bones are lateral to the shelves rather than within them as in mammals. Thus, in birds and lizards, palatine processes present during ontogeny persist in the adult as processes of soft tissue, unsupported by bone. In contrast, the testudine embryo shows no palatine processes (Fig. 1C’). The ossification centers for the palatine bones are forming inferior to the nasal cavity and these will be described in detail below. The crocodilian fetus shows a complete separation of the oral and nasal cavities (Fig. 1D’) with ossification for the palatine process of the maxillary bone extending across the midline. The palatine bones will subsequently articulate with the cranial base and pterygoid bones posteriorly.
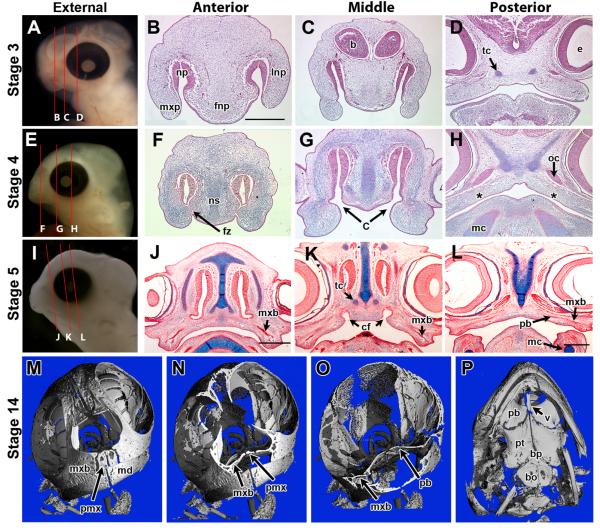
Since turtle embryogenesis is less familiar than traditional model organisms such as birds and mice, we generated serial sections through the head of stage 3–5 embryos in the transverse (Fig. 2A–L, see fly-through animations in Supplementary Movies S1–S3). At stage 3, the primary palate of E. subglobosa is unfused and consists of the frontonasal process, maxillary prominences, and lateral nasal prominence (Fig. 2A,B) similar to all amniotes. There are no signs of skeletogenic condensations with the exception of the trabeculae cranii; the first chondrocranial elements to differentiate in this species (Fig. 2D). This very early initiation of the chondrocranium is precocious compared to birds (Bellairs, ’58).
Figure 2.
Ontogeny of E. subglobosa palate. E. subglobosa embryos at stage 3, 4, and 5 (Werneburg et al., 2009), were sectioned in the transverse plane as indicated by the red lines in panels A,E,I. Planes of section are anterior to the choanae (B,F,J), middle or within the choanae (C,G,K) or posterior to the choanae (D,H,L). Unfused facial prominences are present at stage 3 (B,C) and trabeculae cranii have differentiated (D). At stage 4 the primary palate has fused (F) but openings for the choana are visible in deeper sections (G). In (H), asterisks represent putative regions where palatal processes should arise. Bone condensations for the maxillary (lateral) and palatine bones (medial) are first detected at stage 5 (J–L). μCT of a stage 14 skull oriented at a frontal view showing the association of the premaxillary and maxillary bones in relation to the nasal cavity (M). Transverse cuts show the shallow nasal cavity and the broad, flat palatine bones separating the oral cavity from the cranial vault, with no nasal cavity in between (N,O). Panel N is anterior to the choana while panel O is posterior. Palatine view of the skull shows the sutures have not fused between the bones of the palate (P). b, Brain; bo, basioccipital; bs, basisphenoid; c, choanae; cf, choanal fissure; e, eye; fnp, frontonasal prominence; mc, Meckel’s cartilage; md mandibular bone; mxb, maxillary bone; mxp, maxillary process; np, nasal pit; ns, nasal septum; pb, palatine bone; pmx, premaxilalry bone; pt, pterygoid; tc, trabeculae cranii; v, vomer. Scale bars: 2 mm (B same as C, E same as F, H same as I). Scale bars: B–D, F–H = 220 μm; J–K = 500 μm.
By stage 4, the frontonasal and maxillary prominences have fused anteriorly (Fig. 2F), similar to birds and mammals. However, the nasal slits reconnect with the oral cavity via paired choanae, immediately posterior to the primary palate (Fig. 2G). The choanae provide us with a landmark for where the secondary palate should be initiated since palatal shelves initiate directly posterior to the choanae beginning at E12.5 in mouse (Tamarin, ’82) and stage 29 in chicken (Tamarin et al., ’84). Therefore, if vestigial palatal shelves do develop in E. subglobosa, they should be initiated in the medial surfaces of the maxillary prominences just posterior to the choanae. However, E. subglobosa does not have vestigial shelves in the expected, posterior position (Fig. 2H). We went on to examine older embryos to localize the condensations for the palatine bones since these are either within the palatal shelves (mammals) or directly adjacent to them (birds and lizards; Fig. 1). In stage 5, E. subglobosa, lateral condensations for the maxillary and palatine bones are visible within the mesenchyme of the stomodeum (the embryonic oral cavity) as flat broad ossifications which form the “hard palate” in adult animals (Fig. 2J–L). The initiation of the ossification centers is precocious compared to the rest of the head development in which morphogenesis is still incomplete.
We performed μCT scans of a late stage (stage 14) embryo in order to establish the positions of palatine ossifications relative to the sensory capsules (Fig. 2M–P). Serial slices through the reconstructed head pass through the premaxilla (Fig. 2N), the maxillary (Fig. 2O) and palatine bones (Fig. 2O). The palatine bones are expanding under the nasal capsule to meet the vomer in the midline. A palatal view (Fig. 2P) demonstrates the relationship of the vomer to the palatine bones. The palatine bones directly articulate with the pterygoid, which in turn articulates with the bones of the cranial base (Fig. 2P). This arrangement is in contrast to mammals in which the palatine bones do not contact the pterygoid plates.
Cellular Dynamics Studies in the Maxillary Mesenchyme
To test our first hypothesis: palatine process regression, we used TUNEL analysis to assess programmed cell death in sections anterior and posterior to the choanae (Fig. S1A–D). There were very few apoptotic cells in the maxillary mesenchyme. In contrast, positive cells were found in the fusing regions of the primary palate (Fig. S1C’), which is also typical for the chicken (Ashique et al., 2002) and mouse (Ferretti et al., 2011). Importantly, no apoptosis was observed at the medial edges or anywhere else in the maxillary prominences. Based on the TUNEL data, we excluded the possibility that E. subglobosa has vestigial palatine processes which regress during development. Instead, it appears that palatine processes never initiate.
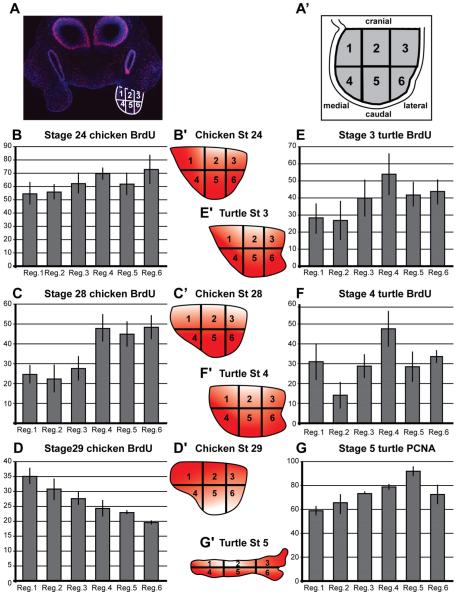
To test our second hypothesis, that processes do not initiate, we used a combination of BrdU and PCNA labeling to analyze proliferation patterns in the maxillary prominences of E. subglobosa in comparison to chicken since chickens are close relatives to turtles from a molecular phylogeny perspective and yet possess palatine processes (Figs. 3A; S3). Previous studies have described proliferation patterns in the maxillary prominence in chicken, however they differ from our methods in several aspects both in labeling and planes of sections (Minkoff and Kuntz, ’78; Bailey et al., ’88).
Figure 3.
Cell proliferation patterns in maxillary prominences of E. subglobosa and chicken Transverse sections posterior to the choanae from comparable stages of turtle and chicken (3,4,5 and 24,28,29, respectively) were stained for proliferating cells using BrdU (B–F) or PCNA (G). The absolute values in G are not directly comparable to those in B–F. (A,A’) The maxillary prominence was divided into regions 1–6 to count the percentage of proliferating cells. Region 1 is where presumptive palatal shelves are thought to arise. (B,E) there is no significant difference in the proliferation index between or within regions in the turtle or chicken. (C) Chicken stage 28, there is an overall drop in proliferation but the cranial maxillary mesenchyme (regions 1–3) have relatively lower levels. (F) Turtle stage 4 shows lower proliferation in region two. (D) In stage 29 chicken, proliferation is relatively higher in region 1 where palatal shelf growth is initiated. (G) Stage 5 of turtle, on the other hand, shows an opposite trend with lower proliferation in regions 1 and 2 compared to the caudal regions (4,5,6). P-values, described in supplementary Tables 1 and 2 were tested using a one-way ANOVA and subsequently analyzed using Tukey’s post hoc testing.
The transverse plane of section used for chickens in our study matched that used for the E. subglobosa and we focused on the anatomical area just posterior to the choana from which palatine processes will arise in chickens. The maxillary prominence was subdivided into five regions in order to identify regional differences in proliferation (Fig. 3A,A’). Stages 3, 4, and 5 in E. subglobosa are approximately equivalent to stage 24, 28, and 29, respectively, in the chicken embryo based on primary and secondary palate formation as well as morphogenesis of the nasal passages. In stage 24 chicken embryos, proliferation was initially even throughout the maxillary prominence (Figs. 3B,B’ and S2A) but polarized differences in proliferation patterns appeared, as the embryos advanced in development (Figs. 3C–D’ and S2B, C; Tables S1 and S2 for P values).
The highest proliferation at stage 28 is at the caudal end of the prominence (regions 4–6, Figs. 3C,C’ and S2B), which correlates with elongation in the cranial-caudal axis. By stage 29, this pattern alters so that the cranial side (regions 1–3) has higher proliferation (Fig. 3D,D’). Moreover, region 1 includes the area nearest to the stomodeum from which the palatine processes grow out (Fig. S2C).
In the stage 3 E. subglobosa, there is significantly higher proliferation on the medial side of the maxillary prominence, rather than a deficiency as might have been predicted (Fig. 3E,E’; Fig. S2D). At stage 4, the medial and caudal regions remain high relative to other regions (Figs. 3F,F’,E and S2E; Table S2). However, at stage 5 the pattern changes such that the highest proliferation is the caudal and central mesenchyme rather than the medial (Figs. 3G,G’,F and S2F). Thus, at later stages where palatine processes should be growing out in E. subglobosa, there is actually lower, rather than higher proliferation. This is a trend that is opposite to the chicken (compare Fig. 3D–G). Thus, we hypothesize that the relatively lower proliferation in the medial regions of E. subglobosa (region 1 in particular) is the likely reason that palatine processes fail to initiate.
Possible Role of BMPs in Mediating the Lack of Medial Proliferation in E. subglobosa
In addition to applying basic histological analyses, we also took a candidate gene approach to identify possible genetic alterations that alter palatal morphogenesis. Previous studies performed by us and several other groups on chicken embryos (Francis-West et al., ’94; Barlow and Francis-West, ’97; Ashique et al., 2002; Hu and Marcucio, 2009b) persuaded us to compare expression of bone morphogenetic proteins—BMP2, 4, and 7 in chicken and E. subglobosa. Specifically, we showed in a previous study from our lab (Ashique et al., 2002) that blocking BMPs with the antagonist Noggin reduced proliferation of maxillary prominence mesenchyme resulting in the specific loss of palatine process.
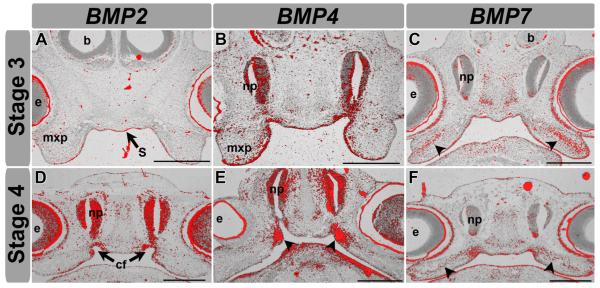
We focused on stages 3 and 4 in E. subglobosa which is just prior to palatal shelf outgrowth in equivalent chicken stages 24 and 28 and when proliferation rates are relatively high. These stages are just prior to the acquisition of major morphological differences in the face. We examined epithelium and mesenchyme since changes could be present in either compartment. In the epithelium of the stomodeum and oral surface of the maxillary prominences, BMP2, BMP4, and BMP7 transcripts are expressed at high levels (Fig. 4A–F). This stomodeal epithelial expression of BMPs in E. subglobosa is similar to the expression in the roof of the oral cavity in chicken and mouse prior to formation of palatal shelves (Francis-West et al., ’94; Ashique et al., 2002; Foppiano et al., 2007; Hu and Marcucio, 2009b). To further investigate the character of the stomodeal epithelium, we also examined PATCHED1 (PTCH1) expression which is a receptor and target of the Hedgehog pathway. Sonic Hedgehog (SHH) is strongly expressed in the chicken stomodeal epithelium (Ashique et al., 2002; Hu and Marcucio, 2009a) and a similar expression pattern is observed for PTCH1 in the E. subglobosa (Fig. S3A–C’).
Figure 4.
Expression of BMPs in E. subglobosa. Radioactive in-situ hybridization on transverse sections through the region directly posterior to the choana of E. subglobosa at stages 3 and 4. Signal from darkfield images has been false colored red and overlaid on top of brightfield images using Adobe Photoshop. (A) BMP2 expression at stage 3 is located in the stomodeal epithelium as well as the medial maxillary prominence epithelium. It is noticeably absent in the mesenchyme. (D) At stage 4, BMP2 is largely expressed in the nasal pits and oral epithelium, in a similar manner to stage 3. Expression is also prominent in the mesenchyme next to the choanal fissures. (B) BMP4 expression is largely restricted to the oral epithelium. (E) BMP4 is also strongly expressed in the mesenchyme around the choanal fissures. Both BMP2 and BMP4 are also strongly expressed in the nasal pits. (C,F) BMP7 expression overlaps with BMP4 expression in its expression pattern within the oral epithelium, although there is no expression in the nasal pit. BMP7 is also expressed in the mesenchyme of the maxillary prominence (arrow heads), possibly implicating it in a role involving palatine ossifications later in ontogeny. b, Brain; cf, choanal fissures; e, eye; mxp, maxillary; np, nasal pit; s, stomodeum. Scale bars: 500 μm A–F.
In the maxillary mesenchyme, we observe a notable difference in expression pattern between the BMPs in chicken as compared to E. subglobosa. We observed a distinct lack of mesenchymal BMP2 expression in E. subglobosa at all stages (Fig. 4A,D) whereas several other studies report mesenchymal BMP2 expression in the chicken maxillary prominence specifically on the medial side (Francis-West et al., ’94, Ashique et al., 2002). Relatively high BMP2 expression is also seen in the embryonic palatal shelves of mice (Baek et al., 2011). This lack of BMP2 in the medial mesenchyme of the embryonic maxillary prominence of E. subglobosa could be one reason for the lack of proliferation which directly leads to failure of palatine process outgrowth.
Pathways other than BMPs could also be regulating outgrowth of palatal shelves. We examined two genes that transduce fibroblast growth factor (FGF) signals, the receptor FGFR2 and the pathway target and antagonist SPRY2. FGFs have previously been implicated in a role in proliferation and in previous work, when we blocked FGF receptor (FGFR) signaling, proliferation in facial mesenchyme was inhibited (Szabo-Rogers et al., 2008). However, there were no major differences in expression of FGFR2 and SPRY2 in the maxillary prominence compared to chicken (Wilke et al., ’97, Szabo-Rogers et al., 2008) suggesting that the FGF signaling pathway is not regulating species differences in the palate (Fig. S4A–J’).
DISCUSSION
In this study, we focused attention on an important stage of development that characterizes all amniotes; that of secondary palate morphogenesis. We showed that embryonic birds and squamates have palatine processes whereas extant turtles do not. Thus, based on the molecular phylogenetic positioning of turtles as a sister group to archosaurs, the most parsimonious assumption is the ancestral lineage leading to turtles also had palatine processes which were subsequently lost. Unfortunately, since palatine processes are comprised of soft tissue, the fossil record is ineffective in helping us ascertain when this occurred in turtles. We established that in the pleurodire turtle E. subglobosa, palatine process outgrowth is never initiated. The lack of budding is likely due to a failure to maintain relatively high proliferation on the medial side of the maxillary prominence; which is the case in chicken. Subsequently, through molecular analyses, we correlated the low proliferation with the lack of mesenchymal BMP2 expression.
Mechanism of Palatal Shelf Budding
We have demonstrated that in the avian embryo, the classic mechanism of budding is being upheld in palatine process formation. The basis of budding is that an area basal to the structure that is growing out will decrease the proliferation index relative to more apical mesenchyme. This change in relative proliferation rates is seen in the limb bud where flank proliferation drops relative to the limb mesenchyme (Saunders, ’48). Similarly, in the avian embryo, the facial prominences bud out from around the embryonic stomodeum based on similar differential proliferation (Minkoff and Kuntz, ’77, ’78). While the relationship to basal mesenchyme near the stomodeum was not the emphasis of our study, the same principle of budding applies to the maxillary prominence. We determined that in the stage 29 chicken, even though maxillary proliferation overall drops relative to younger stages, the medial mesenchyme from which the palatal shelf derives retains a higher proliferative index than lateral or caudal regions. By comparison, others (Bailey et al., ’88) did not find any significant differences in proliferation between different regions of maxillary prominence at stages 28–29. However, these authors did cite the lack of variation in proliferation as anomalous, since they expected to see a region of higher proliferation where palatal shelf outgrowth initiates. A possible reason for the disparity in results between their study and ours may be the plane of section in the previous study, which appears to be more posterior than ours. In the chicken, the more posterior regions of the maxillary prominence do not form palatine processes. In our study we were careful to focus on the region directly posterior to the primary palate and choanal openings, where palatine processes arise. In the larger context of amniotes, our chicken data agree with trends seen in the medial versus lateral mouse maxillary prominence (Iwabe et al., 2005). Although these authors did not comment specifically on the relevance of proliferation to normal outgrowth, we conclude that the mechanism of budding is conserved in all species in which palatal shelves develop.
Precocious Chondrocranial and Dermatocranial Development in Turtles Relative to Birds
Another significant observation from our study is the very early onset of the chondrocranium and intramembranous bone formation in turtles relative to birds. In chickens it is not until the beak has begun to extend (stage 32) that bone formation begins (Romanoff, ’60; Murray, ’63). With histological analyses, we were able to show that in E. subglobosa, ossification begins shortly after fusion of the primary palate and prior to formation of the beak. The precocious development in turtles may allow the palatine bones (palatine process of the maxillary, palatine, vomer, and pterygoid) to achieve a large size relative to the size of the oral cavity. Palatal heterochrony has been described in tetrapods in detail and these authors predict that precocious embryonic development would be associated with larger palatine bones (Kimmel et al., 2009). Interestingly, the maxillary bones of crocodilians seem to exhibit a superficial similarity to those of turtles in that ossification occurs in the same plane as the cranial base. It will be interesting to determine how ossification centers for the palatine processes of the maxillary bones in crocodilians relate to their palatal processes.
Stomodeal Markers Are Expressed in the Roof of the Mouth of the Turtle
We began the study of E. subglobosa ontogeny with histological analyses and determined that palatal processes were not present in the expected anatomical location. The histological data are supported by the expression of the stomodeal markers PTCH1 and BMP7. The expression of SHH in the stomodeum of the P. sinensis turtle (Tokita et al., 2012) is also consistent with our data. These molecular data offer further proof that in turtles, the roof of the embryonic stomodeum is never covered over by palatal shelves as it is in mammals, crocodilians, lizards and birds. Instead the stomodeum of turtles differentiates into the roof of the oral cavity supported by the maxillary, palatine and pterygoid bones. Fate maps of the maxillary prominence and osseous derivatives in the chicken embryo suggest that palatine bones are derived from mesencephalic neural crest cells (Couly et al., ’93; Kontges and Lumsden, ’96). The turtle palatine bones may originate, not from mesencephalic mesenchyme but instead from midline mesenchyme derived originally from forebrain neural crest cells. Neural crest cell fate maps are needed to test these ideas.
The Lack of Mesenchymal BMP2 Is the Likely Cause of Palatine Process Loss in Turtle
The molecular mechanisms that support localized outgrowth of the palatine processes likely involve epithelial–mesenchymal interactions and possibly signals in the BMP pathway. Furthermore, we posit that BMP signals regulate proliferation in E. subglobosa mesenchyme. In chicken embryos, BMP2 and BMP4 protein-soaked beads induce cell proliferation in facial mesenchyme in vivo (Barlow and Francis-West, ’97) and BMP7 protein stimulates proliferation in primary cell cultures (Mina et al., 2002). Moreover, inhibiting the BMP pathway in chicken maxillary prominence using Noggin beads resulted in decreased proliferation (Ashique et al., 2002). Here, not only was proliferation inhibited at early stages, but the resulting phenotype was the failure to form a palatine process on the treated side. Interestingly, overexpression of Noggin in the epithelium does not seem to cause palatal clefting in mice (He et al., 2010). However, based on our data, it would be necessary to use a different transgene in which Noggin was overexpressed in maxillary mesenchyme. Nevertheless, there are several informative mouse genetic experiments in which Bmps or their receptors were deleted in the mesenchyme and palate phenotypes were produced.
Germline knockout of Bmp7 in mice resulted in cleft palate but no proliferation deficiencies were detected (Kouskoura et al., 2013). Interestingly, strictly mesenchymal or strictly epithelial conditional deletion did not result in clefts; therefore deletion in both tissue components is necessary to generate the Bmp7 phenotype. Conditional knockout of Bmp4 in cranial neural crest cells also causes cleft palate in mouse with many of the genes effected being directly involved in proliferation (Bonilla-Claudio et al., 2012). Bmps signal through a complex of type I and type II receptors (Massague, 2012) and various knockouts of the components of the pathway have also affected palatogenesis. Three studies have targeted BMP receptor Bmpr1a and showed that palatogenesis was impaired (Liu et al., 2005; Baek et al., 2011; Li et al., 2011). Furthermore all these studies showed that the inactivation of Bmpr1a decreases mesenchymal proliferation in the palatal shelves. Our findings in E. subglobosa are consistent with a major role for BMPs in regulating formation of the secondary palate. In addition, our data follow the idea that differences in expression of BMPs underlie species-specific jaw form in birds (Abzhanov et al., 2004; Wu et al., 2004, 2006) and in cichlid fishes (Albertson et al., 2005).
Correspondence Between the Vertebrate Phylotypic Stage and Craniofacial Gene Expression Patterns in E. subglobosa
Recent studies (Irie and Kuratani, 2011) and (Wang et al., 2013), showed that transcriptomes of a variety of vertebrates (frogs, fish, birds, mammals, turtles) are less similar in early developmental stages, then become more similar when embryos exhibit similar body plans and subsequently diverge when species-specific morphology is emerging. This period of greater similarity is termed the “phylotypic” stage and corresponds to Pelodiscus stages TK11 (Wang et al., 2013), stage 2–3 in E. subglobosa (Werneburg et al., 2009) or stage 16 of the chicken embryo (Hamburger and Hamilton, ’51; Irie and Kuratani, 2011). This pattern of molecular divergence forms an hourglass shape when gene expression levels are plotted against developmental stage. When only P. sinensis and chicken transcriptomes are compared, the hourglass has a narrower constriction (indicating less divergence), attributed to the relatively close relationship between the two groups.
In our study, we mapped expression of several genes in the craniofacial region. The stages we analyzed, E. subglobosa stage 3 (~TK12–13), stage 4 (~TK14–15), and stage 5 (~TK17), appear to be outside of the narrowest constriction of the hourglass and are just at the start of the period of molecular divergence. Five of the six key craniofacial signaling genes analyzed (FGFR2, SPRY2, PTCH1, BMP4, BMP7), had almost identical expression patterns in chicken and E. subglobosa suggesting that the non-coding regulatory sequences are also conserved. However, BMP2 was not expressed in the E. subglobosa maxillary prominence while in the chicken there is strong expression (Ashique et al., 2002). Looking more broadly, there are additional mesenchymal expression domains for BMP2 in the frontonasal mass and mandibular prominences of chicken embryos and these are also lacking in E. subglobosa. Thus BMP2 is a potentially important gene that could define species-specific jaw shape. The detailed results of in our in situ hybridization studies not only complement the broader approach of the whole genome analysis, but also highlight that discrete regions of the embryo could have slightly different phylotypic stages when analyzed separately. Based on E. subglobosa and chicken morphogenesis, we propose that the craniofacial phylotypic stages will span nasal placode formation (coincident with whole embryo phylotypic stage) and may extend to primary palate formation, but will not extend into the secondary palate stages when morphogenesis diverges.
Evolutionary Significance
Mounting molecular evidence supports the phylogenetic position of turtles as a sister clade to archosaurs (birds and crocodilians); nested higher up within the amniote clade as opposed to being a basal lineage (Zardoya and Meyer, ’98; Crawford et al., 2012; Fong et al., 2012; Shaffer et al., 2013; Wang et al., 2013). The implication of this phylogenetic position suggests that a secondary loss of palatine processes occurred after, rather than before the split between the squamates and archosaurs. Mammals, archosaurs (birds and crocodilians) (Ferguson, ’88) and squamates (Buchtová et al., 2007) all have palatine processes (as we also show in this study), therefore the most parsimonious conclusion is that palatine processes are an ancestral trait which was once present in the stem amniote but is now entirely lost in turtles. Our study helps to shed light on the putative mechanism by which this loss has occurred. Turtles have likely lost mesenchymal BMP2 expression in their embryonic maxillary prominences, whereas birds retain this expression and are able to induce proliferation and grow palatine processes.
It is important to note that the anatomy of several aquatic species of turtles is different than that of E. subglobosa in that a short secondary palate is present anteriorly (Hirayama, ’94; Meylan et al., 2000; Wyneken, 2001; Parham and Pyenson, 2010). Additionally, several extinct lineages of turtles such as Sandownia harrisi, Osteopygis emarginatus, as well as several members of the pleurodire genus Bairdemys, possessed relatively well-developed secondary palates (Meylan et al., 2000; Hirayama and Tong, 2003; Gaffney et al., 2008). Thus we should be cautious about generalizing our findings to all turtles. We leave open the possibility that such as the aforementioned extinct species, as well as select sea and mud turtles may have re-evolved a secondary palate, although the role of palatine processes in the ontogeny of these palates is unknown.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Dr. Andrew Lentini for diligently providing us with E. subglobosa eggs from the Toronto Zoo, Dr. John Whitlock for helpful discussions on reptilian paleontology, Gregory Handrigan for help in cloning the E. subglobosa cDNAs and Katherine Fu for generous assistance with in situ hybridization experiments. We would also like to thank Dr. Ruth Elsey of the Rockefeller Wildlife Refuge and Dr. Chris Austin of LSU for facilitating the acquisition of alligator samples. Finally, we would like to thank the two anonymous reviewers whose suggestions greatly improve this manuscript. This work was funded by an NSERC discovery grant 326908 to J.M.R. J.A. is a recipient of a NIH Ruth L. Kirschstein NRSA Postdoctoral Fellowship (award no.: 1 F32 DE022999-01).
Grant sponsor: NSERC Discovery Grant; grant number: 326908; grant sponsor: NIH Ruth L. Kirschstein NRSA Postdoctoral Fellowship; grant number: 1 F32 DE022999-01.
Footnotes
SUPPORTING INFORMATION Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
LITERATURE CITED
- Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ. Bmp4 and morphological variation of beaks in Darwin’s finches. Science. 2004;305:1462–1465. doi: 10.1126/science.1098095. [DOI] [PubMed] [Google Scholar]
- Albertson RC, Streelman JT, Kocher TD, Yelick PC. Integration and evolution of the cichlid mandible: the molecular basis of alternate feeding strategies. Proc Natl Acad Sci USA. 2005;102:16287–16292. doi: 10.1073/pnas.0506649102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashique AM, Fu K, Richman JM. Endogenous bone morphogenetic proteins regulate outgrowth and epithelial survival during avian lip fusion. Development. 2002;129:4647–4660. doi: 10.1242/dev.129.19.4647. [DOI] [PubMed] [Google Scholar]
- Baek JA, Lan Y, Liu H, et al. Bmpr1a signaling plays critical roles in palatal shelf growth and palatal bone formation. Dev Biol. 2011;350:520–531. doi: 10.1016/j.ydbio.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey LJ, Minkoff R, Koch WE. Relative growth rates of maxillary mesenchyme in the chick embryo. J Craniofac Genet Dev Biol. 1988;8:167–177. [PubMed] [Google Scholar]
- Barlow AJ, Francis-West PH. Ectopic application of recombinant BMP-2 and BMP-4 can change patterning of developing chick facial primordia. Development. 1997;124:391–398. doi: 10.1242/dev.124.2.391. [DOI] [PubMed] [Google Scholar]
- Bellairs AD. The early development of the interorbital septum and the fate of the anterior orbital cartilages in birds. J Embryol Exp Morphol. 1958;6:68–85. [PubMed] [Google Scholar]
- Bonilla-Claudio M, Wang J, Bai Y, et al. Bmp signaling regulates a dose-dependent transcriptional program to control facial skeletal development. Development. 2012;139:709–719. doi: 10.1242/dev.073197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusatte SL, Benton MJ, Desojo JB, Langer MC. A higher-level phylogeny of Archosauria (Tetrapoda: Diapsida) J Syst Palaeontol. 2010;8:3–47. [Google Scholar]
- Buchtová M, Boughner JC, Fu K, Diewert VM, Richman JM. Embryonic development of Python sebae—II: craniofacial microscopic anatomy, cell proliferation and apoptosis. Zoology (Jena) 2007;110:231–251. doi: 10.1016/j.zool.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Bush JO, Jiang R. Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development. Development. 2012;139:231–243. doi: 10.1242/dev.067082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development. 1993;117:409–429. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- Crawford NG, Faircloth BC, McCormack JE, et al. More than 1000 ultraconserved elements provide evidence that turtles are the sister group of archosaurs. Biol Lett. 2012;8:783–786. doi: 10.1098/rsbl.2012.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davit-Beal T, Tucker AS, Sire JY. Loss of teeth and enamel in tetrapods: fossil record, genetic data and morphological adaptations. J Anat. 2009;214:477–501. doi: 10.1111/j.1469-7580.2009.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson MW. The structure and development of the palate in Alligator mississippiensis. Arch Oral Biol. 1981;26:427–443. doi: 10.1016/0003-9969(81)90041-8. [DOI] [PubMed] [Google Scholar]
- Ferguson MW. Reproductive biology and embryology of the crocodilians. In: Gans C, Billet F, Maderson P, editors. Biology of the reptilia. John Wiley & Sons; New York: 1985. pp. 331–491. [Google Scholar]
- Ferguson MW. Palate development. Development. 1988;103:41–60. doi: 10.1242/dev.103.Supplement.41. [DOI] [PubMed] [Google Scholar]
- Ferretti E, Li B, Zewdu R, et al. A conserved Pbx-Wnt-p63-Irf6 regulatory module controls face morphogenesis by promoting epithelial apoptosis. Dev Cell. 2011;21:627–641. doi: 10.1016/j.devcel.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong JJ, Brown JM, Fujita MK, Boussau B. A phylogenomic approach to vertebrate phylogeny supports a turtle-archosaur affinity and a possible paraphyletic lissamphibia. PLoS ONE. 2012;7:e48990. doi: 10.1371/journal.pone.0048990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foppiano S, Hu D, Marcucio RS. Signaling by bone morphogenetic proteins directs formation of an ectodermal signaling center that regulates craniofacial development. Dev Biol. 2007;312:103–114. doi: 10.1016/j.ydbio.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis-West PH, Tatla T, Brickell PM. Expression patterns of the bone morphogenetic protein genes Bmp-4 and Bmp-2 in the developing chick face suggest a role in outgrowth of the primordia. Dev Dyn. 1994;201:168–178. doi: 10.1002/aja.1002010207. [DOI] [PubMed] [Google Scholar]
- Gaffney ES. Comparative cranial morphology of recent and fossil turtles. Bull Am Mus Nat Hist. 1979;164:69–376. [Google Scholar]
- Gaffney ES, Scheyer TM, Johnson KG, Bocquentin J, Aguilera OA. Two new species of the side necked turtle genus, Bairdemys (Pleurodira, Podocnemididae), from the Miocene of Venezuela. Paleontol Zeitschrift. 2008;82:209–229. [Google Scholar]
- Gilbert SF, Loredo GA, Brukman A, Burke AC. Morphogenesis of the turtle shell: the development of a novel structure in tetrapod evolution. Evol Dev. 2001;3:47–58. doi: 10.1046/j.1525-142x.2001.003002047.x. [DOI] [PubMed] [Google Scholar]
- Greenbaum E, Carr JL. Staging criteria for embryos of the spiny softshell turtle, Apalone spinifera (Testudines: Trionychidae) J Morphol. 2002;254:272–291. doi: 10.1002/jmor.10036. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton H. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Handrigan GR, Leung KJ, Richman JM. Identification of putative dental epithelial stem cells in a lizard with life-long tooth replacement. Development. 2010;137:3545–3549. doi: 10.1242/dev.052415. [DOI] [PubMed] [Google Scholar]
- He F, Xiong W, Wang Y, et al. Modulation of BMP signaling by Noggin is required for the maintenance of palatal epithelial integrity during palatogenesis. Dev Biol. 2010;347:109–121. doi: 10.1016/j.ydbio.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieronymus TL, Witmer LM. Homology and evolution of avian compound rhamphotecae. Auk. 2010;127:590–604. [Google Scholar]
- Hirasawa T, Nagashima H, Kuratani S. The endoskeletal origin of the turtle carapace. Nat Commun. 2013;4:2107. doi: 10.1038/ncomms3107. doi: 10.1038/ncomms3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama R. Phylogenetic systematics of chelonioid sea turtles. Isl Arc: 1994;3:270–284. [Google Scholar]
- Hirayama R, Tong H. Osteopygis (Testudines: Ccheloniidae) from the lower tertiary of the Ouled Abdoun Phosphate Basin, Morocco. Paleontology. 2003;46:845–856. [Google Scholar]
- Hu D, Marcucio RS. A SHH-responsive signaling center in the forebrain regulates craniofacial morphogenesis via the facial ectoderm. Development. 2009a;136:107–116. doi: 10.1242/dev.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Marcucio RS. Unique organization of the frontonasal ectodermal zone in birds and mammals. Dev Biol. 2009b;325:200–210. doi: 10.1016/j.ydbio.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie N, Kuratani S. Comparative transcriptome analysis reveals vertebrate phylotypic period during organogenesis. Nat Commun. 2011;2:248. doi: 10.1038/ncomms1248. doi: 10.1038/ncomms1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabe N, Hara Y, Kumazawa Y, et al. Sister group relationship of turtles to the bird-crocodilian clade revealed by nuclear DNA-coded proteins. Mol Biol Evol. 2005;22:810–813. doi: 10.1093/molbev/msi075. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Sidlauskas B, Clack JA. Linked morphological changes during palate evolution in early tetrapods. J Anat. 2009;215:91–109. doi: 10.1111/j.1469-7580.2009.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontges G, Lumsden A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122:3229–3242. doi: 10.1242/dev.122.10.3229. [DOI] [PubMed] [Google Scholar]
- Kouskoura T, Kozlova A, Alexiou M, et al. The etiology of cleft palate formation in BMP7-deficient mice. PLoS ONE. 2013;8:e59463. doi: 10.1371/journal.pone.0059463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MSY. The evolution of beaks in reptiles: a proposed evolutionary constraint. Evol Theory Rev. 1997;11:249–254. [Google Scholar]
- Li C, Wu XC, Rieppel O, Wang LT, Zhao LJ. An ancestral turtle from the Late Triassic of southwestern China. Nature. 2008;456:497–501. doi: 10.1038/nature07533. [DOI] [PubMed] [Google Scholar]
- Li L, Lin M, Wang Y, et al. BmprIa is required in mesenchymal tissue and has limited redundant function with BmprIb in tooth and palate development. Dev Biol. 2011;349:451–461. doi: 10.1016/j.ydbio.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Sun X, Braut A, et al. Distinct functions for Bmp signaling in lip and palate fusion in mice. Development. 2005;132:1453–1461. doi: 10.1242/dev.01676. [DOI] [PubMed] [Google Scholar]
- Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan P, Moody RTJ, Walker CA, Sandra D. Sandownia harrisi, a highly derived trionychoid turtle (Testudines: Cryptodira) from the Early Cretaceous of the Isle of Wight, England. J Vert Paleont. 2000;3:522–532. [Google Scholar]
- Mina M, Wang YH, Ivanisevic AM, Upholt WB, Rodgers B. Region- and stage-specific effects of FGFs and BMPs in chick mandibular morphogenesis. Dev Dyn. 2002;223:333–352. doi: 10.1002/dvdy.10056. [DOI] [PubMed] [Google Scholar]
- Minkoff R, Kuntz AJ. Cell proliferation during morphogenetic change; analysis of frontonasal morphogenesis in the chick embryo employing DNA labeling indices. J Embryol Exp Morphol. 1977;40:101–113. [PubMed] [Google Scholar]
- Minkoff R, Kuntz AJ. Cell proliferation and cell density of mesenchyme in the maxillary process and adjacent regions during facial development in the chick embryo. J Embryol Exp Morphol. 1978;46:65–74. [PubMed] [Google Scholar]
- Moustakas JE. Development of the carapacial ridge: implications for the evolution of genetic networks in turtle shell development. Evol Dev. 2008;10:29–36. doi: 10.1111/j.1525-142X.2007.00210.x. [DOI] [PubMed] [Google Scholar]
- Murray PDF. Adventitious (secondary) cartilage in the chick embryo, and the development of certain bones and articulation in the chick skull. Aust J Zool. 1963;11:368–430. [Google Scholar]
- Natchev N, Lemell P, Heiss E, Beisser C, Weisgram J. Aquatic feeding in a terrestrial turtle: a functional-morphological study of the feeding apparatus in the Indochinese box turtle Cuora galbinifrons (Testudines, Geoemydidae) Zoomorphology. 2010;129:111–119. [Google Scholar]
- Natchev N, Heiss E, Singer K, et al. Structure and function of the feeding apparatus in the common musk turtle Sternotherus odoratus (Chelonia, Kinosternidae) Contrib Zool. 2011;80(2):143–156. Available online at: http://dpc.uba.uva.nl/ctz/vol80/nr02/art04. [Google Scholar]
- Parham JF, Pyenson ND. New sea turtle from the Miocene of Peru and the iterative evolution of feeding ecomorphologies since the Cretaceous. J Paleont. 2010;2:231–247. [Google Scholar]
- Richman JM, Buchtová M, Boughner JC. Comparative ontogeny and phylogeny of the upper jaw skeleton in amniotes. Dev Dyn. 2006;235:1230–1243. doi: 10.1002/dvdy.20716. [DOI] [PubMed] [Google Scholar]
- Romanoff A. Structural and functional development. Macmillan Company; New York: 1960. The avian embryo. [Google Scholar]
- Rowe A, Richman JM, Brickell PM. Development of the spatial pattern of retinoic acid receptor-beta transcripts in embryonic chick facial primordia. Development. 1992;114:805–813. doi: 10.1242/dev.114.3.805. [DOI] [PubMed] [Google Scholar]
- Sánchez-Villagra MR, Muller H, Sheil CA, et al. Skeletal development in the Chinese soft-shelled turtle Pelodiscus sinensis (Testudines: Trionychidae) J Morphol. 2009;270:1381–1399. doi: 10.1002/jmor.10766. [DOI] [PubMed] [Google Scholar]
- Saunders JW., Jr. The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. J Exp Zool. 1948;108:363–403. doi: 10.1002/jez.1401080304. [DOI] [PubMed] [Google Scholar]
- Shaffer HB, Minx P, Warren DE, et al. The western painted turtle genome, a model for the evolution of extreme physiological adaptations in a slowly evolving lineage. Genome Biol. 2013;14:1–22. doi: 10.1186/gb-2013-14-3-r28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheil CA. Osteology and skeletal development of Apalone spinifera (Reptilia: Testudines: Trionychidae) J Morphol. 2003;256:42–78. doi: 10.1002/jmor.10074. [DOI] [PubMed] [Google Scholar]
- Sheil CA. Skeletal development of Macrochelys temminckii (Reptilia: Testudines: Chelydridae) J Morphol. 2005;263:71–106. doi: 10.1002/jmor.10290. [DOI] [PubMed] [Google Scholar]
- Szabo-Rogers HL, Geetha-Loganathan P, Nimmagadda S, Fu KK, Richman JM. FGF signals from the nasal pit are necessary for normal facial morphogenesis. Dev Biol. 2008;318:289–302. doi: 10.1016/j.ydbio.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Tamarin A. The formation of the primitive choanae and the junction of the primary and secondary palates in the mouse. Am J Anat. 1982;165:319–337. doi: 10.1002/aja.1001650308. [DOI] [PubMed] [Google Scholar]
- Tamarin A, Crawley A, Lee J, Tickle C. Analysis of upper beak defects in chicken embryos following with retinoic acid. J Embryol exp Morph. 1984;84:105–123. [PubMed] [Google Scholar]
- Tokita M, Kuratani S. Normal embryonic stages of the Chinese softshelled turtle Pelodiscus sinensis (Trionychidae) Zool Sci. 2001;18:705–715. [Google Scholar]
- Tokita M, Chaeychomsri W, Siruntawineti J. Developmental basis of toothlessness in turtles: insight into convergent evolution of vertebrate morphology. Evolution. 2012;67:260–273. doi: 10.1111/j.1558-5646.2012.01752.x. [DOI] [PubMed] [Google Scholar]
- Tokita M, Chaeychomsri W, Siruntawineti J. Skeletal gene expression in the temporal region of the reptilian embryos: implications for the evolution of reptilian skull morphology. Springer Plus. 2013;2:336. doi: 10.1186/2193-1801-2-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulenko FJ, Sheil CA. Formation of the chondrocranium of Trachemys scripta (Reptilia: Testudines: Emydidae) and a comparison with other described turtle taxa. J Morphol. 2007;268:127–151. doi: 10.1002/jmor.10487. [DOI] [PubMed] [Google Scholar]
- Wang Z, Pascual-Anaya J, Zadissa A, et al. The draft genomes of soft-shell turtle and green sea turtle yield insights into the development and evolution of the turtle-specific body plan. Nat Genet. 2013;45:701–706. doi: 10.1038/ng.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneburg I, Hugi J, Muller J, Sánchez-Villagra MR. Embryogenesis and ossification of Emydura subglobosa (Testudines, Pleurodira, Chelidae) and patterns of turtle development. Dev Dyn. 2009;238:2770–2786. doi: 10.1002/dvdy.22104. [DOI] [PubMed] [Google Scholar]
- Wilke TA, Gubbels S, Schwartz J, Richman JM. Expression of fibroblast growth factor receptors (FGFR1, FGFR2, FGFR3) in the developing head and face. Dev Dyn. 1997;210:41–52. doi: 10.1002/(SICI)1097-0177(199709)210:1<41::AID-AJA5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Wise PA, Vickaryous MK, Russell AP. An embryonic staging table for in ovo development of Eublepharis macularius, the leopard gecko. Anat Rec (Hoboken) 2009;292:1198–1212. doi: 10.1002/ar.20945. [DOI] [PubMed] [Google Scholar]
- Wu P, Jiang TX, Suksaweang S, Widelitz RB, Chuong CM. Molecular shaping of the beak. Science. 2004;305:1465–1466. doi: 10.1126/science.1098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Jiang TX, Shen JY, Widelitz RB, Chuong CM. Morphoregulation of avian beaks: comparative mapping of growth zone activities and morphological evolution. Dev Dyn. 2006;235:1400–1412. doi: 10.1002/dvdy.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyneken J. The Anatomy of Sea Turtles. U.S. Department of Commerce NOAA Technical Memorandum NMFS-SEFSC-470. 2001:1–172. [Google Scholar]
- Yntema CL. A series of stages in the embryonic development of Chelydra serpentina. J Morphol. 1968;125:219–251. doi: 10.1002/jmor.1051250207. [DOI] [PubMed] [Google Scholar]
- Zardoya R, Meyer A. Complete mitochondrial genome suggests diapsid affinities of turtles. Proc Natl Acad Sci. 1998;95:14226–14231. doi: 10.1073/pnas.95.24.14226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.