Abstract
We tested the hypothesis that IL-19, a putative member of the type 2 helper T-cell family of anti-inflammatory interleukins, can attenuate intimal hyperplasia and modulate the vascular smooth muscle cell (VSMC) response to injury. Ligated carotid artery of IL-19 knockout (KO) mice demonstrated a significantly higher neointima/intima ratio compared with wild-type (WT) mice (P = 0.04). More important, the increased neointima/intima ratio in the KO could be reversed by injection of 10 ng/g per day recombinant IL-19 into the KO mouse (P = 0.04). VSMCs explanted from IL-19 KO mice proliferated significantly more rapidly than WT. This could be inhibited by addition of IL-19 to KO VSMCs (P = 0.04 and P < 0.01). IL-19 KO VSMCs migrated more rapidly compared with WT (P < 0.01). Interestingly, there was no type 1 helper T-cell polarization in the KO mouse, but there was significantly greater leukocyte infiltrate in the ligated artery in these mice compared with WT. IL-19 KO VSMCs expressed significantly greater levels of inflammatory mRNA, including IL-1β, tumor necrosis factor α, and monocyte chemoattractant protein-1 in response to tumor necrosis factor α stimulation (P < 0.01 for all). KO VSMCs expressed greater adhesion molecule expression and adherence to monocytes. Together, these data indicate that IL-19 is a previously unrecognized counterregulatory factor for VSMCs, and its expression is an important protective mechanism in regulation of vascular restenosis.
Despite aggressive dietary modification, lipid-lowering medications, and other medical therapy, vascular disease continues to account for 50% of all mortality in the United States. It is a significant systemic problem contributing to mortality of multiple diseases, including myocardial infarction, stroke, renal failure, and peripheral vascular disease, and will worsen with an increasing growing number of patients with comorbidities, such as obesity, metabolic syndrome, and type 2 diabetes mellitus (conditions linked with atherosclerotic vascular disease). Intimal hyperplasia subsequent to mechanical injury remains a clinically significant obstacle limiting the success of vascular intervention.1,2 Even though intracoronary stents are more effective than percutaneous transluminal coronary angioplasty alone in decreasing restenosis, in up to 35% of cases, in-stent restenosis occurs between 6 and 9 months.3–5 Furthermore, the incidence of clinical restenosis in selected patient populations, such as diabetics and those with complex lesions, can exceed 50%, significantly limiting the success of this modality.
As part of the response to injury, vascular smooth muscle cells (VSMCs) migrate from the media into the lumen of the vessel, where they proliferate and synthesize cytokines, which they respond to in an autocrine fashion, sustaining the progression of intimal hyperplasia.6,7 VSMC migration, proliferation, and matrix deposition are responsible for most of the obliterative arterial intimal thickening present in mechanically induced intimal hyperplasia and cardiac allograft vasculopathy, and may be the most critical cellular events in neointima development.6–8 The deleterious effects of proinflammatory cytokines on VSMC pathophysiological characteristics and development of many vascular diseases, ranging from atherosclerosis to transplant vasculopathy, have been well documented. A gap in our knowledge remains concerning the role of anti-inflammatory cytokines in vascular biology, particularly with respect to direct effects of these cytokines on VSMC pathophysiological characteristics. Most of the emphasis on secretion of inflammatory mediators has understandably been placed on leukocytes. The role of nonimmune cells in this process is poorly understood, but nevertheless intriguing, especially considering VSMC phenotypic plasticity. Identification of anti-inflammatory factors, which also inhibit the VSMC response to injury, is of obvious clinical importance.
IL-19 was first described in 2001.9 The first known IL-10–related cytokines were incorporated into the IL-10 family in 2001 without any knowledge of their biological functions,10 and IL-19 is now considered to be in a subfamily that includes IL-19, IL-20, and IL-24. IL-19 is functionally distinct from these subfamily members and IL-10.11,12 We have previously described several studies that point to an anti-inflammatory role of IL-19 outside the immune system, with direct suppressive effects on VSMCs in particular.13–15
We have shown that adenoviral delivery of IL-19 reduces intimal hyperplasia in the rat balloon angioplasty model. Although exogenously delivered IL-19 is protective in vascular injuries, no studies have taken advantage of genetically modified mice to characterize a precise cause-and-effect role of this interleukin in the VSMC response to injury.13 In this study, we tested the hypothesis that IL-19 expression would regulate development of intimal hyperplasia in a murine common carotid artery ligation model of vascular restenosis. This study reports that targeted disruption of the IL-19 gene exacerbates intimal hyperplasia, which can be reversed by addition of IL-19. Addition of exogenous IL-19 can reduce intimal hyperplasia, and we suggest several cellular and molecular mechanisms that may drive these effects. This suggests that, in addition to immune-modulating effects, IL-19 can impart a suppressive, anti-inflammatory, type 2 helper T-cell (Th2)–like phenotype to VSMCs.
Materials and Methods
Animals
IL-19 knockout (KO) mice were generated as described.16,17 Homozygous il-19−/− mice were identified by genotyping of tail DNA by PCR using specific primers and crossed into the C57Bl/6 background.17 Wild-type (WT) FVB mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Age- and sex-matched male and female littermates were used for these studies. The partial ligation model of injury was performed as we described.18 Briefly, mice were anesthetized by injection of ketamine and xylazine. The left common carotid artery was dissected and ligated near the bifurcation. After 28 days, mice were euthanized and tissue was prepared for immunohistochemistry (IHC) and morphological analysis. Severity of hyperplasia is known to be strain dependent; the FVB lineage develops a robust, and the C57/B6 develops a more limited, intimal hyperplasia in response to carotid ligation.19,20 Some mice were injected i.p. with 10 ng/g per day murine recombinant IL-19 (rIL-19; eBioscience, San Diego, CA) or an equivalent volume of PBS 5 days per week for the duration of the study, as we described.21 All animal procedures followed protocols approved by the Temple University Institutional Animal Care and Use Committee (Philadelphia, PA).
IHC and Quantitative Morphological Characteristics
Digitized images of H&E-stained carotid artery cross sections were measured and averaged from at least three representative stained tissue sections (5 μm thick) at least 75 to 100 μm apart per carotid artery using Image Pro Plus (Media Cybernetics, Rockville, MD), as we have described.14,18 At least six mice per group were used for morphological and IHC analyses. The circumference of the lumen, the area-encircled internal elastic lamina (IEL), and the external elastic lamina were quantitated. The medial area was calculated by subtracting the area defined by the IEL from the area defined by the external elastic lamina, and the intimal area was calculated as the difference between the area inside the IEL and the luminal area. Tissue fixation, processing, IL-19, bromodeoxyuridine (BrdU), SMCα actin, antibody, and IHC staining were performed as described.21 CD45 antibody was from Lab Vision, Inc (Fremont, CA). Immunofluorescence was performed as described.21 Briefly, primary antibody incubation was followed by 30-minute incubation with secondary antibody conjugated to Alexa Fluor 568 (red) and Alexa Fluor 488 (green) (Molecular Probes, Inc., Eugene, OR). BrdU staining was quantitated as percentage positive of all cells. VSMC proliferation was counted as the number of BrdU-positive cells that also stained positive for SMCα actin.
VSMC Culture, Proliferation, Migration, and Adhesion Assay
Abdominal aortas from WT and IL-19 KO mice were excised, the endothelial layer was removed, and VSMCs were isolated as described.18 VSMCs were cultured in Dulbecco's modified Eagle's medium supplemented with 15% fetal calf serum (FCS). Greater than 95% of isolated cells were SMC actin positive, and VSMCs from passage 3 to 5 were used. Two different proliferation assays were performed. Briefly, equal numbers of VSMCs were seeded into 24-well plates at a density of 5000 cells/mL, in the presence or absence of 100 ng/mL murine rIL-19 (eBioscience). Medium was changed on the fourth day, and after 1, 4, and 7 days, cells were trypsinized and counted in triplicate using a standard hemocytometer, as described.14,18 Proliferation was also assayed by flow cytometry using the CellTrace CFSE Cell Proliferation Kit (Invitrogen, Grand Island, NY), according to the manufacturer's directions.22 Carboxyfluorescein diacetate succinimyl ester incorporation was calculated by the FloJo proliferation platform software version 2.0. Two different migration assays were performed.15 Briefly, 6.5-mm-diameter transwell Boyden chamber plates (Costar, Grand Island, NY) with an 8-μm polycarbonate membrane pore size were seeded with VSMCs in medium containing 0.5% FCS. Platelet-derived growth factor (PDGF) at 40 ng/mL was placed in the lower chamber, and cells were incubated for 2 hours at 37°C, at which time cells were fixed and stained. VSMCs that migrated to the lower surface of the membrane were quantitated by counting four high-powered fields (HPFs) per membrane. For directional migration, scratch wounding was performed, as previously described, with wound area quantitated by image analysis.15 Briefly, WT or KO VSMCs were grown to 80% confluence in a four-chamber slide in growth medium. A 2-mm uniform scratch was made using a cell scraper, which was cut to 2 mm. For some samples, the medium was replaced with medium plus IL-19. The chamber slide was then returned back to a cell culture incubator for 0 to 24 hours. The slide was then fixed with paraformaldehyde and stained with H&E. Images were captured at ×4 magnification. Experiments were performed in triplicate from VSMCs isolated from three different KO and WT mice. For adhesion, WT or IL-19 KO VSMCs were cultured on glass slides and stimulated with tumor necrosis factor (TNF) α for 16 hours; then, lBCECF/AM-labeled THP1 monocytes were incubated with the monolayers, and adherent cells were quantitated by microscopy, as we described.23 In some experiments, 10 μg/mL anti-intercellular adhesion molecule (ICAM) 1, vascular cell adhesion molecule (VCAM) 1, or IgG1 antibody was added to VSMC monolayers 1 hour before the addition of THP1 cells, as described.24
RNA Extraction and Quantitative RT-PCR
For quantification of gene expression, VSMCs were serum starved in 0.5% FCS for 48 hours, then stimulated with 10 ng/mL TNFα for the indicated times. RNA from cultured VSMCs was isolated and reverse transcribed into cDNA, as we have described, and target genes were amplified using an Eppendorf Realplex4 Mastercycler (New York, NY).13 Multiple mRNAs (CT values) were quantitated simultaneously by the Eppendorf software version 4.0. Primer pairs were purchased from Integrated DNA Technologies (Coralville, IA), and SYBR Green was used for detection. The following primer pairs were used: mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-GCAAGGACACTGAGCAAGAG-3′ (forward) and 5′-GGGTCTGGGATGGAAATTGT-3′ (reverse); mouse monocyte chemoattractant protein (MCP) 1, 5′-TTAAAAACCTGGATCGGAACCAA-3′ (forward) and 5′-GCATTAGCTTCAGATTTACGGGT-3′ (reverse); mouse IL-1β, 5′-CTAATAGGCTCATCTGGGATCC-3′ (forward) and 5′-GGTCCGTCAACTTCAAAGAAC-3′ (reverse); and mouse TNFα, 5′-CTTCTGTCTACTGAACTTCGGG-3′ (forward) and 5′-CAGGCTTGTCACTCGAATTTTG-3′ (reverse). For VSMC phenotype analysis, equal numbers of WT or IL-19−/− VSMCs were serum starved in 0.5% FCS for 48 hours, then stimulated with 15% FCS for 6 or 24 hours. RNA was reverse transcribed, and target genes were amplified and quantitated by Applied Biosystems 7500 software (Foster City, CA), as described.25 Probe sets for murine SM22α (reference Mm00441660-m1), murine SMCα-actin (reference Mm01546133-m1), calponin (reference Mm00487032-m1) were purchased from Applied Biosystems.
Western Blot Analysis
Protein extracts from cultured VSMCs were made as described,13,14,18 separated by SDS-PAGE, transferred to a nitrocellulose membrane, and incubated with a 1:4000 dilution of primary antibody [IL-19, heme oxygenase-1 (HO-1), ICAM1, VCAM1, TNFα, MCP-1, and IL-1β; Santa Cruz, Inc., Dallas, TX] and a 1:6000 dilution of secondary antibody. Equal loading of protein extracts on gels was verified by Ponceau S staining of the membrane, and blotting with the housekeeping protein anti-GAPDH (1:7000 dilution; Biogenesis, Inc., Poole, UK), and reactive proteins were visualized using enhanced chemiluminescence. The intensity of each band was quantitated using ImageJ software version 1.48 (NIH, Bethesda, MD).
Statistical Analysis
Results are expressed as means ± SEM. Differences between groups were evaluated with the use of analysis of variance to evaluate differences between individual mean values or by t tests, where appropriate. Differences were considered significant at P < 0.05.
Results
Lack of IL-19 Exacerbates Intimal Hyperplasia
To define a fundamental role for IL-19 in neointimal hyperplasia, we used IL-19 KO mice. The left common carotid artery of age- and sex-matched IL-19−/− and WT littermates in the C57Bl/6 background was subject to carotid artery ligation near the carotid bifurcation. After 28 days, arteries were recovered and divided into sections, and vascular compartments were quantitated. The neointima/intima (N/I) ratio was significantly less in WT mice (0.50 ± 0.12) compared with IL-19 KO mice (0.83 ± 0.09) (n = 9 for WT and 13 for IL-19−/−; P < 0.05) (Figure 1A). Similarly, the percentage stenosis was also significantly less in WT (49.04% ± 8.1%) compared with IL-19 KO (74.59% ± 4.55%) mice (P < 0.01). The robust response to ligation injury in the IL-19 KO mice is noteworthy considering the C57B/6 strain is particularly unresponsive to this type of injury.18,19 This suggests that lack of IL-19 by targeted disruption exacerbates intimal hyperplasia.
Figure 1.
IL-19 regulates neointimal hyperplasia. A:IL-19−/− mice demonstrate exacerbated response to ligation injury compared with WT age- and sex-matched littermates. Quantitative morphological analysis determines a significantly increased N/I ratio in IL-19−/− (n = 13) compared with WT (n = 9) mice. B: Injection of recombinant IL-19 into IL-19−/− mice can rescue the IL-19−/− response to injury phenotype. Quantitative morphological analysis determined significantly decreased N/I ratio in mice injected with 10 ng/g per day rIL-19 i.p. (n = 11) or PBS (n = 10) 5 days per week for 28 days. C: IL-19 reduces intimal hyperplasia in FVB WT mice. Injection of recombinant IL-19 significantly decreased the N/I ratio in mice injected with 10 ng/g per day rIL-19 i.p. (n = 11) or PBS (n = 10) 5 days per week for 28 days (P < 0.01). Representative photomicrographs are stained with H&E. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Original magnification, ×200.
Injection of Recombinant IL-19 into IL-19−/− Mice Reduces/Rescues Intimal Hyperplasia
To further confirm the role of IL-19 in regulation of neointimal hyperplasia, IL-19 KO mice were subject to carotid artery ligation. Some mice were injected i.p. with 10 ng/g per day rIL-19 5 days per week, others with an equivalent volume of PBS as controls, and vascular compartments were quantitated after 28 days. IL-19 KO mice injected with IL-19 had a significantly lower N/I ratio (0.40 ± 0.11) compared with PBS control mice (1.20 ± 0.37) (n = 11 for rIL-19 and 10 for PBS; P < 0.05) (Figure 1B). Likewise, percentage stenosis was significantly less in IL-19–injected mice (38.36% ± 5.86%) compared with PBS control mice (60.60% ± 5.96%) (P = 0.01). Because addition of IL-19 to IL-19−/− mice can reduce or rescue intimal hyperplasia, these data reinforce the hypothesis that IL-19 can regulate carotid artery ligation–induced carotid artery intimal hyperplasia.
Injection of Recombinant IL-19 Reduces Intimal Hyperplasia in WT Mice
We hypothesized that IL-19 would decrease intimal hyperplasia subsequent to carotid artery ligation. FVB WT mice were used for these studies because it has been shown that this particular strain has a robust response to this type of arterial injury.19,20 One cohort of mice was injected i.p. with 10 ng/g per day of rIL-19 5 days per week, and the other with an equivalent volume of PBS as controls. Quantitative morphological analysis determined a significant decrease in N/I ratio in IL-19–injected mice (0.87 ± 0.20) compared with PBS controls (2.14 ± 0.33) (n = 10 for rIL-19 and 9 for PBS; P < 0.01) (Figure 1C). The percentage stenosis was significantly decreased in IL-19–injected mice (62.00% ± 4.9%) compared with PBS controls (85.01% ± 3.6%) (P = 0.001).
IL-19 Polarization of Adaptive Immunity
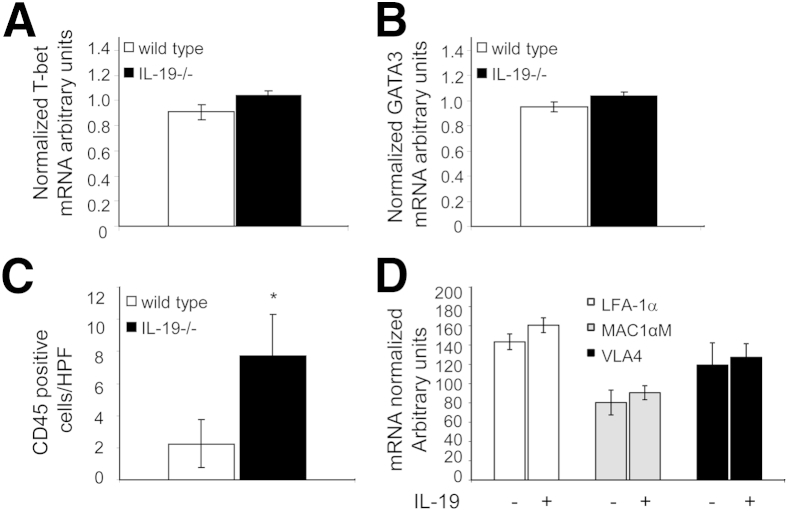
IL-19 is a putative Th2 IL, and considering immune cells participate in vascular injury, we hypothesized that polarization of adaptive immunity to the Th2 phenotype might account for decreased intimal hyperplasia observed in IL-19–treated mice.26 The global immunological status of these mice was determined by quantitation of type 1 helper T-cell (Th1) and Th2 lymphocyte marker expression in splenocytes removed immediately from mice at the termination of the study. Quantitative RT-PCR of the Th1 marker gene T-bet expression in WT C57BL/6 mice was not significantly different compared with IL-19−/− mice (0.91 ± 0.06 versus 1.04 ± 0.04) (Figure 2A). Similarly, mRNA levels of the Th2 marker GATA3 were not significantly different between the two groups of mice (0.95 ± 04 versus 1.04 ± 0.03) (Figure 2B). This suggests that IL-19 KO mice are not polarized toward a proinflammatory, Th1-biased immune response, suggesting that this is not a major mechanism for reduced intimal hyperplasia in IL-19–treated mice.
Figure 2.
IL-19−/− mice are not Th1 polarized. Spleen removed from age-matched WT and IL-19−/− littermates 28 days after carotid ligation; mRNA was extracted and reverse transcribed, and amplified using the primer pairs listed in Materials and Methods. No significant difference was noted in mRNA abundance for T-bet (A) or GATA3 (B) (n = 8 spleens in each group). C: Lack of IL-19 results in increased leukocyte infiltrate in ligated arteries. Serial sections were immunostained using anti-CD45 antibody. Positive cells were counted from at least four stained tissue sections from four different mice, and values expressed are positive cells per HPF. D: IL-19 does not increase CAM expression in monocytes. THP1 cells were treated with rIL-19, and CAM mRNA expression was quantitated by quantitative RT-PCR. There is no significant difference in mRNA expression between IL-19 and untreated THP1 cells. ∗P < 0.05.
IL-19 Decreases Inflammatory Cell Infiltrate in Neointima
To characterize inflammatory cell infiltrate in neointima from these mice, serial sections were immunostained with the pan-leukocyte marker CD45. Significantly more CD45-positive cells were observed in IL-19 KO mice compared with WT mice (7.75 ± 2.06 versus 2.25 ± 1.5 cells per HPF; P = 0.05) (Figure 2C). These differences in leukocyte infiltrate in ligated arteries were unexpected, considering that there were no global differences in adaptive immunity in WT versus IL-19−/−. To determine whether IL-19 could affect leukocyte adhesiveness, we treated the human monocyte line THP-1 with IL-19, and quantitated expression of leukocyte adhesion molecules. IL-19 treatment had no effect on abundance of mRNA for LFA-1α, MAC1αM, or VLA4, all counterreceptors for endothelial adhesion molecules (Figure 2D). Together, these data suggested increased localized inflammation at the site of injury in these mice, and perhaps direct anti-inflammatory effects of IL-19 on VSMCs. In subsequent experiments, we turned our attention to direct effects of IL-19 on VSMCs.
IL-19 Is Expressed in Stimulated Murine VSMCs
VSMCs in the restenotic lesion synthesize and respond to numerous cytokines, which initiates and maintains VSMC proliferation and migration. WT VSMCs were challenged with various stimuli, and expression of IL-19 in these cells was assessed by using Western blot analysis. Although most stimuli increase IL-19 expression higher than basal levels, significant increases were only noted with TNFα and T-cell conditioned medium, which is a mix of several inflammatory cytokines (Figure 3A). This suggests IL-19 expression is responsive to inflammatory cytokines and may participate in intimal hyperplasia subsequent to vascular injury.
Figure 3.
A: IL-19 is expressed in murine VSMCs. Representative immunoblot of mouse VSMCs explanted from abdominal aortas, which were serum starved, then stimulated with the factors shown. Expression was quantitated by densitometry and normalized to GAPDH expression from three identical experiments. B: IL-19 regulates VSMC proliferation. Equal numbers of age-matched WT or IL-19−/− VSMCs were counted at the indicated days after seeding. rIL-19 (100 ng/mL) was added to some samples. Addition of rIL-19 significantly reduced proliferation of IL-19−/− VSMCs. C: Proliferation assayed by fluorescent label flow cytometry using the CellTrace CFSE Cell Proliferation Kit, with CFSE incorporation calculated by the proliferation platform of the FloJo software program. D: IL-19 increases proliferation in vivo. BrdU was injected into ligated WT or IL-19−/− mice. Mice were sacrificed 14 days after ligation, and BrdU incorporation was detected by anti-BrdU antibody. Positive cells were counted from at least four stained tissue sections from four different mice, and values expressed are positive cells per HPF. E: IL-19 regulates VSMC proliferation in vivo. Representative photomicrograph of BrdU/SMCα actin dual IHC. Arrows indicate examples of dual staining. Values are expressed as number of BrdU-positive cells that also stained positive for SMCα actin per HPF. ∗P < 0.05, ∗∗P < 0.01. TCM, T-cell–conditioned media.
IL-19−/− VSMCs Proliferate More Rapidly than WT VSMCs
VSMCs are the major effector cell involved in the development of intimal hyperplasia. Three different, but complementary, approaches were used to determine IL-19 effects on VSMC proliferation. First, VSMC cultures were established from explants from aortas from age-matched WT or IL-19−/− mice, and equal numbers were seeded into 24-well plates and grown in Dulbecco's modified Eagle's medium. Some were treated with IL-19. At 4 and 7 days, cells were recovered and counted. VSMCs isolated from IL-19 KO mice proliferate more rapidly than do WT VSMCs at both 4 and 7 days after seeding (24.2 ± 3.1 × 104 versus 34.2 ± 1.7 × 104 cells per mL for WT versus IL-19−/−; P < 0.01, for 7 days) (Figure 3B), which could be significantly inhibited by the addition of recombinant IL-19 to cultures (34.2 ± 1.7 × 104 versus 25.07 ± 2.0 × 104 cells per mL for IL-19−/− and IL-19−/− VSMCs plus 100 ng/mL rIL-19; P < 0.05). Addition of recombinant IL-19 could also reduce proliferation of WT VSMCs (24.2 ± 3.1 × 104 versus 19.07 ± 1.6 × 104 cells per mL), but not significantly (P = 0.20). In a second assay, VSMCs were fluorescently labeled with CFSE, then stimulated with 10% FCS for 72 hours. Fluorescent label incorporation was quantitated by flow cytometry. IL-19−/− VSMCs proliferate significantly more rapidly than do WT VSMCs [3461 ± 228 versus 2528 ± 110 (P < 0.05), for IL-19−/− and WT VSMCs, respectively]. More important, addition of rIL-19 to WT cells also significantly decreases their proliferation (P < 0.05).
To determine whether IL-19 regulated proliferation in vivo, in a third experiment, WT and IL-19−/− mice were injected with the nucleotide analogue BrdU. Fourteen days after ligation injury, carotid arteries were recovered and immunostained, and positive cells were quantitated. IL-19−/− mice had a significantly increased number of proliferating cells in the neointima compared with WT mice (20.55% ± 12% versus 55.78% ± 4.2%, for WT and IL-19−/−, respectively; P < 0.01) (Figure 3D). To determine the number of BrdU-positive cells that were VSMCs, sections were costained with anti-BrdU and SMCα actin antibody. Although IL-19−/− mice had a significantly increased number of proliferating cells, there were also significantly more proliferating cells that were SMCα actin positive in IL-19−/− compared with WT mice (5.55 ± 2.6 versus 15.78 ± 4.2, per HPF, for WT and IL-19−/−, respectively; P < 0.05) (Figure 3E).
VSMCs are plastic and can assume a synthetic phenotype in response to injury and stimulation. WT and IL-19−/− VSMCs were serum starved, then stimulated with FCS. Expression of several SMC phenotype markers was determined by quantitative RT-PCR. IL-19−/− VSMCs do not express significantly different amounts of SMC phenotype markers compared with WT VSMCs (Figure 4A). Nevertheless, both in vivo and ex vivo experiments point to direct anti-proliferative effects of IL-19 on proliferation of VSMC.
Figure 4.
IL-19 regulates VSMC migration, but not VSMC phenotype. A: IL-19 does not modify VSMC phenotype. WT or IL-19−/− VSMCs were serum starved, then stimulated with FCS. SMC phenotype marker expression, quantitated by quantitative RT-PCR, indicates no significant difference between WT and IL-19−/− VSMCs for any marker. B: WT or IL-19−/− VSMCs were seeded onto the top chamber of a modified Boyden chamber in medium containing 0.2% bovine serum albumin, with or without 40 ng/mL PDGF as a chemoattractant in the lower chamber. Values are means from three experiments performed in triplicate from three independent groups of VSMCs. IL-19 reduces VSMC wound healing. C: Scratch wound migration of IL-19−/− VSMCs. Cells were stained with hematoxylin. Dashed lines define original scratched area. Photomicrograph is representative of three independent experiments. Quantitative analysis of scratch wound area is from three different groups of treated VSMCs. ∗P < 0.05, ∗∗∗P < 0.001 versus control. Original magnification, ×40.
IL-19−/− VSMCs Migrate More Rapidly than WT VSMCs
Medial to intimal migration of VSMCs is an important cellular event in the development of neointimal hyperplasia. To determine differences in migration between WT and IL-19−/− VSMCs, cultures were established from explants from aortas from age-matched WT or IL-19−/− mice. VSMCs were seeded into Boyden chambers, and differences in chemokinesis were quantitated by counting cells that migrated in response to PDGF. A significant difference in migration between IL-19−/− VSMCs and WT VSMCs (49.2 ± 5.1 versus 33.3 ± 1.3 VSMCs per HPF; P < 0.001) was observed (Figure 4B). Interestingly, IL-19−/− VSMCs migrate more rapidly than WT, even in the absence of chemotactic stimuli (39.2 ± 3.2 versus 8.6 ± 0.95 VSMCs per HPF; P < 0.001). In a second assay, scratch wounding of VSMC monolayers was performed. VSMCs from IL-19−/− mice migrate into the wound area significantly more rapidly than do WT VSMCs (568 ± 69 versus 243 ± 49 for wound area of WT and KO, respectively; P < 0.05) (Figure 4C). Together, this indicates that IL-19 expression plays a role in regulation of VSMC migration.
Lack of IL-19 Increases Inflammatory Gene Expression in VSMCs
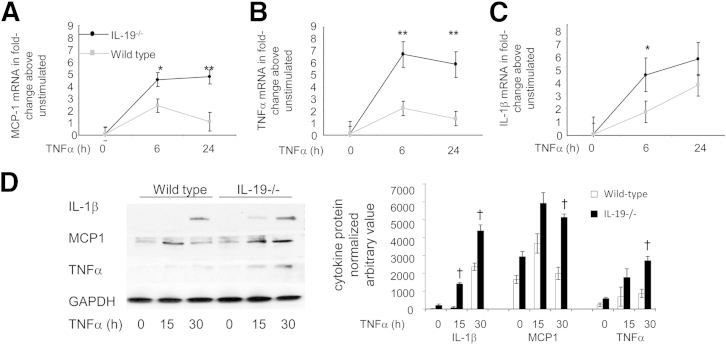
Increased inflammatory cell infiltrate without immune system polarization suggested direct anti-inflammatory effects of IL-19 on VSMCs. We tested if VSMCs from IL-19 KO mice responded to inflammatory stimuli more robustly than WT VSMCs. Aortic VSMCs were isolated from age- and sex-matched littermates, cultured, and then challenged with the proinflammatory cytokine, TNFα. Inflammatory cytokine mRNA expression was determined by quantitative RT-PCR. Expression levels of TNFα, IL-1β, and MCP-1, all potent proinflammatory and chemotactic cytokines, were significantly increased in VSMCs explanted from IL-19 KO mice compared with VSMCs from WT mice (Figure 5, A–C). Similarly, each cytokine protein tested from IL-19 KO mice was significantly increased at 30 hours after TNFα stimulation compared with WT VSMCs (Figure 5D). Together, these data suggest an enhanced response of IL-19−/− VSMCs to inflammatory stimuli.
Figure 5.
Increased inflammatory gene expression in IL-19−/− VSMCs. VSMCs from WT and IL-19−/− mice were serum starved for 48 hours, then stimulated with 10 ng/mL TNFα for the times indicated, at which time total RNA was reverse transcribed and target mRNA was quantitated by quantitative RT-PCR. MCP-1 (A), TNFα (B), and IL-1β (C) mRNA was normalized to GAPDH. Differences in WT versus IL-19−/− VSMCs are significant, where indicated. D: Representative immunoblot and densitometric quantification of cytokine protein expression in WT and IL-19 VSMCs. ∗P < 0.05, ∗∗P < 0.01 (n = 3; B), for all targets at that time, unless otherwise noted; †P < 0.05, quantitated from three different Western blot analyses of IL-19−/− versus WT control VSMCs (D).
IL-19 Decreases CAM Expression in VSMCs
Leukocyte entrapment in neointimal VSMCs contributes to development of restenosis.27 We previously determined that IL-19 decreases CAM expression in human endothelial cells (ECs).23 To determine whether IL-19 affected leukocyte adhesion to VSMCs, several experiments were performed. First, VSMCs isolated from WT and IL-19−/− aortas were challenged with TNFα, and ICAM1 and VCAM1 expression levels were determined by using Western blot analysis. A significant increase in both CAMs in IL-19−/− VSMCs compared with WT VSMCs was observed (Figure 6A). The second experiment used a VSMC monolayer adhesion assay. WT or IL-19 KO VSMCs were cultured on glass slides and stimulated with TNFα for 16 hours, then labeled THP1 monocytes were incubated with the monolayers, and adherent cells were quantitated by microscopy. Significantly more monocytes adhered to IL-19−/− VSMCs compared with WT VSMCs (89.1 ± 15.3 versus 47.3 ± 14.1 adherent cells per HPF for KO and WT, respectively; P < 0.05) (Figure 6B). In a third experiment, anti-ICAM1 or VCAM1 antibody was added to the adhesion assay using IL-19−/− VSMCs. Anti-ICAM1 antibody significantly inhibits THP1-VSMC adherence (99.1 ± 10.1 versus 57.9 ± 14.3 adherent cells per HPF for control and ICAM1 antibody, respectively; P < 0.05) (Figure 6C). Anti-VCAM1 antibody decreased adhesion, but not significantly (P = 0.068). In a fourth experiment, primary human VSMCs were treated with 100 ng/mL recombinant IL-19. IL-19 treatment significantly decreased CAMs abundance in human VSMCs (Figure 6D). Together, these data indicate that IL-19−/− VSMCs are more adhesive than WT, and suggest that IL-19 plays a role in regulation of adhesion molecule expression and leukocyte adhesion.
Figure 6.
IL-19 reduces ICAM-1 and VCAM-1 expression, and IL-19 reduces leukocyte–smooth muscle cell interaction. A: ICAM-1 and VCAM-1 expression is significantly increased in IL-19−/− VSMCs. WT or IL-19−/− VSMCs were serum starved, then stimulated with TNFα for the times indicated. Extracts were blotted with the indicated antibodies, and protein expression was quantitated by densitometry and normalized to GAPDH expression from three experiments. A representative of three Western blot analyses is shown. B: Monocyte adhesion is significantly increased in IL-19−/− VSMCs. VSMCs were grown on glass cover slides and stimulated with TNFα for 16 hours; labeled THP1 monocytes were incubated with the monolayers, and adherent cells were quantitated by counting per HPF. C: Enhanced leukocyte-VSMC adhesion in IL-19−/− VSMCs is reduced by anti-CAM antibody. Anti-ICAM1, VCAM1, or IgG1 antibody (10 mg/mL) was added to VSMC monolayers 1 hour before addition of THP1 cells. D: IL-19 significantly reduces ICAM-1 and VCAM-1 protein abundance in primary human VSMCs. VSMCs were pretreated with IL-19, then stimulated with TNF-α for 24 hours. Western blot analysis was quantitated by densitometry from at least three experiments, ∗P < 0.05 for times indicated, and is representative of at least three experiments. Original magnification, ×200 (B).
Discussion
To our knowledge, this is the first study to use IL-19−/− mice to investigate a role for IL-19 in the development of intimal hyperplasia indicative of vascular restenosis. The major finding is the identification of IL-19 as an integral regulator of the VSMC response to carotid artery ligation injury. Lack of IL-19 leads to increased intimal hyperplasia, which is noteworthy considering that, compared with other strains, C57Bl/6 mice generally demonstrate a more limited response to ligation injury relative to other strains.19,20 More important, IL-19 KO mice injected with rIL-19 demonstrated significantly less intimal hyperplasia compared with control mice injected with PBS. This rescue effect established the specificity of effect of IL-19 in the development of intimal hyperplasia. Addition of recombinant IL-19 to WT FVB-strain mice significantly reduces intimal hyperplasia. The observation that addition of IL-19 was effective in reducing intimal hyperplasia is particularly striking considering that relative to other strains, FVB mice respond to ligation injury in a robust manner.19,20 IL-19 expression can be induced by inflammatory factors in cultured VSMCs. Because lack of IL-19 is deleterious, and exogenous addition of IL-19 is protective, these in vivo experiments support the hypothesis that IL-19 expression in injured arteries may be a compensatory, counterregulatory mechanism to reduce the vascular response to injury.
Little pertinent literature exists on other Th2 ILs in the development of restenosis, and data are somewhat contradictory. Although considered to be anti-atherosclerotic, studies focusing on a role for IL-10 in the development of intimal hyperplasia subsequent to arterial injury are inconclusive. For example, IL-10−/− mice subject to carotid artery ligation demonstrated no significant differences in neointimal hyperplasia compared with WT controls.28 In further contrast to our study, WT mice injected daily with 1 μg rIL-10, which is much more than the 10 ng/g per day IL-19 used in this study, also demonstrated neointimal hyperplasia to the same degree as PBS controls. This same group reported that mice injected with adenoviral IL-10 developed intimal hyperplasia to the same degree as control mice receiving empty adenovirus. On the other hand, hypercholesterolemic rabbits treated with 50 μg rIL-10 showed significantly less neointimal hyperplasia and inflammatory cell infiltrate after balloon angioplasty.29 The differences noted between IL-19 and IL-10 efficacy in response to vascular injury may reflect differences in the etiology of atherosclerosis and ligation-induced neointimal hyperplasia.
In humans, a significant decrease in Th2-dependent IL concentrations was observed after percutaneous coronary angioplasty, suggesting an acute Th1-biased environment immediately after the procedure, consistent with the response to injury hypothesis.30 In a previous study, we reported that similar i.p. injection of rIL-19 into LDLR−/− mice for 12 weeks could polarize the immune response to the Th2 phenotype.21 Similarly, another study reported increased proinflammatory responses in IL-19−/− mice, but in our hands, the IL-19−/− mouse did not demonstrate statistically significant Th1 polarization. This could reflect differences in the type of injury or inflammatory insult, or that 28 days' injection is not sufficient time for IL-19 to polarize T-cell phenotype. Because IL-19 polarization of adaptive immunity appeared not to be a major mechanism of protection, along with the observation that IL-19 did not decrease expression of leukocyte adhesion molecules, we focused our attention to direct effects of IL-19 on VSMCs.
As part of the vascular response to injury, activated VSMCs proliferate and synthesize cytokines to which they respond to in an autocrine manner, sustaining the progression of intimal hyperplasia.6,7 The addition of recombinant IL-19 to cultured human VSMCs is anti-proliferative for human VSMCs, and this study extends that finding both in cultured VSMCs and in vivo.13 Consistent with a counterregulatory response mechanism, lack of IL-19 results in an increased rate of VSMC proliferation. More important, similar to the in vivo rescue experiment, increased proliferation of IL-19−/− VSMCs can be significantly reduced by the addition of IL-19 to culture media. Two studies have reported anti-proliferative effects of IL-10 on VSMCs, where it was proposed that the observed growth-inhibitory effects were due to inhibition of NF-κB. In previous studies, we did not observe any inhibition of NF-κB activation in cultured VSMCs, an important distinction that contrasts the anti-proliferative mechanism of IL-19 from IL-10.13,14
Migration of normally quiescent VSMCs from the media into the lumen of the vessel is a major cellular event in the etiology of vascular restenosis. We have previously reported that addition of recombinant IL-19 to cultured human VSMCs reduces their migration.15 This study using IL-19−/− VSMCs in both Boyden chambers and the scratch wound assay extends that finding and is particularly interesting in that it demonstrates that VSMCs lacking IL-19 can migrate more rapidly, even in the absence of PDGF, suggesting that IL-19 is an important mediator of VSMC motility.
Similar to many vascular interventional procedures in humans, ligation of the murine carotid artery elicits expression of growth factors and inflammatory cytokines from resident vascular cells and infiltrating inflammatory cells. This response to injury initiates and maintains VSMC proliferation and migration. We were surprised to find that there were significantly more inflammatory cells in ligated carotid arteries in IL-19−/− mice compared with WT mice, especially considering that IL-19 did not polarize adaptive immunity in these animals. Together with the potent suppressive effects of IL-19 on VSMCs, this suggested that local inflammation would be regulated by VSMCs themselves, which prompted us to determine whether lack of IL-19 resulted in an enhanced response of VSMCs to inflammatory stimuli. Consistent with a compensatory anti-inflammatory mechanism, IL-19−/− VSMCs expressed more IL-1β, TNFα, and MCP-1 mRNA. Expression of inflammatory genes by activated VSMCs could contribute to intimal hyperplasia in multiple ways. First, these cytokines are all mitogenic for VSMCs, and their expression could drive autocrine proliferation of VSMCs in the restenotic lesion. Second, chemokine expression by vascular cells participates in leukocyte recruitment to the atherosclerotic lesion. Enhanced expression of these cytokines may account for the greater leukocyte burden in carotid artery from IL-19−/− mice. Third, these chemokines could also contribute to autocrine migration of VSMCs into the developing neointima. In both ECs and VSMCs, adhesion molecule expression is driven by chemokine expression. Leukocyte entrapment in neointimal VSMCs contributes to development of restenosis; thus, a fourth mechanism is demonstrated by the finding that IL-19−/− VSMCs express more ICAM1 and VCAM1 compared with WT VSMCs. Addition of IL-19 can reduce expression of both ICAM1 and VCAM1 in human ECs.23 The importance of these adhesion molecules is illustrated by the finding that addition of anti-CAM antibody can reduce the enhanced adhesive properties of IL-19−/−. Although this does not imply that these are the only two CAMs that may play a role in adhesion, it does demonstrate that they are important in mediation of the enhanced adhesiveness displayed by IL-19−/− VSMCs. Consequently, increased cytokine expression in VSMCs could result in increased expression of adhesion molecules, which could explain the observed increases in leukocyte infiltrate in ligated arteries from IL-19 mice. The HO-1 is a cytoprotective protein shown to have anti-restenotic effects, and we have previously reported that IL-19 could induce HO-1 expression in primary human VSMCs.31 In this study, however, we observed no difference in HO-1 expression between WT and IL-19 VSMCs (data not shown).
In summary, IL-19 can reduce the vascular response to injury, primarily by attenuating VSMC migration, proliferation, and inflammatory gene expression. Expression of IL-19 by activated VSMCs may represent a compensatory, autoregulatory, autocrine, or paracrine counterregulatory feedback mechanism to promote resolution of the vascular response to injury by direct suppression of VSMC activation.
Footnotes
Supported by the National Heart, Lung, and Blood Institute/NIH grants HL090885 and HL115575 (M.V.A.), American Heart Association grant 13GRNT1685003 (M.V.A.), predoctoral fellowship 12PRE12040331 (S.E.), and postdoctoral fellowship 11POST7530001 (K.G.).
S.E. and K.G. contributed equally to this work.
Disclosures: None declared.
References
- 1.Welt F.G., Rogers C. Inflammation and restenosis in the stent era. Arterioscler Thromb Vasc Biol. 2002;22:1769–1776. doi: 10.1161/01.atv.0000037100.44766.5b. [DOI] [PubMed] [Google Scholar]
- 2.Dangas G.D., Claessen B.E., Caixeta A., Sanidas E.A., Mintz G.S., Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56:1897–1907. doi: 10.1016/j.jacc.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 3.El-Omar M.M., Dangas C., Iakovou I., Mehran R. Update on in-stent restenosis. Curr Interv Cardiol Rep. 2001;3:296–305. [PubMed] [Google Scholar]
- 4.Anis R.R., Karsch K.R., Oberhoff M. An update on clinical and pharmacological aspects of drugeluting stents. Cardiovasc Hematol Disord Drug Targets. 2006;6:245–255. doi: 10.2174/187152906779010755. [DOI] [PubMed] [Google Scholar]
- 5.Slavin L., Chhabra A., Tobis J.M. Drug-eluting stents: preventing restenosis. Cardiol Rev. 2007;15:1–12. doi: 10.1097/01.crd.0000200844.16899.fc. [DOI] [PubMed] [Google Scholar]
- 6.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 7.IP J., Fuster V., Badimon L., Badimon J., Taubman M., Chesebro J. Syndromes of accelerated atherosclerosis: role of vascular injury and smooth muscle cell proliferation. J Am Coll Cardiol. 1990;15:1667–1687. doi: 10.1016/0735-1097(90)92845-s. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz R.S., Murphy J.G., Edwards W.D., Camrud A.R., Vlietstra R.E., Holmes D.R. Restenosis after balloon angioplasty: a practical proliferative model in porcine coronary arteries. Circulation. 1990;82:2190–2200. doi: 10.1161/01.cir.82.6.2190. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher G., Dickensheets H., Eskdale J., Izotova L.S., Mirochnitchenko O.V., Peat J.D., Vazquez N., Pestka S., Donnelly R.P., Kotenko S.V. Cloning, expression and initial characterization of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10) Genes Immun. 2000;1:442–450. doi: 10.1038/sj.gene.6363714. [DOI] [PubMed] [Google Scholar]
- 10.Pestka S., Krause C.D., Sarkar D., Walter M.R., Shi Y., Fisher P.B. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 11.Oral H., Kotenko S., Yilmaz M., Mani O., Zumkehr J., Blaser K., Akdis C., Akdis M. Regulation of T cells and cytokines by the interleukin-10 (IL-10)-family cytokines IL-19, IL-20, IL-22, IL-24 and IL-26. Eur J Immunol. 2006;36:380–388. doi: 10.1002/eji.200425523. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher G. Interleukin-19: multiple roles in immune regulation and disease. Cytokine Growth Factor Rev. 2010;21:345–352. doi: 10.1016/j.cytogfr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Cuneo A.A., Herrick D., Autieri M.V. IL-19 reduces VSMC activation by regulation of mRNA regulatory factor HuR and reduction of mRNA stability. J Mol Cell Cardiol. 2010;49:647–654. doi: 10.1016/j.yjmcc.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian Y., Sommerville L.J., Cuneo A., Kelemen S.E., Autieri M.V. Expression and suppressive effects of interleukin-19 on vascular smooth muscle cell proliferation, signaling, and development of intimal hyperplasia. Am J Pathol. 2008;173:901–909. doi: 10.2353/ajpath.2008.080163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabunia K., Jain S., England R.N., Autieri M.V. The anti-inflammatory cytokine interleukin-19 inhibits smooth muscle cell migration and activation of cytoskeletal regulators of VSMC motility. Am J Physiol Cell Physiol. 2011;300:C896–C906. doi: 10.1152/ajpcell.00439.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valenzuela D.M., Murphy A.J., Frendewey D., Gale N.W., Economides A.N., Auerbach W., Poueymirou W.T., Adams N.C., Rojas J., Yasenchak J., Chernomorsky R., Boucher M., Elsasser A.L., Esau L., Zheng J., Griffiths J.A., Wang X., Su H., Xue Y., Dominguez M.G., Noguera I., Torres R., Macdonald L.E., Stewart A.F., DeChiara T.M., Yancopoulos G.D. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat Biotechnol. 2003;21:652–659. doi: 10.1038/nbt822. [DOI] [PubMed] [Google Scholar]
- 17.Azuma Y.T., Matsuo Y., Kuwamura M., Yancopoulos G.D., Valenzuela D.M., Murphy A.J., Nakajima H., Karow M., Takeuchi T. Interleukin-19 protects mice from innate-mediated colonic inflammation. Inflamm Bowel Dis. 2010;16:1017–1028. doi: 10.1002/ibd.21151. [DOI] [PubMed] [Google Scholar]
- 18.Sommerville L.J., Kelemen S.E., Autieri M.V. Increased smooth muscle cell activation and neointima formation in response to injury in AIF-1 transgenic mice. Arterioscler Thromb Vasc Biol. 2008;28:47–53. doi: 10.1161/ATVBAHA.107.156794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harmon K.J., Couper L.L., Lindner V. Strain-dependent vascular remodeling phenotypes in inbred mice. Am J Pathol. 2000;156:1741–1748. doi: 10.1016/S0002-9440(10)65045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhel D.G., Zhu B., Witte D.P., Hui D.Y. Distinction in genetic determinants for injury-induced neointimal hyperplasia and diet-induced atherosclerosis in inbred mice. Arterioscler Thromb Vasc Biol. 2002;22:955–960. doi: 10.1161/01.atv.0000017994.77066.75. [DOI] [PubMed] [Google Scholar]
- 21.Ellison S., Gabunia K., Kelemen S.E., England R.N., Scalia R., Richards J.M., Orr W., Traylor J.G., Jr., Rogers T., Cornwell W., Berglund L.M., Goncalves I., Gomez M.F., Autieri M.V. Attenuation of experimental atherosclerosis by interleukin-19. Arterioscler Thromb Vasc Biol. 2013;33:2316–2324. doi: 10.1161/ATVBAHA.113.301521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyons A.B. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Methods. 2000;243:147–154. doi: 10.1016/s0022-1759(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 23.England R.N., Preston K.J., Scalia R., Autieri M.V. Interleukin-19 decreases leukocyte-endothelial cell interactions by reduction in endothelial cell adhesion molecule mRNA stability. Am J Physiol Cell Physiol. 2013;305:C255–C265. doi: 10.1152/ajpcell.00069.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michigami T., Shimizu N., Williams P.J., Niewolna M., Dallas S.L., Mundy G.R., Yoneda T. Cell-cell contact between marrow stromal cells and myeloma cells via VCAM-1 and alpha(4)beta(1)-integrin enhances production of osteoclast-stimulating activity. Blood. 2000;96:1953–1960. [PubMed] [Google Scholar]
- 25.Ailawadi G., Moehle C.W., Pei H., Walton S.P., Yang Z., Kron I.L., Lau C.L., Owens G.K. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J Thorac Cardiovasc Surg. 2009;138:1392–1399. doi: 10.1016/j.jtcvs.2009.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallagher G., Eskdale J., Jordan W., Peat J., Campbell J., Boniotto M., Lennon G.P., Dickensheets H., Donnelly R.P. Human interleukin-19 and its receptor: a potential role in the induction of Th2 responses. Int Immunopharmacol. 2004;4:615–626. doi: 10.1016/j.intimp.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Zeiffer U., Schober A., Lietz M., Liehn E.A., Erl W., Emans N., Yan Z.Q., Weber C. Neointimal smooth muscle cells display a proinflammatory phenotype resulting in increased leukocyte recruitment mediated by P-selectin and chemokines. Circ Res. 2004;94:776–784. doi: 10.1161/01.RES.0000121105.72718.5C. [DOI] [PubMed] [Google Scholar]
- 28.Rectenwald J.E., Minter R.M., Moldawer L.L., Abouhamze Z., La Face D., Hutchins E., Huber T.S., Seeger J.M., Ozaki C.K. Interleukin-10 fails to modulate low shear stress-induced neointimal hyperplasia. J Surg Res. 2002;102:110–118. doi: 10.1006/jsre.2001.6283. [DOI] [PubMed] [Google Scholar]
- 29.Feldman L.J., Aguirre L., Ziol M., Bridou J.P., Nevo N., Michel J.B., Steg P.G. Interleukin-10 inhibits intimal hyperplasia after angioplasty or stent implantation in hypercholesterolemic rabbits. Circulation. 2000;101:908–916. doi: 10.1161/01.cir.101.8.908. [DOI] [PubMed] [Google Scholar]
- 30.Brunetti N.D., Pepe M., Munno I., Tiecco F., Quagliara D., De Gennaro L., Gaglione A., Di Biase M., Favale S. Th2-dependent cytokine release in patients treated with coronary angioplasty. Coron Artery Dis. 2008;19:133–137. doi: 10.1097/MCA.0b013e3282f3fbcb. [DOI] [PubMed] [Google Scholar]
- 31.Gabunia K., Ellison S.P., Singh H., Datta P., Kelemen S.E., Rizzo V., Autieri M.V. Interleukin-19 (IL-19) induces heme oxygenase-1 (HO-1) expression and decreases reactive oxygen species in human vascular smooth muscle cells. J Biol Chem. 2012;287:2477–2484. doi: 10.1074/jbc.M111.312470. [DOI] [PMC free article] [PubMed] [Google Scholar]