Abstract
Hyaluronan (HA), a major component of the extracellular matrix, is enriched in skin tissues, particularly the epidermis. HA binds to a ubiquitous, abundant, and functionally important family of cell surface receptors, CD44. This article reviews the current evidence for HA/CD44-mediated activation of RhoGTPase signaling and calcium mobilization, leading to the regulation of keratinocyte activities and various epidermal functions. It further discusses the role of HA-mediated CD44 interactions with unique downstream effectors, such as RhoGTPases (RhoA and Rac1), Rho-kinase, protein kinase-Nγ, and phosphoinositide-specific phospholipases (phospholipases Cε and Cγ1) in coordinating certain intracellular signaling pathways, such as calcium mobilization, phosphatidylinositol 3-kinase–AKT activation, cortactin-actin binding, and actin-associated cytoskeleton reorganization; generating the onset of important keratinocyte activities, such as cell adhesion, proliferation, migration, and differentiation; and performing epidermal functions. Topical application of selective HA fragments (large versus small HA) to the skin of wild-type mice (but not CD44 knockout mice) improves keratinocyte-associated epidermal functions and accelerates permeability barrier recovery and skin wound healing. Consequently, specific HA fragment (large versus small HA)–mediated signaling events (through the CD44 receptor) are required for keratinocyte activities, which offer new HA-based therapeutic options for patients experiencing epidermal dysfunction and skin damage as well as aging-related skin diseases, such as epidermal thinning (atrophy), permeability barrier dysfunction, and chronic nonhealing wounds.
CME Accreditation Statement: This activity (“ASIP 2014 AJP CME Program in Pathogenesis”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“ASIP 2014 AJP CME Program in Pathogenesis”) for a maximum of 48 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
In the epidermis, extracellular matrix (ECM) components form an integral part of the hemidesmosomes and mediate keratinocyte attachment to the underlying basement membrane. Matrix hyaluronan (HA) is the major glycosaminoglycan in the ECM of most mammalian tissues, including epidermis and dermis,1,2 and HA has been implicated in several skin epidermal functions.1,2 However, the cellular and molecular mechanism by which keratinocytes respond to HA is not fully understood.
HA and CD44 in Epidermal Keratinocytes
The predominant receptor for HA on the cell surface of keratinocytes is CD44.3 CD44 is encoded by a single gene that contains 19 exons.4 The most common form, CD44 standard form, contains exons 1 to 5 (N-terminal 150 amino acids), exons 15 and 16 (membrane proximal 85 amino acids), exon 17 (transmembrane domain), and a portion of exons 17 and 19 (cytoplasmic tail, 70 amino acids).4 Of the 19 exons, 12 can be alternatively spliced.4 Most often, the alternative splicing occurs between exons 5 and 15, leading to an insertion in tandem of one or more variant exons (exon 6 to exon 14; v1 to v10) within the membrane proximal region of the extracellular domain.4 For example, keratinocytes contain the additional exons v3 to v10 inserted into the CD44 standard form transcripts.5 This isoform has been designated as CD44v3-10 (or Epican).5 Several lines of evidence indicate that the HA-CD44 interaction selects unique downstream effectors and coordinates intracellular signaling pathways that initiate a concomitant onset of multiple cellular functions.6
Transgenic mice expressing an antisense construct to CD44 in their skin or traditional CD44 knockout mice show a significant reduction in endogenous HA on the keratinocyte cell surface and the loss of certain keratinocyte functions.7 Both CD44 and endogenous HA are expressed in the epidermal keratinocytes of CD44 wild-type mouse skin.7 No CD44 and little endogenous HA can be detected in the epidermal keratinocytes of CD44 knockdown or CD44 knockout mouse skin.7 Furthermore, several keratinocyte differentiation markers, such as involucrin and filaggrin, appear to be significantly reduced in the skin of CD44 knockdown or CD44 knockout mice, compared with CD44 wild-type mice.7 These findings suggest that both HA and CD44 play an important role in normal epidermal physiological and keratinocyte functions (eg, differentiation). The CD44 deficiency is accompanied by a reduction in HA staining in CD44 knockout mouse skin and marked alterations in keratinocyte barrier function, proliferation, differentiation, and lipid synthesis, resulting in altered barrier function.7 Down-regulation of CD44 in cultured keratinocytes (using CD44 siRNA) also significantly inhibits HA-mediated keratinocyte differentiation and lipid synthesis.7 These observations indicate the importance of both CD44 and HA for several key epidermal keratinocyte functions.
HA/CD44-Mediated RhoA and Rac1 Signaling in Keratinocytes
Members of the Rho subclass of the Ras superfamily [small-molecular-weight GTPases (eg, RhoA, Rac1, and Cdc42)] act as molecular switches that alternate between GTP- and GDP-bound states. The activated GTP-bound enzymes preferentially interact with downstream effector molecules that modulate effector activities.8 For example, activation of RhoA and Rac1 signaling has been shown to be involved in cell growth, survival, cytoskeleton-associated migration, and cell-cell adhesion, as well as keratinocyte differentiation.9–11
RhoA-Activated ROK Signaling Events
Previous work has indicated that HA promotes the interaction between CD44 and several Rho-specific guanine nucleotide exchange factors (eg, p115RhoGEF12 and LARG13) that up-regulate RhoA (a member of the Rho subclass of the Ras superfamily), leading to several important cellular functions.12,13 Several enzymes have been identified as possible downstream targets for RhoGTPases (eg, RhoA) regulating cytoskeleton-mediated cell motility.9–11 One such enzyme is Rho-kinase (ROK; also called Rho-binding kinase), a serine-threonine kinase.14,15 ROK interacts with RhoA in a GTP-dependent manner14,15 and is composed of four functional domains: a kinase domain (catalytic site), a coiled-coil domain, a Rho-binding (RB) domain, and a pleckstrin-homology (PH) domain.14,15 Both the kinase and RB domains share a large percentage of sequence homology with a family of related kinases known to bind Rho GTPase and participate in cell motility and cytoskeleton functions.16 Truncation of the kinase domain at the N-terminal region results in the inhibition of kinase activity, loss of stress fibers, and reduction in focal adhesion complexes.14,15 Point mutations of either RB or PH domains at the C-terminal region of ROK have been shown to block the formation of stress fiber and focal adhesion.14–16 These observations suggest that the RB or PH domain plays an important role in regulating ROK activation.
RhoA/ROK-Regulated Cytoskeleton Pathway
The actin cytoskeleton has been shown to coordinate and stabilize adhesive structures and modulate epidermal shape changes and motility.17 ROK activity is essential for ROK's involvement in promoting stress fiber formation and focal adhesion complexes.16 Microinjection of an expression vector encoding ROK results in the formation of stress fibers and focal adhesion complexes in certain cell types.14–16 In keratinocytes, the polarized cytoskeleton is dependent on RhoA-activated ROK to orchestrate polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium.17 ROK has also been shown to phosphorylate several cellular substrates, including myosin light chain phosphatase18 and LIM kinase.19 Phosphorylation of myosin light chain phosphatase and LIM kinase by RhoA-activated ROK results in actomyosin contractility and actin assembly-disassembly, respectively18,19 (Figure 1A). Keratinocyte migration and differentiation require RhoA-activated ROK signaling.10,11,20 Loss of RhoA signaling significantly decreases directed keratinocyte migration.11 Further analyses indicate that the N-terminal region of ROK is needed for the proper regulation of its activity.14–16 Overexpression of either the RB domain or the PH domain (dominant-negative forms) of ROK by transfecting cells with RB cDNA or PH cDNA blocks HA/CD44-specific phenotypic changes.21,22 These observations indicate that the RB or PH domain plays an important role in regulating ROK activation. In addition, inhibition of RhoA-activated ROK by Y27632 treatment effectively blocks the HA/CD44-induced keratinocyte signaling and functions.23 Selective activation of CD44 signaling by different sizes of HA fragments has been shown to induce RhoA-ROK pathway-specific effects on keratinocyte functions (eg, proliferation and/or migration).23 RhoA-activated ROK also phosphorylates the cytoplasmic domain of the CD44v3 isoform and up-regulates the interaction between the CD44v3 isoform and the cytoskeletal protein, ankyrin, during breast tumor cell migration.21 Thus, ROK is one of the important signaling molecules participating in HA-mediated CD44 function.21
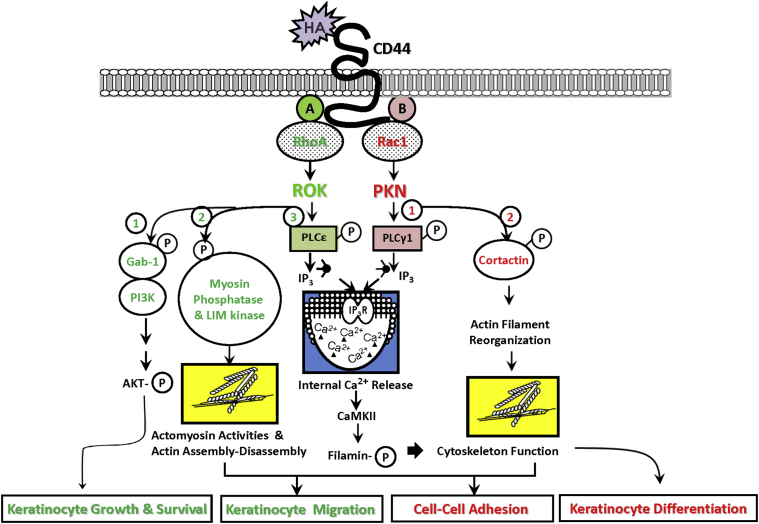
Figure 1.
A proposed model for HA/CD44-mediated signaling in keratinocytes. The binding of HA to the extracellular domain of CD44 promotes activation of RhoGTPases [eg, RhoA (A) and Rac1 (B)], which then generate specific signaling cascades described as follows. A: RhoA-specific signaling pathways. HA-CD44 interaction (left, 1) activates RhoA-ROK signaling, promotes the membrane localization of Gab-1, and activates certain isoforms of PI3K, leading to AKT activation and keratinocyte cell growth and survival. The binding of HA to CD44 induces activation of ROK, which, in turn, phosphorylates myosin light chain phosphatase and LIM kinase, thereby generating actomyosin contractility and actin assembly-disassembly required for keratinocyte migration (left, 2). HA-CD44–mediated ROK signaling also phosphorylates PLCε (left, 3) which, in turn, produces IP3 production and IP3 receptor–triggered intracellular Ca2+ mobilization, resulting in CaMKII activation. CaMKII then phosphorylates the cytoskeletal protein, filamin, leading to cytoskeleton reorganization and keratinocyte migration. B: Rac1-specific signaling pathways. HA-CD44 interaction activates Rac1 signaling and PKN activity (right, 1). Activated PKN then phosphorylates certain cellular proteins, including PLC-γ1, which, in turn, produces IP3 production and IP3 receptor–triggered intracellular Ca2+ mobilization, resulting in CaMKII activation. CaMKII then phosphorylates the cytoskeletal protein, filamin, leading to cytoskeleton function and keratinocyte differentiation. The binding of HA to CD44 also promotes PKN-mediated phosphorylation of cytoskeletal protein, cortactin, thereby generating actin filament reorganization and keratinocyte cell-cell adhesion (right, 2).
RhoA/ROK-Regulated Gab-1, PI3K, and AKT Pathway
The epidermis continuously undergoes self-renewal, proliferation, survival, and differentiation. Grb-2–associated binder-1 (Gab-1), a member of the insulin receptor substrate family,12,24 and phosphatidylinositol 3-kinase (PI3K)24 are key mediators in regulating epidermal cellular functions, such as proliferation and cell survival.12,24 Specifically, Gab-1 functions as one of the major adapter molecules downstream of growth factor signaling.24,25 Gab-1 also possesses multiple phosphorylation sites that could act as docking sites for PI3K, which is known to consist of a catalytic subunit p110 (α, β, and δ) and a regulatory subunit p85 (α, β, and p55γ), or the catalytic subunit p110γ and the regulatory subunit p101.24,25 PI3K signaling to AKT promotes keratinocyte differentiation.26
A previous study also found a positive link between HA-CD44 interaction and Gab-1–associated PI3K activation during the stimulation of cellular transformation.12 Specifically, HA activates PI3K-AKT pathways, leading to cell motility and cell survival signaling pathways.12 The active mutant of the p110 subunit of PI3K exerts its action on the cleavage of CD44 during cell migration.27 These findings suggest PI3K activation is closely coupled with HA-mediated CD44 signaling. The binding of HA to cells stimulates ROK activity, which, in turn, increases serine/threonine phosphorylation of the adaptor protein, Gab-1.24 Phosphorylated Gab-1 promotes PI3K recruitment to CD44v3.12 Subsequently, PI3K is activated (particularly, α, β, and γ forms, but not the δ form of the p110 catalytic subunit), AKT signaling occurs, and cell growth and survival are up-regulated. HA/CD44-mediated PI3K activity and AKT activation can be effectively blocked by a PI3K inhibitor (LY294002).12 These observations support the notion that PI3K activation and HA/CD44 signaling are functionally coupled. Finally, overexpression of a dominant-negative form of ROK (by transfection of cells with ROK's RB domain cDNA) not only inhibits HA/CD44-mediated RhoA-ROK activation and Gab-1 phosphorylation, but also down-regulates oncogenic signaling events (eg, Gab-1/PI3K-CD44 association and PI3K-mediated AKT activation) and cellular behaviors (eg, cell growth and survival).12
CD44v3 contains not only HA binding sites, but also displays the heparin sulfate addition site, which involves the binding of growth factors, cytokines, and chemokines. It is likely that HA/CD44-regulated ROK (under the influence of other growth factors, cytokines, and chemokines) plays a pivotal role in the phosphorylation of both CD44v3 and Gab-1 phosphorylation, leading to ankyrin binding and Gab-1–PI3K membrane localization and AKT signaling, respectively, during HA-mediated functions (Figure 1A).
Rac1-Activated PKN Signaling Events
HA also promotes the interaction between CD44 and several Rac1-specific guanine nucleotide exchange factors (eg, Tiam128 and Vav229) that up-regulate Rac1 (another member of the Rho subclass of the Ras superfamily), leading to altered cytoskeleton-mediated cell functions.28,29 Several enzymes have been identified as possible downstream effectors for Rac1 signaling. One such enzyme is protein kinase N (PKN), which is closely related to the protein kinase C family in their conservative catalytic domains (C-terminal regions) while differing in their N-terminal regulatory domains.30–33 The three known isoforms, PKN 1, 2, and 3, are closely related, exhibiting greatest variation with their regulatory domains. In particular, PKN2 (alias PRK2) belongs to a family of serine-threonine kinases known to interact with Rac1 in a GTP-dependent manner.30–33 The N-terminal region of PKN2 contains three homologous sequences of approximately 70 amino acids (relatively rich in charged residues), which form an antiparallel coiled-coil fold (ACC domain).30–33 This ACC domain interacts with RhoGTPases, such as RhoA and Rac1 (and, to a lesser extent, with Cdc42).30–33 Moreover, PKN has been found to regulate intermediate filaments, such as vimentin, glial fibrillary acidic protein, and other neurofilament proteins.34 In keratinocytes, Rho-activated PKN (PRK2) has been found to be involved in Fyn/Src kinase–regulated cell-cell adhesion during Ca2+-induced differentiation.35 Rac1-PKN2 (to a lesser extent RhoA-PKN2) appears to be preferentially activated by HA-CD44 signaling. However, RhoA-PKN2 appears to be stimulated by high Ca2+ treatment. Because HA (but not high Ca2+) is naturally present in the skin, we believe that HA-induced Rac1-PKN2 is more physiologically relevant to keratinocytes.
HA-CD44 Interaction Regulates Intracellular Ca2+ Signaling and Keratinocyte Functions
The epidermis is characterized by a polarized pattern of keratinocyte growth (ie, dividing cells detected at the basal layer of the epidermis) and differentiation (ie, differentiated cells distributed as multiple overlying layers throughout the epidermis). Mouse primary keratinocytes cultured in low Ca2+ medium (0.03 to 0.07 mmol/L) proliferate actively, with little contact inhibition, and resemble undifferentiated basal epidermal cells.36 Keratinocyte differentiation can be induced by adding high concentrations of Ca2+ (0.12 to 2.0 mmol/L) to these cultures.36 Previous studies indicated that a multiphasic increase in intracellular Ca2+ concentration after the addition of exogenous 1 to 2 mmol/L Ca2+ to keratinocytes is required for Ca2+-induced keratinocyte differentiation.36 Inhibition of intracellular Ca2+ mobilization by treatment with a Ca2+ chelator, 1,2-bis (aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid, prevents Ca2+-induced differentiation.36,37 These findings suggest that intracellular Ca2+ mobilization plays a critical role in regulating keratinocyte differentiation. The early events of keratinocyte differentiation (within hours of the Ca2+ switch) involve cytoskeletal rearrangement and the expression of keratinocyte differentiation markers, including keratins K1, profilaggrin (the precursor of filaggrin, an intermediate filament-associated protein), involucrin, and loricrin (a precursor for the cornified envelop). Within 24 to 48 hours after the Ca2+ switch, transglutaminase is activated and tightly cross-linked with loricrin, involucrin, and other proteins into the insoluble cornified envelop of mouse keratinocytes.36
In addition to externally added Ca2+, keratinocyte growth and differentiation can also be regulated by several agents, including phorbol esters, retinoic acid, vitamin D3, and matrix HA. In particular, HA-CD44 binding appears to stimulate the elevation of intracellular Ca2+ levels in keratinocytes and induces keratinocyte differentiation.32 Recent studies indicate that RhoGTPases are also involved in the regulation of intracellular Ca2+ levels in normal keratinocytes32 and transformed keratinocytes13 during cellular functions.
HA/CD44-Mediated RhoA Activation and Ca2+ Signaling
A previous study showed that HA binding to CD44 induces RhoA-specific guanine nucleotide exchange factor, the leukemia-associated Rho guanine nucleotide exchange factor (LARG), and RhoA signaling in transformed keratinocytes.13 In searching for possible new cellular targets of LARG-activated RhoA, phosphoinositide-specific phospholipase Cε (PLCε) was identified. Specifically, it has been observed that HA-CD44 binding stimulates LARG-catalyzed RhoA signaling, which, in turn, activates the RhoA association with PLCε in a GTP-dependent manner and promotes PLCε-mediated inositol 1,4,5 triphosphate (IP3) production and Ca2+ mobilization.13 Subsequently, the up-regulation of Ca2+/calmodulin-dependent kinase-II (CaMKII) occurs, leading to phosphorylation of the cytoskeletal protein, filamin. The phosphorylation of filamin reduces its interaction with filamentous actin, promoting tumor cell migration. Further analyses show that the PDZ domain of LARG binds to CD44 directly. Transfection of the head and neck tumor cells (HSC-3 cells) with LARG-PDZ cDNA significantly reduces LARG association with CD44. Overexpression of the LARG-PDZ domain functions as a dominant-negative mutant (similar to the PLC/CaMKII inhibitor effects) by blocking HA/CD44-mediated signaling events (eg, RhoA activation, PLCε-mediated IP3 production, intracellular Ca2+ mobilization, CaMKII activity, filamin phosphorylation, and filamin-actin binding) and abrogating tumor cell migration13 (Figure 1A). These findings indicate that CD44 interaction with LARG plays a pivotal role in RhoA activation and PLCε-Ca2+ signaling required for CaMKII-mediated cytoskeleton function, resulting in transformed keratinocytes.13
HA/CD44-Mediated Rac1 Activation and Ca2+ Signaling
HA-CD44 interaction also induces Rac1-PKN phosphorylation of PLCγ1 and cortactin in cultured keratinocytes.32 Specifically, the Rac1-activated PKN (induced by HA-CD44 binding) increases threonine (but not serine) phosphorylation of PLCγ1 and up-regulates PLCγ1 activity, leading to the onset of intracellular Ca2+ mobilization. HA/CD44-activated Rac1-PKN also phosphorylates the cytoskeletal protein, cortactin, at serine/threonine residues.32 The phosphorylation of cortactin by Rac1-PKN attenuates its ability to cross-link filamentous actin in vitro. Further analyses indicate that the N-terminal ACC domains of PKN interact directly with Rac1 in a GTP-dependent manner. The binding of HA to CD44 induces PKN association with endogenous Rac1 and its activity in keratinocytes. Transfection of keratinocytes with PKN-ACC cDNA reduces HA-mediated recruitment of endogenous Rac1 to PKN and blocks PKN activity. These findings demonstrate that the PKN-ACC fragment acts as a potent competitive inhibitor of endogenous Rac1 binding to PKN in vivo. Most important, the PKN-ACC fragment functions as a strong dominant-negative mutant that effectively inhibits HA/CD44-mediated PKN phosphorylation of PLCγ1 and cortactin, as well as keratinocyte signaling (eg, Ca2+ mobilization and cortactin-actin binding) and cellular functioning (eg, cell-cell adhesion and differentiation). Taken together, these findings strongly suggest that HA-CD44 interaction with Rac1-PKN plays a pivotal role in PLCγ1-regulated Ca2+ signaling (Figure 1B) and cortactin-cytoskeleton function (Figure 1B), both of which are required for keratinocyte cell-cell adhesion and differentiation. Because large HA fails to activate Rho-PKN2, it is not clear what the role of Rho-PKN2 is in large HA-CD44 signaling. It is possible that other sizes of HA (eg, small or intermediate sizes of HA) are involved in the selective activation of Rho-PKN2 and downstream targets (PLCγ1). The question of whether these two pathways (Rac1-PKN2 versus Rho-PKN2) represent complementary cross talk or competition awaits further investigation.
HA and CD44 in Normal and Aged Epidermis
Skin aging is a universal and inevitable process characterized by physiological alterations in keratinocyte activities and epidermal functions, as well as dermal changes.38 Aged skin is often associated with retraction of rete ridges/pegs and a flattening of the epidermal-dermal junction.7,23,38 In particular, thinning of the epidermis in aged skin is closely associated with decreased keratinocyte proliferation.23,39 Skin aging also results in delayed wound healing, partially due to reduced keratinocyte migration.40 Although the stratum corneum maintains a relatively constant thickness throughout life, aged epidermis is often characterized by abnormal barrier function, impaired lipid synthesis, and aberrant lamellar body formation and secretion.7,23,41 Epidermal dysfunction and abnormal keratinocyte activities in aged skin often lead to debilitating clinical consequences [eg, epidermal thinning (atrophy), barrier dysfunction, xerosis/xerotic eczema, delayed wound healing, and inflammation] and increased morbidity in elderly persons, which includes altered drug permeability, increased susceptibility to ulceration, and irritant contact dermatitis.38 At the present time, the cellular and molecular mechanisms causing epidermal dysfunction associated with skin aging are not well understood. However, recent studies now reveal that abnormal HA metabolism may be involved in the changes associated with keratinocyte activities, permeability barrier homeostasis, and wound healing during skin aging processes and disease progression.
HA Metabolism and Skin Aging
HA within the epidermis (and, to a lesser extent, the dermis) has been found to rapidly turn over.42,43 These observations suggest that epidermis is able to catabolize HA that is closely coordinated with its synthesis and degradation. Under physiological conditions, HA is synthesized by several HA synthases,44 and HA fragments of low molecular mass are produced by hyaluronidases or oxidation.45 One general concept that has emerged from these studies is that HA fragments [small HAs (HAS)] and their larger precursor molecules [large HAs (HAL)] may include distinct biological activities.23,46–48 This process may involve the recruitment of biologically active ECM fragment (HAS with a mol. wt. of approximately 1 × 105 to 1 × 104 Da) from the intact ECM (HAL with a mol. wt. of >1 × 106 Da) during periods of proliferation, migration, differentiation, and development, as well as injury-related repairs.41–45 Several studies indicate that HAL (>1 × 106 Da) promotes transcriptional activation and differentiation, whereas HAS (1 × 105 to 1 × 104 Da) induces proliferative genes and migration.41 HA fragmentation may also play a key role in the sequential phases of tissue injury and repair,46 but the cellular mechanisms underlying their action are not clearly defined. An age-related decrease in HA production has been found in both rodent42 and human aged skin.43 Age-related changes in the sizes of HA have also been reported previously.23,42,43 It is well known that aging is associated with impaired wound healing40 and the delayed resolution of a variety of skin diseases.38–41 All of these observations are consistent with the notion that both low levels of HA deposition and HA size modifications could contribute to skin aging, impaired wound healing, and disease progression.
Topical Application of HA Fragments and Anti-Skin Aging Effects
Most research on skin aging has focused on the effects of photoaging on the dermis. However, effective therapeutic options available to correct the aging-related changes in epidermal keratinocyte activities remain limited. A recent study indicates that small HA fragments (HA tetrasacchatides) are capable of inducing epidermal differentiation through phosphorylation of CD44 in keratinocyte culture in vivo.49 However, the question of whether these HA tetrasacchatides can promote CD44 signaling and improve epidermal function during skin aging is not known. A previous study by Brown et al50 showed that topical application of HA fragments (labeled by [3H]-hyaluronan) can penetrate through both mouse and human skin via active transport (not passive diffusion). Thus, the penetration of HA fragments in mouse skin does not present a technical problem. Therefore, it is possible to design corrective HA-based therapeutic strategies for the treatment of skin aging-related diseases.
Currently, the cellular and molecular mechanisms involved in epidermal dysfunction and skin aging are not well understood. Nevertheless, HA and its catabolic products are known to selectively activate CD44-mediated keratinocyte signaling that regulates keratinocyte proliferation, migration, differentiation, lamellar body formation/secretion, and wound healing, all of which involve RhoGTPases (eg, RhoA and Rac1).23 As previously mentioned, a recent study indicates that changes in the HA size distribution and CD44 expression are closely associated with reduced RhoA/Rac signaling and marked alterations in epidermal functions, resulting in deleterious consequences for the permeability barrier function and wound healing in aged epidermis. Further analyses indicate that HAS activates CD44-dependent RhoA/ROK signaling, whereas HAL activates CD44-specific Rac1/PKN signaling, leading to distinctly different keratinocyte functions and responses. Down-regulation of ROK and PKN by treating the mouse skin with Y27632 and Ro31-8220, respectively, also greatly reduces sequential HA (HAS→HAL)-mediated epidermal functions and permeability barrier recovery.23
Furthermore, topical application of HAS promotes keratinocyte proliferation and increases skin thickness, but it fails to up-regulate keratinocyte differentiation or permeability barrier repair in aged mouse skin (Figure 2A). In contrast, HAL induces only minimal changes in keratinocyte proliferation and skin thickness, but restores keratinocyte differentiation and improves permeability barrier function in aged epidermis23 (Figure 2B). Because neither HAS nor HAL corrects these epidermal defects in aged CD44 knockout mice, it is likely that CD44 mediates HA-associated epidermal functions in aged mouse skin. Finally, the blockade of Rho-kinase activity with Y27632 (Figure 2A) or protein kinase-N activity with Ro31-8220 (Figure 2B) significantly decreased the HA (HAS or HAL)-mediated changes in epidermal function in aged mouse skin. These findings demonstrate that HA application of different sizes regulates epidermal proliferation, differentiation, and barrier function in aged mouse skin; and that manipulation of matrix (HA) interaction with CD44 and RhoGTPase signaling could provide novel therapeutic approaches targeted at the treatment of various aging-related skin disorders.
Figure 2.
A proposed model for selective effects of HAS versus HAL on CD44-mediated epidermal functions during skin aging. A: HAS-mediated RhoA-ROK signaling in aged skin: In epidermis, HAS binding to CD44 promotes RhoA-ROK signaling, resulting in proliferation and migration, leading to an increase in epidermal thickness and acceleration of wound healing in aged skin. ROK inhibitor, Y27632, effectively reduces HAS-mediated RhoA-ROK signaling and a variety of keratinocyte functions (eg, proliferation, migration, epidermal thickness, and skin wound healing) in aged mouse skin. B: HAL-mediated Rac1-PKN signaling in aged skin: In epidermis, HAL binding to CD44 promotes Rac1-PKN signaling, resulting in cell-cell adhesion, differentiation, and lipid synthesis, leading to restoration of permeability barrier functions in aged skin. PKN inhibitor, Ro31-8220, effectively reduces HAL-mediated Rac1-PKN signaling and a variety of keratinocyte functions (eg, cell-cell adhesion, differentiation, lipid synthesis, and permeability barrier function) in aged mouse skin. Both skin aging and CD44 deficiency in knockout (k/o) mice cause down-regulation of HAS-mediated RhoA-ROK signaling and HAL-induced Rac1-PKN activation events, resulting in abnormal epidermal structure and function.
Conclusions
In summary, we suggest that HA activation of CD44 signaling induces pathway (RhoA-ROK versus Rac-PKNγ)-specific effects on diverse epidermal processes (eg, Gab-1–associated PI3K-AKT activation, Ca2+ signaling, actomyosin activities, and actin-filament reorganization), leading to a variety of epidermal functions (eg, proliferation, survival, migration, cell-cell adhesion, differentiation, and epidermal barrier formation) in keratinocytes. Furthermore, activation of CD44 signaling (via HAS versus HAL or HAS→HAL) induces pathway (RhoA-ROK versus Rac-PKN)-specific effects on diverse epidermal processes (eg, epidermal proliferation, skin thickness, differentiation, and epidermal barrier formation) in aged mouse epidermis. These newly discovered HA/CD44-signaling events provide the opportunity to develop novel HA-based therapeutic approaches for use in the treatment of patients experiencing several aging-related skin diseases (eg, skin atrophy, psoriasis, atopic dermatitis, actinic keratoses, and chronic nonhealing wounds). Signaling perturbation agents (eg, Y27623, a ROK inhibitor) may also be applied to patients with certain skin diseases involving up-regulation of keratinocyte proliferation (eg, psoriasis and actinic keratosis) r to correct the imbalance between RhoA-ROK signaling and Rac1-PKNγ activation during epidermal aging and various skin diseases.
Acknowledgment
We thank Dr. Gerard J. Bourguignon (Mill Valley, CA) for the preparation and review of the manuscript.
Footnotes
Supported by US Department of Veterans Affairs Merit Review AwardsRR and D-1I01 RX000601 and BLR and D-5I01 BX000628, US Public Health grant R01 CA66163, and US Department of Defense grant W8IXWH-11-2-0189. L.Y.W.B. is a VA Senior Research Career Scientist.
Disclosures: None declared.
References
- 1.Tammi R., Ripellino J.A., Margolis R.U., Tammi M. Localization of epidermal hyaluronic acid using the hyaluronate binding region of cartilage proteoglycan as a specific probe. J Invest Dermatol. 1988;90:412–414. doi: 10.1111/1523-1747.ep12456530. [DOI] [PubMed] [Google Scholar]
- 2.Tammi R., MacCallum D., Hascall V.C., Pienimaki J.P., Hyttinen M., Tammi M. Hyaluronan bound to CD44 on keratinocytes is displaced by hyaluronan decasaccharides and not hexasaccharides. J Biol Chem. 1998;273:28878–28888. doi: 10.1074/jbc.273.44.28878. [DOI] [PubMed] [Google Scholar]
- 3.Underhill C. CD44: the hyaluronan receptor. J Cell Sci. 1992;103:293–298. doi: 10.1242/jcs.103.2.293. [DOI] [PubMed] [Google Scholar]
- 4.Screaton G.R., Bell M.V., Jackson D.G., Cornelis F.B., Gerth U., Bell J.I. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci U S A. 1992;89:12160–12164. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haggerty J.G., Bretton R.H., Milstone L.M. Identification and characterization of a cell surface proteoglycan on keratinocytes. J Invest Dermatol. 1992;99:374–380. doi: 10.1111/1523-1747.ep12616087. [DOI] [PubMed] [Google Scholar]
- 6.Turley E.A., Nobel P.W., Bourguignon L.Y. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- 7.Bourguignon L.Y., Ramez M., Singleton P., Man M.Q., Crumrine D., Elias P.M., Feingold K.R. Hyaluronan-CD44 interaction stimulates keratinocyte differentiation, lamellar body formation/secretion and epidermal barrier function. J Invest Dermatol. 2006;126:1356–1365. doi: 10.1038/sj.jid.5700260. [DOI] [PubMed] [Google Scholar]
- 8.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 9.Braga V.M., Machesky L.M., Hall A., Hotchin N.A. The small GTPase Rho and Rac are required the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMullan R., Lax S., Robertson V.H., Radford D.J., Broad S., Watt F.M., Rowles A., Croft D.R., Olson M.F., Hotchin N.A. Keratinocyte differentiation is regulated by the Rho and ROCK signaling pathway. Curr Biol. 2003;13:2185–2189. doi: 10.1016/j.cub.2003.11.050. [DOI] [PubMed] [Google Scholar]
- 11.Jackson B., Peyrollier K., Pedersen E., Basse A., Karlsson R., Wang Z., Lefever T., Ochsenbein A.M., Schmidt G., Aktories K., Stanley A., Quondamatteo F., Ladwein M., Rottner K., van Hengel J., Brakebusch C. RhoA is dispensable for skin development, but crucial for contraction and directed migration of keratinocytes. Mol Biol Cell. 2011;22:593–605. doi: 10.1091/mbc.E09-10-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourguignon L.Y., Singleton P., Zhu H., Diedrich F. Hyaluronan-mediated CD44 interaction with RhoGEF and Rho-Kinase promotes Grb2-associated binding-1 phosphorylation and phosphatidylinositol 3-kinase signaling leading to cytokine (macrophage-colony stimulating factor) production and breast tumor progression. J Biol Chem. 2003;278:29420–29434. doi: 10.1074/jbc.M301885200. [DOI] [PubMed] [Google Scholar]
- 13.Bourguignon L.Y., Gilad E., Brightman A., Diedrich F., Singleton P. Hyaluronan-CD44 interaction with leukemia-associated RhoGEF and epidermal growth factor receptor promotes Rho/Ras co-activation, phospholipase C epsilon-Ca2+ signaling, and cytoskeleton modification in head and neck squamous cell carcinoma cells. J Biol Chem. 2006;281:14026–14040. doi: 10.1074/jbc.M507734200. [DOI] [PubMed] [Google Scholar]
- 14.Matsui T., Amano M., Yamamoto T., Chihara K., Nakafuku M., Ito M., Nakano T., Okawa K., Iwamatsu A., Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- 15.Amano M., Chihara K., Nakamura N., Kaneko T., Matsuura Y., Kaibuchi K. The COOH terminus of Rho-kinase negatively regulates rho-kinase activity. J Biol Chem. 1999;274:32418–32424. doi: 10.1074/jbc.274.45.32418. [DOI] [PubMed] [Google Scholar]
- 16.Amano M., Chihara K., Kimura K., Fukata Y., Nakamura N., Matsuura Y., Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;27:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 17.Vaezi A., Bauer C., Vasioukhin V., Fuchs E. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev Cell. 2002;3:367–381. doi: 10.1016/s1534-5807(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 18.Kimura K., Ito M., Amano M., Chihara K., Fukata Y., Nakafuku M., Yamamori B., Feng J., Nakano T., Okawa K., Iwamatsu A., Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 19.Amano T., Tanabe K., Eto T., Narumiya S., Mizuno K. LIM-kinase 2 induces formation of stress fibers, focal adhesions and membrane blebs, dependent on its activation by Rho-associated kinase-catalysed phosphorylation at threonine-505. Biochem J. 2001;354:149–159. doi: 10.1042/0264-6021:3540149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lock F.E., Hotchin N.A. Distinct roles for ROCK1 and ROCK2 in the regulation of keratinocyte differentiation. PLoS One. 2009;4:e8190. doi: 10.1371/journal.pone.0008190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourguignon L.Y., Zhu H., Shao L., Zhu D., Chen Y.W. Rho-Kinase (ROK) promotes CD44v3,8-10-ankyrin interaction and tumor cell migration in metastatic breast cancer cells. Cell Motil Cytoskeleton. 1999;43:269–287. doi: 10.1002/(SICI)1097-0169(1999)43:4<269::AID-CM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Singleton P.A., Bourguignon L.Y. CD44v10 interaction with Rho-Kinase (ROK) activates inositol 1,4,5-triphosphate (IP3) receptor-mediated Ca2+ signaling during hyaluronan (HA)-induced endothelial cell migration. Cell Motil Cytoskeleton. 2002;53:293–316. doi: 10.1002/cm.10078. [DOI] [PubMed] [Google Scholar]
- 23.Bourguignon L.Y., Wong G., Xia W., Mao-Qiang M., Holleran W.M., Elias P.M. Selective matrix (hyaluronan) interaction with CD44 and RhoGTPase signaling promotes keratinocyte functions and overcomes age-related epidermal dysfunction. J Dermatol Sci. 2013;72:32–44. doi: 10.1016/j.jdermsci.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holgado-Madruga M., Emlet D.R., Moscatello D.K., Godwin A.K., Wong A.J. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 25.Katso R., Okkenhaug K., Ahmadi K., White S., Timms J., Waterfield M.D. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 26.Calautti E., Li J., Saoncella S., Brissette J.L., Goetinck P.F. Phosphoinositide 3-kinase signaling to Akt promotes keratinocyte differentiation versus death. J Biol Chem. 2005;280:32856–32865. doi: 10.1074/jbc.M506119200. [DOI] [PubMed] [Google Scholar]
- 27.Kawano Y., Okamoto I., Murakami D., Itoh H., Yoshida M., Ueda S., Saya H.J. Ras oncoprotein induces CD44 cleavage through phosphoinositide 3-OH kinase and the rho family of small G proteins. J Biol Chem. 2000;275:29628–29635. doi: 10.1074/jbc.M002440200. [DOI] [PubMed] [Google Scholar]
- 28.Bourguignon L.Y., Zhu H., Shao L., Chen Y.W. CD44 interaction with Tiam1 promotes Rac1 signaling and hyaluronic acid (HA)-mediated breast tumor cell migration. J Biol Chem. 2000;275:1829–1838. doi: 10.1074/jbc.275.3.1829. [DOI] [PubMed] [Google Scholar]
- 29.Bourguignon L.Y., Zhu H., Zhou B., Diedrich F., Singleton P.A., Hung M.C. Hyaluronan promotes CD44v3-Vav2 interaction with Grb2-p185HER2 and induces Rac1 and Ras signaling during ovarian tumor cell migration and growth. J Biol Chem. 2001;276:48679–48692. doi: 10.1074/jbc.M106759200. [DOI] [PubMed] [Google Scholar]
- 30.Vincent S., Settleman J. The PRK2 kinase is a potential effector target of both Rho and Rac GTPases and regulates actin cytoskeletal organization. Mol Cell Biol. 1997;17:2247–2256. doi: 10.1128/mcb.17.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flynn P., Mellor H., Palmer R., Panayotou G., Parker P.J. Multiple interactions of PRK1 with RhoA: functional assignment of the Hr1 repeat motif. J Biol Chem. 1998;273:2698–2705. doi: 10.1074/jbc.273.5.2698. [DOI] [PubMed] [Google Scholar]
- 32.Bourguignon L.Y., Singleton P.A., Diedrich F. Hyaluronan-CD44 interaction with Rac1-dependent protein kinase N-gamma promotes phospholipase Cgamma1 activation, Ca(2+) signaling, and cortactin-cytoskeleton function leading to keratinocyte adhesion and differentiation. J Biol Chem. 2004;279:29654–29669. doi: 10.1074/jbc.M403608200. [DOI] [PubMed] [Google Scholar]
- 33.Bourguignon L.Y., Gilad E., Peyrollier K., Brightman A., Swanson R.A. Hyaluronan-CD44 interaction stimulates Rac1 signaling and PKN gamma kinase activation leading to cytoskeleton function and cell migration in astrocytes. J Neurochem. 2007;101:1002–1017. doi: 10.1111/j.1471-4159.2007.04485.x. [DOI] [PubMed] [Google Scholar]
- 34.Mukai H., Toshimori M., Shibata H., Kitagawa M., Shimakawa M., Miyahara M., Sunakawa H., Ono Y. PKN associates and phosphorylates the head-rod domain of neurofilament protein. J Biol Chem. 1996;271:9816–9822. doi: 10.1074/jbc.271.16.9816. [DOI] [PubMed] [Google Scholar]
- 35.Calautti E., Grossi M., Mammucari C., Aoyama Y., Pirro M., Ono Y., Li J., Dotto G.P. Fyn tyrosine kinase is a downstream mediator of Rho/PRK2 function in keratinocyte cell-cell adhesion. J Cell Biol. 2002;156:137–148. doi: 10.1083/jcb.200105140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuspa S.H., Hennings H., Tucker R.W., Jaken S., Kilkenny A.E., Roop D.R. Signal transduction for proliferation and differentiation in keratinocytes. Ann N Y Acad Sci. 1988;548:191–196. doi: 10.1111/j.1749-6632.1988.tb18806.x. [DOI] [PubMed] [Google Scholar]
- 37.Li L., Tucker R., Hennings H., Yuspa S. Chelation of intracellular Ca2+ inhibits murine keratinocyte differentiation in vitro. J Cell Physiol. 1995;163:105–114. doi: 10.1002/jcp.1041630112. [DOI] [PubMed] [Google Scholar]
- 38.Waller J.M., Maibacj H.I. Age and skin structure and function, a quantitative approach (I): blood flow, pH, thickness, and ultrasound echogenicity. Skin Res Technol. 2005;11:221–235. doi: 10.1111/j.0909-725X.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 39.Kaya G., Tran C., Sorg O., Hotz R., Grand D., Carraux P., Didierjean L., Stamenkovic I., Saurat J.H. Hyaluronate fragments reverse skin atrophy by a CD44-dependent mechanism. PLoS Med. 2006;3:2291–2303. doi: 10.1371/journal.pmed.0030493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nuccitelli R., Nuccitelli P., Li C., Narsing S., Pariser D.M., Lui K. The electric field near human skin wounds declines with age and provides a noninvasive indicator of wound healing. Wound Repair Regen. 2011;19:645–655. doi: 10.1111/j.1524-475X.2011.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghadially R., Brown B.E., Sequeira-Martin S.M., Feingold K.R., Elias P.M. The aged epidermal permeability barrier. J Clin Invest. 1995;95:2281–2290. doi: 10.1172/JCI117919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sobel H. Metabolism of hyaluronic acid in the skin of aging mice. J Gerontol. 1971;26:555–557. doi: 10.1093/geronj/26.4.555. [DOI] [PubMed] [Google Scholar]
- 43.Tzellos T.G., Klagas I., Vahtsevanos K., Triaridis S., Printza A., Kyrgidis A., Karakiulakis G., Zouboulis C.C., Papakonstantinou E. Extrinsic ageing in the human skin is associated with alterations in the expression of hyaluronic acid and its metabolizing enzymes. Exp Dermatol. 2009;18:1028–1035. doi: 10.1111/j.1600-0625.2009.00889.x. [DOI] [PubMed] [Google Scholar]
- 44.Weigel P.H., Hascall V.C., Tammi M. Hyaluronan synthases. J Biol Chem. 1997;272:13997–14000. doi: 10.1074/jbc.272.22.13997. [DOI] [PubMed] [Google Scholar]
- 45.Stern R., Jedrzejas M.J. Hyaluronidase: their genomics, structures, and mechanism of action. Chem Rev. 2006;106:818–839. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noble P.W. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002;25:25–29. doi: 10.1016/s0945-053x(01)00184-6. [DOI] [PubMed] [Google Scholar]
- 47.Ghersetich I., Lotti T., Campanile G., Grappone C., Dini G. Hyaluronic acid in cutaneous intrinsic aging. Int J Dermatol. 1994;33:119–122. doi: 10.1111/j.1365-4362.1994.tb01540.x. [DOI] [PubMed] [Google Scholar]
- 48.Bourguignon L.Y., Wong G., Earle C.A., Xia W. Interaction of low molecular weight hyaluronan with CD44 and toll-like receptors promotes the actin filament-associated protein 110-actin binding and MyD88-NFκB signaling leading to proinflammatory cytokine/chemokine production and breast tumor invasion. Cytoskeleton (Hoboken) 2011;68:671–693. doi: 10.1002/cm.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kage M., Tokudome Y., Matsunaga Y., Hariya T., Hashimoto F. Effect of hyaluronan tetrasaccharides on epidermal differentiation in normal human epidermal keratinocytes. Int J Cosmet Sci. 2014;36:109. doi: 10.1111/ics.12105. [DOI] [PubMed] [Google Scholar]
- 50.Brown T.J., Alcorn D., Fraser J.R. Absorption of hyaluronan applied to the surface of intact skin. J Invest Dermatol. 1999;113:740–746. doi: 10.1046/j.1523-1747.1999.00745.x. [DOI] [PubMed] [Google Scholar]