Abstract
The attachment between tendon and bone occurs across a complex transitional tissue that minimizes stress concentrations and allows for load transfer between muscles and skeleton. This unique tissue cannot be reconstructed following injury, leading to high incidence of recurrent failure and stressing the need for new clinical approaches. This review describes the current understanding of the development and function of the attachment site between tendon and bone. The embryonic attachment unit, namely, the tip of the tendon and the bone eminence into which it is inserted, was recently shown to develop modularly from a unique population of Sox9- and Scx-positive cells, which are distinct from tendon fibroblasts and chondrocytes. The fate and differentiation of these cells is regulated by transforming growth factor beta and bone morphogenetic protein signaling, respectively. Muscle loads are then necessary for the tissue to mature and mineralize. Mineralization of the attachment unit, which occurs postnatally at most sites, is largely controlled by an Indian hedgehog/parathyroid hormone-related protein feedback loop. A number of fundamental questions regarding the development of this remarkable attachment system require further study. These relate to the signaling mechanism that facilitates the formation of an interface with a gradient of cellular and extracellular phenotypes, as well as to the interactions between tendon and bone at the point of attachment.
Keywords: musculoskeletal development, enthesis, tendon insertion, bone eminence, cartilage, bone, SCX, BMP4, PTHrP, IHH
Introduction
Body movement requires efficient transfer of force from contracting muscles to bone. This is achieved in the musculoskeletal system across a complex attachment system that includes the myotendinous junction and the tendon–bone junction, connected in series. Although the myotendinous junction is rarely injured, tendon must often be repaired to bone to treat tendon injuries. Unfortunately, the specialized tissue that forms at the attachment of tendon to bone during fetal development and postnatal maturation is not regenerated during tendon-to-bone healing (Rodeo et al., 1993; Thomopoulos et al., 2002, 2003b). Two examples of tendon–bone junctions prone to injury are the rotator cuff in the shoulder and the anterior cruciate ligament in the knee. Surgical repair of these tissues is particularly difficult because the surgeon must overcome the challenge of attaching two materials (tendon and bone) with vastly different mechanical properties. In the rotator cuff, this contributes to documented rates of re-rupture as high as 20% for minor tears and up to 94% for massive tears (Harryman et al., 1991; Galatz et al., 2004). A better understanding of the cellular and molecular mechanisms that drive development of the attachment tissue may allow researchers to develop therapeutic interventions for enhanced tendon-to-bone healing.
The formation of an attachment between a bone and a tendon starts during embryonic development. Throughout musculoskeletal system assembly, the tendon–bone attachment unit forms a complex structure, which includes the distal end of the tendon, the transitional zone across which tendon inserts into bone, and the mineralized side of the attachment. Tendons often insert into bone eminences, projections that grow on bone surfaces, and exhibit a large variety of shapes and sizes (Gray and Lewis, 1918; Hill, 1964). Bone eminences are termed according to their form: a broad, rough elevation is called a tuberosity, protuberance, or process; a small, rough prominence is called a tubercle; a sharp, slender, pointed eminence is termed a spine; and a narrow, rough elevation running along the surface is a ridge, crest, or line (Gray and Lewis, 1918). These superstructures provide a stable anchoring point for tendons, increase the moment arm for more effective muscle force transfer, and dissipate stresses at the tendon–bone interface. This results in a more effective muscle attachment and facilitates movement (Biewener et al., 1996; Benjamin et al., 2002; Genin et al., 2009; Liu et al., 2012b; Lu and Thomopoulos, 2013; Thomopoulos et al., 2013).
The structure of the attachment tissue is mechanically complex. Stress must be transferred between two materials that differ in material stiffness by two orders of magnitude. Bone is a stiff, brittle material relative to tendon, with a material stiffness of approximately 20 GPa (Bostrom et al., 2000). In contrast, tendon is tough and extensible when compared with bone, with a material stiffness of approximately 200 MPa in tension (Woo et al., 2000). The attachment of two dissimilar materials results in stress singularities at their interface and a subsequent increased risk of failure. To overcome this inherent challenge, the tendon–bone attachment consists of transitional tissue with structural and compositional gradients that give rise to graded tissue mechanical properties and reduced stress concentrations (Genin et al., 2009; Liu et al., 2011, 2012b; Thomopoulos et al., 2006, 2003a).
Despite their functional importance, the cellular origin of tendon–bone attachment tissue and the mechanisms that regulate its development have only recently been studied. This review will describe the current state of knowledge on the development of the attachment tissue, from fetal time points through postnatal maturity. Here, we refer to the embryonic tendon–bone attachment tissue as attachment unit, whereas the mature tissue is referred to as the enthesis.
The Origin of the Cells at the Tendon Enthesis
DEVELOPMENT AND MODULARITY OF THE TENDON–BONE ATTACHMENT UNIT
The mechanisms by which the tendon–bone attachment unit is established were initially studied by looking at the development of the bone eminences onto which the tendons insert. The development of the appendicular skeleton is initiated when a subset of mesenchymal cells, originating in the lateral plate mesoderm, amasss and is specified as chondroprogenitors. In mice, the differentiation of chondroprogenitors is completed and cartilaginous templates of future bone form by embryonic day (E) 12.5. However, it was recently shown that bone eminences appear only 2 days after this primary template has been established (Blitz et al., 2013). This finding has raised intriguing questions regarding the process by which bone eminences and tendon–bone attachment units form. Genetic lineage experiments have probed the question of cellular origin of bone eminences. Recently, it was revealed that bone eminence cells are not descendants of chondrocytes that populate the primary cartilaginous template, but rather an external module derived by a pool of progenitors that are added onto the already existing primary template. The differentiation state of the cartilaginous template at murine E12.5 revealed fields of undifferentiated cells at locations of presumptive bone eminences, next to the differentiated chondrocytes that formed the primary template. Additional sets of genetic lineage experiments demonstrated that the modularity had already begun at the specification stage, as eminence progenitors were specified separately and later than those of the primary cartilage (Blitz et al., 2013).
The development of the bone eminence as a distinct module is also manifested at the molecular level. Gene expression analyses showed that, unlike progenitors of the primary cartilage that express only Sox9, eminence progenitors at various attachment sites along with the skeleton express both Sox9 and scleraxis (Scx; Figure 1; Blitz et al., 2013; Sugimoto et al., 2013). The coexpression of Sox9 and Scx by attachment unit progenitors was also demonstrated by cell lineage analyses, showing that cells at tendon insertion sites originate from a Sox9-positive lineage (Akiyama et al., 2005) and that eminence chondrocytes originate from an Scx-positive lineage (Sugimoto et al., 2013). Sox9 is a key molecule in differentiation of mesenchymal cells to chondrocytes (Akiyama et al., 2002). This gene is continuously expressed by all chondroprogenitors and chondrocytes during chondrogenesis (Ng et al., 1997; Zhao et al., 1997; Dy et al., 2012). Scx is a bHLH transcription factor that is expressed by progenitors and cells of all tendinous tissues and regulates their differentiation (Cserjesi et al., 1995; Schweitzer et al., 2001; Murchison et al., 2007).
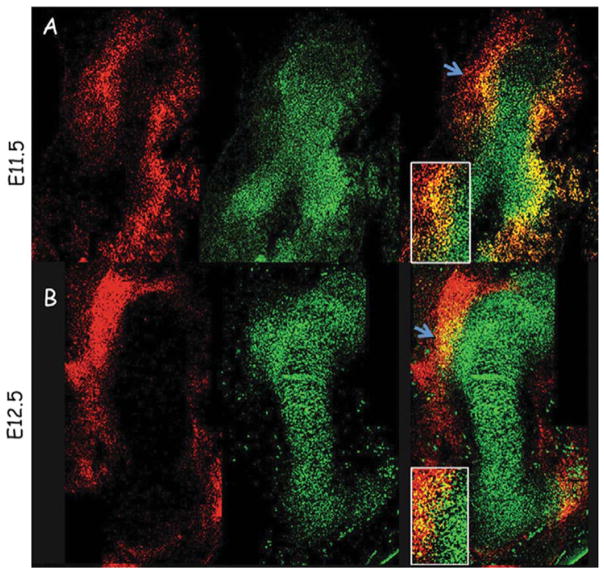
FIGURE 1.
Bone eminence progenitors coexpress Sox9 and Scx. A (top row) and B (bottom row): Double fluorescence in situ hybridization of sagittal humerus sections from E11.5 to E12.5 wild-type mice, using antisense complementary RNA probes for Sox9 (green) and Scx (red). Blue arrows demarcate a field from which the deltoid and great tuberosities develop; box shows enlargement of eminence progenitors expressing both Sox9 and Scx (Blitz et al., 2013).
The development of the tendon attachment unit as a separate module may offer several benefits. Mechanistically, modularity provides a checkpoint to control the assembly of the musculoskeletal system. This checkpoint may increase the robustness of the assembly process, as a modular component can coordinate between the bone and the attaching tendon without interference with the construction of the entire bone. The modular strategy of bone morphogenesis also has an evolutionary advantage, as modules can be easily added or removed, instead of reshaping the whole structure of the bone.
There are still many open questions regarding the newly discovered modularity in attachment unit development. Elucidating the embryonic origin of the modular pool will inform us whether it is derived from the lateral plate, as limb cartilage and tendons, or from another compartment in the embryo. Identification of additional genetic markers for eminence cell lineage would provide a powerful tool for studying the origin of the cells at the tendon–bone interface, as well as signaling between cartilage and tendons during their assembly.
THE “SEGREGATION” MODEL OF TENDON–BONE ATTACHMENT UNIT FORMATION
Modularity offers a new perspective on the fundamental question of how tendons and bones are assembled into one unit. During development, the connection of cartilage and tendon may be formed following differentiation. This would require a complex signaling mechanism to direct tendon to its designated insertion site. Another possibility is that both tissues of the unit are derived from a common pool of progenitor cells, which through differentiation diverges into tendon or cartilage (Figure 2). The advantage of such a strategy, to which we refer as the segregation model, is that it does not require a guidance mechanism for tendon, as both tissues form in situ. Furthermore, this strategy provides a high level of cellular plasticity, which is necessary to form the extremely diverse and complex morphology of the tendon–bone attachment. The notion of pluripotent “tenochondral” progenitor cells was previously suggested in a study on Sox5−/−Sox6−/− double-mutant mouse embryos, in which cartilage differentiation was compromised by the expansion of Scx expression in the early sclerotome (Brent et al., 2005).
FIGURE 2.
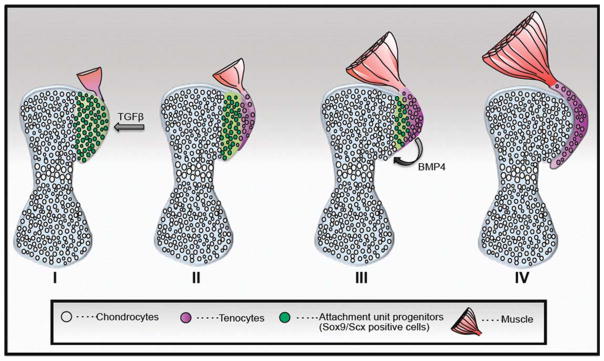
Schematic model for attachment unit formation in situ by segregation of a common progenitor pool to tenocytes and chondrocytes. At the onset (I), the bone anlage comprises differentiated chondrocytes (gray), and the attachment unit domain contains Sox9/Scx-positive progenitors (green). Next (II and III), progenitor cells gradually differentiate to tendon cells from one side (purple) and cartilage cells on the other side (gray) and form the attachment unit (IV). Although specification of attachment unit is regulated by TGFβ signaling (I), their differentiation to chondrocytes is regulated by BMP4 signaling from tendon progenitor cells (Blitz et al., 2013).
The segregation model requires the existence of a common pool of Scx- and Sox9-positive progenitors. In addition to the previously mentioned lineage analyses, loss-of-function studies further support this notion. Conditional knockout experiments demonstrated that Sox9 expression by Scx-positive cells is essential for the establishment of the tendon–bone attachment (Blitz et al., 2013; Sugimoto et al., 2013). Another prediction of the segregation model is that the common pool of Scx- and Sox9-positive progenitors should be gradually reduced, as cells differentiate to become tenocytes or chondrocytes. Indeed, expression analyses showed that the large fields of Scx- and Sox9-positive attachment progenitors seen at E11.5 reduce in size during development. By E13.5, when the population has completely segregated to Scx-expressing tenocytes and Sox9-expressing chondrocytes, the double-positive cells were no longer detectable (Blitz et al., 2013).
The segregation model suggests a number of research topics for future studies. These include the molecular regulation of the lineage divergence process and the interactions between Scx and Sox9 during specification and differentiation of the common progenitor pool.
The Molecular Mechanisms That Regulate Tendon–Bone Attachment Unit Development
The discovery of a new pool of Scx- and Sox9-positive progenitors that forms the tendon–bone attachment module prompted a search for the molecular mechanisms that regulates these cells. One approach for identifying molecular players in attachment unit formation is to analyze mutants that lack bone eminences. Indeed, this approach has uncovered several pathways that are suspected to regulate attachment unit development.
Conditional knockout of TgfβrII in limb mesenchyme demonstrated that the TGFβ signaling pathway regulates specification of eminence progenitors (Blitz et al., 2013). The role of TGFβs in skeletogenesis has long been controversial. Gain-of-function studies in cell culture models have suggested that TGFβ triggers chondrogenesis (Kulyk et al., 1989; Carrington et al., 1991; Leonard et al., 1991; Chimal-Monroy and Diaz de Leon, 1997; Merino et al., 1998; Verrecchia and Mauviel, 2002). However, ablation of Tgfβ receptor in limb mesenchyme in vivo had a mild effect on chondrocyte differentiation and on joint formation in the digits (Verrecchia and Mauviel, 2002; Seo and Serra, 2007; Spagnoli et al., 2007). The finding that TGFβ signaling regulates specification of eminence progenitors may resolve the uncertainty surrounding the role of this pathway in skeletogenesis. Although this pathway regulates chondrogenesis, its influence is limited to the secondary pools of progenitors that establish the cartilaginous side of the tendon attachment. The fact that TGFβ signaling regulates eminence progenitors exclusively supports the existence of two distinct, separately regulated pools of progenitors that contribute to the formation of the long bone, and thereby reinforce the concept of modularity.
Previous studies indicated that TGFβ signaling is also necessary for tendon formation in the limb. Disruption of TGFβ signaling by genetic ablation of the receptor TgfβrII in limb mesenchyme of mouse embryos, or by ablation of Tgfβ1 and Tgfβ2, resulted in the complete loss of all tendon tissue (Pryce et al., 2009). Moreover, TGFβ was suggested to coordinate cartilage and tendon differentiation during limb development (Lorda-Diez et al., 2009). It is therefore tempting to assume that by regulating both tendon and bone eminence progenitors, TGFβ signaling is a key regulator of tendon–bone attachment unit formation.
Another molecular pathway that was shown to be involved in bone eminence formation is BMP4 signaling. Conditional knockout of Bmp4 in limb mesenchyme blocked the differentiation of bone eminence progenitors to cartilage (Figure 3; Blitz et al., 2013). Another study showed that Bmp4 expression under the regulation of Scx at the tendon–cartilage junction induces bone eminence formation (Blitz et al., 2013). Both of these studies suggest that the SCX/BMP4 pathway plays a major role in bone eminence and tendon–bone attachment unit development.
FIGURE 3.
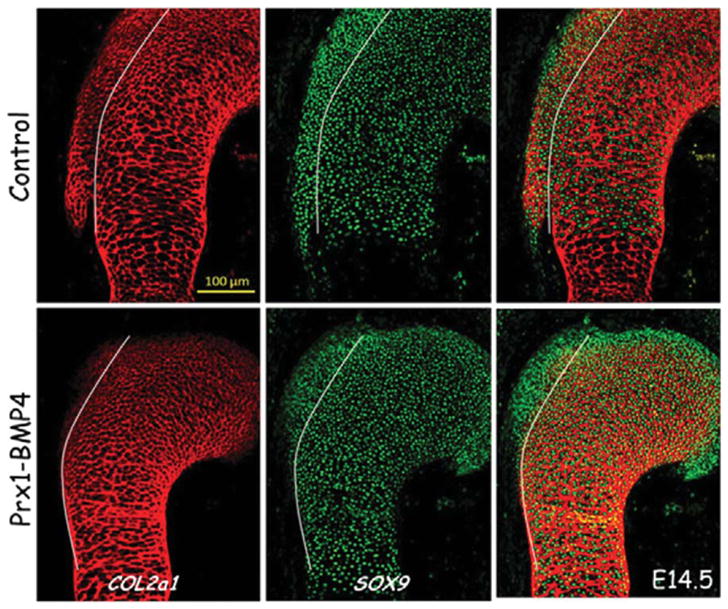
Immunofluorescence staining of humeral sections using anticollagen II (COL2A1) is shown in red, and anti-SOX9 antibodies in green indicates the presence of eminence progenitors at the deltoid tuberosity of the humeral head. Sections from E14.5 control and Prx1-Bmp4 mutants show that conditional knockout of Bmp4 in limb mesenchyme blocked the differentiation of bone eminence progenitors to cartilage. White lines mark the progenitor pool from which the deltoid tuberosity develops (Blitz et al., 2013).
There are still many open questions regarding the molecular pathways involved in tendon attachment unit formation. Given that not all bone eminences were lost in Bmp4-depleted limbs, it is possible that other BMPs are involved in the regulation of attachment unit development at different stages. Promising candidates are BMP2 and BMP7. Abrogation of the expression of their receptor Bmpr1a in limb mesenchyme led to the formation of a humerus devoid of eminences, such as the deltoid tuberosity (Ovchinnikov et al., 2006). BMP5 should also be considered, as dominant-negative Bmp5 mutation in mice has led to alteration in bone eminence formation (Ho et al., 2008). Additionally, other molecules that might be involved in tendon–bone attachment unit development are the fibroblast growth factors (FGFs), which have been implicated in skeletal morphogenesis and tendon formation. Inactivation of both Fgfr1 and Fgfr2 in limb mesenchyme led to skeletal malformation (Yu and Ornitz, 2008). Other studies in mouse and chick have shown that FGF signaling is involved in tendon development by inducing Scx expression in the limb during embryogenesis (Edom-Vovard et al., 2002; Pryce et al., 2009). Moreover, the expression of Fgf8 and Fgf4 in tendon insertion into muscles and the induction of Scx expression in muscle-less limbs by exogenous FGF imply a role for FGF in tendon differentiation through muscles (Edom-Vovard et al., 2002; Eloy-Trinquet et al., 2009).
The role of these candidate molecules and others in tendon–bone attachment unit formation and bone eminence development can be illuminated by examination of various mutants that lack bone eminences and by gene profiling of cells at the interface. It will also be interesting to study the interactions of those molecules with TGFβ and SCX-BMP4 signaling.
Mineralization of the Tendon–Bone Attachment Unit
The fetal events described above define the cell populations and specification of eminences that lead to the mineralized tendon enthesis seen in the mature attachment. Mineralization of the tendon attachment unit occurs via mechanisms similar to those described for the growth plate. In the mouse, a mineral gradient is evident near the maturing rotator cuff enthesis as early as 1 week after birth (Schwartz et al., 2012). The mineral gradient coincides with the mineralizing front of the secondary ossification center in the humeral head (Schwartz et al., 2012). During early stages of postnatal enthesis maturation, the mineral gradient becomes separated from the developing tendon by a region of epiphyseal cartilage that has yet to be mineralized. In a murine model, the gradient gradually moves into the developing transitional tissue of the tendon–bone attachment unit as the epiphyseal cartilage is mineralized between the first 2 weeks of postnatal growth (Figure 4). This process of endochondral ossification is likely regulated by the autocrine/paracrine signaling of Indian hedgehog (Ihh) and parathyroid hormone-related protein (PTHrP; Vortkamp et al., 1996; St. Jacques et al., 1999; Broadus et al., 2007). Ihh is expressed by prehypertrophic and early hypertrophic chondrocytes entering the early stages of terminal differentiation. This molecule stimulates the proliferating chondrocytes by binding the membrane receptor Patched (Ptch), which activates the membrane receptor Smoothened (Smo) and stimulates synthesis of PTHrP. PTHrP expression then blocks further expression of IHH, establishing a negative feedback loop to provide fine control over the rate of chondrocyte proliferation and maturation. Precise spatial and temporal control over these and other extracellular signaling molecules and transcription factors is critical to endochondral mineralization.
FIGURE 4.
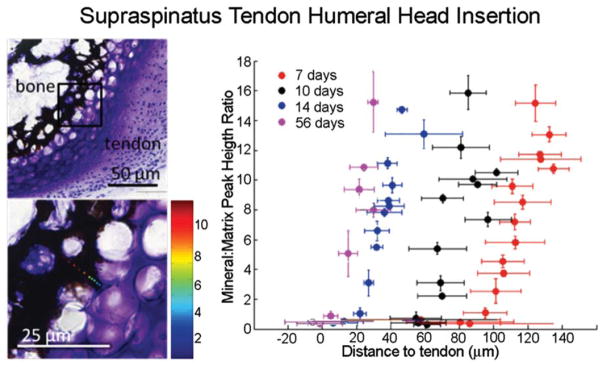
Spatial gradients in mineral (as determined using Raman spectroscopy) form between tendon and bone at the developing entheses from the onset of endochondral ossification (7 days in the mouse supraspinatus tendon enthesis, as shown in the Von Kossa/Toluidine Blue stained sections on the left). Reproduced with permission from Schwartz et al., 2012.
The role of IHH/PthrP signaling for mineralization of the tendon–bone attachment unit has been explored using a number of mouse models. Factors important to growth plate function, including PTHrP, IHH, SOX9, and type X collagen, have been identified at the developing enthesis and likely influence the development of an attachment with a gradient in mineral (Bland and Ashhurst, 1997, 2001; Fujioka et al., 1997; Chen et al., 2006; Galatz et al., 2007). PTHrP has been shown to localize at tendon and ligament entheses during postnatal time points (Chen et al., 2006, 2007). More specifically, PTHrP localizes within a group of fibrochondroblast-like cells in the intermediate zone between the tendon proper and the transitional tissue that inserts into the underlying cortical bone (Chen et al., 2006). Furthermore, PTHrP has been generally localized to periosteal cells in addition to cells that will form the secondary ossification center of long bones (Chen et al., 2006). Elevated expression of PTHrP at tendon-to-bone entheses suggests that PTHrP plays an important role in modeling the mineralized interface during skeletal maturation. In the growth plate, PTHrP maintains chondrocyte proliferation and inhibits maturation and mineralization (Provot and Schipani, 2005). Consistent with this, conditional deletion of PTHrP in SCX-expressing cells led to impaired modeling at multiple enthesis sites (Wang et al., 2013). Without PTHrP, the ability for osteoclasts to excavate and/or migrate with fibrous entheses during linear growth of the long bones was limited (Wang et al., 2013). For example, migration of the medial collateral ligament, a normally fibrous enthesis, was arrested during growth when PTHrP was deleted. In this case, the ligament prematurely anchored to the tibia and a large, hypermineralized tuberosity forms instead of the normal tibial crest (Figure 5). The authors concluded that PTHrP is deployed at developing tendon/ligament attachment sites as a modeling tool that directs osteoclasts to excavate the root system by which these sites attach to the cortical surface.
FIGURE 5.
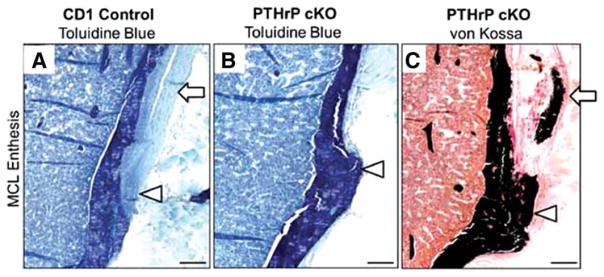
Conditional deletion of PTHrP from Scx-expressing cells led to defects in medial collateral ligament (MCL) enthesis mineralization. A normal MCL enthesis is shown in (A). Note the tuberosity and distortion in (B) and (C) and the mineralization within the tuberosity and tendon itself in (C). The MCL tendon is identified by arrows in (A) and (C), and the enthesis site by arrowheads in (A)–(C). Reproduced with permission from Wang et al., 2013.
Similarly, expression of Ihh and related molecules have been identified at the developing tendon-to-bone attachment (Blitz et al., 2009; Liu et al., 2012a; Liu et al., 2013). To test for a functional role of Hedgehog signaling, Liu et al. (2013) targeted Smo in Scx-expressing cells. Constitutive activation of Ihh in Scx-expressing cells caused enthesis markers, such as type II collagen, biglycan, and tenascin-C, to be expressed in the tendon midsubstance, where they are not normally expressed. In contrast, deletion of Smo throughout the development in Scx-expressing cells impaired the formation of a fibrocartilaginous tendon-to-bone attachment. This was associated with downregulation of genes involved in chondrogenesis and mineralization at the enthesis, as well as downregulation of enthesis markers. Functionally, patellar tendons that developed in the absence of Ihh signaling were weaker than normal. These studies demonstrate a critical role for PthrP/IHH signaling for the maturation of a functional tendon enthesis.
The Role of Mechanical Loading on Tendon–Bone Attachment Unit Initiation, Growth, and Mineralization
A complex synergy between biophysical cues and biological processes gives rise to the complex structure and composition of tendon–bone attachment units. Biophysical cues drive developmental patterning and growth in the fetal and postnatal musculoskeletal system (Carter et al., 2007). Bones, tendons, muscles, and joints are patterned in utero and maturation continues through the early postnatal period. The impact of muscle loading on embryonic development has been examined in several animal models. Muscle contractions in utero begin early in embryonic development and are crucial for the development of sesamoid bones, the knee meniscus, and proper bone and joint formation (Nowlan et al., 2010). The magnitude of in utero forces increases dramatically when increases in muscle volume are coupled with the forces that result from bone elongation (Sharir et al., 2011). In the absence of muscle forces, defects in bone size, shape, and mineralization can occur (Mikic et al., 2000; Osborne et al., 2002; Gomez et al., 2007; Sharir et al., 2011). For example, joint cavitation does not initiate, leading to bone fusion (Mikic et al., 2000; Kahn et al., 2009).
Muscle contraction also has a role in the formation of tendon–bone attachment tissue. The involvement of muscle-induced mechanical load in bone eminence formation was first demonstrated by transplantation experiments, in which bone eminences, such as the humeral deltoid tuberosity, reduced in size when the humerus was transplanted to the umbilical cord (Hamburger, 1938, 1939; Hamburger and Waugh, 1940). Other studies on immobilized chick embryos (Hall and Herring, 1990; Hosseini and Hogg, 1991) and on mice that lacked skeletal muscles or muscle contractility reported loss of the deltoid tuberosity (Pai, 1965; Tremblay et al., 1998; Rot-Nikcevic et al., 2006). The effect of mechanical load on bone eminences extends beyond embryonic development, as human patients with deltoid muscle contracture exhibit enlarged deltoid tuberosity (Ogawa et al., 1999).
The necessity of muscle loading on musculoskeletal development and the specific finding that bone eminences are initiated as cartilaginous elements early during development suggested the involvement of mechanical load in attachment unit formation. Surprisingly, using muscle-less and paralyzed mice, it was demonstrated that muscle contraction does not control the initial steps of specification and differentiation during bone eminence development. However, muscle force did regulate eminence growth by controlling cell proliferation (Blitz et al., 2009; Kahn et al., 2009), implying that bone eminences develop in a biphasic process of initiation and growth (Figure 6). During the initiation phase, eminence progenitors are specified and differentiate to cartilage, whereas during the growth phase, chondrocytes proliferate and extend into an eminence. The reason for the selection of such a biphasic developmental process may lie in the need of the attachment site to supply sufficient initial anchoring capabilities prior to muscle growth and increased force generation. Thus, the regulation of bone eminence initiation is predetermined. Once anchoring is achieved, muscle forces can be transmitted across the attachment without rupturing the connection, and regulation of growth and mineralization can be coupled to muscle activity. This sequence allows for tight and dynamic coordination between the applied physical stress and anchoring and force dissipation capabilities.
FIGURE 6.
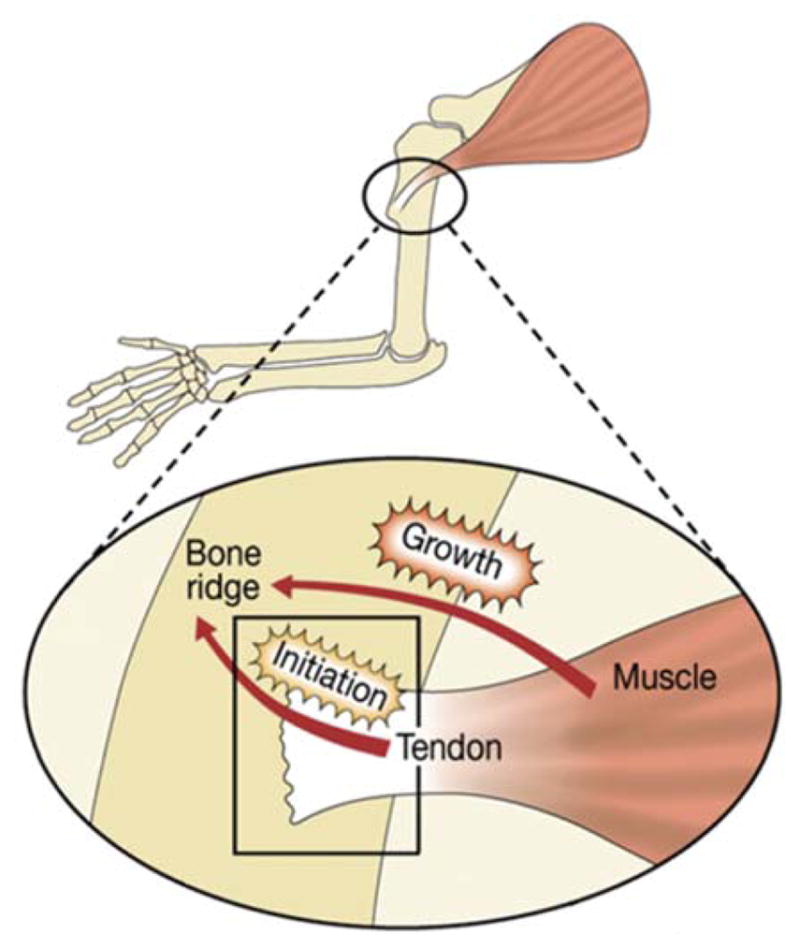
Blitz et al. (2009) suggested a model for the contribution of both tendons and muscles to bone eminence formation. Through a biphasic process, tendons regulate bone eminence initiation, and muscles control its subsequent growth. Further research is necessary to determine the mechanism whereby muscle contraction regulates eminence development. Reproduced with permission from Blitz et al., 2009.
As tendon growth and maturation require the presence of muscles (Kieny and Chevallier, 1979; Kardon, 1998; Schweitzer et al., 2001; Edom-Vovard et al., 2002; Bonnin et al., 2005; Brent et al., 2005), the role of tendons in the second phase of growth is yet to be determined. It is possible that tendons secrete specific factors in response to muscle contraction. Another conjecture is that tendons passively transmit muscle-induced mechanical load to eminences and thereby stimulate their growth.
The final phase of tendon enthesis formation involves mineralization of the tendon–bone attachment unit. The role of mechanical loading on this process has been studied extensively in the rotator cuff attachments to the humeral head. When examining the developing rotator cuff of the shoulder, the supraspinatus neotendon was evident adjacent to the developing humeral head bone at E15.5 (Galatz et al., 2007). In contrast, the mature insertion, defined by the appearance of a mineralized fibrocartilaginous transitional tissue, was not identified until after birth (Galatz et al., 2007; Schwartz et al., 2012). Paralysis of the rotator cuff muscles at birth led to substantial decreases in muscle volume and force across the developing tendon–bone attachment unit when compared with controls, as well as striking changes in tendon enthesis maturation (Figure 7; Thomopoulos et al., 2007; Kim et al., 2009, 2010; Das et al., 2011; Schwartz et al., 2013). Unloading caused severe mineralization defects in the humeral head, including reduced overall volume, morphological changes, and a shift in mineral crystal characteristics (Thomopoulos et al., 2007; Schwartz et al., 2012). Removal of muscle loading also affected the development of a fibrocartilaginous transition at the enthesis. Based on histological analysis, little to no fibrocartilage was observed in the enthesis after 8 weeks of paralysis (Thomopoulos et al., 2007). Collagen fiber alignment indicated that fibers were more disorganized in unloaded shoulders when compared with saline controls of mice (Schwartz et al., 2013). Impaired mineralization, disordered fiber alignment, and a loss of fibrocartilage transitional tissue likely contribute to the overall inferior mechanical properties of tendon entheses that developed in the absence of postnatal muscle loads (Schwartz et al., 2013). Structural mechanical properties (e.g., maximum force and stiffness) and material mechanical properties (e.g., maximum stress and modulus) were decreased in animals with paralyzed shoulders. Thus, perturbations in mechanical cues, even after birth, can have a dramatic influence on the development and maturation of the tendon enthesis.
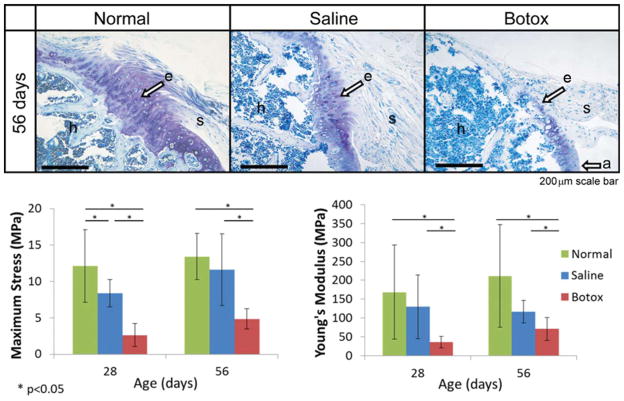
FIGURE 7.
Muscle paralysis dramatically impaired the development of the supraspinatus tendon-to-bone enthesis in mice. Top: A mature, compositionally graded enthesis (“e”) is seen 56 days postnatally in normal mice (scale bar =200 μm). In contrast, the enthesis in paralyzed shoulders appears disorganized, without a graded fibrocartilaginous transition between the supraspinatus tendon (“s”) and the humeral head bone (“h”). Reproduced with permission from Thomopoulos et al., 2007. Bottom: Maximum stress and modulus were significantly lower in the paralyzed group when compared with the normal and saline groups. Reproduced with permission from Schwartz et al., 2013.
Future studies may uncover the molecules that sense and transduce mechanical load to induce the growth of bone eminences and the formation of the tendon enthesis. Candidate molecules include members of many distinct families implicated in mechanotransduction pathways and tendon–bone attachment unit development. These include growth factors such as TGFβ, BMP, and FGF, hedgehog family members, such as IHH, matrix metalloproteinases, such as MMP-1 and MMP-13, and angiogenic factors, such as VEGF (Henderson and Carter, 2002). For example, Ihh expression in chondrocyte cultures is upregulated in response to tensile stretching and is required for increased proliferation (Wu et al., 2001). This mechanically driven response is mediated downstream by BMP-2 and BMP-4 and does not involve PTHrP (Wu et al., 2001). Additionally, PTHrP has also been implicated in mechanotransduction pathways independent of interactions with IHH (Chen et al., 2007; Xu et al., 2013). Chen et al. (2007) observed a significant decrease in PTHrP expression following tendon enthesis unloading via tail suspension or tendon transection. Additionally, PTHrP may be regulated by both magnitude and duration of load. For example, cyclic tensile loading in chondrocyte cultures implicated this molecule in the regulation of chondrocyte behavior during prehypertrophic and hypertrophic phases (Xu et al., 2013).
PERSPECTIVES/CONCLUSIONS
The formation of a functional tendon–bone attachment unit requires both biomolecular and biophysical cues. A number of critical molecular signals have been identified, including TGFβ and BMP for initiation of growth and IHH/PTHrP for mineralization and maturation. Furthermore, experiments at fetal and postnatal time points have demonstrated the importance of muscle loading in the formation of a functional tendon–bone attachment unit. Spatial gradients in these signals across the attachment unit are likely necessary to initiate and maintain the gradients in cell phenotypes, extracellular matrix composition, and subsequent mechanical properties of the mature enthesis.
The development of the attachment unit has only recently been studied. Although numerous experiments have been conducted over the past few decades on bone, muscle, cartilage, and tendon development, an integrative perspective of these tissues and their interactions has been lacking. Studies in the past few years have elucidated the signaling between tendon and presumptive bone that leads to the formation of an attachment unit. However, despite the recent appreciation and study of attachments, a number of questions remain about how this attachment system forms and how we might use that knowledge to enhance tendon-to-bone healing in the clinical setting:
What is the embryonic origin of the modular pool of cells that initiates eminence and tendon attachment unit formation? Are these cells derived from the lateral plate like limb cartilage and tendons or are they derived from another compartment in the embryo?
How do gradients in cell phenotypes and extracellular matrix composition develop? Furthermore, how are these gradients maintained in the mature enthesis? Using endochondral ossification as a model, one would expect the chondrocytes that mineralize the enthesis to hypertrophy and undergo apoptosis. In contrast to the sequence of events described for endochondral ossification, the cells that populate the mature enthesis maintain their phenotype and a gradient of mineral through maturity.
What are the particular paracrine signals that drive development, and what role does mechanical loading play in the process? The recent identification of a pool of Scx-Sox9-positive cells at the developing attachment unit leads to the question of how specification and differentiation are regulated in these cells.
Are the cells that form the tendon attachment unit and populate the mature enthesis unique from tenocytes, chondrocytes, and osteoblasts? It was previously understood that compressive stresses at the tendon–bone interface prompted local tendon cells to become “fibrochondrocytes” (Benjamin and Ralphs, 1998). Although compression of adult tendon fibroblasts does indeed lead to production of cartilage-like extracellular matrix, recent work indicates that a progenitor cell population expressing markers of tendoprogenitors and chondroprogenitors populate the enthesis (Blitz et al., 2013; Sugimoto et al., 2013). It remains unclear, however, if these enthesis cells have a unique phenotype or if they are subpopulations of the classically described tenocyte, chondrocyte, and osteoblast lineages.
Finally, how can this information be used to regenerate the enthesis in the adult repair setting? Can a progenitor cell population be delivered to the repair site with the appropriate molecular signals to form an attachment unit following the developmental program?
Answers to these and related questions may allow researchers to propose novel biological and engineering solutions for enthesis injuries and pathologies. As current clinical care of these injuries is unsatisfactory, new treatment modalities could have a significant impact on clinical care.
NOMENCLATURE
In this review, we refer the embryonic attachment tissue as attachment unit, whereas the mature tissue is referred to as enthesis.
Tendon enthesis: The attachment point of tendon to mature bone.
Tendon–bone attachment unit: The fetal structure of tendon precursor attaching to bone precursor.
Bone eminence: A protuberance or projection from the surface of a bone (e.g., ridge and tuberosity).
Chondroprogenitor: A cell that is fated to differentiate into a chondrocyte.
Tenoprogenitor: A cell that is fated to differentiate into a tenocyte.
SCX: Scleraxis is a member of the basic helix-loop-helix superfamily and is a transcription factor necessary for tendon development.
SOX9: (Sex-determining region Y)-box 9 is a transcription factor that is necessary for chondrocyte differentiation.
TGF-β: Transforming growth factor-β is a family of growth factors that is involved in cell differentiation, proliferation, and other functions.
BMP4: Bone morphogenetic protein 4 is a member of the transforming growth factor superfamily and is involved in endochondral bone formation.
IHH: Indian hedgehog is a member of the hedgehog family of secreted signaling molecules and is involved in bone growth and differentiation.
PTHrP: Parathyroid hormone-related protein is a member of the parathyroid hormone family and is involved in endochondral bone development.
Acknowledgments
Supported by a grant from the National Institutes of Health (AR055580).
Footnotes
The authors have no conflicts of interest with the material reviewed in this article.
References
- Akiyama H, Chaboissier MC, Martin JF, et al. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Kim JE, Nakashima K, et al. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci USA. 2005;102:14665–14670. doi: 10.1073/pnas.0504750102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments—an adaptation to compressive load. J Anat. 1998;193 (Part 4):481–494. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Kumai T, Milz S, Boszczyk BM, et al. The skeletal attachment of tendons—tendon “enthuses”. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:931–945. doi: 10.1016/s1095-6433(02)00138-1. [DOI] [PubMed] [Google Scholar]
- Biewener AA, Fazzalari NL, Konieczynski DD, Baudinette RV. Adaptive changes in trabecular architecture in relation to functional strain patterns and disuse. Bone. 1996;19:1–8. doi: 10.1016/8756-3282(96)00116-0. [DOI] [PubMed] [Google Scholar]
- Bland YS, Ashhurst DE. Fetal and postnatal development of the patella, patellar tendon and suprapatella in the rabbit; changes in the distribution of the fibrillar collagens. J Anat. 1997;190 (Part 3):327–342. doi: 10.1046/j.1469-7580.1997.19030327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland YS, Ashhurst DE. The hip joint: the fibrillar collagens associated with development and ageing in the rabbit. J Anat. 2001;198 (Part 1):17–27. doi: 10.1046/j.1469-7580.2001.19810017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz E, Sharir A, Akiyama H, Zelzer E. Tendon–bone attachment unit is formed modularly by a distinct pool of Scx-and Sox9-positive progenitors. Development. 2013;140:2680–2690. doi: 10.1242/dev.093906. [DOI] [PubMed] [Google Scholar]
- Blitz E, Viukov S, Sharir A, et al. Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon–skeleton junction. Dev Cell. 2009;17:861–873. doi: 10.1016/j.devcel.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin MA, Laclef C, Blaise R, et al. Six1 is not involved in limb tendon development, but is expressed in limb connective tissue under Shh regulation. Mech Dev. 2005;122:573–585. doi: 10.1016/j.mod.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Bostrom MPG, Boskey A, Kauffman JK, Einhorn TA. Form and function of bone. In: Buckwalter JA, Einhorn T, Simon SR, editors. Orthopaedic basic science. 2. American Academy of Orthopaedic Surgeons; Rosemont, IL: 2000. pp. 319–370. [Google Scholar]
- Brent AE, Braun T, Tabin CJ. Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse development. Development. 2005;132:515–528. doi: 10.1242/dev.01605. [DOI] [PubMed] [Google Scholar]
- Broadus AE, Macica C, Chen X. The PTHrP functional domain is at the gates of endochondral bones. Ann NY Acad Sci. 2007;1116:65–81. doi: 10.1196/annals.1402.061. [DOI] [PubMed] [Google Scholar]
- Carrington JL, Chen P, Yanagishita M, Reddi AH. Osteogenin (bone morphogenetic protein-3) stimulates cartilage formation by chick limb bud cells in vitro. Dev Biol. 1991;146:406–415. doi: 10.1016/0012-1606(91)90242-u. [DOI] [PubMed] [Google Scholar]
- Carter DR, Beaupré GS, Beaupre GS. Skeletal function and form: mechanobiology of skeletal development, aging, and regeneration. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Chen X, Macica C, Nasiri A, et al. Mechanical regulation of PTHrP expression in entheses. Bone. 2007;41:752–759. doi: 10.1016/j.bone.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Macica CM, Dreyer BE, et al. Initial characterization of PTH-related protein gene-driven lacZ expression in the mouse. J Bone Miner Res. 2006;21:113–123. doi: 10.1359/JBMR.051005. [DOI] [PubMed] [Google Scholar]
- Chimal-Monroy J, Diaz de Leon L. Differential effects of transforming growth factors β1, β2, β3 and β5 on chondrogenesis in mouse limb bud mesenchymal cells. Int J Dev Biol. 1997;41:91–102. [PubMed] [Google Scholar]
- Cserjesi P, Brown D, Ligon KL, et al. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development (Suppl) 1995;121:1099–1110. doi: 10.1242/dev.121.4.1099. [DOI] [PubMed] [Google Scholar]
- Das R, Rich J, Kim HM, et al. Effects of botulinum toxin-induced paralysis on postnatal development of the supraspinatus muscle. J Orthop Res. 2011;29:281–288. doi: 10.1002/jor.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dy P, Wang W, Bhattaram P, et al. Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev Cell. 2012;22:597–609. doi: 10.1016/j.devcel.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edom-Vovard F, Schuler B, Bonnin MA, et al. Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev Biol. 2002;247:351–366. doi: 10.1006/dbio.2002.0707. [DOI] [PubMed] [Google Scholar]
- Eloy-Trinquet S, Wang H, Edom-Vovard F, Duprez D. Fgf signaling components are associated with muscles and tendons during limb development. Dev Dyn. 2009;238:1195–1206. doi: 10.1002/dvdy.21946. [DOI] [PubMed] [Google Scholar]
- Fujioka H, Wang GJ, Mizuno K, et al. Changes in the expression of type-X collagen in the fibrocartilage of rat Achilles tendon attachment during development. J Orthop Res. 1997;15:675–681. doi: 10.1002/jor.1100150508. [DOI] [PubMed] [Google Scholar]
- Galatz L, Rothermich S, VanderPloeg K, et al. Development of the supraspinatus tendon-to-bone insertion: localized expression of extracellular matrix and growth factor genes. J Orthop Res. 2007;25:1621–1628. doi: 10.1002/jor.20441. [DOI] [PubMed] [Google Scholar]
- Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- Genin GM, Kent A, Birman V, et al. Functional grading of mineral and collagen in the attachment of tendon to bone. Bio-phys J. 2009;97:976–985. doi: 10.1016/j.bpj.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez C, David V, Peet NM, et al. Absence of mechanical loading in utero influences bone mass and architecture but not innervation in Myod-Myf5-deficient mice. J Anat. 2007;210:259–271. doi: 10.1111/j.1469-7580.2007.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray H, Lewis WH. Anatomy of the human body. Philadelphia, PA: Lea & Febiger; 1918. [Google Scholar]
- Hall BK, Herring SW. Paralysis and growth of the musculoskeletal system in the embryonic chick. J Morphol. 1990;206:45–56. doi: 10.1002/jmor.1052060105. [DOI] [PubMed] [Google Scholar]
- Hamburger V. Morphogenetic and axial self-differentiation of transplanted limb primordia. J Exp Zool. 1938;77:379–399. [Google Scholar]
- Hamburger V. The development and innervation of transplanted limb primordia of chick embryos. J Exp Zool. 1939;80:347–389. [Google Scholar]
- Hamburger V, Waugh M. The primary development of the skeleton in nerveless and poorly innervated limb transplants of chick embryos. Physiol Zool. 1940;13:367–384. [Google Scholar]
- Harryman DT, II, Mack LA, Wang KY, et al. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73:982–989. [PubMed] [Google Scholar]
- Henderson JH, Carter DR. Mechanical induction in limb morphogenesis: the role of growth-generated strains and pressures. Bone. 2002;31:645–653. doi: 10.1016/s8756-3282(02)00911-0. [DOI] [PubMed] [Google Scholar]
- Hill WCO. Cebidae, Part A. Vol. 4. Edinburgh: University Press; 1964. Primates, comparative anatomy and taxonomy. [Google Scholar]
- Ho AM, Marker PC, Peng H, et al. Dominant negative Bmp5 mutation reveals key role of BMPs in skeletal response to mechanical stimulation. BMC Dev Biol. 2008;8:35. doi: 10.1186/1471-213X-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini A, Hogg DA. The effects of paralysis on skeletal development in the chick embryo. II. Effects on histogenesis of the tibia. J Anat. 1991;177:169–178. [PMC free article] [PubMed] [Google Scholar]
- Kahn J, Shwartz Y, Blitz E, et al. Muscle contraction is necessary to maintain joint progenitor cell fate. Dev Cell. 2009;16:734–743. doi: 10.1016/j.devcel.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Kardon G. Muscle and tendon morphogenesis in the avian hind limb. Development. 1998;125:4019–4032. doi: 10.1242/dev.125.20.4019. [DOI] [PubMed] [Google Scholar]
- Kieny M, Chevallier A. Autonomy of tendon development in the embryonic chick wing. J Embryol Exp Morphol. 1979;49:153–165. [PubMed] [Google Scholar]
- Kim HM, Galatz LM, Das R, et al. Musculoskeletal deformities secondary to neurotomy of the superior trunk of the brachial plexus in neonatal mice. J Orthop Res. 2010;28:1391–1398. doi: 10.1002/jor.21128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HM, Galatz LM, Patel N, et al. Recovery potential after postnatal shoulder paralysis. An animal model of neonatal brachial plexus palsy. J Bone Joint Surg Am. 2009;91:879–891. doi: 10.2106/JBJS.H.00088. [DOI] [PubMed] [Google Scholar]
- Kulyk WM, Rodgers BJ, Greer K, Kosher RA. Promotion of embryonic chick limb cartilage differentiation by transforming growth factor-β. Dev Biol. 1989;135:424–430. doi: 10.1016/0012-1606(89)90191-7. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Fuld HM, Frenz DA, et al. Role of transforming growth factor-β in chondrogenic pattern formation in the embryonic limb: stimulation of mesenchymal condensation and fibronectin gene expression by exogenenous TGF-β and evidence for endogenous TGF-β-like activity. Dev Biol. 1991;145:99–109. doi: 10.1016/0012-1606(91)90216-p. [DOI] [PubMed] [Google Scholar]
- Liu CF, Aschbacher-Smith L, Barthelery NJ, et al. Spatial and temporal expression of molecular markers and cell signals during normal development of the mouse patellar tendon. Tissue Eng Part A. 2012a;18:598–608. doi: 10.1089/ten.tea.2011.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CF, Breidenbach A, Aschbacher-Smith L, et al. A role for hedgehog signaling in the differentiation of the insertion site of the patellar tendon in the mouse. PLoS One. 2013;8:e65411. doi: 10.1371/journal.pone.0065411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Birman V, Chen C, et al. Mechanisms of bimaterial attachment at the interface of tendon to bone. J Eng Mater Technol. 2011;133:281–288. doi: 10.1115/1.4002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YX, Thomopoulos S, Birman V, et al. Bi-material attachment through a compliant interfacial system at the tendon-to-bone insertion site. Mech Mater. 2012b;44:83–92. doi: 10.1016/j.mechmat.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorda-Diez CI, Montero JA, Martinez-Cue C, et al. Transforming growth factors β coordinate cartilage and tendon differentiation in the developing limb mesenchyme. J Biol Chem. 2009;284:29988–29996. doi: 10.1074/jbc.M109.014811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HH, Thomopoulos S. Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu Rev Biomed Eng. 2013;15:201–226. doi: 10.1146/annurev-bioeng-071910-124656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino R, Ganan Y, Macias D, et al. Morphogenesis of digits in the avian limb is controlled by FGFs, TGFβs, and noggin through BMP signaling. Dev Biol. 1998;200:35–45. doi: 10.1006/dbio.1998.8946. [DOI] [PubMed] [Google Scholar]
- Mikic B, Johnson TL, Chhabra AB, et al. Differential effects of embryonic immobilization on the development of fibrocartilaginous skeletal elements. J Rehabil Res Dev. 2000;37:127–133. [PubMed] [Google Scholar]
- Murchison ND, Price BA, Conner DA, et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- Ng LJ, Wheatley S, Muscat GE, et al. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev Biol. 1997;183:108–121. doi: 10.1006/dbio.1996.8487. [DOI] [PubMed] [Google Scholar]
- Nowlan NC, Sharpe J, Roddy KA, et al. Mechanobiology of embryonic skeletal development: insights from animal models. Birth Defects Res C Embryo Today. 2010;90:203–213. doi: 10.1002/bdrc.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Yoshida A, Inokuchi W. Deltoid contracture: a radiographic survey of bone and joint abnormalities. J Shoulder Elbow Surg. 1999;8:22–25. doi: 10.1016/s1058-2746(99)90049-6. [DOI] [PubMed] [Google Scholar]
- Osborne AC, Lamb KJ, Lewthwaite JC, et al. Short-term rigid and flaccid paralyses diminish growth of embryonic chick limbs and abrogate joint cavity formation but differentially preserve pre-cavitated joints. J Musculoskelet Neuronal Interact. 2002;2:448–456. [PubMed] [Google Scholar]
- Ovchinnikov DA, Selever J, Wang Y, et al. BMP receptor type IA in limb bud mesenchyme regulates distal outgrowth and patterning. Dev Biol. 2006;295:103–115. doi: 10.1016/j.ydbio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Pai AC. Developmental genetics of a lethal mutation, muscular dysgenesis (Mdg), in the mouse. II. Developmental analysis. Dev Biol. 1965;11:93–109. doi: 10.1016/0012-1606(65)90039-4. [DOI] [PubMed] [Google Scholar]
- Provot S, Schipani E. Molecular mechanisms of endochondral bone development. Biochem Biophys Res Commun. 2005;328:658–665. doi: 10.1016/j.bbrc.2004.11.068. [DOI] [PubMed] [Google Scholar]
- Pryce BA, Watson SS, Murchison ND, et al. Recruitment and maintenance of tendon progenitors by TGFβ signaling are essential for tendon formation. Development. 2009;136:1351–1361. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeo SA, Arnoczky SP, Torzilli PA, et al. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75:1795–1803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- Rot-Nikcevic I, Reddy T, Downing KJ, et al. Myf5−/−: MyoD−/− amyogenic fetuses reveal the importance of early contraction and static loading by striated muscle in mouse skeletogenesis. Dev Genes Evol. 2006;216:1–9. doi: 10.1007/s00427-005-0024-9. [DOI] [PubMed] [Google Scholar]
- Schwartz AG, Lipner JH, Pasteris JD, et al. Muscle loading is necessary for the formation of a functional tendon enthesis. Bone. 2013;55:44–51. doi: 10.1016/j.bone.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AG, Pasteris JD, Genin GM, et al. Mineral distributions at the developing tendon enthesis. PLoS One. 2012;7:e48630. doi: 10.1371/journal.pone.0048630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer R, Chyung JH, Murtaugh LC, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- Seo HS, Serra R. Deletion of Tgfbr2 in Prx1-cre expressing mesenchyme results in defects in development of the long bones and joints. Dev Biol. 2007;310:304–316. doi: 10.1016/j.ydbio.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharir A, Stern T, Rot C, et al. Muscle force regulates bone shaping for optimal load-bearing capacity during embryogenesis. Development. 2011;138:3247–3259. doi: 10.1242/dev.063768. [DOI] [PubMed] [Google Scholar]
- Spagnoli A, O’Rear L, Chandler RL, et al. TGF-β signaling is essential for joint morphogenesis. J Cell Biol. 2007;177:1105–1117. doi: 10.1083/jcb.200611031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y, Takimoto A, Akiyama H, et al. Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development. 2013;140:2280–2288. doi: 10.1242/dev.096354. [DOI] [PubMed] [Google Scholar]
- Thomopoulos S, Genin GM, Birman V, editors. Structural interfaces and attachments in biology. New York: Springer; 2013. p. 386. [Google Scholar]
- Thomopoulos S, Hattersley G, Rosen V, et al. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J Orthop Res. 2002;20:454–463. doi: 10.1016/S0736-0266(01)00144-9. [DOI] [PubMed] [Google Scholar]
- Thomopoulos S, Kim HM, Rothermich SY, et al. Decreased muscle loading delays maturation of the tendon enthesis during postnatal development. J Orthop Res. 2007;25:1154–1163. doi: 10.1002/jor.20418. [DOI] [PubMed] [Google Scholar]
- Thomopoulos S, Marquez JP, Weinberger B, et al. Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. J Biomech. 2006;39:1842–1851. doi: 10.1016/j.jbiomech.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Thomopoulos S, Williams GR, Gimbel JA, et al. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res. 2003a;21:413–419. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003b;125:106–113. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Dietrich S, Mericskay M, et al. A crucial role for Pax3 in the development of the hypaxial musculature and the long-range migration of muscle precursors. Dev Biol. 1998;203:49–61. doi: 10.1006/dbio.1998.9041. [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Mauviel A. Transforming growth factor-β signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- Vortkamp A, Lee K, Lanske B, et al. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- Wang M, VanHouten JN, Nasiri AR, et al. PTHrP regulates the modeling of cortical bone surfaces at fibrous insertion sites during growth. J Bone Miner Res. 2013;28:598–607. doi: 10.1002/jbmr.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SL, An K, Frank CB, et al. Anatomy, biology, and biomechanics of tendon and ligament. In: Buckwalter JA, Einhorn T, Simon SR, editors. Orthopaedic basic science. 2. American Academy of Orthopaedic Surgeons; Rosemont, IL: 2000. pp. 581–616. [Google Scholar]
- Wu Q, Zhang Y, Chen Q. Indian hedgehog is an essential component of mechanotransduction complex to stimulate chondrocyte proliferation. J Biol Chem. 2001;276:35290–35296. doi: 10.1074/jbc.M101055200. [DOI] [PubMed] [Google Scholar]
- Xu T, Yang K, You H, et al. Regulation of PTHrP expression by cyclic mechanical strain in postnatal growth plate chondrocytes. Bone. 2013;56:304–311. doi: 10.1016/j.bone.2013.06.027. [DOI] [PubMed] [Google Scholar]
- Yu K, Ornitz DM. FGF signaling regulates mesenchymal differentiation and skeletal patterning along the limb bud proximodistal axis. Development. 2008;135:483–491. doi: 10.1242/dev.013268. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Eberspaecher H, Lefebvre V, De Crombrugghe B. Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev Dyn. 1997;209:377–386. doi: 10.1002/(SICI)1097-0177(199708)209:4<377::AID-AJA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]