Abstract
Background
Transient gene expression via Agrobacterium-mediated DNA transfer offers a simple and fast method to analyze transgene functions. Although Arabidopsis is the most-studied model plant with powerful genetic and genomic resources, achieving highly efficient and consistent transient expression for gene function analysis in Arabidopsis remains challenging.
Results
We developed a highly efficient and robust Agrobacterium-mediated transient expression system, named AGROBEST (Agrobacterium-mediated enhanced seedling transformation), which achieves versatile analysis of diverse gene functions in intact Arabidopsis seedlings. Using β-glucuronidase (GUS) as a reporter for Agrobacterium-mediated transformation assay, we show that the use of a specific disarmed Agrobacterium strain with vir gene pre-induction resulted in homogenous GUS staining in cotyledons of young Arabidopsis seedlings. Optimization with AB salts in plant culture medium buffered with acidic pH 5.5 during Agrobacterium infection greatly enhanced the transient expression levels, which were significantly higher than with two existing methods. Importantly, the optimized method conferred 100% infected seedlings with highly increased transient expression in shoots and also transformation events in roots of ~70% infected seedlings in both the immune receptor mutant efr-1 and wild-type Col-0 seedlings. Finally, we demonstrated the versatile applicability of the method for examining transcription factor action and circadian reporter-gene regulation as well as protein subcellular localization and protein–protein interactions in physiological contexts.
Conclusions
AGROBEST is a simple, fast, reliable, and robust transient expression system enabling high transient expression and transformation efficiency in Arabidopsis seedlings. Demonstration of the proof-of-concept experiments elevates the transient expression technology to the level of functional studies in Arabidopsis seedlings in addition to previous applications in fluorescent protein localization and protein–protein interaction studies. In addition, AGROBEST offers a new way to dissect the molecular mechanisms involved in Agrobacterium-mediated DNA transfer.
Keywords: Agrobacterium, Arabidopsis, Transient transformation, Gene expression, Innate immunity, Gain-of-function
Background
Agrobacterium-mediated DNA transfer is currently the most facile and versatile method to deliver gene constructs into the nucleus for gene function analysis in diverse plant species [1-3]. Although stable integration of physiologically active and regulated transgenes is the ultimate goal, transient gene expression via Agrobacterium-mediated DNA transfer in different plant tissues offers a simple and fast method to analyze transgene functions, which is amenable for high-throughput screens. The transient expression assay is also ideal for systematic dissection of the exquisite and complex processes of Agrobacterium–plant interactions and DNA transfer events [4-7].
Agrobacterium tumefaciens is a soil phytopathogen that naturally infects plant wound sites and causes crown gall disease via delivery of transferred (T)-DNA from bacterial cells into host plant cells through a bacterial type IV secretion system (T4SS) [8]. Although Agrobacterium is considered a wound-associated pathogen, it can transfer DNA into diverse host cells or tissues under unwounded conditions [9-13]. Interestingly, most of the Arabidopsis mutants that are resistant to Agrobacterium transformation identified by root explant assays remain highly transformable by floral dip transformation [14]. The mechanisms and plant factors involved in Agrobacterium-mediated transformation may differ between wounded and unwounded cells or different tissues. However, the mechanisms underlying Agrobacterium infection in unwounded cells/tissues have not been explored.
In plant biology research, Arabidopsis mesophyll-protoplast transfection [15,16] and Agrobacterium-mediated leaf infiltration in Nicotiana benthamiana[17] are the well-established and commonly used platforms for transient gene expression analysis. The Arabidopsis mesophyll-protoplast transient expression system allows for versatile and high-throughput analyses of diverse gene functions and signal transduction pathways; advanced skills with training and practice are essential for successful use of this powerful tool for gene function studies [16,18,19]. Agrobacterium-mediated transient expression methods by leaf infiltration have been developed for a wide range of plants including Nicotiana, lettuce, tomato, and Arabidopsis[20-23]. However, the use of 4- to 5-week-old adult plants with manual infiltration has limited application in high-throughput analyses. Furthermore, although Arabidopsis is the most-studied model plant with superbly annotated genome sequences and powerful genetic and genomic resources mostly available for the Columbia (Col) accession, achieving highly efficient and consistent transient expression in Col by adult leaf infiltration is challenging [22,24].
The use of young seedlings for Agrobacterium-mediated transient expression assays will greatly simplify and amplify the power of the method. Indeed, Agrobacterium-mediated transient expression in Arabidopsis seedlings has been recently developed for fast and robust analysis of protein subcellular localization and protein–protein interactions [25-27]. The system’s requirement for high-density Agrobacterium cells and vacuum infiltration [27] or chemical treatment (e.g., the addition of surfactant Silwet L-77) [26] to achieve high cellular transformation efficiency could induce innate immunity and stress responses in plants, which globally alters cellular, physiological, and signaling processes and severely retards growth [28,29]. Thus, developing a system that circumvents a plant defense barrier may be a key to enhance transient expression efficiency in Arabidopsis seedlings. Furthermore, such a fast, robust, and highly efficient transient expression system could support gain-of-function studies of diverse genes and signaling pathways in planta.
Pattern-triggered immunity (PTI) induced by a microbe- or pathogen-associated molecular pattern (MAMP or PAMP) is the first line of active defense in both plants and animals against pathogens [28-30]. Previous studies have suggested that Agrobacterium-mediated transformation efficiency may be compromised when plants recognize Agrobacterium MAMPs by corresponding pattern-recognition receptors (PRRs) to trigger PTI and block Agrobacterium infection [22,24]. The elongation factor Tu (EF-Tu) receptor mutant efr-1, which cannot sense EF-Tu MAMP, showed increased Agrobacterium-mediated transient expression efficiency, as did transgenic Arabidopsis expressing a potent bacterial effector AvrPto to suppress PTI signaling with agroinfiltration of 4- to 5-week-old leaves [22,24]. However, whether these immune-compromised Arabidopsis plants are amenable to increase Agrobacterium-mediated transient expression efficiency in young seedlings has not been tested. Defining the condition for reliable and highly efficient transformation in healthy Col-0 seedlings will be extremely valuable but has never been achieved.
In this study, we systematically investigated various biological factors and growth variances to define a combination of key factors that contribute to the unprecedentedly high transient transformation and reporter gene expression efficiency in Arabidopsis seedlings. As a result of these investigations, we developed an optimized AGROBEST (Agrobacterium-mediated enhanced seedling transformation) method that enabled high transient transformation and expression efficiency in both efr-1 mutant and Col-0 Arabidopsis seedlings. Importantly, we demonstrated the versatile applicability of AGROBEST in gain-of-function studies for the MYB75 transcription factor in specific target-gene activation and for GIGANTEA (GI) reporter gene expression regulated by the Arabidopsis circadian clock. The AGROBEST method is a fast, simple, reliable, and versatile tool for systematic gene function analysis and a new tool for dissecting the Agrobacterium-mediated DNA transfer processes.
Results
Cotyledons of young Arabidopsis EF-TU receptor mutant is highly susceptible to Agrobacterium-mediated transient transformation
Environmental and biological factors such as growth conditions, host plants, and Agrobacterium strains can affect the transformation efficiency. We first evaluated the transient expression efficiency of selected Arabidopsis ecotypes and mutants defective in pattern-recognition receptors (PRRs) with a disarmed A. tumefaciens strain C58C1(pTiB6S3ΔT-DNA) [31] containing a pCH32 helper plasmid [32] and abbreviated as C58C1(pTiB6S3ΔT)H. The T-DNA vector pBISN1 harboring the gusA-intron[12] was transformed into C58C1(pTiB6S3ΔT)H to infect 4-d-old seedlings, and β-glucuronidase (GUS) activity was determined to monitor transient expression efficiency at 3 days post-infection (dpi). We consistently observed 100% of analyzed EF-Tu receptor mutant efr-1 seedlings were successfully transformed, with strong and homogenous GUS staining in cotyledons, with 4-fold higher GUS activity in efr-1 than wild-type Col-0 seedlings (Figure 1A and B, Additional file 1: Table S1). The flagellin receptor mutant, fls2, and the Ws ecotype that is highly susceptible to Agrobacterium transformation in the root explant [14] and a natural fls2 variant [33] showed similar transient GUS expression efficiency as the Col-0, so the fls2 mutant contributes little to enhancing Agrobacterium-mediated transient transformation (Figure 1A and B). Our seedling transient expression results confirm and further support that EFR but not FLS2 is an important factor limiting Agrobacterium-mediated transient expression efficiency previously observed by agroinfiltration of Arabidopsis adult leaves [24,34,35].
Figure 1.
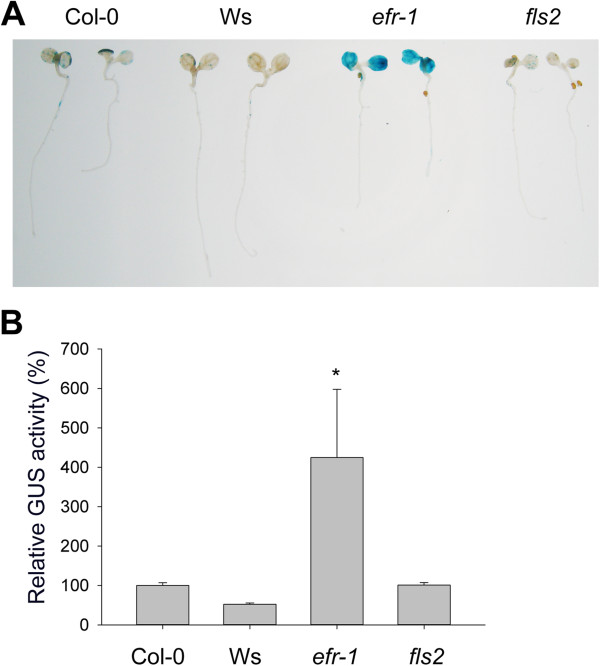
Transient transformation assays in different Arabidopsis ecotype/genotypes. Four-day-old Arabidopsis seedlings were infected with Agrobacterium strain C58C1(pTiB6S3ΔT)H carrying pBISN1, which was pre-incubated in AB-MES (pH5.5) supplemented with 200 μM acetosyringone (AS) to induce vir gene expression. Seedlings were co-cultivated with pre-induced A. tumefaciens cells with final OD600 = 0.02 in the MS medium (1/2 MS, 0.5% sucrose (w/v), pH 5.5) containing 50 μM AS and determined for transient GUS expression levels by overnight GUS staining (A) and quantitative GUS activity (B) at 3 dpi. The GUS activity obtained from Col-0 seedlings was set to 100% and that of Ws, efr-1, and fls2 is relative to that of Col-0. Data are mean ± SD GUS activity from two biological replicates. Similar results were obtained from at least two independent experiments. Values significantly different from that obtained with Col-0 are denoted (*P = 0.058 by Student’s t test).
Buffered medium at pH 5.5 with AB salts is critical for high transient expression efficiency
To exploit this transient expression system for higher efficiency, we tested several factors including pre-induction and co-cultivation conditions. Pre-induction with acetosyringone (AS) in AB-MES medium (ABM50 and ABM200 methods) and continuous addition of AS to stimulate vir gene expression during the infection process are required for efficient transient GUS expression. Because AB-MES medium (pH 5.5) is the optimized medium for vir gene induction [36,37], we tested whether mixing AB-MES (pH 5.5) with an equal volume of commonly used plant culture MS medium (1/2 MS, 0.5% sucrose (w/v), pH 5.5), named ABM-MS (1/2 AB-MES, 1/4 MS, 0.25% sucrose (w/v), pH 5.5) in the presence of AS could produce high transient expression efficiency. Strikingly, GUS activity was strongly expressed in all seedlings and was 20-fold higher with co-cultivation in ABM-MS than in MS medium alone (Figure 2A, Additional file 1: Table S1). To avoid over-staining, the reaction time for histological GUS staining shown in Figure 2 was limited to 6 hr instead of overnight for the result in Figure 1A.
Figure 2.
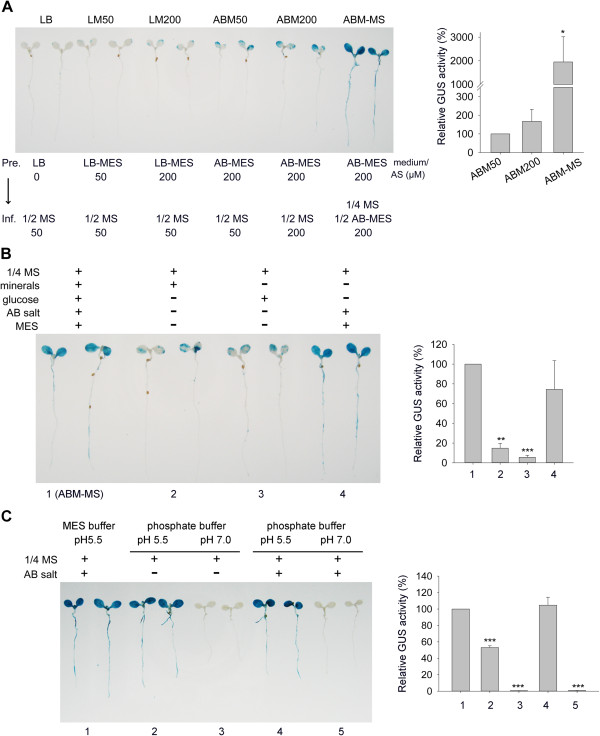
Optimization of Agrobacterium pre-culture and infection media for efficient transient expression efficiency. Four-day-old Arabidopsis efr-1 seedlings infected with Agrobacterium C58C1(pTiB6S3ΔT)H carrying pBISN1 were grown in various pre-culture and co-cultivation media to test their effects on transient GUS expression efficiency measured by GUS staining and quantitative GUS activity. (A) Various pre-culture and infection media in the absence or presence of vir gene inducer AS at the indicated concentration. (B) Effect of factors in AB-MES medium on increased transient expression efficiency. (C) Effect of AB salts, pH and buffering systems on transient GUS expression efficiency. Data for relative quantitative GUS activity are mean ± SD of 3 independent experiments. Values significantly different from that infected by ABM50 (A) or condition 1 (B and C) are denoted (*P < 0.05, **P < 0.01, ***P < 0.005 by Student’s t test).
Key components in AB-MES are AB salt (17.2 mM K2HPO4, 8.3 mM NaH2PO4, 18.7 mM NH4Cl, 2 mM KCl), minerals (1.25 mM MgSO4, 100 μM CaCl2, 10 μM FeSO4), glucose (2% w/v), and buffering with MES (50 mM) to pH 5.5. We thus tested whether one of these components is responsible for the increased transient expression efficiency. The addition of AB salts with MES buffered at pH 5.5 in MS medium was sufficient to result in comparable levels of GUS expression as with ABM-MS (Figure 2B). Therefore, AB salts alone, pH 5.5 buffered by MES, or both, are critical for the increased transient expression efficiency. Strikingly, all MS media with the addition of AB salts buffered with MES or sodium phosphate at pH 5.5 showed comparable and strong GUS activity as that with ABM-MS (Figure 2C). However, omitting AB salts resulted in ~50% reduction in GUS activity, and no GUS activity was detected with MS medium buffered with sodium phosphate at pH 7.0 in the presence or absence of AB salts. Thus, buffered pH at 5.5 and the presence of AB salts in MS co-cultivation medium are the two key factors for this high transient expression efficiency. We named this optimized infection method AGROBEST (Agrobacterium-mediated enhanced seedling transformation).
Disarmed Agrobacterium strain C58C1(pTiB6S3ΔT)H enables highly efficient AGROBEST-mediated transient expression in Col-0 seedlings
Next, we tested whether the AGROBEST method optimized with efr-1 seedlings could also improve Agrobacterium-mediated transient transformation in wild-type Col-0 seedlings. Because the use of C58C1(pTiB6S3ΔT)H as compared with other disarmed or virulent A. tumefaciens strains produced higher transient expression levels with leaf agroinfiltration of various plants [23], we also tested whether C58C1(pTiB6S3ΔT)H is a more superior strain in our system. We compared C58C1(pTiB6S3ΔT)H with the wild-type virulent strain C58 or C58-derived disarmed strain GV3101(pMP90) [38] for their transient expression efficiency in efr-1 and Col-0 seedlings using both sub-optimal ABM50 and optimized AGROBEST methods. Remarkably, Col-0 seedlings infected by all transfer-competent strains achieved significantly higher transient expression efficiency by AGROBEST than ABM50 (Figure 3A and B). Moreover, Col-0 seedlings infected by AGROBEST showed higher transient expression than efr-1 seedling infected by ABM50 (Figure 3A and B). No GUS stains could be detected in control seedlings without infection (MOCK) or infected with ΔvirB2, a strain lacking the key component of the type IV secretion system (T4SS) essential for T-DNA/effector translocation [8,39]. Therefore, the GUS activity detected was indeed from T-DNA gene expression inside the plant cells. Strikingly, 5- to 15-fold higher GUS activity was observed in efr-1 or Col-0 seedlings infected with C58C1(pTiB6S3ΔT)H than with C58 or GV3101(pMP90) (Figure 3A and B). The root length was significantly shorter for Arabidopsis seedlings infected with C58, ΔvirB2, or GV3101(pMP90) at 3 dpi in all combinations or with C58C1(pTiB6S3ΔT)H by the AGROBEST method as compared with uninfected seedlings (MOCK) (Figure 3C). Notably, seedlings infected with C58C1(pTiB6S3ΔT)H showed no or little inhibition of root elongation in efr-1 seedlings with the AMB50 method, which achieves fair although not the highest transient expression efficiency.
Figure 3.
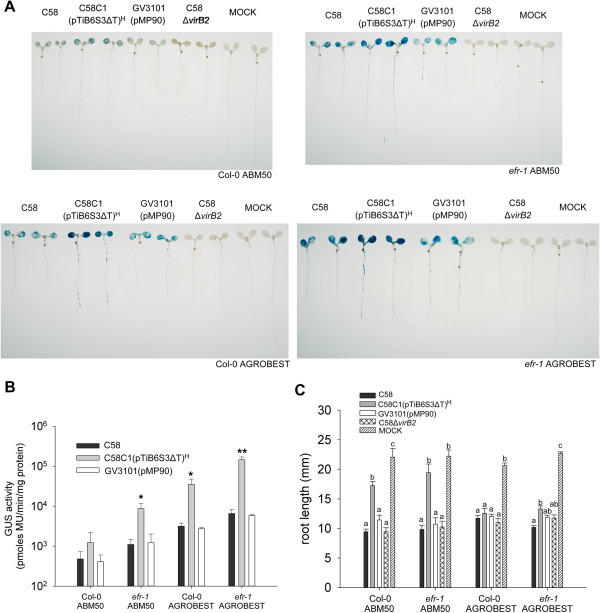
Transient transformation of the Arabidopsis seedlings by various Agrobacterium strains. Four-day-old Arabidopsis Col-0 and efr-1 seedlings infected with different Agrobacterium stains carrying pBISN1 by ABM50 or ABM-MS (named as AGROBEST) were compared by GUS staining (A), quantitative GUS activity (B), and root length (C) at 3 days post-inoculation (dpi). Data for quantitative GUS activity are mean ± SD of at least 4 biological replicates from 2 independent experiments. Values significantly different from that infected with wild-type C58 are denoted (*P < 0.05, **P < 0.01 by Student’s t test). Data for root length measurement are mean ± SEM of 4-6 biological replicates from 2–4 independent experiments. Statistics was analyzed by ANOVA and means annotated with the same letter (a-c) are not significantly different; those with different letters are significantly different (P < 0.05). Seedlings grown in the same co-cultivation medium without Agrobacterium infection are indicated (MOCK).
AGROBEST achieves higher transient expression efficiency than existing methods in both efr-1 and Col-0 seedlings
We also compared AGROBEST with previously developed methods [26,27] for their transient expression efficiency in both Col-0 and efr-1 seedlings. Remarkably, all Col-0 seedlings infected by the AGROBEST showed ~10-fold increased transient expression efficiency than with two existing methods, the FAST method [26] and the method by Marion et al. [27] with either GUS (Figure 4A) or luciferase (LUC2) (Figure 4B) used as reporters. Interestingly, both AGROBEST and the Marion et al. method achieved significantly higher transient expression activity in efr-1 than in Col-0, efr-1 seedlings remained poorly transformed by the FAST method (Figure 4A and B). As a result, AGROBEST conferred at least 40-fold and 3-fold higher transient expression efficiency in efr-1 seedlings than with FAST and the Marion et al. methods, respectively (Figure 4A and B). However, we detected no increased transient expression activity in infected seedlings of the dexamethasone (DEX)-induced AvrPto transgenic line than in Col-0 seedlings with the AGROBEST method (Figure 4C), despite a significantly higher transient expression efficiency than Col-0 detected in adult leaves by agroinfiltration [22]. The AvrPto transgenic line germinated at the same rate and grew to a similar size as Col-0 and efr-1, but growth was arrested with the addition of DEX at 3 days old. This finding is consistent with previous studies showing that overexpression of AvrPto can also interfere with growth hormone signals and trigger cell death by interrupting the diverse functions of BAK1 and BKK1 in multiple receptor complexes, not restricted to PRRs [40].
Figure 4.
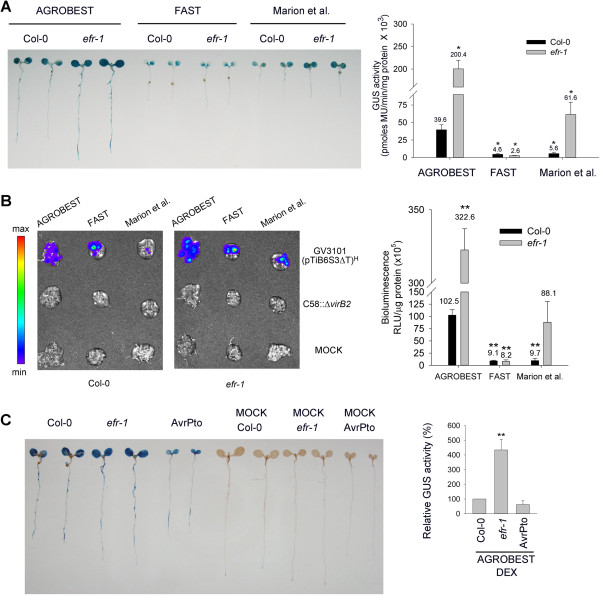
AGROBEST enables high transient expression levels in Col-0. Four-day-old Arabidopsis seedlings were infected with Agrobacterium strain C58C1(pTiB6S3ΔT)H carrying pBISN1 (A and C) or 35S::LUC2(B), and transient expression activity was determined at 3 dpi. (A) Transient GUS expression efficiency of Col-0 and efr-1 seedlings by AGROBEST, FAST and Marion et al. methods. Data for quantitative GUS activity are mean ± SD of 3 biological replicates. Values significantly different from those obtained with Col-0 by AGROBEST are denoted (*P < 0.05 by Student’s t test). (B) Transient luciferase expression efficiency of Col-0 and efr-1 seedlings by AGROBEST, FAST and Marion et al. methods. Seedlings infected by C58ΔvirB2 carrying 35S::LUC2 were used as a background control and those without Agrobacterium infection are indicated as MOCK. Luciferase activity of Col-0 obtained by AGROBEST was set to 100% and that of others is relative to activity of Col-0 by AGROBEST. Data are mean ± SD of 3 biological replicates. Values significantly different from those obtained with Col-0 by AGROBEST are denoted (**P < 0.01, by Student’s t test). (C) Transient GUS expression efficiency of Col-0, AvrPto transgenic line, and efr-1 by AGROBEST. For dexamethasone (DEX) treatment, 3-d-old seedlings were treated with 10 μM DEX for 1 day and the following 3 days infected by the AGROBEST method. Quantitative GUS activity from DEX-induced Col-0 seedlings by AGROBEST was set to 100% and that of others is relative to activity of DEX-induced Col-0 seedlings with AGROBEST. Data are mean ± SD GUS activity from 4 repeats (2 biological repeats from each of 2 independent experiments). Values significantly different from that obtained with Col-0 are denoted (**P < 0.01 by Student’s t test). Seedlings grown in the same co-cultivation medium without Agrobacterium infection are indicated (MOCK).
Impact of seedling age and infection time on transient expression efficiency of AGROBEST in efr-1 seedlings
Because the highest transient expression efficiency in efr-1 seedlings can be achieved by infection with C58C1(pTiB6S3ΔT)H by AGROBEST, we chose this combination to test the versatility and applicability of AGROBEST. For example, dissecting the minimal infection time (from 1–5 days) and range of seedling age (from 3- to 6-d-old) applicable for efficient transient expression is of interest. We tested different ages of Arabidopsis efr-1 seedlings infected at different dpi and noted that GUS signals were barely detectable at 1 dpi but gradually reached a plateau at 3 or 4 dpi (Figure 5A). When 5- or 6-d-old seedlings were infected, we observed transient GUS expression in true leaves at 3 or 4 dpi. Although strong GUS staining could still be detected in seedlings at 4 or 5 dpi, these seedlings often showed bleached lesions in cotyledons (Figure 5B), which explained the lack of GUS expression in part of the cotyledons at 4 or 5 dpi (Figure 5A). At 3 dpi, the bleached lesions were more visible when the transformation was performed with 5- or 6-d-old seedlings than with 3- or 4-d-old seedlings. Thus, the use of younger seedlings for AGROBEST may be more desirable for maintaining plants in healthy and physiological conditions.
Figure 5.
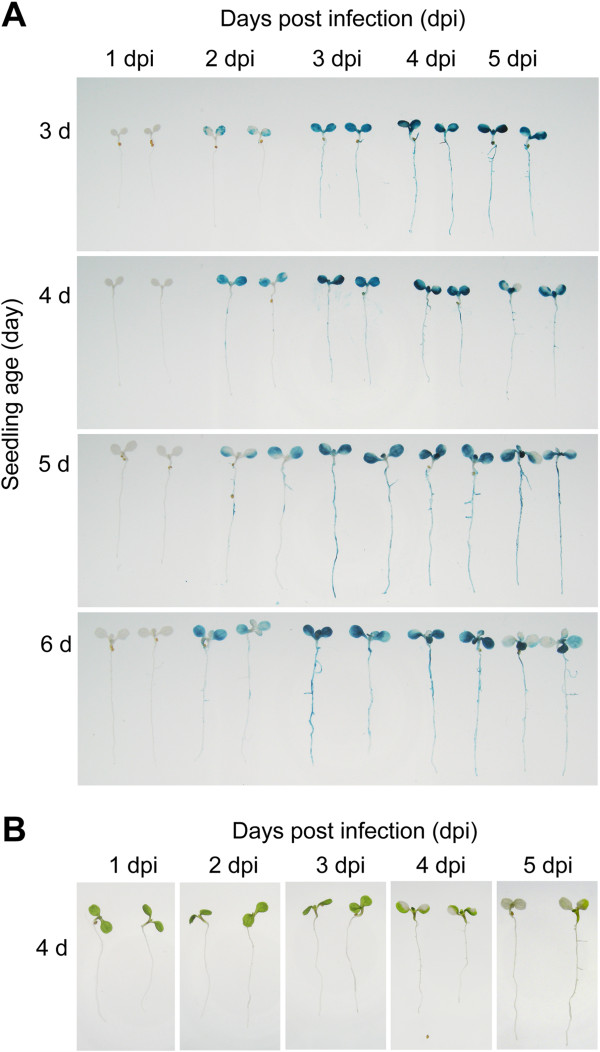
Impact of seedling age and infection time on transient expression. (A and B) Different ages of Arabidopsis efr-1 seedlings were infected with C58C1(pTiB6S3ΔT)H carrying pBISN1 by the AGROBEST method and analyzed for GUS activity (A) and morphologic features (B) at different dpi.
To test the minimal infection time for GUS detection and to avoid plant damage due to prolonged Agrobacterium infection, the co-cultivation medium was replaced with fresh medium containing antibiotics (100 μM Timentin) at 1 or 2 dpi to inhibit bacterial growth. In 4-d-old seedlings, we detected low levels of GUS signals with an additional 2 or 3 days of cultivation after Timentin treatment at 1 dpi (Figure 6). Importantly, with Timentin treatment at 2 dpi, seedlings with 1 to 3 days of additional cultivation remained healthy (without bleached lesions) and showed strong GUS signals in cotyledons. Because Agrobacterium cells were mostly killed when true leaves emerged from infected seedlings, the newly grown true leaves were not efficiently transformed. Therefore, the use of 4-d-old seedlings infected for 2 days followed by an additional 1 to 3 days of cultivation with Timentin is the optimal condition to transiently express genes for functional studies.
Figure 6.
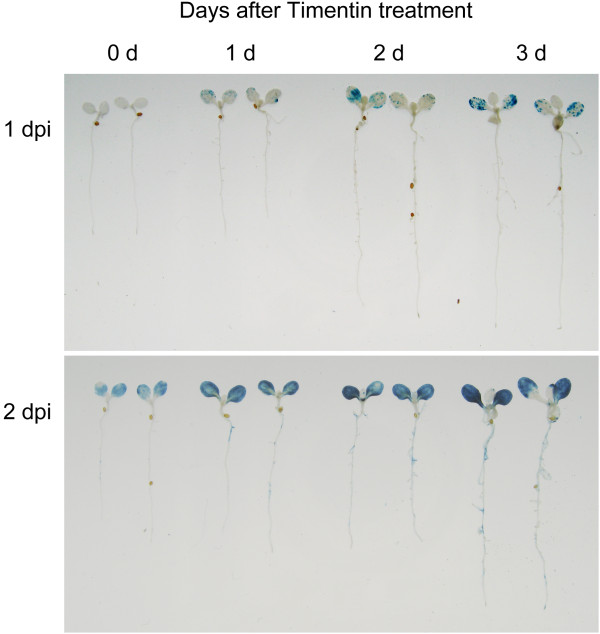
Impact of Timentin treatment on transient GUS expression efficiency. Four-day-old Arabidopsis efr-1 seedlings were infected with Agrobacterium C58C1(pTiB6S3ΔT)H carrying pBISN1 by the AGROBEST method at 1 or 2 dpi before Timentin treatment. GUS staining was performed at 0 to 3 days after Timentin treatment.
Widespread transient transformation events in different organs and cell types
The high transient expression efficiency with AGROBEST was mostly evident with strong and homogeneous GUS signals detected in cotyledons of 100% infected Col-0 or efr-1 seedlings (Figures 3A, 4A and 7A, Additional file 1: Table S1). When 7-d-old seedlings were used for infection, strong GUS signals were also detected in true leaves, as shown in efr-1 seedlings (Figure 7B). However, in roots, GUS signals could be detected in ~70% of Col-0 or efr-1 seedlings infected by AGROBEST (Additional file 1: Table S1) and mostly appeared in lateral root initiation sites or in the elongation zone (Figure 7C and D). In addition to analyzing the GUS reporter, we determined the expression of fluorescent proteins as reporters at cellular and subcellular levels using efr-1 seedlings. With expression of the Venus-intron or NLS-RFP driven by the CaMV 35S promoter, fluorescent protein signals were widely detected in cotyledon cells (Figure 7E and F), mainly in epidermal pavement cells but also in guard cells and mesophyll cells (Figure 7G-I). For roots, epidermal cells consistently showed fluorescent protein signals (Figure 7J). Therefore, the AGROBEST seedling transformation system allows for high transient gene expression and, potentially, functional analysis in diverse tissues and cell types in Arabidopsis seedlings.
Figure 7.
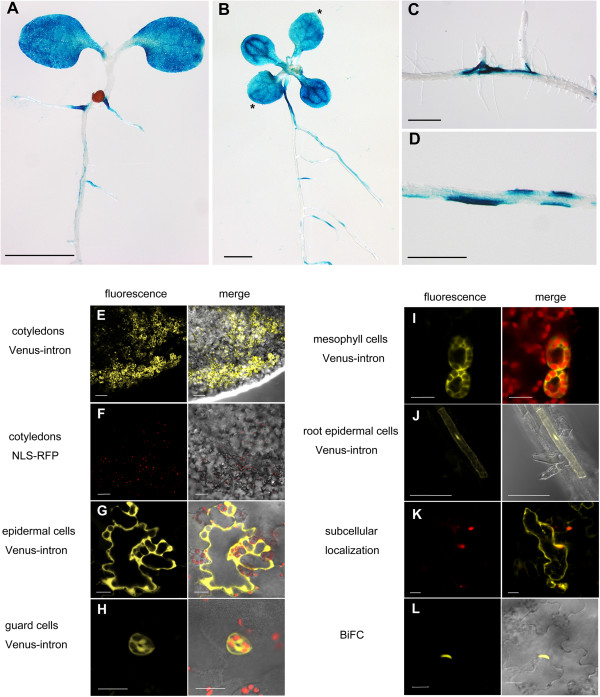
Transient transformation events in different organs and cell types. (A-D) Four-day-old (A and C-D) or 7-d-old (B)Arabidopsis efr-1 seedlings were infected with C58C1(pTiB6S3ΔT)H carrying pBISN1 by the AGROBEST method and analyzed for GUS staining. GUS staining was detected in true leaves (B, indicated by asterisk), cotyledons (A and B), main roots near lateral initiation site (C), and elongation zone (D). (E-L) Confocal microscopy of 4-day-old Arabidopsis efr-1 seedlings infected with C58C1(pTiB6S3ΔT)H carrying various vectors for transient expression of indicated fluorescent proteins by the ABM200 method. Fluorescence signals for 35S::Venus-intron or 35S::NLS-RFP were detected in cotyledons (E and F). Venus-intron signals were detected in different types of cells, including epidermal cells (G), guard cells (H), mesophyll cells (I) of cotyledon, and root epidermal cells (J). (K) Subcellular localization of Venus-intron and NLS-RFP by co-infection of 2 Agrobacterium strains expressing 35S::Venus-intron or 35S::NLS-RFP. (L) Protein–protein interaction by BiFC of nYFP-ASK1 and TIR1-cYFP. Images show fluorescence alone (K) and/or merged with bright field (E, F, J and L) or chloroplast fluorescence (G-I). Scale bars are 2 mm (A and B), 0.5 mm (C and D), 100 μm (E, F and J), 50 μm (L) and 20 μm (G-I and K). BiFC, bimolecular fluorescence complementation.
Studies of protein subcellular localization and protein–protein interactions
Because Arabidopsis plants are less amenable for transient expression analysis, both fluorescent protein localization and bimolecular fluorescence complementation (BiFC) studies are often conducted in protoplasts via transfection or in N. benthamiana leaves via agroinfiltration because of the high transient expression efficiency [16,17]. Here, we co-infected two A. tumefaciens strains carrying a binary vector for 35S::Venus-intron or 35S::NLS-RFP in efr-1 seedlings and detected both cytoplasmic and nuclear fluorescence signals for Venus and nuclear localization of NLS-RFP in separate or the same cells (Figure 7K). Our assay is also feasible for BiFC studies, which is supported by the interaction of two known interacting proteins, F-box protein TIR1 (transport inhibitor response 1) and ASK1 (Arabidopsis Skp1-like protein) [41], in the nucleus (Figure 7L). Thus, AGROBEST is an ideal system for subcellular localization and protein-protein interaction studies.
AGROBEST for the expression analysis of a circadian clock reporter gene
Encouraged by the high transient expression efficiency with AGROBEST, we next tested its applicability in gene function/regulation study in physiological contexts. Most Arabidopsis genes express rhythmically under various thermocycles, photocycles, or circadian clock conditions [42]. Reporter genes driven by promoters of the circadian genes are commonly used to monitor the regulation of circadian genes. To test whether circadian rhythm could be monitored in transiently transformed seedlings, a circadian reporter (GI::LUC2) constructed by fusing the promoter of the circadian gene GIGANTEA (GI) with the luciferase gene (LUC2) [43] was used. Four-day-old Arabidopsis efr-1 seedlings were infected with Agrobacterium delivering GI::LUC2 for 3 days under 16-h/8-h light/dark cycles and then transferred to MS medium in the presence of 100 μM Timentin and 0.5 mM luciferin under continuous light to monitor real-time bioluminescence for 5 days. In contrast to constant low levels of bioluminescence from seedlings infected with a vector control, Arabidopsis seedlings infected with Agrobacterium delivering GI::LUC2 showed clear circadian oscillation at slightly lengthened period for at least 5 days (Figure 8). The observed transiently expressed GI circadian cycle is indistinguishable from the stable GI expression in GI::LUC2 transgenic Arabidopsis plant (TP) [43], although with lower amplitude. The comparable circadian oscillation between the stable and transient expression of the GI::LUC2 indicated that the slightly longer period we observed was unlikely a result of the Agrobacterium infection. This result indicated the applicability of AGROBEST for transient expression of circadian rhythm reporter in Arabidopsis seedlings without detectable interference by Agrobacterium infection.
Figure 8.
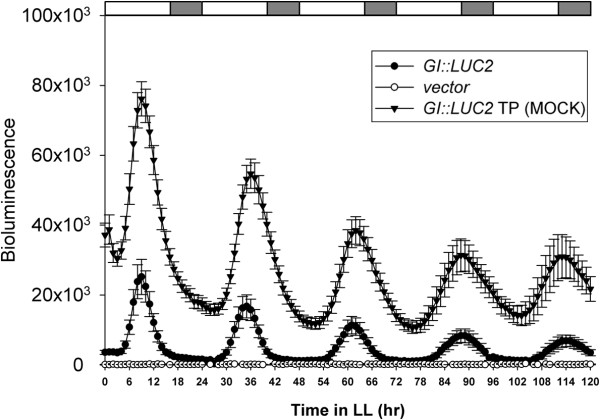
Monitoring Arabidopsis circadian rhythm by transient expression of GIGANTEA::luciferase (GI::LUC2). Four-day-old Arabidopsis efr-1 seedlings were infected with Agrobacterium C58C1(pTiB6S3ΔT)H carrying a vector (pCAMBIA1390) or p1390-GI-LUC2 by the AGROBEST method for 3 days in a 16-h/8-h light/dark cycle (75 μmol m-2 s-1), then transferred to 1/2 MS liquid medium in the presence of 100 μM Timentin and 0.5 mM luciferin and grown under continuous light at 40 μmol m-2 s-1 for up to 5 days. The GI::LUC2 transgenic Arabidopsis plant (TP) cultured in identical conditions without Agrobacterium infection was a positive control. Real-time bioluminescence signals were photographed and the luciferase intensity is shown as mean ± SEM from 12 seedlings expressing GI::LUC2. Similar results were obtained from at least 3 independent experiments. The white and gray regions indicate subjective light and dark periods, respectively.
AGROBEST for functional assays of transcription factor MYB75
Next, we tested AGROBEST for gain-of-function studies. For a proof of concept, we transiently expressed a transcription factor MYB75 because of its well-established function in anthocyanin accumulation by upregulating a key gene encoding chalcone synthase (CHS) in the anthocyanin synthesis pathway [44]. Four-day-old efr-1 Arabidopsis seedlings were infected for 3 days after Timentin treatment for an additional 3 days to determine the effect on MYB75 transient expression. MYB75 mRNA level in infected seedlings was 60-, 400-, and 200-fold higher when the expression was driven by single (1X35S) and double (2X35S) CaMV 35S promoter and super promoter, respectively, than in seedlings expressing control vectors (Figure 9A). CHS mRNA level was increased 4- and 3-fold in 2X35S::MYB75 and super::MYB75 seedlings, respectively (Figure 9B). However, CHS expression was not increased in 1X35S::MYB75 seedlings despite its 60-fold higher MYB75 expression, which suggests a threshold expression level or the requirement of other MYB75-modulated co-activators for CHS activation. Importantly, consistent with increased CHS expression, high level of anthocyanin (purple coloration) was readily detectable in cotyledons of 2X35S::MYB75 and super::MYB75 seedlings but not in seedlings infected with a vector control, 1X35S::MYB75, or super::gusA-intron (Figure 9C). No increase of anthocyanin accumulation from super::gusA-intron seedlings indicated that the specificity of the observed anthocyanin phenotype was due to the transient expression of MYB75 rather than a secondary effect from the infection or the overexpression of any foreign protein. Importantly, AGROBEST also enables the transient expression of MYB75 to monitor its downstream CHS expression and anthocyanin accumulation in Col-0 seedlings. We show that transient expression of MYB75 driven by the 2X35S promoter results in significantly higher of MYB75 mRNA levels than vector control in Col-0 seedlings (Figure 10A). Remarkably, CHS mRNA levels were also upregulated in 2X35S::MYB75 seedlings (Figure 10B), in which a moderate increase in anthocyanin accumulation was also detected (Figure 10C). This result strongly suggested the broad application of AGROBEST for gain-of-function studies not limited to the immune-compromised mutant.
Figure 9.
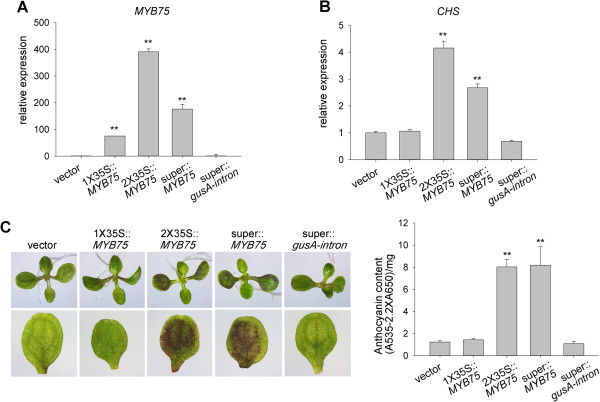
Transient expression of MYB75 increases anthocyanin accumulation. Four-day-old Arabidopsis efr-1 seedlings were infected with Agrobacterium C58C1(pTiB6S3ΔT)H carrying a vector (pCAMBIA1390), 35S::MYB75, 2X35S::MYB75, super::MYB75, or super::gusA-intron by the AGROBEST method. At 3 dpi, co-cultivation medium was replaced with MS medium containing 100 μM Timemtin for additional incubation for 3 days. qRT-PCR of relative expression of MYB75(A) and CHS(B) with representative data shown with mean ± SD from 3 technical repeats. Similar results were obtained from three independent experiments. Zeiss inverted microscopy of anthocyanin accumulation in seedlings (upper panels) and cotyledons (lower panels) and quantification (C). Data for anthocyanin content are mean ± SD from 4 repeats (2 biological repeats from each of 2 independent experiments, 20–30 seedlings for each biological repeat), Values significantly different from that obtained with vector are denoted (**P < 0.01, by Student’s t test).
Figure 10.
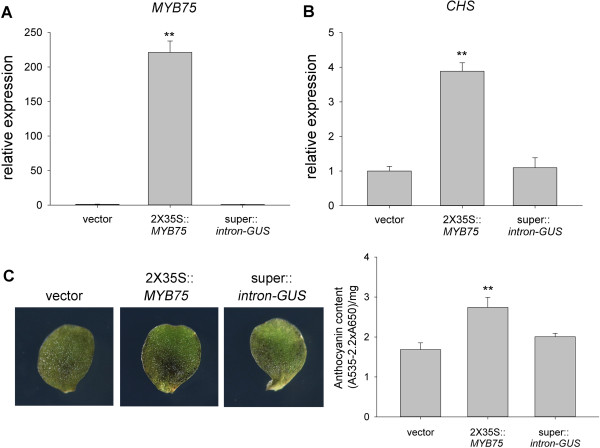
Transient expression of MYB75 increases anthocyanin accumulation in Col-0 seedlings. Four-day-old Arabidopsis Col-0 seedlings were infected with Agrobacterium C58C1(pTiB6S3ΔT)H carrying a vector (pCAMBIA1390), 2X35S::MYB75, or super::gusA-intron by the AGROBEST method. At 3 dpi, co-cultivation medium was replaced with MS medium containing 100 μM timemtin for additional incubation for 3 days. qRT-PCR of relative expression of MYB75(A) and CHS(B) with representative data shown with mean ± SD from 3 technical repeats. Similar results were obtained from three independent experiments. Zeiss inverted microscopy of anthocyanin accumulation in seedlings (upper panels) and cotyledons (lower panels) and quantification (C). Data for anthocyanin content are mean ± SD from 3 independent experiments (20–30 seedlings for each biological repeat, 3 biological repeats for each independent experiment). Values significantly different from those obtained with vector are denoted (**P < 0.01, by Student’s t test).
Discussion
AGROBEST enables high transient transformation and expression efficiency in intact Arabidopsis young seedlings
In this study, we developed a simple, fast, reliable, and robust transient expression system named AGROBEST and uncovered the key factors enabling 100% of infected seedlings with high transgene expression efficiency in Arabidopsis seedlings. Remarkably, AGROBEST appears to achieve the highest transient expression efficiency in the EF-Tu receptor efr-1 mutant as compared to the wild-type Col-0, flagellin receptor mutant fls2, and DEX-inducible AvrPto transgenic line. This result is consistent with a previous finding in agroinfiltrated Arabidopsis adult leaves showing increased transient GUS expression efficiency in efr-1[24]. Because of no detectable phenotype impairing the growth and development in the efr mutant [24], the use of the efr mutant has an advantage over DEX-inducible AvrPto in seedling stages. Thus, more selected elimination of specific PRRs such as EFR with minimal effects on hormonal signaling, cell death and seedling growth may be a preferred system for Agrobacterium-mediated high transient expression efficiency. Interestingly, N. benthamiana leaves, which are commonly used for Agrobacerium-mediated transient transformation, also lack the EFR receptor [45].
Unexpectedly, we discovered that AGROBEST also enables high transient expression efficiency in wild-type Col-0 seedlings. The significantly higher transient expression activity by AGROBEST than the FAST and Marion et al. methods likely accounts for the success of our gain-of-function experiments, which have not been shown previously [26,27]. Of note, efr-1 seedlings remained poorly transformed by FAST method as compared with the significantly increased transient expression in efr-1 by AGROBEST or the Marion et al. method. The reason underlying this discrepancy is unknown, but the yellowish and retarded-growth seedlings after co-cultivation with Agrobacterium in the dark for 2 days by the FAST method may contribute to the observed phenotype. Our AGROBEST method, applying a lower density of Agrobacterium cells (OD600 0.02 as opposed to OD600 0.5 for the FAST method and OD600 2 for the Marion et al. method) for co-cultivation with seedlings without any mechanical treatment (e.g., vacuum infiltration) or chemical treatment (e.g., the addition of surfactant Silwet L-77) offers advantages to maintain infected seedlings with normal growth and a physiological state without injury. The success of transiently expressing the circadian rhythm reporter in Arabidopsis seedlings may open a new platform to rapidly test the circadian behaviors of Arabidopsis mutants, bypassing the process of introducing a circadian reporter gene into the mutants by crossing. Most remarkably, AGROBEST allows for high transient expression of the MYB75 transcription factor and subsequently upregulates the expression of its downstream gene CHS in both efr-1 and Col-0 seedlings. This result suggested the broad application of AGROBEST to study transcription factor action.
Widespread and differential transient transformation events in different organs and cell types
AGROBEST has a breakthrough performance by enabling high and homogeneous transient GUS expression efficiency in shoots of 100% infected Col-0 or efr-1 seedlings. The successful transient expression in roots, although with less efficient transformation events (~70% of seedlings with GUS staining in roots), is also remarkable and not previously detected [26,27]. Interestingly, preferential transformation events occurring at the initiation sites of lateral roots or the root elongation zone of infected intact seedlings were also previously detected in wounded Arabidopsis roots [46]. High transformation of Arabidopsis roots may require further loosening or opening of cell walls or wounding, which was not included in our infection conditions. Because we observed similar transient expression levels and transformation efficiency in roots of Col-0 and efr-1 seedlings (Additional file 1: Table S1), EFR may play no or little role in seedling root transformation efficiency under our infection conditions. Consistently, EFR is expressed at low levels in Col-0 seedling roots [47], which were not responsive to the EF-Tu peptide elf26, as evidenced by limited induction of immune marker genes and callose deposition in the roots of Col-0 seedlings [48]. Because the flg22 peptide derived from Agrobacterium flagellin is inactive in Arabidopsis[24,34,35] and the flagellin receptor mutant exhibited similar transformation efficiency as Col-0 in our seedling assays, the flagellin receptor FLS2 may not be involved in Agrobacterium-triggered plant innate immune responses and therefore did not compromise Agrobacterium-mediated transient gene expression. Future investigations could examine whether the absence of the peptidoglycan receptor [49] or yet-to-be identified receptors in recognizing additional MAMPs such as polysaccharides [50] could increase the transformation efficiency in seedling roots.
Key factors for high transient transformation/expression efficiency
During this course of our method development, we also uncovered new factors critical for the high transient transformation/expression efficiency in Arabidopsis seedlings. One factor is the addition of AB salts in MS medium buffered with acidic pH 5.5 during Agrobacterium infection, which allows for significantly higher transient expression efficiency than in MS medium alone. Another breakthrough is the use of the disarmed A. tumefaciens strain C58C1(pTiB6S3ΔT)H, which offers the highest transient expression efficiency with the least adverse impact on plant growth over other tested strains. Root growth was severely inhibited on infection with other tested A. tumefaciens strains including the transfer-incompetent ΔvirB2. These data indicate that the transport of T-DNA and T4SS effectors into plant cells by a virulent C58 strain may not suppress host immune responses like that observed in T3SS effectors from Pseudomonas syringae[51]. We observed that C58C1(pTiB6S3ΔT)H achieved higher transient expression efficiency in both Col-0 and efr-1 seedlings than other A. tumefaciens strains tested. The agent also had little impact on root growth inhibition of infected seedlings by the ABM50 method (Figure 3). The results suggested that the A. tumefaciens strain C58C1(pTiB6S3ΔT)H is the main factor affecting the root growth difference. EFR may play a minor role in root growth inhibition because we observed slightly stronger root growth inhibition in Col-0 than efr-1 seedlings infected with C58C1(pTiB6S3ΔT)H. This finding is consistent with limited root growth inhibition detected in Col-0 seedlings in response to EF-Tu peptide elf18 as compared with strong root growth inhibition induced by flg22 [47]. The observed inverse association of root growth inhibition and transient expression efficiency suggested that C58C1(pTiB6S3ΔT)H may circumvent a plant defense barrier to enable high transient expression levels in Arabidopsis seedlings. However, interestingly, root length was significantly lower in Col-0 and efr-1 seedlings with C58C1(pTiB6S3ΔT)H infection than in uninfected seedlings (MOCK), despite the significantly higher transient expression efficiency with the AGROBEST than the ABM50 method (Figure 3). Thus, although C58C1(pTiB6S3ΔT)H remains a strain causing the least inhibition in seedling root growth as compared to other A. tumefaciens strains, whether the observed root growth inhibition results from PTI contributing to reduce transient expression efficiency requires future investigation. Other factors in addition to PTI may contribute to the enhanced transient expression efficiency by AGROBEST.
C58C1(pTiB6S3ΔT)H has been known to achieve high transformation efficiency in several plant species including Arabidopsis, but the underlying mechanism is not known. The nomenclature of Agrobacterium strains used in plant transformation experiments is often simplified, which causes confusion and could sometimes be misleading. C58C1(pTiB6S3ΔT)H is often simplified as C58C1 in the plant community. C58C1 is in fact named after curing pTiC58 from the wild-type virulent strain C58, and rifampicin (Rif)-resistant strains are selected from C58C1 for convenient use to acquire various disarmed Ti plasmids transferred from different Agrobacterium strains [52,53]. Therefore, C58C1(pTiB6S3ΔT)H is a Rif-resistant C58C1 harboring the octopine-type Ti plasmid pTiB6S3 with the removal of the T-DNA region [31] and containing a pCH32 helper plasmid with increased expression of virulence genes virG and virE2[32]. GV3101(pMP90) is a C58-derived disarmed strain, in which pMP90 is a nopaline-type Ti plasmid, pTiC58, with the removal of T-DNA [38]. Therefore, in theory, C58C1(pTiB6S3ΔT)H should share the same chromosomal background with GV3101(pMP90) and only differ in the use of different Ti plasmids and the presence of the helper plasmid pCH32. Future work to determine which genetic factor(s) contribute to increased transient expression efficiency with less growth inhibition by C58C1(pTiB6S3ΔT)H will shed light on understanding the molecular mechanisms underlying the observed high transient transformation and expression efficiency.
Conclusions
In this study, we developed a valuable and novel method, named AGROBEST, and uncovered the key factors enabling this unprecedented high transient transformation and reporter gene expression efficiency in the immune receptor mutant efr-1 and in wild-type Col-0 Arabidopsis seedlings. The applicability for transient expression of MYB75 in activating downstream gene expression in a Col-0 background further suggested that AGROBEST may be a feasible method to use in examining transcription factor actions or gain-of-function studies in different Arabidopsis ecotypes/genotypes. Because most plants do not harbor EFR, which is only present in Brassicaceae[24], the established method may be applicable in other plant species. This fast, sensitive, and quantitative assay was routinely used with culture plates, which are easily scaled up for quick and systematic screens. Importantly, this method nicely compliments the commonly used Arabidopsis mesophyll-protoplast transfection [15,16] and Agrobacterium-mediated leaf infiltration in N. benthamiana[17] for gene functional studies and provides advantages for its high reproducibility without advanced skills. Furthermore, AGROBEST may be an alternative method for evaluating Agrobacterium virulence and discovering and dissecting gene functions involved in various steps of Agrobacterium-mediated DNA transfer. The method may help unravel the mechanisms underlying Agrobacterium infection in unwounded cells/tissues.
Methods
Materials and growth condition
Strains, plasmids, and primer sequences used in this study are in Additional file 2: Table S2 and Additional file 3: Table S3. The bacterial growth conditions and procedures for plasmid and mutant constructions are described in Additional file 4: Methods S1. Arabidopsis thaliana plants included ecotype Columbia-0 (Col-0), Wassilewskija (Ws-2), T-DNA insertion mutants efr-1 (SALK_044334) and fls2 (SALK_093905) and the DEX-inducible AvrPto transgenic line generated in a Col-0 background were obtained from the Arabidopsis Biological Resource Center (Ohio). Seeds were sterilized in 50% bleach (v/v) containing 0.05% Triton X-100 (v/v) for 10 min, rinsed 5 times with sterile water, and incubated at 4°C for 3 days. For germination, 10 seeds were transferred to 1 ml 1/2 MS liquid medium (1/2 MS salt supplemented with 0.5% sucrose (w/v), pH 5.5 [pH adjusted to 5.7 by KOH but pH 5.5 after autoclaving], in each well of a 6-well plate. Germination and growth took place in a growth room at 22°C under a 16-hr/8-hr light–dark cycle (75 μmol m-2 s-1).
Agrobacterium infection in Arabidopsis seedlings
For AGROBEST infection assay, A. tumefaciens was freshly streaked out from -80°C glycerol stock onto a 523 agar plate for 2-day incubation at 28°C. A fresh single colony from the plate was used to inoculate 5 ml of 523 liquid medium containing appropriate antibiotics for shaking (220 rpm) at 28°C for 20–24 hr. For pre-induction of A. tumefaciens vir gene expression, A. tumefaciens cells were pelleted and re-suspended to OD600 0.2 in various liquid media including LB, LB-MES (LB with 10 mM MES, pH 5.7) [53,54] or AB-MES (17.2 mM K2HPO4, 8.3 mM NaH2PO4, 18.7 mM NH4Cl, 2 mM KCl, 1.25 mM MgSO4, 100 μM CaCl2, 10 μM FeSO4, 50 mM MES, 2% glucose (w/v), pH 5.5) [37] with different concentrations of acetosyringone (AS; 0, 50 or 200 μM) without antibiotics, then shaken (220 rpm) at 28°C for 12–16 hr. Before infection, A. tumefaciens cells were pelleted and re-suspended in desired co-cultivation liquid media to OD600 0.02. The growth medium of Arabidopsis seedlings was replaced with 1 ml A. tumefaciens cells freshly prepared above and incubated in the same growth room until further analysis. Three-day-old seedlings were treated with 10 μM DEX for 1 day before infection for the following 3 days. When the removal of Agrobacterium cells was required, co-cultivation medium was removed after the chosen infection time and replaced with 1 ml freshly prepared MS medium containing 100 μM Timentin and incubated for additional days before analysis. The procedures for the seedling transient transformation assay using the method optimized by Marion et al. and FAST Method developed by Li et al. were performed [26,27] and described in Additional file 4: Methods S1. Unless indicated, 10 seedlings grown in each well were infected and 3 biological repeats were performed in each independent experiment.
Plant RNA extraction and quantitative RT-PCR
RNA was extracted from Arabidopsis seedlings as described [55]. An amount of 4 μg total RNA was used to synthesize first-strand cDNA with SuperScript III Reverse Transcriptase (Invitrogen) and oligo dT primer. Quantitative PCR involved the Applied Biosystems QuantStudio 12 K Flex Real Time PCR machine and Power SYBRR Green PCR Master Mix (Invitrogen). Arabidopsis ACTIN 2 (At3g18780) or UBC21 (At5g25760) was an internal control.
GUS staining and activity assays
Seedlings were stained with 5-bromo-4-chloro-3-indolyl glucuronide (X-Gluc) at 37°C for 6 hr unless indicated or quantified with a fluorescence substrate (4-methylumbelliferyl-β-D-glucuronide [MUG]) as described [56]. For MUG assay, fluorescence was determined using a 96 microtiter-plate reader (Bio-Tek Synergy Mx, 356 nm excitation 455 nm emission with ±20 nm filter) and calculation of specific GUS enzyme activity was based on the standard curve of 0.5–500 pmole (0.5, 5, 50 and 500 pmole) 4-MU standards obtained from the same microtiter plate. For relative GUS activity, the fluorescence signal value was normalized by an equal amount of proteins with subtraction of the background fluorescence signal detected by the mock control.
Confocal microscopy
Fluorescence signals were observed by use of a Zeiss LSM 510 Meta Confocal microscope. Venus signals were observed at 488-nm excitation with an HFT 488/514-nm filter and emission with NTF 515- and BP 505- to 530-nm filters. RFP signals were observed at 488-nm excitation with an HFT 405/488-nm filter and emission with NFT 545 and LP 650 filters.
Transient expression of MYB75 and anthocyanin content assay
Four-day-old seedlings were infected with A. tumefaciens strain C58C1(pTiB6S3ΔT)H carrying the control or MYB75-expressing binary vector in ABM-MS liquid medium for 3 days. The co-cultivation medium was then replaced with 1 ml fresh MS medium (1/2 MS, 2% sucrose (w/v), pH adjusted to 5.7 by KOH but pH 5.5 after autoclaving) containing 100 μM Timemtin and then incubated for 3 days. For anthocyanin content assay, seedlings were blot-dried briefly, weighed, ground into powder with liquid nitrogen and mixed with 1 ml extraction buffer (0.12 M HCl, 18% isopropanol (v/v)). The mixture was boiled for 90 sec and centrifuged at 16000 × g for 15 min. The supernatant was collected and measured at OD535 (A535) and OD650 (A650). Anthocyanin content was calculated as A535 - (2.2 × A650)/fresh weight (g) [57].
Transient expression of GI::LUC2 and bioluminescence measurement
Four-day-old seedlings were infected with A. tumefaciens strain C58C1(pTiB6S3ΔT)H carrying p1390-GI::LUC2 or empty vector (pCAMBIA1390) in ABM-MS co-cultivation medium. At 3 dpi, each seedling was transferred to MS medium (1/2 MS, pH adjusted to 5.7 by KOH but pH 5.5 after autoclaving) containing 100 μM Timentin and 0.5 mM luciferin in a black 96-well plate. Bioluminescence activity was measured and analyzed as described [43].
Luciferase activity assay
Arabidopsis seedlings after infection were surface sterilized with 1% bleach (0.05% sodium hypochlorite) for 5–10 min and washed with sterile water 3 times to remove bacteria before assay. The washing step is essential to minimize the background signals expressed in bacteria because of the use of intron-less LUC2 reporter. For photography, 10 seedlings infected by each method were placed in a clean 15-cm square Petri dish and covered with 100 μl 1 mM luciferin. Luciferase intensity was imaged by use of the XENOGEN IVIS lumina system with 5-sec exposure time. Bioluminescence assay involved the luciferase assay system (Promega). Briefly, 10–15 seedlings after a washing were blot-dried with tissue paper before being frozen with liquid nitrogen and stored at -80°C. Seedlings were ground into fine powder by liquid nitrogen, mixed with 300 μl cell-culture lysis reagent (Promega), and centrifuged at 16000 × g for 10 min at 4°C. Supernatant was 100× diluted with cell-culture lysis reagent. In total, 20 μl cell lysate was mixed with 100 μl Luciferase Assay Reagent and the signal was detected by use of lumat LB 9507 (Berthold Technologies). The bioluminescence signal was normalized to the protein amount of each sample quantified by the Bradford protein assay (Bio-Rad).
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HYW participated the experimental designs, performed most of the experiments, analyzed data, and drafted the manuscript. KHL and WLC participated in method optimization. YCW and JFW performed experiments. CYC participated in data analysis. SHW and JS participated in experimental designs and helped drafting the manuscript. EML conceived of the study, participated in method optimization and experimental designs, coordinated the project, and wrote the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Transient transformation efficiency of shoots and roots of Arabidopsis Col-0 and efr-1 seedlings.
Bacterial strains and plasmids.
Primer information.
Methods for bacterial strains and plasmids and infection method by Li et al. (FAST method) and by Marion et al.
Contributor Information
Hung-Yi Wu, Email: r92633011@ntu.edu.tw.
Kun-Hsiang Liu, Email: khliu@molbio.mgh.harvard.edu.
Yi-Chieh Wang, Email: apowerled@yahoo.com.tw.
Jing-Fen Wu, Email: fenny@gate.sinica.edu.tw.
Wan-Ling Chiu, Email: wchiu@vcu.edu.
Chao-Ying Chen, Email: Chcychen@ccms.ntu.edu.tw.
Shu-Hsing Wu, Email: shuwu@gate.sinica.edu.tw.
Jen Sheen, Email: sheen@molbio.mgh.harvard.edu.
Erh-Min Lai, Email: emlai@gate.sinica.edu.tw.
Acknowledgements
The authors thank Hau-Hsuan Hwang, Lay-Sun Ma, Jer-Sheng Lin, and Po-Yuan Shih for discussion and critical reading of the manuscript; and Yajie Niu and Hoosun Chung for preliminary transient expression tests on different seedling ages. We thank Dr. Inhwan Hwang for NLS-RFP; Drs. Stanton Gelvin and Lan-Ying Lee for pBISN1, Venus-intron and BiFC vectors; and Ms. Mei-Jane Fang from the Cell Biology Core Laboratory at the Institute of Plant and Microbial Biology, Academia Sinica, for excellent technical support on confocal microscopy. This work was supported by research grants from the National Science Council of Taiwan (NSC 99-2918-I-001-005 and NSC 101-2321-B-001-033 to E. M. Lai, NSC100-2311-B-001-028-MY3 to S. H. Wu) and the US National Institutes of Health (R01GM60493 and R01GM70567 to J. Sheen).
References
- Dandekar AM, Fisk HJ. Plant transformation: Agrobacterium-mediated gene transfer. Methods Mol Biol. 2005;286:35–46. doi: 10.1385/1-59259-827-7:035. [DOI] [PubMed] [Google Scholar]
- Gelvin SB. Agricultural biotechnology: gene exchange by design. Nature. 2005;433:583–584. doi: 10.1038/433583a. [DOI] [PubMed] [Google Scholar]
- Tzfira T, Citovsky V. Agrobacterium-mediated genetic transformation of plants: biology and biotechnology. Curr Opin Biotechnol. 2006;17:147–154. doi: 10.1016/j.copbio.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Gelvin SB. Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev. 2003;67:16–37. doi: 10.1128/MMBR.67.1.16-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelvin SB. Plant proteins involved in Agrobacterium-mediated genetic transformation. Annu Rev Phytopathol. 2010;48:45–68. doi: 10.1146/annurev-phyto-080508-081852. [DOI] [PubMed] [Google Scholar]
- Gelvin SB. Traversing the cell: Agrobacterium T-DNA’s journey to the host genome. Front Plant Sci. 2012;3:52. doi: 10.3389/fpls.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullen CA, Binns AN. Agrobacterium tumefaciens and plant cell interactions and activities required for interkingdom macromolecular transfer. Annu Rev Cell Dev Biol. 2006;22:101–127. doi: 10.1146/annurev.cellbio.22.011105.102022. [DOI] [PubMed] [Google Scholar]
- Fronzes R, Christie PJ, Waksman G. The structural biology of type IV secretion systems. Nat Rev Microbiol. 2009;7:703–714. doi: 10.1038/nrmicro2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brencic A, Angert ER, Winans SC. Unwounded plants elicit Agrobacterium vir gene induction and T-DNA transfer: transformed plant cells produce opines yet are tumour free. Mol Microbiol. 2005;57:1522–1531. doi: 10.1111/j.1365-2958.2005.04763.x. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Escudero J, Hohn B. Transfer and integration of T-DNA without cell injury in the host plant. Plant Cell. 1997;9:2135–2142. doi: 10.1105/tpc.9.12.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhulu SB, Deng XB, Sarria R, Gelvin SB. Early transcription of Agrobacterium T-DNA genes in tobacco and maize. Plant Cell. 1996;8:873–886. doi: 10.1105/tpc.8.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu CM, Anand A, Kang L, Mysore KS. Agrodrench: a novel and effective agroinoculation method for virus-induced gene silencing in roots and diverse Solanaceous species. Plant J. 2004;40:322–331. doi: 10.1111/j.1365-313X.2004.02211.x. [DOI] [PubMed] [Google Scholar]
- Mysore KS, Kumar CT, Gelvin SB. Arabidopsis ecotypes and mutants that are recalcitrant to Agrobacterium root transformation are susceptible to germ-line transformation. Plant J. 2000;21:9–16. doi: 10.1046/j.1365-313x.2000.00646.x. [DOI] [PubMed] [Google Scholar]
- Sheen J. Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol. 2001;127:1466–1475. [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc. 2007;2:1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]
- Vaghchhipawala Z, Rojas CM, Senthil-Kumar M, Mysore KS. Agroinoculation and agroinfiltration: simple tools for complex gene function analyses. Methods Mol Biol. 2011;678:65–76. doi: 10.1007/978-1-60761-682-5_6. [DOI] [PubMed] [Google Scholar]
- Kim J, Somers DE. Rapid assessment of gene function in the circadian clock using artificial microRNA in Arabidopsis mesophyll protoplasts. Plant Physiol. 2010;154:611–621. doi: 10.1104/pp.110.162271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JF, Chung HS, Niu Y, Bush J, McCormack M, Sheen J. Comprehensive protein-based artificial microRNA screens for effective gene silencing in plants. Plant Cell. 2013;25:1507–1522. doi: 10.1105/tpc.113.112235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapila J, De Rycke R, Van Montagu M, Angenon G. An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci. 1997;122:101–108. [Google Scholar]
- Sheludko YV, Sindarovska YR, Gerasymenko IM, Bannikova MA, Kuchuk NV. Comparison of several Nicotiana species as hosts for high-scale Agrobacterium-mediated transient expression. Biotechnol Bioeng. 2007;96:608–614. doi: 10.1002/bit.21075. [DOI] [PubMed] [Google Scholar]
- Tsuda K, Qi Y, Nguyen LV, Bethke G, Tsuda Y, Glazebrook J, Katagiri F. An efficient Agrobacterium-mediated transient transformation of Arabidopsis. Plant J. 2012;69:713–719. doi: 10.1111/j.1365-313X.2011.04819.x. [DOI] [PubMed] [Google Scholar]
- Wroblewski T, Tomczak A, Michelmore R. Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis. Plant Biotechnol J. 2005;3:259–273. doi: 10.1111/j.1467-7652.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–760. doi: 10.1016/j.cell.2006.03.037. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Baek K, Park CM. Optimization of conditions for transient Agrobacterium-mediated gene expression assays in Arabidopsis. Plant Cell Rep. 2009;28:1159–1167. doi: 10.1007/s00299-009-0717-z. [DOI] [PubMed] [Google Scholar]
- Li JF, Park E, von Arnim AG, Nebenfuhr A. The FAST technique: a simplified Agrobacterium-based transformation method for transient gene expression analysis in seedlings of Arabidopsis and other plant species. Plant Methods. 2009;5:6. doi: 10.1186/1746-4811-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion J, Bach L, Bellec Y, Meyer C, Gissot L, Faure JD. Systematic analysis of protein subcellular localization and interaction using high-throughput transient transformation of Arabidopsis seedlings. Plant J. 2008;56:169–179. doi: 10.1111/j.1365-313X.2008.03596.x. [DOI] [PubMed] [Google Scholar]
- Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- Tena G, Boudsocq M, Sheen J. Protein kinase signaling networks in plant innate immunity. Curr Opin Plant Biol. 2011;14:519–529. doi: 10.1016/j.pbi.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonzac C, Zipfel C. Activation of plant pattern-recognition receptors by bacteria. Curr Opin Microbiol. 2011;14:54–61. doi: 10.1016/j.mib.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Deblaere R, Bytebier B, De Greve H, Deboeck F, Schell J, Van Montagu M, Leemans J. Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res. 1985;13:4777–4788. doi: 10.1093/nar/13.13.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton CM. A binary-BAC system for plant transformation with high-molecular-weight DNA. Gene. 1997;200:107–116. doi: 10.1016/s0378-1119(97)00388-0. [DOI] [PubMed] [Google Scholar]
- Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, Felix G, Boller T. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428:764–767. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]
- Bauer Z, Gomez-Gomez L, Boller T, Felix G. Sensitivity of different ecotypes and mutants of Arabidopsis thaliana toward the bacterial elicitor flagellin correlates with the presence of receptor-binding sites. J Biol Chem. 2001;276:45669–45676. doi: 10.1074/jbc.M102390200. [DOI] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999;18:265–276. doi: 10.1046/j.1365-313x.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- Gelvin SB. Agrobacterium virulence gene induction. Methods Mol Biol. 2006;343:77–84. doi: 10.1385/1-59745-130-4:77. [DOI] [PubMed] [Google Scholar]
- Lai EM, Kado CI. Processed VirB2 is the major subunit of the promiscuous pilus of Agrobacterium tumefaciens. J Bacteriol. 1998;180:2711–2717. doi: 10.1128/jb.180.10.2711-2717.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz C, Schell J. The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Genet Genomics. 1986;204:383–396. [Google Scholar]
- Berger BR, Christie PJ. The Agrobacterium tumefaciens virB4 gene product is an essential virulence protein requiring an intact nucleoside triphosphate-binding domain. J Bacteriol. 1993;175:1723–1734. doi: 10.1128/jb.175.6.1723-1734.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan L, He P, Li J, Heese A, Peck SC, Nurnberger T, Martin GB, Sheen J. Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe. 2008;4:17–27. doi: 10.1016/j.chom.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Liang CS, Kao AL, Yang CC. HHP1, a novel signalling component in the cross-talk between the cold and osmotic signalling pathways in Arabidopsis. J Exp Bot. 2010;61:3305–3320. doi: 10.1093/jxb/erq162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael TP, Mockler TC, Breton G, McEntee C, Byer A, Trout JD, Hazen SP, Shen R, Priest HD, Sullivan CM, Givan SA, Yanovsky M, Hong F, Kay SA, Chory J. Network discovery pipeline elucidates conserved time-of-day-specific cis-regulatory modules. PLoS Genet. 2008;4:e14. doi: 10.1371/journal.pgen.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu JF, Nakamichi N, Sakakibara H, Nam HG, Wu SH. LIGHT-REGULATED WD1 and PSEUDO-RESPONSE REGULATOR9 form a positive feedback regulatory loop in the Arabidopsis circadian clock. Plant Cell. 2011;23:486–498. doi: 10.1105/tpc.110.081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C. Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell. 2000;12:2383–2394. doi: 10.1105/tpc.12.12.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe S, Rougon-Cardoso A, Sherwood E, Peeters N, Dahlbeck D, van Esse HP, Smoker M, Rallapalli G, Thomma BP, Staskawicz B, Jones JD, Zipfel C. Interfamily transfer of a plant pattern-recognition receptor confers broad-spectrum bacterial resistance. Nat Biotechnol. 2010;28:365–369. doi: 10.1038/nbt.1613. [DOI] [PubMed] [Google Scholar]
- Yi H, Mysore KS, Gelvin SB. Expression of the Arabidopsis histone H2A-1 gene correlates with susceptibility to Agrobacterium transformation. Plant J. 2002;32:285–298. doi: 10.1046/j.1365-313x.2002.01425.x. [DOI] [PubMed] [Google Scholar]
- Ranf S, Eschen-Lippold L, Pecher P, Lee J, Scheel D. Interplay between calcium signalling and early signalling elements during defence responses to microbe- or damage-associated molecular patterns. Plant J. 2011;68:100–113. doi: 10.1111/j.1365-313X.2011.04671.x. [DOI] [PubMed] [Google Scholar]
- Millet YA, Danna CH, Clay NK, Songnuan W, Simon MD, Werck-Reichhart D, Ausubel FM. Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell. 2010;22:973–990. doi: 10.1105/tpc.109.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmann R, Lajunen HM, Erbs G, Newman M-A, Kolb D, Tsuda K, Katagiri F, Fliegmann J, Bono J-J, Cullimore JV, Jehle AK, Götz F, Kulik A, Molinaro A, Lipka V, Gust AA, Nürnberger T. Arabidopsis lysin-motif proteins LYM1 LYM3 CERK1 mediate bacterial peptidoglycan sensing and immunity to bacterial infection. Proc Natl Acad Sci USA. 2011;108:19824–19829. doi: 10.1073/pnas.1112862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow M, Newman MA, von Roepenack E. The induction and modulation of plant defense responses by bacterial lipopolysaccharides. Annu Rev Phytopathol. 2000;38:241–261. doi: 10.1146/annurev.phyto.38.1.241. [DOI] [PubMed] [Google Scholar]
- He P, Shan L, Lin NC, Martin GB, Kemmerling B, Nurnberger T, Sheen J. Specific bacterial suppressors of MAMP signaling upstream of MAPKKK in Arabidopsis innate immunity. Cell. 2006;125:563–575. doi: 10.1016/j.cell.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Hellens R, Mullineaux P, Klee H. Technical focus:a guide to Agrobacterium binary Ti vectors. Trends Plant Sci. 2000;5:446–451. doi: 10.1016/s1360-1385(00)01740-4. [DOI] [PubMed] [Google Scholar]
- Van Larebeke N, Engler G, Holsters M, Van den Elsacker S, Zaenen I, Schilperoort RA, Schell J. Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature. 1974;252:169–170. doi: 10.1038/252169a0. [DOI] [PubMed] [Google Scholar]
- Burch-Smith TM, Schiff M, Liu Y, Dinesh-Kumar SP. Efficient virus-induced gene silencing in Arabidopsis. Plant Physiol. 2006;142:21–27. doi: 10.1104/pp.106.084624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AO, Larkins BA. Influence of ionic strength, pH, and chelation of divalent metals on isolation of polyribosomes from tobacco leaves. Plant Physiol. 1976;57:5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-W, Franceschi V, Davin L, Lewis N. In: Methods in Molecular Biology, Arabidopsis Protocols. Volume 323. Salinas J, Sanchez-Serrano J, editor. Totowa, New Jersey, USA: Humana Press; 2006. β-Glucuronidase as reporter gene; pp. 263–273. [DOI] [PubMed] [Google Scholar]
- Lange H, Shropshire W, Mohr H. An analysis of phytochrome-mediated anthocyanin synthesis. Plant Physiol. 1971;47:649–655. doi: 10.1104/pp.47.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transient transformation efficiency of shoots and roots of Arabidopsis Col-0 and efr-1 seedlings.
Bacterial strains and plasmids.
Primer information.
Methods for bacterial strains and plasmids and infection method by Li et al. (FAST method) and by Marion et al.


