Lymphotoxin expressed by RORγt+ innate lymphoid cells is critical for natural killer cell development.
Abstract
Natural killer (NK) cell development relies on signals provided from the bone marrow (BM) microenvironment. It is thought that lymphotoxin (LT) α1β2 expressed by the NK cell lineage interacts with BM stromal cells to promote NK cell development. However, we now report that a small number of RORγt+ innate lymphoid cells (ILCs), and not CD3−NK1.1+ cells, express LT to drive NK development. Similar to LT−/− or RORγt−/− mice, the mice conditionally lacking LTα1β2 on RORγt+ ILCs experience a developmental arrest at the immature NK stages, between stages of NK development to the mature NK cell stage. This developmental block results in a functional deficiency in the clearance of NK-sensitive tumor cells. Reconstitution of Thy1+ ILCs from BM or purified RORγt+ ILCs from lamina propria lymphocytes into LT-deficient RORγt+ BM cultures rescues NK cell development. These data highlight a previously undiscovered role of RORγt+ ILCs for NK cell development and define LT from ILCs as an essential molecule for the stromal microenvironment supporting NK cell development.
NK cells play a critical role in host defense against some pathogens and play an essential role in clearing tumor cells (Biron et al., 1999; Cerwenka et al., 2001; Vivier et al., 2012). The BM is the key site for multiple stages of NK development, but the precise mechanisms that regulate the transition between various stages of NK development remain elusive. Currently, it is established that NK cells develop from common lymphoid progenitors (CLPs), which possess precursor potential for T, B, and NK cells (Ramirez and Kee, 2010; Vosshenrich and Di Santo, 2013). CLPs lack the markers of hematopoietic lineages but are distinguished based on their expression of low levels of c-Kit, Sca1, and IL7Rα (Kondo et al., 1997). Under support from stromal cells, CLPs are directed toward the NK fate through several stages defined by patterns of expression of CD122 (IL-2 and IL-15 receptor–β chain), NK1.1 (an activating NKR), and DX5 (integrin α2 and CD49b; Kim et al., 2002; Lian and Kumar, 2002; Ramirez and Kee, 2010). As CLPs develop into NK progenitors, they begin to express CD122 while remaining negative for other lineage markers (Ter119, CD3, CD19, and Gr1; Di Santo, 2006). Acquisition of NK1.1 occurs at the immature NK (iNK) cell stage, characterized by expression of multiple NKRs and IL-15 dependence (Vosshenrich et al., 2005). Transient expression of integrin αν (CD51) and TRAIL also occurs at this stage (Kim et al., 2002). Further maturation into mature NK (mNK) cells is accompanied by increased expression of DX5, CD11b, and CD43 and the loss of CD51 and TRAIL (Kim et al., 2002; Vosshenrich et al., 2005; Chiossone et al., 2009). Although distinct stages in the progression of CLPs to the development of mNK cells have been identified, how those key developmental programs are regulated is currently unappreciated.
Lymphotoxin (LT), in its trimeric form (LTα1β2), is expressed by activated lymphocytes and binds to LTβR expressed primarily on myeloid, parenchymal, and stromal cell populations (Fu et al., 1998; Murphy et al., 1998; Fu and Chaplin, 1999). LT is thought to be essential for the development of secondary lymphoid tissues (Fu and Chaplin, 1999). We and others have reported that the loss of LT (LTα or LTβ gene) causes a dramatic reduction of the number of NK cells in the spleen and BM and impairment of antitumor activity caused by defective NK cell activities (Iizuka et al., 1999; Ito et al., 1999; Smyth et al., 1999; Wu et al., 2001). Therefore, it is possible that LT delivers an essential signal to the LTβR-expressing stromal cells to promote NK cell development and maturation (Iizuka et al., 1999; Wu et al., 2001; Lian et al., 2004). We have further observed that NK cell development of RAG1−/− mice is also reduced after prolonged blockade of LT signaling (Wu et al., 2001). These data have supported a model in which LT from NK lineage cells is required for optimal NK cell development.
NK cells are considered to be the founding members of the innate lymphoid cell (ILC) family, having shared immunological and developmental characteristics. However, recent studies have unearthed the existence of ILCs, which is a heterogeneous family of innate effector cells that have critical roles in the generation and maintenance of innate immune responses. One subset of ILCs expressing retinoic acid receptor–related orphan receptor γt (RORγt) is essential in lymphoid tissue formation and immune defense in an LT-dependent fashion (Cherrier and Eberl, 2012; Spits and Cupedo, 2012; Upadhyay and Fu, 2013). Studies argue that NK cells (NK1.1+, CD3−) never express RORγt throughout their life and that IL-15–deficient mice have defective NK cells but normal numbers for RORγt+ ILCs (Sawa et al., 2010; Pandiyan et al., 2012). Therefore, it is thought that NK cells are a completely distinct lineage from RORγt+ ILCs.
Upon profiling the BM of wild-type mice, we unexpectedly have found that RORγt+ ILCs express a significant level of surface LT, whereas NK cells expressed virtually undetectable levels of LT, casting doubt on the model in which NK cells directed their own development through LT expression. We therefore explored the possibility that RORγt+ ILCs, and not NK cells, provided the LT signal to LTβR-expressing stromal cells necessary for NK cell development. Using various RORγt or LT-deficient animal models as well as in vitro BM cell culture systems, we now report that LTα1β2 expressed on RORγt+ ILCs in BM plays a crucial role in promoting a microenvironment for the development of iNK into mNK cells and therefore have uncovered a close interaction between NK cell development and RORγt+ ILCs.
RESULTS
LT from RORγt+ cells plays a critical role in NK cell development
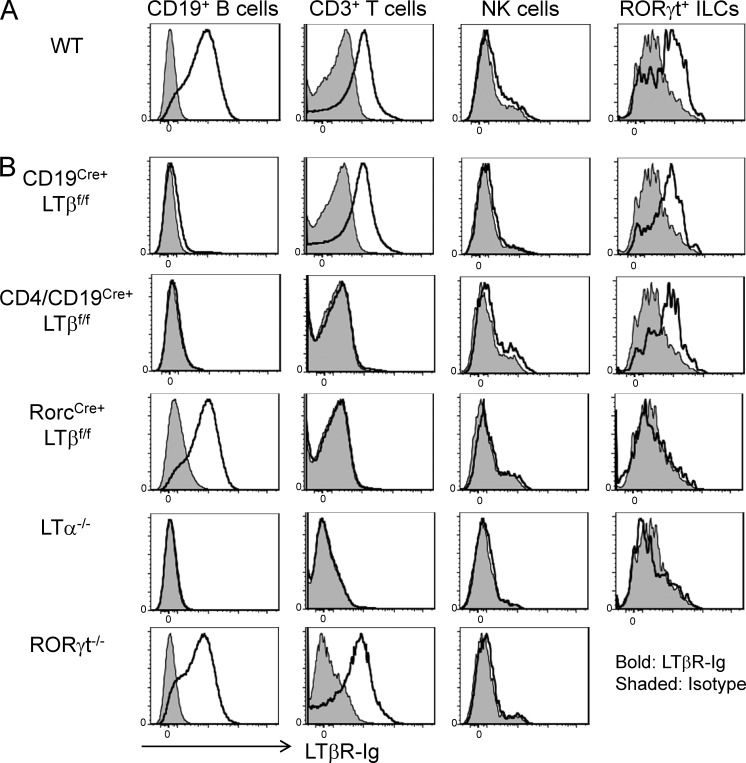
NK cell development occurs independently of adaptive immunity, and therefore, it has been speculated that LT from NK cells is essential for NK cell development (Iizuka et al., 1999; Smyth et al., 1999; Wu et al., 2001). LT was originally described as expressed on T, B, and NK cells (Ware et al., 1995), and these findings raised doubts over the ability of NK cells to regulate their own development using the LT pathway. However, using the biotinylated decoy receptor LTβR-Ig, we have only been able to detect membrane LT on T and B cells but not NK cells (Fig. 1 A). Inspired by recent findings demonstrating LT expression on the RORγt+ ILCs (Finke, 2005), we compared LT expression in RORγt+ ILCs and CD3−NK1.1+ NK cells. In contrast to the absence of expression of LT on conventional CD3−NK1.1+ NK cells, RORγt+ ILCs appeared to express LTα1β2, which is capable of binding to LTβR (Fig. 1 A). To further determine the precise role of specific LT-positive lymphocyte subsets in NK cell development, we crossed mice containing a floxed allele of LTβ (LTβf/f) to mice expressing Cre recombinase in B cells (CD19Cre+), T cells (CD4Cre+), both B and T cells (CD19Cre+CD4Cre+), or RorcCre+.
Figure 1.
RORγt+ ILCs but not NK cells express LT. (A and B) Splenocytes from wild-type (A) and CD19Cre+LTβf/f, CD4/CD19Cre+LTβf/f, RorcCre+LTβf/f, LTα−/−, and RORγt−/− (B) mice were stimulated with 20 ng/ml PMA for 20 h. Stimulated cells were then labeled with biotinylated LTβR-Ig for LT staining and assessed by flow cytometry to detect LT expression. Data are representative of two independent experiments.
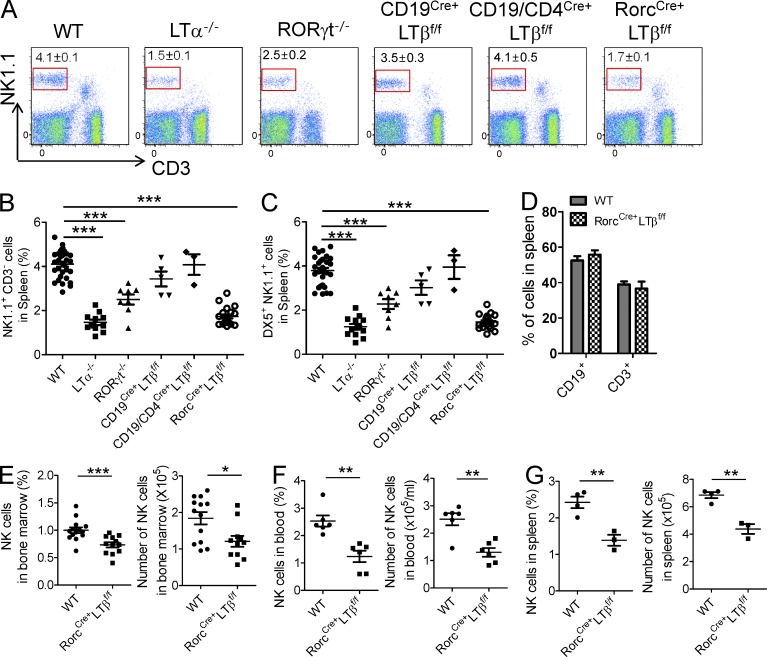
As seen in Fig. 1 B, specific deletion of LT was observed in B (CD19Cre+) or both B and T (CD19Cre+CD4Cre+) cells. As expected, LT expression was lacking on RORγt+ ILCs in RorcCre+LTβf/f mice. As additional controls, LTα-deficient mice did not show any visible expression of surface LT in all subsets tested, whereas RORγt−/− mice demonstrated normal LT expression in B and T cells (no RORγt+ ILCs found in these mice). We next examined whether these mice displayed developmental NK cell defects. It has been shown previously that LT is expressed on RORγt+ T cells and that ILCs can control the generation of secondary lymphoid tissues (Chiang et al., 2009; Cherrier and Eberl, 2012; Spits and Cupedo, 2012). LTα-deficient mice were shown to have severely diminished NK cells (Iizuka et al., 1999; Smyth et al., 1999), and as such, we saw dramatic reduction of CD3−NK1.1+ NK cells in the spleen of LTα−/− mice (Fig. 2 A). Similar to LTα−/− mice, NK cell frequency in RORγt−/− mice were severely reduced, raising the possibility that LT on RORγt+ cells could be essential for NK cell development. In contrast, no significant reduction of NK cell populations was noted in CD19Cre+LTβf/f, CD4Cre+LTβf/f, and double CD19/CD4Cre+LTβf/f mice (Fig. 2, A–C; not depicted for CD4 single knockout). Interestingly, the analysis of RorcCre+LTβf/f mice revealed a severe reduction of both CD3−NK1.1+ and DX5+NK1.1+ cells in the spleen, similar to that observed in LTα−/− mice (Fig. 2, A–C). However, splenic CD3+ and CD19+ cells from RorcCre+LTβf/f mice were not significantly different from those in wild-type mice (Fig. 2 D). Not only the percentage of CD3−NK1.1+ cells but also their absolute numbers were significantly reduced in RorcCre+LTβf/f mice (Fig. 2, E and F). Furthermore, these defects were apparent in 3-wk-old mice (Fig. 2 G), demonstrating a true developmental defect for NK cells in these mice. Together, these data confirm that LT on RORγt+ cells is essential for NK cell development and homeostasis in mice.
Figure 2.
LT on RORγt+ cells regulates NK cells homeostasis. (A–C) Cells from spleen of 6–12-wk-old wild-type (n = 32), LTα−/− (n = 11), RORγt−/− (n = 8), CD19Cre+LTβf/f (n = 5), CD19/CD4Cre+LTβf/f (n = 3), and RorcCre+LTβf/f (n = 20) mice were isolated and stained for NK1.1+, CD3−. (A) Dot plot represents NK cells in spleen. (B and C) Frequency of splenic NK (NK1.1+, CD3−) and mNK (DX5+, NK1.1+) cell populations as assessed by flow cytometry. (D) Frequency of splenic CD3+ and CD19+ cell populations from wild-type and RorcCre+LTβf/f mice as assessed by flow cytometry (each group, n = 4). (E and F) Cells from BM (each group, n = 13) or blood (each group, n = 6) of 6–12-wk-old wild-type and RorcCre+LTβf/f mice were assessed by flow cytometry for the percentage and total cell numbers of NK cells. (G) Cells from spleen of 3-wk-old wild-type and RorcCre+LTβf/f mice were assessed for the frequency and cell numbers (each group, n = 3 or 4). Data are from at least three independent experiments. All data are presented as the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
RORγt+ ILCs regulate NK cell development
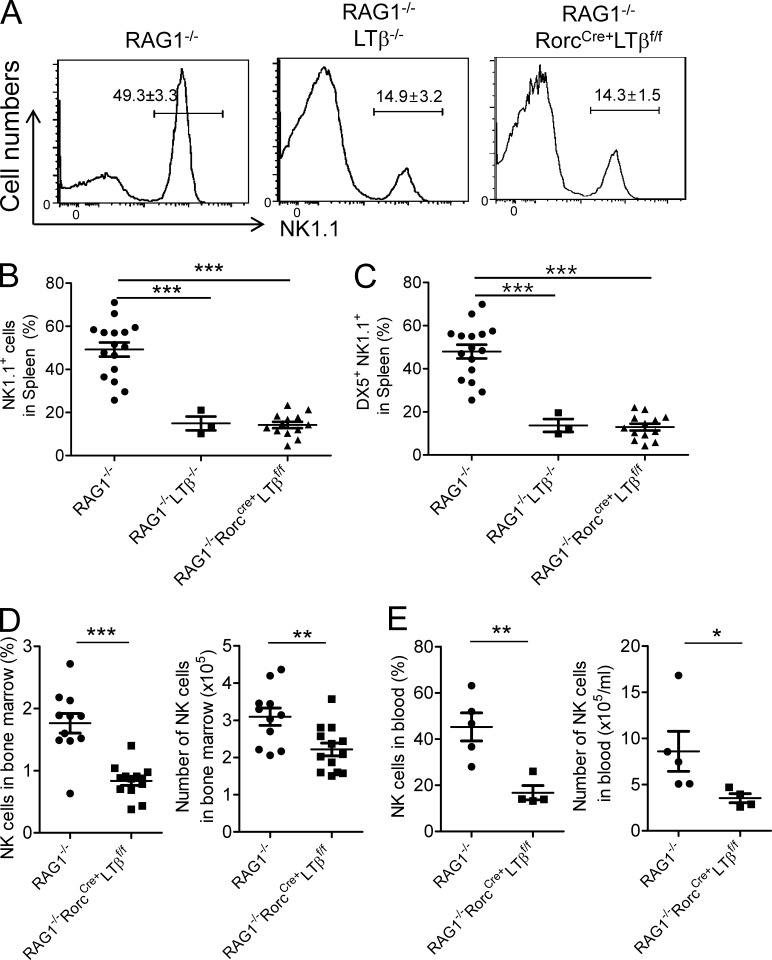
Because RORγt is expressed on both T cells and subsets of ILCs, it is possible that RORγt+ T cells contribute to the development of NK cells in addition to ILCs. To address this possibility, we crossed RorcCre+LTβf/f mice to the RAG1−/− background and analyzed the proportion of NK cells in the absence of T and B cells. As can be seen in Fig. 3 A, the percentage of NK1.1+ cells in the spleen of RAG1−/−RorcCre+LTβf/f mice was significantly diminished to <30% of that observed in RAG1−/− mice. Similar impairment was detected in RAG1−/−LTβ−/− mice (Fig. 3 A), suggesting that LT on ILCs, but not on T or B cells, was critical for the normal development of NK cells. Both NK1.1+ and DX5+NK1.1+ cell populations in the spleen were reduced in RAG1−/−RorcCre+LTβf/f and RAG1−/−LTβ−/− mice (Fig. 3, B and C). Similar to RorcCre+LTβf/f mice, reduction of the NK cell percentage and numbers was also observed in the BM and blood of RAG1−/−RorcCre+LTβf/f mice (Fig. 3, D and E). These data clearly demonstrate that LT signaling from RORγt+ ILCs but not T cells is essential for NK cell development.
Figure 3.
LT on RORγt-expressing innate cells influences NK cells homeostasis. (A–C) Cells from spleen of 6–12-wk-old RAG1−/− (n = 16), RAG1−/−LTβ−/− (n = 3), and RAG1−/−RorcCre+LTβf/f (n = 13) mice were isolated and stained for NK cells (NK1.1+). Splenocytes from RAG1−/−, RAG1−/−LTβ−/−, and RAG1−/−RorcCre+LTβf/f mice were stained for NK cells (NK1.1+, CD3−) and mNK cell marker (DX5+, NK1.1+) and analyzed by flow cytometry. (D and E) Cells from BM (each group, n = 11) and blood (each group, n = 5 or 4) of 6–12-wk-old RAG1−/− and RorcCre+LTβf/fRAG1−/− mice were assessed for the percentage and cell number of NK cells by flow cytometry. Data are from at least three independent experiments. All data are presented as the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
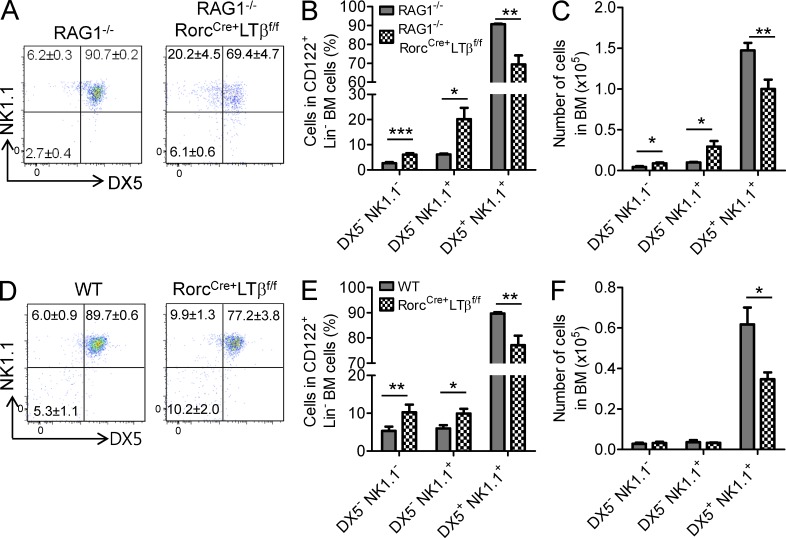
LTα1β2 on RORγt+ ILCs is important for early NK development
We next investigated whether RORγt+ ILCs influence the maturation stage of NK cell development. NK cell maturation can be staged from CLP → pre-NKp → NKp → iNK → mNK cells (Kim et al., 2002), and we monitored these stages using recently described cell surface markers: CD122, NK1.1, and DX5 (Bezman et al., 2011). NKp cells are designated as CD122+, Lin−, NK1.1−, DX5−, whereas iNK cells and mNK cells are found to express CD122+, Lin−, NK1.1+, DX5− and CD122+, Lin−, NK1.1+, DX5+, respectively (Rosmaraki et al., 2001; Kim et al., 2002). Therefore, we exploited these surface markers to evaluate a potential block that may have been occurring in the NK developmental pathway in RAG1−/−RorcCre+LTβf/f mice. As shown in Fig. 4, there are even increased percentages of NKp (CD122+, Lin−, NK1.1−, DX5−) and iNK (CD122+, Lin−, NK1.1+, DX5−) cells present in the BM of mice conditionally lacking LTβ on RORγt+ ILCs. Coordinately, the absence of LTβ on this cell type resulted in a significantly diminished mNK cell (CD122+, Lin−, NK1.1+, DX5+) frequency and cell numbers in BM (Fig. 4, A–C), demonstrating a developmental block at the NKp stage. These defects in RAG1−/−RorcCre+LTβf/f mice were also observed in RorcCre+LTβf/f mice that are immunocompetent (Fig. 4, D–F), suggesting that adaptive immune T and B cells did not play a significant role in NK cell development. These data indicate that LT signaling from RORγt+ ILCs controls a key stage of NK cell development before final maturation.
Figure 4.
LT on RORγt+ ILCs is important for the transitional stage of NK cells. (A–C) Flow cytometric analysis of RAG1−/− (n = 5) and RAG1−/−RorcCre+LTβf/f (n = 5) mice in BM. (A) Dot plots indicate the frequency of NKp (CD122+, Lin−, NK1.1−, DX5−), iNK (CD122+, Lin−, NK1.1+, DX5−), and mNK cells (CD122+, Lin−, NK1.1+, DX5+). (B and C) Bar graphs indicate the percentage and number of NKp (NK1.1−, DX5−), iNK (NK1.1+, DX5−), and mNK (NK1.1+, DX5+) cell populations. (D–F) Flow cytometric analysis of BM from LTβf/f (n = 5) and RorcCre+LTβf/f (n = 5) mice. (D) Dot plots indicate the frequency of NKp (CD122+, Lin−, NK1.1−, DX5−), iNK (CD122+, Lin−, NK1.1+, DX5−), and mNK cells (CD122+, Lin−, NK1.1+, DX5+). (E and F) Bar graphs indicate the percentage and number of NKp (NK1.1−, DX5−), iNK (NK1.1+, DX5−), and mNK (NK1.1+, DX5+) cell populations. Data are from at least five independent experiments. All data are presented as the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
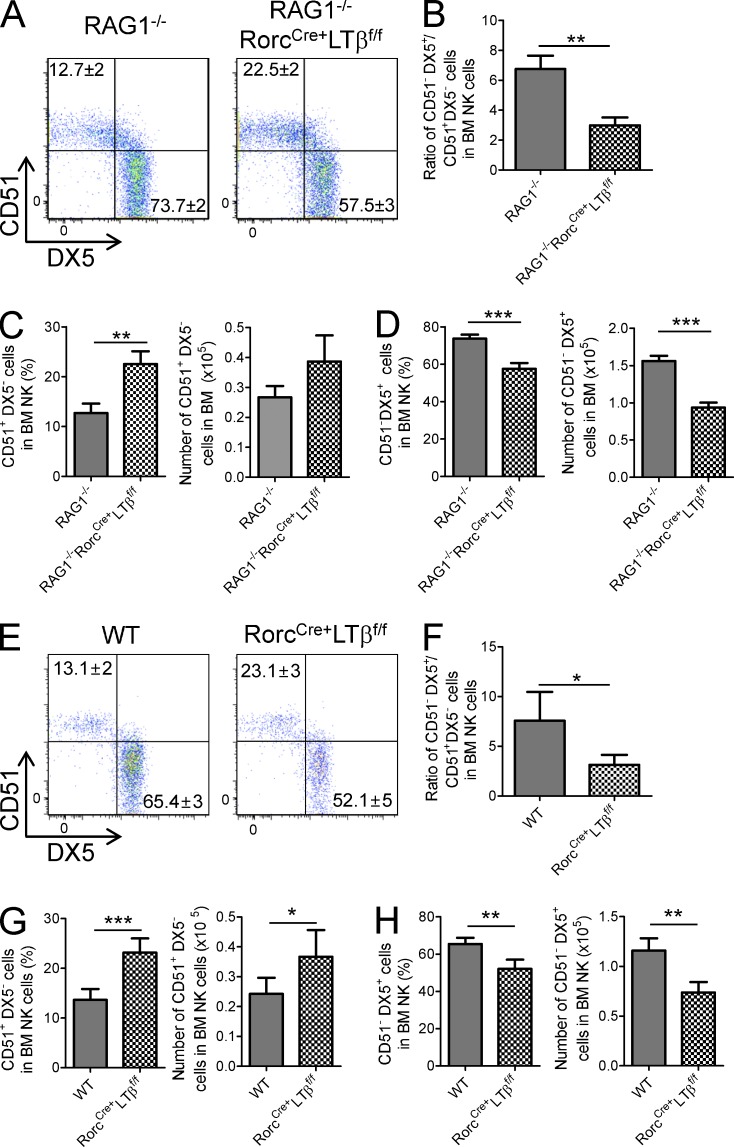
To confirm our findings, we compared the NK maturation status in these mice according to the expression of DX5 and CD51, as markers for mNK and iNK cells, respectively (Kim et al., 2002). As shown in Fig. 5 (A–D), the CD51+, DX5− (iNK) frequency and cell number are shown to be significantly increased in the BM of RAG1−/−RorcCre+LTβf/f mice, whereas the CD51−, DX5+ population (mNK) is largely decreased (Fig. 5, A, C, and D). The ratio of mNK and iNK cells was significantly decreased in RorcCre+LTβf/f mice in the immunocompromised host, supporting developmental arrest at this stage (Fig. 5 B). Like in RAG1−/− background host, the CD51+, DX5− NK cell (immature) frequency and cell number are shown to be significantly increased in the BM of RorcCre+LTβf/f mice. However, the CD51−, DX5+ NK cell (mature) frequency and cell number were significantly decreased (Fig. 5, E, G, and H). The ratio of mNK and iNK cells was significantly reduced in RorcCre+LTβf/f mice in the immunocompetent host (Fig. 5 F). Together, these results highlight the role of LTα1β2 signaling from ILCs facilitating NK cell development.
Figure 5.
Absence of LT on RORγt+ ILCs leads to accumulation of the CD51+DX5− iNK cell population. (A) Dot plots indicate the percentage of iNK (CD51+, DX5−) and mNK (CD51−, DX5+) cell populations in BM of RAG1−/− or RAG1−/−RorcCre+LTβf/f mice. (B) Bar graph indicates ratio of iNK (CD51+, DX5−) to mNK (CD51−, DX5+) cells in the indicated mouse strains (each group, n = 10). (C and D) Bar graphs represent the mean percentage ± SEM of frequency or cell number of iNK (CD51+, DX5−) and mNK (CD51−, DX5+) cells. (E) Dot plot indicates the percentage of iNK (CD51+, DX5−) and mNK (CD51−, DX5+) cells in BM of the indicated mouse strains on the B6 background. (F) Bar graph indicates the ratio of iNK (CD51+, DX5−) to mNK (CD51−, DX5+) cells in the indicated mouse strains on the B6 background (each group, n = 9). (G and H) Bar graphs represent percentage and cell number of iNK (CD51+, DX5−) and mNK (CD51−, DX5+) cells. Data are from at least nine independent experiments. All data are presented as the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
LTα1β2 from RORγt+ ILCs controls NK cell development in vitro
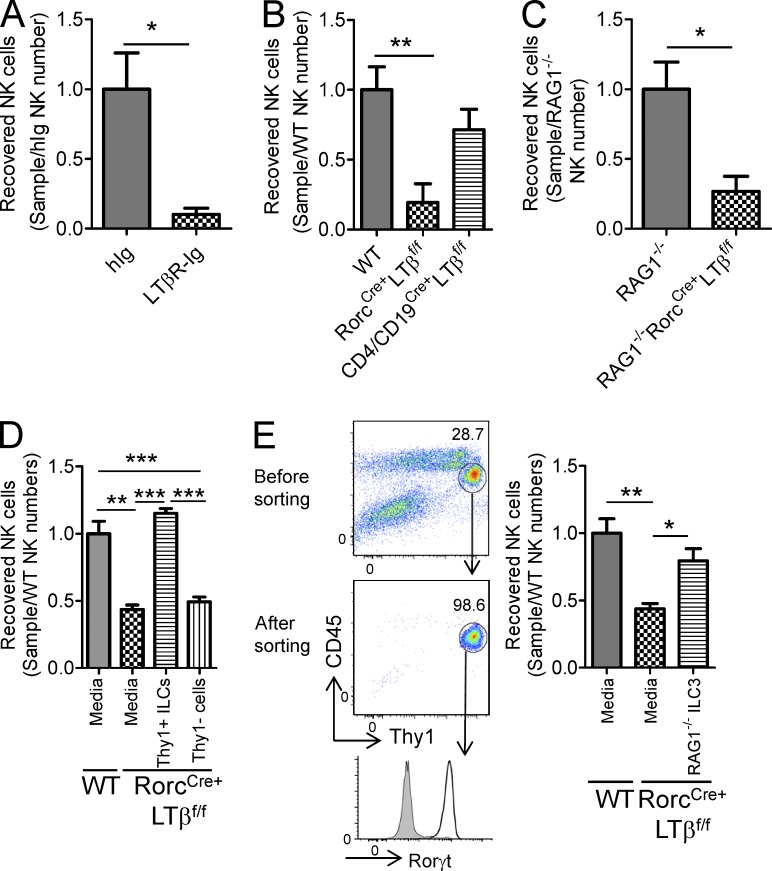
ILCs from mucosal tissues are thought to play various roles in host defense (Wang et al., 2010; Tumanov et al., 2011), but the role of ILCs within the BM is poorly defined. To study whether ILCs from BM cells can regulate NK cell development and avoid NK cell tracking between the periphery and BM, a BM cell culture system was developed to model NK cell development inside BM without interference of their trafficking. We first tested whether blockade of LT signaling using the decoy receptor LTβR-Ig impairs NK development in wild-type BM. BM cells were initially cultured in the absence of IL-15 for 10–14 d to eliminate all mNK cells. IL-15 was then added into the BM culture system to drive NKp cells for their maturation and development. After an additional 10 d of culture following IL-15 treatment, we assessed NK cell number using the markers described above.
As previously demonstrated, in vitro BM culture successfully generated mNK cells (Fig. 6) in both immunocompetent and immunocompromised mice. However, blocking LTβR signaling by the addition of LTβR-Ig to the culture significantly decreased NK cell recovery compared with the human-Ig (hIg) control group (Fig. 6 A). These data indicate that LT signaling within the cultured BM microenvironment is necessary and sufficient for NK cell development. To determine whether LTα1β2 on RORγt+ ILCs is important for NK cell development, we compared the number of NK cells generated from wild-type and RorcCre+LTβf/f BM cultures. As seen in Fig. 6 (B and C), the number of NK cells generated from RorcCre+LTβf/f BM cultures was significantly reduced compared with those obtained from wild-type BM cultures, regardless of the presence or absence of adaptive immune cells (Fig. 6, B and C). These data strongly suggest the pivotal role of LT on RORγt+ ILCs in the early stage of NK cell development.
Figure 6.
ILCs could restore defective LT-mediated early NK cell development. Fresh isolated BM cells were cultured without IL-15 cytokine for 10 d and then with 20 ng/ml IL-15 cytokine for an additional 10 d. NK cells were harvested and counted. (A) hIg (n = 4) and LTβR-Ig (n = 4) were added to fresh isolated BM cells from B6 mice at 0, 3, and 6 d. (B and C) BM cells from wild-type, RorcCre+LTβf/f, and CD4/CD19Cre+LTβf/f mice from the B6 (B) or RAG1−/− (C) backgrounds were cultured for 20 d (each group, n = 4). (D) Isolated Lin−, NK1.1−, Thy1+ or Lin−, NK1.1−, Thy1− ILCs from wild-type BM cells were added into the RorcCre+LTβf/f BM culture group at 0 d (each group, n = 4). (E, left) Flow cytometry gating strategy. RORγt+ ILCs from LPLs were isolated from RAG1−/− mice. (right) Isolated RORγt+ ILCs from RAG1−/− intestine LPLs were added into RorcCre+LTβf/f BM cultures at day 0, and recovered NK cells were enumerated on day 20 (each group, n = 3 or 4). Data are representative of three (E) or at least four (A–D) independent experiments. All data are presented as the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Our in vivo data demonstrated that LT signaling on RORγt+ ILCs is essential for NK cell development, and therefore, we next investigated whether ILCs from wild-type mice could rescue the NK cell defect in RorcCre+LTβf/f mice. To investigate whether Lin−NK1.1−Thy1+ ILCs could contribute to the NK cell development, we isolated Lin−NK1.1−Thy1+ ILCs from wild-type BM cells using a magnetic beads system. We then supplemented the BM cultures of RorcCre+LTβf/f with these cells at the beginning of the culture. As depicted in Fig. 6 D, addition of wild-type ILCs from BM completely rescued the ability of RorcCre+LTβf/f BM cultures to generate NK cells. Therefore, Lin−NK1.1−Thy1+ ILCs rescue NK cell development in an LT-dependent fashion in the in vitro BM culture system. To address the possibility that IL-15 was being supplemented from the Lin−NK1.1−Thy1+ subset, Lin−NK1.1−Thy1− cell populations were cultured in the presence of IL-15; supplementing IL-15 to the Lin−NK1.1−Thy1− cells failed to rescue the NK cell defect observed from RorcCre+LTβf/f BM (Fig. 6 D). To rule out the potential contamination of NKps during the cell transfer, RORγt+ ILC populations were isolated from intestinal lamina propria lymphocytes (LPLs) using cell sorting and added to the BM cultures of RorcCre+LTβf/f. As shown in Fig. 6 E, addition of RAG1−/− ILC3 from LPLs could successfully restore NK cell generation in the RorcCre+LTβf/f BM culture group. Together, these data confirm that NK cells generated from the addition of wild-type ILCs in RorcCre+LTβf/f BM cultures were not likely from the contaminated wild-type CLP or NKp, but rather from the LT signaling provided by wild-type ILCs themselves.
Lack of LT on ILCs significantly impairs NK-mediated tumor clearance
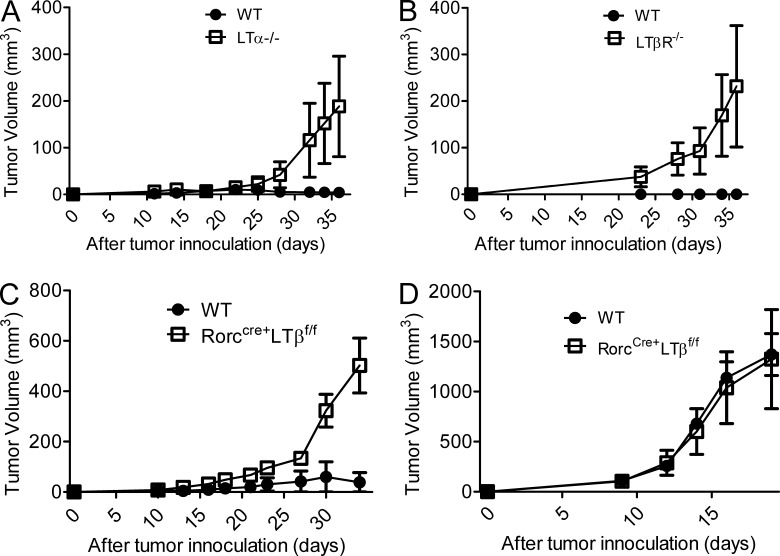
NK cells have been observed to play a critical role in the scavenging of hematopoietic cells that have undergone malignant transformation (Dong et al., 2009). Therefore, we hypothesized that impaired LT signaling on ILCs could affect the ability of NK cells in providing the host protection from malignant hematopoietic cells. To test this, we injected an NK-sensitive tumor line, RMA-S (MHC class I–deficient T cell lymphoma; van den Broek et al., 1995; Smyth et al., 1998), into the right flank of LTα−/−, LTβR−/−, and wild-type mice and monitored tumor growth every 3–4 d. Although wild-type mice controlled RMA-S tumors almost completely, LTα−/− and LTβR−/− mice failed to control tumor growth (Fig. 7, A and B). To examine whether LT signaling directly affects tumor clearance, we injected LTβR-Ig (150 µg/each, one time, 0 d) into wild-type mice to block LT signaling in mice. No significant difference in tumor growth was obtained between hIg- and the LTβR-Ig–treated group, arguing that the defect seen in mice lacking the LT pathway occurred indirectly through a developmental defect present in these mice (not depicted). Given that active LT signaling did not protect mice from MHC I–deficient tumor cells, we challenged mice lacking LT from ILCs with RMA-S and RMA, which differ by expression of MHC class I and subsequent sensitivity to NK killing. As depicted in Fig. 7 (C and D), RorcCre+LTβf/f mice were susceptible to the RMA-S tumor line, whereas RMA tumor growth was similar between WT and RorcCre+LTβf/f mice (Fig. 7, C and D). These data confirm that LT signaling is critical for the clearance of MHC class I–deficient tumor cells and immune surveillance provided by NK cells in vivo. Furthermore, it suggests that LT from RORγt+ ILCs creates a unique microenvironment for NK cell development that renders the host resistant to some tumors.
Figure 7.
Absence of LT on RORγt+ ILCs significantly impaired NK-mediated tumor surveillance. (A–C) RMA-S cells (5 × 105) were injected s.c. into wild-type (n = 4), LTα−/− (n = 4), LTβR−/− (n = 3), or RorcCre+LTβf/f (n = 5) mice. (D) RMA cells (5 × 105) were injected s.c. into wild-type and RorcCre+LTβf/f mice (each group, n = 3). Tumor volume was assessed at the indicated time points. Data are representative of two (B and D) or at least three (A and C) independent experiments. All data are presented as the mean ± SEM.
DISCUSSION
Although the developmental stages of NK cells within the BM have been identified, how the BM stromal microenvironment supports NK cell development is not well defined. It has been shown that LT-deficient mice exhibit a developmental arrest of NK cells (Iizuka et al., 1999; Ito et al., 1999; Smyth et al., 1999; Wu et al., 2001). Furthermore, LTβR signaling on BM stromal cells is required for optimal NK development in the absence of T and B cells (Wu et al., 2001). It is thought that membrane-bound LT expressed on NK cell–like precursors is required for NK development. In this study, we revisit this hypothesis and refine this concept by discovering a new population of LT-expressing ILCs within BM, which control the development and maturation of NK cells: (a) RORγt−/− mice as well as RorcCre+LTβf/f mice yield reduced NK cells, providing compelling evidence that LTs on RORγt+ ILCs are important players in NK cell development; (b) depletion of ILCs blocked the development of NK cell; (c) adoptive transfer of LT-expressing ILCs rescued NK cell development in LT-deficient BM cells, suggesting that ILCs, not NK precursors, are the population essential for NK cell development.
The ILC population can be broadly divided into classical NK cells and three newly defined distinct populations of ILCs. Group 1 ILCs are T-bet–expressing ILCs producing IFN-γ and are associated with cell-mediated immunity, whereas group 2 ILCs are dependent on the transcription factor RORα, express the transcription factor GATA3, and produce the Th2-associated cytokines IL-5 and IL-13. Group 3 ILCs consist of RORγt+ ILCs that include LTi-like, ILC17, and NCR22 cells. All ILCs derive from an Id2-dependent lymphoid precursor and respond to γc cytokines, which play important roles in lymphoid cell development and function (Cherrier and Eberl, 2012; Mjösberg et al., 2012; Spits and Cupedo, 2012). RORγt+ group 3 ILCs partially depend on Aryl hydrocarbon receptor (AhR) signaling for development and function, express the IL-23R, and can produce IL-17A, IL-17F, and IL-22. RORγt+ ILCs and NK cells are derived from same CLP (Mebius et al., 2001; Yoshida et al., 2001) and develop into common precursor NK (NKp) cells. But recent lineage mapping suggests that they are a distinct population. NK cells (NK1.1+) never express RORγt through their life (Sawa et al., 2010). IL-15–deficient mice have defective NK cells but normal numbers of RORγt+ ILCs (Pandiyan et al., 2012). In various LT-deficient mice, such as RorcCre+LTβf/f mice with RAG1−/− or B6 background, generation of NKp appears to be normal within BM. However, the number of mNK is significantly reduced, whereas that of iNK cells, characterized by a CD51+NK1.1+CD3−DX5− cell population, is elevated. Therefore, it appears that the NK cell development process in the RorcCre+LTβf/f mice is arrested at the stage of iNK after surface CD51 expression. This increased iNK cell population is also observed in the secondary lymphoid organs, including spleen, blood, and lung, suggesting that the iNK cells are able to emigrate from the BM at the immature state. The defect in iNK development persists even after addition of IL-15 in the BM culture in vitro. These data highlight the critical role of LT signaling from ILCs in guiding iNK cells to further develop into the NK1.1+DX5+ stage before the IL-15–dependent phase.
NK cells can be divided into four different stages according to the expression patterns of cell surface CD27 and CD11b (Chiossone et al., 2009; Vosshenrich and Di Santo, 2013). Upon analysis of mNK cells in the spleen, no significant difference was observed in the percentage of CD27- and CD11b-expressing NK cells between wild-type and RorcCre+LTβf/f mice. Therefore, the remaining small numbers of mNK cells developing independently of LT signaling are normal in LT-deficient mice. Experiments are in progress to identify the mechanism controlling LT-independent developmental pathways of NK cells.
Upon analysis of NK effector function, we found a significant defect in eliminating NK-sensitive class Ilow RMA-S tumors in the absence of LT signaling. RMA-S tumor burdens are significantly bigger in LTα−/−, LTβR−/−, and RorcCre+LTβf/f mice than wild-type mice, indicating that NK cell function is heavily suppressed in vivo. This defect is largely caused by the defect in NK cells because NK-insensitive class I+ RMA tumor burden was not statistically different between WT and RorcCre+LTβf/f mice. Therefore, these data demonstrate that LT is essential, not only in the maturation process within BM but also the antitumor effector functions.
Collectively, our data present a new paradigm of NK cell developmental process by incorporating a critical role of ILCs expressing LT; upon generation of iNK cells, membrane-bound LT on RORγt+ ILCs provides signal LTβR on BM stromal cells, which stimulate NF-κB signaling for chemokines and cytokines necessary for iNK maturation within the BM microenvironment. In the absence of LT-dependent signaling, NK cell development was arrested at the iNK stage expressing CD51 and resulted in functional impairment. However, interactions among newly defined ILCs and NK cells for their development and function have not been well defined and should be important aspects of future studies. Thus, it is fascinating that such a small number of RORγt+ ILCs can play a critical role for NK cell development and that we have uncovered a close interaction between NK cell development and RORγt+ ILCs. Our study further defines membrane LT as an essential molecule from RORγt+ ILCs in controlling the development of NK cells within the BM microenvironment.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from Harlan Laboratories, Inc. RAG1−/−, LTα−/−, LTβR−/−, and RORγt−/− mice were bred in our animal facility at the University of Chicago. CD4, CD19Cre+LTβf/f mice were intercrossed as previously described (Tumanov et al., 2002, 2003). RorcCre+LTβf/f mice were generated by crossing LTβ floxed with Rorc-Cre transgenic mice (Eberl and Littman, 2004). Animal care and use were in accordance with institutional and National Institutes of Health guidelines, and all experiments were approved by the Animal Care and Use Committee of the University of Chicago.
Flow cytometric analysis and antibodies.
The cells were treated with anti-CD16/CD32 antibody (clone 2.4G2) before surface staining. Antibodies conjugated to FITC, PE, Percp-cy5.5, PE-cy7, APC, APC-cy7, Pacific blue, and biotin for the following antigens were used for this study: NK1.1 (PK136), CD3e (145-2C11), CD19 (1D3), CD49b (DX5), CD122 (TMβ1), CD51 (RMV-7), TER-119 (TER-119), Ly-6G/Ly-6C (RB6-8C5), CD45R/B220 (RA3-6B2), CD45 (30-F11), and CD90.2 (30-H12). These antibodies were purchased from BioLegend and eBioscience. Live cells were gated based on the 7AAD-negative cell population. For RORγt staining, cells were fixed and permeabilized with the Foxp3 staining buffer set (eBioscience). For detection of LT, splenocytes from mice were stimulated with 20 ng/ml PMA for 20 h. After stimulation, cells were stained with LTβR-biotin antibodies, and then we stained streptavidin-conjugated APC antibodies. Cells were analyzed with an LSR Fortessa (BD). Acquired data were analyzed with FlowJo software (Tree Star).
BM in vitro culture.
BM cells were obtained from the tibias and femurs of mice. Fresh isolated BM cells were harvested from wild-type or Rorccre+LTβf/f mice and cultured in vitro for 10 d without exogenous cytokines, and 10 or 20 ng/ml IL-15 (BioLegend) was then added for the last 10 d. LTβR-Ig was added into each well at day 0 for neutralizing LT. Thy1+Lin−NK1.1− cells were isolated from BM using MACS magnetic beads (Miltenyi Biotec). RORγt+ ILCs were isolated from intestinal LPLs using cell sorting. Absolute NK cell (NK1.1+, CD3−) numbers were counted by flow cytometry based cell bead assay (Invitrogen), and acquired data were analyzed with FlowJo software.
Isolation of RORγt+ ILCs from intestinal LPLs.
RORγ+ ILCs from LPLs were isolated by modification of a previous study (Guo et al., 2014). Mice were sacrificed, and small and large intestines were removed. These intestines were cut open longitudinally, thoroughly washed in PBS, and cut into small pieces. Intestines were incubated in 1× HBSS supplemented with 5% FBS, 10 mM Hepes, 5 mM EDTA, and 1 mM DTT at 37°C for 20 min. After washing, the intestines were digested in RPMI 1640 supplemented with 0.05% DNase I (Sigma-Aldrich) and 0.1 mg/ml Liberase (Roche) at 37°C for 20 min. The digested intestines were homogenized by a gentleMACS dissociator (Miltenyi Biotec) and passed through a 70-µm strainer. Mononuclear cells were obtained from a Percoll gradient. After washing, RORγt+ ILCs were obtained by cell sorting using a FACSAria cell sorter (BD).
Tumor cell lines and tumor model.
RMA-S (MHC class I negative) and RMA (MHC class I positive) cell lines were cultured in RPMI 1640 supplemented with 10% FBS, 1% nonessential amino acid, 1% Hepes, and 1% penicillin/streptomycin. RMA-S or RMA cells were injected s.c. into the lateral flank of mice. Tumor volumes were determined along three orthogonal axes (A, B, and C) and calculated as tumor volume = ABC/2. Tumor volume was measured every 3–4 d.
Statistical analysis.
The statistical significances were determined by Student’s t test. The p-values were defined as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Statistical analyses were performed using Prism version 5.01 (GraphPad Software).
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (grants NRF-2007-00107 and NRF-2013M3A9D3045719) and the Converging Research Center Program (grant 2013K000268) awarded to K.-M. Lee. T.-J. Kim is additionally supported by a grant from the NRF (NRF-2013R1A1A2013308). This research was supported in part by the US National Institutes of Health (grants CA141975 and CA134563-01).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- CLP
- common lymphoid progenitor
- ILC
- innate lymphoid cell
- iNK
- immature NK
- LPL
- lamina propria lymphocyte
- LT
- lymphotoxin
- mNK
- mature NK
References
- Bezman N.A., Chakraborty T., Bender T., Lanier L.L. 2011. miR-150 regulates the development of NK and iNKT cells. J. Exp. Med. 208:2717–2731 10.1084/jem.20111386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron C.A., Nguyen K.B., Pien G.C., Cousens L.P., Salazar-Mather T.P. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189–220 10.1146/annurev.immunol.17.1.189 [DOI] [PubMed] [Google Scholar]
- Cerwenka A., Baron J.L., Lanier L.L. 2001. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc. Natl. Acad. Sci. USA. 98:11521–11526 10.1073/pnas.201238598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier M., Eberl G. 2012. The development of LTi cells. Curr. Opin. Immunol. 24:178–183 10.1016/j.coi.2012.02.003 [DOI] [PubMed] [Google Scholar]
- Chiang E.Y., Kolumam G.A., Yu X., Francesco M., Ivelja S., Peng I., Gribling P., Shu J., Lee W.P., Refino C.J., et al. 2009. Targeted depletion of lymphotoxin-α–expressing TH1 and TH17 cells inhibits autoimmune disease. Nat. Med. 15:766–773 10.1038/nm.1984 [DOI] [PubMed] [Google Scholar]
- Chiossone L., Chaix J., Fuseri N., Roth C., Vivier E., Walzer T. 2009. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 113:5488–5496 10.1182/blood-2008-10-187179 [DOI] [PubMed] [Google Scholar]
- Di Santo J.P. 2006. Natural killer cell developmental pathways: a question of balance. Annu. Rev. Immunol. 24:257–286 10.1146/annurev.immunol.24.021605.090700 [DOI] [PubMed] [Google Scholar]
- Dong Z., Cruz-Munoz M.E., Zhong M.C., Chen R., Latour S., Veillette A. 2009. Essential function for SAP family adaptors in the surveillance of hematopoietic cells by natural killer cells. Nat. Immunol. 10:973–980 10.1038/ni.1763 [DOI] [PubMed] [Google Scholar]
- Eberl G., Littman D.R. 2004. Thymic origin of intestinal αβ T cells revealed by fate mapping of RORγt+ cells. Science. 305:248–251 10.1126/science.1096472 [DOI] [PubMed] [Google Scholar]
- Finke D. 2005. Fate and function of lymphoid tissue inducer cells. Curr. Opin. Immunol. 17:144–150 10.1016/j.coi.2005.01.006 [DOI] [PubMed] [Google Scholar]
- Fu Y.X., Chaplin D.D. 1999. Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 17:399–433 10.1146/annurev.immunol.17.1.399 [DOI] [PubMed] [Google Scholar]
- Fu Y.X., Huang G., Wang Y., Chaplin D.D. 1998. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin α–dependent fashion. J. Exp. Med. 187:1009–1018 10.1084/jem.187.7.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Qiu J., Tu T., Yang X., Deng L., Anders R.A., Zhou L., Fu Y.X. 2014. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity. 40:25–39 10.1016/j.immuni.2013.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka K., Chaplin D.D., Wang Y., Wu Q., Pegg L.E., Yokoyama W.M., Fu Y.X. 1999. Requirement for membrane lymphotoxin in natural killer cell development. Proc. Natl. Acad. Sci. USA. 96:6336–6340 10.1073/pnas.96.11.6336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D., Back T.C., Shakhov A.N., Wiltrout R.H., Nedospasov S.A. 1999. Mice with a targeted mutation in lymphotoxin-alpha exhibit enhanced tumor growth and metastasis: impaired NK cell development and recruitment. J. Immunol. 163:2809–2815 [PubMed] [Google Scholar]
- Kim S., Iizuka K., Kang H.S., Dokun A., French A.R., Greco S., Yokoyama W.M. 2002. In vivo developmental stages in murine natural killer cell maturation. Nat. Immunol. 3:523–528 10.1038/ni796 [DOI] [PubMed] [Google Scholar]
- Kondo M., Weissman I.L., Akashi K. 1997. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 91:661–672 10.1016/S0092-8674(00)80453-5 [DOI] [PubMed] [Google Scholar]
- Lian R.H., Kumar V. 2002. Murine natural killer cell progenitors and their requirements for development. Semin. Immunol. 14:453–460 10.1016/S1044532302000805 [DOI] [PubMed] [Google Scholar]
- Lian R.H., Chin R.K., Nemeth H.E., Libby S.L., Fu Y.X., Kumar V. 2004. A role for lymphotoxin in the acquisition of Ly49 receptors during NK cell development. Eur. J. Immunol. 34:2699–2707 10.1002/eji.200425394 [DOI] [PubMed] [Google Scholar]
- Mebius R.E., Miyamoto T., Christensen J., Domen J., Cupedo T., Weissman I.L., Akashi K. 2001. The fetal liver counterpart of adult common lymphoid progenitors gives rise to all lymphoid lineages, CD45+CD4+CD3− cells, as well as macrophages. J. Immunol. 166:6593–6601 10.4049/jimmunol.166.11.6593 [DOI] [PubMed] [Google Scholar]
- Mjösberg J., Bernink J., Peters C., Spits H. 2012. Transcriptional control of innate lymphoid cells. Eur. J. Immunol. 42:1916–1923 10.1002/eji.201242639 [DOI] [PubMed] [Google Scholar]
- Murphy M., Walter B.N., Pike-Nobile L., Fanger N.A., Guyre P.M., Browning J.L., Ware C.F., Epstein L.B. 1998. Expression of the lymphotoxin β receptor on follicular stromal cells in human lymphoid tissues. Cell Death Differ. 5:497–505 10.1038/sj.cdd.4400374 [DOI] [PubMed] [Google Scholar]
- Pandiyan P., Yang X.P., Saravanamuthu S.S., Zheng L., Ishihara S., O’Shea J.J., Lenardo M.J. 2012. The role of IL-15 in activating STAT5 and fine-tuning IL-17A production in CD4 T lymphocytes. J. Immunol. 189:4237–4246 10.4049/jimmunol.1201476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez K., Kee B.L. 2010. Transcriptional regulation of natural killer cell development. Curr. Opin. Immunol. 22:193–198 10.1016/j.coi.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmaraki E.E., Douagi I., Roth C., Colucci F., Cumano A., Di Santo J.P. 2001. Identification of committed NK cell progenitors in adult murine bone marrow. Eur. J. Immunol. 31:1900–1909 [DOI] [PubMed] [Google Scholar]
- Sawa S., Cherrier M., Lochner M., Satoh-Takayama N., Fehling H.J., Langa F., Di Santo J.P., Eberl G. 2010. Lineage relationship analysis of RORγt+ innate lymphoid cells. Science. 330:665–669 10.1126/science.1194597 [DOI] [PubMed] [Google Scholar]
- Smyth M.J., Kelly J.M., Baxter A.G., Körner H., Sedgwick J.D. 1998. An essential role for tumor necrosis factor in natural killer cell–mediated tumor rejection in the peritoneum. J. Exp. Med. 188:1611–1619 10.1084/jem.188.9.1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth M.J., Johnstone R.W., Cretney E., Haynes N.M., Sedgwick J.D., Korner H., Poulton L.D., Baxter A.G. 1999. Multiple deficiencies underlie NK cell inactivity in lymphotoxin-alpha gene-targeted mice. J. Immunol. 163:1350–1353 [PubMed] [Google Scholar]
- Spits H., Cupedo T. 2012. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 30:647–675 10.1146/annurev-immunol-020711-075053 [DOI] [PubMed] [Google Scholar]
- Tumanov A., Kuprash D., Lagarkova M., Grivennikov S., Abe K., Shakhov A., Drutskaya L., Stewart C., Chervonsky A., Nedospasov S. 2002. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity. 17:239–250 10.1016/S1074-7613(02)00397-7 [DOI] [PubMed] [Google Scholar]
- Tumanov A.V., Grivennikov S.I., Shakhov A.N., Rybtsov S.A., Koroleva E.P., Takeda J., Nedospasov S.A., Kuprash D.V. 2003. Dissecting the role of lymphotoxin in lymphoid organs by conditional targeting. Immunol. Rev. 195:106–116 10.1034/j.1600-065X.2003.00071.x [DOI] [PubMed] [Google Scholar]
- Tumanov A.V., Koroleva E.P., Guo X., Wang Y., Kruglov A., Nedospasov S., Fu Y.X. 2011. Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe. 10:44–53 10.1016/j.chom.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay V., Fu Y.X. 2013. Lymphotoxin signalling in immune homeostasis and the control of microorganisms. Nat. Rev. Immunol. 13:270–279 10.1038/nri3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek M.F., Kägi D., Zinkernagel R.M., Hengartner H. 1995. Perforin dependence of natural killer cell-mediated tumor control in vivo. Eur. J. Immunol. 25:3514–3516 10.1002/eji.1830251246 [DOI] [PubMed] [Google Scholar]
- Vivier E., Ugolini S., Blaise D., Chabannon C., Brossay L. 2012. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 12:239–252 10.1038/nri3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshenrich C.A., Di Santo J.P. 2013. Developmental programming of natural killer and innate lymphoid cells. Curr. Opin. Immunol. 25:130–138 10.1016/j.coi.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Vosshenrich C.A., Samson-Villéger S.I., Di Santo J.P. 2005. Distinguishing features of developing natural killer cells. Curr. Opin. Immunol. 17:151–158 10.1016/j.coi.2005.01.005 [DOI] [PubMed] [Google Scholar]
- Wang Y., Koroleva E.P., Kruglov A.A., Kuprash D.V., Nedospasov S.A., Fu Y.X., Tumanov A.V. 2010. Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune responses against mucosal bacterial infection. Immunity. 32:403–413 10.1016/j.immuni.2010.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware C.F., VanArsdale T.L., Crowe P.D., Browning J.L. 1995. The ligands and receptors of the lymphotoxin system. Curr. Top. Microbiol. Immunol. 198:175–218 [DOI] [PubMed] [Google Scholar]
- Wu Q., Sun Y., Wang J., Lin X., Wang Y., Pegg L.E., Fütterer A., Pfeffer K., Fu Y.X. 2001. Signal via lymphotoxin-βR on bone marrow stromal cells is required for an early checkpoint of NK cell development. J. Immunol. 166:1684–1689 10.4049/jimmunol.166.3.1684 [DOI] [PubMed] [Google Scholar]
- Yoshida H., Kawamoto H., Santee S.M., Hashi H., Honda K., Nishikawa S., Ware C.F., Katsura Y., Nishikawa S.I. 2001. Expression of α4β7 integrin defines a distinct pathway of lymphoid progenitors committed to T cells, fetal intestinal lymphotoxin producer, NK, and dendritic cells. J. Immunol. 167:2511–2521 10.4049/jimmunol.167.5.2511 [DOI] [PubMed] [Google Scholar]