Insight from Bertrand Boisson (left) and Jean-Laurent Casanova (right)
In this issue, Rodgers et al. report a new substrate of the linear ubiquitin assembly complex (LUBAC) and a new function of this complex in mice. Linear ubiquitination has been implicated as a key regulator of the NF-κB pathway. LUBAC consists of three components: HOIL-1, HOIP, and Sharpin. It adds linear ubiquitin to NEMO, driving the formation of a TNF-R–IL-1R signaling complex and leading to the nuclear translocation of NF-κB. Linear ubiquitination thus plays an important role in the regulation of immunity via the transduction of NF-κB–dependent signals, such as those mediated by the TLR/IL-1 and TNF receptor families.
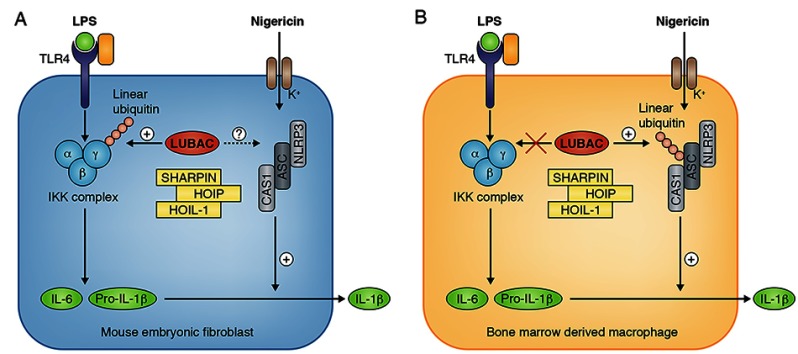
Rodgers et al. now show that LUBAC is essential for NF-κB activation in mouse embryonic fibroblasts (MEFs) stimulated with LPS, but dispensable in bone marrow–derived macrophages (BMDMs). They also demonstrate that the activation of caspase 1 by the complex formed by NLRP3 (NLR family, pyrin domain containing 3, encoded by CIAS1) and ASC (apoptosis-associated speck-like protein containing CARD) is dependent on LUBAC in LPS-primed, nigericin-stimulated macrophages. Using different approaches, they show that the formation of the NLPR3–ASC complex in response to nigericin stimulation involves the linear ubiquitination of ASC. Linear ubiquitination is required for the transformation of pro–IL-1β into secreted and bioactive IL-1β by caspase 1. HOIL1-deficient mice are protected against LPS toxicity in vivo.
Cell specificity of linear ubiquitin dependent activation of IKK complex and inflammasome in mice. In mouse embryonic fibroblasts (A), expression of IL-6 and pro–IL-1β upon LPS is dependent of LUBAC activity. In BMDM (B), expression of IL-6 and pro–IL-1β upon LPS is independent of LUBAC activity but IL-1β secretion is dependent of linear ubiquitination of ASC.
This work reveals new aspects of linear ubiquitination in mice. First, it reveals a cell specificity of LUBAC in the activation of NF-κB. It shows, for the first time, that LUBAC is dispensable for NF-κB activation in certain cell types, although the mechanism involved remains elusive. Second, it shows that LUBAC plays an essential role in the activation of caspase-1 upon stimulation of the NLRP3-ASC inflammasome. This finding extends the cellular function of LUBAC and linear ubiquitination to processes independent of NEMO and the NF-κB pathway.
If extended to human cells, these findings would provide a novel angle from which to tackle the pathogenesis of the autoinflammation, immunodeficiency, and amylopectinosis observed in patients with inherited HOIL-1 deficiency. Surprisingly, these patients also display a cell-specific pattern of response to IL-1β, with hyperresponsive monocytes and hyporesponsive fibroblasts. LUBAC-dependent NLRP3 activation would also pave the way for the targeting of linear ubiquitination in patients with autoinflammatory diseases due to mutations of NLRP3, such as cryopyrin-associated periodic syndrome (CAPS) and Muckle-Wells syndrome.
References
- Rodgers M.A., et al. 2014. J. Exp. Med. 10.1084/jem.20132486. [DOI] [Google Scholar]