CD1c self-reactive T cells recognize a novel class of self-lipids that are accumulated on leukemia cells.
Abstract
T cells that recognize self-lipids presented by CD1c are frequent in the peripheral blood of healthy individuals and kill transformed hematopoietic cells, but little is known about their antigen specificity and potential antileukemia effects. We report that CD1c self-reactive T cells recognize a novel class of self-lipids, identified as methyl-lysophosphatidic acids (mLPAs), which are accumulated in leukemia cells. Primary acute myeloid and B cell acute leukemia blasts express CD1 molecules. mLPA-specific T cells efficiently kill CD1c+ acute leukemia cells, poorly recognize nontransformed CD1c-expressing cells, and protect immunodeficient mice against CD1c+ human leukemia cells. The identification of immunogenic self-lipid antigens accumulated in leukemia cells and the observed leukemia control by lipid-specific T cells in vivo provide a new conceptual framework for leukemia immune surveillance and possible immunotherapy.
CD1-restricted T lymphocytes recognize lipid antigens presented by the nonpolymorphic, MHC class I–related family of CD1 molecules (Porcelli and Modlin, 1999). CD1-restricted T cells can respond to lipid antigens derived from microbial cells and may exert protective roles during host infection (Moody et al., 2000, 2004; Amprey et al., 2004; Gilleron et al., 2004; Kinjo et al., 2005; Sriram et al., 2005; Wu et al., 2005; Montamat-Sicotte et al., 2011). A striking characteristic of many CD1-restricted T cells is autoreactivity against different types of APCs even in the absence of microbial antigens, implying that they can also recognize endogenous self-lipid molecules (Dellabona et al., 1993; Mattner et al., 2005; Vincent et al., 2005). Autoreactive T cells recognize different types of self-lipids present in cell membranes and synthesized within different cellular compartments (Shamshiev et al., 1999, 2000; Gumperz et al., 2000; Wu et al., 2003; De Libero et al., 2005). CD1a- and CD1c-autoreactive T cells are relatively abundant among circulating T cells in healthy individuals (de Jong et al., 2010; de Lalla et al., 2011) and might become activated by host antigens in autoimmune diseases and cancer. Lipid-specific T cells can control cancer cell growth in mouse models (Berzofsky and Terabe, 2009) as well as in human patients (Dhodapkar and Richter, 2011; Metelitsa, 2011), but it remains unknown whether they recognize unique lipids expressed by tumor cells.
Acute leukemia comprises a heterogeneous group of hematological disorders characterized by blood and bone marrow accumulation of immature and abnormal cells derived from hematopoietic precursors (Pui et al., 2004; Rubnitz et al., 2008). Current therapy for acute leukemia is based on polychemotherapy and allogeneic hematopoietic stem cell (HSC) transplantation (HSCT). A major cause of treatment failure and area of substantial unmet need in HSCT is posttransplant regrowth of residual leukemia blasts that survive the conditioning regimen (Wingard et al., 2011). Donor-derived T cells transferred into patients may induce a beneficial graft versus leukemia (GVL) reaction capable of maintaining remission (Kolb, 2008), but grafted T cells are also capable of killing patient cells in nonhematopoietic tissues to induce detrimental graft versus host disease (GVHD; Socié and Blazar, 2009). A promising therapeutic strategy is the selective targeting of T cell responses against malignant hematopoietic cells, while maintaining hematopoietic capacity among grafted cells and preserving organ functions in recipient patients (Kolb, 2008). Because CD1 molecules are both nonpolymorphic and preferentially expressed by mature hematopoietic cells (Porcelli and Modlin, 1999; Brigl and Brenner, 2004), targeting tumor-associated lipid antigens presented by CD1 molecules might provide opportunities to improve the efficacy of HSCT. Immune recognition of tumor-associated lipid antigens may also complement ongoing antitumor responses mediated by protein antigens.
Here we have identified the novel self-lipid antigen that stimulates CD1c autoreactive T cells to destroy tumor cell lines and primary human leukemia cells. We report that both group 1 CD1 molecules and a novel class of tumor-associated lipids are broadly expressed by different types of acute leukemia. In addition to killing CD1c+ leukemia cell lines and primary blasts in vitro, the CD1c-restricted T cells also displayed therapeutic efficacy in a mouse xenograft model of human leukemia. Our findings provide proof-of-concept evidence that T cell responses against lipids accumulated in acute leukemia could be exploited for leukemia immunotherapy.
RESULTS
Identification of CD1c-presented antigenic lipids in leukemia cells
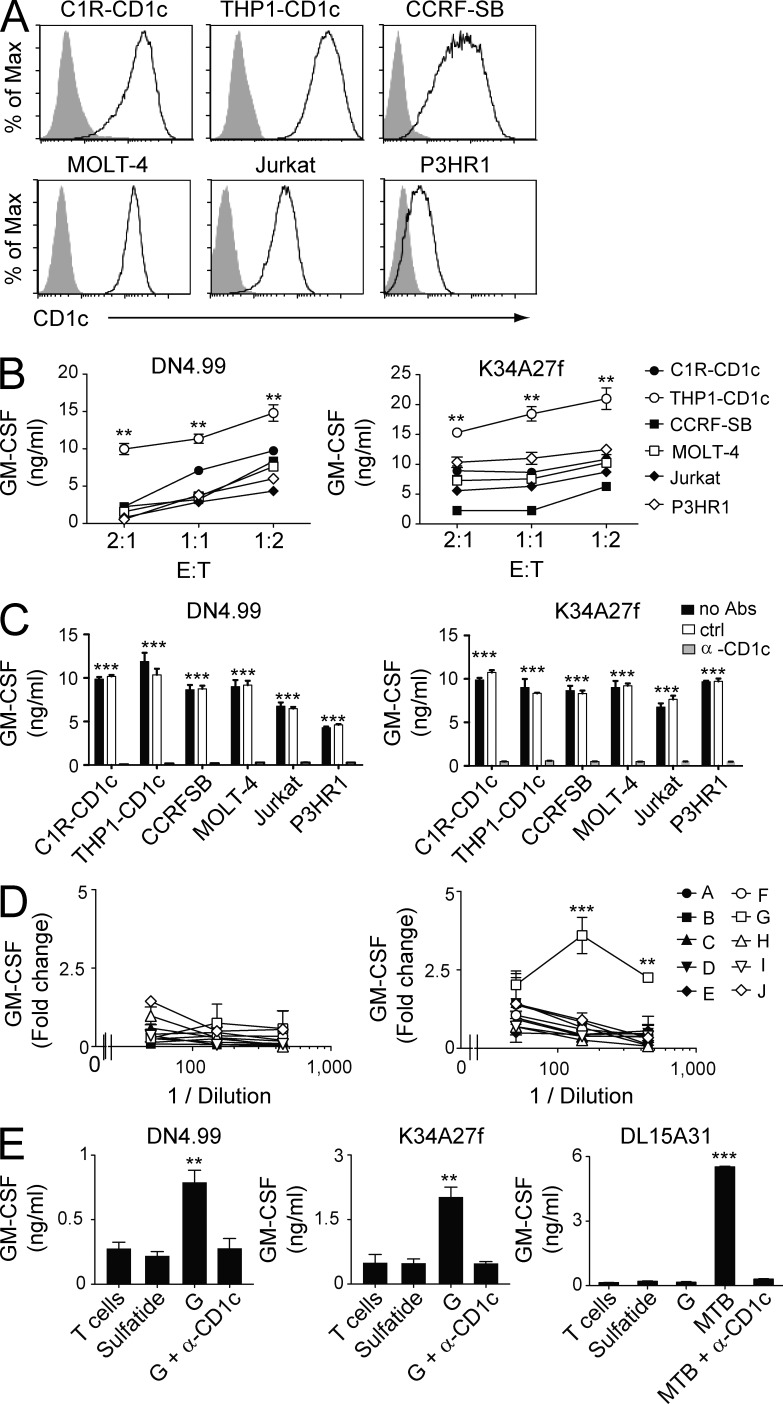
Autoreactive T cells restricted to CD1c are abundant in the peripheral blood of healthy donors (de Lalla et al., 2011) and are activated in the absence of exogenous antigens, suggesting that they target cells expressing endogenous molecules presented by CD1c. Because CD1c is exclusively expressed on hematopoietic cells, we initially evaluated the capacity of different tumor cell lines of hematopoietic origin to activate CD1c autoreactive T lymphocytes. Two CD1c self-reactive T cell clones isolated from separate donors were stimulated with CD1c gene-transfected C1R cells (C1R-CD1c, representative of Epstein-Barr virus–transformed lymphoblastoid cells) and THP1 cells (THP1-CD1c, representative of acute myeloid leukemia [AML]) and with four other cell lines that naturally expressed CD1c: CCRF-SB (a B cell acute lymphoblastic leukemia [B-ALL]), MOLT-4 and Jurkat (established from T cell acute lymphoblastic leukemia [T-ALL]), and P3HR1 (a Burkitt’s lymphoma; Fig. 1 A). All six tumor cell lines induced T cell production of GM-CSF (Fig. 1 B) and IFN-γ (not depicted) in a CD1c-dependent manner, as indicated by full inhibition of target recognition with blocking anti-CD1c mAbs (Fig. 1 C). Hence, CD1c self-reactive T cells were stimulated by a broad range of hematological malignancies, raising the question as to which type of common self-lipid antigens stimulate these cells.
Figure 1.
Recognition of leukemia cell lines and leukemia-derived lipids by CD1c autoreactive T cells. (A) Flow cytometry detection of CD1c surface protein in C1R and THP1 cell lines transfected with CD1c cDNA and other leukemia cell lines constitutively expressing CD1c. Gray histograms represent staining with isotype-matched control mAbs. (B) Recognition of CD1c-expressing leukemia cell lines by CD1c self-reactive T cell clones DN4.99 (left) and K34A27f (right) at the indicated E/T ratios. T cell activation was assessed by measurements of GM-CSF release. (C) Anti-CD1c blocking mAbs (α-CD1c) inhibit the recognition of CD1c-expressing leukemia cell lines by DN4.99 (left) and K34A27f (right). T cell reactivity in the absence of antibodies (no Abs) and presence of matched isotype control mAbs (ctrl) is shown. T cell activation was evaluated my measuring GM-CSF release. (D) Fractions (A–J) of THP1 lipids extracted from the organic (left) and aqueous phase (right) were used to stimulate DN4.99 T cells in the presence of fixed THP1-CD1c cells. T cell activation was assessed by measurement of GM-CSF release and expressed as fold change over background. (E) CD1c self-reactive T cell clones DN4.99 (left) and K34A27f (middle) or CD1c-restricted M. tuberculosis–specific DL15A31 T cells (right) were cultured either alone (T cells) or in the presence of plastic-bound sCD1c molecules loaded with sulfatide, fraction G from aqueous extraction (G), or M. tuberculosis sonicate (MTB) with or without anti-CD1c blocking mAbs (α-CD1c). T cell activation was assessed by measurement of GM-CSF release. All data are represented as mean ± SD. Results are representative of three (B, C, and E) and seven (D) independent experiments. **, P < 0.01; ***, P < 0.001, determined by two-tailed Student’s t test. P-values in C refer to both groups “no Abs” and “ctrl” individually compared with the group “α-CD1c.”
To identify the common lipid antigens, we used combined biochemical approaches in conjunction with T cell activation assays. Total lipids were extracted from the THP1 cell line, which most efficiently stimulated both CD1c-restricted T cell clones (Fig. 1 C). Separation of the total lipid mixture based on polarity and charge (Facciotti et al., 2012) revealed only one lipid fraction (G) that was capable of supporting the dose-dependent activation of self-reactive T cells by fixed CD1c+ APCs (Fig. 1 D). Fraction G also stimulated T cells when presented by plastic-bound recombinant soluble CD1c (sCD1c), whereas anti-CD1c mAb inhibited T cell activation in these assays (Fig. 1 E, left and middle). Control antigen sulfatide (Shamshiev et al., 2002) failed to stimulate T cells in these experiments, and an irrelevant CD1c-restricted T cell clone (specific for Mycobacterium tuberculosis lipids) did not respond to fraction G, thus confirming the antigen specificity of the response (Fig. 1 E, right).
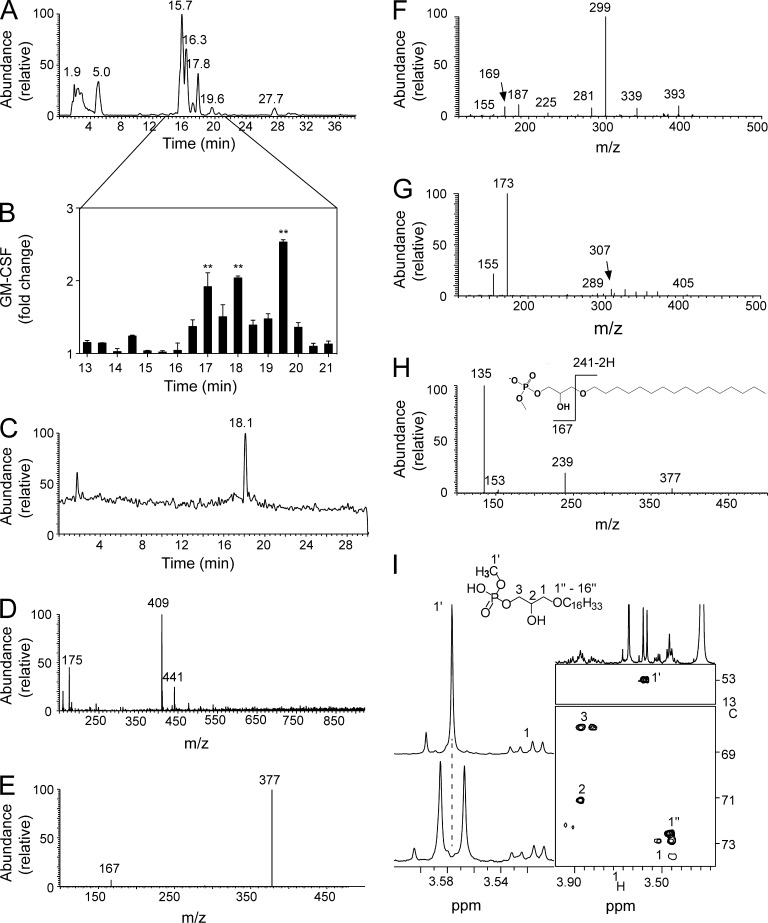
HPLC purification of the lipids in fraction G (Fig. 2 A) indicated that the lipids eluted at minutes 17, 18, and 19.5 were capable of stimulating CD1c self-reactive T cells (Fig. 2 B). Liquid chromatography (LC)–tandem mass spectrometry (MS [LC-MS-MS]) was then used to reanalyze the lipids obtained at min 17 to better determine the structure of the tumor-associated lipid antigens. A single lipid was eluted at min 17 (Fig. 2 C), and this compound exhibited a mass/charge (m/z) value of 409 in negative ion mode (Fig. 2 D). Because this mass did not match any known lipids, a detailed biochemical investigation was performed to identify the novel structure. Tandem MS (MS2) fragmentation of the novel compound yielded an ion of m/z 377 (Fig. 2 E), corresponding to the loss of methanol (32 D). The detection of an additional ion of m/z 167 in the low-mass region of the MS2 spectra indicated the presence of a phosphate moiety (signature ion m/z 153; Pulfer and Murphy, 2003) and an associated methyl group (m/z 14). Based on these data, we assigned a methylated phosphate head to the structure of the antigenic lipid.
Figure 2.
Identification of mLPA as a CD1c-restricted T cell antigen. (A) LC-MS total ion profile of fraction G derived from aqueous extract. (B) Activation of DN4.99 T cells by fixed THP1-CD1c cells in the presence of HPLC-MS lipid subfractions (minutes 13–21) derived from fraction G. GM-CSF levels are expressed as fold change over background. Error bars represent SD. **, P < 0.01, determined by two-tailed Student’s t test. Data are representative of three independent experiments. (C) Profile of LC-MS rechromatography of the active subfraction collected at 17 min. (D) Mass spectrum of the compound eluted at 18.1 min, showing a single molecular species with m/z 409 in negative ion mode. (E) MS2 spectrum of ion m/z 409. (F) MS2 spectrum of ion m/z 411 from fraction G as analyzed in positive mode. The presence of a methylated phosphate head group was confirmed by the detection of a major peak at m/z 299 (caused by the loss of 112 D from parent ion m/z 411) and presence of ions at m/z 187 and 169. A low intensity ion at m/z 225 was attributable to a hexadecyl-carbocation, which supported the existence of a C16 alkyl chain (as predicted by the negative mode analysis). (G) MS2 spectrum of the unmodified LPA 1-O-9-(Z)-octadecenyl-sn-glycero-3-phosphate. The spectrum showed signature ions for a phosphate head at m/z 155 and 173. These ions were shifted to m/z 169 and 187 in the spectrum presented in F because of the presence of an additional methyl group in the polar head. (H) MS3 spectrum of ion m/z 377 and structure and fragmentation pattern of C16 mLPA. (I) Part of the 1H-NMR profile of the fraction collected at 17 min either without (bottom) or with (top) broadband decoupling on the 31P channel. Sections of the 13C-1H HSQC spectrum showing the relevant cross peaks for the assignment of mLPA (right).
We next used positive ion mode analyses to confirm the candidate structure proposed by the negative ion mode data. The protonated ion of the novel compound (m/z 411) generated a fragment of m/z 299 and associated loss of 112 D in mass, corresponding to the loss of a methyl hydrogen phosphate moiety (Fig. 2 F). Ions with m/z of 169 and 187 were detected in the low mass region (Fig. 2 F), consistent with the presence of a 14-D methylated head group bound to phospholipids of 155 or 173 D (Fig. 2 G). Additional experiments were then performed using a triple quadrupole analyzer to confirm the presence of a phospho-methyl group within the novel lipid antigen. Upon collision-induced dissociation, the deprotonated ion of m/z 409 generated ions of m/z 377 and 111 (not depicted). An excess mass of 14 D again pointed to the presence of a methylated phosphate group (m/z 97 + 14).
To elucidate the structure of the hydrophobic chain of the novel lipid antigen, we used triple-stage MS (MS3) in negative mode with an ion-trap instrument. Fragmentation of ion m/z 377 generated a further ion of m/z 239 (Fig. 2 H), indicative of an ether-bonded C16 alkyl or alkenyl chain. However, prominent peaks indicative of alkenyl chains (Han and Gross, 1996; Hsu and Turk, 2007) were absent in the earlier MS2 analysis, thereby excluding this structural feature. The presence of a reproducible, low-intensity hexadecyl-carbocation at m/z 225 in the MS2 spectra of the protonated molecule further supported the presence of an alkyl chain (Fig. 2 F). Together, the MS data defined the lipid antigen eluted at minute 17 as a lyso-glycero-methyl hydrogen phosphate with an ether-bonded C16 alkyl chain (1-O-hexadecyl-sn-glycero-3-phosphoric acid methyl ester, or methyl-lysophosphatidic acid [LPA; mLPA]; Fig. 2 H). High-resolution MS analysis supported this conclusion by revealing a protonated molecular mass of 411.2872 D, compatible with the theoretical mass of the proposed molecule to within 0.5 parts per million (ppm) error (not depicted).
To confirm the proposed structure of the novel lipid, full assignment and structural elucidation of the compound were undertaken using one- and two-dimensional nuclear magnetic resonance (NMR) spectroscopy. A prominent signal was detected at 3.58 ppm in the proton NMR spectrum, corresponding to the methoxy group on the phosphate portion of the novel lipid. The resonance reading for this phosphate moiety appeared as a doublet because of scalar coupling to the 31P nucleus (3JHP = 10.9 Hz), but the doublet was resolved by decoupling (Fig. 2 I). Correlation spectroscopy (COSY) unraveled the complex pattern of the five glycerol protons (not depicted). We then applied a two-dimensional 13C-1H heteronuclear single quantum coherence spectroscopy correlation (HSQC) to identify and assign all carbons and protons within the molecule (Fig. 2 I, right inset) and validated this assignment using a 31P-1H heteronuclear multiple bond correlation (HMBC) to demonstrate the interaction of the phosphorus atom with the methoxy group and the H3 protons (not depicted). In support of these data, we prepared synthetic mLPA analogues that showed identical chemical shifts in the 1H, 13C, and 31P spectra and recorded spectra from 1H-1H nuclear Overhauser effect spectroscopy (NOESY) and long-range 13C-1H HMBC that were consistent with the postulated structure (not depicted).
A similar structure was also identified in the second active subfraction of THP1 lipid extract collected at min 18 (Fig. 2 B). Analysis of this molecule revealed a second lyso-glycero-methyl hydrogen phosphate that contained an ether-bonded C18-alkyl chain (not depicted), which was longer than the C16 alkyl chain present in the compound eluted at minute 17. Indeed, we characterized the stimulatory compound collected at minute 18 as a C18 mLPA (not depicted).
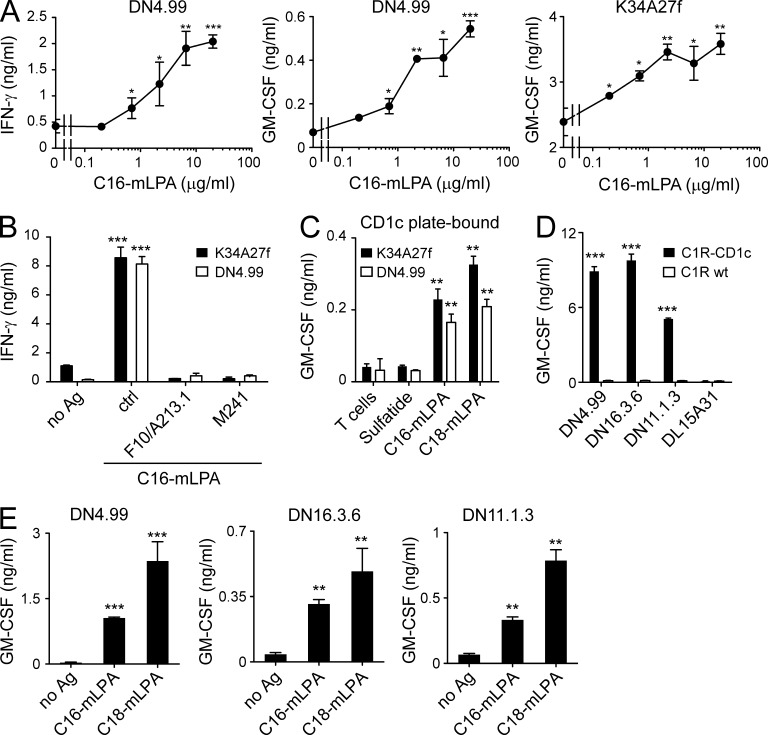
We next used synthetic analogues of the naturally occurring molecules (identical mass spectra and NMR behavior; not depicted) to test the antigenic potency of the novel lipids. The synthetic compounds very efficiently activated T cells when presented by CD1c+ APCs with potency ranging between 0.5 and 2 µg/ml (Fig. 3 A). Two anti-CD1c mAbs completely inhibited the response (Fig. 3 B). The synthetic analogues also stimulated T cells when loaded onto plastic-bound sCD1c molecules, thus confirming the recognition of the CD1c–mLPA complexes (Fig. 3 C). The same T cells were very weakly stimulated by other more common phospholipids (LPA, lysophosphatidylcholine [LPC], and lysophosphatidylethanolamine [LPE]) only at higher doses, >50 µg/ml, suggesting high specificity for mLPA (not depicted). Two additional CD1c self-reactive T cell clones isolated from a further two healthy donors (Fig. 3 D) and bearing different TCR α/β chains were also capable of recognizing the synthetic analogues (Fig. 3 E), indicating that T cells specific for the novel lipids are present in healthy individuals. Collectively, these data identified the previously unknown self-lipid antigen mLPA as a potent agonist for CD1c-restricted human T cells.
Figure 3.
mLPA-specific T cells recognize synthetic mLPA analogues. (A) T cell clones DN4.99 (left and middle) and K34A27f (right) were stimulated with synthetic C16 mLPA presented by purified primary B cells. (B) DN4.99 and K34A27f T cells were stimulated with purified primary B cells unloaded (no Ag) or loaded with 10 µg/ml C16 mLPA in the presence or not (ctrl) of two different anti-CD1c mAbs (F10/A213.1 or M241). (C) The same T cells were either cultured alone (T cells) or stimulated with plastic-bound sCD1c molecules loaded with control lipid (sulfatide) or synthetic mLPA (C16 or C18). (D) Recognition of C1R-CD1c but not C1R WT by CD1c self-reactive T cell clones DN4.99, DN16.3.6, and DN11.1.3, each obtained from a different donor. The CD1c-restricted M. tuberculosis–specific DL15A31 T cell clone was used as a negative control for irrelevant specificity. (E) Activation of T cell clones DN4.99 (left), DN16.3.6 (middle), and DN11.1.3 (right) in the presence of purified primary B cells and synthetic C16 or C18 mLPA (or without antigen, no Ag). T cell activation was evaluated by measurement of IFN-γ and/or GM-CSF release, expressed as mean ± SD. Results are representative of at least three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001, determined by two-tailed Student’s t test.
Primary leukemia blasts express CD1 molecules
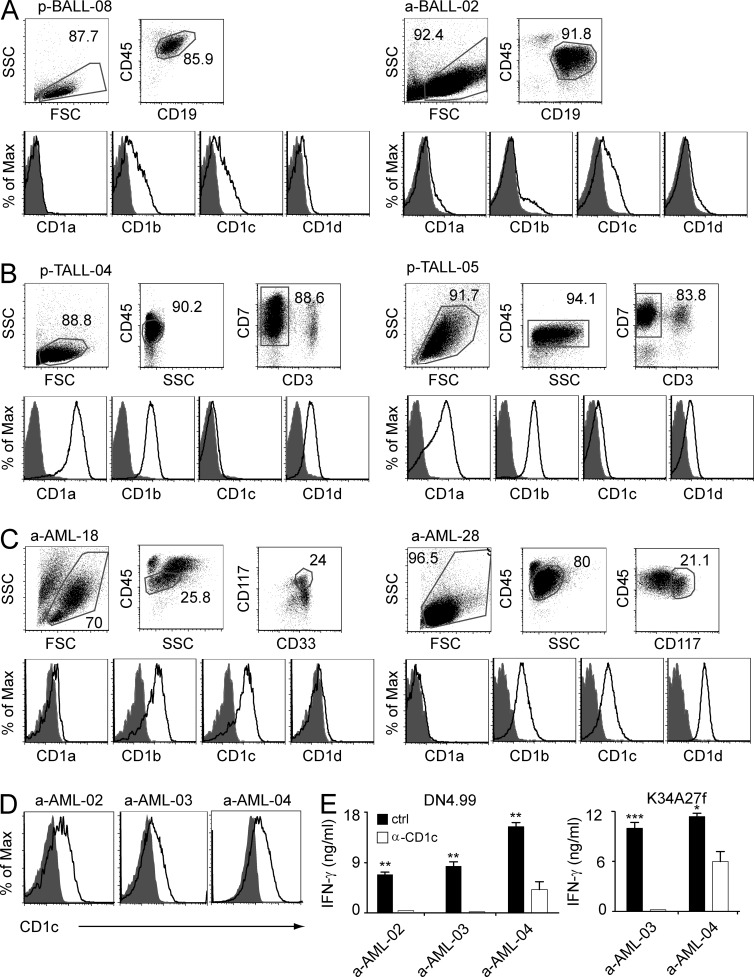
Having identified a novel self-lipid antigen that was presented by CD1c in leukemia cell lines, we next investigated the frequency of CD1c expression by leukemia blasts in patients. Peripheral blood and bone marrow samples obtained from AML, B-ALL, and T-ALL patients (Tables S1 and S2) were analyzed for the expression of different CD1 isoforms. Malignant cells were identified according to specific leukemia-associated cellular phenotypes (Fig. 4). All leukemia blasts expressed CD1 molecules, although at different levels in different patients (Table 1 and Fig. 4). AML blasts from pediatric patients expressed CD1b (45% of patients) and CD1c (45%) but lacked CD1a and CD1d (Table 1). Pediatric B-ALL blasts expressed CD1b (22%) and CD1c (26%) but rarely expressed either CD1a (4%) or CD1d (4%; Table 1 and Fig. 4 A). Pediatric T-ALL blasts expressed CD1a (75%), CD1b (75%), and CD1d (50%) but displayed CD1c at much lower frequency (12%; Table 1 and Fig. 4 B). In adult patients, AML blasts expressed CD1b (54%), CD1c (51%), and CD1d (24%) but little CD1a (3%; Table 1 and Fig. 4 C). B-ALL blasts expressed CD1c (71%) and CD1d (29%), whereas CD1a and CD1b were undetectable (Table 1 and Fig. 4 A). CD1 molecules are thus frequently present on leukemia blasts. The expression of CD1 molecules was not associated with disease risk or particular clinical, cytogenetic, and molecular prognostic features (Tables S1 and S2; Döhner et al., 2010; Pui et al., 2011).
Figure 4.
Primary leukemia blasts express CD1 molecules and stimulate mLPA-specific T cells. (A) CD1 expression by primary B-ALL blasts from a pediatric patient (p-BALL-08) and an adult patient (a-BALL-02). (B) CD1 expression by primary T-ALL blasts from two pediatric patients (p-TALL-04 and p-TALL-05). (C) CD1 expression by primary AML blasts from two adult patients (a-AML-18 and a-AML-28). Gating strategies to identify the patient-specific leukemia phenotypes are shown in the top panels. White histograms indicate specific staining of the gated populations with CD1-specific mAbs, and gray histograms indicate the negative isotype-matched control staining (bottom). (D) CD1c expression by primary AML blasts from three adult patients (a-AML-02, a-AML-03, and a-AML-04). (E) Specific recognition of the same primary AML blasts by T cell clones DN4.99 (left) and K34A27f (right) at a 1:10 E/T ratio, in the presence of irrelevant mAb (ctrl) or anti-CD1c blocking mAbs (α-CD1c). T cell activation was assessed by measuring IFN-γ release and was repeated twice with frozen aliquots of each leukemia sample. Data are expressed as mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001, determined by two-tailed Student’s t test.
Table 1.
CD1 expression by primary leukemia blasts
| Malignancy | Number | Patients with CD1-expressing blasts | |||
| CD1a | CD1b | CD1c | CD1d | ||
| % | % | % | % | ||
| Pediatric patients | |||||
| AML | 9 | 0 | 45 | 45 | 0 |
| B-ALL | 23 | 4 | 22 | 26 | 4 |
| T-ALL | 8 | 75 | 75 | 12 | 50 |
| Adult patients | |||||
| AML | 33 | 3 | 54 | 51 | 24 |
| B-ALL | 7 | 0 | 0 | 71 | 29 |
Data refer to circulating blasts. Similar CD1 expression frequencies were observed in bone marrow biopsy samples.
Differential recognition of primary leukemia blasts and healthy hematopoietic cells by mLPA-specific T cells
Because CD1c is expressed by a large proportion of acute leukemia blasts, we studied whether mLPA-specific T cells also recognize primary leukemia cells in a CD1c-dependent manner. Primary CD1c+ AML blasts freshly isolated from three different patients (Fig. 4 D) induced IFN-γ release by two mLPA-specific T cell clones, and their recognition was blocked by anti-CD1c mAbs (Fig. 4 E).
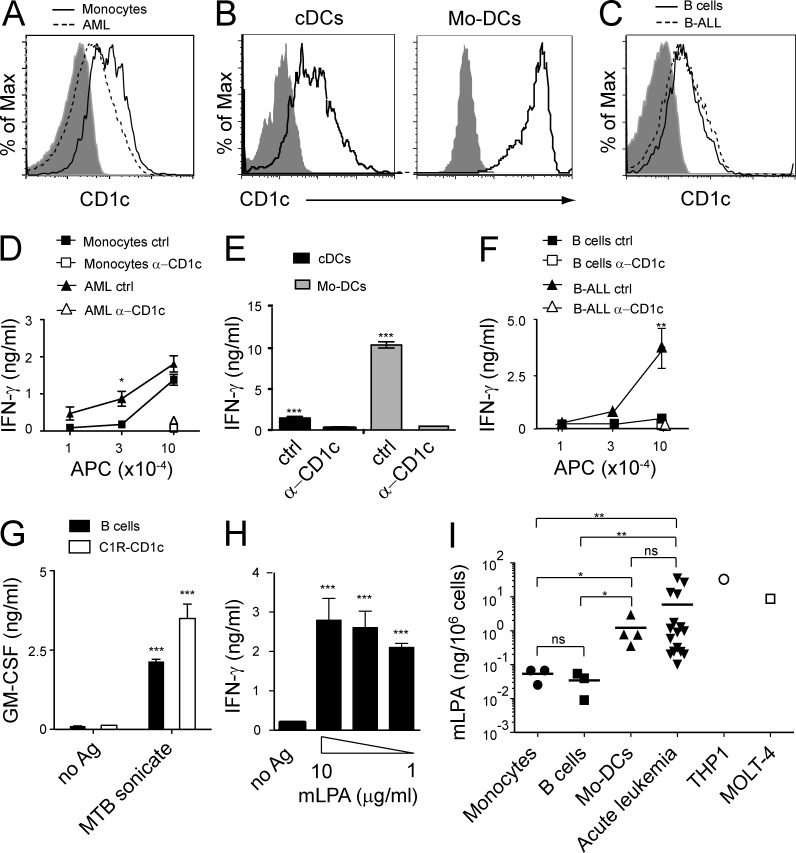
We next assessed whether mLPA-specific T cells recognized cancer as well as normal CD1c+ cells. Initially, we examined the myeloid compartment. The selected AML blasts expressed lower levels of CD1c than normal circulating monocytes and DCs (cDCs; Fig. 5, A and B); nevertheless, they elicited higher levels of T cell activation than normal monocytes (Fig. 5 D) and similar to that of cDCs (Fig. 5 E), suggesting that AML blasts act as potent stimulatory cells. In vitro–differentiated monocyte-derived DCs (Mo-DCs) displayed high expression of CD1c (Fig. 5 B) and also exhibited strong T cell stimulatory activity (Fig. 5 E). The recognition of all four cell types was fully inhibited by anti-CD1c mAbs (Fig. 5, D and E).
Figure 5.
Differential recognition of malignant and healthy hematopoietic cells by mLPA-reactive T cells. (A–C) Expression of CD1c by healthy donor-derived primary monocytes and primary AML blasts (A), primary DCs purified from circulating mononuclear cells (cDCs) and Mo-DCs (B), and B cells from healthy donors and primary B-ALL blasts (C). (D–F) The same cell types were used to stimulate DN4.99 T cells in the presence of anti-CD1c mAbs (α-CD1c) or isotype-matched control mAbs (ctrl). IFN-γ release by T cells was measured and expressed as mean ± SD. (G) Normal B cells efficiently presented M. tuberculosis lipid antigen (MTB sonicate) to CD1c-restricted M. tuberculosis–specific T cells (DL15A31). C1R-CD1c lymphoblastoid cells were used as control. T cell activation was assessed by measuring release of GM-CSF, expressed as mean ± SD. (H) Normal B cells efficiently presented C16 mLPA to CD1c self-reactive T cells (DN4.99). T cell activation was evaluated by measuring release of IFN-γ, expressed as mean ± SD. (A–H) Results are representative of three (A–G) and four (H) independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001, two-tailed Student’s t test. (I) C16 mLPA content in normal primary circulating monocytes (n = 3) and B cells from healthy donors (n = 3), in vitro–differentiated Mo-DCs (n = 4), primary circulating B-ALL and AML blasts (acute leukemia, n = 5 and n = 11, respectively), AML cell line THP1, and T-ALL cell line MOLT-4. In primary acute leukemia samples the percentage of blasts ranged between 75 and 97%. The mLPA concentration value of each sample represents the mean value of two to three independent determinations performed with the same sample. Horizontal bars represent the mean values of the primary samples. *, P < 0.05; **, P < 0.01; ns, not statistically different, two-tailed Student’s t test.
Next, we determined CD1c-restricted T cell recognition of transformed and normal B cells. Primary CD1c+ B-ALL blasts and healthy B cells displayed comparable CD1c expression levels (Fig. 5 C), yet the leukemic cells induced markedly higher production of IFN-γ by mLPA-reactive T cell clones (Fig. 5 F). Also in this case, anti-CD1c mAbs completely blocked the recognition of B-ALL (Fig. 5 F). Normal B cells were able to present exogenously loaded synthetic mLPA or bacterial-derived lipids to specific CD1c-restricted T cell clones (Fig. 5, G and H), thus ruling out the possibility of an impaired lipid antigen presentation capacity of these cells. Collectively, these data indicated that mLPA-specific T cells recognize malignant cells more efficiently than normal primary hematopoietic cells.
Accumulation of mLPA in leukemic cells
We next quantified the amount of mLPA present in primary leukemia blasts and in acute leukemia cell lines. C16 mLPA was detected in all the primary leukemia samples tested (11 AML and 5 B-ALL; range 0.58–35.22 ng/106 cells) in THP1 and MOLT-4 cells (31.68 and 8.76 ng/106 cells, respectively; Fig. 5 I), thereby confirming that mLPA is commonly present in leukemic cells. C16 mLPA was also detected in large amounts in Mo-DCs (range 0.35–2.96 ng/106 cells), in agreement with the stimulatory capacity of these cells (Fig. 5 E). Very low quantities of C16 mLPA were detected in normal circulating monocytes and B cells (range 0.03–0.07 and 0.01–0.05 ng/106 cells, respectively; Fig. 5 I), in agreement with their poor T cell stimulatory activity (Fig. 5, D and F). These data suggested that mLPA accumulates in leukemia cells but is poorly present in normal hematopoietic cells, with the exception of in vitro–differentiated Mo-DCs.
mLPA-reactive T cells kill leukemic cells and limit tumor growth in vivo
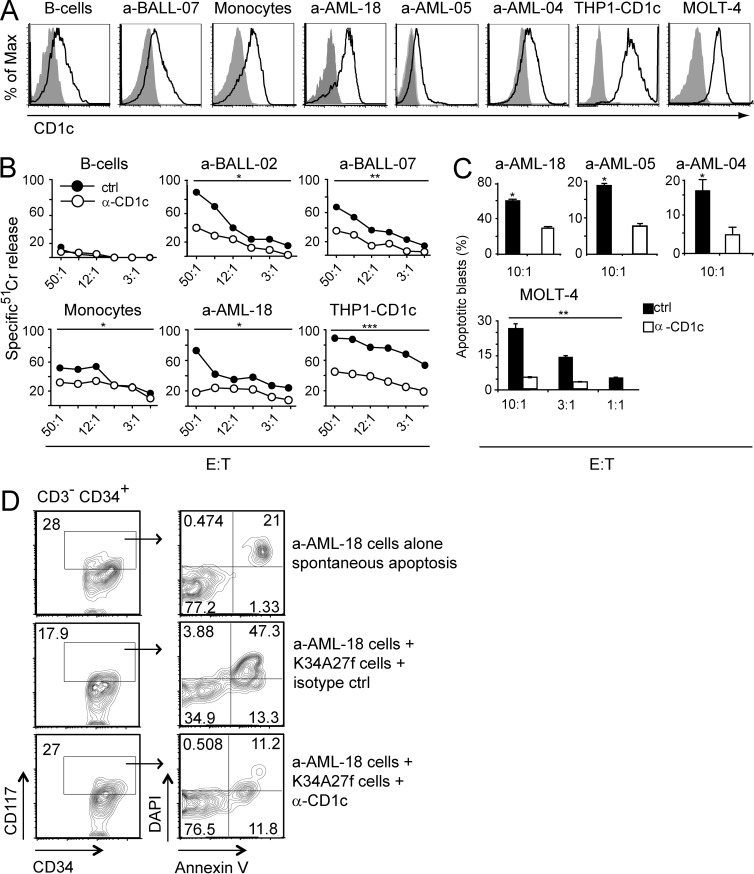
To assess the relevance of leukemia recognition by mLPA-specific T cells, we investigated the ability of these cells to restrict the growth of CD1c+ acute leukemia cells in different settings. T cell clones specific for mLPA were capable of killing CD1c+ primary AML and B-ALL blasts and acute leukemia cell lines in a CD1c-dependent manner in vitro (Fig. 6, A–C). mLPA-specific T cell clones did not kill normal B cells, unlike leukemic B cells, whereas they killed normal monocytes, albeit less efficiently than AML blasts (Fig. 6 B).
Figure 6.
mLPA-specific T cells kill CD1c+ acute leukemia cells. (A) CD1c expression by the indicated primary normal monocytes, primary AML blasts, and leukemia cell lines. (B) Killing of 51Cr-labeled primary AML and B-ALL blasts and normal purified monocytes and B cells. Cell targets were cultured with mLPA-specific T cells (K34A27f) at increasing E/T ratio in the presence of isotype control (ctrl) or CD1c blocking mAbs (α-CD1c). 51Cr-labeled THP1-CD1c cells were used as positive control. Data are representative of two independent experiments. Two-tailed paired Student’s t test was used to compare the curves: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) The indicated cell targets were cultured with mLPA-specific T cells (K34A27f) at the indicated E/T ratios in the presence of isotype control (ctrl) or CD1c blocking mAbs (α-CD1c). The percentages of apoptotic blasts were determined by flow cytometry using Annexin V and DAPI staining. Columns indicate mean ± SD and are representative of two and three independent experiments with primary AML blasts and leukemia cell lines, respectively. *, P < 0.05; **, P < 0.01, determined by two-tailed Student’s t test. (D) FACS analysis of apoptotic primary AML blasts (a-AML-18) upon co-culture with mLPA-specific T cells (K34A27f) in the presence of isotype control mAbs (IgG1 isotype ctrl, middle) or anti-CD1c mAbs (α-CD1c, bottom). Spontaneous apoptosis of AML blasts in the absence of T cells is also shown (top). Leukemic cells responsible for the minimal residual disease in treated patients were identified by coexpression of CD34 and CD117 according to their specific LAIP (left). Apoptotic cells were defined by combined staining with DAPI and Annexin V (right). Data are representative of two independent experiments.
Remarkably, the mLPA-specific T cells efficiently killed the CD34+ CD117+ myeloid leukemia blasts identified by the expression of the specific leukemia-associated immune phenotype (LAIP; Vidriales et al., 2003), which are responsible for the minimal residual disease in treated patients (Fig. 6 D). The control M. tuberculosis–specific CD1c-restricted T cell clone failed to kill acute leukemia primary cells and cell lines but efficiently killed M. tuberculosis–infected target cells (not depicted).
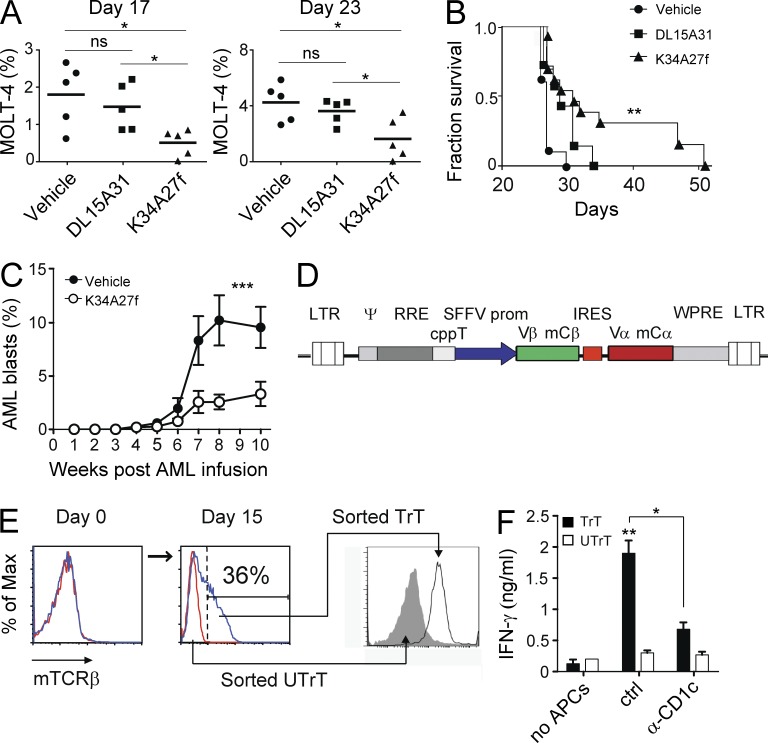
In a second type of experiment, the capacity of mLPA-specific T cells to control leukemia cells in vivo was investigated. The transfer of mLPA-specific T cells delayed the growth of CD1c+ MOLT-4 acute leukemia cells grafted into immunodeficient nonobese diabetic (NOD)/scid mice (Fig. 7 A), which displayed significantly increased survival compared with control groups that received either T cells with irrelevant specificity (CD1c-restricted and M. tuberculosis–specific) or vehicle alone (Fig. 7 B). MOLT-4 cells were used because they express CD1c (Figs. 1 A and 6 A), contain high amounts of mLPA (Fig. 5 I), and efficiently graft in these immunocompromised animals (not depicted). The two transferred T cell clones displayed a similar persistence in vivo. They both declined from a mean of 10% of the mouse PBMCs on day 1 after transfer to undetectable levels on day 7. The adoptive transfer of the same mLPA-specific T cell clone also significantly delayed the progression of primary CD1c+ AML blasts, purified from the peripheral blood of a patient and transferred into immunocompromised NOD/scid/common γ chain−/− (NSG) mice (Fig. 7 C). In all the mice, MOLT-4 cells retained CD1c expression upon progression, whereas the primary AML blasts reduced CD1c expression at week 5 after transfer independently of the transfer of mLPA-specific T cells (not depicted). Collectively, these findings indicate that (a) the long-term control of leukemia by the mLPA-specific T cells is probably ascribed to killing functions exerted in the first week after adoptive transfer, (b) progression of both leukemias was not linked to CD1c down-regulation as escape mechanisms, and (c) the maintenance of CD1c gene expression in primary AML blasts transferred in NSG mice depended on specific environmental cues.
Figure 7.
mLPA-specific T cells are promising tools for T cell adoptive immunotherapy of CD1c+ acute leukemia. (A) Percentage of circulating MOLT-4 cells at different time points in mice inoculated with CD1c-restricted T cell clones specific for mLPA (K34A27f) or M. tuberculosis (DL15A31) or with saline only (vehicle). Horizontal bars represent the mean values. *, P < 0.05; ns, not significant, two-tailed Student’s t test. (B) Survival of mice that received either T cells or vehicle alone. Data show cumulative results from three independent experiments including five mice per group. Mice that received mLPA-specific T cells showed increased survival. **, P < 0.01, Mantel-Cox test. (C) Progression of human primary CD1c+ AML blasts in NSG mice that received mLPA-specific K34A27f T cells (n = 5) or vehicle only (n = 5). The percentage of AML blasts was monitored by flow cytometry in PBMCs at the indicated times. Bars indicate mean ± SD. Results are representative of two independent experiments. ***, P < 0.001, nonparametric Student’s t test. (D) Map of the bicistronic lentivirus vector pHR-SINEGFP carrying the chimeric TCR V-(D)-J segments from the mLPA-specific T cell clone DN4.99, fused with the mouse TCR Cα or Cβ cDNAs. Both chains are expressed from the internal spleen focus forming virus (SSFV) promoter. (E) Generation of mLPA-specific T cell lines in vitro upon transduction of polyclonal T cells with the lentivirus described in D. TCR expression by transduced (TrT, blue lines) and untransduced (UTrT, red lines) T cells at days 0 and 15 and after sorting. TCR expression was detected using mouse Cβ-specific (mTCRβ) mAbs. Data are representative of three experiments. (F) Recognition C1R-CD1c lymphoblastoid cells by T cells transduced with the DN4.99 TCR (TrT) but not by untransduced ones (UTrT). T cells were cultured without (no APCs) or with C1R-CD1c cells in the presence (α-CD1c) or absence (ctrl) of anti-CD1c blocking mAbs. Bars indicate mean ± SD of IFN-γ production of triplicate cultures. Results are representative of three experiments. *, P < 0.05; **, P < 0.01; ns, not statistically different, two-tailed Student’s t test.
Collectively, these experiments underscored the capacity of mLPA-specific T cells to limit leukemia progression in vivo, suggesting the possibility to harness these T cells for the adoptive immunotherapy of CD1c+ acute leukemia. To directly assess this possibility, we generated primary T cell lines endowed with CD1c self-reactivity by transducing PBMCs from healthy donors with a lentivirus expressing the TCR α and β chains of a CD1c self-reactive mLPA-specific T cell clone (Fig. 7 D). Only the T cells displaying surface expression of the transduced TCR chains, and not the untransduced ones sorted from the same cell line (Fig. 7 E), recognized a CD1c-expressing tumor cell line (Fig. 7 F). Anti-CD1c mAbs inhibited recognition. These results indicated that mLPA-specific T cells may be generated and expanded for adoptive immunotherapy of acute leukemia.
Absence of CD1c expression in hematopoietic precursors and in nonhematopoietic tissues of healthy individuals
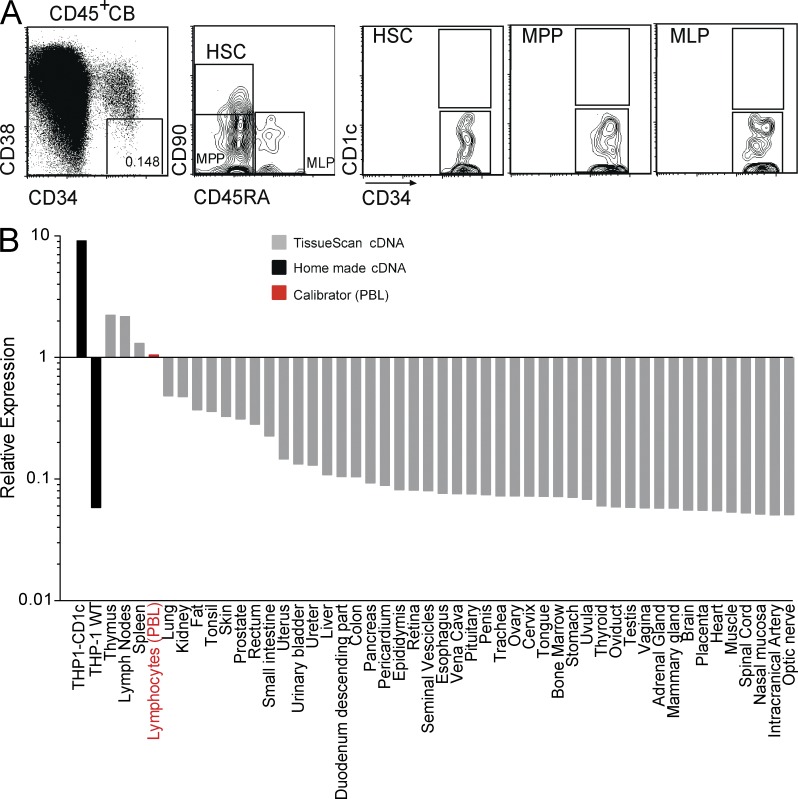
We finally investigated the expression of CD1c in hematopoietic precursors and other not lymphoid solid tissues to determine whether they might be targets of mLPA-specific CD1c-restricted T cells. CD1c was undetectable by flow cytometry on relevant subsets of primary CD34+ HSCs and immature precursors contained in cord blood (Fig. 8 A), suggesting that HSC recognition by CD1c self-reactive T cells is unlikely to occur in healthy individuals. CD1c mRNA was also almost undetectable in several parenchymatous organs and nonlymphoid tissues (Fig. 8 B), confirming that tissues do not express CD1c (Porcelli and Modlin, 1999; Brigl and Brenner, 2004). These results suggested that hematopoietic cell precursors as well as solid organs are unlikely to be targeted by mLPA-specific CD1c-restricted T cells.
Figure 8.
CD1c expression pattern in human hematopoietic precursors and healthy organs. (A) Normal hematopoietic precursors lack CD1c expression. Cord blood (CB) cells from healthy donors were stained with the indicated mAbs (CD34-FITC, anti–CD45-APCCy7, CD38-PECy7, CD45RA-PB, CD90-APC, and anti–CD1c-PE). The boxes represent the gates used to identify HSCs, multipotent progenitors (MPPs), and multilymphoid progenitors (MLPs), according to Doulatov et al. (2012). Representative dot plots from at least three independent experiments are shown. (B) CD1c mRNA expression in human tissues. RT-qPCR for CD1c expression was performed on cDNAs from human tissues contained in the TissueScan Real-Time Human Major Tissue Panels II (OriGene), according to the manufacturer’s instructions. We added two custom cDNAs, produced by us from THP1-CD1c and THP1 WT cell lines, as positive and negative controls for CD1c expression. The data obtained were normalized against a calibrator signal, provided by the level of CD1c cDNA detection in PBMCs. CD1c expression was detected in lymph nodes, spleen, and thymus, whereas it was almost undetectable in all of the other tissues.
DISCUSSION
The tumor-associated antigens identified so far consist primarily of proteins that stimulate specific T cells only when processed into small peptides presented by HLA molecules (Speiser and Romero, 2010; Seremet et al., 2011). In this study, we identified a unique class of lipid self-antigens, exemplified by mLPA, which may represent promising novel targets for immunotherapy in human leukemia. We readily detected mLPA in primary human leukemia cells and confirmed that this antigen is potently stimulatory for CD1c-restricted T cell clones, which restrained leukemia progression in mice xenografted with human tumor cells.
mLPA is an unusual lysoglycerophosphate molecule never described before. It includes at least two different alkyl chains and displays two prominent structural features. The first is the presence of a methyl group on the phosphate moiety, which is shared in common with lysophosphomethanol (LPM). Both LPM and mLPA are methylated LPAs, but where LPM incorporates an sn-1 ester-bonded alkyl chain, mLPA instead features an ether bond. LPM is generated by autotaxin-mediated cleavage of LPC and accumulates in mouse cells to promote the proliferation and migration of tumors (Endo et al., 2009). The close structural similarity of mLPA and LPM suggests that these molecules could exert similar biological effects in tumor cells.
The second prominent structural feature of mLPA is the ether bond that links the alkyl chain to glycerol, which is a peroxisomal hallmark of ether-bonded lipid synthesis (Wanders et al., 2010). Peroxisome numbers increase in tumor cells (Cha et al., 2009) and increased β oxidation of fatty acids in peroxisomes is critical for tumor cell growth (Schrader and Fahimi, 2006). Because augmented peroxisome frequency and activity are characteristic of altered tumor cell metabolism, it is conceivable that the immune system recognizes accumulated peroxisome-derived lipids as tumor antigens. Indeed, a recent study has reported that a peroxisome-derived lipid antigen promotes thymic expansion and peripheral homeostasis of natural killer T cells (Facciotti et al., 2012). Peroxisomes thus represent an important source of lipids that can stimulate specific T cell responses in immunoregulation and tumor surveillance and may therefore also contribute to host protection against leukemias.
A key finding of our study was that mLPA can be detected in large amounts in different types of primary leukemic blasts and in established tumor cell lines of different hematopoietic lineages. We also measured high levels of mLPA in Mo-DCs, induced upon culturing with the inflammatory cytokines GM-CSF and IL-4, whereas it was detected at trace levels in normal circulating monocytes and B cells, consistent with their different stimulatory activity for T cells. In this respect, the cellular distribution of mLPA resembles that of other tumor-associated antigens that accumulate in tumor cells but are also present in healthy cells (Jäger et al., 2001; Boon et al., 2006). LPC, another lysophosphoglycerolipid, was also found to accumulate in the plasma in myeloma patients and to stimulate CD1d-restricted type II NKT cells. These latter cells were proposed to regulate chronic inflammation in cancer patients (Chang et al., 2008). These data support a similar link between oncogenetic stress, lysophospholipid biosynthesis, and CD1-restricted T cell responses.
Lipolytic release and remodeling of free fatty acids are associated with metabolic changes in tumor cells (Hsu and Sabatini, 2008; Vander Heiden et al., 2009) and could contribute to mLPA accumulation in cancers. Tumor cells often express increased levels of monoacylglycerol lipase, an enzyme which also generates lysophospholipids (Nomura et al., 2010) and induces accumulation of LPA. LPA is structurally similar to mLPA and can rescue cancer cells from migration defects and promote oncogenesis via activation of G protein–coupled receptors (Dorsam and Gutkind, 2007). LPA also promotes cytoskeletal reorganization, chemotaxis, and cell growth (Mills and Moolenaar, 2003), as well as inducing CXCL1 secretion, tumor progression, vascular remodeling, and reversal of cell differentiation (van Meeteren and Moolenaar, 2007). mLPA may confer similar advantages on rapidly growing cells, which could explain the accumulation of this lipid in tumors.
Our findings raise important questions concerning the physiological role of mLPA-specific T cells in healthy individuals versus cancer patients. This particular antigen specificity might “jump-start” immune responses by promoting rapid DC maturation and accelerating the expansion of antigen-specific B and T cells (Vincent et al., 2002; Cerundolo et al., 2009). Recognition of accumulating mLPA might represent a mechanism by which the host immune system recognizes self-lipid molecules as markers of cellular stress (De Libero et al., 2005). In addition, mLPA-specific T cells may participate in tumor immune surveillance, using their cytolytic capacity to eliminate potentially tumorigenic cells undergoing uncontrolled proliferation. Further investigation is warranted to unravel whether in patients the extent of the leukemia burden might have reduced T cells involved in leukemia immune surveillance.
The value of mLPA-specific T cells as novel immunotherapeutic tools is supported by their protective effect in a mouse xenograft model of human leukemia. These experiments suggested that mLPA-specific T cells may target leukemic cells in human patients in vivo. Adoptive T cell immunotherapy strategies that selectively target malignant cells without inducing GVHD hold promise for the control of tumor recurrence after HSCT in acute leukemia (Kolb, 2008). Targeting CD1c molecules and tumor-associated lipid antigens for immunotherapy might ideally integrate strategies that target protein antigens with remarkable advantages. Unlike peptide antigens (Matsushita et al., 2012), lipid antigens do not undergo structural changes when subject to the strong selective pressure of specific immune responses. Alterations of entire lipid biosynthetic pathways are often incompatible with cell survival, thus making loss of lipid antigens in tumor cells an unlikely mechanism of cancer immune evasion. Furthermore, the nonpolymorphic nature of CD1c and high frequency of CD1c+ leukemia (∼50%) might make adoptive immunotherapy with mLPA-specific T cells applicable in a large number of patients. The adoptive transfer of mLPA-specific T cells might complement immunotherapy with HLA-restricted T cells in HLA-haploidentical transplants, in which the leukemia blasts relapse and escape immune recognition after loss of HLA (Vago et al., 2009).
We showed that mLPA-specific T cells able to recognize leukemia blasts in a CD1c-dependent manner could be generated in vitro upon transduction of mLPA-specific TCR genes. This strategy might represent a relatively safe approach for immunotherapy of acute leukemia. Indeed, allogeneic T cells from stem cell donors might be transduced and their potential alloreactivity might be prevented by editing the endogenous TCR genes (Canderan et al., 2010; Provasi et al., 2012). An issue that deserves careful evaluation is that mLPA-specific T cells killed normal monocytes in vitro. In the case of mLPA-specific adoptive T cell therapy in the course of HSCT, this side effect might be tolerated by patients during the peritransplant period, when the control of minimal residual leukemia is paramount. Importantly, the absence of CD1c in hematopoietic cell precursors and its almost undetectable expression in parenchymatous organs and nonlymphoid tissues might prevent harmful reactions of transferred mLPA-specific T cells. Transduced cells might also be engineered with suicide genes, currently used in the clinics (Ciceri et al., 2009), to prevent or halt GVHD.
In conclusion, we have identified mLPA as a novel lipid antigen that potently activates CD1c-restricted human T cells. The broad expression of CD1 molecules by a large number of primary acute leukemias, combined with the wide distribution of mLPA in leukemias and the presence of mLPA-specific T cells in blood of healthy individuals, could provide new opportunities for cellular immunotherapy in human patients.
MATERIALS AND METHODS
Cells.
The following human cell lines were obtained from the ATCC: C1R (Epstein-Barr virus–transformed B lymphoblastoid), THP1 (myelomonocytic leukemia), MOLT-4 and Jurkat (T-ALL), CCRF-SB (B-ALL), and P3HR1 (Burkitt’s lymphoma). THP1-CD1c cells were generated by transfection of the human CD1c gene construct as previously described (Manolova et al., 2003). Primers used for PCR amplification were as follows: forward XhoI, 5′-CTCGAGGACATGCTGTTTCTGCAGTTTC-3′; and reverse NotI, 5′-GCGGCCGCTCACAGGATGTCCTGATATGAGC-3′. C1R-CD1c cells were provided by S. Porcelli (Albert Einstein College of Medicine, Bronx, New York). Bone marrow and peripheral blood samples containing primary leukemia blasts were obtained after written informed consent from adult patients or from the parents of pediatric patients in accordance with the Declaration of Helsinki. The study was approved by the Institutional Review Boards of San Raffaele Scientific Institute, Milan, and Fondazione IRCCS Policlinico San Matteo, Pavia. Primary AML, T-ALL, and B-ALL leukemia samples were provided by Unità Trapianto Midollo Osseo (San Raffaele, Milan), Northern Italy Leukemia Group (Bergamo), and A. Wodnar-Filipowicz (University of Basel, Basel, Switzerland). T cell clones were generated and maintained in culture as previously described (Shamshiev et al., 1999; de Lalla et al., 2011). Normal monocytes and B cells were purified (>90% purity) from PBMCs obtained from healthy volunteers using anti-CD14 and anti-CD19 immunomagnetic beads, respectively, according to the manufacturer’s instructions (Miltenyi Biotec). cDCs were enriched from PBMCs using human DC enrichment beads (Invitrogen), and the unbound fraction was sorted according to the expression of CD64 and CD11c. The resulting CD64+CD11c+ cDCs (>95% purity) uniformly expressed CD1c. Mo-DCs were differentiated in vitro from purified CD14+ monocytes in the presence of GM-CSF and IL-4 as described previously (Shamshiev et al., 1999).
Flow cytometry.
CD1 expression was determined using mAbs anti–CD1a-PE (clone HI149; BD), anti–CD1b-PE (clone 0249; Santa Cruz Biotechnology, Inc.), anti–CD1c-PE (clone L161; Santa Cruz Biotechnology, Inc.), and anti–CD1d-PE (clone 42.1; BD). Leukemia blasts were identified using anti–CD19-FITC or CD19-PerCPCy5.5 (both clone HIB19) and anti–CD14-FITC (clone HCD14; all from BioLegend); anti–CD3-FITC (clone UCHT1), anti–CD45-APC (clone HI30), and anti–CD117-PerCpCy5.5 (clone 2B8; all from BD); anti–CD7-PC5 (clone 8H8.1), anti–CD10-PC5 (clone ALB1), anti–CD33-FITC or CD33-PC5 or anti–CD33-APC (all clone D3HL60-251), anti–CD34-FITC or CD34-PC5 or anti–CD34-APC (all clone 581), and anti–CD45-PC7 (clone J33; all from Beckman Coulter). cDCs were sorted using anti-CD64 FITC (clone 22; Beckman Coulter) and anti–CD11c-APC (clone B-ly6; BD). Samples were acquired on CyAn ADP (Dako) or FACSCanto (BD) flow cytometers. Cell sorting experiments were performed using a MoFlo sorter (Beckman Coulter). Dead cells were excluded on the basis of propidium iodide and DAPI staining. All data were analyzed using FlowJo software (Tree Star). Relative fluorescent intensity (RFI) of CD1c expression on leukemia cells was calculated dividing the mean CD1c fluorescence intensity by the mean fluorescence intensity of the corresponding isotype control mAb.
T cell activation assays.
T cell activation assays were performed by coculturing the indicated numbers of target cells (5 × 104/well unless otherwise indicated) with T cells (5 × 104/well) in 200-µl volume in duplicate or triplicate cultures. In some experiments, mouse anti-CD1c (L161; Abcam) or mouse IgG1 isotype control mAbs were added and incubated for 30 min before the addition of T cells. Supernatants were collected after 24–48 h, and cytokines released by T cells (GM-CSF and/or IFN-γ) were measured by ELISA. In assays using fractionated lipids, THP1-CD1c cells (5 × 104/well) were fixed with 0.05% glutaraldehyde for 20 s and used as APCs. Fold change in cytokine release was calculated using the following formula: GM-CSF release with lipid-pulsed APC/GM-CSF release with unpulsed APC. In some experiments, normal B cells purified from PBMCs (5 × 104/well) were used as APCs without prior fixation. APCs were pulsed with lipids for 4 h at the indicated dilutions before addition of T cells. sCD1c plate-bound assays were performed using MaxiSorp plates (Thermo Fisher Scientific) coated with anti-BirA mAb (Nowbakht et al., 2005) to capture sCD1c and were then incubated with lipids for 6 h. The wells were extensively washed before addition of T cells. Each stimulation was performed in triplicate. Killing assays were performed using target primary blasts or established leukemia cell lines (2 × 104 cells/ml) incubated either alone or with T cells at different E/T ratios for 24 h, in the presence or absence of anti-CD1c mAb (L161). Killing of 51Cr-labeled targets was performed as described previously (Montagna et al., 2006). The mean spontaneous release ranged between 15 and 22% of total release. In the killing assays performed by flow cytometry, the target cells were stained with PE–Annexin V (BD) and DAPI (Sigma-Aldrich), according to published protocols (Jedema et al., 2004). Leukemia blasts were identified by their specific LAIPs and then assessed for apoptosis as follows: Annexin V+ DAPI+ = advanced apoptosis and Annexin V− DAPI+ = necrosis. The percentage of apoptotic + necrotic cells in the absence of T cells (spontaneous apoptosis) was subtracted from the percentage of apoptotic + necrotic cells in the presence of T cells (induced apoptosis) to obtain the proportion of blasts killed by T cells.
T cell transduction with lentivirus encoding the mLPA-specific TCR genes.
The generation of the bicistronic lentivirus vector pHR-SINEGFP (provided by V. Cerundolo, University of Oxford, Oxford, England, UK) encoding the chimeric TCR α and β genes from the mLPA-specific T cell clone DN4.99 was performed as described previously (Canderan et al., 2010). To generate the chimeric TCR chains, the TRAV38-TRAJ44 and the TRBV28-TRBJ2-7 segments were fused with the mouse TRAC and TRBC cDNA, respectively, by PCR. T cells were purified form healthy donors and activated with anti-CD3/CD28 immunomagnetic beads (Invitrogen) in RPMI-FCS complete medium supplemented with 100 U/ml hrIL-2 and 10 ng/ml IL-7 (R&D Systems). 2 d after activation, 4 × 105 T cells were infected in 500 µl complete medium at a multiplicity of infection of 5. After 15 d, transduced T cells were sorted for mouse TCR-β expression. Both positive and negative cells were stimulated with anti-CD3/CD28 immunomagnetic beads and cytokines and tested for antigen recognition after 10 d.
Production of purified sCD1c.
Recombinant soluble human CD1c tagged with BirA peptide (sCD1c), was obtained and purified using techniques similar to those previously described (Garcia-Alles et al., 2006). The following primers were used to PCR amplify the extracellular domain of human CD1c: forward XhoI, 5′-CTCGAGGACATGCTGTTTCTGCAGTTTC-3′; and reverse ClaI, 5′-CCATCGATGGGGTTTCTCCAGTAGAGGA-3′. sCD1c was purified from J558-transfected cell culture supernatants by affinity chromatography using anti-CD1c mAb (10C3; BD).
Lipid extraction and analysis.
Total lipids were extracted from a dry pellet of 109 THP1 cells with water/methanol/chloroform (1:1:2 vol/vol/vol). The chloroform layer was collected, centrifuged, dried, and dissolved in chloroform/methanol (9:1 vol/vol). Highly polar lipids were extracted from the remaining aqueous phase using butanol (1 vol added) and then centrifuged, dried, and redissolved in chloroform/methanol (9:1 vol/vol). Both organic and aqueous extractions were separately loaded on an amino-propyl Sep-Pak cartridge (Waters Corporation), and lipids were fractionated into 10 fractions as previously described (Facciotti et al., 2012). No structural modifications were observed in a mixture of standard lipids subjected to the same experimental procedures, thus excluding qualitative effects of the solvents and the amino-propyl cartridges on the eluted lipids. Subfractionation of fraction G was performed by HPLC using a reverse-phase C18 polar end-capped column (3-µm particle size, 3-mm ID, 125-mm length; MACHEREY-NAGEL) as previously described (Facciotti et al., 2012). LC-MS-MS was performed using a Rheos Allegro pump (Flux Instruments) and HTC PAL injector with a 20-µl loop (CTC Analytics AG). MSn were acquired using an LXQ ion trap mass spectrometer equipped with a heated electrospray ionization (ESI) source (Thermo Fisher Scientific) controlled by Xcalibur software (Thermo Fisher Scientific). Source conditions were as follows: spray voltage, 5 kV; capillary voltage, 12 V; capillary temperature, 265°C; tube lens offset voltage, 100 V; and sheath gas flow rate, 15 U. Helium was used as the damping gas. CID spectra were acquired on an API 4000 LC-MS-MS system with a triple quadrupole analyzer (Applied Biosystems/MDS Sciex). Exact mass measurements were performed with LTQ MS (Thermo Fisher Scientific). All lipid standards were purchased from Avanti Polar Lipids, Inc.
To quantify the amounts of C16 mLPA contained in leukemic and healthy cells, polar lipids were extracted with a one-step methanol-based method (Zhao and Xu, 2010). In brief, dry cell pellets were frozen in liquid nitrogen (5 min) and then kept on ice for 3 min and finally brought to room temperature in water for 2 min. Samples were vortexed (30 s), added to 50 µl water, vortexed for an additional 30 s, added to 450 µl methanol, vortexed again (30 s), and finally sonicated for 3 min. After centrifugation (10 min, 10,000 g), the upper phase was collected and dried under the fume hood. Dry lipid extracts were resuspended in 30 µl methanol, and 5 µl was injected into the ultra-performance LC (UPLC) tandem MS (UPLC-MS-MS) for analysis. UPLC was performed on a Waters ACQUITY UPLC system (Waters Corporation) using a 2.1 mm × 100 mm × 1.7 µm ACQUITY UPLC BEH C18 column with a flow rate of 0.20 ml/min. The column temperature was held at 30°C. The solvent system applied was mobile phase A (2 mM ammonium formate in Milli-Q water) and mobile phase B (acetonitrile, HPLC grade) with a gradient starting at 70% A–30% B and ramping to 100% B over 10 min. MS detection was performed using a Xevo TQS Tandem MS detector (Waters Corporation) equipped with an ESI source operating in negative ionization and multiple reaction monitoring (MRM) mode. The desolvation gas flow rate was set to 800 liter/h at a temperature of 350°C. The capillary voltage was set to 2,500 V. The precursor to product ion transitions (quantification and target ions), dwell times, cone voltage, and collision energy for C16 mLPA and the internal standard C17 mLPA are listed in Table S3. Data were acquired and processed with MassLynx software version 4.1 (Waters Corporation) using peak area ratio for quantification. C16 and C17 mLPA molecules were synthesized in house.
NMR.
mLPA spectra were recorded on an Avance III spectrometer (Bruker) operating at 14.1 Tesla and 298K. The spectrometer was equipped with a triple-resonance (1H, 13C, 31P), 1.7-mm micro probe head with a self-shielded z-gradient coil. All mLPA experiments were performed in sodium formate–buffered deuterated methanol (99.96% D; Cambridge Isotopes Laboratories). All synthetic samples were prepared in deuterated solvents (>99.8% D; Cambridge Isotope Laboratories). The NMR experiments conducted on synthetic samples were performed using a DRX–600 MHz NMR spectrometer (equipped with a self-shielded z-axis pulsed field gradient dual-channel broadband inverse 5-mm probe head; Bruker). Chemical shifts were referenced to residual solvent peaks.
mLPA was dissolved in 30 µl solvent, syringe-transferred to a 1.7-mm-diameter NMR sample tube, and then sealed. The two-dimensional experiments were recorded with 2,048 data points in the direct dimension, and increments in the indirect dimension numbering 1,024 (COSY and HSQC) or 256 (31P-HMBC), resulting in acquisition times of 285 (F2) and 142 ms (F1) for the COSY, 142 and 20.5 ms for the HSQC, and 142 and 10.5 ms for the 31P-HMBC. The COSY was performed with eight scans per increment, leading to a total experiment time of 3 h, 21 min; for the HSQC these parameters were 144 scans and 64 h, 17 min; and for the 31P-HMBC, eight scans and 59 min. A 31P decoupling 180° pulse was applied during the 13C evolution period of the sensitivity-enhanced Echo-Antiecho HSQC experiment. The long-range delay in the 31P-HMBC was set to 62.5 ms. All other NMR investigations were routine experiments.
Human leukemia xenograft model.
We generated a human leukemia xenograft model in immune-compromised mice according to published protocols (Bondanza et al., 2006; Provasi et al., 2012). TMβ-1 anti–mouse IL-2Rβ blocking mAb (clone TM-1; provided by K. Tanaka, Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan) was injected i.p. 24 h before the start of the experiment in 6–8-wk-old female NOD/scid mice (Charles River) to neutralize residual NK cell activity. On day 0, mice were conditioned by 175 cGy sublethal irradiation and then immediately infused i.v. with 106 MOLT-4 cells engineered to express EGFP via lentivirus transfer. After 48 h, the mice were divided into three groups (five animals/group) that received vehicle alone, mLPA-specific CD1c-restricted T cells (K34A27f), or M. tuberculosis–specific CD1c-restricted T cells (DL15A31; 107 cells/mouse). The proliferation of human leukemia cells in blood was monitored weekly by flow cytometry. MOLT-4 cells were identified as human-CD45/EGFP double-positive cells. Moribund mice were euthanized for ethical reasons. For experiments with primary human leukemia blasts, AML blasts were purified from the blood of an AML patient and injected i.v. (8 × 106/animal) into 14 NSG mice. 2 d later, 1.5 × 107 K34A27f T cells were injected into half of the mice, and leukemia progression was monitored weekly by flow cytometry of PBMCs. The area under the curve describing the kinetic of AML expansion in peripheral blood was calculated for each mouse infused with K34A27f cells or with vehicle only and values derived from the two groups of animals compared. Animal experiments were approved by the Institutional Animal Care and Use Committee of the San Raffaele Scientific Institute (IACUC no. 370).
Statistical analyses.
Cytokine assay data were analyzed using a two-tailed Student’s t test. Data describing the presence of leukemia cells in injected mice were analyzed with two-tailed nonparametric Student’s t test. Animal survival data were analyzed using log-rank (Mantel-Cox) test.
Online supplemental material.
Tables S1 and S2 show the clinical characteristics of pediatric and adult patients, respectively. Table S3 shows the C16 and C17 mLPA MRM transitions. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20140410/DC1.
Supplementary Material
Acknowledgments
We thank L. Angman for technical assistance and D. Moskau (Bruker, Fällanden, Switzerland) for support and providing the 1.7-mm, triple-resonance NMR probe head. We also thank D. Campana and J.-P. Abastado for critical reading and N. McCarthy of Insight Editing London for editing/revising the manuscript.
This work was supported by grants from the Swiss National Foundation (3100AO-122464/1 and Sinergia CRS133-124819) awarded to G. De Libero, Oncosuisse awards to L. Mori and G. De Libero, grants from the Association for International Cancer Research to P. Dellabona, G. Casorati, L. Mori, and G. De Libero, grants from the Italian Ministry of Health and the Italian Association for Cancer Research (AIRC-IG-5804, AIRC-IG-11523, Special Program 5x1000 9965) to P. Dellabona and G. Casorati, AIRC Special Program 5x1000 9962 to F. Locatelli, and Yunnan High-End Technology Professionals Introduction Program (2010CI117) to C. Xia.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- AML
- acute myeloid leukemia
- B-ALL
- B cell acute lymphoblastic leukemia
- COSY
- correlation spectroscopy
- GVHD
- graft versus host disease
- HMBC
- heteronuclear multiple bond correlation
- HSC
- hematopoietic stem cell
- HSCT
- HSC transplantation
- HSQC
- heteronuclear single quantum coherence spectroscopy correlation
- LAIP
- leukemia-associated immune phenotype
- LC
- liquid chromatography
- LPA
- lysophosphatidic acid
- LPC
- lysophosphatidylcholine
- LPM
- lysophosphomethanol
- mLPA
- methyl-LPA
- Mo-DC
- monocyte-derived DC
- MS
- mass spectrometry
- m/z
- mass/charge
- NMR
- nuclear magnetic resonance
- NOD
- nonobese diabetic
- NSG
- NOD/scid/common γ chain−/−
- sCD1c
- soluble CD1c
- T-ALL
- T cell acute lymphoblastic leukemia
- UPLC
- ultra-performance LC
References
- Amprey J.L., Im J.S., Turco S.J., Murray H.W., Illarionov P.A., Besra G.S., Porcelli S.A., Späth G.F. 2004. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J. Exp. Med. 200:895–904 10.1084/jem.20040704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzofsky J.A., Terabe M. 2009. The contrasting roles of NKT cells in tumor immunity. Curr. Mol. Med. 9:667–672 10.2174/156652409788970706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondanza A., Valtolina V., Magnani Z., Ponzoni M., Fleischhauer K., Bonyhadi M., Traversari C., Sanvito F., Toma S., Radrizzani M., et al. 2006. Suicide gene therapy of graft-versus-host disease induced by central memory human T lymphocytes. Blood. 107:1828–1836 10.1182/blood-2005-09-3716 [DOI] [PubMed] [Google Scholar]
- Boon T., Coulie P.G., Van den Eynde B.J., van der Bruggen P. 2006. Human T cell responses against melanoma. Annu. Rev. Immunol. 24:175–208 10.1146/annurev.immunol.24.021605.090733 [DOI] [PubMed] [Google Scholar]
- Brigl M., Brenner M.B. 2004. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 22:817–890 10.1146/annurev.immunol.22.012703.104608 [DOI] [PubMed] [Google Scholar]
- Canderan G., Gruarin P., Montagna D., Fontana R., Campi G., Melloni G., Traversari C., Dellabona P., Casorati G. 2010. An efficient strategy to induce and maintain in vitro human T cells specific for autologous non-small cell lung carcinoma. PLoS ONE. 5:e12014 (published erratum appears in PLoS ONE. 2011. 6:10.1371/annotation/df559170-4836-4bc7-a16d-44c7c6339cda) 10.1371/journal.pone.0012014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerundolo V., Silk J.D., Masri S.H., Salio M. 2009. Harnessing invariant NKT cells in vaccination strategies. Nat. Rev. Immunol. 9:28–38 10.1038/nri2451 [DOI] [PubMed] [Google Scholar]
- Cha M.K., Suh K.H., Kim I.H. 2009. Overexpression of peroxiredoxin I and thioredoxin1 in human breast carcinoma. J. Exp. Clin. Cancer Res. 28:93 10.1186/1756-9966-28-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D.H., Deng H., Matthews P., Krasovsky J., Ragupathi G., Spisek R., Mazumder A., Vesole D.H., Jagannath S., Dhodapkar M.V. 2008. Inflammation-associated lysophospholipids as ligands for CD1d-restricted T cells in human cancer. Blood. 112:1308–1316 10.1182/blood-2008-04-149831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciceri F., Bonini C., Stanghellini M.T., Bondanza A., Traversari C., Salomoni M., Turchetto L., Colombi S., Bernardi M., Peccatori J., et al. 2009. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 10:489–500 10.1016/S1470-2045(09)70074-9 [DOI] [PubMed] [Google Scholar]
- de Jong A., Peña-Cruz V., Cheng T.Y., Clark R.A., Van Rhijn I., Moody D.B. 2010. CD1a-autoreactive T cells are a normal component of the human αβ T cell repertoire. Nat. Immunol. 11:1102–1109 10.1038/ni.1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lalla C., Lepore M., Piccolo F.M., Rinaldi A., Scelfo A., Garavaglia C., Mori L., De Libero G., Dellabona P., Casorati G. 2011. High-frequency and adaptive-like dynamics of human CD1 self-reactive T cells. Eur. J. Immunol. 41:602–610 10.1002/eji.201041211 [DOI] [PubMed] [Google Scholar]
- De Libero G., Moran A.P., Gober H.J., Rossy E., Shamshiev A., Chelnokova O., Mazorra Z., Vendetti S., Sacchi A., Prendergast M.M., et al. 2005. Bacterial infections promote T cell recognition of self-glycolipids. Immunity. 22:763–772 10.1016/j.immuni.2005.04.013 [DOI] [PubMed] [Google Scholar]
- Dellabona P., Casorati G., Friedli B., Angman L., Sallusto F., Tunnacliffe A., Roosneek E., Lanzavecchia A. 1993. In vivo persistence of expanded clones specific for bacterial antigens within the human T cell receptor alpha/beta CD4-8- subset. J. Exp. Med. 177:1763–1771 10.1084/jem.177.6.1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar M.V., Richter J. 2011. Harnessing natural killer T (NKT) cells in human myeloma: progress and challenges. Clin. Immunol. 140:160–166 10.1016/j.clim.2010.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döhner H., Estey E.H., Amadori S., Appelbaum F.R., Büchner T., Burnett A.K., Dombret H., Fenaux P., Grimwade D., Larson R.A., et al. European LeukemiaNet. 2010. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 115:453–474 10.1182/blood-2009-07-235358 [DOI] [PubMed] [Google Scholar]
- Dorsam R.T., Gutkind J.S. 2007. G-protein-coupled receptors and cancer. Nat. Rev. Cancer. 7:79–94 10.1038/nrc2069 [DOI] [PubMed] [Google Scholar]
- Doulatov S., Notta F., Laurenti E., Dick J.E. 2012. Hematopoiesis: a human perspective. Cell Stem Cell. 10:120–136 10.1016/j.stem.2012.01.006 [DOI] [PubMed] [Google Scholar]
- Endo T., Kano K., Motoki R., Hama K., Okudaira S., Ishida M., Ogiso H., Tanaka M., Matsuki N., Taguchi R., et al. 2009. Lysophosphatidylmethanol is a pan lysophosphatidic acid receptor agonist and is produced by autotaxin in blood. J. Biochem. 146:283–293 10.1093/jb/mvp068 [DOI] [PubMed] [Google Scholar]
- Facciotti F., Ramanjaneyulu G.S., Lepore M., Sansano S., Cavallari M., Kistowska M., Forss-Petter S., Ni G., Colone A., Singhal A., et al. 2012. Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nat. Immunol. 13:474–480 10.1038/ni.2245 [DOI] [PubMed] [Google Scholar]
- Garcia-Alles L.F., Versluis K., Maveyraud L., Vallina A.T., Sansano S., Bello N.F., Gober H.J., Guillet V., de la Salle H., Puzo G., et al. 2006. Endogenous phosphatidylcholine and a long spacer ligand stabilize the lipid-binding groove of CD1b. EMBO J. 25:3684–3692 10.1038/sj.emboj.7601244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleron M., Stenger S., Mazorra Z., Wittke F., Mariotti S., Böhmer G., Prandi J., Mori L., Puzo G., De Libero G. 2004. Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1-restricted T cells during infection with Mycobacterium tuberculosis. J. Exp. Med. 199:649–659 10.1084/jem.20031097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumperz J.E., Roy C., Makowska A., Lum D., Sugita M., Podrebarac T., Koezuka Y., Porcelli S.A., Cardell S., Brenner M.B., Behar S.M. 2000. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 12:211–221 10.1016/S1074-7613(00)80174-0 [DOI] [PubMed] [Google Scholar]
- Han X., Gross R.W. 1996. Structural determination of lysophospholipid regioisomers by electrospray ionization tandem mass spectrometry. J. Am. Chem. Soc. 118:451–457 10.1021/ja952326r [DOI] [Google Scholar]
- Hsu F.F., Turk J. 2007. Differentiation of 1-O-alk-1′-enyl-2-acyl and 1-O-alkyl-2-acyl glycerophospholipids by multiple-stage linear ion-trap mass spectrometry with electrospray ionization. J. Am. Soc. Mass Spectrom. 18:2065–2073 10.1016/j.jasms.2007.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P.P., Sabatini D.M. 2008. Cancer cell metabolism: Warburg and beyond. Cell. 134:703–707 10.1016/j.cell.2008.08.021 [DOI] [PubMed] [Google Scholar]
- Jäger D., Jäger E., Knuth A. 2001. Immune responses to tumour antigens: implications for antigen specific immunotherapy of cancer. J. Clin. Pathol. 54:669–674 10.1136/jcp.54.9.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedema I., van der Werff N.M., Barge R.M., Willemze R., Falkenburg J.H. 2004. New CFSE-based assay to determine susceptibility to lysis by cytotoxic T cells of leukemic precursor cells within a heterogeneous target cell population. Blood. 103:2677–2682 10.1182/blood-2003-06-2070 [DOI] [PubMed] [Google Scholar]
- Kinjo Y., Wu D., Kim G., Xing G.W., Poles M.A., Ho D.D., Tsuji M., Kawahara K., Wong C.H., Kronenberg M. 2005. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 434:520–525 10.1038/nature03407 [DOI] [PubMed] [Google Scholar]
- Kolb H.J. 2008. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 112:4371–4383 10.1182/blood-2008-03-077974 [DOI] [PubMed] [Google Scholar]
- Manolova V., Hirabayashi Y., Mori L., De Libero G. 2003. CD1a and CD1b surface expression is independent from de novo synthesized glycosphingolipids. Eur. J. Immunol. 33:29–37 10.1002/immu.200390004 [DOI] [PubMed] [Google Scholar]
- Matsushita H., Vesely M.D., Koboldt D.C., Rickert C.G., Uppaluri R., Magrini V.J., Arthur C.D., White J.M., Chen Y.S., Shea L.K., et al. 2012. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 482:400–404 10.1038/nature10755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattner J., Debord K.L., Ismail N., Goff R.D., Cantu C., III, Zhou D., Saint-Mezard P., Wang V., Gao Y., Yin N., et al. 2005. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 434:525–529 10.1038/nature03408 [DOI] [PubMed] [Google Scholar]
- Metelitsa L.S. 2011. Anti-tumor potential of type-I NKT cells against CD1d-positive and CD1d-negative tumors in humans. Clin. Immunol. 140:119–129 10.1016/j.clim.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills G.B., Moolenaar W.H. 2003. The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer. 3:582–591 10.1038/nrc1143 [DOI] [PubMed] [Google Scholar]
- Montagna D., Maccario R., Locatelli F., Montini E., Pagani S., Bonetti F., Daudt L., Turin I., Lisini D., Garavaglia C., et al. 2006. Emergence of antitumor cytolytic T cells is associated with maintenance of hematologic remission in children with acute myeloid leukemia. Blood. 108:3843–3850 10.1182/blood-2006-05-021535 [DOI] [PubMed] [Google Scholar]
- Montamat-Sicotte D.J., Millington K.A., Willcox C.R., Hingley-Wilson S., Hackforth S., Innes J., Kon O.M., Lammas D.A., Minnikin D.E., Besra G.S., et al. 2011. A mycolic acid–specific CD1-restricted T cell population contributes to acute and memory immune responses in human tuberculosis infection. J. Clin. Invest. 121:2493–2503 10.1172/JCI46216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody D.B., Ulrichs T., Mühlecker W., Young D.C., Gurcha S.S., Grant E., Rosat J.P., Brenner M.B., Costello C.E., Besra G.S., Porcelli S.A. 2000. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature. 404:884–888 10.1038/35009119 [DOI] [PubMed] [Google Scholar]
- Moody D.B., Young D.C., Cheng T.Y., Rosat J.P., Roura-Mir C., O’Connor P.B., Zajonc D.M., Walz A., Miller M.J., Levery S.B., et al. 2004. T cell activation by lipopeptide antigens. Science. 303:527–531 10.1126/science.1089353 [DOI] [PubMed] [Google Scholar]
- Nomura D.K., Long J.Z., Niessen S., Hoover H.S., Ng S.W., Cravatt B.F. 2010. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 140:49–61 10.1016/j.cell.2009.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowbakht P., Ionescu M.C., Rohner A., Kalberer C.P., Rossy E., Mori L., Cosman D., De Libero G., Wodnar-Filipowicz A. 2005. Ligands for natural killer cell–activating receptors are expressed upon the maturation of normal myelomonocytic cells but at low levels in acute myeloid leukemias. Blood. 105:3615–3622 10.1182/blood-2004-07-2585 [DOI] [PubMed] [Google Scholar]
- Porcelli S.A., Modlin R.L. 1999. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 17:297–329 10.1146/annurev.immunol.17.1.297 [DOI] [PubMed] [Google Scholar]
- Provasi E., Genovese P., Lombardo A., Magnani Z., Liu P.Q., Reik A., Chu V., Paschon D.E., Zhang L., Kuball J., et al. 2012. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat. Med. 18:807–815 10.1038/nm.2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pui C.H., Relling M.V., Downing J.R. 2004. Acute lymphoblastic leukemia. N. Engl. J. Med. 350:1535–1548 10.1056/NEJMra023001 [DOI] [PubMed] [Google Scholar]
- Pui C.H., Carroll W.L., Meshinchi S., Arceci R.J. 2011. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J. Clin. Oncol. 29:551–565 10.1200/JCO.2010.30.7405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulfer M., Murphy R.C. 2003. Electrospray mass spectrometry of phospholipids. Mass Spectrom. Rev. 22:332–364 10.1002/mas.10061 [DOI] [PubMed] [Google Scholar]
- Rubnitz J.E., Gibson B., Smith F.O. 2008. Acute myeloid leukemia. Pediatr. Clin. North Am. 55:21–51: ix (ix.) 10.1016/j.pcl.2007.11.003 [DOI] [PubMed] [Google Scholar]
- Schrader M., Fahimi H.D. 2006. Peroxisomes and oxidative stress. Biochim. Biophys. Acta. 1763:1755–1766 10.1016/j.bbamcr.2006.09.006 [DOI] [PubMed] [Google Scholar]
- Seremet T., Brasseur F., Coulie P.G. 2011. Tumor-specific antigens and immunologic adjuvants in cancer immunotherapy. Cancer J. 17:325–330 10.1097/PPO.0b013e3182326004 [DOI] [PubMed] [Google Scholar]
- Shamshiev A., Donda A., Carena I., Mori L., Kappos L., De Libero G. 1999. Self glycolipids as T-cell autoantigens. Eur. J. Immunol. 29:1667–1675 [DOI] [PubMed] [Google Scholar]
- Shamshiev A., Donda A., Prigozy T.I., Mori L., Chigorno V., Benedict C.A., Kappos L., Sonnino S., Kronenberg M., De Libero G. 2000. The αβ T cell response to self-glycolipids shows a novel mechanism of CD1b loading and a requirement for complex oligosaccharides. Immunity. 13:255–264 10.1016/S1074-7613(00)00025-X [DOI] [PubMed] [Google Scholar]
- Shamshiev A., Gober H.J., Donda A., Mazorra Z., Mori L., De Libero G. 2002. Presentation of the same glycolipid by different CD1 molecules. J. Exp. Med. 195:1013–1021 10.1084/jem.20011963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socié G., Blazar B.R. 2009. Acute graft-versus-host disease: from the bench to the bedside. Blood. 114:4327–4336 10.1182/blood-2009-06-204669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser D.E., Romero P. 2010. Molecularly defined vaccines for cancer immunotherapy, and protective T cell immunity. Semin. Immunol. 22:144–154 10.1016/j.smim.2010.03.004 [DOI] [PubMed] [Google Scholar]
- Sriram V., Du W., Gervay-Hague J., Brutkiewicz R.R. 2005. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur. J. Immunol. 35:1692–1701 10.1002/eji.200526157 [DOI] [PubMed] [Google Scholar]
- Vago L., Perna S.K., Zanussi M., Mazzi B., Barlassina C., Stanghellini M.T., Perrelli N.F., Cosentino C., Torri F., Angius A., et al. 2009. Loss of mismatched HLA in leukemia after stem-cell transplantation. N. Engl. J. Med. 361:478–488 10.1056/NEJMoa0811036 [DOI] [PubMed] [Google Scholar]
- van Meeteren L.A., Moolenaar W.H. 2007. Regulation and biological activities of the autotaxin-LPA axis. Prog. Lipid Res. 46:145–160 10.1016/j.plipres.2007.02.001 [DOI] [PubMed] [Google Scholar]
- Vander Heiden M.G., Cantley L.C., Thompson C.B. 2009. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 324:1029–1033 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidriales M.B., Pérez J.J., López-Berges M.C., Gutiérrez N., Ciudad J., Lucio P., Vazquez L., García-Sanz R., del Cañizo M.C., Fernández-Calvo J., et al. 2003. Minimal residual disease in adolescent (older than 14 years) and adult acute lymphoblastic leukemias: early immunophenotypic evaluation has high clinical value. Blood. 101:4695–4700 10.1182/blood-2002-08-2613 [DOI] [PubMed] [Google Scholar]
- Vincent M.S., Leslie D.S., Gumperz J.E., Xiong X., Grant E.P., Brenner M.B. 2002. CD1-dependent dendritic cell instruction. Nat. Immunol. 3:1163–1168 10.1038/ni851 [DOI] [PubMed] [Google Scholar]
- Vincent M.S., Xiong X., Grant E.P., Peng W., Brenner M.B. 2005. CD1a-, b-, and c-restricted TCRs recognize both self and foreign antigens. J. Immunol. 175:6344–6351 10.4049/jimmunol.175.10.6344 [DOI] [PubMed] [Google Scholar]
- Wanders R.J., Ferdinandusse S., Brites P., Kemp S. 2010. Peroxisomes, lipid metabolism and lipotoxicity. Biochim. Biophys. Acta. 1801:272–280 10.1016/j.bbalip.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Wingard J.R., Majhail N.S., Brazauskas R., Wang Z., Sobocinski K.A., Jacobsohn D., Sorror M.L., Horowitz M.M., Bolwell B., Rizzo J.D., Socié G. 2011. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J. Clin. Oncol. 29:2230–2239 10.1200/JCO.2010.33.7212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D.Y., Segal N.H., Sidobre S., Kronenberg M., Chapman P.B. 2003. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J. Exp. Med. 198:173–181 10.1084/jem.20030446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Xing G.W., Poles M.A., Horowitz A., Kinjo Y., Sullivan B., Bodmer-Narkevitch V., Plettenburg O., Kronenberg M., Tsuji M., et al. 2005. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc. Natl. Acad. Sci. USA. 102:1351–1356 10.1073/pnas.0408696102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Xu Y. 2010. An extremely simple method for extraction of lysophospholipids and phospholipids from blood samples. J. Lipid Res. 51:652–659 10.1194/jlr.D001503 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.