NK cells lacking proapoptotic factor Bim show impaired contraction phase after MCMV infection, leading to impaired memory cell maturation and a less effective responses to viral rechallenge.
Abstract
Apoptosis is critical for the elimination of activated lymphocytes after viral infection. Proapoptotic factor Bim (Bcl2l11) controls T lymphocyte contraction and the formation of memory T cells after infection. Natural killer (NK) cells also undergo antigen-driven expansion to become long-lived memory cells after mouse cytomegalovirus (MCMV) infection; therefore, we examined the role of Bim in regulating the MCMV-driven memory NK cell pool. Despite responding similarly early after infection, Bcl2l11−/− Ly49H+ NK cells show impaired contraction and significantly outnumber wild-type (WT) cells after the expansion phase. The inability to reduce the effector pool leads to a larger Bcl2l11−/− NK memory subset, which displays a less mature phenotype (CD11blo, CD27+) and lower levels of NK cell memory-associated markers KLRG1 and Ly6C. Bcl2l11−/− memory NK cells demonstrate a reduced response to m157-mediated stimulation and do not protect as effectively as WT memory NK cells in an MCMV challenge model. Thus, Bim-mediated apoptosis drives selective contraction of effector NK cells to generate a pool of mature, MCMV-specific memory cells.
Although NK cells are traditionally classified as innate cells, recent evidence indicates that they may also acquire immunological memory (Paust and von Andrian, 2011; Min-Oo et al., 2013). Work by several groups has uncovered memory-like properties of NK cells, including antigen-specific recall response to haptens and viral-like particles (Paust et al., 2010), cytokine-induced memory (Cooper et al., 2009), and enhanced secondary response to mouse CMV (MCMV; Sun et al., 2009). An expanded and persistent population of NK cells bearing the NKG2C receptor has been found after infection by human CMV, suggesting the existence of memory in human NK cells (Gumá et al., 2004; Lopez-Vergès et al., 2011). Resistance to MCMV is dependent on the NK cell response and is mediated in C57BL/6 mice by the activating Ly49H receptor (Brown et al., 2001; Lee et al., 2001). NK cells undergo robust expansion upon encountering infected cells expressing m157, the MCMV-encoded ligand for Ly49H. Ly49H+ NK cell expansion peaks and is followed by a contraction phase (Sun and Lanier, 2011). A small pool of Ly49H+ NK cells persists for >90 d after infection; importantly, these cells show enhanced response to secondary challenge (Sun et al., 2009). A previous study has established an important role for cytokine signaling during the expansion phase (Sun et al., 2012), but no work has examined the mechanism driving contraction.
The induction of lymphocyte apoptosis is a key mechanism regulating the immune response after viral infection (Prlic and Bevan, 2008; Kurtulus et al., 2010). Failure to control the number of activated lymphocytes can result in fatal immune-mediated pathology. Apoptosis is stimulated through two distinct pathways: death receptor signaling and mitochondrial apoptosis triggered by BH3-only proteins (Strasser, 2005). Bim, a BH3-only family member (O’Connor et al., 1998), binds the prosurvival molecule Bcl-2 and regulates apoptotic signaling through Bax and Bak (Strasser, 2005). Bim regulates the T cell response by reducing the effector T cell pool, in both acute and latent models of viral infection (Kurtulus et al., 2010).
Huntington et al. (2007) described Bim-deficient NK cells to be more mature than WT NK cells, but with no defects in cytotoxicity or cytokine production. After MCMV, Bim-deficient mice had an increased number of NK cells. However, Bcl2l11−/− mice exhibit hematopoietic abnormalities in leukocyte homeostasis (Bouillet et al., 1999), which might impact host response to infection independently of NK cells. Therefore, we examined the cell-intrinsic effect of Bim deficiency in Ly49H+ NK cells on the antigen-specific response to MCMV and the generation of memory NK cells.
RESULTS AND DISCUSSION
Bim-deficient NK cells expand normally but show reduced contraction
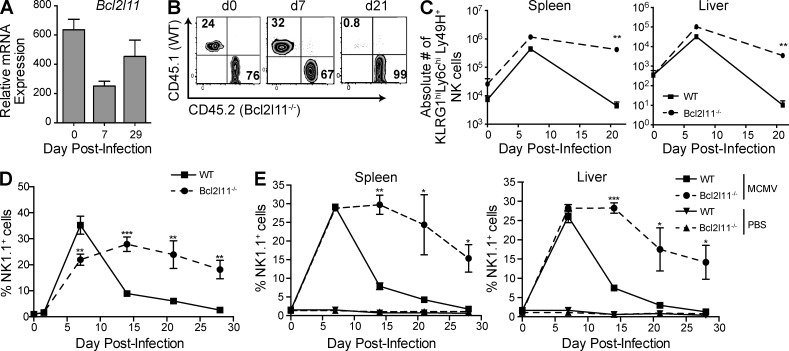
Data generated by the ImmGen Consortium (Bezman et al., 2012) revealed that Bim mRNA expression drops after MCMV-driven expansion and remains low in Ly49H+ memory NK cells, likely reflecting the loss of cells expressing high levels of Bim (Fig. 1 A). To determine the role of Bim in the development and function of NK cells, we generated mixed BM chimeric mice reconstituted with 50% Bcl2l11−/− and 50% WT BM cells. Bcl2l11−/− cells reconstituted the recipient mouse to the same extent as WT cells, although a skewing toward Bcl2l11−/− cells was observed at 8–10 wk after reconstitution (Fig. 1 B and not depicted). We infected chimeric mice with MCMV, which induced a comparable expansion of Bcl2l11−/− and WT Ly49H+ NK cells by day 7, demonstrating that Bim is not essential for expansion (Fig. 1 B). However, by day 21 we observed a preferential selection of Bcl2l11−/− NK cells within the Ly49H+ subset, accounting for >90% of the population (Fig. 1 B). This was consistent with a difference in the absolute number of KLRG1hiLy6ChiLy49H+ NK cells in the spleen and liver, markers shown to be associated with MCMV-specific memory NK cells (Fig. 1 C; Sun et al., 2009; Bezman et al., 2012).
Figure 1.
Bcl2l11−/− Ly49H+ NK cells expand normally but demonstrate impaired contraction. (A) Levels of Bim mRNA are shown as relative levels for Ly49H+ NK cells after MCMV infection. (B) Plots show ratios of Bcl2l11−/− and WT Ly49H+ cells in the spleen of mixed BM chimeric mice after MCMV infection (days 0, 7, and 21) of representative mice. (C) Absolute numbers of the WT and Bcl2l11−/− KLRG1+Ly6ChiLy49H+ cells are plotted. (D) Adoptively transferred Bcl2l11−/− (CD45.2) and WT (CD45.1) Ly49H+ NK cells in peripheral blood were analyzed at days 1.5, 5, 7, 14, 21, and 28 after MCMV and are expressed as a percentage of NK1.1+TCRβ− NK cells. (E) Tissue lymphocytes were analyzed at days 7, 21, and 28; graph shows Bcl2l11−/− and WT Ly49H+ cells expressed as a percentage of NK1.1+TCRβ− NK cells. For all panels, n = 3 mice per time point, and data are representative of two to three independent experiments. Error bars signify the standard error of the mean. ***, P < 0.0005; **, P < 0.005; *, P < 0.05 (paired Student’s t test).
To further investigate the NK cell response to MCMV in the absence of Bim, we used a competitive adoptive transfer system into Ly49H-deficient mice (Sun et al., 2009). We measured the expansion and contraction of Bcl2l11−/− and WT Ly49H+ NK cells in the peripheral blood after infection (Fig. 1 D). Bcl2l11−/− and WT NK cells showed similar kinetics during the proliferative phase, between days 2 and 7 postinfection (p.i.), but Bcl2l11−/− NK cells did not subsequently contract (days 14, 21, and 28; Fig. 1 D). Bcl2l11−/− Ly49H+ NK cells showed a reduced contraction and greatly outnumbered WT Ly49H+ cells by day 14 in both spleen and liver (Fig. 1 E). The preferential survival of Bcl2l11−/− Ly49H+ NK cells continued into the memory phase, with Bcl2l11−/− cells accounting for 90% of detectable long-lived cells at day 28.
Bcl2l11−/− Ly49H+ NK cells are activated and proliferate normally in response to MCMV
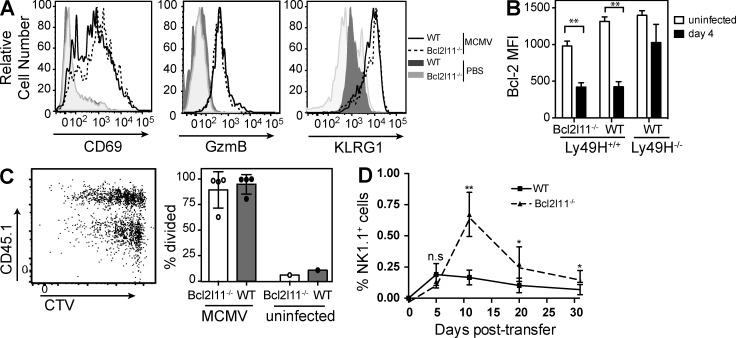
To eliminate the possibility that Bcl2l11−/− NK cells display an early defect in response to MCMV, we assessed Bcl2l11−/− Ly49H+ NK cells at early time points after infection in the adoptive transfer model. The percentage of Bcl2l11−/− NK cells that expressed the activation markers CD69 and KLRG1, as well as increased expression of granzyme B, was equal to WT NK cells in spleen (Fig. 2 A) and liver (not depicted). Bcl-2 is down-regulated after activation of lymphocytes (Kurtulus et al., 2010). We observed significantly decreased levels of Bcl-2 in both Bcl2l11−/− and WT Ly49H+ cells at day 4 p.i., with median fluorescence intensity reduced from ∼1,200 to ∼400 (Fig. 2 B; P < 0.01). A slight difference in Bcl-2 levels at the basal state has been reported in Bcl2l11−/− T cells and is likely caused by the absence of Bim, which is sequestered by Bcl-2 (Hildeman et al., 2002).
Figure 2.
Bcl2l11−/− Ly49H+ NK cells respond normally to MCMV and proliferate similarly to WT NK cells. (A–D) Adoptively transferred Bcl2l11−/− and WT Ly49H+ NK cells were analyzed at days 3 (B) and 4 (A, C, and D) after MCMV. Splenocytes were gated on NK1.1+TCRβ−Ly49H+ cells. (A) Representative histogram of the expression of CD69, GzmB, and KLRG1 on Bcl2l11−/− (dashed lines) and WT (solid lines) NK cells, with uninfected mice shown as control (shaded). (B) Bar graphs depict median fluorescence intensity (MFI) of intracellular Bcl-2 levels in Bcl2l11−/− and WT Ly49H+ and Ly49H− NK cells at day 4 p.i. compared with uninfected controls. n = 4 mice per group. (C) Cell division was analyzed using dilution of CellTrace violet. A representative dot plot (left) and bar graph (right) are shown (n = 4 mice). (D) Bcl2l11−/− and WT cells (1:1) NK cells transferred into Rag2−/−Il2rg−/− mice were tracked in peripheral blood and plotted as a percentage of NK1.1+TCRβ− NK cells (n = 3 mice per time point). Data in each panel are representative of two independent experiments. Error bars signify the standard error of the mean. **, P < 0.005; *, P < 0.05.
To assess the proliferative capacity of Bcl2l11−/− Ly49H+ NK cells, we labeled NK cells with CellTrace violet before transfer. Our results showed slightly increased proliferation at day 4 in the WT (CD45.1+) Ly49H+ NK cells in the spleen (Fig. 2 C) and liver (not depicted), but this was not significant across all mice. Overall, Bcl2l11−/− Ly49H+ NK cells were able to proliferate to a similar extent as WT cells within the same mouse. This correlates with the rapid expansion of both the Bcl2l11−/− and WT NK cells in the first 7 d p.i. (Fig. 1). Moreover, using a model of homeostatic proliferation in which WT NK cells adoptively transferred into Rag2−/−Il2rg−/− recipient mice expand to fill an empty lymphocyte niche (Prlic et al., 2003; Sun et al., 2011), Bcl2l11−/− NK cells showed no impairment in early expansion but reduced contraction. As a result, Bcl2l11−/− NK cells were found at greater numbers than WT at day 10 after transfer (Fig. 2 D) and were still detectable at day 70 (not depicted). Moreover, long-lived Bcl2l11−/− Ly49H+ NK cells were still able to respond robustly to MCMV in immune-deficient mice (not depicted).
Reduced contraction is caused by decreased apoptosis of Bcl2l11−/− Ly49H+ NK cells
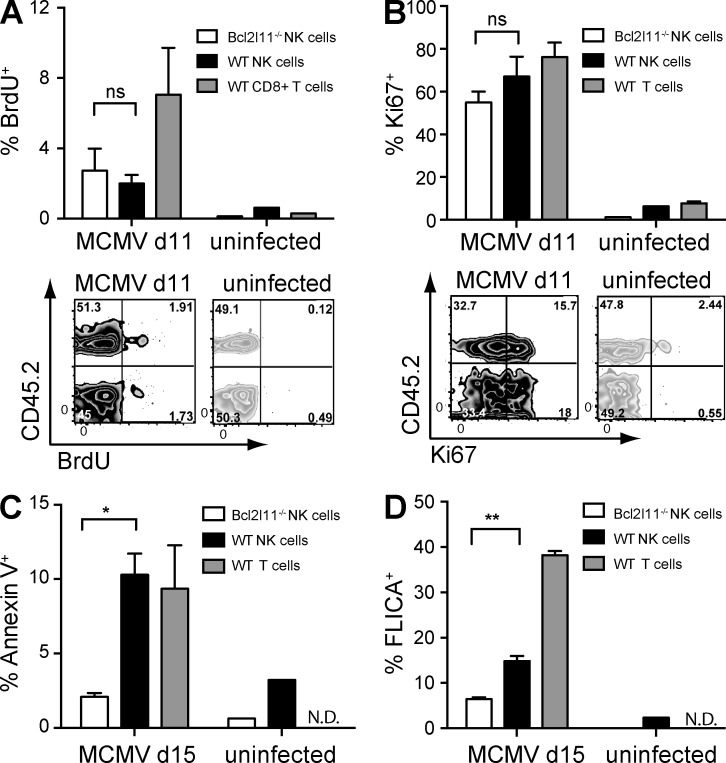
The dramatic shift of the WT/Bcl2l11−/− ratio toward Bcl2l11−/− Ly49H+ NK cells after the peak of expansion could be caused by either continued proliferation or reduced apoptosis. We assessed proliferation during the contraction phase using a 2-h BrdU pulse and the proliferation marker Ki67, when the WT/Bcl2l11−/− ratio had not yet skewed dramatically. At day 11 p.i., very few Ly49H+ NK cells were dividing, and no differences were seen between Bcl2l11−/− and WT cells in the spleen (Fig. 3 A) or liver (not depicted). CD8+ T cells, which were still dividing, are shown for comparison. Ki67 was not significantly different between Bcl2l11−/− and WT transferred NK cells (Fig. 3 B) and was similar to T cells. Because Bcl2l11−/− NK cells were not undergoing continued proliferation after the peak of expansion, we measured apoptosis at day 15, at which time Bcl2l11−/− outnumbered WT Ly49H+ NK cells. The percentage of annexin V+ WT Ly49H+ cells was significantly (P = 0.03) higher than Bcl2l11−/− Ly49H+ NK cells, within each mouse (Fig. 3 C), suggesting that Bcl2l11−/− Ly49H+ cells are more resistant to apoptosis. We confirmed this result using a probe for activated caspases (FLICA), which also showed significantly (P = 0.008) higher levels of caspase activity in WT Ly49H+ NK cells at 15 d p.i. (Fig. 3 D). As a control, WT T cells at this time demonstrated very high levels of apoptosis.
Figure 3.
Impaired contraction in Bcl2l11−/− NK cells reflects decreased apoptosis. (A) The percentages of adoptively transferred Bcl2l11−/− or WT BrdU+NK1.1+TCRβ−Ly49H+ NK cells or BrdU+TCRβ+CD8+ T cells are plotted for infected and uninfected mice at day 11 after MCMV, after a 2-h in vivo pulse of BrdU. Representative flow cytometry plots are shown for CD45.2+ (Bcl2l11−/−) and CD45.2− (WT) cells (n = 3 mice per time point). (B) The percentages of Ki67+NK1.1+TCRβ−Ly49H+ NK cells or Ki67+TCRβ+ T cells are shown for infected and uninfected mice at day 11. Representative flow cytometry plots are shown for CD45.2+ (Bcl2l11−/−) and CD45.2− (WT) cells. (C and D) The percentage of annexin V+ (C) and FLICA+ (D) NK1.1+TCRβ−Ly49H+ NK cells is shown for the Bcl2l11−/− and WT NK cell subsets in infected and uninfected mice at day 15 after MCMV. TCRβ+ T cells are shown as a control (n = 3 mice per group). Data in each panel are representative of two to three independent experiments. Error bars signify the standard error of the mean. **, P < 0.01; *, P < 0.05.
Immature CD27+CD11bloLy49H+ NK cells accumulate and alter the phenotype of the memory NK pool
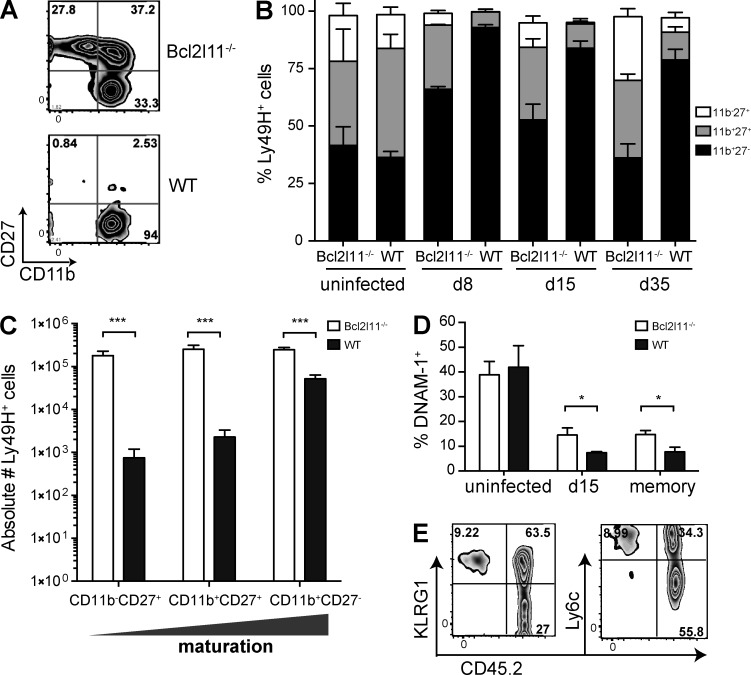
We explored which subset of Ly49H+ NK cells is most sensitive to Bim-driven apoptosis by assessing markers of maturation on the transferred Ly49H+ NK cells throughout infection and at the memory phase. WT Ly49H+ NK cells showed a marked change in the proportion of mature compared with immature cells, as defined by CD27 and CD11b; immature cells (CD27+CD11blo) disappeared during the expansion and contraction phase, making up <5% of the memory pool (Fig. 4 A). Bcl2l11−/− Ly49H+ NK cells displayed a greater proportion of cells with a less differentiated phenotype at the peak of expansion (day 8; Fig. 4 B), and this increased by day 35; the higher proportion at day 35 versus day 8 likely reflects decreasing total numbers of Ly49H+ NK cells. It has been previously shown that MCMV infection of Bcl2l11−/− mice resulted in a higher proportion of immature NK cells than in WT mice (Huntington et al., 2007). Despite the decreased proportion of mature Ly49H+ cells in the Bcl2l11−/− subset, the absolute number of mature Bcl2l11−/− CD27−CD11bhiLy49H+ NK cells was still significantly greater than WT (Fig. 4 C; P < 0.001). Interestingly, the proportion of Bcl2l11−/− NK cells expressing DNAM-1 was higher during contraction (day 15) and at the memory phase (day 35) than in WT cells, which were ∼95% DNAM-1− during the memory phase (Fig. 4 D). KLRG1 and Ly6C have been identified as stably marking MCMV memory NK cells (Bezman et al., 2012). The Bcl2l11−/− Ly49H+ NK cell subset (CD45.2+, right) at the memory time point showed a higher proportion of KLRG1−Ly6Clo cells (Fig. 4 E) than WT cells (CD45.1−, left). Furthermore, transferred Bcl2l11−/− memory NK cells were detectable at day 150 p.i. in spleen and liver, displaying a similar phenotype (not depicted).
Figure 4.
Bim deficiency alters the phenotype of memory NK cells. Ly49H+ memory NK cells generated by adoptive transfer into Ly49H-deficient mice were assessed at day 35 after MCMV. (A) Representative plots show the expression of CD11b and CD27 on WT and Bcl2l11−/− Ly49H+ memory NK cells. (B) The proportions of CD11b−CD27+, CD11b+CD27−, and CD11b+CD27−Ly49H+ NK1.1+TCRβ− NK cells are presented in a bar graph in uninfected mice and at days 8, 15, and 35 p.i. (C) The absolute number of Ly49H+ NK cells in each maturation state was enumerated in the spleen at day 35 after MCMV. (D) The percentage of DNAM-1+Ly49H+ NK cells was quantified in the Bcl2l11−/− and WT compartment. (E) Representative plots showing the expression of KLRG1 and Ly6C on Bcl2l11−/− (CD45.1) and WT (CD45.2) Ly49H+ memory NK cells are presented. Data in each panel are representative of two to three independent experiments. Error bars signify the standard error of the mean. ***, P < 0.001; *, P < 0.05.
Bim deficiency alters the function and reduces the protective capacity of memory Ly49H+ NK cells
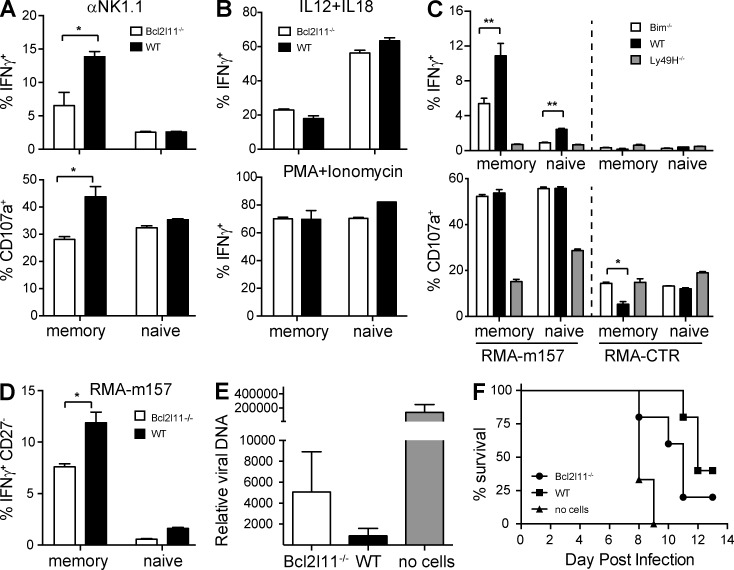
MCMV-induced memory NK cells have heightened responses to stimulation through the Ly49H and NK1.1 receptors in vitro (Sun et al., 2009). Splenic and hepatic memory NK cells were stimulated with anti-NK1.1 antibody, IL-12 and IL-18, or PMA plus ionomycin. Bcl2l11−/− Ly49H+ memory NK cells showed significantly reduced degranulation and IFN-γ production in response to NK1.1 engagement (Fig. 5 A), but a similar response to cytokines and PMA plus ionomycin (Fig. 5 B).
Figure 5.
Bim-deficient Ly49H+ memory NK cells are functionally impaired. Bcl2l11−/− and WT Ly49H+ memory NK cells generated by adoptive transfer were assessed at day 35 after MCMV. (A) Percentage of IFN-γ+ or CD107A+ Bcl2l11−/− or WT memory and naive NK cells after stimulation with plate-bound anti-NK1.1 is shown. (B) Percentage of IFN-γ+ Bcl2l11−/− or WT memory and naive NK cells after stimulation by IL12 + IL18 or PMA + ionomycin is shown. (C) Percentage of IFN-γ+ or CD107A+ Bcl2l11−/− or WT memory and naive NK cells after co-culture with m157-RMA or untransfected RMA cells is shown. Ly49H− NK cells are shown as a control. (D) The percentage of IFN-γ+CD27− Bcl2l11−/− or WT memory and naive NK cells is shown after stimulation by RMA-m157 cells. (E) 20,000 Bcl2l11−/− or WT Ly49H+ memory NK cells, or no Ly49H+ cells, were transferred into secondary Ly49H-deficient recipients and challenged with MCMV. Relative viral burden is shown in blood at day 3 p.i. (F) Survival of MCMV-infected Rag2−/− Ly49H-deficient mice receiving either Bcl2l11−/− or WT Ly49H+ memory NK cells, or no cells. Open bars represent Bcl2l11−/− Ly49H+ NK cells; black bars represent WT Ly49H+ NK cells (n = 4 mice for each condition). Data in each panel represent results from two to three independent experiments. Error bars signify the standard error of the mean. **, P < 0.01; *, P < 0.05.
To compare WT versus Bcl2l11−/− memory NK cells in an antigen-specific assay, we co-cultured memory NK cells with m157-transfected RMA target cells. Memory Ly49H+ NK cells produced significantly higher levels of IFN-γ than naive cells (Fig. 5 C). Bcl2l11−/− memory NK cells showed an impaired response to Ly49H stimulation, despite higher levels of basal degranulation when cultured with control RMA cells. Ly49H− NK cells are shown as an internal negative specificity control. Furthermore, we examined the mature CD27−Ly49H+ memory NK cells and observed a similarly impaired m157-mediated response in the Bcl2l11−/− memory NK cells (Fig. 5 D). These findings suggest that the increased proportion of NK cells with an immature phenotype in the Bcl2l11−/− NK cell subset cannot solely explain the decreased response to Ly49H stimulation.
We assessed the functional impact of Bim deficiency in memory NK cells using an MCMV challenge model; we transferred equal numbers (2 × 104) of WT or Bcl2l11−/− memory NK cells into secondary Ly49H− recipients and infected with 2 × 104 PFU MCMV and quantified viral titers in the blood at days 3 and 4 p.i. Critically, Bcl2l11−/− memory NK cells did not protect as well as WT memory cells. Despite high variability in blood viral titers between individual mice, titers were noticeably increased in mice receiving Bcl2l11−/− cells in two independent experiments (Fig. 5 E). Moreover, transfer of Bcl2l11−/− memory NK cells did not protect Rag2−/− Ly49H-deficient mice from MCMV infection as efficiently as WT memory NK cells, as assessed by survival (Fig. 5 F). These data suggest that the contraction phase of Ly49H+ cells is critical for the enhanced protective capacity of MCMV memory NK cells to secondary challenge.
A hallmark feature of memory NK cells is longevity; lymphocyte lifespan and homeostasis are intimately linked to appropriate signaling through proapoptotic or antiapoptotic pathways (Bouillet and O’Reilly, 2009). Although the Bim-induced apoptotic pathway has been documented in T cell responses after viral infection (Kurtulus et al., 2010), the importance of this pathway during antigen-specific NK cell responses and memory formation had not been addressed. We show that apoptotic signaling through Bim is essential for regulation of the size, phenotype, and functional properties of the antigen-specific Ly49H+ NK cells after MCMV infection. Our findings suggest that apoptotic signaling removes more immature effector cells and/or influences responsiveness to cytokines that drive maturation and survival of antigen-specific NK cells.
Lymphocyte apoptosis can be induced through various mechanisms and may use either the intrinsic (mitochondrial) or extrinsic (death receptor) pathways (Strasser et al., 1995). IL-15 is a critical survival cytokine of NK cells through its action on Bcl-2 and is required for NK cell homeostasis (Cooper et al., 2002; Prlic et al., 2003). In NK cells, IL-15 suppresses Bim through PI(3)K and Akt signaling (Huntington et al., 2007). Bcl2l11−/− NK cells are resistant to death induced by IL-15 withdrawal in vitro and survive after transfer into Il15−/− hosts (Huntington et al., 2007), whereas WT NK cells do not (Cooper et al., 2002; Prlic et al., 2003). IL-12 is critical for Ly49H+ NK cell expansion (Sun et al., 2012) and may contribute to survival and/or apoptosis in certain subsets of NK cells through STAT4 signaling. In T cells, IL-12 induction of STAT4 regulates Bcl-2–associated proteins and promotes survival of CD8+ T cells (Li et al., 2006). Importantly, we observed increased expansion of Bcl2l11−/− NK cells and reduced contraction in the homeostatic model, in the absence of infection-induced inflammatory cytokines, suggesting that IL-15 plays a more critical role during the contraction phase. Moreover, Il12rb2−/− Ly49H+ NK cells show a similar induction of Bim protein after infection compared with WT NK cells (unpublished data).
The accumulation of immature CD27+CD11bhiKLRG1−Ly49H+ cells in the absence of Bim suggests these cells may normally receive a stronger activation signal that triggers cell death during contraction. Alternatively, Bcl2l11−/− NK cells are less sensitive to IL-15 and may not up-regulate maturation markers as quickly as WT cells. Immature NK cells have an increased capacity for proliferation (Prlic et al., 2003) and cytokine production (Chiossone et al., 2009). Our findings demonstrate that Bcl2l11−/− memory NK cells are not impaired in response to cytokine stimulation compared with WT memory NK cells, although neither memory subset responds as potently as more immature naive NK cells.
Signaling through Ly49H during infection may change the balance of survival versus apoptotic signals. Ly49H− NK cells do not down-regulate Bcl-2, at the RNA or protein level, to the same extent as Ly49H+ NK cells after MCMV infection (Fig. 3). This suggests that although Bim mRNA levels are similarly regulated in both Ly49H+ and Ly49H− NK cells, modulation of Bcl-2 levels may contribute to differences in apoptosis. TCR ligation in T cells inhibits IL-7–mediated homeostatic survival and initiates a distinct survival program through changes in levels of Bcl-2 and Bim (Koenen et al., 2013). ITAM-driven signaling through Ly49H may similarly contribute to the Bim versus Bcl-2 balance.
We observed a functional impact of Bim deficiency on Ly49H+ memory NK cells, which reflects improper elimination of a particular subset of NK cells; however, the number of KLRG1hiLy6ChiLy49H+ memory cells remains the same, if not higher. Similarly, Bcl2l11−/− Th1 cells are not effectively eliminated in the transition from effector to memory and show decreased sensitivity to antigen (Jay et al., 2013). Our findings reveal a similar situation for Ly49H+ memory NK cells; Bim is required for the removal of NK cells with impaired functional response to antigen stimulation. On a per cell basis, Ly49H+ memory NK cells that have not undergone appropriate contraction do not protect as well against secondary challenge with MCMV with respect to controlling viral burden or overall survival in mice lacking T and B lymphocytes. Overall, appropriate signaling through the intrinsic mitochondrial pathway is critically important to generate a small, but MCMV-specific memory NK cell pool.
MATERIALS AND METHODS
Mice and MCMV infection.
C57BL/6 and congenic C57BL/6-CD45.1 mice were purchased from the National Cancer Institute. Rag2−/−IL2rg−/− mice were purchased from Taconic. Bcl2l11−/− (O’Connor et al., 1998) and Ly49H-deficient (Klra8−/−) C57BL/6 mice were gifts from G. Evan (University of California, San Francisco [UCSF], San Francisco, CA) and S. Vidal (McGill University, Montreal, Quebec, Canada), respectively. Rag2−/−Klra8−/− mice were generated at UCSF. Mice were maintained in accordance with the guidelines of the UCSF Institutional Animal Care and Use Committee. Bcl2l11−/− (CD45.2), and WT (CD45.1) BM cells were mixed at a 1:1 ratio and used to reconstitute lethally irradiated C57BL/6 mice (Sun et al., 2009). After 8–10 wk, mice were infected by intraperitoneal injections of 104 PFU of MCMV Smith strain.
NK cell adoptive transfer.
NK cells were enriched by staining splenocytes with rat mAbs against mouse CD4, CD5, CD8, CD19, Gr-1, and Ter119, followed by anti–rat IgG antibodies conjugated to magnetic beads (QIAGEN). 500,000–1,000,000 Bcl2l11−/− (CD45.2) and WT (CD45.1) Ly49H+ NK cells (1:1) were injected intravenously into Ly49H-deficient recipient mice on the day before MCMV infection. Enriched NK cells were labeled with 10 µM CellTrace violet (Invitrogen) for 30 min at 37°C and then injected intravenously into Ly49H-deficient mice. Homeostatic proliferation was assessed after transfer of 106 Bcl2l11−/− and WT cells (1:1) NK cells into Rag2−/−Il2rg−/− mice.
MCMV challenge and viral quantification.
Bcl2l11−/− or WT Ly49H+ memory NK cells were enriched and enumerated at day 28 after MCMV. 20,000 Ly49H+ memory NK cells were adoptively transferred into secondary Ly49H-deficient or Rag2−/− Ly49H-deficient mice and were challenged with 2 × 104 PFU MCMV Smith strain. Viral titers were quantified by quantitative PCR in the blood at days 3 and 4 p.i. DNA was prepared from 50 µl of blood (Promega), and the relative copy number of MCMV IE1 was determined by quantitative PCR analysis with an SYBR green master mix reagent (Invitrogen) using the following primer pair: MCMV IE1 forward, 5′-AGCCACCAACATTGACCACGCAC-3′; and MCMV IE1 reverse, 5′-GCCCCAACCAGGACACACAACTC-3′. Mice were monitored daily for weight loss and survival.
Flow cytometry, proliferation, and apoptosis assays.
Fc receptors were blocked with anti-CD16+CD32 mAb (clone 2.4G2) before staining with the indicated mAbs (BD, eBioscience, or BioLegend). We assessed apoptosis by staining freshly isolated splenocytes and hepatic lymphocytes with PE-conjugated annexin V (BD) or using a probe for activated caspase (FLICA; Immunochemistry Technologies). Cell division was measured by injecting mice intravenously with 200 µg BrdU in PBS (BD) 2 h before tissue harvest. Samples were analyzed on an LSRII (BD) using FlowJo software (Tree Star).
Ex vivo stimulation of NK cells.
Cell culture 96-well plates treated with N-[1-(2,3-dioleoyloxyl)propyl]-N,N,N-trimethylammonium methylsulphate (Sigma-Aldrich) were coated with 10 µg/ml anti-NK1.1 (PK136). Enriched NK cells (naive or memory) were incubated with plate-bound antibody or 20 ng/ml mouse IL-12 + 10 ng/ml IL-18 (R&D Systems) or 20 ng/ml PMA + 200 ng/ml ionomycin for 6 h at 37°C in the presence of PE-conjugated anti-CD107a and GolgiStop (BD), followed by staining for surface markers and intracellular cytokines. For the co-culture assay, 105 enriched NK cells were incubated for 6 h at 37°C with 105 RMA or m157-transduced RMA cells in the presence of PE–anti-CD107a and GolgiStop (BD), followed by staining for surface markers and intracellular cytokines.
Transcriptional profiling.
Transcriptional profiling was performed by the Immunological Genome Consortium (Bezman et al., 2012).
Statistical methods.
Paired (co-transfer experiments) and unpaired Student’s t tests or two-way ANOVA was used to compare results; P < 0.05 was considered statistically significant. Bar graphs represent the mean, and error bars signify the standard error of the mean.
Acknowledgments
L.L. Lanier is an American Cancer Society Professor and is supported by National Institutes of Health (NIH) grant AI068129. G. Min-Oo is a Bisby Postdoctoral Fellow of the Canadian Institutes of Health Research. J.C. Sun is supported by the Searle Scholars Program, the Cancer Research Institute, and NIH grants AI085034 and AI100874.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- MCMV
- mouse CMV
- p.i.
- postinfection
References
- Bezman N.A., Kim C.C., Sun J.C., Min-Oo G., Hendricks D.W., Kamimura Y., Best J.A., Goldrath A.W., Lanier L.L.; Immunological Genome Project Consortium. 2012. Molecular definition of the identity and activation of natural killer cells. Nat. Immunol. 13:1000–1009 10.1038/ni.2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P., O’Reilly L.A. 2009. CD95, BIM and T cell homeostasis. Nat. Rev. Immunol. 9:514–519 10.1038/nri2570 [DOI] [PubMed] [Google Scholar]
- Bouillet P., Metcalf D., Huang D.C., Tarlinton D.M., Kay T.W., Köntgen F., Adams J.M., Strasser A. 1999. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 286:1735–1738 10.1126/science.286.5445.1735 [DOI] [PubMed] [Google Scholar]
- Brown M.G., Dokun A.O., Heusel J.W., Smith H.R., Beckman D.L., Blattenberger E.A., Dubbelde C.E., Stone L.R., Scalzo A.A., Yokoyama W.M. 2001. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 292:934–937 10.1126/science.1060042 [DOI] [PubMed] [Google Scholar]
- Chiossone L., Chaix J., Fuseri N., Roth C., Vivier E., Walzer T. 2009. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 113:5488–5496 10.1182/blood-2008-10-187179 [DOI] [PubMed] [Google Scholar]
- Cooper M.A., Bush J.E., Fehniger T.A., VanDeusen J.B., Waite R.E., Liu Y., Aguila H.L., Caligiuri M.A. 2002. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 100:3633–3638 10.1182/blood-2001-12-0293 [DOI] [PubMed] [Google Scholar]
- Cooper M.A., Elliott J.M., Keyel P.A., Yang L., Carrero J.A., Yokoyama W.M. 2009. Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. USA. 106:1915–1919 10.1073/pnas.0813192106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumá M., Angulo A., Vilches C., Gómez-Lozano N., Malats N., López-Botet M. 2004. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 104:3664–3671 10.1182/blood-2004-05-2058 [DOI] [PubMed] [Google Scholar]
- Hildeman D.A., Zhu Y., Mitchell T.C., Bouillet P., Strasser A., Kappler J., Marrack P. 2002. Activated T cell death in vivo mediated by proapoptotic Bcl-2 family member Bim. Immunity. 16:759–767 10.1016/S1074-7613(02)00322-9 [DOI] [PubMed] [Google Scholar]
- Huntington N.D., Puthalakath H., Gunn P., Naik E., Michalak E.M., Smyth M.J., Tabarias H., Degli-Esposti M.A., Dewson G., Willis S.N., et al. 2007. Interleukin 15–mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat. Immunol. 8:856–863 10.1038/ni1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay D.C., Mitchell D.M., Williams M.A. 2013. Bim mediates the elimination of functionally unfit Th1 responders from the memory pool. PLoS ONE. 8:e67363 10.1371/journal.pone.0067363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen P., Heinzel S., Carrington E.M., Happo L., Alexander W.S., Zhang J.-G., Herold M.J., Scott C.L., Lew A.M., Strasser A., Hodgkin P.D. 2013. Mutually exclusive regulation of T cell survival by IL-7R and antigen receptor-induced signals. Nat Commun. 4:1735 10.1038/ncomms2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtulus S., Tripathi P., Opferman J.T., Hildeman D.A. 2010. Contracting the ‘mus cells’—does down-sizing suit us for diving into the memory pool? Immunol. Rev. 236:54–67 10.1111/j.1600-065X.2010.00920.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Girard S., Macina D., Busà M., Zafer A., Belouchi A., Gros P., Vidal S.M. 2001. Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nat. Genet. 28:42–45 10.1038/ng0501-42 [DOI] [PubMed] [Google Scholar]
- Li Q., Eppolito C., Odunsi K., Shrikant P.A. 2006. IL-12-programmed long-term CD8+ T cell responses require STAT4. J. Immunol. 177:7618–7625 10.4049/jimmunol.177.11.7618 [DOI] [PubMed] [Google Scholar]
- Lopez-Vergès S., Milush J.M., Schwartz B.S., Pando M.J., Jarjoura J., York V.A., Houchins J.P., Miller S., Kang S.-M., Norris P.J., et al. 2011. Expansion of a unique CD57+NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA. 108:14725–14732 10.1073/pnas.1110900108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min-Oo G., Kamimura Y., Hendricks D.W., Nabekura T., Lanier L.L. 2013. Natural killer cells: walking three paths down memory lane. Trends Immunol. 34:251–258 10.1016/j.it.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor L., Strasser A., O’Reilly L.A., Hausmann G., Adams J.M., Cory S., Huang D.C. 1998. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 17:384–395 10.1093/emboj/17.2.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paust S., von Andrian U.H. 2011. Natural killer cell memory. Nat. Immunol. 12:500–508 10.1038/ni.2032 [DOI] [PubMed] [Google Scholar]
- Paust S., Gill H.S., Wang B.-Z., Flynn M.P., Moseman E.A., Senman B., Szczepanik M., Telenti A., Askenase P.W., Compans R.W., von Andrian U.H. 2010. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat. Immunol. 11:1127–1135 10.1038/ni.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prlic M., Bevan M.J. 2008. Exploring regulatory mechanisms of CD8+ T cell contraction. Proc. Natl. Acad. Sci. USA. 105:16689–16694 10.1073/pnas.0808997105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prlic M., Blazar B.R., Farrar M.A., Jameson S.C. 2003. In vivo survival and homeostatic proliferation of natural killer cells. J. Exp. Med. 197:967–976 10.1084/jem.20021847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A. 2005. The role of BH3-only proteins in the immune system. Nat. Rev. Immunol. 5:189–200 10.1038/nri1568 [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris A.W., Huang D.C., Krammer P.H., Cory S. 1995. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 14:6136–6147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.C., Lanier L.L. 2011. NK cell development, homeostasis and function: parallels with CD8+ T cells. Nat. Rev. Immunol. 11:645–657 10.1038/nri3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.C., Beilke J.N., Lanier L.L. 2009. Adaptive immune features of natural killer cells. Nature. 457:557–561 10.1038/nature07665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.C., Beilke J.N., Bezman N.A., Lanier L.L. 2011. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J. Exp. Med. 208:357–368 10.1084/jem.20100479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.C., Madera S., Bezman N.A., Beilke J.N., Kaplan M.H., Lanier L.L. 2012. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J. Exp. Med. 209:947–954 10.1084/jem.20111760 [DOI] [PMC free article] [PubMed] [Google Scholar]