Insight from William Muller
In this issue, Canault et al. report for the first time a point mutation in the RAS guanyl-releasing protein 2 (RASGRP2) gene that results in a severe bleeding defect in humans.
The study of inherited platelet disorders has shed light on the molecular mechanisms of physiologic thrombosis and hemostasis and led to the development of several therapies to prevent pathologic clotting. The RASGRP2 gene encodes the guanine nucleotide exchange factor (GEF) calcium- and DAG-regulated GEF 1 (CalDAG-GEFI), which is crucial for activation of Rap1, a small GTPase that regulates integrin-mediated activation and granule secretion in platelets and other cells. The function of CalDAG-GEFI has been studied in vitro and in mice—mice lacking CalDAG-GEFI have impaired thrombi formation by platelets as well as a defect in neutrophil function—but to date, no pathological RASGRP2 mutations have been identified in humans.
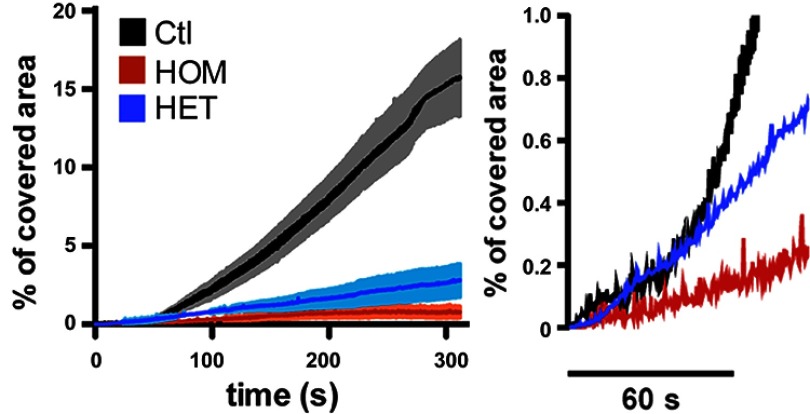
Adhesion of calcein-AM–labeled platelets to fibrillar collagen under flow (750 s-1) from a homozygous (HOM), heterozygous (HET), and healthy (Ctl) subject. Percentage of covered area was assessed over 300 seconds (left). The initial 60 seconds are magnified (right).
Now, Canault et al. have investigated the cause of an inherited platelet disorder in three siblings from a consanguineous marriage that are all affected by a severe bleeding disorder. Whole genome sequencing was used to identify a mutation (cG742T) in the RASGRP2 gene. This mutation reduces Rac1 GTP binding (secondary to decreased Rap1 activation), impairing the ability of platelets to aggregate in response to a variety of stimuli, to form thrombi under flow, and to undergo normal spreading. Heterozygotes also have platelets that fail to adhere normally under flow and have a spreading defect (see figure) but they do not suffer from bleeding because their platelets aggregate normally. The functional deficiency induced by the mutation was confined to platelets and megakaryocytes with no obvious alteration in leukocytes. This is probably because other GEFs in leukocytes are able to activate Rap1 or, as the authors speculate, that CalDAG-GEFI with this point mutation is still able to function in leukocytes. In fact, even in platelets, the aggregation defects can be overcome by high doses of agonists in vitro, so while CalDAG-GEFI may be the preferred way for platelets to activate Rap1, it is clearly not the only way.
Mutations in CalDAG-GEFI had previously been reported to be responsible for leukocyte adhesion deficiency type III, which was later found to be caused by the absence of kindlin-3. To demonstrate that the mutation in CalDAG-GEFI is truly responsible for the phenotype, the authors showed that cells transfected with wild-type RASGRP2 can activate Rap1, whereas those transfected with the mutation found in the affected siblings cannot.
Looking at these data from a different perspective, the presence of a single normal allele is sufficient to prevent bleeding, but not platelet adhesion to collagen. This suggests that partial inhibition of CalDAG-GEFI might be a novel and potentially safe therapeutic target to prevent thrombosis without causing bleeding—a holy grail of vascular medicine and surgery.
References
- Canault M., et al. 2014. J. Exp. Med. 10.1084/jem.20130477. [DOI] [Google Scholar]