Abstract
A new series of new 1,4-naphthoquinone derivatives containing carbazole-6,11-dione moiety, which has not been reported yet, has been synthesized from 1,4-naphthoquinone and 4-aminophenylsulfone involving a Michael addition, benzoylation, and Pd-catalyzed coupling. This set of compounds has been evaluated for in vitro antibacterial studies against different Gram-positive and Gram-negative bacteria, and most of the synthesized compounds exhibited good antibacterial activity and the minimum inhibitory concentrations (MICs) are compared with the standard drugs used. Compound 7 exhibited good antibacterial activity among all the molecules studied with the best MIC of 2.1 μg/mL against Bacillus subtilis. To understand the molecular interactions with targeted proteins, the molecular docking of all the synthesized compounds were carried out; between 14 molecules docked, compound 7 was the one with the best glide and E model score of −7.73 and −95.37, respectively. In all docked molecules, compound 5 exhibited least glide and E model score of −4.55 and −101.56, respectively.
Figure.

ᅟ
Keywords: 1,4-Naphthoquinone; Bacillus subtilis; Carbazole; Molecular docking; Water as solvent
Introduction
During the past two decades, the resistance to multiple antibiotics of Gram-positive and Gram-negative bacterial strains, methicillin-resistant Staphylococcus aureus (MRSA), is considered as an important clinical problem. The vancomycin and teicoplanin of glycopeptide antibiotics, quinupristin/dalfopristin, and linezolid of new antibiotics are clinically identified and used for the treatment of MRSA infections, but the structural complexity and side effects of these antibiotics have prompted increased efforts to develop novel antibiotics. Thus, the discovery and development of new classes of antibacterial drugs are essentially of great concern due to the rapid acquirement of multidrug resistance by Gram-positive and Gram-negative pathogens [1].
Quinones are a large group of compounds with a wide range of biological activities which includes anticancer, antibacterial, or antimalarial drugs as well as fungicides [2–4]. The heterocyclic derivatives of -1,4-naphthoquinones have been identified that have potent biological activities towards viral [5], molluscidal [6], malarial [7], leishmanial [8], cancer [9], and bacterial and fungal diseases [10], due to their redox potentials [11].
The present work reveals synthesis of heterocyclic 1,4-naphthoquinone derivatives and their characterization. The compounds were tested for antibacterial activity against two Gram-positive and five Gram-negative bacteria by agar dilution method. To understand the molecular interactions with targeted proteins, the molecular simulation was carried out by using Schrödinger software version 8.5 and reported in this article.
Experimental
Melting points (degree Celsius, uncorrected) of the synthesized compounds were checked in open capillary tubes by using digital automelting point apparatus (Labtronics 110, India) and found uncorrected. All the chemicals and solvents were purchased from Sigma-Aldrich and Merck, India. All reactions were carried out under atmospheric air and the products were checked by thin layer chromatography on TLC silica gel 60 F254 using eluting solvents such as ethyl acetate and hexane (1:1). The synthesized compounds were purified by column chromatography using column silica gel 100–200 mesh (ethyl acetate/hexane 1:2). All the compounds were characterized by UV–Vis spectrophotometer (UV-1800, Shimadzu, Japan) using acetone as solvent, FT-IR spectrometer (IR 8400, Shimadzu, Japan) using KBr pellets, 1H NMR spectroscopy in DMSO (400 MHz, Bruker), 13C NMR spectroscopy in DMSO (100 MHz, Bruker) using tetramethylsilane (TMS) as internal standard. Coupling constants (J values) are reported in hertz. Mass spectra were measured by Electron Impact (EI) method (Jeol GC-Mate 2). In vitro antibacterial studies of all the compounds were studied by agar dilution method. Molecular docking studies of all the synthesized compounds were studied by GLIDE program (version 8.5, Schrodinger, LLC, New York, 2010).
General procedures for synthesis of 2-[4-(4-amino-benzene sulfonyl)-phenyl amino]-[1,4] naphthoquinone (1)
Method A [12]
A solution of 1,4-naphthoquinone (1.581 g, 10 mmol) in 95 % of ethyl alcohol (40 mL) was gradually added over a period of 30 min to a solution of 4-aminophenyl sulfone (2.048 g, 10 mmol) in glacial acetic acid (10–30 mL) and stirred for 30 min. Then, the mixture was refluxed for 1 h. The reaction mixture was cooled and left overnight at room temperature. The black precipitate formed was separated by filtration. Water was added to the filtrate, the brownish material formed was filtered, washed with hot water (200 mL), dried at 80 °C, and crystallized from 95 % ethyl alcohol to give 1 (3.692 g, 91 %) as orange crystals.
Method B [13]
4-Aminophenyl sulfone (2.048 g, 10 mmol) was added to a solution of 1,4-naphthoquinone (1.581 g, 10 mmol) in water (100 mL) and refluxed for 4 h. The reaction mixture was cooled at room temperature and the brownish precipitate was filtered and washed with hot water (200 mL). The precipitate was dried at 80 °C and crystallized from 95 % ethyl alcohol to give 1 (1.657 g, 41 %) as orange crystals; mp >300 °C; UV–Vis (acetone)—451.45 nm; IR (KBr)—1,294, 1,633, 3,381, and 3,475 cm−1; 1H NMR (400 MHz, DMSO-d6) δ—6.14 (s, 2H), 6.34 (s, 1H), 6.61 (d, 2H, J = 8.8 Hz), 7.53 (d, 2H, J = 8.8 Hz), 7.56 (d, 2H, J = 8.8 Hz), 7.70–7.80 (m, 4H), 7.95 (d, 1H, J = 7.2 Hz), 8.06 (d, 1H, J = 7.3 Hz), and 9.41 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ—104.4, 112.9, 122.6, 125.2, 125.5, 126.1, 127.8, 129.2, 130.3, 132.2, 132.8, 134.8, 138.2, 142.3, 144.8, 153.5, 181.2, and 183.0; MS (EI)—m/z 403.49 (M - 1, 8 %), 257 (80), 180.80 (75), 157.78 (45), 142.78 (60), and 122.83 (100).
General procedure for synthesis of N-{4-[4-(1,4-dioxo-1,4-dihydro-naphthalene-2-ylamino) benzenesulfonyl]-phenyl} benzamides (2–7)
Substituted benzoyl chloride (1 mmol) was added to a solution of 1 (0.404 g, 1 mmol) in acetone (100 mL). After refluxing for 30 min, the reaction mixture was filtered and concentrated in vacuo to give pure samples of 2–7 which required no further purification.
N-{4-[4-(1,4-dioxo-1,4-dihydro-naphthalene-2-ylamino)-benzenesulfonyl]-phenyl} benzamide (2)
Orange solid; reaction time—25 min (0.501 g, 99 %); mp > 300 °C; UV–Vis (acetone)—447.36 nm; IR (KBr)—1,296, 1,631, 1,676, and 3,400 cm−1; 1H NMR (400 MHz, DMSO-d6) δ—6.39 (s, 1H), 7.54 (d, 2H, J = 6.8 Hz), 7.61–8.07 (m, 15H), 9.45 (s, 1H), and 10.62 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ—104.9, 120.2, 122.6, 125.2, 126.2, 127.7, 128.3, 128.4, 128.5, 130.3, 131.9, 132.1, 132.9, 134.2, 134.8, 135.2, 136.2, 143.1, 143.7, 144.6, 166.0, 181.1, and 183.0; MS (EI)—m/z 508.028 9 (M+, 12 %), 444.63 (55), 300.89 (60), 224.76 (100), and 123.07 (60).
N-{4-[4-(1,4-dioxo-1,4-dihydro-naphthalene-2-ylamino)-benzenesulfonyl]-phenyl}-3-methyl-benzamide (3)
Red-brown solid; reaction time—25 min (0.515 g, 99 %); mp > 300 °C; UV–Vis (acetone)—445.23 nm; IR (KBr)—1,298, 1,616, 1,680, 2,922, and 3,309 cm−1; 1H NMR (400 MHz, DMSO-d6) δ—2.08 (s, 3H), 6.40 (s, 1H), 7.36–8.08 (m, 16H), 9.49 (s, 1H), and 10.61 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ—21.3, 105.4, 120.7, 123.1, 125.4, 125.7, 126.7, 126.9, 128.7, 128.8, 128.9, 130.1, 130.8, 131.2, 132.7, 133.0, 133.4, 134.7, 135.7, 138.3, 143.6, 144.3, 167.8, 181.7, and 183.6; MS (EI)—m/z 521.60 (M − 1, 15 %), 499.17 (25), 457.80 (10), 274.85 (50), 257.85 (25), 175.06 (97), 114.09 (100), and 100.07 (76).
N-{4-[4-(1,4-dioxo-1,4-dihydro-naphthalene-2-ylamino)-benzenesulfonyl]-phenyl}-4-methyl-benzamide (4)
Crimson red solid; reaction time—30 min (0.512 g, 98 %); mp > 300 °C; UV–Vis (acetone)—448.38 nm; IR (KBr)—1,294, 1,616, 1,680, 2,945, 3,307, and 3,360 cm−1; 1H NMR (400 MHz, DMSO-d6) δ—2.30 (s, 3H), 6.38 (s, 1H), 7.27 (d, 2H, J = 8.0 Hz), 7.32 (d, 2H, J = 8.0 Hz), 7.61 (d, 2H, J = 8.8 Hz), 7.77–8.06 (m, 10H), 9.45 (s, 1H), and 10.53 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ—20.9, 104.8, 120.1, 122.6, 125.2, 126.1, 127.8, 128.3, 128.9, 129.2, 130.3, 131.3, 132.1, 132.9, 134.8, 135.1, 136.3, 142.1, 143.1, 143.8, 144.6, 165.8, 181.1, and 183.0; MS (EI)—m/z 521.54 (M − 1, 45 %), 456.86 (15), 250.75 (80), and 184.42 (100).
N-{4-[4-(1,4-dioxo-1,4-dihydro-naphthalene-2-ylamino)-benzene sulfonyl]-phenyl}-3-nitro-benzamide (5)
Red-brown solid; reaction time—25 min (0.545 g, 99 %); mp > 300 °C; UV–Vis (acetone)—447.36 nm; IR (KBr)—1,274, 1,348, 1,529, 1,616, 1,687, and 3,412 cm−1; 1H NMR (400 MHz, DMSO-d6) δ—6.40 (s, 1H), 7.63 (d, 2H, J = 7.2 Hz), 7.82 (d, 2H, J = 7.2 Hz), 7.85–8.06 (m, 7H), 8.34 (d, 1H, J = 7.3 Hz), 8.40 (d, 1H, J = 7.3 Hz), 8.47 (d, 1H, J = 7.8 Hz), 8.62 (s, 1H), 9.50 (s, 1H), 10.96 (s, 1H), and 13.71 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ—105.4, 121.0, 123.1, 123.2, 124.1, 125.7, 126.7, 127.7, 128.9, 129.1, 130.7, 130.8, 131.2, 132.6, 132.9, 133.4, 135.3, 135.8, 136.1, 143.8, 145.1, 148.2, 164.4, 181.6, and 183.6; MS (EI)—m/z 553.12 (M+, 13 %), 507.98 (15), 440.31 (38), 366.39 (100), 293.45 (41), and 232.44 (40).
N-{4-[4-(1,4-dioxo-1,4-dihydro-naphthalene-2-yl amino)-benzene sulfonyl]-phenyl}-4-nitro-benzamide (6)
Orange solid; reaction time—25 min (0.543 g, 98 %); mp > 300 °C; UV–Vis (acetone)—453.49 nm; IR (KBr)—1,273, 1,350, 1,529, 1,614, 1,680, 3,248, and 3,315 cm−1; 1H NMR (400 MHz, DMSO-d6) δ—6.39 (s, 1H), 7.62 (d, 2H, J = 8.0 Hz), 8.79 (t, 1H, J = 7.8 Hz), 7.86 (t, 1H, J = 7.8 Hz), 7.95 (d, 2H, J = 8.0 Hz), 7.98 (d, 2H, J = 8.0 Hz), 8.02 (d, 2H, J = 8.0 Hz), 8.06 (d, 1H, J = 7.8 Hz), 8.14 (d, 1H, J = 7.8 Hz), 8.18 (d, 2H, J = 8.0 Hz) 8.37 (d, 2H, J = 8.0 Hz), 9.46 (s, 1H), and 10.92 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ—104.9, 120.4, 122.6, 123.5, 125.2, 126.2, 126.2, 128.4, 128.6, 130.3, 132.1, 132.9, 134.3, 135.3, 135.6, 135.8, 136.1, 143.2, 144.6, 149.3, 164.4, 181.1, and 183.0; MS (EI)—m/z 551.85 (M − 2, 10 %), 528.20 (30), 510.51 (25), 268.46 (75), 252.45 (98), 191.36 (100), and 177.40 (52).
N-{4-[4-(1,4-dioxo-1,4-dihydro-naphthalene-2-yl amino)-benzene sulfonyl]-phenyl}-3, 5-dinitro-benzamide (7)
Red-brown solid; reaction time—30 min (0.589 g, 98 %); mp > 300 °C; UV–Vis (acetone)—446.12 nm; IR (KBr)—1,271, 1,346, 1,541, 1,624, 1,691, 3,334, and 3,400 cm−1; 1H NMR (400 MHz, DMSO-d6) δ—6.39 (s, 1H), 7.62 (d, 2H, J = 8.8 Hz), 7.78 (t, 1H, J = 6.8 Hz), 7.82 (t, 1H, J = 6.8 Hz) 7.93 (d, 2H, J = 7.2 Hz), 7.93–8.05 (m, 8H), 9.17 (s, 1H), 9.45 (s, 1H), and 11.16 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ—104.9, 120.7, 121.4, 122.6, 125.2, 126.1, 128.1, 128.6, 130.3, 132.1, 132.9, 134.8, 134.8, 136.2, 136.8, 142.8, 143.2, 144.6, 148.0, 148.2, 161.9, 181.1, and 183.0; MS (EI)—m/z 596.51 (M − 2, 55 %), 566.45 (20), 537.60 (25), 473.23 (15), 399.43 (100), 382.51 (46), and 218.53 (45).
General procedure for synthesis of 2-(4-amino-phenylsulfonyl)-5H-benzo [b]carbazole-6,11-diones (1a-7a)
Method C [14, 15]
A mixture of 1–7 (0.5 mmol) in glacial acetic acid (60 mL) and palladium (II) acetate (0.112 g, 0.5 mmol) were refluxed for 2 h, and the reaction mixture was cooled at room temperature and poured into ice-cold water. The precipitate was filtered, dried at 60 °C and crystallized from acetone to give 1a–7a.
2-(4-Amino-phenylsulfonyl)-5H-benzo[b]carbazole-6, 11-dione (1a)
Yellow solid; reacton time—2 h (0.150 g, 75 %); mp > 300 °C; UV–Vis (acetone)—278.71 nm; IR (KBr)—1,288, 1,629, 1,651, 3,384, and 3,478 cm−1; 1H NMR (400 MHz, DMSO-d6) δ—6.13 (s, 2H), 6.60 (d, 2H, J = 8.8 Hz), 7.55 (d, 2H, J = 8.8 Hz), 7.70 (d, 1H, J = 8.8 Hz), 7.80–7.90 (m, 3H), 8.09 (d, 1H, J = 5.2 Hz), 8.10 (d, 1H, J = 5.2 Hz), 8.60 (s, 1H), and 13.4 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ—113.5, 118.4, 121.9, 123.6, 125.0, 126.2, 126.7, 129.8, 130.3, 133.0, 134.0, 134.3, 135.0, 139.1, 139.8, 140.0, 151.0, 154.0, 177.9, and 180.7; MS (EI)—m/z 402.40 (M+, 5 %), 342.75 (8), 250.87 (50), 205.06 (98), 162.06 (100), 117.10 (60), and 75.10 (45).
N-[4-(6,11-dioxo-6,11-dihydro-5H-benzo[b]carbazole-2-sulfonyl)-phenyl]-benzamide (2a)
Light yellow solid; reaction time—2 h (0.195 g, 77 %); mp > 300 °C; UV–Vis (acetone)—278.79 nm; IR (KBr)—1,244, 1,589, 1,668, and 3,375 cm−1; 1H NMR (400 MHz, DMSO-d6) δ—7.58 (t, 2H, J = 8.0 Hz), 7.60 (t, 1H, J = 8.0 Hz), 7.76 (d, 1H, J = 8.8 Hz), 7.81–7.92 (m, 5H), 7.90 (d, 2H, J = 8.2 Hz), 8.03 (d, 2H, J = 8.2 Hz), 8.10–8.16 (m, 2H), 8.76 (d, 1H, J = 1.2 Hz), 10.61 (s, 1H), and 13.47 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ—115.3, 117.9, 120.3, 122.2, 123.2, 123.3, 124.8, 126.2, 127.7, 128.4, 131.9, 132.4, 132.5, 133.5, 133.7, 134.2, 134.5, 135.3, 136.8, 139.4, 139.8, 143.7, 166.0, 177.4, and 180.2; MS (EI)—m/z 505.84 (M-1, 5 %), 491.09 (35), 474.60 (10), 450.66 (15), 259.18 (20), 218.31 (60), 198.36 (45), 125.41 (35), and 81.36 (100).
N-[4-(6,11-dioxo-6,11-dihydro-5H-benzo[b]carbazole-2-sulfonyl)-phenyl]-3-methyl-benzamide (3a)
Light yellow solid; reaction time—2 h (0.199 g, 77 %); mp > 300 °C; UV–Vis (acetone)—339.39 nm; IR (KBr)—1,244, 1,589, 1,668, 2,922, and 3,255 cm−1; 1H NMR (400 MHz, DMSO-d6) δ—2.38 (s, 3H), 7.40 (m, 2H, J = 7.8 Hz), 7.70–8.20 (m, 12H), and 8.77 (s, 1H), 10.59 (s, 1H), 13.54 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ—21.3, 115.8, 120.7, 122.7, 125.4, 126.7, 128.7, 128.8, 128.9, 132.4, 133.0, 133.5, 134.0, 134.2, 134.7, 135.0, 135.8, 137.3, 138.2, 139.9, 140.3, 142.6, 143.2, 166.6, 178.1, and 181.0; MS (EI)—m/z 518.45 (M − 2, 15 %), 496.07 (15), 477.41 (20), 300.78 (12), 226.92 (35), 171.95 (100), 110.98 (75), and 96.96 (52).
N-[4-(6,11-dioxo-6,11-dihydro-5H-benzo[b]carbazole-2-sulfonyl)-phenyl]-4-methyl-benzamide (4a)
Brick red color solid; reaction time—2 h (0.201 g, 78 %); mp > 300 °C; UV–Vis (acetone)—346.67 nm; IR (KBr)—1,242, 1,651, 1,666, 2,922, and 3,352 cm−1; 1H NMR (400 MHz, DMSO-d6) δ—2.36 (s, 3H), 7.31 (d, 2H, J = 8.0 Hz), 7.75–8.16 (m, 12H), 8.75 (s, 1H), 10.51 (s, 1H), 13.50 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ—20.9, 115.3, 117.9, 120.2, 122.2, 123.1, 124.8, 126.2, 127.8, 128.3, 128.9, 131.3, 131.5, 132.4, 133.5, 133.7, 134.5, 135.2, 136.8, 139.4, 139.8, 142.1, 143.8, 165.8, and 177.4, 180.2; MS (EI)—m/z 521.85 (M + 1, 25 %), 471 (75), 250.37 (30), 184 (100), and 81.79 (65).
N-[4-(6,11-dioxo-6,11-dihydro-5H-benzo[b]carbazole-2-sulfonyl)-phenyl]-3-nitro-benzamide (5a)
Pale yellow solid; reaction time—2 h (0.212 g, 77 %); mp > 300 °C; UV–Vis (acetone)—277.58 nm; IR (KBr)—1,251, 1,350, 1,525, 1,591, 1,670, and 3,257 cm−1; 1H NMR (400 MHz, DMSO-d6) δ—7.70–8.20 (m, 15H) 10.94 (s, 1H), and 13.55 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ—106.9, 119.1, 122.9, 123.2, 124.5, 125.6, 125.6, 125.3, 125.1, 126.4, 127.1, 129.1, 131.2, 132.4, 132.5, 132.9, 133.2, 134.1, 134.4, 134.9, 136.0, 137.1, 139.2, 146.1, 163.4, 180.2, and 181.0; MS (EI)—m/z 550.65 (M − 1, 15 %), 536.40 (30), 507.28 (10), 437.65 (10), 408.63 (15), 351.84 (38), 289.86 (95), 233.83 (100), and 222.08 (18).
N-[4-(6,11-dioxo-6,11-dihydro-5H-benzo[b]carbazole-2-sulfonyl)-phenyl]-4-nitro-benzamide (6a)
Yellow solid; reaction time—2 h (0.215 g, 78 %); mp > 300 °C; UV–Vis (acetone)—258.18 nm; IR (KBr)—1,319, 1,348, 1,529, 1,591, 1,678, and 3,437 cm−1; 1H NMR (400 MHz, DMSO-d6) δ—7.64–8.39 (m, 15H), 9.81 (s, 1H), and 10.80 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ—117.1, 119.0, 122.0, 122.1, 123.5, 124.6, 126.1, 126.3, 126.8, 127.8, 128.4, 129.0, 131.0, 132.1, 132.7, 132.8, 133.4, 135.1, 135.2, 136.4, 138.0, 140.0, 163.1, 180.7, and 182.4; MS (EI)—m/z 548.56 (M − 3, 5 %), 527.29 (20), 509.61 (20), 251.56 (80), 190.52 (100), and 164.77 (18).
N-[4-(6,11-dioxo-6,11-dihydro-5H-benzo[b]carbazole-2-sulfonyl)-phenyl]-3,5-dinitro-benzamide (7a)
Yellow solid; reaction time—2 h (0.228 g, 77 %); mp > 300 °C; UV–Vis (acetone)—269.09 nm; IR (KBr)—1,244, 1,344, 1,535, 1,629, 1,660, and 3,300 cm−1; 1H NMR (400 MHz, DMSO-d6) δ—7.75–8.15 (m, 10H), 8.76 (d, 1H, J = 1.2 Hz), 8.98 (t, 1H, J = 2.0 Hz), 9.10 (d, 2H, J = 2.0 Hz), 11.14 (s, 1H), and 13.50 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ—115.3, 117.9, 120.8, 212.4, 211.3, 123.2, 124.8, 126.1, 128.1, 128.5, 132.4, 133.5, 133.7, 134.4, 136.3, 136.5, 136.8, 139.4, 139.8, 142.8, 148.0, 148.9, 161.9, 177.4, and 180.2; MS (EI)—m/z 595.42 (M − 1, 35 %), 562.58 (15), 543.04 (40), 407.72 (45), 386.74 (60), 328.63 (100), 249.72 (75), and 214.68 (32).
Molecular docking studies
To understand the interaction of all the synthesized molecules (1–7, 1a–7a) with Bacillus substilis, the crystal structure of YmaH from B. subtilis [16] were downloaded from protein data bank and the molecular docking studies were performed using the GLIDE program [17] (version 8.5, Schrodinger, LLC). To analyze the docking results and execute the protocol, the maestro user interface (version 8.5, Schrodinger, LLC) was employed and the validation of the protocol was evaluated by redocking. YmaH (PDB ID: 3HSB) was selected for docking studies as a reference sample and was prepared for docking through a protein preparation wizard. Structures of 1–7, 1a–7a were sketched using ACD/chemsketch (Freeware version). The GLIDE grid generation wizard has been used to define the docking space. Docking was performed using XP (Extra Precision mode) docking protocol.
In vitro antibacterial activity
All the synthesized compounds were studied for their antibacterial activity against clinically isolated two Gram-positive bacteria (Bacillus subtilis and Klebsiella pneumoniae) and five Gram-negative bacilli (Staphylococcus aureus, Escherichia coli, Proteus vulgaris, Salmonella typhi, and Pseudomonas aureus) using conventional agar dilution method [18, 19]. The minimum inhibitory concentrations (MICs) values were calculated by comparison to Sparfloxacin and Norfloxacin as the reference bacterial drugs and they are presented in Table 2. All the cultures were prepared by Muller Hinton agar, and the turbidity of all the bacterial cultures was adjusted to 0.5 McFarland Standard by preparing a bacterial suspension of three to five well-isolated colonies of the same morphological type selected from an agar plate culture. The cultures were further diluted 1,000-fold to get an inoculums size of 1.5 × 105 CFU/mL. The synthesized compounds and standard bacterial drugs (50 mg) were dissolved in dimethyl formamide (DMF) (0.5 mL) and the solution was diluted with water (4.5 mL) to get a stock solution of 10,000 mg/L of each compound. Further progressive double dilution with Muller–Hinton broth was performed to obtain the required concentrations of 2,500–2.1 μg/mL [20]. To ensure that the solvent had no effect on the bacterial growth, a control test was performed with a test medium supplemented with DMF at the same dilutions as used in the experiment.
Table 2.
In vitro antibacterial activity of synthesized compounds against Gram-positive and Gram-negative bacteria (MICs in μg/mL)
| Compounds | MIC (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| B. subtilis (clinically isolated) | S. aureus (clinically isolated) | E. coli (clinically isolated) | P.vulgaris (clinically isolated) | S. typhi (clinically isolated) | P. aureus (clinically isolated) | K. pneumoniae (clinically isolated) | |
| 1 | 8.7 | 3.6 | 621.3 | 3.4 | –a | 127.4 | 892.5 |
| 2 | 7.2 | 4.3 | 592.2 | 3.1 | 848.3 | 132.6 | 243.6 |
| 3 | 9.1 | 53 | 536 | 6.8 | 789.2 | 92.5 | 874.6 |
| 4 | 11.2 | 33.8 | 541.9 | 4.3 | –a | 24.8 | 992.6 |
| 5 | 7.3 | 29 | 638.2 | –a | 549.2 | 27.4 | 1,891.4 |
| 6 | 4.3 | 4.2 | –a | 9.2 | 881.2 | 32 | 532.6 |
| 7 | 2.1 | 3.1 | 46.7 | 4.6 | –a | 72.1 | 578.9 |
| 1a | 8.3 | 3.7 | –a | 3.6 | –a | 128.6 | –a |
| 2a | 8.3 | 27.1 | 457 | 3.7 | 834.2 | 89.6 | 982.5 |
| 3a | 11 | 32.1 | 541 | 2.4 | 634.2 | 23 | –a |
| 4a | 8.9 | 41.7 | –a | 5.9 | 611.7 | 26 | 1,732.6 |
| 5a | 6.1 | 32 | 635.4 | 12.7 | 456.6 | 29.5 | –a |
| 6a | 6.1 | 2.8 | 472.6 | 11.5 | 941.4 | –a | 592.1 |
| 7a | 2.5 | 3.8 | 432 | 4.8 | 892 | 67.3 | 678.7 |
| Sparfloxacinb | 9.76 | 4.87 | 156.3 | 4.8 | 2,500 | 156.3 | 2,500 |
| Norfloxacinb | –a | 39.06 | 625 | –a | 627 | 39.06 | <1.2 |
Lower MIC values indicates that higher antimicrobial activity
aNo inhibition observed
bStandard antibacterial drugs
In each microwell inoculated with 75 μL of the serial dilutions, 75 μL of the bacterial suspension was added in a series of 12 microwells. Incubation of the cultures overnight at 37 °C was done and the growth measured. The MICs of the test compounds and the standard control drugs are tabulated in Table 2.
Results and discussion
Chemistry
1,4-Naphthoquinone reacts with 4-aminophenyl sulfone to generate 2-[4-(4-amino-benzenesulfonyl)-phenylamino]-[1,4]naphthoquinone (1). Though this conversion has already been effected in glacial acetic acid under reflux [12], it is now found that the reaction takes place smoothly in water medium without the aid of the acid. It is found that the yield in this method is only marginal (41 %) (method B) [13], but when the reaction is conducted in a mixture of ethanol and acetic acid, the reaction led to a very good yield (91 %) (method A). However, performing the reaction in ethanol alone is not at all successful, the yield being very poor (35 %). Compound 1 is then reacted with several aromatic acid chlorides to give N-{4-[4-(1,4-dioxo-1,4-dihydro naphthalene-2-yl amino)-benzene sulfonyl]-phenyl}-aryl benzamide derivatives (2–7) by conventional method in acetone and yield between 98 and 99 %. Finally, carbazole-6,11-dione derivatives (1a–7a) are synthesized via a typical intramolecular cyclization with palladium (II) acetate in acetic acid (method C).
This the first report on the synthesis of N-{4-[4-(1,4-dioxo-1,4-dihydronaphthalen-2-yl amino)-benzenesulfonyl]-phenyl}-aryl benzamide derivatives (2–7) and 2-(4-amino-benzosulfonyl)-5H-benzo[b]carbazole-6,11-dione derivatives (1a–7a) to the best of our knowledge. Only few reports are available in literature wherein compounds with carbazoloquinone nuclei on naphthoquinone and N-dansyl carbazoloquinone have been found to exhibit antituberculosis [12] apart from chemical and electrochemical fluorescent activities [14]. However, the molecular docking and antibacterial studies have not been tested on these systems so far (Scheme 1).
Scheme 1.
The synthesis of 2-[4-(4-amino-benzenesulfonyl)-phenylamino]-[1,4] naphthoquinone (1), N-{4-[4-(1,4-dioxo-1,4-dihydro-naphthalene-2-ylamino)-benzenesulfonyl]-phenyl}-aryl benzamides (2–7) and carbazole-6,11-dione derivatives (1a–7a)
Biology
Molecular docking studies
To understand the interaction of bacterial protein receptor with synthesized molecules (1–7, 1a–7a), the crystal structure of YmaH from B. subtilis was downloaded from protein data bank and studied with the glide program. All the glide and E model scores are compared to the MIC of B. subtilis and the details of docked compounds are presented in Table 1.
Table 1.
Molecular docking studies of 14 analogues taken for study with Bacillus subtilis (BS) (PDB ID: 3HSB)
| Compounds | Molecular docking | |||
|---|---|---|---|---|
| Glide score (kcal/mol) | E model score | No. of H bonds | MICs of BS (μg/mL) | |
| 1 | −4.65 | −61.47 | Hydrophobic interaction | 8.7 |
| 2 | −5.46 | −79.16 | 3 (GLN63, HIE268, LYS179) | 7.2 |
| 3 | −5.22 | −85.08 | 1 (LYS179) | 9.1 |
| 4 | −5.85 | −84.29 | 3 (LYS179, HIE268, GLN63) | 11.2 |
| 5 | −4.55 | −101.56 | 4 (LYS179, HIE268, ASN273, TRP58) | 7.3 |
| 6 | −7.70 | −88.56 | 2 (LYS179, ASN273) | 4.3 |
| 7 | −7.73 | −95.3 | 2 (LYS179, TRP58) | 2.1 |
| 1a | −4.90 | −59.51 | 2 (ASN273, GLN63) | 8.3 |
| 2a | −5.86 | −62.74 | 2 (GLN63, GLN208) | 8.3 |
| 3a | −5.58 | −74.34 | 1 (ASN273) | 11 |
| 4a | −5.48 | −76.24 | 1 (GLN63) | 8.9 |
| 5a | −6.06 | −72.78 | 3 (GLN63, GLN208, LYS179) | 6.1 |
| 6a | −5.86 | −78.76 | 2 (GLN63, GLN208) | 6.1 |
| 7a | −5.92 | −82.73 | 2 (GLN63, GLN208) | 2.5 |
| Sparfloxacin | –a | –a | –a | 9.76 |
| Norfloxacin | –b | |||
Bold letters indicate the best activity among all studied compounds
aDocking studies not carried out
bNo inhibition observed
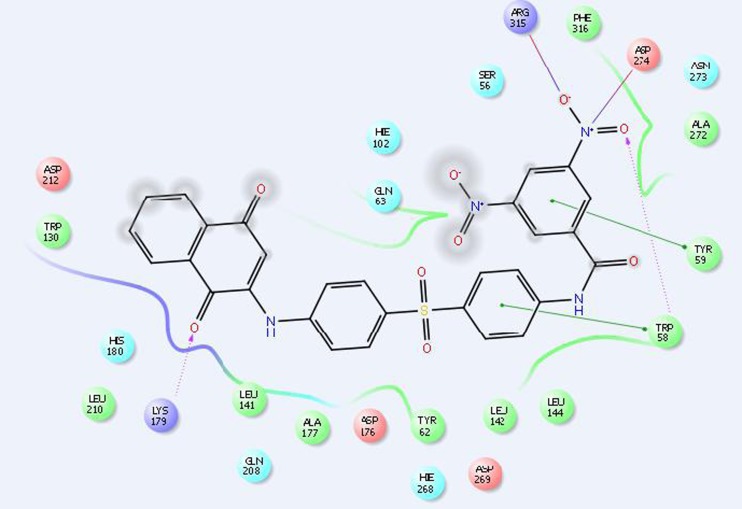
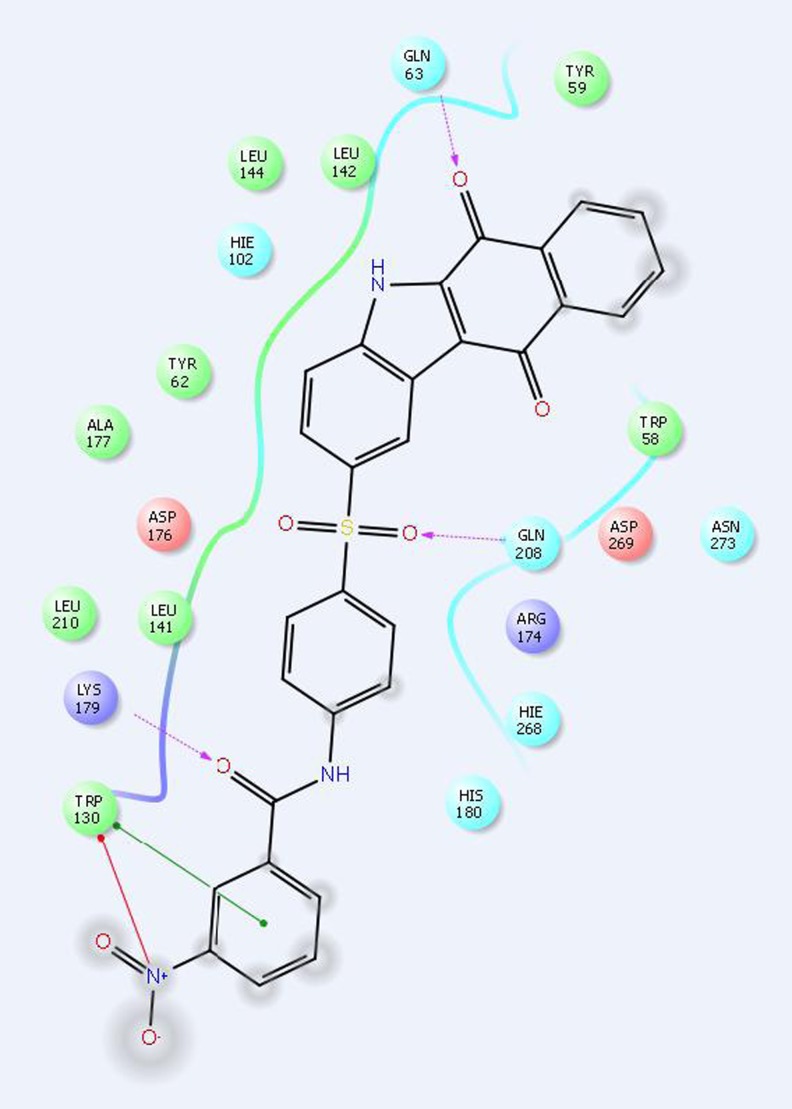
The use of glide and E model scores for ranking the different derivatives within a series is always not dependable. The molecular docking and in vitro antibacterial study results show that the glide scores and MIC values of the synthesized compounds do not have any correlation. The glide scores are mainly used to identify the active and inactive compounds. In addition, glide is primarily concerned with generating an accurate pose for each ligand and enrichment (the separation of actives from inactives) [17] (Figs. 1, 2, and 3).
Fig. 1.
Docking model structure of compound 7 into the YmaH binding pocket
Fig. 2.
Docking model structure of compound 6 into the YmaH binding pocket
Fig. 3.
Docking model structure of compound 5a into the YmaH binding pocket
In vitro antibacterial studies of quinone derivatives
All the synthesized compounds were tested against two Gram-positive and five Gram-negative bacteria. All the compounds (1–7, 1a–7a) exhibited good bacterial activity against Gram-positive bacteria of K. pneumoniae than the standard drugs used (Sparfloxacin and Norfloxacin). Compound 7 exhibit good activity against most of the Gram-positive and Gram-negative microorganisms due to the presence of the two nitro groups at the third and fifth position of the aromatic system of the benzoyl unit. Compound 6a exhibit better activity against S. aureus (2.8 μg/mL) than Sparfloxacin (4.8 μg/mL) and Norfloxacin (39.06 μg/mL) due to the presence of electron withdrawing nitro group in the molecule. Compound 7 (46.7 μg/mL) exhibit better activity against E. coli than the standard drug Sparfloxacin (156.3 μg/mL) and Norfloxacin (625 μg/mL). Compound 3a (2.4 μg/mL) was exhibit better bacterial activity against P. vulgaris than Sparfloxacin (4.8 μg/mL) and compound 5a (456.6 μg/mL) exhibit better antibacterial activity against S. typhi than the standards of Sparfloxacin (2,500 μg/mL) and Norfloxacin (627 μg/mL) used. Compound 3a (23 μg/mL) exhibit good antibacterial activity against P. aureus than Sparfloxacin (156.3 μg/mL) and Norfloxacin (39.06 μg/mL). Compounds 7 (2.1 μg/mL), 3a (2.4 μg/mL), and 6a (2.8 μg/mL) exhibited very good antibacterial activity due to the presence of methyl and nitro functional groups, among all the molecules synthesized against B. subtilis, P. aureus, and S. aureus than Sparfloxacin and Norfloxacin. Standard drug Norfloxacin did not exhibit any activity against B. subtilis and P. vulgaris microorganisms. Compound 1 did not exhibit any inhibition against E. coli, S. typhi, K. pneumoniae, and compound 4a did not exhibit any inhibition against E. coli (Table 2).
Conclusions
In summary, a new series of novel 2-(4-aminophenylsulfonyl)-5H-benzo[b]carbazole-6,11-dione derivatives were synthesized and characterized by FT-IR, 1H NMR, 13C NMR, and mass (MS-EI) spectral analyses. All the molecules were studied for their interactions with YmaH by molecular docking protocol. Among the tested molecules, compound 7 exhibited a good glide score value of −7.73 with E model value of −95.37. In vitro antibacterial activity of the tested compounds shows improved activity against all the microorganisms used. In particular, compound 3a exhibits marked activity against two microorganisms. In conclusion, molecules posses with electron withdrawing groups exhibits better interactions and good antibacterial activities against Gram-positive and Gram-negative pathogens.
Acknowledgments
The authors thank the management and the authorities of Karunya University, Coimbatore, for their kind support, constant encouragement, and also for providing KSJF fellowship to PR. Our thanks are also extended to SAIF, IIT, Madras, India for NMR and mass spectral analysis.
References
- 1.Shin DY, Kim SM, Chae JH, Hyun SS, Seo SY, Lee YS, Lee KO, Kim SH, Lee YS, Jeong JM, Choi NS, Suh YG. Syntheses and anti-MRSA activities of the C3 analogs of mansonone F, a potent anti-bacterial sesquiterpenoid: insights into its structural requirements for anti-MRSA activity. Bioorg Med Chem Lett. 2004;14:4519–4523. doi: 10.1016/j.bmcl.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 2.Batra M, Kriplani P, Batra C, Ojha KG. An efficient synthesis and biological activity of substituted p-benzoquinones. Bioorg Med Chem. 2006;14:8519–8526. doi: 10.1016/j.bmc.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 3.Anthony RA, Greg GO, Udo B, Peter S, Larry WR. Metabolic activation of PCBs to quinones: reactivity toward nitrogen and sulfur nucleophiles and influence of superoxide dismutase. Chem Res Toxicol. 1996;9:623–629. doi: 10.1021/tx950117e. [DOI] [PubMed] [Google Scholar]
- 4.Brien JOP. Molecular mechanisms of quinone cytotoxicity. Chem Biol Interact. 1991;80:1–41. doi: 10.1016/0009-2797(91)90029-7. [DOI] [PubMed] [Google Scholar]
- 5.Ganapaty S, Thomas PS, Karagianis G, Waterman PG, Brun R. Antiprotozoal and cytotoxic naphthalene derivatives from Diospyros assimilis. Phytochemistry. 2006;67:1950–1956. doi: 10.1016/j.phytochem.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 6.Silva TMS, Camara CS, Barbosa TP, Soares AZ, Cunha LC, Pinto AC, Vargas MD. Molluscicidal activity of synthetic lapachol amino and hydrogenated derivatives. Bioorg Med Chem. 2005;13:193–196. doi: 10.1016/j.bmc.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 7.Biot C, Bauer H, Schirmer RH, Charret ED. 5-Substituted tetrazoles as bioisosteres of carboxylic acids. Bioisosterism and mechanistic studies on glutathione reductase inhibitors as antimalarials. J Med Chem. 2004;47:5972–5983. doi: 10.1021/jm0497545. [DOI] [PubMed] [Google Scholar]
- 8.Mantyla A, Rautio JTG, Nevalainen T, Vepsalainen J, Koskinen A, Croft SI, Jarvinen T. Synthesis, in vitro evaluation, and antileishmanial activity of water-soluble prodrugs of buparvaquone. J Med Chem. 2004;47:188–195. doi: 10.1021/jm030868a. [DOI] [PubMed] [Google Scholar]
- 9.Tandon VK, Maurya HK, Tripathi A, ShivaKesva GB, Shukla PK, Srivastava A, Srivastava P, Panda D. 2,3-Disubstituted-1,4-naphthoquinones, 12H-benzo[b]phenothiazine-6,11-diones and related compounds: synthesis and biological evaluation as potential antiproliferative and antifungal agents. Eur J Med Chem. 2009;44:1086–1092. doi: 10.1016/j.ejmech.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Tandon VK, Maurya HK, Yadav DB, Tripathi A, Kumar M, Shukla PK. Naphtho[2,3-b][1,4]-thiazine-5,10-diones and 3-substituted-1,4-dioxo-1,4-dihydronaphthalen-2-yl-thioalkanoate derivatives: synthesis and biological evaluation as potential antibacterial and antifungal agents. Bioorg Med Chem Lett. 2006;16:5883–5887. doi: 10.1016/j.bmcl.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez PI. Mechanism(s) of bioreductive activation: the example of diaziquinone (AZQ) Free Radic Biol Med. 1989;6:405–445. doi: 10.1016/0891-5849(89)90087-7. [DOI] [PubMed] [Google Scholar]
- 12.Osman SAA, Abdalla AA, Alaib MO. Synthesis of Sulfanilamido-Naphthoquinones as potential antituberculous agents. J Pharm Sci. 1983;72:68–71. doi: 10.1002/jps.2600720116. [DOI] [PubMed] [Google Scholar]
- 13.Ravichandiran P, Kannan R, Ramasubbu A, Muthusubramanian S, Samuel VK. Green synthesis of 1,4-quinone derivatives and evaluation of their fluorescent and electro chemical properties. J Saudi Chem Soc. 2012 [Google Scholar]
- 14.Illos RA, Shamir D, Shimon LJW, Zilbermann I, Bittner S. N-Dansyl-carbazoloquinone; a chemical and electrochemical fluorescent switch. Tetrahedron Lett. 2006;47:5543–5546. doi: 10.1016/j.tetlet.2006.05.139. [DOI] [Google Scholar]
- 15.Bernardo PH, Chai CLL, Guen ML, Smith GD, Waring P. Structure–activity delineation of quinones related to the biologically active Calothrixin B. Bioorg Med Chem Lett. 2007;17:82–85. doi: 10.1016/j.bmcl.2006.09.090. [DOI] [PubMed] [Google Scholar]
- 16.Someya T, Baba S, Fujimoto M, Kawai G, Kumasaka T, Nakamura K. Crystal structure of YmaH (Hfq) from Bacillus subtilis in complex with an RNA aptamer. Nucleic Acids Res. 2012;40:1856–1867. doi: 10.1093/nar/gkr892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friesner RAO, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein–ligand complexes. J Med Chem. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- 18.Baron EJ, Finegold SM. Bailey and Scott’s diagnostic microbiology. 8. St. Louis: C.V. Mosby; 1990. pp. 184–188. [Google Scholar]
- 19.Massah AR, Adibi H, Khodarahmi R, Abiri R, Majnooni MB, Shahidi S, Asadi B, Mehrabi M, Zolfigol MA. Synthesis, in vitro antibacterial and carbonic anhydrase II inhibitory activities of N-acylsulfonamides using silica sulfuric acid as an efficient catalyst under both solvent-free and heterogeneous conditions. Bioorg Med Chem. 2008;16:5465–5472. doi: 10.1016/j.bmc.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Andrews JMJ. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48:5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]