Abstract
Sudden cardiac death (SCD) remains a daunting problem. It is a major public health issue for several reasons: from its prevalence (20% of total mortality in the industrialized world) to the devastating psycho-social impact on society and on the families of victims often still in their prime, and it represents a challenge for medicine, and especially for cardiology. This text summarizes the discussions and opinions of a group of investigators with a long-standing interest in this field. We addressed the occurrence of SCD in individuals apparently healthy, in patients with heart disease and mild or severe cardiac dysfunction, and in those with genetically based arrhythmic diseases. Recognizing the need for more accurate registries of the global and regional distribution of SCD in these different categories, we focused on the assessment of risk for SCD in these four groups, looking at the significance of alterations in cardiac function, of signs of electrical instability identified by ECG abnormalities or by autonomic tests, and of the progressive impact of genetic screening. Special attention was given to the identification of areas of research more or less likely to provide useful information, and thereby more or less suitable for the investment of time and of research funds.
Keywords: Sudden cardiac death, Risk stratification, Electrical instability, Autonomic nervous system, Cardiac function , Genetics
Translational Perspective.
Sudden cardiac death (SCD) continues to be a major public health challenge, representing close to one-fifth of all mortality in industrialized countries and making about half of its victims among people not previously diagnosed with heart disease. Currently, applied techniques of risk assessment identify only a very small portion of all future cardiac arrests with sufficient specificity to justify defibrillator therapy and even in this small subgroup medical and economic reasons call for improving the positive predictive value of our methods. Better information about the incidence of SCD in different populations is urgently needed. Cardiovascular risk scores must be applied routinely in middle-aged persons and research is necessary to determine which further tests are effective in people without cardiovascular symptoms when risk scores remain high despite treatment. Integrated models for SCD risk must be developed and tested that combine various risk markers as identified for instance in the ECG, in autonomic tests, in cardiac function tests and in genetic profiles, where progress is rapid.
Introduction
Death from cardiac disease has been diminishing in the industrialized world during the last two decades.1–4 Nevertheless, ∼20% of all deaths still occur suddenly and unexpectedly, most often caused by ventricular fibrillation (VF) or asystole. However, precise figures are lacking for many regions of the world.5 It is estimated that sudden cardiac death (SCD) claims ∼10 times as many lives as do traffic accidents in the EU and USA combined, emphasizing the importance of this societal challenge and of efforts to improve SCD risk stratification.
Sudden cardiac death occurs in different population groups: (i) a large subset without a prior diagnosis of heart disease; (ii) patients with a history of heart disease with no or mild cardiac dysfunction; (iii) patients with a history of heart disease and severe cardiac dysfunction; and (iv) those diagnosed with a defined genetically based cause for a life-threatening cardiac arrhythmia (Table 1). The majority of SCD victims is not known to have had heart disease, or have heart disease with a normal or mildly impaired cardiac function (left ventricular ejection fraction, LVEF >40%). Our ability to recognize their risk before the event is severely limited. This raises the question whether our methods for prediction of SCD can be improved now and in the future, in order to develop appropriate preventive measures for this large number of potential victims.
Table 1.
The different groups that contribute to the total number of sudden cardiac deaths and our current ability to identify possible candidates before the event
| % of all SCD | Predictability | |
|---|---|---|
| Not diagnosed with heart disease | 45 | Poor |
| History of heart disease: LVEF >40% | 40 | Limited |
| History of heart disease: LVEF <40% | 13 | Possible |
| Genetically based arrhythmic disease | 2 | Limited |
SCD, sudden cardiac death; LVEF, left ventricular ejection fraction.
In the following sections, we will consider how the risk of SCD can be assessed in those without a history of cardiac disease, how signs of electrical instability on the ECG may predict SCD, how results of autonomic nervous system (ANS) tests may be used, how pump function influences SCD risk and how genetic screening may help predict SCD.
No previous history of cardiac disease
Between 45 and 50% of SCD victims are not previously diagnosed with heart disease.6–8 Most have coronary artery disease (CAD), while at younger age cardiomyopathies and ion-channelopathies play a major role in cardiac arrest and SCD. The risk of developing a CAD substrate, or expression of a coronary event, can be assessed by using risk scores (such as Framingham, or SCORE) based on age, gender, smoking, blood pressure, total and LDL cholesterol, body mass index, and diabetes.9,10 Some risk scores have added other lipid measurements, socio-economic factors or a family history of heart disease. It should be appreciated that the relation between levels of LDL cholesterol or blood pressure and subsequent cardiac events varies across countries.11 Therefore, SCORE provides different tables for high- and low-risk countries in Europe.12 The decision which risk calculator should be used has an important impact on risk categorization and absolute risk estimation, with broad implications for guidelines recommending therapies.13 Moreover, as time passes, it is likely that country-specific risk patterns may change, in parallel with emerging socio-economic and healthcare patterns.
On-line access has made these risk scores readily accessible for physician and layman. Primary prevention of cardiovascular disease using validated risk scores is possible, although questions remain about the appropriate intensity of intervention in relation to the level of risk.14,15 The parameters used in the current risk scores are derived from population studies and have limited power for individual risk stratification, with possible exceptions in the highest risk subgroups such as diabetics who smoke and have high blood pressure and LDL cholesterol levels (although having diabetes already puts patients in the high or very high-risk category). Also, these risk markers do not specifically predict SCD, but rather combined cardiac events (Framingham) or cardiovascular mortality (SCORE).
At present risk scores can be used to identify subjects who are most likely to achieve a statistical benefit from preventive medical therapy12 and to motivate individuals to reduce correctable risk factors. This emphasizes the need for public education initiatives creating awareness of easily accessible risk assessment models on the Internet. As a general approach a risk score should be routinely performed around the age of 40 years in males and in post-menopausal women,12 by a first line healthcare professional. Repeat risk assessments are appropriate depending on the presence and severity of risk markers.
The incremental value of a family history of heart disease or SCD and biochemical and genetic markers reflecting atherosclerosis, coagulation, endothelial function, inflammation, oxidative stress, renal function, neuro-humoral status, and cardiac pump function16 to predict cardiovascular events, particularly SCD, needs to be evaluated as a function of age, gender and ethnic background. Risk assessment should be dynamic, repeated over time.17 The risk level at which more advanced diagnostic methods should be performed in the person without a history of heart disease needs to be better defined in the context of clinical and economic efficacy.
The fact that most SCDs occur in individuals without a history of heart disease emphasizes the importance of routinely using accepted risk scores to identify people who will benefit from lifestyle improvement and medical interventions, to motivate them to correct risk factors and to refer them to a cardiologist for additional testing when corrective measures are insufficient.
Electrical instability and sudden cardiac death
A plethora of studies has examined the value of different ECG-derived risk parameters, both in the general population18 and in patients with different cardiac diseases. Their practical value is sometimes unclear because of small sample size or limited follow-up duration, and studies determining which parameter combinations provide strong risk predictors are lacking. Table 2 lists their availability in the 12 lead ECG or Holter recording, and their reported value in people without diagnosed heart disease or in the presence of heart disease with either a preserved or diminished LVEF. When the absolute risk is low, the predictive value of a single test will also be low, although relative risks up to three have been reported for some parameters. Identification of the optimal combination of ECG risk markers remains a challenge, realizing that some give information about an arrhythmia substrate, and others about possible arrhythmia triggers, neural influences, and genetic background.
Table 2.
ECG-derived risk stratifiers reported to have prognostic value in different clinical settings
| No HD diagnosis | preLVEF | redLVEF | 12-lead ECG | Holter | |
|---|---|---|---|---|---|
| Sinus rhythm | |||||
| Resting rate, profile during exercise tolerance test | + | + | ? | + | + |
| Heart rate variability | +/− | + | + | − | + |
| Heart rate turbulence | ? | + | + | − | + |
| Deceleration capacity | ? | + | + | − | + |
| Intra-atrial conduction delay | + | + | + | + | − |
| Atrial dilatation | + | + | + | + | − |
| Atrial fibrillation | + | + | + | + | + |
| AV conduction | |||||
| Site of AV block | + | + | + | + | + |
| Presence of accessory AV pathways | + | ? | ? | + | + |
| QRS | |||||
| Width >100 ms | ? | + | + | + | + |
| Left bundle branch block | + | + | + | + | − |
| Notching, fractionation | ? | + | + | + | − |
| Number and location of Q waves | ? | + | + | + | − |
| Reduced voltage (limb leads) | ? | + | + | + | − |
| Signal-averaged ECG | ? | + | + | + | + |
| Left ventricular hypertrophy | + | + | + | + | − |
| Mean QRS—T angle | + | + | + | + | − |
| QT interval | |||||
| Duration | + | + | + | + | + |
| Dispersion | ? | + | + | + | − |
| Dynamicity | ? | + | + | − | + |
| ST segment | |||||
| Elevation/depression | +/− | ? | ? | + | − |
| Early repolarization (infero-lateral leads) | + | ? | ? | + | − |
| T-wave | |||||
| Axis | + | + | + | + | − |
| Negativity | + | + | + | + | + |
| T-wave alternans | ? | + | + | − | + |
| Tpeak–Tend interval in V5 | ? | + | + | + | − |
| T amplitude V1 and aVR | + | ? | ? | + | − |
| Ventricular ectopy | |||||
| Width and site of origin | + | + | + | + | +/− |
| VPB coupling interval | +/− | + | + | + | + |
| Frequent VPBs | +/− | + | + | + | + |
| Increase during exercise | + | + | + | − | + |
| Non-sustained VT | − | + | + | − | + |
| Sustained VT | ? | + | + | − | + |
No HD DIAGN, no diagnosis of heart disease; LVEF, left ventricular ejection fraction; preLVEF, cardiac disease, preserved LVEF; redLVEF, cardiac disease, reduced LVEF; VPB, ventricular premature beat. The majority of these parameters has been shown to predict mortality, not specifically SCD.
Among a population without clinical evidence of cardiovascular disease sinus rate at rest, during and after exercise or during mental stress,19–22 have been helpful in recognizing SCD risk over a long follow-up period. Risk was markedly elevated (Hazard Ratio between 2 and 6) with a high resting heart rate (>75 b.p.m.), a limited heart rate increase during exercise (<89 b.p.m.), or a sluggish heart rate recovery (<25 b.p.m.). This was interpreted as an impaired ability to increase not only vagal but also sympathetic activity to appropriate levels.19
The usefulness of corrected QT interval (QTc) has been evaluated in prospective cohort studies23 and in subjects >55 years of age a prolonged QTc (>450 ms in men and >470 ms in women) was associated with a three-fold increased risk of SCD.24 A prolonged Tpeak–Tend interval measured in lead V5 seems independently associated with SCD, even when the QTc is normal.25 In a study of 10 864 middle-aged subjects from Finland, prevalence and prognostic significance of early repolarization in the inferior/lateral leads of the 12-lead ECG over a mean follow-up of 30 years was determined.26 When stratified according to the degree of J-point elevation (≥0.2 mV) in inferior and/or lateral leads, there was a three-fold higher risk for both death from cardiac causes and from arrhythmias. In the same database, T-wave inversions, wide QRS-T angle, and prolonged QRS duration due to either left bundle branch block (LBBB) or intraventricular conduction delay but not right bundle branch block, predicted SCD. In similar studies of general population samples27–29 essentially the same ECG variables predicted all-cause mortality and also SCD,18 yet none of them usefully predicts individual risk.
In patients with cardiovascular disease but preserved LV function, several ECG parameters predict the occurrence of cardiac death. This patient group is of particular importance as it generates ∼30–40% of all SCDs. In particular, heart rate turbulence (HRT) and deceleration capacity were shown to select patients with preserved LV function who have similarly high risk of SCD as those with depressed LV function.30 In the combined Finnish and German database of survivors of acute myocardial infarction (AMI), non-sustained ventricular tachycardia (VT) and several measures of heart rate variability predicted SCD among those with preserved left ventricular function.31 However, none of these variables was associated specifically with the occurrence of SCD since they were stronger predictors of non-SCD. Similarly, microvolt T-wave alternans (MTWA) carries prognostic value in patients with preserved LV function,32 but results cannot be obtained in a large group that does not show the required heart rate increase or is in atrial fibrillation. Based on these and similar studies, the REFINE-implantable cardioverter-defibrillator (ICD) trial is currently randomizing infarct survivors with a LVEF between 36 and 49%, reduced HRT and positive MTWA to standard therapy or the ICD.
In patients with cardiovascular disease and impaired LV function parameters such as QRS duration, particularly in the presence of LBBB, HRT, baroreflex sensitivity (BRS), or T-wave alternans are independently associated with increased risk for SCD. The latter may have a particularly high negative predictive value potentially useful in selecting patients unlikely to benefit from device therapy.32
There are limitations of current ECG-derived risk predictors. For instance, some of the more promising methodologies such as HRT or MTWA assessment cannot be applied to patients in atrial fibrillation (which by itself is a risk predictor for SCD). The REFINE and CARISMA33 trials have demonstrated that risk stratification in patients after an acute ischaemic event should be done 6–8 weeks later as tests performed earlier lack strong predictive power, likely due to the fact that there is remodelling of the arrhythmogenic substrate during the early weeks after myocardial infarction. However, this may not apply to autonomic parameters, as demonstrated by the validity of risk stratification performed by BRS assessed 2–3 weeks post-MI in ATRAMI and by similarly early assessment of HRT and deceleration capacity.30,34 Finally, there is a lack of dynamic risk assessment studies over time.17 At present, it remains largely unknown for which time interval a given risk assessment will optimally predict individual risk for SCD.
In patients with genetically based causes for lethal arrhythmias, the evidence that ECG parameters are helpful in risk stratification is clear, particularly for the long QT syndrome (LQTS), and partially for the Brugada syndrome. This is well established for the magnitude of QT interval prolongation,35 whereas the evidence of a risk predictive role for heart rate is more recent and limited to specific genetic subgroups.36
To prospectively determine the best risk score using ECG parameters, listed in Table 2 for the four groups from Table 1, remains a challenge. It seems that static, ECG-based parameters as well as dynamic metrics of RR interval patterns, and of ventricular repolarization can be useful for risk stratification in all groups. Further research is needed into the dynamic behaviour of parameters and into the predictive power of parameter combinations.
Risk prediction based on autonomic abnormalities
The ANS helps maintain body homoeostasis. Autonomic nervous system abnormalities may be caused directly by autoimmune diseases and metabolic neuropathies, such as diabetes mellitus,37,38 or indirectly by the ANS response to disturbed homoeostasis, as in congestive heart failure (HF).39 Autonomic nervous system imbalance may cause clinically relevant perturbations in cardiac physiology. In cardiac patients, the strength of the association between ANS abnormalities, poor outcome, and cause-specific mortality, namely SCD, likely depends upon the underlying cardiac condition and upon the presence of diabetes or renal insufficiency.40 Although obviously important, genetic predisposition to ANS abnormalities is still poorly understood. Experimental and clinical data show that enhanced parasympathetic influence on the heart is generally antiarrhythmic and antifibrillatory41,42 while increased sympathetic influence is generally pro-arrhythmic.42–44 In particular, sympathetic dominance combined with other pro-arrhythmic processes such as myocardial ischaemia leads to increased probability of VF and thus SCD risk.43 Specific autonomic tests distinguishing the risk of arrhythmic vs. asystolic SCD are desirable but not developed.
Autonomic tests45 include recording and analysis of spontaneous RR intervals, simple or complex provocations such as the Valsalva manoeuvre, handgrip test, cold face test, carotid massage, neck suction, low body pressure test, and baroreflex testing. While exploring different reflexes, involving different aspects of autonomic modulation, all of them measure RR interval and blood pressure responses.
Clinical exploration of autonomic markers for SCD risk stratification has been largely obtained from studies involving autonomic modulation of RR interval changes following either provoked or spontaneously occurring blood pressure changes to assess baroreflex responsiveness,46,47 and has been fostered mainly by pioneering studies of BRS in a conscious post-MI canine model for SCD.48 A particularly powerful risk prediction for cardiac death and life-threatening arrhythmias in ischaemic heart disease and HF has been obtained with BRS48–51 and with tests separating the sympathetic and vagal control of sinus node activity, such as with HRT and deceleration capacity.30,52,53 Autonomic nervous system function can also be assessed from heart rate responses to exercise testing. Heart rate recovery after exercise has prognostic significance for both total and sudden cardiac mortality.54,55
Validated models of spectral analysis of RR intervals permit assessing the strength of cardiac sympathetic and vagal modulations, especially when measuring responses to well-defined provocations.56,57 The environmental data stability and accuracy of the analyses, including rejection of ectopic beats, are essential in such studies.
Despite the large number of existing ANS function tests, little comparative data exist regarding their predictive value. No guidance exists to which ANS test or combination of tests is most appropriate in different clinical conditions. Advanced age decreases but not eliminates the value of ANS tests in predicting poor outcome, as shown with baroreflex testing in elderly post-MI patients.58 Autonomic nervous system tests are not influenced by usual clinical doses of beta-blockers,52,59 whereas sleep abnormalities affect autonomic testing based on full 24-h data.60
A consensus exists that the risk prediction power of ANS tests increases when quantifying autonomic responses to specific provocations rather than studying unprovoked baseline ANS function. Not only the provocations underlying a test but also its environmental conditions must be standardized: while the predictive power of 24-h RR interval variability in hospitalized cardiac patients has been well documented, similar analysis of recordings in truly ambulating out-of-hospital patients is of little value because of the differences in environmental challenges to which the ANS responds. Short-duration tests should become the standard for ANS testing in cardiac patients for risk prediction.61,62 For studies of RR interval dynamicity, a combination of sufficiently long-resting period (e.g. 20–30 min) with a strictly standardized staged exercise test (including its recovery phase or tilting) might prove useful,63,64 as well as tilt or simple postural provocations.
Autonomic nervous system-based risk assessment has been attempted in patients without cardiovascular disease but its predictive value is likely too low to make such studies useful for first line SCD risk-screening.
The predictive value of ANS testing in cardiac patients appears independent of ventricular function65 and can be thus used in patients with and without compromised LVEF.30,46 Tests involving RR interval measurements (either alone or in combination with blood pressure measurements), however, require normal sinus node function.
Indices of the QT interval variability and other ventricular repolarization markers may allow investigating ANS influence at the ventricular level and can be used to quantify autonomic function66–68 providing information probably complementary to that derived from heart rate variability. The measurement of QT variability, alone or simultaneously with RR variability, may improve risk stratification in patients with life-threatening arrhythmic diseases, specifically those with the LQTS.69
Autonomic nervous system-based risk assessment in patients with genetically based cardiac diseases is in its infancy but preliminary encouraging data are being obtained. Hyperreactive ANS reflexes leading to very abrupt RR interval changes in either direction appear to pose an increased risk in those LQTS patients (LQT1) whose genetic mutations impair the IKs current and who are thereby unable to appropriately adapt their QT interval to abrupt heart rate changes.70 This is an example of how ANS responses may dangerously interact with underlying genetic abnormalities.
Regional abnormalities in cardiac sympathetic innervation are associated with increased arrhythmic risk and they can be quantified by imaging techniques. Recently, the 123-I MIBG defect score was found to independently predict ventricular arrhythmias causing appropriate ICD shocks.71 The combination of traditional markers of ANS activity with imaging techniques quantifying the degree of sympathetic denervation at ventricular level appears promising.
Autonomic nervous system markers contribute to SCD risk stratification. Arrhythmic risk is enhanced whenever markers of vagal activity decrease or markers of sympathetic activity increase. Both RR and QT variability may provide useful and complementary information. Reflex autonomic responses are more informative than baseline measurements. The limited use of ANS tests is partially due to their complexity. Analyses derived from tests as simple as an exercise stress test are more likely to impact on clinical practice.
Role of cardiac function
Left ventricular function as reflected by ejection fraction, as well as functional capacity, expressed by a variety of parameters (NYHA class, maximum oxygen uptake, and exercise duration) are directly related to both total mortality and SCD in patients with heart disease. As such, LVEF has been used as a primary entry criterion for multiple clinical trials. In addition, NYHA functional class has been added to LVEF as an entry criterion in trials of SCD in patients with HF. Easy to measure, but with significant errors depending on the method used, LVEF now occupies a central position in guidelines for use of ICDs when recommended for primary prevention of SCD. The rationale is that LVEF significantly modifies the effects of all other variables that impact on both total mortality and SCD risk. However, there are multiple limitations to the use of LVEF as the sole determinant of high risk and, therefore, as indication for ICD: (i) the population with HF and reduced (<30 or 35%) LVEF (HFrLVEF) accounts for <20% of all SCDs;8,72 (ii) there is no evidence that LVEF bears a direct causal relation to arrhythmia mechanisms; (iii) LVEF exhibits considerable spontaneous variability in some individuals; (iv) measurement of LVEF in practice is often less than precise. Furthermore, recent data indicate that most of SCD victims did not have a previously documented depressed LVEF, and would have not been candidates for ICD therapy.73,74
Among survivors of AMI whether in the pre- or post-reperfusion era,75 low LVEF (cutoffs 30–40%) has accounted for 22–72% of SCD cases. The relation between LVEF and mode of death (SCD vs. non-SCD) in patients with CAD shows no significant difference in per cent of SCD for any range of LVEF.8 Similar findings characterize patients with HF in the CHARM programme.76 Thus, LVEF lacks both sensitivity and specificity for prediction of SCD risk.77,78
Multiple factors other than LVEF and NYHA class, and their interaction, may contribute useful information towards prediction of SCD risk. Another important caveat is that not all SCDs are due to arrhythmia. Non-arrhythmic causes are found by autopsy in 50% of SCD cases in patients with recent MI, and there is autopsy evidence of acute coronary events in 54% of SCD cases with CAD and even in 5% of SCD cases in patients without CAD.79 In 97% of cases, the AMI was not diagnosed clinically ante-mortem. Of interest in relation to the diagnostic accuracy of autopsy is that post-mortem MRI results in a higher diagnosis of peracute infarction as possible cause of SCD.80 This also indicates the feasibility of a post-mortem MRI in the absence of consent for clinical autopsy. Also the severity of HF, as judged by NYHA class, does not correlate with mode of death if one examines only patients with LVEF ≤40%.81 However, the relative incidence of SCD ( vs. death due to progressive HF) in patients with HF and preserved LVEF (HFpLVEF) is roughly half of that in patients with HF and reduced LVEF (HFrLVEF).82,83 In contrast, the CHARM studies showed no significant difference in relative incidence of SCD vs. progressive HF death for patients with HFpLVEF vs. HFrLVEF.76 On balance, it appears that total mortality and SCD rates are somewhat lower in patients with HFpLVEF than in patients with HFrLVEF.84
Other demographic characteristics of SCD are also important to place the relative importance of HF in perspective. In the Maastricht prospective registry, only 26% of SCD cases had a history of HF.72,74 The underlying cause of HF (coronary vs. non-CAD cardiomyopathy) influences mechanisms of arrhythmias, and mortality risk, with most studies finding significantly higher mortality in patients with CAD.85 Unfortunately, although approximately 50% of SCDs occur in persons with HF and an LVEF >35%, there are little data in this group and no variables have proved useful to identify individuals at increased risk specifically for SCD.82,86–88 However, several small studies of patients with recent AMI and LVEF >35–40% have reported results that suggest potential utility for risk stratification for mortality and possibly SCD using additional diagnostic tests such as T-wave alternans,89 BRS,46 and HRT.90
Predictors of SCD in patients with HFrLVEF have been sought for years. Single risk factors in this population have limited utility, while analysis of multiple risk factors performs far better for prediction of total mortality and SCD, as it happens in patients with CAD.91 The most widely used model is the Seattle Heart Failure Model. This algorithm uses multiple readily available clinical variables to predict mortality.92 Retrospectively applied to a large population of patients enrolled in six clinical trials of pharmacological HF therapy,93 it did identify patients at increased risk for total mortality but it had limited accuracy to predict SCD.
Of interest is the emergence of biochemical markers as potential risk stratifiers in patients with HF or CAD. A number of studies evaluated the relation between BNP and/or N-terminal of the prohormone brain natriuretic peptide (NTproBNP) and SCD in both HF populations and post-MI patients94,95 and noted significant associations between BNP and SCD or ventricular arrhythmias detected by ICD.96 Of note, a retrospective analysis of patients who had received ICDs for primary prevention of SCD found not only a significant association between NTproBNP and appropriate ICD therapies, but patients with higher levels of BNP had a significantly higher increase in their risk for appropriate ICD intervention than in their mortality risk.96 This suggests BNP may have utility in identifying patients specifically at risk for SCD and sustained VT. Other biochemical markers being actively investigated for utility in risk stratification of HF or post-MI patients include markers of collagen turnover, and C-reactive protein.97 There is far less clinical experience with these markers than BNP, and more data are needed before their potential role is clear.
In view of our current inaccurate SCD prediction, apart from LVEF and NYHA class, the role of other factors needs to be evaluated prospectively. This holds both for the HF patient with reduced or preserved LVEF.
Role of genetics
Genetic screening has the potential of contributing significantly to the identification of individuals at greater risk for SCD. A few concepts need clarification before examining its role in genetically based disorders, in myocardial ischaemia-related SCD, and in the general population.
Genetic variants may be rare (<1 in 1000), common (> 1 in 100), or of intermediate frequency. The greatest disease effect is caused by rare variants (mutations), whereas the common ones (single nucleotide polymorphisms, SNPs) have been associated with modest or minimal effects. Genetically based disorders—such as LQTS, Brugada syndrome, and catecholaminergic polymorphic VT—are due to disease-causing mutations, whereas in complex diseases (MI and CHF) an interaction likely takes place between the arrhythmogenic substrate (scar and dilated ventricles) and several SNPs, each contributing to a decrease or increase in cardiac electrical stability. These relatively common genetic variants capable of modifying the propensity to life-threatening arrhythmias are referred to as ‘modifier genes’. Their effects may help understanding why patients with the same disease and similar clinical parameters may either survive long term or die suddenly.
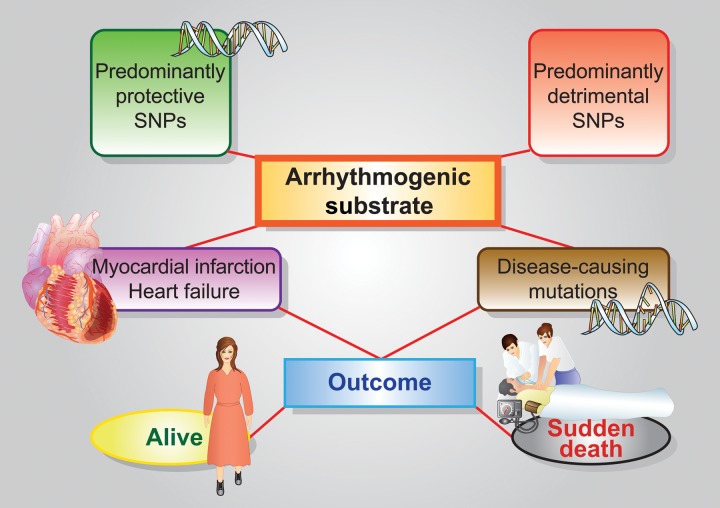
Genetic variants are not immutable. There is growing evidence, encompassed by epigenetics, that environmental factors (age, food, pollution, and radiation) may modify clinical expression of SNPs. A critical concept is that of the algebraic summation of different SNPs. Each SNP has, by definition, a small effect and is thus unlikely to play a major role by itself. The number of SNPs potentially interacting with either the disease-causing mutation or with the arrhythmogenic substrate is large, however, and a cumulative effect must be expected. Protective and arrhythmogenic SNPs may annul each other but if an individual inherits a series of SNPs acting predominantly in one direction, there may be an important effect on clinical outcome (Figure 1), and initial examples have been published.98 This cumulative effect, depending on which SNPs are inherited, represents the unpredictable play of chance. The progressive identification of SNPs acting on cardiac electrical stability will allow to significantly refine risk stratification by the use of ‘clusters of SNPs’. This should be an area of expanding research.
Figure 1.
Illustration of the potential impact on outcome (survival vs. sudden death) of the interaction between two arrhythmogenic substrates (acute myocardial infarction or heart failure, and mutations causing arrhythmogenic diseases) and predominantly protecting or damaging clusters of common genetic variants (SNPs). As the cluster of SNPs of a given individual reflects the inheritance by the parents, this interaction is clearly governed by chance.
Two additional concepts are relevant. The probability of identifying modifier genes increases whenever the disease-causing mutation is the same in the population under study, as it happens in the so-called ‘founder populations’, because this approach corrects for the different effects produced by different individual mutations.99 Also, research in this field must deal with clean phenotypes. Regarding SCD, this requires that the population studied must be at risk of dying because of a primary lethal arrhythmia related to a single trigger (e.g. acute myocardial ischaemia) and not as a consequence of VF resulting from progressively worsening cardiac function.
Based upon this conceptual foundation, we can discuss where we stand and where we should go for a more fruitful approach to risk stratification for SCD.
Long QT syndrome, with 80–85% success in positive genotyping, is the best understood among the genetically based channelopathies100 and serves as an appropriate paradigm for SCD. Risk stratification in LQTS has progressively incorporated clinical parameters (e.g. previous occurrence of syncope, QTc >500 ms), specific genetic subgroups (LQT1 vs. LQT2 vs. LQT3), the mutation site (e.g. transmembrane vs. C-terminal), the type of mutation (missense vs. non-missense), the biophysical function (dominant negative vs. haploinsufficiency), and mutation-specific consequences. The focus is now on the interaction between the disease-causing mutations and the SNPs which have already been found to modify the arrhythmic risk. Most of them increase risk,101 and sometimes QT interval duration, but recently also ‘protective’ SNPs have been recognized.102,103 This is a critically important area of research because of its implications for individual risk prediction, both for the inherited channelopathies and SCD generally.
Although a strong genetic component exists in the risk for SCD, typically occurring in individuals over 40 years with cardiac ischaemia or infarction,104–108 the underlying genetic variation remains unknown. Key barriers to the identification of these genetic factors have been restricted sample sizes, heterogeneity of the associated cardiac substrate, and the difficulty of ascertaining adequate phenotype information after cardiac arrest.109 Especially heterogeneity of the substrate hampers successful gene finding in population sample studies as within every individual substrate the interplay of many different pro-arrhythmic factors involves too many potentially causative genes.110 By contrast, VF in the setting of a first AMI is most likely based on re-entry and as such has one underlying arrhythmogenic mechanism. To date, four genome-wide association studies (GWAS) on VF/SCD have been published111–114 and one of the two SNPs that were identified in GWAS studies comes from AGNES, a study in patients with and without VF in the setting of a first AMI.111 A stark contrast is represented by studies based on mixed phenotypes as in ICD patients using appropriate shocks as endpoint.115–117 Such patients are at risk of VF through many different mechanisms (pump failure in a scarred ventricle, different medications, electrolyte disorders, etc.) and the hope to identify single genetic variants predicting SCD in these populations is probably naive. Future research and funding should go for studies with a clean phenotype and a homogeneous underlying arrhythmia mechanism likely to provide the highest yield.
An alternative approach to unravel the genetic basis of SCD is to identify genetic variants associated with phenotypes that are considered as risk factors for SCD, i.e. ‘intermediate phenotypes’, such as heart rate, QRS width, and QT interval. Genome-wide association studies have identified numerous ‘hits’,110 but attempts to link them to SCD have so far not been successful.112,117,118
Genetic screening has a high potential to contribute to the identification of individuals at risk for SCD, as has already been proved for genetically based diseases. There is a strong rationale for expecting significant insights also for SCD in the general population but this will require studying homogeneous phenotypes. The use of novel technologies including next generation sequencing119 and the smart analysis of clusters of common genetic variants, as risk modifiers, hold significant promise.
Conclusions and recommendations
Both national and regional SCD registries are needed with data about medical history, gender, and race. They must allow the evaluation of the effects of different measures taken to reduce out of hospital cardiac arrest mortality to increase insight of their value.
For the population without a history of heart disease, more attention should be given to public education initiatives, addressing awareness of risk for development of coronary disease as a cause of early death, including SCD.
Primary healthcare providers should be strongly encouraged to apply risk scores routinely, both in men over 40 years of age and in women after the onset of menopause. There is also need for a comparison of the value of the different risk score methods. The estimated level of risk can be used in a dynamic way17 to determine whether and when advanced diagnostics are appropriate. The incremental value of bio- and genetic markers reflecting atherosclerosis, coagulation, inflammation, neuro-humoral status, and ventricular function requires evaluation in this population, in the context of clinical and cost efficiency.
Current guidelines recommend 12-lead ECG screening for certain high-risk occupations and for professional athletes, but not for all asymptomatic middle-aged subjects. Since many of ECG indexes have the potential to identify future arrhythmic death, the utility of routine screening of the 12-lead ECG should be explored from existing databases, especially considering value and cost of analysing a 12-lead ECG in addition to a standard risk score. The main rationale of this approach is the identification of subclinical cardiac disease, such as asymptomatic myocardial infarction, left ventricular hypertrophy, and fibrosis, which are common autopsy observations in victims of SCD even in the absence of previously diagnosed cardiac disease.
In patients with cardiac disease and preserved or reduced ventricular function, we need large registries and randomized trials to determine the value of ECG markers for risk assessment.
Autonomic balance affects the cardiac response to acute challenges such as ischaemia or tachyarrhythmia, thus importantly influencing outcome. The large number of proposed tests and the inability to standardize acquisition and analysis of parameters have contributed to preventing ANS-based tests becoming routinely used for the clinical assessment of SCD risk. This is unfortunate because some tests, especially those quantitating reflex responses, are of proven value. Future approaches should focus on well-standardized tests that are easily obtained and can be readily interpreted. Long-term ambulatory monitoring no longer represents a favoured approach for ANS assessment. Autonomic responses during exercise testing or other provocative manoeuvers under standardized circumstances will likely provide optimal information but this requires prospective evaluation.
Measurement of resting systolic function (LVEF) and assessment of clinical severity of HF (NYHA Class) are the only risk stratifiers currently in widespread use. While they are good predictors of total mortality, their sensitivity and specificity for prediction of SCD is not adequate. Current practice guidelines for implantation of ICDs for primary prevention of SCD, based on these parameters, have demonstrated their clinical and cost-effectiveness but they do not impact the majority of patients who will die suddenly as a first cardiac event or during the onset of AMI.74,120 Apart from LVEF and NYHA Class, many other factors are probably important for the SCD risk profile, such as age, gender, ethnicity, blood pressure and heart rate, ischaemic vs. non-ischaemic cause, diabetes, kidney function, as well as findings during cardiac imaging, electrical instability, ANS balance, biochemical markers, and the genetic profile. Whether the significance of these factors is affected by treatment and by time needs to be evaluated.
At present, there is scant information about SCD risk in patients with HFpLVEF. Newer functional markers of pump failure, such as BNP, show promise as indicators of SCD risk, but prospectively well-designed clinical trials evaluating their ability to guide ICD therapy are needed, before they can be recommended.
While individual risk markers often lack the power to influence clinical practice, it seems important to investigate integrated risk models, combining parameters that characterize properties of the arrhythmia substrate, the triggers and the ANS.
The genetic variants, identified thus far either as modulators of ECG traits or of SCD, have proved useful for genetically based diseases such as LQTS, but are not ready to be used clinically for assessing genetic susceptibility to SCD in the general population. Yet, genetic factors can contribute significantly to this huge health problem. The areas where research should expand, for further refinement of risk stratification, include the use of clusters of relatively common or even rare genetic variants (SNPs) capable of increasing or decreasing the risk for SCD associated either to a disease-causing mutation or to an arrhythmogenic substrate.
Worldwide collaboration is needed in order to increase sample sizes and consequently statistical power for detecting more variants. Currently, the published efforts to gain statistical power have been at the expense of phenotypic homogeneity (unexplained SCD in the community or post-MI ICD patients). Instead, to further the field it will probably be ideal to use whole exome or whole genome sequencing in large cohorts with a clean phenotype associated with SCD, possibly through just one or two well-defined arrhythmogenic mechanisms.
When designing prognostic models for assessing SCD risk or clinical studies for demonstrating ICD benefit, it is important to consider that risk factors may change over time and that patients may be at a substantial risk of dying from other causes than the one intended to be treated. Modern statistical techniques such as time-dependent covariates in Cox proportional hazards models and cause-specific hazard (competing risks) modelling must be used to address such challenges in risk stratification.
Table 3 summarizes the currently recommended approach to SCD risk stratification as well as our suggestions for areas of research. We feel that better methods for identification of high SCD risk are especially needed following AMI, either by combining presently available risk markers or by developing new ones. Genetic studies already provided markers for arrhythmia risk but thus far only in small subgroups of the population.
Table 3.
Our current approach to sudden cardiac death risk stratification and areas for research
| Based on what we know today | |
| No diagnosis of cardiovascular disease |
|
| History of heart disease, no or mild dysfunction |
|
| History of heart disease, reduced LV function |
|
| Genetically based pro-arrhythmic disorders |
|
| What we recommend for research | |
| In general |
|
| No diagnosis of cardiovascular disease |
|
| History of heart disease, no or mild dysfunction |
|
| History of heart disease, reduced LV function |
|
| Genetically based pro-arrhythmic disorders | Further define different phenotypes, improve risk stratification, knowledge of modifiers, and gene therapy |
It is obvious that we still have a lot to learn on how to make a dependable risk profile for individual patients. When realizing our current inability to identify most cardiac arrest victims before the event, we should also consider the development of better strategies to improve results of SCD resuscitation attempts. This will require public education and training, better emergency response systems, and probably the development of minimally invasive devices that upon detection of VF sound an audible alarm and provide the victim's location to emergency services.121 Meanwhile, progress will also continue to depend on the willingness of governments worldwide, through their funding agencies, as well as philanthropic foundations and interested corporate sectors, to accelerate a well-focused effort in this direction.
Funding
This study was supported by Sigrid Juselius Foundation, Helsinki Finland, and the Foundation for Cardiovascular Research, Helsinki, Finland (H.V.H.); CTMM, the Center for Translational Molecular Medicine (www.ctmm.nl), project COHFAR (grant 01C-203) (A.A.W.). Funding to pay the Open Access publication charges for this article was provided by Medtronic.
Conflict of interest: member of the advisory board of Sorin (A.A.W.). Consultancy to Medtronic and St Jude (H.J.J.W.). Retired Medtronic employee, Medtronic shareholder (F.W.L.).
Acknowledgements
The authors are grateful to Pinuccia De Tomasi for her expert editorial support and effort in coping with the many versions of this manuscript.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela MB, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nicol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein JL, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Executive Summary: Heart Disease and Stroke Statistics-2013 Update. A report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe: epidemiological update. Eur Heart J. 2013;34:3028–3034. doi: 10.1093/eurheartj/eht356. [DOI] [PubMed] [Google Scholar]
- 3.Nichols M, Townsend N, Scarborough P, Rayner M. Trends in age-specific coronary heart disease mortality in the European Union over three decades: 1980–2009. Eur Heart J. 2013;34:3017–3027. doi: 10.1093/eurheartj/eht159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danchin N, Puymirat E, Simon T. The (possibly) deceptive figures of decreased coronary heart disease mortality in Europe. Eur Heart J. 2013;34:3014–3016. doi: 10.1093/eurheartj/eht362. [DOI] [PubMed] [Google Scholar]
- 5.Pagidipati NJ, Gaziano TA. Estimating deaths from cardiovascular disease. A review of global methodologies of mortality measurement. Circulation. 2013;127:749–756. doi: 10.1161/CIRCULATIONAHA.112.128413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death: structure, function, and time dependence of risk. Circulation. 1992;85(Suppl. 1):2–10. [PubMed] [Google Scholar]
- 7.De Vreede-Swagemakers J, Gorgels A, Dubois-Arbouw W, Daemen M, van Ree J, Houben L, Wellens HJJ. Out-of-Hospital cardiac arrest in the 1990's. A population based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol. 1997;30:1500–1505. doi: 10.1016/s0735-1097(97)00355-0. [DOI] [PubMed] [Google Scholar]
- 8.Stecker EC, Vickers C, Waltz J, Sosatearu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon sudden unexpected death study. J Am Coll Cardiol. 2006;47:1161–1166. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd-Jones DM, Wilson PW, Larson MG. Framingham risk score and prediction of life time risk for coronary artery disease. Am J Cardiol. 2004;94:20–24. doi: 10.1016/j.amjcard.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Laslett LJ, Alagona P, Clark III BA, Drozda JP, Saldivar F, Wilson SR, Poe C, Hart M. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60(Suppl. S):1–49. doi: 10.1016/j.jacc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Menotti A, Lanti M, Kromhout D, Blackburn H, Jacobs D, Nissinen A, Dontas A, Kafatos A, Nedelikovic S, Adachi H. Homogeneity in the relationship of serum cholesterol to coronary deaths across different cultures: 40-year follow-up of the seven countries study. Eur J Cardiovasc Prev Rehabil. 2008;15:719–725. doi: 10.1097/HJR.0b013e328315789c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvänne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG) European guidelines on cardiovascular disease prevention in clinical practice (version 2012) Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 13.Allan GM, Nouri F, Korownyk C, Kolber MR, Vandermeer B, McCormack J. Agreement among cardiovascular disease risk calculators. Circulation. 2013;127:1948–1956. doi: 10.1161/CIRCULATIONAHA.112.000412. [DOI] [PubMed] [Google Scholar]
- 14.Willis A, Davies M, Yates T, Khunti K. Primary prevention of cardiovascular disease using validated risk scores: a systematic review. J R Soc Med. 2012;105:348–356. doi: 10.1258/jrsm.2012.110193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avery CL, Loehr LR, Baggett C, Chang PP, Kucharska-Newton AM, Matsushita K, Rosamund WD, Heiss G. The population burden of heart failure attributable to modifiable risk factors: the ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol. 2012;60:1640–1646. doi: 10.1016/j.jacc.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Kimmenade RRJ, Januzzi JL., Jr Emerging biomarkers in heart failure. Clin Chem. 2012;58:127–138. doi: 10.1373/clinchem.2011.165720. [DOI] [PubMed] [Google Scholar]
- 17.Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. 2012;125:1043–1052. doi: 10.1161/CIRCULATIONAHA.111.023846. [DOI] [PubMed] [Google Scholar]
- 18.Soliman EZ, Prineas RJ, Case LD, Russell G, Rosamund W, Rea T, Sotoodehnia N, Post WS, Siscovick D, Psaty BM, Burke GL. Electrocardiographic and clinical predictors separating atherosclerotic sudden cardiac death from incident coronary heart disease. Heart. 2011;97:1597–1601. doi: 10.1136/hrt.2010.215871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–1958. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 20.Jouven X, Empana JP, Escolano S, Buyck JF, Tafflet M, Desnos M, Ducimetiere P. Relation of heart rate at rest and long-term (>20 years) death rate in initially healthy middle-aged men. Am J Cardiol. 2009;103:279–283. doi: 10.1016/j.amjcard.2008.08.071. [DOI] [PubMed] [Google Scholar]
- 21.Jouven X, Schwartz PJ, Escolano S, Straczek C, Tafflet M, Desnos M, Empana JP, Ducimetiere P. Excessive heart rate increase during mild mental stress in preparation for exercise predicts sudden death in the general population. Eur Heart J. 2009;30:1703–1710. doi: 10.1093/eurheartj/ehp160. [DOI] [PubMed] [Google Scholar]
- 22.Greenland P, Daviglus ML, Dyer AR, Liu K, Huang C, Goldberger J, Stamler J. Resting heart rate is a risk factor for cardiovascular and noncardiovascular mortality: the Chicago Heart Association Detection Project in Industry. Am J Epidemol. 1999;149:853–862. doi: 10.1093/oxfordjournals.aje.a009901. [DOI] [PubMed] [Google Scholar]
- 23.Montanez A, Ruskin JN, Hebert PR, Lamas GA, Hennekens CH. Prolonged QTc interval and risks of total and cardiovascular mortality and sudden death in the general population. A review and qualitative overview of the prospective cohort studies. Arch Intern Med. 2004;164:943–948. doi: 10.1001/archinte.164.9.943. [DOI] [PubMed] [Google Scholar]
- 24.Straus SMJM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MCJM, Stricker BHC, Witteman JCM. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–367. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 25.Panikkath R, Reinier K, Uy-Evanado A, Teodorescu C, Hattenhauer J, Mariani R, Gunson K, Jui J, Chugh SS. Prolonged Tpeak-to-Tend interval on the resting ECG is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol. 2011;4:441–447. doi: 10.1161/CIRCEP.110.960658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–2537. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- 27.Aro AL, Anttonen O, Tikkanen JT, Junttila MJ, Kerola T, Rissanen H, Reunanen A, Huikuri HV. Intraventricular conduction delay in a standard 12-lead electrocardiogram as a predictor of mortality in general population. Circ Arrhythm Electrophysiol. 2011;4:704–710. doi: 10.1161/CIRCEP.111.963561. [DOI] [PubMed] [Google Scholar]
- 28.Aro AL, Anttonen O, Kerola T, Tikkanen JT, Junttila MJ, Rissanen H, Reunanen A, Huikuri HV. Prevalence and prognostic significance of T-Wave inversions in right precordial leads of a 12-lead electrocardiogram in the middle-aged subjects. Circulation. 2012;125:2572–2577. doi: 10.1161/CIRCULATIONAHA.112.098681. [DOI] [PubMed] [Google Scholar]
- 29.Aro AL, Huikuri HV, Tikkanen JT, Junttila MJ, Rissanen HA, Reunanen A, Anttonen O. QRS-T angle as a predictor of sudden cardiac death in a middle-aged general population. Europace. 2012;14:872–8712. doi: 10.1093/europace/eur393. [DOI] [PubMed] [Google Scholar]
- 30.Bauer A, Barthel P, Schneider R, Ulm K, Müller A, Joeinig A, Stich R, Kiviniemi A, Hnatkova K, Huikuri H, Schömig A, Malik M, Schmidt G. Improved Stratification of Autonomic Regulation for risk prediction in post-infarction patients with preserved left ventricular function (ISAR-Risk) Eur Heart J. 2009;30:576–583. doi: 10.1093/eurheartj/ehn540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mäkikallio TH, Barthel P, Schneider R, Bauer A, Tapanainen JM, Tulppo MP, Schmidt G, Huikuri HV. Prediction of sudden cardiac death after acute myocardial infarction: role of Holter monitoring in the modern treatment era. Eur Heart J. 2005;26:762–769. doi: 10.1093/eurheartj/ehi188. [DOI] [PubMed] [Google Scholar]
- 32.Merchant FM, Ikeda T, Pedretti RF, Salerno-Uriarte JA, Chow T, Chan PS, Bartone C, Hohnloser SH, Cohen RJ, Armoundas AA. Clinical utility of microvolt T-wave alternans testing in identifying patients at high or low risk of sudden cardiac death. Heart Rhythm. 2012;8:1256–1264. doi: 10.1016/j.hrthm.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huikuri HV, Exner DV, Kavanagh KM, Aggarwal SG, Mitchell LB, Messier MD, Becker D, Sheldon RS, Bloch Thomsen PE CARISMA and REFINE Investigators. Attenuated recovery of heart rate turbulence early after myocardial infarction identifies patients at high risk for fatal or near-fatal arrhythmic events. Heart Rhythm. 2010;7:229–235. doi: 10.1016/j.hrthm.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 34.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ for the ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 35.Moss AJ, Schwartz PJ, Crampton RS, Tzivoni D, Locati EH, MacCluer J, Hall WJ, Weitkamp L, Vincent GM, Garson A, Jr, Robinson JL, Benhorin J, Choi S. The long QT syndrome: prospective longitudinal study of 328 families. Circulation. 1991;84:1136–1144. doi: 10.1161/01.cir.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 36.Brink PA, Crotti L, Corfield V, Goosen A, Durrheim G, Hedley P, Heradien M, Geldenhuys G, Vanoli E, Bacchini S, Spazzolini C, Lundquist AL, Roden DM, George AL, Jr, Schwartz PJ. Phenotypic variability and unusual clinical severity of congenital long QT syndrome in a founder population. Circulation. 2005;112:2602–2610. doi: 10.1161/CIRCULATIONAHA.105.572453. [DOI] [PubMed] [Google Scholar]
- 37.Kanda T. Biology of the blood-nerve barrier and its alteration in immune mediated neuropathies. J Neurol Neurosurg Psychiatry. 2013;84:208–212. doi: 10.1136/jnnp-2012-302312. [DOI] [PubMed] [Google Scholar]
- 38.Fleischer J. Diabetic autonomic imbalance and glycemic variability. J Diabetes Sci Technol. 2012;6:1207–1215. doi: 10.1177/193229681200600526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eckberg DL, Drabinsky M, Braunwald E. Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med. 1971;285:877–883. doi: 10.1056/NEJM197110142851602. [DOI] [PubMed] [Google Scholar]
- 40.Barthel P, Bauer A, Müller A, Junk N, Huster KM, Ulm K, Malik M, Schmidt G. Reflex and tonic autonomic markers for risk stratification in patients with type 2 diabetes surviving acute myocardial infarction. Diabetes Care. 2011;34:1833–1837. doi: 10.2337/dc11-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68:1471–1481. doi: 10.1161/01.res.68.5.1471. [DOI] [PubMed] [Google Scholar]
- 42.Lown B, Verrier RL. Neural activity and ventricular fibrillation. N Engl J Med. 1976;294:1165–1170. doi: 10.1056/NEJM197605202942107. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz PJ, La Rovere MT, Vanoli E. Autonomic nervous system and sudden cardiac death. Experimental basis and clinical observations for post-myocardial infarction risk stratification. Circulation. 1992;85(Suppl. I):I77–I91. [PubMed] [Google Scholar]
- 44.Vaseghi M, Ajijola OA, Mahajan A, Shivkumar K. Sympathetic innervation, denervation, and cardiac arrhythmias. Cardiac electrophysiology. 2014:409–417. In Zipes DP, Jalife J, eds From Cell to Bedside, 6th ed. Philadelphia: Elsevier Saunders. [Google Scholar]
- 45.Malik M. Clinical Guide to Cardiac Autonomic Tests. Dordrecht, The Netherlands: Kluwer Academic Publisher; 1998. (Ed). ISBN: 978-90-481-5071-7. [Google Scholar]
- 46.La Rovere MT, Schwartz PJ. Baroreflex sensitivity. In: Zipes DP, Jalife J, editors. Cardiac electrophysiology. From Cell to Bedside. 3rd ed. Philadelphia: WB Saunders Co.; 2000. pp. 771–781. [Google Scholar]
- 47.Barthel P, Bauer A, Muller A, Huster KM, Kanters JK, Paruchuri V, Yang X, Ulm K, Malik M, Schmidt G. Spontaneous baroreflex sensitivity: prospective validation trial of a novel technique in survivors of acute myocardial infarction. Heart Rhythm. 2012;9:1288–1294. doi: 10.1016/j.hrthm.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 48.Billman GE, Schwartz PJ, Stone HL. Baroreceptor reflex control of heart rate: a predictor of sudden cardiac death. Circulation. 1982;66:874–880. doi: 10.1161/01.cir.66.4.874. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz PJ, Vanoli E, Stramba-Badiale M, De Ferrari GM, Billman GE, Foreman RD. Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without a myocardial infarction. Circulation. 1988;78:969–979. doi: 10.1161/01.cir.78.4.969. [DOI] [PubMed] [Google Scholar]
- 50.La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, Bigger JT, Jr, Camm AJ, Schwartz PJ on behalf of the ATRAMI Investigators. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias. Implications for clinical trials. Circulation. 2001;103:2072–2077. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- 51.La Rovere MT, Pinna GD, Maestri R, Robbi E, Caporotondi A, Guazzotti G, Sleight P, Febo O. Prognostic implications of baroreflex sensitivity in heart failure patients in the beta-blocking era. J Am Coll Cardiol. 2009;53:193–199. doi: 10.1016/j.jacc.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 52.Bauer A, Malik M, Schmidt G, Barthel P, Bonnemeier H, Cygankiewicz I, Guzik P, Lombardi F, Müller A, Oto A, Schneider R, Watanabe M, Wichterle D, Zareba W. Heart rate turbulence: standards of measurement, physiological interpretation, and clinical use: International Society for Holter and Noninvasive Electrophysiology Consensus. J Am Coll Cardiol. 2008;52:1353–1365. doi: 10.1016/j.jacc.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 53.Bauer A, Kantelhardt JW, Barthel P, Schneider R, Mäkikallio T, Ulm K, Hnatkova K, Schömig A, Huikuri H, Bunde A, Malik M, Schmidt G. Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: cohort study. Lancet. 2006;367:1674–1681. doi: 10.1016/S0140-6736(06)68735-7. [DOI] [PubMed] [Google Scholar]
- 54.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 55.Johnson NP, Goldberger JJ. Prognostic value of late heart rate recovery after treadmill exercise. Am J Cardiol. 2012;110:45–49. doi: 10.1016/j.amjcard.2012.02.046. [DOI] [PubMed] [Google Scholar]
- 56.Pomeranz M, Macaulay RJB, Caudill MA, Kutz I, Adam D, Gordon D, Kilborn KM, Barger AC, Shannon DC, Cohen RJ, Benson M. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248:H151–H153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 57.Montano N, Gnecchi-Ruscone T, Porta A, Lombardi F, Pagani M, Malliani M. Power spectrum analysis of heart rate variability to assess the changes in sympatho-vagal balance during graded orthostatic tilt. Circulation. 1994;90:1826–1831. doi: 10.1161/01.cir.90.4.1826. [DOI] [PubMed] [Google Scholar]
- 58.De Ferrari GM, Sanzo A, Bertoletti A, Specchia G, Vanoli E, Schwartz PJ. Baroreflex sensitivity predicts long-term cardiovascular mortality after myocardial infarction even in patients with preserved left ventricular function. J Am Coll Cardiol. 2007;50:2285–2290. doi: 10.1016/j.jacc.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 59.Wichterle D, Simek J, La Rovere MT, Schwartz PJ, Camm AJ, Malik M. Prevalent low-frequency oscillation of heart rate: novel predictor of mortality after myocardial infarction. Circulation. 2004;110:1183–1190. doi: 10.1161/01.CIR.0000140765.71014.1C. [DOI] [PubMed] [Google Scholar]
- 60.Stein PK, Pu Y. Heart rate variability, sleep and sleep disorders. Sleep Med Rev. 2012;16:47–66. doi: 10.1016/j.smrv.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 61.La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, Ferrari R, Franchini M, Gnemmi M, Opasich C, Riccardi PG, Traversi E, Cobelli F. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107:565–570. doi: 10.1161/01.cir.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- 62.Malik M. Autonomic tests to detect cardiac risk and their clinical practicality. J Cardiovasc Electrophysiol. 2011;22:128–130. doi: 10.1111/j.1540-8167.2010.01996.x. [DOI] [PubMed] [Google Scholar]
- 63.Crotti L, Spazzolini C, Porretta AP, Dagradi F, Taravelli E, Petracci B, Vicentini A, Pedrazzini M, La Rovere MT, Vanoli E, Goosen A, Heradien M, George AL, Jr, Brink PA, Schwartz PJ. Vagal reflexes following an exercise stress test: a simple clinical tool for gene-specific risk stratification in the long QT syndrome. J Am Coll Cardiol. 2012;60:2515–2524. doi: 10.1016/j.jacc.2012.08.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Falcone C, Buzzi MP, Klersy C, Schwartz PJ. Rapid heart rate increase at onset of exercise predicts adverse cardiac events in patients with coronary artery disease. Circulation. 2005;112:1959–1964. doi: 10.1161/CIRCULATIONAHA.105.545111. Erratum: 2005;112:e295) [DOI] [PubMed] [Google Scholar]
- 65.Odemuyiwa O, Malik M, Farrell T, Bashir Y, Poloniecki J, Camm AJ. Comparison of the predictive characteristics of heart rate variability index and left ventricular ejection fraction for all-cause mortality, arrhythmic events and sudden death after acute myocardial infarction. Am J Cardiol. 1991;68:434–439. doi: 10.1016/0002-9149(91)90774-f. [DOI] [PubMed] [Google Scholar]
- 66.Berger RD, Kasper EK, Baughman KL, Marban E, Calkins H, Tomaselli GF. Beat-to-beat QT interval variability: novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation. 1997;96:1557–1565. doi: 10.1161/01.cir.96.5.1557. [DOI] [PubMed] [Google Scholar]
- 67.Porta A, Tobaldini E, Gnecchi-Ruscone T, Montano N. RT variability unrelated to heart period and respiration progressively increases during graded head-up tilt. Am J Physiol. 2010;298:H1406–H1414. doi: 10.1152/ajpheart.01206.2009. [DOI] [PubMed] [Google Scholar]
- 68.Zabel M, Acar B, Klingenheben T, Franz MR, Hohnloser SH, Malik M. Analysis of 12-lead T-wave morphology for risk stratification after myocardial infarction. Circulation. 2000;102:1252–1257. doi: 10.1161/01.cir.102.11.1252. [DOI] [PubMed] [Google Scholar]
- 69.Bari V, Valencia JF, Vallverdú M, Girardengo G, Marchi A, Bassani T, Caminal P, Cerutti S, George AL, Jr, Brink PA, Crotti L, Schwartz PJ, Porta A. Multiscale complexity analysis of the cardiac control identifies asymptomatic and symptomatic patients in long QT syndrome type 1; PLoS One 2014;9:e93808. doi: 10.1371/journal.pone.0093808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwartz PJ, Vanoli E, Crotti L, Spazzolini C, Ferrandi C, Goosen A, Hedley P, Heradien M, Bacchini S, Turco A, La Rovere MT, Bartoli A, George AL, Jr, Brink PA. Neural control of heart rate is an arrhythmia risk modifier in long QT syndrome. J Am Coll Cardiol. 2008;51:920–929. doi: 10.1016/j.jacc.2007.09.069. [DOI] [PubMed] [Google Scholar]
- 71.Boogers MJ, Borleffs CJ, Henneman MM, van Bommel RJ, van Ramshorst J, Boersma E, Dibbets-Schneider P, Stokkel MP, van der Wall EE, Schalij MJ, Bax JJ. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. J Am Coll Cardiol. 2010;55:2769–2777. doi: 10.1016/j.jacc.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 72.Narayanan K, Reinier K, Uy-Evanado A, Teodorescu C, Chugh H, Marijon E, Gunson K, Jui J, Chugh SS. Frequency and determinants of implantable cardioverter defibrillator deployment among primary prevention candidates with subsequent sudden cardiac arrest in the community. Circulation. 2013;128:1733–1738. doi: 10.1161/CIRCULATIONAHA.113.002539. [DOI] [PubMed] [Google Scholar]
- 73.Albert CM, Stevenson WG. Implantable cardioverter-defibrillators for primary prevention of sudden cardiac death: too little and too late? Circulation. 2013;128:1721–1723. doi: 10.1161/CIRCULATIONAHA.113.005832. [DOI] [PubMed] [Google Scholar]
- 74.Gorgels APM, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJJ. Out-of-hospital cardiac arrest-the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24:1204–1209. doi: 10.1016/s0195-668x(03)00191-x. [DOI] [PubMed] [Google Scholar]
- 75.Ross AM, Coyne KS, Moreyra E, Reiner JS, Greenhouse SW, Walker PL, Simoons ML, Draoui YC, Califf RM, Topol EJ, Van de Werf F, Lundergan CF. Extended mortality benefit of early postinfarction reperfusion. GUSTO-I Angiographic Investigators. Global utilization of streptokinase and tissue plasminogen activator for occluded coronary arteries trial. Circulation. 1998;97:1549–1556. doi: 10.1161/01.cir.97.16.1549. [DOI] [PubMed] [Google Scholar]
- 76.Solomon SD, Wang D, Finn P, Skali H, Zornoff L, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Pocock S, Pfeiffer MA. Effect of candesartan on cause-specific mortality in heart failure patients. The Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) Program. Circulation. 2004;110:2180–2183. doi: 10.1161/01.CIR.0000144474.65922.AA. [DOI] [PubMed] [Google Scholar]
- 77.Yap YG, Duong T, Bland JM, Malik M, Torp-Pedersen C, Kober L, Gallagher MM, Camm AJ. Optimising the dichotomy limit for left ventricular ejection fraction in selecting patients for defibrillator therapy after myocardial infarction. Heart. 2007;93:832–836. doi: 10.1136/hrt.2006.102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dagres N, Hindricks G. Risk stratification after myocardial infarction: is left ventricular ejection fraction enough to prevent sudden cardiac death? Eur Heart J. 2013;34:1964–1971. doi: 10.1093/eurheartj/eht109. [DOI] [PubMed] [Google Scholar]
- 79.Pouleur AC, Barkoudah E, Uno H, Skali H, Finn PV, Zelenkofske SL, Belenkov Y, Mareev V, Velazques EJ, Rouleau JL, Maggioni AP, Kober L, Califf RM, McMurray JJ, Pfeffer MA, Solomon SD. Pathogenesis of sudden unexpected death in a clinical trial of patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. Circulation. 2010;122:597–602. doi: 10.1161/CIRCULATIONAHA.110.940619. [DOI] [PubMed] [Google Scholar]
- 80.Jackowski C, Schwendener N, Grabherr S, Persson A. Post-mortem cardiac 3-T magnetic resonance imaging visualization of sudden cardiac death? J Am Coll Cardiol. 2013;62:617–629. doi: 10.1016/j.jacc.2013.01.089. [DOI] [PubMed] [Google Scholar]
- 81.Uretsky BF, Thygesen K, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Packer M, Poole-Wilson PA, Ryden L. Acute coronary findings at autopsy in heart failure patients with sudden death. Results from the Assessment of Treatment with Lisinopril and Survival (ATLAS) Trial. Circulation. 2000;102:611–616. doi: 10.1161/01.cir.102.6.611. [DOI] [PubMed] [Google Scholar]
- 82.MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 83.Adabag S, Smith LG, Anand IS, Berger AK, Luepker RV. Sudden cardiac death in heart failure patients with preserved ejection fraction. J Card Fail. 2012;18:749–754. doi: 10.1016/j.cardfail.2012.08.357. [DOI] [PubMed] [Google Scholar]
- 84.Hamaguchi S, Kinugawa S, Sobirin MA, Goto D, Tsuchihashi-Makaya M, Yamada S, Yokoshiki H, Tsutsui H. Mode of death in patients with heart failure and reduced vs. preserved ejection fraction: report from the registry of hospitalized heart failure patients. Circ J. 2012;76:1662–1669. doi: 10.1253/circj.cj-11-1355. [DOI] [PubMed] [Google Scholar]
- 85.Chan MM, Lam CS. How do patients with heart failure with preserved ejection fraction die? Eur J Heart Fail. 2013;15:604–613. doi: 10.1093/eurjhf/hft062. [DOI] [PubMed] [Google Scholar]
- 86.Packer DL, Prutkin JM, Hellkamp AS, Mitchell LB, Bernstein RC, Wood F, Boehmer JP, Carlson MD, Frantz RP, McNulty SE, Rogers JG, Anderson J, Johnson GW, Walsh MN, Poole JE, Mark DB, Lee KL, Bardy GH. Impact of implantable cardioverter-defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure: analysis from the sudden cardiac death in heart failure trial. Circulation. 2009;120:2170–2176. doi: 10.1161/CIRCULATIONAHA.109.853689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Al-Khatib SM, Shaw LK, O'Connor C, Kong M, Califf RM. Incidence and predictors of sudden cardiac death in patients with diastolic heart failure. J Cardiovasc Electrophysiol. 2007;18:1231–1235. doi: 10.1111/j.1540-8167.2007.00957.x. [DOI] [PubMed] [Google Scholar]
- 88.Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, Lopez-Sendon J, Teerlink JR, White M, McMurray JJ, Komajda M, Mackelvie R, Ptaszynska A, Hetzel SJ, Massie BM, Carson PE. Mode of death in patients with heart failure and a preserved ejection fraction: results from the irbesartan in heart failure with preserved ejection fraction study (I-Preserve) trial. Circulation. 2010;121:1393–1405. doi: 10.1161/CIRCULATIONAHA.109.909614. [DOI] [PubMed] [Google Scholar]
- 89.Ikeda T, Yoshino H, Sugi K, Tanno K, Shimizu H, Watanabe J, Kasamaki Y, Yoshida A, Kato T. Predictive value of microvolt T-wave alternans for sudden cardiac death in patients with preserved cardiac function after acute myocardial infarction: results of a collaborative cohort stud. J Am Coll Cardiol. 2006;48:2268–2274. doi: 10.1016/j.jacc.2006.06.075. [DOI] [PubMed] [Google Scholar]
- 90.Bauer A, Barthel P, Muller A, Ulm K, Huikuri H, Malik M, Schmidt G. Risk prediction by heart rate turbulence and deceleration capacity in postinfarction patients with preserved left ventricular function retrospective analysis of 4 independent trials. J Electrocardiol. 2009;42:597–601. doi: 10.1016/j.jelectrocard.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 91.Buxton A, Lee K, Hafley G, Pires LA, Fisher JD, Gold MR, Josephson ME, Lehmann MH, Prystowski EN. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease. Lessons from the MUSTT study. J Am Coll Cardiol. 2007;50:1150–1157. doi: 10.1016/j.jacc.2007.04.095. [DOI] [PubMed] [Google Scholar]
- 92.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton p, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 93.Mozaffarian D, Anker SD, Anand I, Linker DT, Sullivan MD, Cleland JG, Carson PE, Maggioni AP, Mann DL, Pitt B, Poole-Wilson PA, Levy WC. Prediction of mode of death in heart failure: the Seattle Heart Failure Model. Circulation. 2007;116:392–398. doi: 10.1161/CIRCULATIONAHA.106.687103. [DOI] [PubMed] [Google Scholar]
- 94.van Veldhuisen DJ, Linssen GC, Jaarsma T, Van Gilst WH, Hoes AW, Tijssen JG, Paulus WJ, Voors AA, Hillege HL. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61:1498–1506. doi: 10.1016/j.jacc.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 95.Scott PA, Barry J, Roberts PR, Morgan JM. Brain natriuretic peptide for the prediction of sudden cardiac death and ventricular arrhythmias: a meta-analysis. Eur J Heart Fail. 2009;11:958–966. doi: 10.1093/eurjhf/hfp123. [DOI] [PubMed] [Google Scholar]
- 96.Levine Y, Mittleman MA, Rosenberg M, Buxton A. N-terminal pro-B-type natruiuretic peptide is a major predictor of appropriate ICD therapy. Heart Rhythm. 2013;10:A240. doi: 10.1016/j.hrthm.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 97.Blangy H, Sadoul N, Dousset B, Radauceanu A, Fay R, Aloit E, Zannad F. Serum BNP, hs-C-reactive protein, procollagen to assess the risk of ventricular tachycardia in ICD recipients after myocardial infarction. Europace. 2007;9:724–729. doi: 10.1093/europace/eum102. [DOI] [PubMed] [Google Scholar]
- 98.Bezzina CR, Barc J, Mizusawa Y, Remme CA, Gourraud JB, Simonet F, Verkerk AO, Schwartz PJ, Crotti L, Dagradi F, Guicheney P, Fressart V, Leenhardt A, Antzelevitch C, Bartkowiak S, Schulze-Bahr E, Zumhagen S, Behr ER, Bastiaenen R, Tfelt-Hansen J, Salling Olesen M, Kaab S, Beckmann BM, Weeke P, Watanabe H, Endo N, Minamino T, Horie M, Ohno S, Hasegawa K, Makita N, Nogami A, Shimizu W, Aiba T, Frogue P, Balkau B, Lantieri O, Wiese C, Weber D, Wolswinke R, Coronel R, Boukens BJ, Charpentier E, Chatel S, Despres A, Gros F, Kyndt F, Lecointe S, Lindenbaum P, Portero V, Violleau J, Gessler M, Tan HL, Roden DR, Christoffels VM, Le Marec H, Wilde AA, Probst V, Schott JJ, Dina C, Redon R. Common variants at SCN5A/SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat Genet. 2013;45:1044–1049. doi: 10.1038/ng.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schwartz PJ. Sudden cardiac death, founder populations and mushrooms. What is the link with gold mines and modifier genes? Heart Rhythm. 2011;8:548–550. doi: 10.1016/j.hrthm.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 100.Schwartz PJ, Ackerman MJ, George AL, Jr, Wilde AM. Impact of genetics on the clinical management of channelopathies. J Am Coll Cardiol. 2013;62:169–180. doi: 10.1016/j.jacc.2013.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Crotti L, Monti MC, Insolia R, Peljto A, Goosen A, Brink PA, Greenberg DA, Schwartz PJ, George AL., Jr NOS1AP is a genetic modifier of the long-QT syndrome. Circulation. 2009;120:1657–1663. doi: 10.1161/CIRCULATIONAHA.109.879643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Amin AS, Giudicessi JR, Tijsen AJ, Spanjaart AM, Reckman YJ, Klemens CA, Tanck MW, Kapplinger JD, Hofman N, Sinner MF, Müller M, Wijnen WJ, Tan HL, Bezzina CR, Creemers EE, Wilde AAM, Ackerman MJ, Pinto YM. Variants in the 3′ untranslated region of the KCNQ1-encoded Kv7.1 potassium channel modify disease severity in patients with type 1 long QT syndrome in an allele-specific manner. Eur Heart J. 2012;33:714–723. doi: 10.1093/eurheartj/ehr473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duchatelet S, Crotti L, Peat R, Denjoy I, Itoh H, Berthet M, Ohno S, Fressart V, Monti MC, Crocamo C, Pedrazzini M, Dagradi F, Vicentini A, Klug D, Brink PA, Goosen A, Swan H, Toivonen L, Lahtinen A, Kontula K, Shimizu W, Horie M, George AL, Trégouët DA, Guicheney P, Schwartz PJ. Identification of a KCNQ1 polymorphism acting as a protective modifier against arrhythmic risk in the long QT syndrome. Circ Cardiovasc Genet. 2013;6:354–361. doi: 10.1161/CIRCGENETICS.113.000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 105.Friedlander Y, Siscovick DS, Weinmann S, Austin MA, Psaty BM, Lemaitre RN, Arbogast P, Raghunathan TE, Cobb LA. Family history as a risk factor for primary cardiac arrest. Circulation. 1998;97:155–160. doi: 10.1161/01.cir.97.2.155. [DOI] [PubMed] [Google Scholar]
- 106.Jouven X, Desnos M, Guerot C, Ducimetière P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999;99:1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 107.Dekker LR, Bezzina CR, Henriques JP, Tanck MW, Koch KT, Alings MW, Arnold AE, de Boer MJ, Gorgels AP, Michels HR, Verkerk A, Verheugt FW, Zijlstra F, Wilde AA. Familial sudden death is an important risk factor for primary ventricular fibrillation: a case–control study in acute myocardial infarction patients. Circulation. 2006;114:1140–1145. doi: 10.1161/CIRCULATIONAHA.105.606145. [DOI] [PubMed] [Google Scholar]
- 108.Kaikkonen KS, Kortelainen ML, Linna E, Huikuri HV. Family history and the risk of sudden cardiac death as a manifestation of an acute coronary event. Circulation. 2006;114:1462–1467. doi: 10.1161/CIRCULATIONAHA.106.624593. [DOI] [PubMed] [Google Scholar]
- 109.Kolder IC, Tanck MW, Bezzina CR. Common genetic variation modulating cardiac ECG parameters and susceptibility to sudden cardiac death. J Mol Cell Cardiol. 2012;52:620–629. doi: 10.1016/j.yjmcc.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 110.Spooner PM, Albert C, Benjamin EJ, Boineau R, Elston RC, George AL, Jr, Jouven X, Kuller LH, MacCluer JW, Marban E, Muller JE, Schwartz PJ, Siscovick DS, Tracy RP, Zareba W, Zipes DP. Sudden cardiac death, genes, and arrhythmogenesis. Consideration of new population and mechanistic approaches from a National Heart, Lung, Blood Institute workshop. Part I. Circulation. 2001;103:2361–2364. doi: 10.1161/01.cir.103.19.2361. and Part II Circulation 2001;103:2447–2452. [DOI] [PubMed] [Google Scholar]
- 111.Bezzina CR, Pazoki R, Bardai A, Marsman RF, de Jong JS, Blom MT, Scicluna BP, Jukema JW, Bindraban NR, Lichtner P, Pfeufer A, Bishopric NH, Roden DM, Meitinger T, Chugh SS, Myerburg RJ, Jouven X, Kääb S, Dekker LR, Tan HL, Tanck MW, Wilde AA. Genome-wide association study identifies a susceptibility locus at 21q21 for ventricular fibrillation in acute myocardial infarction. Nat Genet. 2010;42:688–694. doi: 10.1038/ng.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Arking DE, Junttila MJ, Goyette P, Huertas-Vazquez A, Eijgelsheim M, Blom MT, Newton-Cheh C, Reinier K, Teodorescu C, Uy-Evanado A, Carter-Monroe N, Kaikkonen KS, Kortelainen ML, Boucher G, Lagacé C, Moes A, Zhao X, Kolodgie F, Rivadeneira F, Hofman A, Witteman JC, Uitterlinden AG, Marsman RF, Pazoki R, Bardai A, Koster RW, Dehghan A, Hwang SJ, Bhatnagar P, Post W, Hilton G, Prineas RJ, Li M, Köttgen A, Ehret G, Boerwinkle E, Coresh J, Kao WH, Psaty BM, Tomaselli GF, Sotoodehnia N, Siscovick DS, Burke GL, Marbán E, Spooner PM, Cupples LA, Jui J, Gunson K, Kesäniemi YA, Wilde AA, Tardif JC, O'Donnell CJ, Bezzina CR, Virmani R, Stricker BH, Tan HL, Albert CM, Chakravarti A, Rioux JD, Huikuri HV, Chugh SS. Identification of a sudden cardiac death susceptibility locus at 2q24.2 through genome-wide association in European ancestry individuals. PLoS Genet. 2011;7:e1002158. doi: 10.1371/journal.pgen.1002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arking DE, Reinier K, Post W, Jui J, Hilton G, O'Connor A, Prineas RJ, Boerwinkle E, Psaty BM, Tomaselli GF, Rea T, Sotoodehnia N, Siscovick DS, Burke GL, Marban E, Spooner PM, Chakravarti A, Chugh SS. Genome-wide association study identifies GPC5 as a novel genetic locus protective against sudden cardiac arrest. PLoS One. 2010;5:e9879. doi: 10.1371/journal.pone.0009879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aouizerat BE, Vittinghoff E, Musone SL, Pawlikowska L, Kwok PY, Olgin JE, Tseng ZH. GWAS for discovery and replication of genetic loci associated with sudden cardiac arrest in patients with coronary artery disease. BMC Cardiovasc Disord. 2011;11:29. doi: 10.1186/1471-2261-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cheng A, Dalal D, Butcher B, Norgard S, Zhang Y, Dickfeld T, Eldadah ZA, Ellenbogen KA, Guallar E, Tomaselli GF. Prospective observational study of implantable cardioverter-defibrillators in primary prevention of sudden cardiac death: study design and cohort description. J Am Heart Assoc. 2013;2:e000083. doi: 10.1161/JAHA.112.000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murray SS, Smith EN, Villarasa N, Nahey T, Lande J, Goldberg H, Shaw M, Rosenthal L, Ramza B, Alaeddini J, Han X, Damani S, Soykan O, Kowal RC, Topol EJ on behalf of the GAME Investigators. Genome-wide association of implantable cardioverter-defibrillator activation with life-threatening arrhythmias. PLoS One. 2012;7:e25387. doi: 10.1371/journal.pone.0025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wieneke H, Spencker S, Svendsen JH, Martinez JG, Strohmer B, Toivonen L, Le Marec H, Garcia J, Kaup B, Soykan O, Corrado D, Siffert W. Polymorphisms associated with ventricular tachyarrhythmias: rationale, design, and endpoints of the ‘diagnostic data influence on disease management and relation of genomics to ventricular tachyarrhythmias in implantable cardioverter/defibrillator patients (DISCOVERY)’ study. Europace. 2010;12:424–429. doi: 10.1093/europace/eup444. [DOI] [PubMed] [Google Scholar]
- 118.Noseworthy PA, Havulinna AS, Porthan K, Lahtinen AM, Jula A, Karhunen PJ, Perola M, Oikarinen L, Kontula KK, Salomaa V, Newton-Cheh C. Common genetic variants, QT interval, and sudden cardiac death in a Finnish population-based study. Circ Cardiovasc Genet. 2011;4:305–311. doi: 10.1161/CIRCGENETICS.110.959049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Crotti L, Schwartz PJ. Drug-induced long QT syndrome and exome sequencing: Chinese shadows link past and future. J Am Coll Cardiol. 2014;63:1438–1440. doi: 10.1016/j.jacc.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 120.Myerburg RJ, Reddy V, Castellanos A. Indications for implantable cardioverter-defibrillators based on evidence and judgment. J Am Coll Cardiol. 2009;54:747–763. doi: 10.1016/j.jacc.2009.03.078. [DOI] [PubMed] [Google Scholar]
- 121.Wellens HJJ, Gorgels AP, de Munter H. Cardiac arrest outside of a hospital: how can we improve results of resuscitation? Circulation. 2003;107:1948–1950. doi: 10.1161/01.CIR.0000067880.57844.62. [DOI] [PubMed] [Google Scholar]