Summary
A consistent pattern of response has been observed when FMS-like tyrosine kinase 3 (FLT3) tyrosine kinase inhibitors (TKIs) have been used as monotherapy to treat patients with relapsed or refractory FLT3- internal tandem duplication (ITD) acute myeloid leukaemia (AML). Circulating blasts are cleared from the peripheral blood, while bone marrow blasts are either unaffected or are cleared from the marrow at a much slower rate. We used an in vitro model of FLT3-ITD AML blasts co-cultured with normal human bone marrow stromal cells to investigate the basis for this dichotomous response pattern to FLT3 inhibitors. We have found that in blasts on stroma, potent FLT3 inhibition predominantly results in cell cycle arrest rather than apoptosis. The anti-apoptotic effect is mediated through a combination of direct cell-cell contact and soluble factors. The addition of exogenous FLT3 ligand (FL) augments the protection, primarily by shifting the 50% inhibitory concentration for FLT3 inhibition upwards. Cytokine-activated extracellular regulated kinase (ERK), rather than STAT5, appears to be the most important downstream signalling protein mediating the protective effect, and inhibition of MEK significantly abrogates stromal-mediated resistance. These findings explain the phenomenon of peripheral blood versus bone marrow blast responses and suggest that the combination of potent FLT3 inhibition and MEK inhibition is a promising strategy for the treatment of FLT3-ITD AML.
Keywords: acute myeloid leukaemia, FLT3-ITD, quizartinib, bone marrow stroma, extracellular regulated kinase
Acute myeloid leukaemia (AML) is a haematological disorder characterized by de-regulated proliferation of myeloid cells. The overall survival for AML patients is generally poor, and is even more dismal for those AML patients harbouring internal tandem duplication (ITD) mutations in the receptor tyrosine kinase FLT3 (FLT3-ITD) (Levis & Small, 2003). FLT3 (FMS-like tyrosine kinase 3) is a class III receptor tyrosine kinase, expressed mainly in CD34+ haematopoietic stem/progenitor cells and differentiated dendritic cells. It can be activated through dimerization upon ligand binding, but mutations such as an ITD (23%) in its juxtamembrane domain or a point mutation (7%) in the activation loop will result in ligand-independent constitutive autophosphorylation. A series of downstream signalling cascades, including STAT5, PI3K-Akt and Ras-ERK pathways, are activated by FLT3, resulting in increased proliferation and blockade of apoptosis and differentiation.
With the identification of mutant FLT3 as a target for AML therapy, several drugs have been advanced into clinical trials, either as monotherapy (e.g., sunitinib,(Fiedler et al, 2005) tandutinib (DeAngelo et al, 2006), lestaurtinib (Smith et al, 2004), midostaurin (Stone et al, 2005), KW-2449 (Pratz et al, 2009), sorafenib (Borthakur et al, 2011)) or in combination with other chemotherapies (Pratz & Levis, 2008; Ravandi et al, 2010; Levis et al, 2011). Despite this effort, the clinical benefits of FLT3 inhibition have been somewhat disappointing to date. The responses to FLT3 inhibitor monotherapy usually consist of rapid clearance of peripheral blasts, but a limited effect on bone marrow blasts. FLT3 inhibitors with greater in vivo potency, such as sorafenib and quizartinib, have recently been developed, and are associated with higher rates of response in the bone marrow (Zarrinkar et al, 2009; Borthakur et al, 2011; Levis et al, 2012). However, as we have recently reported, there is now evidence to suggest that the high level of FLT3 inhibition induced by these newer agents is associated not with rapid apoptosis of marrow blasts, but rather with cell-cycle arrest and terminal differentiation of blasts over several weeks (Sexauer et al, 2012). The responses remain short-lived (from a few weeks to several months), primarily due to the emergence of resistance-conferring FLT3 point mutations (Smith et al, 2012). The combination of FLT3 inhibition with either chemotherapy or other targeted therapies has the potential for a more rapid therapeutic effect and to prevent the development of resistance.
It has been well-established that the bone marrow microenvironment confers some degree of protection for leukaemia cells against virtually any type of therapy, either through various cytokines/growth factors (e.g., PDGF, VEGF, EGF, SCF, etc.) or through cell-cell surface contact (e.g., SDF1/CXCR4, VLA4/VCAM1, CD44/E-selectin, etc.) (Manabe et al, 1994; Wilson & Trumpp, 2006; Weisberg et al, 2008; Zeng et al, 2009; Sison & Brown, 2011). It has been reported that FLT3-ITD leukaemia stem/progenitor cells are better protected by bone marrow than FLT3 wild type leukaemia cells (Parmar et al, 2011). Moreover, FLT3-ITD AML cells are still responsive to exogenous FLT3 ligand (FL), which impedes the efficacy of FLT3 inhibitors (Sato et al, 2011). FL levels are routinely elevated in AML patients undergoing therapy, and so the protective effects of the combination of bone marrow stroma and FL represents a significant obstacle to achieving a meaningful clinical benefit from FLT3 inhibition (Sato et al, 2011). To begin to address these issues, we have studied the effects of FLT3 inhibitors on AML blasts co-cultured with bone marrow stromal cells and exogenous FL. Our results indicate that extracellular regulated kinase (ERK) represents a central nexus in the mechanism of resistance conferred by stroma and FL towards FLT3 inhibition, and that targeting MEK (MAPK/ERK Kinase), which activates ERK, abrogates much of this microenvironmental resistance. Our findings suggest that the combination of FLT3 and MEK inhibition, therefore, represents a potentially important novel approach to this disease that could be explored clinically.
Methods
Cell culture and reagents
All cell lines and primary blast samples were cultured as described (Pratz et al, 2010a). Molm14 cells were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ; Braunschweig, Germany). Quizartinib, sorafenib, and PD0325901were dissolved in dimethyl sulfoxide (DMSO) at stock concentrations of 10 mmol/l. Sequential dilutions were made with RPMI medium (DMSO ≤ 0·1%). Quizartinib was supplied by Ambit Biosciences, Inc. (La Jolla, CA, USA). Sorafenib was obtained from LC Laboratories, Inc. (Woburn, MA, USA). PD0325901 and hydrocortisone was ordered from Sigma-Aldrich Co. (St. Louis, MO, USA). Trametinib (GSK1120212) was obtained from Selleck (Houston, TX, USA). L-Glutamine and Penicillin/Streptomycin were from Invitrogen (Carlsbad, CA, USA). Antibodies for flow cytometry analysis [phycoerythrin (PE) mouse anti-human CD33, allophycocyanin (APC) mouse anti-human CD45, etc.] and fluorescein isothiocyanate (FITC) Annexin V were purchased from BD Pharmingen, BD Biosciences (San Jose, CA, USA). Anti-FLT3 and anti-STAT antibodies were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA, USA).
Patient samples
Whole blood and bone marrow aspirates from healthy donors and leukaemia patients were collected and banked separately as part of an institutional protocol supported by the Regional Oncology Research Center Grant # 2 P30 CA 006973-44. All patients gave informed consent according to the Declaration of Helsinki. Leukaemia blasts were separated by centrifugation over a layer of Ficoll-paque PLUS (GE Healthcare, Fairfield, CT, USA), collected as the buffy coat and stored in freezing medium [fetal bovine serum (FBS) with 10% DMSO] in a liquid nitrogen tank.
Bone marrow stromal culture
Bone marrow stromal cultures were prepared in a manner similar to that described by others (Kaneko et al, 1982; Chamberlain et al, 2007; Brinchmann, 2008). Unused bone marrow from healthy donor harvests was collected and diluted with phosphate-buffered saline (PBS). After centrifugation over a layer of Ficoll-paque PLUS, the buffy coat of mononuclear cells was collected and washed with PBS. Cells were re-suspended in stroma medium (RPMI medium/10% FBS/1% L-Glutamine/1% Penicillin-Streptomycin/1 μmol/l Hydrocortisone) and cells counted on a Coulter counter (Beckman Coulter, Indianapolis, IN, USA). Then cells were plated into a T-75 (75 cm2) tissue culture flask with an average of 60 × 106 cells/flask in 15 ml RPMI culture medium and incubated at 33°C in 5% CO2. After 24 h, medium containing non-adherent cells was removed and replaced with 15 ml of fresh stroma medium in each flask. The culture medium was replenished every 2 weeks until 95–100% cell confluence was reached (in 1~2 months). At that point, the stromal layers were harvested with 0·25% Trypsin-EDTA (Sigma, St. Louis, MO, USA) and the cells seeded into 6- or 96-well plates (Becton Dickinson, Franklin Lakes, NJ, USA) for future assays. The stromal cell composition was analysed by flow cytometry after being stained for CD73, CD105, CD33 and CD45 at 2, 4 and 6 months of culturing. Cells harvested in this manner stained positive for CD73 and CD105 at these time points. The cells were negative for CD33 and CD45 for at least the first 4 months, but at 6 months, some low-level surface staining of CD33 and CD45 could be detected. For the experiments in this report, bone marrow from more than five different donors was used for cell signalling, apoptosis and cell cycle assays. Most stromal cultures were 2–4 months old. Leukaemia cells, when added to established stromal cultures, adhere to the stromal layers tightly enough such that they are not dislodged by gentle swirling of the plate (as visualized by light microscopy), but can be dislodged by pipetting up and down. This pipetting does not result in removal of stromal cells, which require trypsinization for removal.
Cytotoxicity assays
Proliferation and cytotoxicity were assessed using dimethylthiazol diphenyl tetrazolium bromide (MTT, Roche, Indianapolis, IN, USA) as previously described (Pratz et al, 2010a). For the assays with bone marrow stromal co-culture, 7000~8000 stromal cells were seeded in each well of the 96-well plate 1 month beforehand to allow stromal attachment and monolayer formation. One plate of stroma was co-cultured with leukaemia cells and one without (serving as background control); each condition was performed in sets of six wells, and MTT agent was added 3 d later. The highest and lowest 570 nm optical density (OD) values of each condition were taken out as outliers and the remaining four readings were averaged. The final results were obtained by subtracting the average background control from the average reading of each condition.
Flow cytometry
Flow cytometric immunophenotyping was performed as described (Sexauer et al, 2012) on Molm14 cells and primary patient blasts treated with quizartinib, sorafenib and PD0325901 in the presence and absence of stromal co-culture. Stromal cells (50 000~100 000/well) were seeded into each well of the 6-well plate 1 month beforehand to allow stromal attachment and monolayer formation. Leukaemia cells were seeded in 6-well plates with or without a confluent stromal cell monolayer at a density of 2 × 106 suspension cells in 8 ml of RPMI medium with different concentrations of inhibitors. After incubation for 2 d (Molm14 cells) or 3 d (primary samples), the leukaemia cells were harvested by pipetting and then centrifuged into pellets and analysed as described for CD33, CD45, propidium iodide, and Annexin V (Levis et al, 2004). Each assay was duplicated with stromal cells from a different donor, and a consistent pattern was observed. One set of data was plotted as a representative figure.
Immunoblotting
In each well of the 6-well plate, Molm14 (3·5 × 106) cells or patient primary blasts (7~8 × 106) were pre-incubated with or without a stromal monolayer in 4 ml RPMI medium at 37°C overnight. Quizartinib solution (4 ml with 2 × concentration) was added and incubated with the cells at 37°C. One hour later, FL (100 ng/ml, 200 μl) or/and PD0325901 (100 μmol/l, 4 μl) was added into the culture medium of designated wells. After incubation at 37°C for an additional 30 min, the cells were lifted and pelleted. Leukaemia cells were lysed and analysed by immunoblotting for FLT3, STAT5, ERK, and AKT as described (Pratz et al, 2010b). Antibodies to AKT and ERK were obtained from Cell Signalling (Bedford, MA, USA). Each Western blot assay was performed in triplicate with bone marrow stroma derived from different donors, and the same pattern was observed.
Antibody arrays
Secreted proteins and membrane-expressed proteins of human bone marrow stromal cells were detected with a Biotin Label-Based Human Antibody Array (AAH-BLG-1-4, RayBio, Norcross, GA, USA), according to the manufacturer’s recommended protocols. The confluent stromal cells in a T-75 flask were incubated in RPMI medium without serum or with 10% FBS. After 2 d, the supernatant medium was collected and the stromal layer was harvested using GIBCO PBS-based cell dissociation buffer (Invitrogen, Carlsbad, CA, USA). The stromal cells were pelleted and washed once with PBS. Hypotonic buffer (25 mmol/l Tris, 2 mmol/l MgCl2, pH 7·5) with protease inhibitors was used to re-suspend the stromal cells, and the suspension was subjected to Dounce homogenization. The homogenized suspension was centrifuged at 400 g for 5 min to remove the cell nuclei. Then the supernatant was collected and centrifuged at 12 000 g for 10 min to enrich for membrane-bound proteins in micelles. The micelles were lysed using the same lysis buffer as the one used in ‘Immunoblotting’. Both the supernatant medium and the membrane-bound protein solution were diluted with 1 × PBS to a final total protein concentration of 250 μg/ml before the microarray studies. The protein solutions were dialysed and biotinylated according to the manufacturer’s protocol. The hybridization was carried out overnight at 4°C. All slides were scanned using a GenePix 4000B Microarray Scanner (Molecular Devices, Sunnyvale, CA, USA) and analysed using the software GenePix Pro 6.0 (Molecular Devices).
Results
Both bone marrow stromal cells and exogenous FL confer a protective effect against FLT3 inhibition
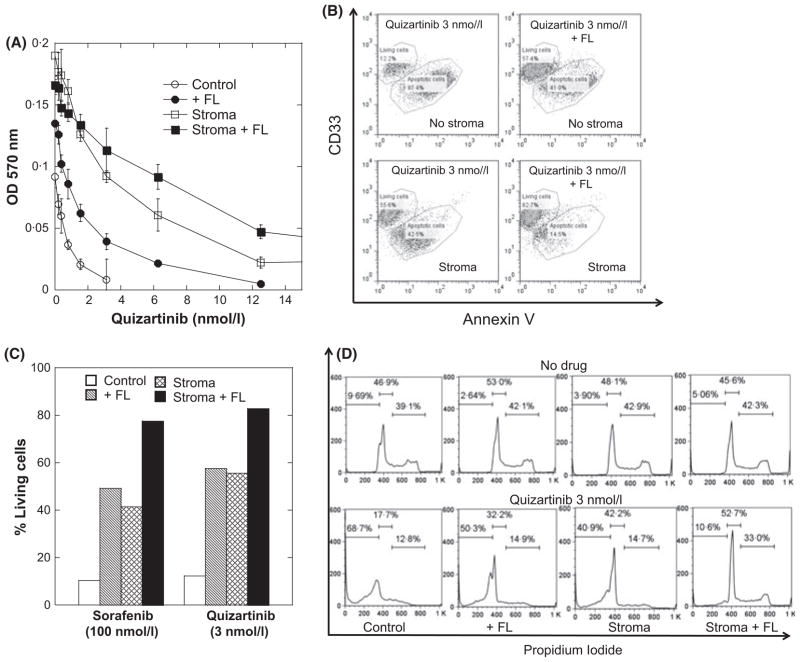
We first used a cell line model to study the protective effects of the microenvironment on AML cells exposed to a FLT3 inhibitor. The Molm14 cell line is an AML cell line derived from a patient with a FLT3-ITD mutation (Matsuo et al, 1997). Using Molm14 cells co-cultured with mesenchymal stromal cells, in the presence and absence of exogenous FL, we examined the effects of FLT3 inhibitors using three different assays for cell viability: MTT/proliferation; Annexin V (apoptosis); and cell cycle analysis with propidium iodide. Stromal cells, and to a somewhat lesser degree FL, augmented the proliferation of Molm14 cells in the absence of FLT3 inhibition (Fig 1A). Both stroma and FL conferred a protective effect against quizartinib in the proliferative assay, with the combination of the two resulting in a significant shift in the dose response curve. The 50% inhibitory concentration (IC50) for inhibition of proliferation of Molm14 cells in suspension was 0·6 nmol/l compared with 4·8 nmol/l for Molm14 cells on stroma with exogenous FL. A similar effect was observed with sorafenib (not shown). In assays measuring Annexin V binding, the presence of stroma, FL or the combination of these resulted in reduced apoptosis in response to quizartinib or sorafenib treatment (Figs 1B and C). Strikingly, quizartinib was considerably more potent at inducing apoptosis than sorafenib (Fig 1C). The results of cell cycle profile analysis (Fig 1D) were consistent with the results from the proliferation and apoptosis assays, with FLT3 inhibition inducing a significant sub-2N DNA population only in cells cultured without stroma or FL.
Fig. 1.
The protective effects of exogenous FL and bone marrow stroma against FLT3 inhibition in Molm14 cells. (A) Molm14 cells were treated with increasing dosage of quizartinib in RPMI medium (10% fetal bovine serum) for 72 h under four conditions: control [no FLT3 ligand (FL) and no stroma], with FL (2·5 ng/ml), on stroma, with FL on stroma. Cell viability was then determined using the MTT assay and measuring the optical density at 570 nmol/l (OD 570 nm). Results are plotted as percentage of dimethyl sulfoxide control. (B) Under the same four conditions as in (A), Molm14 cells were exposed to a fixed dose of quizartinib (3 nmol/l) for 48 h. The CD33+ CD45+ cell population was gated on for Annexin V staining analysis. (C) Molm14 cells were exposed to fixed doses of sorafenib (100 nmol/l) or quizartinib (3 nmol/l) for 48 h and the CD33+ CD45+ cell population was gated on for Annexin V staining analysis as in (B). The results were plotted as percentage of living cells (Annexin V-negative). (D) Comparison of propidium iodide staining results of Molm14 cells with or without quizartinib (3 nmol/l) under all four conditions as in (B).
ERK phosphorylation is maintained in cells co-cultured with stroma and/or FL despite FLT3 inhibition
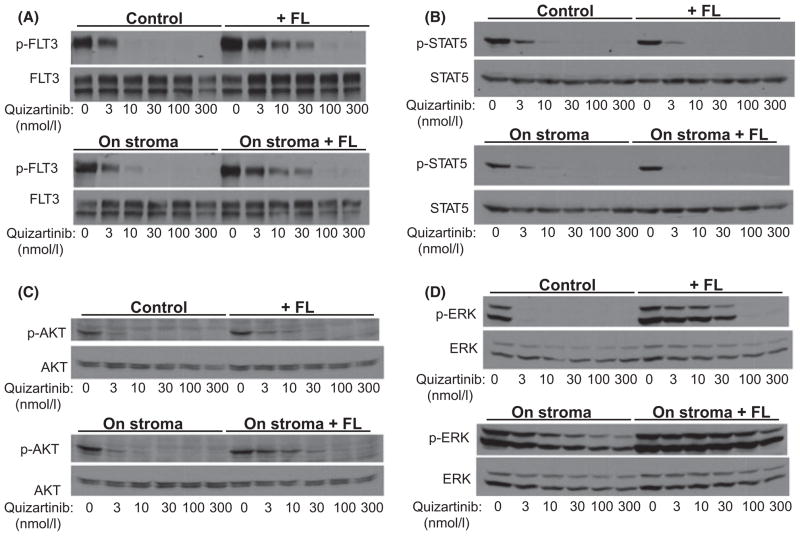
Given that both bone marrow stroma and exogenous FL conferred a degree of resistance to FLT3 inhibition, we next wished to examine the effects of these two conditions on FLT3 signalling. We therefore examined FLT3 autophosphorylation and the phosphorylation of proteins downstream in the FLT3 signalling pathway. Consistent with what we reported previously, FL impeded the ability of quizartinib to inhibit FLT3 autophosphorylation (Fig 2A) (Sato et al, 2011). Co-culture of Molm14 cells with bone marrow stroma had no significant effect on the IC50 for inhibition of FLT3. Interestingly, while exogenous FL shifted the dose response to quizartinib upwards, this did not impact on the de-phosphorylation of STAT5 (Fig 2B). This is consistent with the findings of Choudhary et al (2009), in which STAT5 was noted to be activated by a mis-localized intracellular form of mutated FLT3 (and therefore would be unaffected by an extracellular ligand). On the other hand, co-culture with stroma and/or with FL had very different effects on AKT and ERK in response to FLT3 inhibition. There were only modest effects on AKT phosphorylation, which mirrored FLT3 phosphorylation in the presence of FL (Fig 2C). The effects of stroma and FL on ERK phosphorylation, however, were particularly striking (Fig 2D). Co-culture with stroma resulted in more pronounced ERK phosphorylation in the absence of FLT3 inhibition, and ERK phosphorylation could not be fully inhibited, even at doses of quizartinib that fully inhibited autophosphorylation of FLT3. This same assay was performed with identical results using sorafenib as well (data not shown). The presence of FL seemed to augment this effect even further. Of note, we were unable to detect any FL secretion by enzyme-linked immunosorbent assay in the supernatant of stromal cultures (data not shown). These findings offered a potential explanation for the failure of FLT3 inhibition to induce apoptosis in Molm14 cells co-cultured with stroma and FL. The anti-apoptotic effects of bone marrow stroma and of FL appear to correlate with persistent activation of ERK (and, to a much lesser degree, AKT). STAT5 activation, in this system, seems to have no relationship to cell survival in response to FLT3 inhibition.
Fig. 2.
The effects of exogenous FL and bone marrow stroma on FLT3 autophosphorylation and downstream signals in Molm14 cells. Molm14 cells were treated with increasing doses of quizartinib under four different conditions: 1- suspension culture (control); 2- stromal co-culture; 3- suspension culture with exogenous FLT3 ligand (FL) at 2·5 ng/ml; and 4- stromal co-culture with exogenous FL at 2·5 ng/ml. (A) Cells exposed to quizartinib under the four different conditions were lysed and FLT3 autophosphorylation (p-FLT3) was analysed by immunoprecipitation and immunoblotting as described in Methods. (B–D) Whole cell lysates from the four treatment conditions in (A) were probed for phospho- (p-) and total STAT5, AKT and ERK as described in Methods.
The protective effect of stroma is mediated through both soluble and membrane-bound cytokines
The bone marrow microenvironment can confer a protective effect against cytotoxic agents through mechanisms mediated by direct contact and/or soluble factors (Meads et al, 2009; Zeng et al, 2009; Straussman et al, 2012). In order to determine the relative contribution of these two mechanisms on the survival benefit against FLT3 inhibition conferred by stromal co-culture, we carried out the experiments using trans-well inserts. Molm14 cells were co-cultured either directly in contact with stroma, or confined to transwell chambers inserted into stroma-containing culture wells. As shown in Figure S1A, the anti-apoptotic effect of stroma in the transwell system was only about half of what was observed when leukaemia cells were incubated in direct contact with stroma. Because we had observed persistent activation of ERK in cells on stroma (Fig 2D), we hypothesized that any factors mediating this survival benefit were probably signalled through activation of the RAS/MEK/ERK pathway. Therefore, we used PD0325901, a small molecule inhibitor of MEK [the kinase upstream of ERK (Brown et al, 2007)] to try and abrogate this protective effect. The MEK inhibitor eliminated more than half of the survival benefit from direct contact, while it had a much less significant effect in the trans-well system (Figure S1A). This suggests that, while one or more soluble factors might contribute to the stromal protective effect, a fraction of the contribution is through signalling pathways other than ERK. The results of immunoblotting analysis (Figures S1B and C) confirm this. The effect of exogenous FL was the same in the transwell system as compared to direct stromal contact (Figures S1B versus S2A). However, the stroma-induced persistence of ERK activation in the presence of quizartinib was much less prominent in the transwell system (compare Figures S1C with S2D).
Following the results of the transwell experiment, we wished to further explore the mechanism of the stroma-induced ERK activation using an antibody-based cytokine microarray. Not surprisingly, the results showed that multiple secreted soluble factors and membrane-bound factors were produced by human bone marrow stroma (Table SI), any number of which might be responsible for downstream ERK activation. Specifically, there were multiple proteins that were consistently secreted by stromal cells into the culture medium. A list of proteins secreted to a detection level of at least 20-fold over background in the absence of serum is shown in Table SI (left column). In addition, the secretion level of a number of additional proteins was up-regulated 4- to 14-fold in presence of 10% serum (Table SI, middle column). Finally, the microarray results indicated that all of the soluble proteins listed in the first two columns of Table SI were detectable in the membrane fraction of the stromal cells. A number of additional proteins were expressed only in membrane-bound form, and a partial list of the most easily detectable of these is shown in Table SI (right column). Of note, the identified cytokines included CXCR4, several angiopoietins, TNF-alpha, multiple different interleukins, G-CSF/GM-CSF/TNF and VEGF/EGF/IGF.
Persistent ERK activation correlates with stroma-induced resistance to FLT3 inhibitors
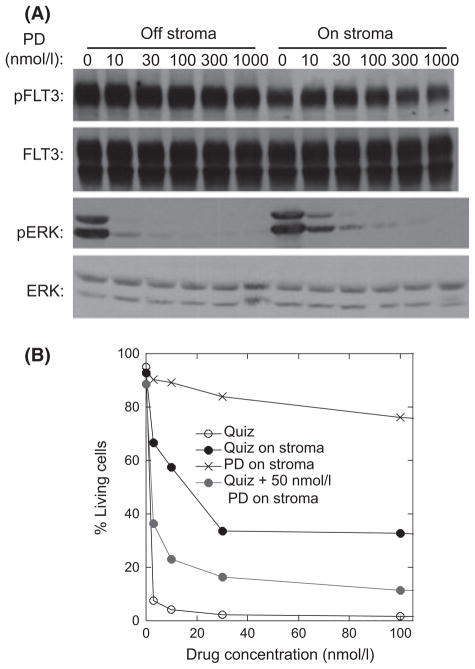
Based on the above findings, we hypothesized that the protective effects of bone marrow stroma and FL were mediated predominantly by multiple different cytokines inducing persistent activation of ERK rather than STAT5 or AKT. If this were true, then inhibition of ERK (rather than trying to target multiple cytokine receptors) should be an efficient way to abrogate the resistance to the effects of FLT3 inhibition under these conditions. To test this, we further examined the anti-proliferative effects of PD0325901 in this system. As shown in Fig 3A, PD0325901 inhibited phosphorylation of ERK (without affecting FLT3) in Molm14 cells both in suspension culture and in co-culture with stroma. PD0325901 by itself induced only minimal degrees of apoptosis (Fig 3B). However, when combined with quizartinib at a concentration that inhibited phosphorylation of ERK, it significantly blunted the protective effect of the stroma. These results were reproduced using both a different MEK inhibitor, trametinib (Ohashi et al, 2012) and a different FLT3 inhibitor, sorafenib (data not shown).
Fig. 3.
Correlation of ERK and STAT5 phosphorylation with Molm14 survival. (A) Molm14 cells were treated with increasing concentration of PD0325901 (PD) on and off stroma. The phosphorylation levels of FLT3 and ERK were determined using immunoblotting. (B) Molm14 cells were treated with quizartinib (Quiz) and/or PD0325901 (PD) on and off stroma. Apoptosis was evaluated with Annexin V staining and flow cytometry.
STAT5 is an important downstream signalling molecule in the FLT3-ITD pathway conferring survival benefit (Mizuki et al, 2000; Rocnik et al, 2006), although its relative importance is somewhat controversial (Parmar et al, 2011). However, we had noted from the experiment shown in Fig 2B that inhibition of STAT5 phosphorylation did not correlate with cytotoxicity in FLT3-ITD blasts on stroma. To determine if activation of STAT5 could confer resistance to apoptosis from FLT3 inhibition, we treated the Molm14 cells with IL3, a cytokine known to activate STAT5 in myeloid cells (Coffer et al, 2000). We first confirmed that IL3 activated STAT5 independently of FLT3 in Molm14 cells on or off stroma (Figure S2A). In suspension culture, IL3 conferred a modest protective effect against quizartinib. However, in cells on stroma, the addition of IL3 failed to provide any additional protection compared to stroma alone (Figure S2B). Therefore, in this model system, persistent activation of ERK, not STAT5, appears to be best correlated with the resistance to FLT3 inhibitors conferred by bone marrow stroma and FL.
The protective effect of FL and stroma on primary blasts from FLT3-ITD AML patients
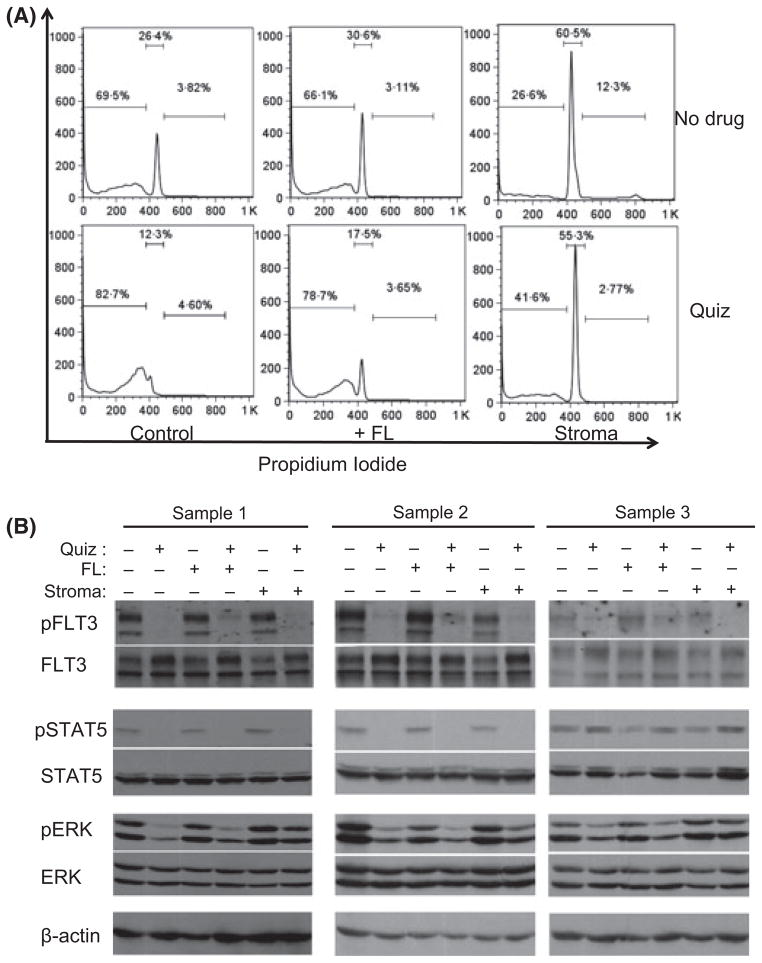
We next wished to validate our findings using primary AML samples. We used peripheral blood blasts collected from three patients with FLT3-ITD AML. Based on our previous findings that FLT3-ITD AML cells from relapsed or refractory patients were more responsive to FLT3 inhibition as compared to cells from newly-diagnosed patients (Pratz et al, 2010a) we chose 2 relapsed/refractory samples and 1 diagnostic sample. Sample 1 was from a 51-year-old man whose disease was refractory to induction therapy with daunorubicin, cytarabine, and etoposide. Sample 2 was from a 26-year-old woman whose disease had relapsed after an allogeneic transplant and was refractory to mitoxantrone, etoposide, and cytarabine. Sample 3 was from a 61-year-old man with newly-diagnosed FLT3-ITD AML. We first performed cell cycle analysis of these samples exposed to 100 nmol/l quizartinib in suspension culture versus co-culture with stroma. We chose this concentration of quizartinib for two reasons. First, plasma trough levels of the drug in trial patients was typically 2 μmol/l or higher (James et al, 2008), which, when accounting for plasma protein binding, corresponds to approximately 100 nmol/l. Second, this concentration of the drug in vitro is sufficient to overcome the impediment of FL (see Fig 2A). Results from Sample 1 are shown in Fig 4A. We and others have previously shown that primary AML samples maintained in suspension culture have a high spontaneous rate of apoptosis (Smith et al, 1998; Pratz et al, 2010a). Indeed, these blasts, when maintained in suspension culture for 3 d, demonstrated a large population of sub-2N cells, with the remaining cells displaying a G1 arrest (Fig 4A, upper left). When co-cultured with bone marrow stroma, however, the blasts displayed a cell cycle profile indicative of viability, with some proliferation. While quizartinib essentially eliminated all viable cells in the suspension culture, even 100 nmol/l of the drug failed to induce significant apoptosis in these blasts on stroma, although it did induce a G1-arrest, with loss of the proliferative fraction. The addition of FL to cells in suspension still provided a modest degree of protection against quizartinib (consistent with our prior findings (Sato et al, 2011)), but the protective effect from stroma was much more prominent.
Fig. 4.
Protective effects of exogenous FL and stroma on primary FLT3-ITD AML samples treated with FLT3 inhibition. (A) Primary AML cells (FLT3-ITD Sample 1) were incubated at 37°C for 3 d in suspension culture, in the presence of FLT ligand (FL, 2·5 ng/ml), or in co-culture with stroma, with or without 100 nmol/l quizartinib (Quiz). The cells were then collected and analysed with propidium iodide staining by flow cytometry. (B) Three primary FLT3-ITD AML samples were incubated in presence or absence of 100 nmol/l quizartinib (Quiz)/FL (2·5 ng/ml)/stroma. The cells were then harvested and lysed for immunoblotting of phosphorylated and total FLT3, STAT5 and ERK as described in ‘Methods’.
As with the Molm14 cells, we examined the effects of stroma and FL on the ability of quizartinib to inhibit FLT3 signalling in these primary samples (Fig 4B). Inhibition of FLT3 autophosphorylation was achieved under all conditions using 100 nmol/l quizartinib, although the presence of FL impeded this somewhat, as expected. This concentration of drug inhibited STAT5 phosphorylation in both of the relapse samples, but in the sample from the newly-diagnosed patient, STAT5 was unaffected. This apparent uncoupling of STAT5 and FLT3-ITD signalling in primary samples has been noted by others (Parmar et al, 2011). Furthermore, the fact that STAT5 phosphorylation was fully inhibited in the two relapse samples co-cultured with stroma (Fig 4B) without the presence of a significant sub-2N population again suggests that STAT5 may not be an essential survival signal in FLT3-ITD AML at diagnosis. In all three samples treated with quizartinib in suspension culture, ERK phosphorylation was uniformly inhibited. On stroma, however, ERK remained persistently phosphorylated, despite complete inhibition of FLT3 autophosphorylation. The AKT signal in these samples was of poor quality and was not interpretable (data not shown).
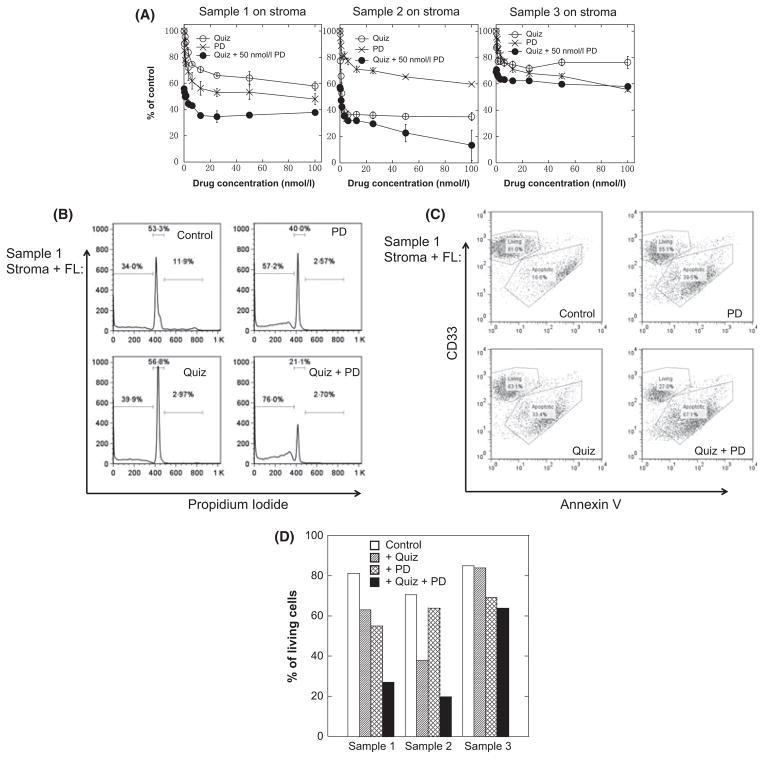
Therefore, as with the Molm14 cells, co-culture of primary FLT3-ITD AML cells with bone marrow stroma was associated with cell cycle arrest and persistent activation of ERK despite FLT3 inhibition. We next wished to determine if MEK inhibition could overcome the protective effect of stroma and/or FL for these primary samples. As shown in Fig 5A, Samples 1 and 2 (relapsed/refractory) displayed a cytotoxic response to both drugs individually, but – in opposite fashion – Sample 1 responded more strongly to MEK inhibition, while Sample 2 responded more to FLT3 inhibition. However, in both samples the combination of both drugs induced synergistic cytotoxicity. Median effect analysis yielded combination indices (CI) for Samples 1 and 2 of 0·484 and 0·058, respectively. Interestingly, and consistent with what we have observed previously with samples from newly-diagnosed patients, Sample 3 displayed only a modest response to either drug, with only an additive effect of the combination (CI = 0·838). The results of cell cycle analysis (Fig 5B) and Annexin V binding (Fig 5C; for Sample 1) confirm the synergistic cytotoxic effect of the combination. Figure 5D summarizes the induction of apoptosis for fixed concentrations of the 2 drugs for all 3 samples co-cultured with stroma and FL, and inhibition of FLT3 autophosphorylation and ERK phosphorylation is displayed in Figure S3. FLT3 autophosphorylation and ERK phosphorylation were inhibited under these conditions, although only the 2 relapsed samples showed a cytotoxic response.
Fig. 5.
Stroma-mediated resistance can be partially overcome through MEK inhibition in primary samples. (A) The cytotoxicity of quizartinib (Quiz), PD0325901 (PD) or the combination on patient samples 1, 2 and 3 on stroma were evaluated using MTT assays according to the description in ‘Methods’. (B) Primary AML cells (FLT3-ITD sample 1) were co-cultured on stroma in presence of FL (2·5 ng/ml) and incubated for 3 d with either 100 nmol/l quizartinib (Quiz), 50 nmol/l PD0325901 (PD) or the combination (Quiz + PD). The cells were then harvested and analysed for propidium iodide staining by flow cytometry. (C) The same cells as in B were analysed for Annexin V binding. (D) Using the same conditions as in (B), all three primary samples were analysed for Annexin V binding. The results are plotted as ‘% of living cells’ (Annexin V negative).
Discussion
Peripheral blood blast clearance with minimal or modest bone marrow blast clearance has been a consistent feature of clinical responses to investigational FLT3 inhibitors in FLT3-ITD AML. Previous work from another group using stromal cell lines was consistent with our findings, indicating that the protective effect conferred by bone marrow stroma was mediated through multiple signalling pathways, suggesting a strategy of combining several kinase inhibitors, such as midostaurin and dasatininib, for overcoming such protection (Weisberg et al, 2008). While such a multi-targeted approach might be efficacious in vitro, tolerability remains an issue when trying to implement this approach in patients. Our data now provides a more specific mechanistic explanation for this phenomenon, assuming that in vitro bone marrow stromal cell cultures provide a reasonable surrogate for the bone marrow microenvironment as it exists in vivo, and suggests that a strategy of highly selective targeting of FLT3 and ERK would be both efficacious and probably much more tolerable. In the absence of stroma (e.g., in the peripheral blood or in suspension culture), a leukaemic cell with addiction to the FLT3-ITD signalling pathway responds to FLT3 inhibition with apoptosis, while direct contact and/or proximity to stromal cells is protective and FLT3 inhibition results only in a cell cycle arrest. We observed this effect in the FLT3-ITD cell line Molm14 and even more prominently in primary AML cells from relapsed/refractory FLT3-ITD AML patients, although the responses in the primary samples were, not surprisingly, more heterogeneous in nature. The majority of the stromal protective effect appears to be mediated through persistent activation of the MAPK/ERK pathway through a combination of direct stromal contact and stromal-derived soluble factors.
We are currently beginning a proteomics-based investigation into the specific stromal factors that potentially activate the MAPK/ERK pathway in these leukaemic cells. The antibody-based microarray assay yielded a number of candidate cytokines that might be responsible for the activation of ERK signalling pathway. Compared with their free form as soluble factors, the membrane-bound versions would be expected to have higher localized concentrations on the cell surface and more sustained activation effects with the hybridizing complex not being endocytosed. This would explain why we observed that direct cell-cell contact was more essential for the persistent ERK activation than exposure to soluble factors. Even though multiple cytokines may be involved in the stroma-mediated resistance, it is possible that some key cytokines could be more important than others (Straussman et al, 2012; Wilson et al, 2012). Further characterization is needed to narrow down the list of potential cytokines mediating the persistent activation of ERK.
Our observation that persistent activation of ERK confers resistance to FLT3 inhibitor-induced apoptosis is consistent with a previous finding of activating mutations of RAS in Molm14 cells that had acquired resistance to FLT3 inhibitors in vitro (Piloto et al, 2007). The RAS mutations resulted in persistent activation of MAPK/ERK in these cells despite FLT3 inhibition. Molm14 cells, therefore, require activation of this pathway for survival. In suspension, ERK activation is maintained by FLT3-ITD signalling, and FLT3 inhibition leads to rapid apoptosis. On bone marrow stroma, other signalling pathways that mediate ERK activation allow Molm14 cells to survive FLT3 inhibition (albeit in G1 arrest). RAS and FLT3 mutations do not typically occur together in AML (Schlenk et al, 2008). Our results, in combination with previous findings (Piloto et al, 2007), suggest that one reason these two mutations are near-mutually exclusive is that both activate the same crucial downstream signalling protein, ERK. While both also activate other signalling proteins, ERK may be the most important of these in stimulating proliferation and inhibiting apoptosis. Interestingly, while we confirmed that STAT5 was activated predominantly by intracellular FLT3 (Choudhary et al, 2009), we were unable to demonstrate an association with STAT5 activation and survival, at least in our in vitro model. The uncoupling of FLT3-ITD signalling and STAT5 activation has been noted in primary AML samples by others (Parmar et al, 2011).
From a practical standpoint, in order for a FLT3 inhibitor to induce a meaningful cytotoxic effect in FLT3-ITD blasts on bone marrow, potency appears to be essential. We noted a threshold of roughly 30–50 nmol/l of quizartinib (and somewhat higher in the presence of exogenous FL) that was required to achieve a maximum effect on stroma, whether it be induction of apoptosis or inhibition of ERK activation. This concentration corresponds to plasma trough levels of 600–1000 nmol/l in patients treated with quizartinib. While these levels are readily achievable with tolerable dosing schemes of quizartinib (James et al, 2008), the relative lesser potency of sorafenib can explain why its response rate in patients is not as high as quizartinib (Borthakur et al, 2011; Levis et al, 2012).
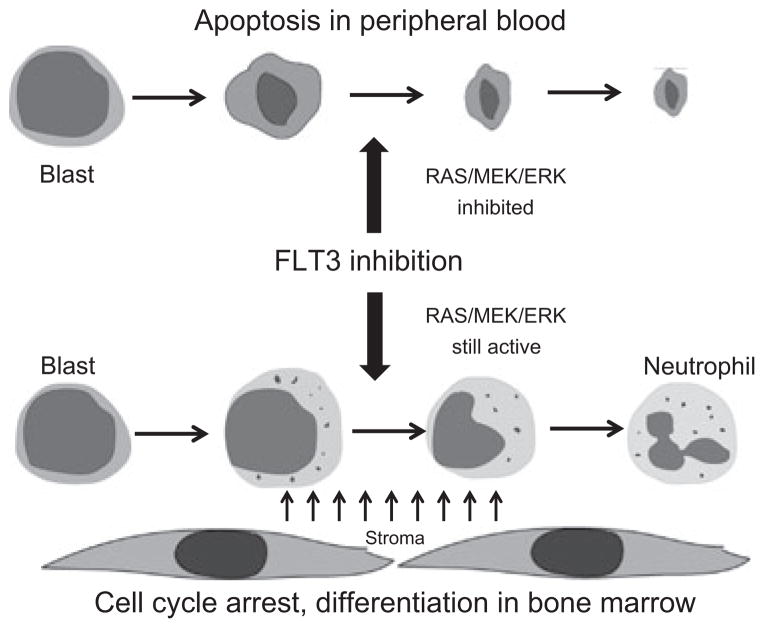
These finding, coupled with our recent work characterizing the differentiation of FLT3-ITD blasts in the marrow of patients treated with quizartinib (Sexauer et al, 2012), allow us to construct a model that explains the difference in effect of FLT3 inhibitors on peripheral blood blasts versus marrow blasts (Fig 6). Finally, our data provide clear evidence for the potential therapeutic benefit of a combination of FLT3 inhibition and MEK inhibition. Like FLT3 inhibitors, MEK inhibitors have been under study for several years now for both solid tumors and leukaemias (Roberts & Der, 2007). If a combination of FLT3 and MEK inhibition proves tolerable, it may result in not only more clinically meaningful responses, but may also broaden the population of AML patients (e.g., FLT3 wild type) that could benefit from these targeted therapies.
Fig. 6.
Proposed model for response of FLT3-ITD AML blasts to potent FLT3 inhibition. Blasts circulating in the peripheral blood maintain ERK activity exclusively via FLT3-ITD signalling. FLT3 inhibition results in apoptosis, which manifests clinically as clearance of blasts from the peripheral blood. Blasts within the microenvironment of the marrow are exposed to stroma-derived cytokines, which contribute to ERK activation along with FLT3-ITD signalling. FLT3 inhibition in these blasts results in cell-cycle arrest and eventual terminal differentiation (Sexauer et al, 2012).
Supplementary Material
Acknowledgments
This work was supported by grants from the NCI (NCI Leukemia SPORE P50 CA100632-06, R01 CA128864) and the American Society of Clinical Oncology (ML). ML is a Clinical Scholar of the Leukemia and Lymphoma Society.
Footnotes
Author contributions
XY and ML designed the study, performed experiments, analysed the data, and wrote the manuscript. AS contributed to the design and analysis.
Conflict of interest
ML is on the clinical advisory board for Ambit Biosciences, Inc., whose product was investigated in this study.
Additional Supporting Information may be found in the online version of this article:
Fig S1. The protective effects of direct contact with stroma versus stroma-derived soluble factors.
Fig S2. A. Molm14 cells were treated with increasing concentrations of quizartinib in the presence or absence of IL-3 (2.5 ng/ml), on and off stroma. The phosphorylation level of STAT5 was determined using immunoblotting. B. In the presence of FL or IL-3, Molm14 cells were exposed to quizartinib (3 nmol/l) on and off stroma for 2 d. The cells were then collected and analyzed for Annexin V binding using flow cytometry. The results were plotted as percentage of living cells (Annexin V negative).
Fig S3. Under the same conditions as in Fig 5, the three samples were exposed to the drugs for 1 h and then the cells were subjected to immunoblotting of phospho- and total-FLT3 and ERK.
Table SI. List of proteins detected in stromal culture medium and stromal cell membrane.
References
- Borthakur G, Kantarjian H, Ravandi F, Zhang W, Konopleva M, Wright JJ, Faderl S, Verstovsek S, Mathews S, Andreeff M, Cortes JE. Phase I study of sorafenib in patients with refractory or relapsed acute leukemias. Haematologica. 2011;96:62–68. doi: 10.3324/haematol.2010.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinchmann JE. Expanding autologous multipotent mesenchymal bone marrow stromal cells. Journal of the Neurological Sciences. 2008;265:127–130. doi: 10.1016/j.jns.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Brown AP, Carlson TC, Loi CM, Graziano MJ. Pharmacodynamic and toxicokinetic evaluation of the novel MEK inhibitor, PD0325901, in the rat following oral and intravenous administration. Cancer Chemotherapy and Pharmacology. 2007;59:671–679. doi: 10.1007/s00280-006-0323-5. [DOI] [PubMed] [Google Scholar]
- Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- Choudhary C, Olsen JV, Brandts C, Cox J, Reddy PN, Bohmer FD, Gerke V, Schmidt-Arras DE, Berdel WE, Muller-Tidow C, Mann M, Serve H. Mislocalized activation of oncogenic RTKs switches downstream signaling outcomes. Molecular Cell. 2009;36:326–339. doi: 10.1016/j.molcel.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Coffer PJ, Koenderman L, de Groot RP. The role of STATs in myeloid differentiation and leukemia. Oncogene. 2000;19:2511–2522. doi: 10.1038/sj.onc.1203479. [DOI] [PubMed] [Google Scholar]
- DeAngelo DJ, Stone RM, Heaney ML, Nimer SD, Paquette RL, Klisovic RB, Caligiuri MA, Cooper MR, Lecerf JM, Karol MD, Sheng S, Holford N, Curtin PT, Druker BJ, Heinrich MC. Phase 1 clinical results with tandutinib (MLN518), a novel FLT3 antagonist, in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome: safety, pharmacokinetics, and pharmacodynamics. Blood. 2006;108:3674–3681. doi: 10.1182/blood-2006-02-005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler W, Serve H, Dohner H, Schwittay M, Ottmann OG, O’Farrell AM, Bello CL, Allred R, Manning WC, Cherrington JM, Louie SG, Hong W, Brega NM, Massimini G, Scigalla P, Berdel WE, Hossfeld DK. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105:986–993. doi: 10.1182/blood-2004-05-1846. [DOI] [PubMed] [Google Scholar]
- James J, Pratz K, Stine A, Apuy J, Insko DE, Armstrong RC, Zarrinkar PP, Small D, Wierenga W, Levis M. Clinical pharmacokinetics and FLT3 phosphorylation of AC220, a highly potent and selective inhibitor of FLT3. Blood. 2008;112:2637a. [Google Scholar]
- Kaneko S, Motomura S, Ibayashi H. Differentiation of human bone marrow-derived fibroblastoid colony forming cells (CFU-F) and their roles in haemopoiesis in vitro. British Journal of Haematology. 1982;51:217–225. doi: 10.1111/j.1365-2141.1982.tb02774.x. [DOI] [PubMed] [Google Scholar]
- Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17:1738–1752. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- Levis M, Pham R, Smith BD, Small D. In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood. 2004;104:1145–1150. doi: 10.1182/blood-2004-01-0388. [DOI] [PubMed] [Google Scholar]
- Levis M, Ravandi F, Wang ES, Baer MR, Perl A, Coutre S, Erba H, Stuart RK, Baccarani M, Cripe LD, Tallman MS, Meloni G, Godley LA, Langston AA, Amadori S, Lewis ID, Nagler A, Stone R, Yee K, Advani A, Douer D, Wiktor-Jedrzejczak W, Juliusson G, Litzow MR, Petersdorf S, Sanz M, Kantarjian HM, Sato T, Tremmel L, Bensen-Kennedy DM, Small D, Smith BD. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood. 2011;117:3294–3301. doi: 10.1182/blood-2010-08-301796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis M, Perl A, Dombret H, Dohner H, Steffen B, Rousselot P, Martinelli G, Estey E, Burnett A, Gammon G, Trone D, Leo E, Cortes J. Final results of a phase 2 open-label, monotherapy efficacy and safety study of quizartinib (AC220) in patients with FLT3-ITD positive or negative relapsed/refractory acute myeloid leukemia after second-line chemotherapy or hematopoietic stem cell transplantation. Blood (ASH Annual Meeting Abstracts) 2012;120:673a. [Google Scholar]
- Manabe A, Murti KG, Coustan-Smith E, Kumagai M, Behm FG, Raimondi SC, Campana D. Adhesion-dependent survival of normal and leukemic human B lymphoblasts on bone marrow stromal cells. Blood. 1994;83:758–766. [PubMed] [Google Scholar]
- Matsuo Y, MacLeod RA, Uphoff CC, Drexler HG, Nishizaki C, Katayama Y, Kimura G, Fujii N, Omoto E, Harada M, Orita K. Two acute monocytic leukemia (AML-M5a) cell lines (MOLM-13 and MOLM-14) with interclonal phenotypic heterogeneity showing MLL-AF9 fusion resulting from an occult chromosome insertion, ins(11;9)(q23; p22p23) Leukemia. 1997;11:1469–1477. doi: 10.1038/sj.leu.2400768. [DOI] [PubMed] [Google Scholar]
- Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nature Reviews Cancer. 2009;9:665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- Mizuki M, Fenski R, Halfter H, Matsumura I, Schmidt R, Muller C, Gruning W, Kratz-Albers K, Serve S, Steur C, Buchner T, Kienast J, Kanakura Y, Berdel WE, Serve H. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood. 2000;96:3907–3914. [PubMed] [Google Scholar]
- Ohashi K, Sequist LV, Arcila ME, Moran T, Chmielecki J, Lin YL, Pan Y, Wang L, de Stanchina E, Shien K, Aoe K, Toyooka S, Kiura K, Fernandez-Cuesta L, Fidias P, Yang JC, Miller VA, Riely GJ, Kris MG, Engelman JA, Vnencak-Jones CL, Dias-Santagata D, Ladanyi M, Pao W. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2127–E2133. doi: 10.1073/pnas.1203530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar A, Marz S, Rushton S, Holzwarth C, Lind K, Kayser S, Dohner K, Peschel C, Oostendorp RA, Gotze KS. Stromal niche cells protect early leukemic FLT3-ITD+ progenitor cells against first-generation FLT3 tyrosine kinase inhibitors. Cancer Research. 2011;71:4696–4706. doi: 10.1158/0008-5472.CAN-10-4136. [DOI] [PubMed] [Google Scholar]
- Piloto O, Wright M, Brown P, Kim KT, Levis M, Small D. Prolonged exposure to FLT3 inhibitors leads to resistance via activation of parallel signaling pathways. Blood. 2007;109:1643–1652. doi: 10.1182/blood-2006-05-023804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratz K, Levis M. Incorporating FLT3 inhibitors into acute myeloid leukemia treatment regimens. Leukaemia & Lymphoma. 2008;49:852–863. doi: 10.1080/10428190801895352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratz KW, Cortes J, Roboz GJ, Rao N, Arowojolu O, Stine A, Shiotsu Y, Shudo A, Akinaga S, Small D, Karp JE, Levis M. A pharmacodynamic study of the FLT3 inhibitor KW-2449 yields insight into the basis for clinical response. Blood. 2009;113:3938–3946. doi: 10.1182/blood-2008-09-177030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratz KW, Sato T, Murphy KM, Stine A, Rajkhowa T, Levis M. FLT3-mutant allelic burden and clinical status are predictive of response to FLT3 inhibitors in AML. Blood. 2010a;115:1425–1432. doi: 10.1182/blood-2009-09-242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratz KW, Cho E, Levis MJ, Karp JE, Gore SD, McDevitt M, Stine A, Zhao M, Baker SD, Carducci MA, Wright JJ, Rudek MA, Smith BD. A pharmacodynamic study of sorafenib in patients with relapsed and refractory acute leukemias. Leukemia. 2010b;24:1437–1444. doi: 10.1038/leu.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravandi F, Cortes JE, Jones D, Faderl S, Garcia-Manero G, Konopleva MY, O’Brien S, Estrov Z, Borthakur G, Thomas D, Pierce SR, Brandt M, Byrd A, Bekele BN, Pratz K, Luthra R, Levis M, Andreeff M, Kantarjian HM. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. Journal of Clinical Oncology. 2010;28:1856–1862. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- Rocnik JL, Okabe R, Yu JC, Lee BH, Giese N, Schenkein DP, Gilliland DG. Roles of tyrosine 589 and 591 in STAT5 activation and transformation mediated by FLT3-ITD. Blood. 2006;108:1339–1345. doi: 10.1182/blood-2005-11-011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Yang X, Knapper S, White P, Smith BD, Galkin S, Small D, Burnett A, Levis M. FLT3 ligand impedes the efficacy of FLT3 inhibitors in vitro and in vivo. Blood. 2011;117:3286–3293. doi: 10.1182/blood-2010-01-266742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, Habdank M, Spath D, Morgan M, Benner A, Schlegelberger B, Heil G, Ganser A, Dohner H. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. New England Journal of Medicine. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- Sexauer A, Perl A, Yang X, Borowitz M, Gocke C, Rajkhowa T, Thiede C, Frattini M, Nybakken GE, Pratz K, Karp J, Smith BD, Levis M. Terminal myeloid differentiation in vivo is induced by FLT3 inhibition in FLT3/ITD AML. Blood. 2012;120:4205–4214. doi: 10.1182/blood-2012-01-402545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sison EA, Brown P. The bone marrow microenvironment and leukemia: biology and therapeutic targeting. Expert Review of Hematology. 2011;4:271–283. doi: 10.1586/ehm.11.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BD, Bambach BJ, Vala MS, Barber JP, Enger C, Brodsky RA, Burke PJ, Gore SD, Jones RJ. Inhibited apoptosis and drug resistance in acute myeloid leukaemia. British Journal of Haematology. 1998;102:1042–1049. doi: 10.1046/j.1365-2141.1998.00854.x. [DOI] [PubMed] [Google Scholar]
- Smith BD, Levis M, Beran M, Giles F, Kantarjian H, Berg K, Murphy KM, Dauses T, Allebach J, Small D. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–3676. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- Smith CC, Wang Q, Chin CS, Salerno S, Damon LE, Levis MJ, Perl AE, Travers KJ, Wang S, Hunt JP, Zarrinkar PP, Schadt EE, Kasarskis A, Kuriyan J, Shah NP. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485:260–263. doi: 10.1038/nature11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD, Grandin W, Lebwohl D, Wang Y, Cohen P, Fox EA, Neuberg D, Clark J, Gilliland DG, Griffin JD. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, Davis A, Mongare MM, Gould J, Frederick DT, Cooper ZA, Chapman PB, Solit DB, Ribas A, Lo RS, Flaherty KT, Ogino S, Wargo JA, Golub TR. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg E, Wright RD, McMillin DW, Mitsiades C, Ray A, Barrett R, Adamia S, Stone R, Galinsky I, Kung AL, Griffin JD. Stromal-mediated protection of tyrosine kinase inhibitor-treated BCR-ABL-expressing leukemia cells. Molecular Cancer Therapeutics. 2008;7:1121–1129. doi: 10.1158/1535-7163.MCT-07-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nature Reviews Immunology. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, Ribas A, Li J, Moffat J, Sutherlin DP, Koeppen H, Merchant M, Neve R, Settleman J. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505–509. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrinkar PP, Gunawardane RN, Cramer MD, Gardner MF, Brigham D, Belli B, Karaman MW, Pratz KW, Pallares G, Chao Q, Sprankle KG, Patel HK, Levis M, Armstrong RC, James J, Bhagwat SS. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML) Blood. 2009;114:2984–2992. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z, Shi YX, Samudio IJ, Wang RY, Ling X, Frolova O, Levis M, Rubin JB, Negrin RR, Estey EH, Konoplev S, Andreeff M, Konopleva M. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;113:6215–6224. doi: 10.1182/blood-2008-05-158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.