Abstract
For cancer chemotherapy, a tumor regression without any surgical resection and severe side effects is greatly preferred to merely slowing down the growth of tumors. Here, we report a formulation composed of irinotecan (IRN) and poly(D,L-lactide-co-glycolide)-b-poly(ethylene glycol)-b-poly(D,L-lactide-co-glycolide) (PLGA-PEG-PLGA). IRN is a clinically used antitumor drug with active and inactive chemical forms in equilibrium, and the major form at physiological conditions is inactive but still has side effects. The aqueous solution of the PLGA-PEG-PLGA is a sol at room temperature and physically gels at body temperature, forming a thermogel. We successfully mixed this moderately soluble drug into the amphiphilic copolymer aqueous solution for the first time. The mixture was subcutaneously injected into nude mice with xenografted SW620 human colon tumors. Excellent in vivo antitumor efficacy was observed in the group that received the IRN-loaded thermogel. The tumor was significantly regressed after being treated with the IRN/thermogel, and the side effects (blood toxicity and body weight decrease) were very mild. These results might be attributed to the ideal sustained release profile and period of release of the drug from the thermogel and to the significant enhancement of the fraction of the active form of the drug by the thermogel.
The new century has witnessed the rapid development of chemotherapy, known as one of the most important therapies in cancer treatment1,2. Nevertheless, most in vivo results of chemotherapy only reduce the growth rate of tumors or relatively decrease tumor sizes compared with those in untreated groups. Therefore, despite extensive preclinical and clinical efforts worldwide, tumor tissues still grow, which eventually leads to death in many cases. A treatment to absolutely regress tumors without any surgical resection is thus strongly desired.
Even for traditional chemotherapeutics, escalating drug doses would lead to better antitumor efficacy and sometimes can even eradicate tumors. However, a very high drug dose means a severe side effect, which is why over-treatment might kill both tumors and patients. There has always been a balance between the efficacy and toxicity of chemotherapy to essentially improve the quality of lives3. Therefore, the key is to find an approach to realize the aim at efficiently decreasing the tumor volume without any increase of side effects. In recent years, many drug delivery systems (DDSs) have emerged4,5,6,7,8,9, and chemotherapy, radiotherapy and other new therapies have been combined to realize the above aim10. Considering that translation into clinical applications is challenging with “so many papers and so few drugs”11, it is, of course, of significant meaning if the aim of increasing drug efficacy without introducing extra side effects could be achieved merely by chemotherapy of a clinically used drug in a simple new DDS. Here, we developed a pharmaceutical formulation of an antitumor drug with a unique biomaterial to decrease tumor volume as well as reduce side effects. Our rational design is based upon our analysis of new biomaterials, of appropriate drugs, and of ideal systems for sustained release.
New biomaterials have been widely investigated in various DDSs12,13,14,15. The amphiphilic copolymer composed of poly(ethylene glycol) (PEG) and biodegradable polyester has been developed in the form of a physical hydrogel for sustained release of drugs using the concept suggested by the Kim group16. Under appropriate compositions and concentrations, the aqueous system of this type of copolymer exhibits a sol-gel transition upon heating, and the resulting hydrogel is therefore called a thermogel. At low temperatures, the material is a sol and can mix with drugs; once injected, it can spontaneously gel at body temperature to form an in situ drug release matrix17.
Camptothecin (CPT) analogs are topoisomerase I inhibitors and constitute one important category of antitumor drugs. Among them, irinotecan (IRN, CPT-11) has a moderate solubility in water, with the apparent solubility of approximately 10 mg/mL, as demonstrated in Supporting Information. The value is higher than that of insoluble drugs (<0.1 mg/mL) and lower than that of soluble drugs (>30 mg/mL) according to the U.S. Pharmacopeia (USP 35). IRN was approved by the US Food and Drug Administration (FDA) in the 1990s and has been used in the clinic for years, especially in the treatment of colon cancers18. It is the only “blockbuster” in the CPT family. In pharmacy, a “blockbuster” denotes a drug with an annual sale close to or above 1 billion US dollars19. The sale of IRN was close to 1 billion in 2007 according to the annual financial report of Pfizer, Inc. before this company lost its exclusivity in 2008. Nearly all the CPT family drugs are in equilibrium between active and inactive forms; the low fraction of the active lactone form leads to lower therapeutic efficacy but higher toxicity20,21. Unfortunately, the inactive form is predominant over the active one under physiological conditions22. Therefore, it would be beneficial if one type of DDS of CPT family drugs could realize a sustained release while maintaining a high fraction of the active form of the drug.
For the sustained release, we believe that two issues should be considered before making efforts to develop a new pharmaceutical formulation. The first issue involves the necessity of sustained release for a specific drug in an appropriate period. Although a longer period of drug release could reduce drug administration frequency and increase drug compliance, we do not accept the doctrine “the longer, the better” because excess exposure to chemotherapeutics may lead to cumulative toxicity23. The second issue involves the drug-material interaction in a sustained release system. It is always a classical dilemma of a small burst release at the initial stage and complete release at the later stage24,25,26,27. For instance, highly water-soluble drugs can easily be released but usually with a significant initial burst24; by contrast, water-insoluble drugs might be released evenly but it is very difficult to ensure the complete release of this type of drug26. In our opinion, selecting a drug with a moderate solubility might be a potential breakthrough to this dilemma.
On the basis of the above analysis, we selected the antitumor drug IRN as the model drug and a thermogel composed of the amphiphilic copolymer poly(D,L-lactide-co-glycolide)-b-poly(ethylene glycol)-b-poly(D,L-lactide -co-glycolide) (PLGA-PEG-PLGA) as the sustained-release carrier, as illustrated in Fig. 1. Although the copolymer PLGA-PEG-PLGA has not yet been approved by the FDA, the blocks of both PLGA and PEG have been applied in clinics for years. While many drugs have been attempted to be encapsulated into different thermogels28,29,30,31,32,33, the IRN/thermogel system has never been reported. We observed that it was, if not extremely difficult, not easy to mix this moderately soluble drug into the aqueous solution of the temperature-sensitive amphiphilic block copolymers.
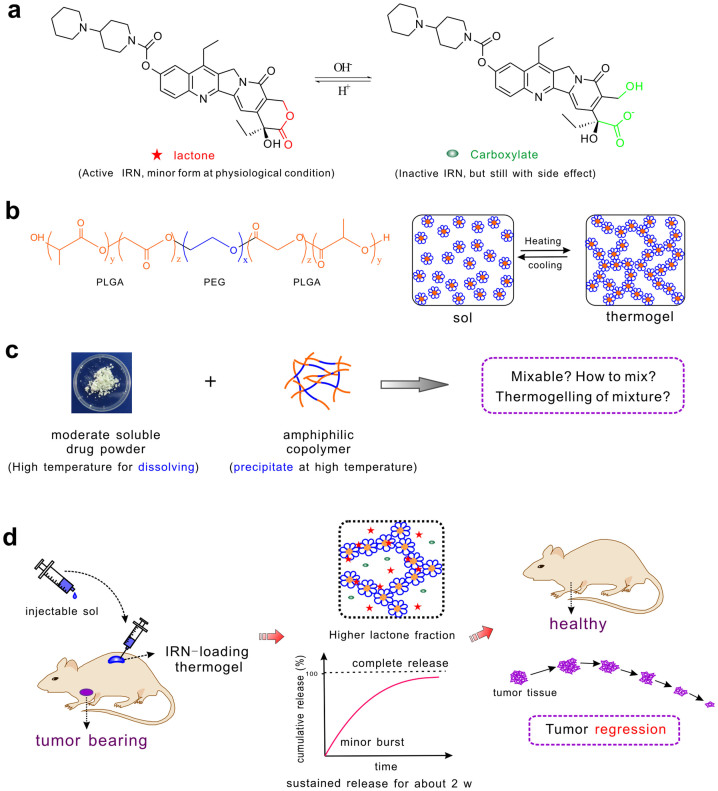
Figure 1. Schematic presentation of chemotherapy with IRN-loaded thermogel.
(a) The chemical structure of IRN with two forms, active lactone and inactive carboxylate. (b) The chemical structure of PLGA-PEG-PLGA triblock copolymer and the temperature-induced sol-gel transition of this amphiphilic copolymer in water. A sol is a suspension of micelles, and a thermogel has a percolated micelle network. A further increase of temperature leads to precipitation. (c) Difficulty in mixing the moderately soluble drug IRN and the amphiphilic block copolymer PLGA-PEG-PLGA. (d) Main procedures of the IRN/thermogel system and its functions in tumor treatment. The IRN-loaded polymer solution was injectable; the injected mixture was gelled in vivo at body temperature. IRN would be released sustainably for approximately 2 weeks without a significant initial burst and with almost complete release. Its active lactone fraction in the thermogel was enhanced significantly, much higher than that in phosphate buffer saline (PBS) solution. The tumor volume eventually decreased compared with the initial size in therapy.
In this study, we prepared a facial roadmap to load IRN into the PLGA-PEG-PLGA thermogel without any organic solvent, as illustrated in Fig. 2. An excellent IRN release profile and period on demand of the potential clinical applications was achieved, as schematically shown in Fig. 1d; even the fraction of the active lactone form of IRN in the thermogel was significantly enhanced. Through an appropriate “mixing” strategy without any modification of the drug molecules, we achieved a decrease of tumor sizes in nude mice bearing human colon cancer. The present paper reports the rational design and the experimental results.
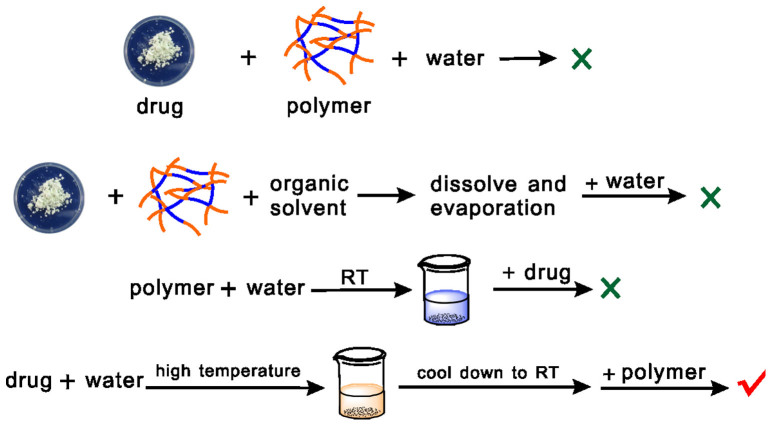
Figure 2. Possible roadmaps of mixing drugs and copolymers to obtain IRN-loaded PLGA-PEG-PLGA aqueous solution.
Here, the “drug” IRN is moderately soluble in water, and the “polymer” PLGA-PEG-PLGA is amphiphilic and sensitive to temperature in water; “RT” and “high temperature” refer to room temperature (approximately 20°C) and 85°C, respectively.
Results
Synthesis of the PLGA-PEG-PLGA triblock copolymer
The amphiphilic copolymer used in this study was synthesized via the ring-opening polymerization of lactide (LA) and glycolide (GA) initiated by the end hydroxy groups of PEG with a molecular weight (MW) of 1500 Da. The MW and polydispersity of the copolymer were characterized by 1H NMR and gel permeation chromatography (GPC). The results are summarized in Table 1 and Supplementary Fig. S1.
Table 1. Molecular parameters of the synthesized copolymer.
a)Molar ratio LA/GA in the PLGA block and the number-average molecular weight (Mn) of the block in the copolymer calculated from 1H NMR;
b)weight-average molecular weight (Mw) over Mn obtained from GPC.
In vitro cytotoxicity of PLGA-PEG-PLGA
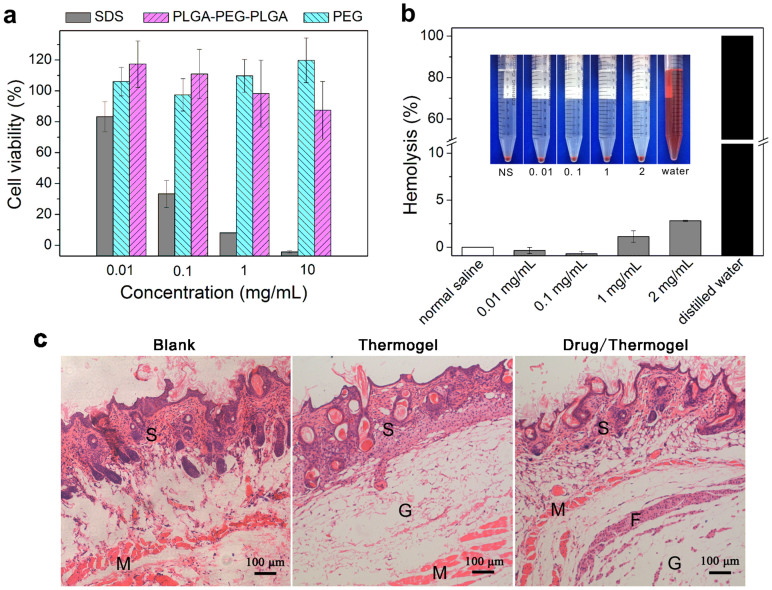
Before trying to load drugs into the biomaterial, we examined the biocompatibility of the block copolymer PLGA-PEG-PLGA synthesized in this study. The in vitro cytotoxicity was first evaluated using a cell counting kit-8 (CCK-8) assay. According to the results in Fig. 3a, the synthetic material exhibited slight cytotoxicity against MC3T3 cells (a mouse osteoblast cell line) with a cell viability of over 80% in the culture medium with polymer concentrations as high as 10 mg/mL. By contrast, cells seldom survived in that concentration of sodium dodecyl sulfate (SDS).
Figure 3. Biocompatibility evaluation of the triblock copolymer PLGA-PEG-PLGA synthesized in this study.
(a) Cytotoxicity of PLGA-PEG-PLGA against the MC3T3 cell line after incubating for 24 h with PEG of number average MW Mn ~ 2000 Da (PEG2000) as the negative control and SDS as the positive control (n = 4). The absorption in the CCK-8 test of the untreated sample (culture medium with 10% fetal bovine serum) was defined as 100%. (b) Hemolysis of rabbit blood cells after being suspended in PLGA-PEG-PLGA aqueous solutions with normal saline as the medium at 37°C for 1 h (n = 3). Complete hemolysis occurred in the group of distilled water, which was defined as 100%. The inserted image shows the medium color of the hemolysis of red blood cells after centrifugation. (c) Optical micrographs of hematoxylin-eosin (HE)-stained slices of surrounding tissues 7 days after subcutaneous injection of the indicated solutions. S: skin tissue; M: muscle tissue; F: fibrous tissue; G: thermogel.
Hematotoxicity of PLGA-PEG-PLGA
The hematotoxicity of PLGA-PEG-PLGA was evaluated using a hemolysis test, following the protocol of ISO 10993 as an international standard for the biological evaluation of medical devices. The hemolysis percentages of blood cells in different concentrations of the polymer solutions are shown in Fig. 3b. The standard of no hematotoxicity usually refers to a hemolysis percentage below 5%. The hemolysis percentage was only 2.8% even in a 2 mg/mL copolymer solution. Therefore, the hemolysis capacity of PLGA-PEG-PLGA was negligible.
Local inflammatory response of PLGA-PEG-PLGA after subcutaneous injection
The local inflammatory response was evaluated by histological observation 7 days after a subcutaneous injection of the copolymer. Some inflammatory cells such as macrophagocytes or monocytes appeared, as shown in Fig. 3c; in some samples, a fibrous tissue surrounding the gel was observed. Nevertheless, the overall inflammatory response was weak. No necrosis of surrounding tissues was observed even in the group in which a mixture of the antitumor drug IRN and copolymer PLGA-PEG-PLGA was implanted.
Phase diagram of PLGA-PEG-PLGA in water
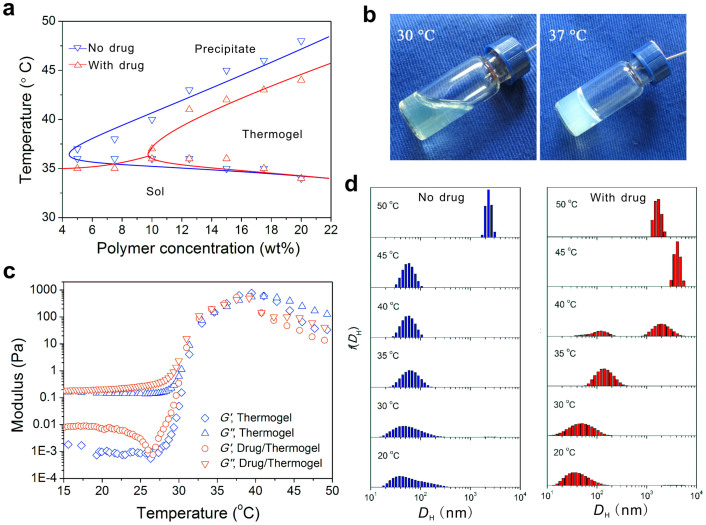
The sol-gel transition of PLGA-PEG-PLGA aqueous solutions was observed using the inverting tube method. The phase diagram is presented in Fig. 4a; some typical images of the sol and gel states are presented in Fig. 4b. The critical gelation concentration (CGC) of the PLGA-PEG-PLGA aqueous solution with normal saline as the medium was approximately 5 wt% and increased to approximately 10 wt% after loading 4 mg/mL IRN. In addition, the transition temperature between the gel and precipitate states decreased 2–4°C after drug loading.
Figure 4. Characterization of the triblock copolymer of PLGA-PEG-PLGA synthesized in this study.
(a) Phase diagram of PLGA-PEG-PLGA aqueous solutions with and without drug loading (IRN, 4 mg/mL). The medium was normal saline. (b) Typical images of the sol and gel states of 20 wt% PLGA-PEG-PLGA aqueous solutions containing 4 mg/mL IRN at the indicated temperatures. (c) Storage modulus G′ and loss modulus G″ of 20 wt% polymer solutions with and without drug loading (IRN, 4 mg/mL). Oscillatory frequency ω: 10 rad/s; Heating rate: 0.5°C/min. (d) Temperature-dependent distributions of hydrodynamic diameters of micelles in 0.1 wt% PLGA-PEG-PLGA aqueous solutions with and without drug loading (IRN, 0.02 mg/mL).
Rheological properties of the thermogelling biomaterials at high concentrations
The sol-gel transition of concentrated polymer solutions was detected using a dynamic rheometer. At lower temperatures, the storage modulus G′ was much lower than the loss modulus G″, indicating a typical sol. With the increase of temperature, both G′ and G″ increased significantly, tracing the process of gelation (Fig. 4c).
Copolymer micellization at low concentrations
The amphiphilic copolymer formed micelles over its critical micelle concentration (CMC) and critical micelle temperature (CMT). The PLGA-PEG-PLGA micelles were detected using a dynamic light scattering spectrophotometer. For the 0.1 wt% polymer solution, the micelle size remained relatively constant when T < 45°C; after drug loading, the enlargement of micelles started early at 35°C, and the light intensity greatly increased, as illustrated in Fig. 4d and Fig. S2.
Degradation of thermogels in vitro
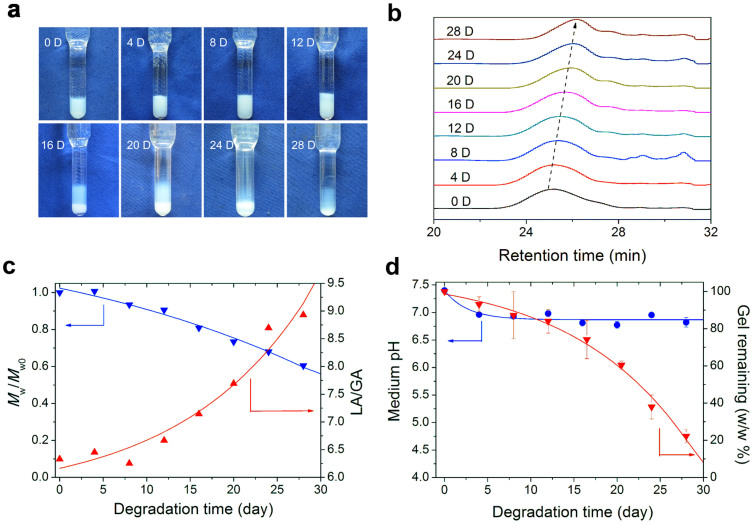
The PLGA-PEG-PLGA aqueous solution underwent hydrolysis. Here, we used phosphate buffer saline (PBS, pH 7.4) as a simulated body fluid to evaluate the degradation behavior of this material in vitro. The gel state of 20 wt% PLGA-PEG-PLGA lasted approximately 2 weeks in PBS at 37°C; then, macroscopic phase separation (precipitation) occurred, as shown in Fig. 5a. During the degradation period, the weight-average MW (Mw) of the copolymer decreased, as illustrated by GPC curves in Fig. 5b. Meanwhile, the LA/GA ratio increased due to the preferred hydrolysis of the repeating unit of glycolic acid compared with that of lactic acid in the copolymer block, and the remaining gel after 28 days was only 20% of the original weight, as shown in Figs. 5c–d.
Figure 5. Degradation of 20 wt% PLGA-PEG-PLGA thermogel in PBS.
(a) Photographs of thermogels in tubes at indicated degradation time in vitro. The volume of PBS was 10 mL in the upper medium per vial with 0.5 mL 20 wt% polymer solution in the bottom. The PBS was refreshed every 4 days. (b) GPC curves of materials at various degradation times. The mobile phase for GPC was tetrahydrofuran with a flow rate of 1 mL/min. (c) Change of Mw of the copolymer and molar ratio of LA/GA during degradation. Mw0 and Mw, the weight-average molecular weight of polymer at day 0 and in degradation, respectively; the values were determined from GPC. The LA/GA molar ratios were obtained from 1H NMR. (d) pH value of the PBS medium and weight percentage of thermogel remaining during degradation.
Drug loading
The drug loading roadmaps examined in this study are summarized in Fig. 2. We observed that direct dissolving both the drug powder and polymer paste in water was not feasible at either room temperature (RT) or high temperature. The high temperature (above 80°C) was necessary for dissolving IRN; however, the amphiphilic block copolymer precipitated at such a high temperature. The commonly used thin film hydration method with dichloromethane as the co-soluble organic solvent of both the drug and polymer was also not sufficient to achieve a high drug loading in this case. First dissolving the polymer paste in water at RT then loading IRN merely through magnetic stirring was not available for this system either, because the drug could not be dissolved at such a temperature.
The available method to co-dissolve the drug and copolymer in water was eventually suggested as follows: IRN powder was first dissolved in normal saline at 85°C to prepare the stock solution; then, the polymer paste was dissolved in the drug stock solution at RT through gently magnetic stirring; the nanoscaled micelles formed along with the copolymer dissolution and drug loading; a clear aqueous dispersion of amphiphilic copolymer micelles was obtained approximately 12 h later.
In vitro drug release from the thermogel
The release profile of IRN was monitored by UV absorption at 370 nm. At this wavelength, the absorption of IRN was least affected by the pH of the medium, as illustrated in Fig. S3, with the standard curve presented in Fig. S4.
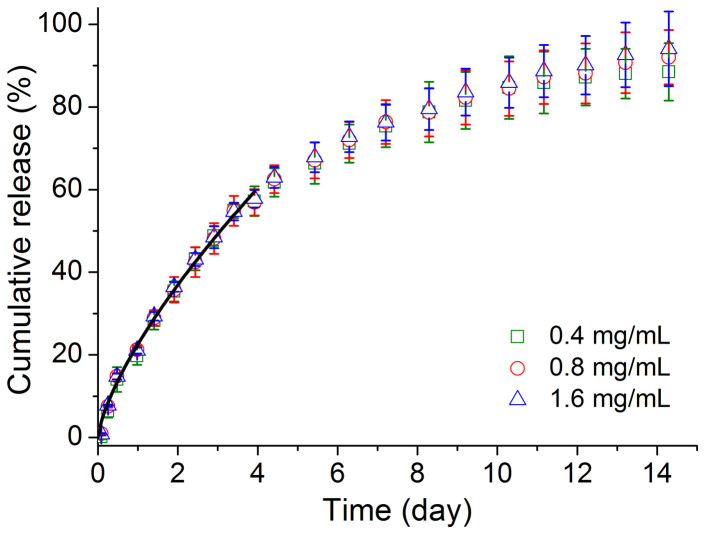
IRN was observed to be released from the thermogel in a sustained manner for approximately 2 weeks. The relative cumulative release seemed not very sensitive to the loading amount of the drug in the detection range, as observed in Fig. 6.
Figure 6. Sustained release of IRN.
Drug release profile of IRN of indicated drug concentrations out of the thermogel with 20 wt% copolymer (n = 3). The solid black line represents the data fitting via equation (1) within a cumulative release <60%.
The data of cumulative release of IRN was evaluated using the Peppas equation34:
 |
where Mt/M∞ refers to the relative cumulative release at time t. This equation is justified within the cumulative release <60%34. The exponent m is determined by the underlying release mechanism: m = 0.5 for diffusion-controlled release, and m = 1 for degradation-controlled release; the intermediate value indicates coexistence of the above two mechanisms. The fitted value of m of the release curves in this work was equal to 0.71 with a squared correlation coefficient R2 of 0.99. Therefore, both drug diffusion and material degradation are responsible for the release of the moderately soluble drug IRN from the thermogel of the amphiphilic block copolymer PLGA-PEG-PLGA.
Lactone fraction of IRN in thermogel
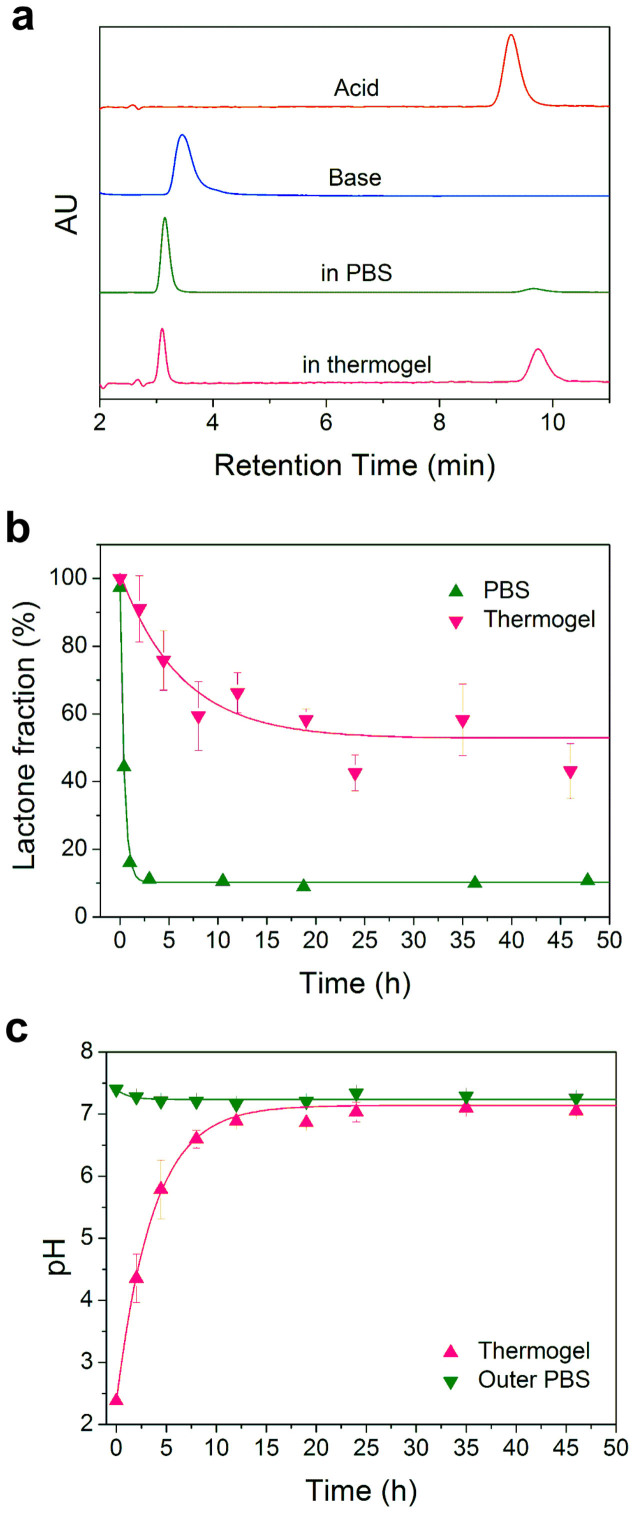
Similar to other CPT analogs, IRN undergoes hydrolysis from the active lactone to the inactive carboxylate at physiological conditions, leading to lower efficacy but still with toxicity35. We separated and quantified the two forms of IRN using high performance liquid chromatography (HPLC). The retention times of these two forms, active lactone and inactive carboxylate, were approximately 9 min and 3 min, respectively, in our experiments (Fig. 7a). The lactone form of IRN was rapidly hydrolyzed to the carboxylate form in PBS, with a final equilibrium lactone fraction of only approximately 10% (Fig. 7b). By contrast, even though the pH within the gel already returned to neutral of about pH 7 after 10 h (Fig. 7c), the lactone fraction in the thermogel still remained as high as 50% (Fig. 7b).
Figure 7. Active lactone fraction of IRN in the thermogel.
(a) HPLC chromatogram of IRN under indicated conditions. The complete lactone form was obtained under an acid condition (pH 3); the complete carboxylate form was obtained under a base condition (pH 10). The equilibrium lactone fraction was much higher in the thermogel than that in PBS only. (b) Lactone fractions of IRN in both PBS and thermogel (20 wt% PLGA-PEG-PLGA with PBS as medium) at 37°C at indicated time points (n = 3). (c) pH values in the thermogel and outer PBS.
Tumor inhibition ratio
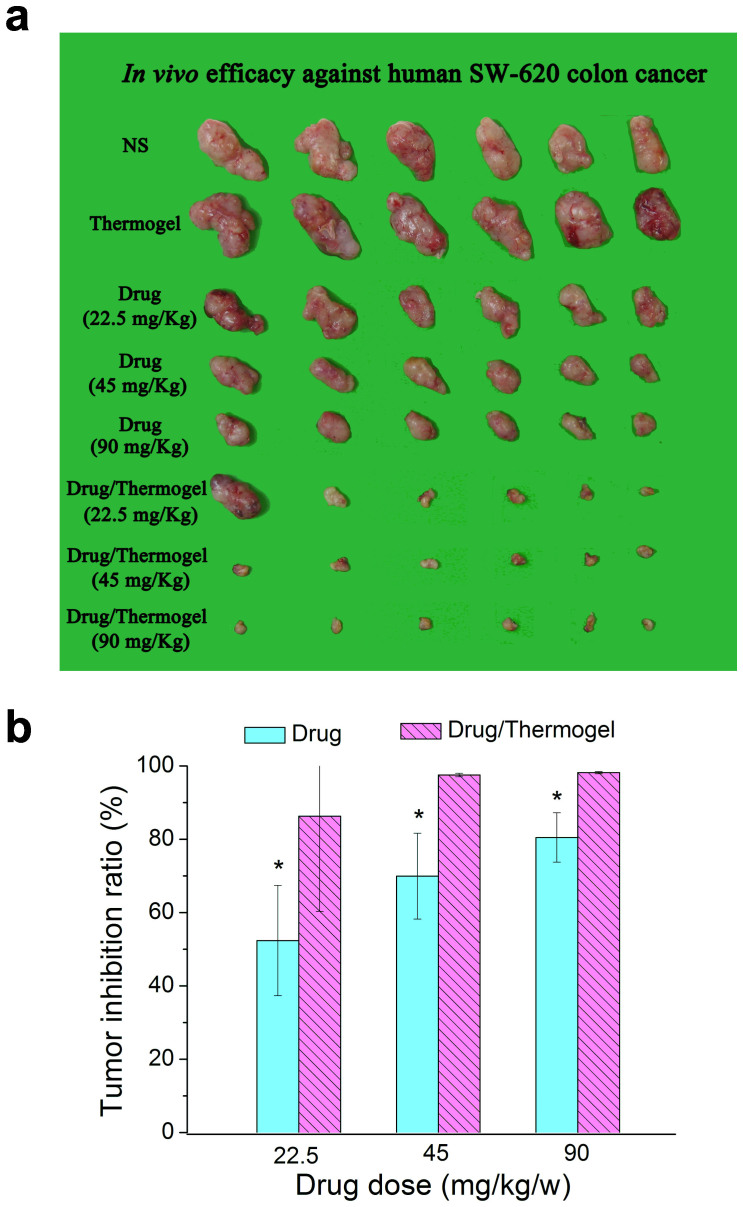
The in vivo antitumor efficacy of this sustained release system of IRN was evaluated in a nude mouse model bearing human SW620 colon tumors. The corresponding treatment information of each group is summarized in Table 2. The tumor tissues from all the mice were imaged after sacrificing the mice on day 21, and the photographs are presented in Fig. 8a.
Table 2. In vivo efficiency against human SW620 colon cancer in nude mice.
| Animal number | ||||||
|---|---|---|---|---|---|---|
| Group | Drug dose (mg/kg/w) | Delivery Route | Start | End | Tumor weight (g) | Tumor inhibition ratio (%)e) |
| NSa) | 0 | s.c. | 6 | 6 | 1.82 ± 0.45 | |
| Thermogelb) | 0 | s.c. | 6 | 6 | 2.21 ± 0.49 | / |
| IRNc) | 22.5 | i.v. | 6 | 6 | 0.87 ± 0.27 | 52.3 |
| IRNc) | 45 | i.v. | 6 | 6 | 0.55 ± 0.21 | 69.9 |
| IRNc) | 90 | i.v. | 6 | 6 | 0.36 ± 0.12 | 80.5 |
| IRN/thermogeld) | 22.5 | s.c. | 6 | 6 | 0.25 ± 0.47 | 86.2 |
| IRN/thermogeld) | 45 | s.c. | 6 | 6 | 0.05 ± 0.01 | 97.5 |
| IRN/thermogeld) | 90 | s.c. | 6 | 6 | 0.03 ± 0.01 | 98.2 |
a)NS: normal saline as the negative control.
b)Thermogel: polymer solutions only with 20 wt% PLGA-PEG-PLGA. A 0.45 mL polymer solution was injected subcutaneously into the back of each mouse on days 0, 7, and 14, with tumor tissues inoculated in the right armpit.
c)IRN: drug solution only (1 mg/mL, 2 mg/mL, or 4 mg/mL) in normal saline. A 0.45 mL drug solution was injected intravenously into each mouse on days 0, 7, and 14.
d)IRN/thermogel: IRN (1 mg/mL, 2 mg/mL, or 4 mg/mL) in polymer solutions (20 wt% PLGA-PEG-PLGA). A 0.45 mL mixture solution of the drug and polymer was injected subcutaneously into the back of each mouse on days 0, 7, and 14.
e)Calculated from equation (2). A tumor inhibition value >40% indicates effectiveness.
Figure 8. In vivo antitumor efficacy against human SW620 tumor.
(a) Tumor tissue extracted from nude mice after a cycle of therapy. The groups are indicated in Table 2. (b) Tumor inhibition ratios of medication groups with or without thermogel. Student t-tests were performed, and p < 0.05 was regarded as a statistically significant difference, denoted by an asterisk. The p values are 0.024, 0.002, 0.001 between the drug/thermogel and drug groups with respect to 22.5, 45, 90 mg/kg/w IRN doses (n = 6 per group), respectively.
The results were quantified by the tumor inhibition ratio as follows:
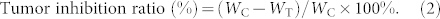 |
where WC is the tumor weight in the negative control group (only normal saline injected), and WT measures the tumor weight in the test group. While the tumor inhibition ratios in the groups receiving IRN only ranged from 52.3% to 80.5% with an increase of drug dose, those in the IRN/thermogel groups were 86.2% to 98.2% at the same dose as in the IRN groups, as presented in Fig. 8b. Therefore, the sustained-release group exhibited superior efficacy.
Tumor growth curves
We also monitored the tumor growth of all the groups, with the results summarized in Table 2. The tumor length (a) and tumor width (b) were measured twice a week during the 3 weeks of drug treatment, without sacrificing mice. The measurement of the tumor depth was not available without extracting tumors, and the value was estimated to be close to b. The tumor volume V was calculated using 1/2 × a × b2 with the assumption of an ellipsoid and the approximation of π/6 ~ 1/2.
The time-dependent volumes were deduced using the following equation:
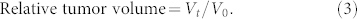 |
where V0 and Vt refer to the tumor volumes at day 0 and day t, respectively. The deduced results are presented in Fig. 9.
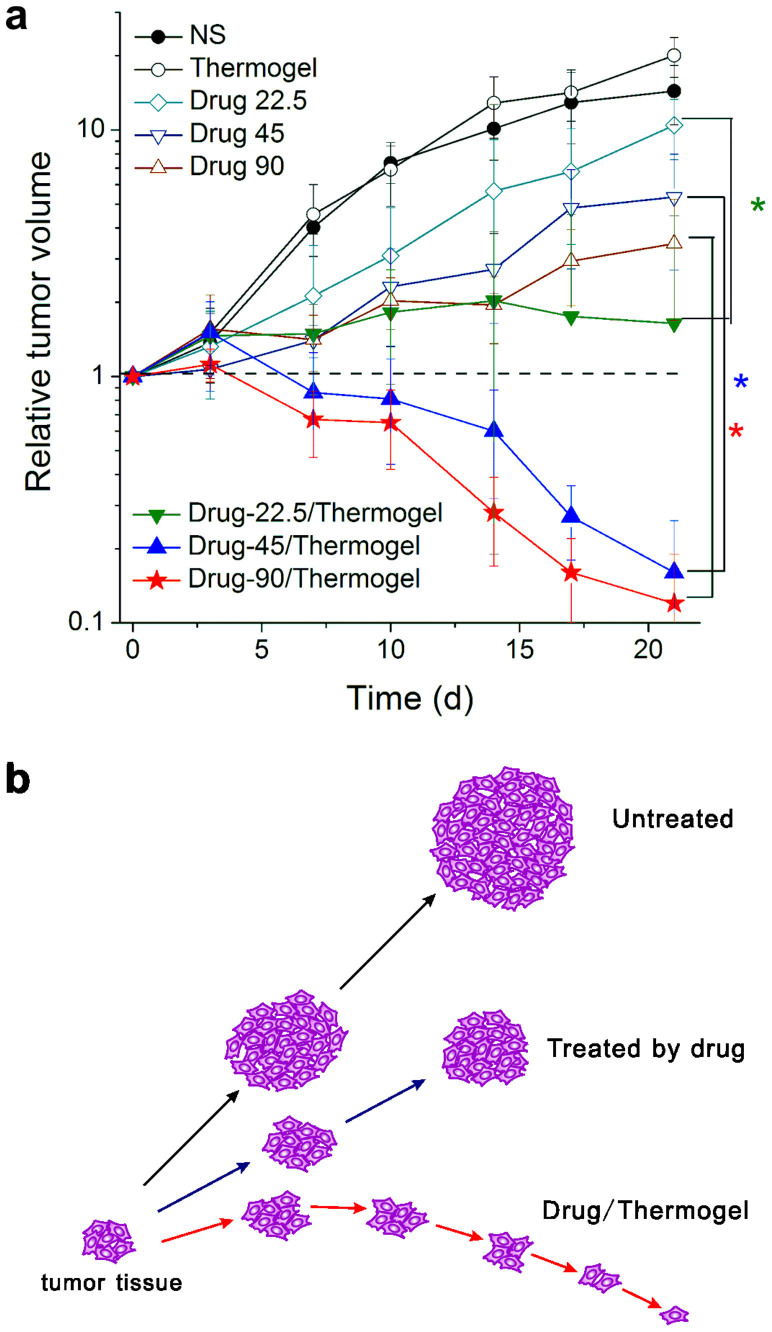
Figure 9. Tumor volume as a function of time in nude mice bearing human SW620 tumors.
(a) The relative tumor volume calculated from equation (3) of the indicated groups (n = 6). The dashed line marks the value of “1”, indicating no change of tumor volume after a cycle of therapy. Student t-tests were conducted on day 21 between the IRN only group and the IRN/thermogel group at the same drug dose, resulting in p values of 0.0003, 0.0048, and 0.0058 for drug doses of 22.5, 45, and 90 mg/kg/w, respectively, all less than 0.05. (b) Scheme of change of tumor volume during therapy. In the untreated group, the tumor volume increased most rapidly; in the IRN-only-treated group, the final tumor volume was smaller than that of the untreated group but still larger than the initial one; in the drug/thermogel group, the tumor volume first slightly increased and then decreased significantly. The final tumor volume was smaller than the initial size.
The initial tumor volumes were all pre-designed to be approximately 100 mm3 before drug administration; the actual experimental values were 96 mm3 on average. The final tumor volume of the NS group was one order of magnitude larger than the initial volume, resulting in the average value of the relative tumor volume being 14.4. For the IRN group, the tumor volume increased continuously during the entire therapy; however, the tumor growth rate was slower than that of the NS group. The values of the relative tumor volume were 10.4 to 3.5 upon increase of drug dose. For the IRN/thermogel groups, the tumor volume first slightly increased and then decreased significantly with the average of the final values of the relative tumor volume being 1.6 to 0.1, indicating significant regression of the tumor tissue after treatment with the IRN/thermogel.
Decrease of body weight
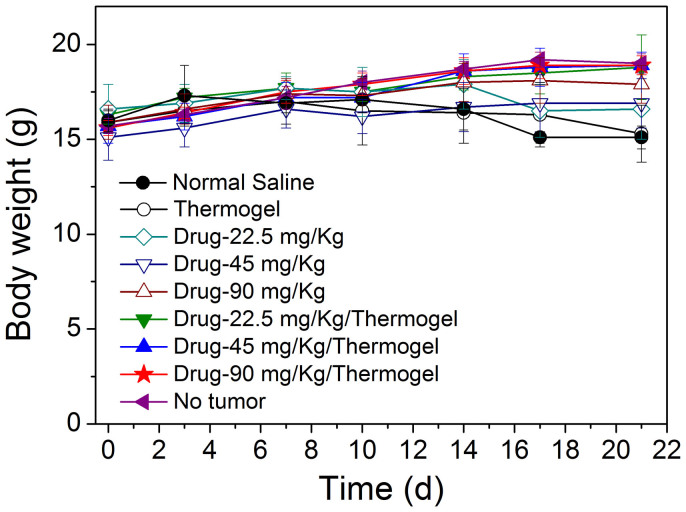
We also examined the possible adverse effects. Chemotherapy often leads to severe side effects such as gastrointestinal toxicity and myelosuppression; the most typical gastrointestinal toxicity of IRN is diarrhea together with body weight loss36. No diarrhea was observed in any of our groups, indicating that the drug doses used in this study were within the tolerable range.
The body weight of the IRN/thermogel groups remained similar to that of the tumor-free group during the treatment period, as shown in Fig. 10. By contrast, for the mice receiving IRN only, the body weights (including tumors) were slightly lower than those of the IRN/thermogel groups in Fig. 10, even though the tumor weights were higher (Table 2).
Figure 10. Possible side effects of body weight.
Nude mice bearing human colon tumor SW620 of the indicated groups in Table 2 were weighed during a cycle of therapy (n = 6).
Decrease of immune cell number
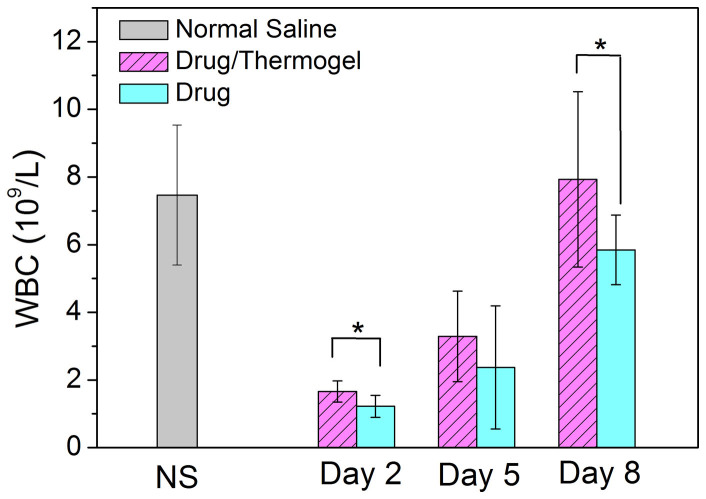
The in vivo hematological toxicity or myelosuppression of IRN was evaluated by counting the white blood cells (WBCs) in Kunming mice bearing murine solid S180 tumors after IRN treatment. Kunming mice were used instead of nude mice in this experiment to verify the possible adverse effects of the antitumor drug on immune cells because such a test is not meaningful for nude mice with immune deficiency. As a famous experimental animal model, the nude mouse is excellent for an in vivo examination of human cells and tissues, and was thus used to examine all of the other effects in this study.
For the negative control group, in which saline was injected, the WBCs were maintained at a high level of 7.5 × 109 per liter blood. On the next day after IRN was injected, the WBC count rapidly decreased below 2 × 109 per liter blood. By contrast, the extent of decrease in the IRN/thermogel group was significantly lower than that in the group receiving IRN only, as shown in Fig. 11. Therefore, the recovery of WBCs was also quicker in the IRN/thermogel group than in the IRN group.
Figure 11. Myelosuppression after medication of IRN with or without thermogel.
WBC number per liter blood in Kunming mice (capable of normal immune system) bearing murine solid tumor S180 after injecting IRN or IRN/thermogel with the same drug dose (45 mg/kg). The p values from t-tests are 0.007 and 0.047 between the IRN and IRN/thermogel groups at day 2 and day 8, respectively. Injection of NS was set as the negative control of myelosuppression. At day 8, the p values between IRN or IRN/thermogel and the NS group are 0.045 and 0.677, respectively. The animal numbers of n are as follows: 10 for the group of NS, 10 for IRN/thermogel (day 2), 10 for IRN (day 2), 6 for IRN/thermogel (day 5), 4 for IRN (day 5), 9 for IRN/thermogel (day 8), and 10 for IRN (day 8).
Discussion
Why is sustained release required for IRN and what is the appropriate release time for an ideal DDS of IRN?
Irinotecan has been used to treat colon cancer for decades18; nevertheless, a sustained release system for IRN is still needed. We believe that the necessity of the sustained release stems mainly from two issues. The first issue is related to its antitumor mechanism: all CPT family members are S-phase cell specific inhibitors, and a longer exposure of S-phase inhibitors is beneficial for their efficacies37. The second issue involves the structure of IRN itself. The chemical structure of IRN has to be converted to 7-ethyl-10-hydroxycamptothecin (SN38) by carboxylesterases in vivo to enable substantial antitumor efficacy38, and this enzyme might be saturated23. While the ratio of the AUC (area under the curve in a plot of concentration of the drug in plasma vs. time) of SN38 to that of IRN was only 3–5% in the treatment schedule of injections every week or every 3 weeks, the value could be significantly increased to 16% under a continuous infusion for 14 days23. The side effects of myelosuppression (reduced WBCs) and gastrointestinal toxicity (diarrhea) were also dose dependent and therefore dose-limiting36,39. Thus, sustained release is highly required for the delivery of IRN while considering the drug efficacy and side effects.
Some new DDSs have been developed to modify the release profiles of IRN, including nanoparticles40, microspheres41, and macroscopic matrices42. In practical trials, this aim is often achieved by increasing the injection times or elongation of infusion time39,43, sometimes even for weeks23, which makes the medication poorly compliant to patients. In addition, there have been reports that a continuous infusion would lead to a cumulative toxicity of IRN if used for longer than 21 days23. Therefore, the release time is not necessarily very long; instead, it should fit the clinical requirements. In our opinion, the ideal release system of IRN must have no significant burst release and be almost completely released with a sustained release profile within a period of less than 3 weeks, usually 1–2 weeks.
Why was the PLGA-PEG-PLGA thermogel used?
Amphiphilic copolymer macromolecules in water form micelles if the copolymer concentration is over the CMC. The PLGA block is hydrophobic, while the PEG block is soluble in water but exhibits some hydrophobic interactions with temperature sensitivity44. The hydrophobic interaction is driven by the decrease of the loss of the orientational entropy of water molecules surrounding the solute groups, resulting in a positive entropy change (ΔS > 0) in a hydrophobic aggregation process. The free energy difference ΔG is related to ΔS via ΔG = ΔH − TΔS, where ΔH and T are the entropy change and absolute temperature, respectively. Therefore, the effect of the hydrophobic aggregation must be strengthened by temperature, which is why the aqueous system of PLGA-PEG-PLGA would, under appropriate compositions, concentrations and salt conditions, undergo a sol-to-gel transition upon heating. The internal structure of the thermogel is a percolated micelle network44,45.
Unlike a concentrated fish soup gelling at low temperatures, the aqueous system of this synthetic block copolymer can be reversibly physically gelled. This unique thermogelling property enables this type of material to act as an injectable medical material: at low temperatures such as room temperature, the aqueous sol is injectable and can mix with drugs; after being injected into the body, it gels rapidly in approximately half a minute to form a drug-releasing matrix in situ if the sol-gel transition temperature Tgel is between refrigerator (usually 4°C) and body temperature (37°C) and the precipitation temperature is above the body temperature17. Thus far, the use of these thermogels has been attempted in drug delivery46,47,48, post-operative antiadhesion49 and other biomedical applications50.
The solubility of drugs is a classic and challenging topic in pharmaceutics51. The mixing of the moderately soluble drug IRN with the temperature-sensitive amphiphilic block copolymer PLGA-PEG-PLGA is problematic, as illustrated in Fig. 1c and Fig. 2. We eventually overcame this difficulty using an appropriate control of temperature for the different processes. The structure of IRN remained intact during the entire dissolving and loading process, as confirmed by a comparison between the characteristic UV absorptions of IRN before and after heating at 85°C for 24 h (Fig. S5) and between HPLC chromatograms of IRN before and after heating (Fig. S6). The IRN loading was observed not to hamper the sol-gel transition of the PLGA-PEG-PLGA thermogel (Fig. 4), although some drug-material interactions occurred. In general, the loading of IRN tended to increase the global “hydrophobicity” of the polymer micelles and caused them to more easily aggregate to form large clusters. As illustrated in Fig. 4d, the micelle size began to increase at lower temperatures compared with the micelles that were free of drug loading. This increase of hydrophobicity caused the thermogel to more easily precipitate, with the transition temperature from the gel to precipitate decreasing 2–4°C in Fig. 4a. In other words, the moderately soluble drug IRN and the amphiphilic copolymer PLGA-PEG-PLGA were mixable, and the aqueous system of the mixture was still thermogelable; however, some drug-material interactions occurred.
Higher in vivo antitumor efficacy of IRN in thermogel
The in vivo antitumor efficacy against human colon cancer was examined in nude mice. The tumor inhibition ratio serves as an indicator of therapeutic efficacy. For the sustained release groups (IRN/thermogel), the tumor inhibition ratio reached 98% at a drug dose of 45 mg/kg. According to the tumor images in Fig. 8a, even the group with a low drug dose (22.5 mg/kg IRN/thermogel) exhibited a good antitumor effect. By contrast, for the groups that received only IRN, the tumor inhibition at the highest dose of 90 mg/kg was still worse than that at the lowest dose in the presence of the copolymer (22.5 mg/kg IRN/thermogel).
The curves of the time-dependent relative tumor volumes are presented in Fig. 9. After injection of IRN/thermogel, the tumor volume first increased slightly and then decreased significantly. The relative tumor volumes on day 21 in the IRN/thermogel groups were 0.2 and 0.1 for drug doses of 45 and 90 mg/kg/w, respectively. The final tumor volume was only 10% of the initial size, indicating a dramatic tumor regression. The “extra” tissue cannot disappear completely, and some “scar” is left after implanting numerous extra cells and materials subcutaneously52. In summary, the sustained release of IRN with the PLGA-PEG-PLGA thermogel exhibits excellent antitumor activity against human SW620 colon cancer.
Reduced side effects of IRN in thermogel
The body weight of nude mice (immune system destroyed) and WBC number per liter of blood of S180-tumor bearing mice (capable of normal immune system) were evaluated as indicators of possible adverse effects of IRN. From the body weight of the mice of all the groups, as shown in Fig. 10, there is no marked difference between the experimental group and tumor-free group. In addition, no diarrhea or other global adverse effects were observed.
For the WBC count, a significant difference was observed between the IRN and IRN/thermogel groups, as shown in Fig. 11. The IRN/thermogel group exhibited a relatively lower WBC decrease than the IRN group, and the recovery was also more rapid in the IRN/thermogel group. Thus, the side effects of IRN were alleviated through the sustained release.
Ideal release profile and period of IRN from the thermogel
Then, what causes the significant therapeutic efficacy and reduced side effects after the drug was encapsulated into the thermogel? Two reasons might account for these excellent results.
First, an ideal release profile and period were designed and achieved, with no significant initial burst and an almost complete release in approximately 2 weeks, as schematically illustrated in Fig. 1d and experimentally illustrated in Fig. 6. It is rare to meet the requirements of these three aspects (slight initial burst, complete release, and sustained release for 2 weeks) in one formulation. Usually, the release profile and time of drugs within this type of thermogel is mainly affected by the solubility of the drug molecules27. For a highly water-soluble small-molecule drug, a significant initial burst release would appear24; in the other extreme, a water-insoluble drug frequently leads to incomplete release26. The apparent solubility of IRN was approximately 10 mg/mL, with the detection procedure described in the Supporting Information, indicating that the drug is moderately soluble in water. Notably, the present combination of the moderately soluble drug IRN with the amphiphilic block copolymer PLGA-PEG-PLGA ideally resolves the classic dilemma in pharmaceutics, which is between the suppression of the initial burst release and maintenance of complete late-stage release24,25,26,27. In addition, the gel network was delicately designed to only last for 2 weeks and then collapse, as illustrated in Fig. 5a, which also guaranteed the complete release of IRN in the late stage. This ideal release profile and period are attributed to the properties of both the drugs and the materials. Interactions between the drugs and materials play an important role in modulating the release profile. This insight might be helpful for the design of other sustained release systems of moderately soluble drugs.
Enhancement of the lactone form of IRN in thermogel
Another important reason for the drug efficacy is related to the chemical form of this drug. Similar to other CPT analogs, IRN has two forms: active lactone and inactive carboxylate. The hydrolysis process of the CPT-family drug consists of two steps: ring-opening of the lactone and ionization of the carboxylate53, written as follows:
 |
The equilibrium depends upon the pH of the medium, as indicated in Fig. 1a. Nevertheless, the much higher lactone fraction of IRN within the thermogel is not mainly due to the pH change in the thermogel, for the interior pH within the thermogel is primarily neutral, as indicated in Fig. 7c. Neither can the dynamic “protection” account for the high lactone fraction. The enhancement in the thermogel system is a thermodynamic phenomenon with a shift of equilibrium, as clearly illustrated in Fig. 7b. The key is the change of the equilibrium constants.
We use a term pH1/2 defined as the pH with respect to the lactone fraction f = 1/254. The lactone fraction equals [L]/([C] + [C−]). Because the concentration of the unionized open-ring form is very low53, f is approximated as follows:
 |
The global equilibrium constant of IRN is written as follows:
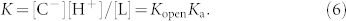 |
Therefore,
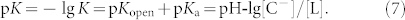 |
The combination of equations (5) and (7) with the definition of pH1/2 leads to
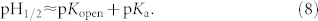 |
The equilibrium lactone fraction of IRN in PBS was only approximately 10% (Fig. 7b), and thus, pH1/2 ≪ 7.4 for IRN in PBS. While pKopen in the first step of the ring-opening in equation (4) is a constant, pKa in the second step of ionization of the carboxylate form for the CPT family is significantly affected by the local hydrophilic/hydrophobic environment55,56. The ionization constant of the moderately soluble drug IRN might be highly sensitive to the local hydrophilicity or hydrophobicity; the addition of the amphiphilic block copolymer PLGA-PEG-PLGA can lead to less ionization of the carboxylate form and thus larger pKa, resulting in a higher pH1/2 due to drug-material interactions. Therefore the equilibrium lactone fraction of IRN might be increased even at a given physiological pH.
In this study, we detected the lactone fraction of IRN in the thermogel during release, as shown in Fig. 7. Although the pH within the gel rapidly returned to neutral within approximately 10 h, the equilibrium lactone fraction of IRN remained as high as approximately 50%. IRN could be kept active during a long release period, whereas in PBS, the lactone fraction rapidly decreased to only 10% within 2 h. Therefore, the enhancement of the active lactone fraction led to a higher drug efficacy with reduced side effects, which is attributed again to the drug-material interactions.
Finally, the present study offers significant improvements compared with our recent work57, although thermogels and antitumor drugs were examined in both studies. First, the present paper is the original report of the tumor regression using a thermogel formulation with an encapsulated antitumor drug. Second, the enhancement of the lactone form of IRN, a CPT analog, due to the drug-material interactions, has not been concerned in the above reference at all, and this issue is one of the key reasons of the excellent antitumer efficacy in the present study. Third, another key reason, namely, an ideal release profile and period of IRN in the present report about IRN in a thermogel is also absent in that report about another antitumor drug doxorubicin (DOX.HCl) in a thermogel. We would like to emphasize that the critical improvement is not simply from a different drug used. Mixing the water-soluble drug DOX.HCl with the thermogel is very easy, but the initial release is burst. By contrast, the release profile of IRN is ideal with a relatively much insignificant initial release, but encapsulating IRN, a typical moderately soluble drug with the thermogel is very difficult with a tricky mixing strategy reported in this paper for the first time.
In summary, the commercialized and nonexclusive antitumor drug IRN was successfully loaded into a thermogel of the synthetic block copolymer PLGA-PEG-PLGA using an appropriate mixing roadmap. The system exhibited rich physics; the drug was released in a sustained manner for approximately 2 weeks, without a significant initial burst and with almost complete release. The active lactone form of IRN was significantly enhanced from approximately 10% in PBS to approximately 50% even at neutral pH within the thermogel. The in vivo antitumor efficacy against SW620 human colon cancer confirmed that after being treated with appropriate IRN/thermogel, the tumor volume initially increased slightly and then decreased significantly, leaving the tumor volume completely reduced compared with the initial size. Possible adverse effects were also evaluated. The sustained release of IRN with the PLGA-PEG-PLGA thermogel relieved myelosuppression compared with an intravenous injection of IRN only. Therefore, simply loading IRN into the PLGA-PEG-PLGA thermogel could both significantly regress the tumor and reduce the side effects.
The present study paves an avenue to resolve a classic dilemma in the chemotherapy of tumors by taking advantage of interactions between drugs and materials. Such a convenient chemotherapy formulation by physically mixing the drug and the copolymer might impact the fields of biomaterials and pharmaceutics. Considering that the drug itself is used clinically and the building blocks of the copolymer have been approved by FDA, a strengthened “blockbuster” is expected, and further investigations are required. The strategy to employ moderately soluble drugs and amphiphilic biomaterials might also stimulate the development of other drugs, biomaterials, and advanced DDSs.
Methods
Materials
Irinotecan hydrochloride was purchased from Shanghai Knowshine Pharmachemicals, Inc. PEG with number average molecular weights of Mn ~ 1500 Da and 2000 Da were products of Sigma-Aldrich. D,L-lactide (LA) and glycolide (GA) were purchased from Purac. All the chemicals were used without any purification.
Polymer synthesis
The block copolymer PLGA-PEG-PLGA was synthesized by the ring-opening polymerization of LA and GA initiated by the hydroxy end groups of PEG. In brief, 20.06 g PEG with Mn ~ 1500 Da was dehydrated under vacuum at 120°C for 4 h. After the temperature decreased to 80°C, 4.21 g GA and 37.18 g LA were added under the protection of argon and dehydrated for another 30 min. Then, 0.15% stannous octoate of the raw material of LA and GA (w/w) was added as a catalyst. After the reaction at 150°C for 12 h, the product was purified by washing in 80°C hot water three times.
In vitro cytotoxicity of PLGA-PEG-PLGA
MC3T3 cells (a mouse osteoblast cell line) were seeded in a 96-well cell culturing plate at a cell density of 1.0 × 104/well. After the cells completely spread on the plate, the upper medium was refreshed with a new medium of 200 μL minimum essential medium α (MEMα) cell culture containing 10% fetal bovine serum and various amounts of polymers or other chemicals, in which PEG with Mn ~ 2000 Da (PEG2000) and SDS were used as negative and positive controls, respectively. After incubating at 37°C in a 5% CO2 atmosphere for another 24 h, 20 μL of CCK-8 stock solution was added into each well. The incubation lasted at 37°C for another 3 h. Then, the absorption of each sample was measured at 492 nm. The cell viability showed a positive correlation with the value of absorption, and the value in the group of culture medium with no polymer was defined as 100%.
Blood toxicity of PLGA-PEG-PLGA
Copolymer solutions of various concentrations were prepared with normal saline. After being filtered with a 0.22-μm filter, 100 μL of fresh blood of New Zealand white rabbit was added into 10 mL of the above solutions, in which normal saline and distilled water were used as negative and positive controls, respectively (n = 3). After being incubated in a 37°C water bath for 1 h, all the samples were centrifuged at 1000 r/min for 5 min. The UV absorption at 540 nm of the upper transparent solution was detected. The hemolysis percentage was calculated using (A-Anc)/(Apc-Anc), where Apc and Anc refer to the absorption of the positive control group (distilled water) and negative control group (normal saline), respectively, and A, of the experimental group.
Histological observation after subcutaneous injection of PLGA-PEG-PLGA aqueous solutions
The surrounding tissue after 7 days of injecting polymer solutions into nude mice subcutaneously were excised out and fixed in 4% paraformaldehyde. After embedded with paraffin, the tissue was sliced and stained by HE. The stained slices were examined using optical microscopy.
Drug loading
IRN stock solution was prepared after dissolving the drug powder in normal saline at 85°C with the final drug concentrations of 0.5, 1, 1.25, 2, 2.5, 5 mg/mL. Then add the polymer paste into above drug solutions. After quickly cooling down to RT, the polymer was dissolved into the drug solution after gentle magnetic stirring for 12 h. Drugs were encapsulated into thermogels due to the temperature-induced sol-gel transition after the sol was injected into mammal body in vivo or heated to the body temperature in vitro.
Phase diagram of PLGA-PEG-PLGA in water
The sol-gel transition of PLGA-PEG-PLGA aqueous solutions (NS as medium) was observed using the inverting-tube method58. Briefly, a series of polymer solutions of various concentrations (5, 7.5, 10, 12.5, 15, 17.5 and 20 wt%) with or without drug loading (IRN, 4 mg/mL) was prepared. Then, 700 μL of each sample was added to a vial, and the vials at each temperature were equilibrated in the water bath for 15 min. The state was defined as a gel if no flow was observed within 30 s after the vials were inverted 180°.
Rheological measurements
The sol-gel transition of the 20 wt% PLGA-PEG-PLGA copolymer solutions were detected using a rheometer (Malvern, Kinexus) equipped with parallel plates (diameter of 60 mm). Cold samples were dropped on the parallel plates with an initial temperature of 15°C. The storage modulus G′ and loss modulus G″ were determined along with the temperature increase to 50°C with a heating rate of 0.5°C/min and an oscillatory frequency ω of 10 rad/s. The detection process was controlled within the linear viscoelastic range.
Dynamic light scattering
The sizes of the PLGA-PEG-PLGA copolymer micelles in normal saline were determined using a NanoZS90 (Malvern) light-scattering spectrophotometer. The dispersions were filtered through a 0.45-μm filter before measurement. The samples were equilibrated at each temperature for 15 min. The scattering data were fitted using the GENERAL approach.
Degradation of thermogels in vitro
A half milliliter of the copolymer solution (20 wt%) was pipetted into the test tube. After equilibrating at 37°C for 10 min, 10 mL PBS (pH 7.4) was added. Every 4 days, three of the thermogel samples were taken out and lyophilized. The freeze-dried samples were carefully weighed; the MW and LA/GA ratio were determined by GPC and 1H NMR. For the other samples, PBS was refreshed to maintain the neutral degradation medium.
Drug release from thermogels
Half milliliters of IRN-loaded polymer solutions were injected into the test tubes. After equilibrating at 37°C for 10 min, 10 mL of the release medium (PBS, pH 7.4) was added. At a predetermined time, 9 mL PBS was taken out and refreshed with the new medium of an equal amount. Then, the old release medium was detected using a UV spectrometer at 370 nm to calculate the drug concentrations according to the pre-calibrated standard curve. The corresponding polymer solution without the drug loaded was used as a control. Each specimen was duplicated 3 times.
Lactone fraction of IRN in thermogels
The lactone fraction of IRN in thermogel was determined by HPLC. Six grams of PLGA-PEG-PLGA copolymer solution (20 wt%) with 1.6 mg/mL IRN loaded was injected into a dialysis with cut-off MW 3500 Da. Then, the solution was transferred into 500 mL 37°C PBS for equilibrating.
At a predetermined time, the pH of the thermogel in dialysis was detected using a pH meter from Aurora Scientific Instruments (Shanghai) Co., Ltd., and some thermogel was taken out for HPLC analysis to detect the lactone fraction. The HPLC was conducted by a 5-μm C18 reverse phase column (150 mm × 4.6 mm, SunFire™) with a mobile phase of 23% methanol, 70% phosphate buffer (25 mM, pH 6.5) and 7% tetrahydrofuran at a flow rate of 1 mL/min. Each sample was measured in triplicate.
In vivo antitumor efficacy
A model of human SW620 solid colon tumor in nude mice (with immune system destroyed) was used to examine the antitumor efficacy of the sustained release system of IRN. Briefly, 5.0 × 106 SW620 colon cancer cells were inoculated in each nude mouse. After the tumor tissue grew, it was removed, cut into small pieces of 1.5 mm3 and inoculated subcutaneously in the right armpit of the experimental nude mice again. After the tumor tissue grew to approximately 100 mm3 (denoted as day 0), sample solutions were injected at day 0, day 7, and day 14. All the mice were sacrificed at day 21. Then, the tumors were taken out and weighed. During this period, the tumor sizes and mice body weights were recorded twice a week.
All the animal tests were performed in accordance with the approved guidelines of the “Principles of Laboratory Animal Care” (NIH publication #85-23, revised 1985) and were approved by the Institute Animal Care and Use Committee at Shanghai Institute of Materia Medica, Chinese Academy of Sciences.
Blood tests
The hematological toxicity of IRN was evaluated via WBC counting in the S180-tumor-bearing Kunming mice after being IRN medicated. S180 is a murine sarcoma, and a Kunming mouse is a standard experimental animal capable of the normal immune system. Briefly, S180 tumor cells were inoculated in the right armpit of the mice at day 0. At day 1, IRN and IRN/thermogel were injected intravenously and subcutaneously, respectively; the intravenous injection of NS was set as the negative control of myelosuppression. At days 2, 5, and 8, the jugular blood was collected for WBC counting by Adicon Clinical Laboratories, Inc. The numbers of available samples for WBC counting were not all the same for the different groups because blood coagulation sometimes occurred during blood collection. NS: n = 10; IRN/thermogel (day 2): 10; IRN (day 2): 10; IRN/thermogel (day 5): 6; IRN (day 5): 4; IRN/thermogel (day 8): 9; IRN (day 8): 10.
Statistical analysis
Data are presented as means plus standard deviation. Student t tests were used to make comparison between two groups. The difference was regarded as significant if p < 0.05.
Author Contributions
J.D.D. suggested the examination of encapsulating antitumor drugs into thermogels to achieve regression of tumors. J.D.D. and T.Y.C. designed the experiments; T.Y.C. performed most of the experiments; T.Y.C. and J.D.D. performed most of the data analysis and manuscript writing. L.C. and L.Y. joined in pertinent experiments and manuscript writing.
Supplementary Material
Supporting Info
Acknowledgments
The authors are grateful for the financial support received from the NSF of China (grants No. 91127028, No. 21034002, and No. 51273046), the Chinese Ministry of Science and Technology (973 program No. 2011CB606203), and the Shanghai Government.
References
- Chabner B. A. & Roberts T. G. Timeline - Chemotherapy and the war on cancer. Nat. Rev. Cancer 5, 65–72 (2005). [DOI] [PubMed] [Google Scholar]
- Galmarini D., Galmarini C. M. & Galmarini F. C. Cancer chemotherapy: A critical analysis of its 60 years of history. Crit. Rev. Oncol./Hematol. 84, 181–199 (2012). [DOI] [PubMed] [Google Scholar]
- Braun M. S. & Seymour M. T. Balancing the efficacy and toxicity of chemotherapy in colorectal cancer. Ther Adv Med Oncol 3, 43–52 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. G., Burdick J. A. & Langer R. Materials science - Smart biomaterials. Science 305, 1923–1924 (2004). [DOI] [PubMed] [Google Scholar]
- Derfus A. M. et al. Remotely triggered release from magnetic nanoparticles. Adv. Mater. 19, 3932–3936 (2007). [Google Scholar]
- Hoffman A. S. The origins and evolution of “controlled” drug delivery systems. J. Control. Release 132, 153–163 (2008). [DOI] [PubMed] [Google Scholar]
- Pitukmanorom P., Yong T. H. & Ying J. Y. Tunable release of proteins with polymer-inorganic nanocomposite microspheres. Adv. Mater. 20, 3504–3509 (2008). [Google Scholar]
- Jay S. M. & Saltzman W. M. Controlled delivery of VEGF via modulation of alginate microparticle ionic crosslinking. J. Control. Release 134, 26–34 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. H. et al. Active scaffolds for on-demand drug and cell delivery. Proc. Natl. Acad. Sci. U. S. A. 108, 67–72 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J. W. et al. Photothermal nanodrugs: potential of TNF-gold nanospheres for cancer theranostics. Sci Rep 3, 1293 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditto V. J. & Szoka F. C. Cancer nanomedicines: So many papers and so few drugs!. Adv. Drug Deliv. Rev. 65, 80–88 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissel T., Li Y. X. & Unger F. ABA-triblock copolymers from biodegradable polyester A-blocks and hydrophilic poly(ethylene oxide) B-blocks as a candidate for in situ forming hydrogel delivery systems for proteins. Adv. Drug Deliv. Rev. 54, 99–134 (2002). [DOI] [PubMed] [Google Scholar]
- Watanabe M. et al. Preparation of camptothecin-loaded polymeric micelles and evaluation of their incorporation and circulation stability. Int. J. Pharm. 308, 183–189 (2006). [DOI] [PubMed] [Google Scholar]
- Bae Y. & Kataoka K. Intelligent polymeric micelles from functional poly(ethylene glycol)-poly(amino acid) block copolymers. Adv. Drug Deliv. Rev. 61, 768–784 (2009). [DOI] [PubMed] [Google Scholar]
- Kopecek J. & Kopeckova P. HPMA copolymers: origins, early developments, present, and future. Adv. Drug Deliv. Rev. 62, 122–149 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong B., Bae Y. H., Lee D. S. & Kim S. W. Biodegradable block copolymers as injectable drug-delivery systems. Nature 388, 860–862 (1997). [DOI] [PubMed] [Google Scholar]
- Yu L. & Ding J. D. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 37, 1473–1481 (2008). [DOI] [PubMed] [Google Scholar]
- Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat. Rev. Cancer 6, 789–802 (2006). [DOI] [PubMed] [Google Scholar]
- Lawrence S. Billion dollar babies - biotech drugs as blockbusters. Nat. Biotechnol. 25, 380–382 (2007). [DOI] [PubMed] [Google Scholar]
- Potmesil M. Camptothecins- from bench research to hospital wards. Cancer Res. 54, 1431–1439 (1994). [PubMed] [Google Scholar]
- Liu J., Jiang Z. Z., Zhang S. M. & Saltzman W. M. Poly(omega-pentadecalactone-co-butylene-co-succinate) nanoparticles as biodegradable carriers for camptothecin delivery. Biomaterials 30, 5707–5719 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke T. G. & Mi Z. The structural basis of camptothecin interactions with human serum albumin: impact on drug stability. J. Med. Chem. 37, 40–46 (1994). [DOI] [PubMed] [Google Scholar]
- Herben V. M. M. et al. Phase I and pharmacokinetic study of irinotecan administered as a low-dose, continuous intravenous infusion over 14 days in patients with malignant solid tumors. J. Clin. Oncol. 17, 1897–1905 (1999). [DOI] [PubMed] [Google Scholar]
- Narasimhan B. & Langer R. Zero-order release of micro- and macromolecules from polymeric devices: the role of the burst effect. J. Control. Release 47, 13–20 (1997). [Google Scholar]
- Huang X. & Brazel C. S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 73, 121–136 (2001). [DOI] [PubMed] [Google Scholar]
- Rao V. M., Haslam J. L. & Stella V. J. Controlled and complete release of a model poorly water-soluble drug, prednisolone, from hydroxypropyl methylcellulose matrix tablets using (SBE)(7 m)-beta-cyclodextrin as a solubilizing agent. J. Pharm. Sci. 90, 807–816 (2001). [DOI] [PubMed] [Google Scholar]
- Lao L. L., Venkatraman S. S. & Peppas N. A. A novel model and experimental analysis of hydrophilic and hydrophobic agent release from biodegradable polymers. J. Biomed. Mater. Res. Part A 90, 1054–1065 (2009). [DOI] [PubMed] [Google Scholar]
- Qiu Y. & Park K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 53, 321–339 (2001). [DOI] [PubMed] [Google Scholar]
- Zentner G. M. et al. Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J. Control. Release 72, 203–215 (2001). [DOI] [PubMed] [Google Scholar]
- Choi S. & Kim S. W. Controlled release of insulin from injectable biodegradable triblock copolymer depot in ZDF rats. Pharm. Res. 20, 2008–2010 (2003). [DOI] [PubMed] [Google Scholar]
- Van Tomme S. R. & Hennink W. E. Biodegradable dextran hydrogels for protein delivery applications. Expert Rev. Med. Devices 4, 147–164 (2007). [DOI] [PubMed] [Google Scholar]
- Elstad N. L. & Fowers K. D. OncoGel (ReGel/paclitaxel) - Clinical applications for a novel paclitaxel delivery system. Adv. Drug Deliv. Rev. 61, 785–794 (2009). [DOI] [PubMed] [Google Scholar]
- Chang G. T., Ci T. Y., Yu L. & Ding J. D. Enhancement of the fraction of the active form of an antitumor drug topotecan via an injectable hydrogel. J. Control. Release 156, 21–27 (2011). [DOI] [PubMed] [Google Scholar]
- Siepmann J. & Peppas N. A. Higuchi equation: derivation, applications, use and misuse. Int. J. Pharm. 418, 6–12 (2011). [DOI] [PubMed] [Google Scholar]
- Beretta G. L. & Zunino F. Relevance of extracellular and intracellular interactions of camptothecins as determinants of antitumor activity. Biochem. Pharmacol. 74, 1437–1444 (2007). [DOI] [PubMed] [Google Scholar]
- Gupta E. et al. Metabolic fate of irinotecan in humans: correlation of glucuronidation with diarrhea. Cancer Res. 54, 3723–3725 (1994). [PubMed] [Google Scholar]
- Burris H. A. et al. Activity of topotecan, a new topoisomerase I inhibitor, against human tumor colony-forming units In vitro. J. Natl. Cancer Inst. 84, 1816–1820 (1992). [DOI] [PubMed] [Google Scholar]
- Mathijssen R. H. J. et al. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin. Cancer Res. 7, 2182–2194 (2001). [PubMed] [Google Scholar]
- Kurita A. et al. Alleviation of side effects induced by irinotecan hydrochloride (CPT-11) in rats by intravenous infusion. Cancer Chemother. Pharmacol. 52, 349–360 (2003). [DOI] [PubMed] [Google Scholar]
- Crcarevska M. S. et al. Definition of formulation design space, in vitro bioactivity and in vivo biodistribution for hydrophilic drug loaded PLGA/PEO-PPO-PEO nanoparticles using OFAT experiments. Eur. J. Pharm. Sci. 49, 65–80 (2013). [DOI] [PubMed] [Google Scholar]
- Tang Y. Q., Czuczman P. R., Chung S. T. & Lewis A. L. Preservation of the active lactone form of irinotecan using drug eluting beads for the treatment of colorectal cancer metastases. J. Control. Release 127, 70–78 (2008). [DOI] [PubMed] [Google Scholar]
- Yohe S. T., Herrera V. L. M., Colson Y. L. & Grinstaff M. W. 3D superhydrophobic electrospun meshes as reinforcement materials for sustained local drug delivery against colorectal cancer cells. J. Control. Release 162, 92–101 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohe Y. et al. Phase I study and pharmacokinetics of CPT-11 with 5-day continuous infusion. J. Natl. Cancer Inst. 84, 972–974 (1992). [DOI] [PubMed] [Google Scholar]
- Li T., Ci T. Y., Chen L., Yu L. & Ding J. D. Salt-induced reentrant hydrogel of poly(ethylene glycol)-poly(lactide-co-glycolide) block copolymers. Polym. Chem. 5, 979–991 (2014). [Google Scholar]
- Zhang H., Yu L. & Ding J. D. Roles of hydrophilic homopolymers on the hydrophobic-association-induced physical gelling of amphiphilic block copolymers in water. Macromolecules 41, 6493–6499 (2008). [Google Scholar]
- He C. L., Kim S. W. & Lee D. S. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J. Control. Release 127, 189–207 (2008). [DOI] [PubMed] [Google Scholar]
- Moon H. J., Ko D. Y., Park M. H., Joo M. K. & Jeong B. Temperature-responsive compounds as in situ gelling biomedical materials. Chem. Soc. Rev. 41, 4860–4883 (2012). [DOI] [PubMed] [Google Scholar]
- Li K. et al. A long-acting formulation of a polypeptide drug exenatide in treatment of diabetes using an injectable block copolymer hydrogel. Biomaterials 34, 2834–2842 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang Z. et al. Biodegradable and thermoreversible PCLA-PEG-PCLA hydrogel as a barrier for prevention of post-operative adhesion. Biomaterials 32, 4725–4736 (2011). [DOI] [PubMed] [Google Scholar]
- Yu L. et al. Poly(lactic acid-co-glycolic acid)–poly(ethylene glycol)–poly(lactic acid-co-glycolic acid) thermogel as a novel submucosal cushion for endoscopic submucosal dissection. Acta Biomater. 10, 1251–1258 (2014). [DOI] [PubMed] [Google Scholar]
- Lipinski C. A., Lombardo F., Dominy B. W. & Feeney P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 23, 3–25 (1997). [DOI] [PubMed] [Google Scholar]
- Willett C. G. et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J. Clin. Oncol. 27, 3020–3026 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassberg J. & Stella V. J. A kinetic and mechanistic study of the hydrolysis of camptothecin and some analogues. J. Pharm. Sci. 81, 676–684 (1992). [DOI] [PubMed] [Google Scholar]
- Ci T. Y. et al. Effects of amphiphilic block copolymers on the equilibrium lactone fractions of camptothecin analogues at different pHs. Biomater. Sci. 1, 1235–1243 (2013). [DOI] [PubMed] [Google Scholar]
- Ci T. Y., Li T., Chang G. T., Yu L. & Ding J. D. Simply mixing with poly(ethylene glycol) enhances the fraction of the active chemical form of antitumor drugs of camptothecin family. J. Control. Release 169, 329–335 (2013). [DOI] [PubMed] [Google Scholar]
- Ci T. Y. et al. Effects of “mature micelle” formation of Pluronic P123 on equilibrium between lactone and carboxylate forms of 10-hydrocamptothecin in water. Polym. Chem. 4, 3245–3255 (2013). [Google Scholar]
- Yu L., Ci T. Y., Zhou S. C., Zeng W. J. & Ding J. D. The thermogelling PLGA-PEG-PLGA block copolymer as a sustained release matrix of doxorubicin. Biomater. Sci. 1, 411–420 (2013). [DOI] [PubMed] [Google Scholar]
- Liu C. D., Zhang Z. X., Liu K. L., Ni X. P. & Li J. Biodegradable thermogelling poly(ester urethane)s consisting of poly(1,4-butylene adipate), poly(ethylene glycol), and poly(propylene glycol). Soft Matter 9, 787–794 (2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Info