Introduction
An “Acute Coronary Syndrome” (ACS) is the most ominous manifestation of coronary artery disease (CAD). The burden of ACS and its impact are striking. Cardiovascular disease is now the most common cause of mortality worldwide and among cardiovascular deaths, the majority are attributable to CAD.1 As a result, while CAD in general is a major global public health concern, ACS is particularly worrisome as it is both prevalent but at the same time portends a poor prognosis. While advanced therapies may alleviate ACS-related morbidity and mortality in well served communities located in affluent countries, many persons in less fortunate situations living in low- and middle-income countries remain exposed to the ravages of this disease.
Despite this outlook, rapid progress is being made in understanding pathology, in prevention and in treatment of ACS. As readers will find even by perusing the headings of the articles in this ACS Compendium, there is a lot to be optimistic about. As Editors of this ACS Compendium, we are privileged to have played a small role in helping to provide the framework for the esteemed authorship groups to leverage their collective expertise and provide for us a definitive overall review of ACS. In working with these world-renown scientists and clinicians on this collection of ACS articles, which also included a cadre of expert reviewers (to who we are especially thankful), we found ourselves in the enviable position of being privy to a deeply insightful, cutting-edge and forward looking appraisal of the current state-of-the-science for ACS. While this fund of knowledge is clearly set out in the articles that follow, several unexpected points arose from these interactions. The most obvious, somewhat surprisingly, was the question of what exactly is an acute coronary syndrome?
What Constitutes an ACS Event, and is this Definition Evolving?
The term “acute coronary syndrome” appeared relatively recently in the medical lexicon. A simple search in Pubmed reveals that the first article to use the term “acute coronary syndrome” in the title or abstract appeared in 1986.2 In this article titled “Flow characteristics of coronary balloon catheters”, the following sentence appeared in the abstract: “Sudden reocclusion leads frequently to an acute coronary syndrome (acute myocardial infarction, hypotension, arrhythmias) that requires emergency surgery and also leads to permanent myocardial damage of various degrees.”2 Clearly, this 1986 use of the term “acute coronary syndrome” (meaning acute myocardial infarction, hypotension and/or arrhythmia) differs significantly from its meaning today. When we used the term only a few years later in 1992 in an article titled “The Pathogenesis of Coronary Artery Disease and The Acute Coronary Syndromes”, we defined ACS as myocardial infarction (MI), unstable angina or ischemic sudden death.3,4 As a historical point in the evolution of the use and meaning of term ACS, it is interesting to note that one of the themes of our 1992 article was the concept that MI, unstable angina and ischemic sudden death are all part of a spectrum of manifestation of the same atherosclerotic coronary artery substrate.3,4 While this is now an established principle of CAD and atherosclerosis, this understanding paved the way for the contemporary use of the term acute coronary syndrome as it unified these “syndromes” through their common pathologic basis. Fast-forward to today, and as a ‘textbook’ definition for ACS we believe most would still agree it is defined as MI, unstable angina and ischemic sudden death. However, as we describe below, this appears to be evolving with several factors now reshaping what is perceived to be an ACS-type event.
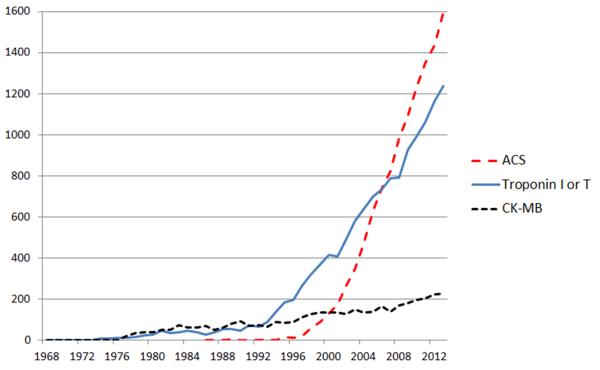
An important and relatively recent change in ACS diagnosis has been the widespread use of troponin assays in favor of the older creatine kinase-MB (CK-MB) assay. The measurement of troponin as a cardiac biomarker is now an objective and important aspect of the diagnosis of non ST-elevation MI (NSTEMI), and we believe that cardiac troponin testing has been influential on the popular use and meaning of “ACS”. Indeed, since the 1980's, the number of articles appearing per year in the medical literature using the terms “acute coronary syndrome” and “troponin” in the title or abstract has run an almost parallel course (Figure). Current generation troponin assays are extremely sensitive for detecting myocardial ischemia and/or infarction, and the measurement of troponin I or T is one of the principal means of formally diagnosing MI according to the latest 2012 iteration of the Universal Definitions.5 As a result, with this now exquisite sensitivity of troponin assays, there has been a progressive reclassification of patients previously thought to have unstable angina, that with high-sensitivity troponin assays are now recognized as having small NSTEMIs.6 Therefore, as compared to the pre-troponin era, in contemporary practice a greater proportion of ACS patients are diagnosed with NSTEMI (troponin-positive), while unstable angina (troponin-negative) is correspondingly less common and somewhat de-emphasized as a clinical entity.6,7
Figure 1.
Number of references appearing in PubMed per year for ACS, troponin or creatine kinase-MB (CK-MB). Articles were identified using PubMed seeking the search terms “acute coronary syndrome”, “troponin” or “creatine kinase-MB” in the title or abstract.
Extending this observation, what could be the potential advantages of considering biomarker-negative angina as a non-ACS entity? While this may be a dubious distinction at the level of the plaque (see article by Drs. Fog Bentzon, Otsuka, Virmani and Falk in this ACS Compendium series) a biomarker-negative chest pain syndrome nevertheless implies a lack of acute vessel occlusion or distal embolization, which may therefore at least partially distinguish troponin-negative from troponin-positive events at a pathological level. Furthermore, by excluding biomarker-negative chest pain syndromes from the ACS definition, and by relying on troponin to diagnose minor events with small biomarker elevations, there can be little if any uncertainty as to what constitutes an ACS-type event, and its diagnosis is greatly simplified. As a practical advantage, this would remove the current uncertainty in ACS diagnosis of interpreting a patient's historical account of their angina and determining if this is stable or otherwise. While “unstable angina” is a subjective clinical symptom, a positive troponin is an objective laboratory result. Indeed, troponin assays are now so sensitive that with paired samples drawn sequentially several hours apart after presentation to an emergency department, the sensitivity of this test for diagnosing MI approaches 100%.8,9 Therefore, particularly in a busy emergency department setting, there is obvious appeal in relying on a troponin assay to rule in/out what is considered to be an “ACS-type event”. Moreover, this also has the advantage of eliminating “false positive” ACS diagnoses in patients who are thought to have unstable angina, but who in reality have stable angina or even non-cardiac chest pain.
What might be the disadvantages of relying on a troponin assay to rule in/out ACS-type events? Most concerning is that patients with truly unstable angina may be managed conservatively, when a more pro-active ACS treatment algorithm would be more appropriate. Nevertheless, as reflected by both the GRACE10 and TIMI11 risk scores and other studies,6,12 biomarker-negative patients are generally at lower risk for adverse events. Therefore, the clinical consequences of conservatively managing a low-risk, troponin-negative ACS patient as stable angina might generally be expected to be minimal. This is supported by current guidelines, with the routine invasive evaluation of low-risk ACS patients being not recommended by the European Society of Cardiology13 and of uncertain benefit according to US guidelines.14 Chest pain observation units make use of this fact and are now increasingly being used in certain centers to rule in/out and initiate management for ACS in the initially low risk patient.
Collectively therefore, we believe that due to a combination of the widespread use of high-sensitivity troponin assays, changing definitions of MI, the generally favorable prognosis of low-risk troponin-negative patients and increased overall simplicity, the implied meaning and practical use of the term “acute coronary syndrome” is slowly evolving to exclude troponin-negative unstable angina. While support is not universal,15 a strong case has been made that in the high-sensitivity troponin era, troponin-negative unstable angina would be better categorized as a sub-type of severe stable CAD rather than together with MI.16 However, we caution that there remains a specific clinical entity of a high-risk unstable angina patient with negative troponin. These patients are characterized by a number of features such as older age, heart failure and low blood pressure.10,11 Therefore, while we believe a gradual shift in the implied meaning of ACS may be occurring, great clinical care is required for certain troponin-negative but high-risk patients.
What is not an ACS Event?
We have set out above the reasons we perceive that biomarker-negative chest pain syndromes are now less commonly considered an ACS. However, clinicians will likely be more familiar with the opposite issue – that patients with any number of non-cardiac problems may have a weakly positive troponin result and are, for practical purposes, labeled as having an ACS. Typically, these are medically unwell patients who present with a broad spectrum of acute illnesses such as heart failure, renal failure, blood loss or sepsis.12 Often, the criteria for MI are not met because EKG changes or chest pain may be absent.5 Therefore, as they have neither MI nor unstable angina, they also do not meet the usual criteria for an ACS. Despite this, as any practicing cardiologist will attest, medically unwell patients are frequently triaged as if they had an ACS event based solely on a weakly positive troponin. These patients may be wrongly admitted to a coronary care unit or cardiology service, which only serves to misdirect resources and medical efforts. This emerging clinical problem is currently the focus of much attention and was recently dubbed “the plague of troponinitis.”17 While it is clear that nobody in the medical community is advocating for these medically unwell patients to be included in the definition of “ACS”, we highlight this concern as an issue yet to be resolved in the accurate triage and management of patients with a true ACS event.
In summary, the definition and practical use of the term “acute coronary syndrome” is in a state of continued evolution. It is not a term which is rigorously defined by a consensus committee such as MI.5 Rather, it is a term whose meaning is to some extent governed by popular use and which is entwined in our increasing knowledge of this disease and improved diagnostic and therapeutic tools.
ACS Compendium
Readers will find the following articles move in a logical sequence through mechanisms of plaque formation, the role of inflammation and lipids, genetics, ACS imaging, diagnosis, invasive and medical treatments and finally global perspectives. As a disclaimer, not every detail of ACS is covered in this Compendium. Broadly, we deliberately omitted material that we felt was either too detailed (e.g. technical details of stent implantation) or which has evolved little in the last decade (e.g. EKG changes of myocardial ischemia). We believe the articles which follow are, collectively, a contemporary and unsurpassed cross-disciplinary review of acute coronary syndromes.
Assuming its rightful place as the first review in this ACS compendium, Drs. Fog Bentzon, Otsuka, Virmani and Falk have, as one reader memorably described it, provided a “Magnum Opus” covering Mechanisms of Plaque Formation and Rupture. The authors logically describe stages of atherosclerotic plaque formation and mechanisms of rupture. In addition, the authors cover numerous other relevant aspects, including atherosclerotic disease activity, scientific areas of uncertainty (e.g. mechanisms of plaque erosion) and opportunities for ACS prevention from a histopathological perspective. This article elegantly sets the stage for this ACS Compendium and lays a foundation of understanding for the articles that follow.
Moving from general mechanisms of plaque formation and rupture, two articles then follow that are dedicated to specific, fundamental aspects of ACS biology: inflammation and lipid metabolism. In the first of these, Drs. Libby, Tabas, Fredman and Fisher review the details of “Inflammation and its resolution as determinants of ACS”. Many important aspects of the inflammatory responses that govern ACS are explored, including defective efferocytosis in advanced plaques that is associated with a large necrotic core – akin to a vascular graveyard of inflammatory, smooth muscle and other cell types leading to rupture-prone lesions that may culminate in ACS. As an emerging aspect of plaque progression, the concept of failure of resolution of inflammation is also broached with “specialized pro-resolving mediators” (SPMs) being novel biologic agents to prevent ACS. While several familiar agents such as aspirin and statins may act as partial SPMs (in addition to their anti-platelet and lipid lowering effects, respectively), the appreciation that enhanced resolution of inflammation may mitigate ACS and CAD has paved the way for novel therapeutic agents targeting highly-specific aspects of vascular inflammation without compromising host immune defense.
In an article that transcends biomarkers, mechanisms and therapy, Drs. Rosenson, Brewer and Rader drill down on a topic at the core of atherosclerosis, CAD and ACS: lipoproteins in the setting of acute coronary syndrome. While on the one hand it could be argued that the great bulk of data regarding lipoproteins is in patients with prior MI or those with stable CAD, Rader and colleagues effectively draw from the existing literature and illustrate the pivotal role of LDL cholesterol, HDL cholesterol and other lipid moieties in ACS. From a treatment perspective this is an especially important article, as it covers the role of statins and introduces novel agents such as PCSK9 inhibitors, which hold hope for the future primary prevention of ACS.
The genetics of CAD and MI has been an area of striking progress in the last decade. While a strong genetic component was previously suspected,18 it was only as recently as 2007 that this field exploded with the discovery of the 9p21 CAD risk variant. In his Compendium review, Dr. Robert Roberts covers the full spectrum of this field. Interestingly, the genome wide association (GWA) data underpinning this research has used the general endpoints of presence of CAD and/or occurrence of MI. There are few, if any data pertaining directly to ACS, and certainly to ST-elevation MI versus NSTEMI or unstable angina. Therefore, as an exception to this ACS Compendium series, Dr. Robert's article is not entirely specific to ACS, but relates more to presence of CAD. This notwithstanding, several fundamental messages are delivered in this article that shape our outlook on CAD, including that the heritable component of common atherosclerosis is driven by the cumulative risk inferred by the inheritance of multiple genetic variants, each conferring only a marginal increment in risk. These variants are common, with over half being present in > 50% of the population. As another major and unexpected finding arising from GWA and other data, it is becoming increasingly clear that HDL cholesterol levels may be unrelated to developing CAD. This article will serve as a concise, interpretive summary of this field as it stands in 2014, capturing both the excitement of these recent genetic insights yet the profound complexity of the genetic and molecular pathways that remain to be unraveled.
A major area of recent progress has been in imaging of atherosclerosis, CAD and ACS.19 This includes non-invasive and invasive (intra-coronary) modalities, which have collectively played a key role in helping to elucidate the pathobiology, natural history and optimal treatment strategies for ACS. Drs. Garcia-Garcia, Jang, Serruys, Kovacic, Narula and Fayad have concisely reviewed this field in their manuscript titled “Imaging Plaques to Predict and Better Manage Patients with Acute Coronary Events”. Of the many important lessons learned through cardiovascular imaging, perhaps one of the most notable is that CAD does not occur in isolation, but rather is part of a systemic disease process affecting the entire arterial tree. In addition, while it is possible to identify “vulnerable” or “rupture-prone” coronary plaques, each lesion has a low overall likelihood of causing an ACS.20 This knowledge has driven interest away from potential interventions seeking to individually treat a specific lesion(s) to prevent ACS. Rather, these imaging data have generally reinforced the need to tackle atherosclerosis, CAD and prevention of ACS at the systemic level by attention to risk factors and optimal medical therapy.
Next we move to a series of three articles that focus on the acute and secondary treatment of ACS. In the first of these treatment-focused articles, Drs. Bagai, Dangas, Stone and Granger review reperfusion strategies in ACS, or in other words, options for reopening the culprit vessel(s) that causes an ACS event. In broad terms, this can be divided into either mechanical reperfusion in a catheterization laboratory with coronary angioplasty and stent implantation (which may be considered for any ACS patient), or intravenous thrombolytic therapy such as tenecteplase or alteplase (which is only efficacious for ACS with ST-segment elevation). As well as reviewing this field and providing an update on current best practice in angioplasty/stenting and thrombolysis, a significant section of this article is appropriately devoted to the logistic aspects of the timely reopening of the culprit ACS vessel. In the context of an ST-segment elevation MI it has been well documented that “Time is Muscle”, and prompt reperfusion to reduce morbidity and mortality is a critical component of current treatment algorithms.
In their review of antiplatelet and anticoagulation therapy for ACS, Drs. Bhatt, Hulot, Moliterno and Harrington provide a focused exposé of what has been a rapidly developing field. While aspirin and warfarin have been in our armamentarium for many years, readers should be aware that almost all of the other agents discussed in this article have appeared in the last 2 decades. Even clopidogrel, which in addition to aspirin is arguably the cornerstone of antiplatelet therapy in ACS, is a relatively new agent. Many readers will recall that is was only in 1999-2000 that clopidogrel replaced ticlopidine (“Ticlid”) as the preferred ‘second agent’ after aspirin,21 and in the brief ensuing period clopidogrel has gone on to become one of the most important cardiovascular agents on the market. In addition to these therapies a diverse array of newer agents that inhibit platelet function are now available and appropriate for use in ACS. The recently emerged anticoagulants targeting thrombin or factor Xa are also now under investigation for their possible utility in ACS, potentially in conjunction with antiplatelet agents. Although perhaps not living up to its promise, the study of the pharmacogenomics of clopidogrel has also been an area of intense interest, and has concurrently served to propel the genetic investigation of response to cardiovascular therapies into the forefront of investigation. While there is still clearly a tradeoff between bleeding risk and potential for thrombosis in ACS, with their improved pharmacologic profiles we believe it is reasonable to surmise that contemporary agents are slowly shifting this trade-off toward a more favorable balance in risk.
In the current era, ‘optimal medical therapy’ has become a mandatory cornerstone of the management of not only ACS, but almost the full spectrum of conditions encountered in clinical cardiology. In their article “Non-Atherothrombotic Medical Options in ACS: Old Agents and New Lines on the Horizon”, Drs. Soukoulis, Boden, Smith and O’Gara deliver a “must read” for those who treat or are interested in ACS. Woven around salient aspects of cardiac physiology during ACS, the authors first comprehensively review the currently available therapeutic options such as β-blockers, nitrates, ACE inhibitors and aldosterone antagonists, with close reference to pivotal trials and current guidelines. This is followed by an exciting look at new and upcoming treatments in late-phase clinical studies and important areas for further investigation. Broadly, newer areas that are now becoming the focus of attention include the prevention of the no-reflow phenomenon, minimizing myocardial reperfusion injury, ischemic preand post-conditioning (and developing important mediators of this process as therapies for ACS), and modulation of inflammation and/or cellular metabolic activity. The approaches being studied are diverse and include sophisticated biologic agents, older agents such as cyclosporine22 or beta-blockers (which may reduce ischemia-reperfusion injury via nitric oxide synthase activation),23,24 remote conditioning by manual blood pressure cuff inflation/deflation while en-route to the catheterization laboratory25 and simple mechanical thrombus aspiration catheters that are used during percutaneous coronary intervention for ACS. Certainly, we perceive these to be very important areas for research moving forwards.
In an article that could have been positioned first rather than last in this Compendium, Drs. Vedanthan, Seligman and Fuster broaden the frame of reference for ACS with their manuscript “Global Perspective on ACS: A Burden on the Young and Poor”. While the earlier articles in this Compendium might read as back-to-back success stories of how high income countries have systematically gone about understanding ACS and translating this understanding into reduced morbidity and mortality from this disease, low- and middle-income countries (LMICs) are still struggling to define the exact magnitude of the problem at hand. It is a telling point that due to the paucity of data on ACS in LMICs, to a certain extent the authors of this article had to extrapolate broader information relating to ischemic heart disease and cardiovascular disease to piece together the global picture of ACS in these nations. As an example of these unknowns, the median age of mortality from ischemic heart disease in males is a decade younger in LMICs than high income countries,26 which may be due to earlier onset of ACS and/or ischemic heart disease, and/or shorter survival after initial presentation. Differences between countries, regions and races add to these uncertainties. Of course, these are but some of the major challenges faced – other key problems to be overcome are carefully detailed in this article. These data sit as a sobering conclusion to this Compendium, highlighting that while much has been achieved, we are only just beginning our global fight against ACS and ischemic heart disease.
As Guest Editors of the ACS Compendium we were honored and humbled by the enthusiasm and contributions of the distinguished authors of these articles. As readers will identify, each authorship team comprises true thought leaders and cutting-edge scientists at the vanguard of these respective disciplines. More than just their contribution in writing these articles it is by their hard work and notable contributions over the last several decades, along with those from their many peers, that we have now reached the current point of understanding of ACS described herein and are making an impact on patient outcomes.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
Valentin Fuster acknowledges research support from AstraZeneca. Jason Kovacic acknowledges research support from the NIH (K08HL111330), The Leducq Foundation (Transatlantic Network of Excellence Award) and AstraZeneca.
Nonstandard Abbreviations and Acronyms
- ACS
acute coronary syndrome
- CAD
coronary artery disease
- CK-MB
creatine kinase-MB
- GWA
genome wide association
- LMIC
low- and middle-income countries
- MI
myocardial infarction
- NSTEMI
non ST-elevation myocardial infarction
- SPM
specialized pro-resolving mediators
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Kovacic JC, Fuster V. From treating complex coronary artery disease to promoting cardiovascular health: Therapeutic transitions and challenges, 2010-2020. Clin Pharmacol Ther. 2011;90:509–518. doi: 10.1038/clpt.2011.173. [DOI] [PubMed] [Google Scholar]
- 2.Angelini P, Leachman R, Heibig J. Flow characteristics of coronary balloon catheters. Texas Heart Institute journal / from the Texas Heart Institute of St. Luke's Episcopal Hospital, Texas Children's Hospital. 1986;13:213–215. [PMC free article] [PubMed] [Google Scholar]
- 3.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med. 1992;326:242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- 4.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (2). N Engl J Med. 1992;326:310–318. doi: 10.1056/NEJM199201303260506. [DOI] [PubMed] [Google Scholar]
- 5.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Reichlin T, Twerenbold R, Reiter M, Steuer S, Bassetti S, Balmelli C, Winkler K, Kurz S, Stelzig C, Freese M, Drexler B, Haaf P, Zellweger C, Osswald S, Mueller C. Introduction of high-sensitivity troponin assays: Impact on myocardial infarction incidence and prognosis. Am J Med. 2012;125:1205–1213. e1201. doi: 10.1016/j.amjmed.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Deckers JW. Classification of myocardial infarction and unstable angina: A re-assessment. Int J Cardiol. 2013;167:2387–2390. doi: 10.1016/j.ijcard.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Weber M, Bazzino O, Navarro Estrada JL, de Miguel R, Salzberg S, Fuselli JJ, Liebetrau C, Woelken M, Moellmann H, Nef H, Hamm C. Improved diagnostic and prognostic performance of a new high-sensitive troponin t assay in patients with acute coronary syndrome. Am Heart J. 2011;162:81–88. doi: 10.1016/j.ahj.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Giannitsis E, Becker M, Kurz K, Hess G, Zdunek D, Katus HA. High-sensitivity cardiac troponin t for early prediction of evolving non-st-segment elevation myocardial infarction in patients with suspected acute coronary syndrome and negative troponin results on admission. Clinical chemistry. 2010;56:642–650. doi: 10.1373/clinchem.2009.134460. [DOI] [PubMed] [Google Scholar]
- 10.Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, Avezum A, Goodman SG, Flather MD, Anderson FA, Jr., Granger CB. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: Prospective multinational observational study (grace). BMJ. 2006;333:1091. doi: 10.1136/bmj.38985.646481.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The timi risk score for unstable angina/non-st elevation mi: A method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 12.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, Pfeffer MA, Braunwald E. Prevention of Events with Angiotensin Converting Enzyme Inhibition Trial I. A sensitive cardiac troponin t assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D. Guidelines ESCCfP. Esc guidelines for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation: The task force for the management of acute coronary syndromes (acs) in patients presenting without persistent st-segment elevation of the european society of cardiology (esc). Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 14.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. Accf/scai/sts/aats/aha/asnc/hfsa/scct 2012 appropriate use criteria for coronary revascularization focused update: A report of the american college of cardiology foundation appropriate use criteria task force, society for cardiovascular angiography and interventions, society of thoracic surgeons, american association for thoracic surgery, american heart association, american society of nuclear cardiology, and the society of cardiovascular computed tomography. J Am Coll Cardiol. 2012;59:857–881. doi: 10.1016/j.jacc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Steg PG, FitzGerald G, Fox KA. Risk stratification in non-st-segment elevation acute coronary syndromes: Troponin alone is not enough. Am J Med. 2009;122:107–108. doi: 10.1016/j.amjmed.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 16.Mueller C. Biomarkers and acute coronary syndromes: An update. Eur Heart J. 2014;35:552–556. doi: 10.1093/eurheartj/eht530. [DOI] [PubMed] [Google Scholar]
- 17.Kramer CM. Avoiding the imminent plague of troponinitis: The need for reference limits for high-sensitivity cardiac troponin t. J Am Coll Cardiol. 2014;63:1449–1450. doi: 10.1016/j.jacc.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330:1041–1046. doi: 10.1056/NEJM199404143301503. [DOI] [PubMed] [Google Scholar]
- 19.Tomey MI, Narula J, Kovacic JC. Year in review: Advances in understanding of plaque composition and treatment options. J Am Coll Cardiol. 2014;63:1604–1616. doi: 10.1016/j.jacc.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 20.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW, Investigators P. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 21.Quinn MJ, Fitzgerald DJ. Ticlopidine and clopidogrel. Circulation. 1999;100:1667–1672. doi: 10.1161/01.cir.100.15.1667. [DOI] [PubMed] [Google Scholar]
- 22.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 23.Aragon JP, Condit ME, Bhushan S, Predmore BL, Patel SS, Grinsfelder DB, Gundewar S, Jha S, Calvert JW, Barouch LA, Lavu M, Wright HM, Lefer DJ. Beta3-adrenoreceptor stimulation ameliorates myocardial ischemia-reperfusion injury via endothelial nitric oxide synthase and neuronal nitric oxide synthase activation. J Am Coll Cardiol. 2011;58:2683–2691. doi: 10.1016/j.jacc.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibanez B, Macaya C, Sanchez-Brunete V, Pizarro G, Fernandez-Friera L, Mateos A, Fernandez-Ortiz A, Garcia-Ruiz JM, Garcia-Alvarez A, Iniguez A, Jimenez-Borreguero J, Lopez-Romero P, Fernandez-Jimenez R, Goicolea J, Ruiz-Mateos B, Bastante T, Arias M, Iglesias-Vazquez JA, Rodriguez MD, Escalera N, Acebal C, Cabrera JA, Valenciano J, Perez de Prado A, Fernandez-Campos MJ, Casado I, Garcia-Rubira JC, Garcia-Prieto J, Sanz-Rosa D, Cuellas C, Hernandez-Antolin R, Albarran A, Fernandez-Vazquez F, de la Torre-Hernandez JM, Pocock S, Sanz G, Fuster V. Effect of early metoprolol on infarct size in st-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: The effect of metoprolol in cardioprotection during an acute myocardial infarction (metocard-cnic) trial. Circulation. 2013;128:1495–1503. doi: 10.1161/CIRCULATIONAHA.113.003653. [DOI] [PubMed] [Google Scholar]
- 25.Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, Pedersen L, Sorensen HT, Botker HE, Investigators C. Improved long-term clinical outcomes in patients with st-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J. 2014;35:168–175. doi: 10.1093/eurheartj/eht369. [DOI] [PubMed] [Google Scholar]
- 26.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.