Abstract
L-Fucose has been found abundantly in human milk oligosaccharides, bacterial lipopolysaccharides, glycolipids, and many N- and O-linked glycans produced by mammalian cells. Fucose-containing carbohydrates have important biological functions. Alterations in the expression of fucosylated oligosaccharides have been observed in several pathological processes such as cancer and atherosclerosis. Chemical formation of fucosidic bonds is challenging due to its acid lability. Enzymatic construction of fucosidic bonds by fucosyltransferases is highly efficient and selective but requires the expensive sugar nucleotide donor guanosine 5′- diphosphate-L-fucose (GDP-Fuc). Here, we describe a protocol for applying a one-pot three-enzyme system in synthesizing structurally defined fucose-containing oligosaccharides from free L-fucose. In this system, GDP-Fuc is generated from L-fucose, adenosine 5′-triphosphate (ATP), and guanosine 5′-triphosphate (GTP) by a bifunctional L-fucokinase/GDP-fucose pyrophosphorylase (FKP). An inorganic pyrophosphatase (PpA) is used to degrade the by-product pyrophosphate (PPi) to drive the reaction towards the formation of GDP-Fuc. In situ generated GDP-Fuc is then used by a suitable fucosyltransferase for the formation of fucosides. The three-enzyme reactions are carried out in one pot without the need for high cost sugar nucleotide or isolation of intermediates. The time for the synthesis is 4–24 hours. Purification and characterization of products can be completed in 2–3 days.
Keywords: enzymatic synthesis, FKP, fucoside, fucose, fucosylation, fucosyltransferase, Lewis x, one-pot multienzyme, sialyl Lewis x
INTRODUCTION
Lewis x [Lex, Galβ1–4(Fucα1–3)GlcNAcβOR] and sialyl Lewis x [sLex, Neu5Acα2–3Galβ1–4(Fucα1–3)GlcNAcβOR] antigens are fucose-containing type II (Galβ1–4GlcNAcβOR) glycans (Figure 1) presented in carbohydrate moieties of glycoconjugates on the human cell surface (Green, 1989). Lex is expressed in deep glands (Sakamoto et al., 1989), while sLex is presented on the surface of white blood cells and has also been found on certain tumor and cancer cells (Takada et al., 1993). SLex is the essential recognition component of E-, P-, and L-selectin ligands (Dube and Bertozzi, 2005; Kannagi, 2002; Lowe, 2003) and an important tumor-associated carbohydrate antigen (TACA) which has been used as a cancer marker for diagnosis and prognosis of cancer metastasis (Kannagi, 2004; Magnani, 2004) and as a lead structure for developing anti-inflammatory agents and cancer vaccines (Danishefsky and Allen, 2000; Ouerfelli et al., 2005; Seeberger and Werz, 2007; Simanek et al., 1998). Lex is also expressed by human pathogenic bacterium Helicobacter pylori which is believed to protect the pathogenic bacterium from immune detection by the host (Chan et al., 1995; Monteiro et al. 1998; Moran et al., 1996).
Figure 1.
Structures of important fucosylated glycans in nature. A, Lewis and sialyl Lewis antigens; B, human blood group H(O) antigens; C, fucosylated LNT and LNnT in human milk oligosaccharides (HMOs).
The L-fucose (or 6-deoxy-L-galactose) in Lex and sLex is a special monosaccharide. It is an L-sugar unlike other monosaccharides in mammalian systems which are commonly D-sugars. It is also the only deoxyhexose in animals (Varki et al., 2008). L-Fucose is commonly found as the terminal monosaccharide in the carbohydrate moiety of many important glycoconjugates in eukaryotes and are believed to be involved in tissue development, angiogenesis, fertilization, cell adhesion, inflammation, and tumor metastasis (Ma et al., 2006; Miyoshi et al., 2008). On the other hand, fucose-containing lipopolysaccharides (LPS) are expressed by some pathogenic bacteria including Helicobacter pylori (Ma et al., 2006), Bacteroides fragilis (Coyne et al., 2005), and E. coli (Guo et al., 2005). They have been suggested to be involved in molecular mimicry, adhesion, colonization, and modulating the host immune response (Ma et al., 2006).
Other than Lex and sLex , L-fucose is also presented in other Lewis antigens and human blood group antigens. Lewis a [Lea, Galβ1–3(Fucα1–4)GlcNAcβOR], sialyl Lewis a [sLea, Neu5Acα2–3Galβ1–3(Fucα1–4)GlcNAcβOR], and Lewis b [Leb, Fucα1–2Galβ1–3(Fucα1–4)GlcNAcβOR] are fucose-containing type I (Galβ1–3GlcNAcβOR) glycans. Lewis a and b antigens are mainly expressed on the surface of epithelial cells. High level of sLea has been found to be associated with tumor progression (Gong et al., 1985), location, gross appearance, invasion, and has been considered as a prognostic factor of gastric carcinoma (Nakamori et al., 1997). Lewis y [Ley, Fucα1–2Galβ1–4(Fucα1–3)GlcNAcβOR] is α1–2-fucosylated Lex. Like Lex, Ley is also expressed in deep glands and has a type II (Galβ1–4GlcNAcβOR) core structure (Green, 1989) (Figure 1A). Blood group ABO(H) antigens are fucose-containing carbohydrates presented on the glycoproteins and glycolipids on the surface of human red blood cells. They are involved in a variety of important biological processes such as blood transfusion, organ transplantation, cell development, differentiation, and oncogenesis (Milland and Sandrin, 2006). The human blood group A and B antigens are synthesized by the transfer of N-acetylgalactosamine (GalNAc) and galactose (Gal), respectively, to the H(O) antigens by two human blood group glycosyltransferases GTA and GTB which differ only at four amino acid residues (Rose et al., 2005). At least five types of H(O) antigens (Figure 1B) have been found in human, including type I, Fucα1–2Galβ1–3GlcNAcβOR; type II, Fucα1–2Galβ1–4GlcNAcβOR; type III, Fucα1–2Galβ1–3GalNAcβOR; type IV, Fucα1–2Galβ1–3GalNAcβOR; and type V, Fucα1–2Galβ1–3GlcβOR (Hakomori, 1999; Letts et al., 2006; Rose et al., 2005).
Furthermore, L-fucose is also an important component of many human milk oligosaccharides (HMOs). HMOs play a potentially protective role in nursing infants. Apart from their prebiotic effects, there is also evidence that human milk oligosaccharides act as receptor analogs to inhibit the adhesion of pathogens on the epithelial surface and interact directly with immune cells (Boehm and Stahl, 2007). The major components of the fucosylated oligosaccharides in human milk are mono- or difucosylated lacto-N-tetraose (LNT) and lacto-N-neo-tetraose (LNnT) (Kunz et al., 2000) (Figure 1C).
Due to the importance of fucosides (fucose-containing structures) in biological systems and their potential applications in treating inflammation, bacterial and viral infection, and cancer, they have been attractive synthetic targets. Although new synthetic reagents, catalysts, and strategies are becoming available (Pudelko et al., 2010), formation of the fucosidic bonds by chemical synthesis is challenging due to its acid lability. Chemical approaches are also limited due to multiple tedious protection/deprotection schemes and the lack of stereoselectivity in the formation of fucosidic linkages. Therefore, chemical synthesis is considered impractical for producing long chain polysaccharides. In addition, it is unsuitable for glycan decoration of glycoproteins. Thus, fucosyltransferase-catalyzed highly efficient and highly selective enzymatic approaches have been and will continue to play indispensable roles in obtaining many biomedically important fucose-containing glycans and glycoconjugates.
Here, we describe a protocol for applying a one-pot three-enzyme system in efficient synthesis of structurally defined naturally occurring fucosides from fucosyltransferase acceptors and L-fucose.
STRATEGIC PLANNING
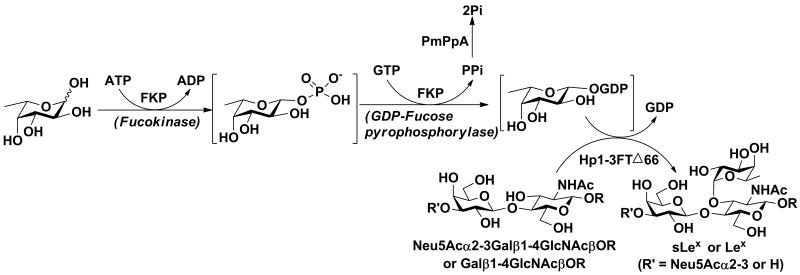
In this protocol, 3-azidopropyl N-acetyllactosamine (LacNAcβProN3 or Galβ1–4GlcNAcβProN3) and its α2–3-linked sialoside (Neu5Acα2–3Galβ1–4GlcNAcβProN3) were chosen as acceptor substrates for an α1–3-fucosyltransferase cloned from Helicobacter pylori (Hpα1–3FTΔ66) (Sugiarto et al., 2011) in a one-pot three-enzyme reaction system (Figure 2) to synthesize Lewis x [Galβ1–4(Fucα1–3)GlcNAcβProN3] and sialyl Lewis x [Neu5Acα2–3Galβ1–4(Fucα1–3)GlcNAcβProN3] antigens, respectively. In this system, a recombinant bifunctional L-fucokinase/GDP-fucose pyrophosphorylase (FKP) from Bacteroides fragilis (Yi et al., 2009) was used to convert free L-fucose to L-fucose-1-phosphate (L-Fuc-1-P) intermediate (catalyzed by the L-fucokinase activity of FKP) which was converted to guanosine 5′-diphosphate-L-fucose (GDP-Fuc) catalyzed by the GDP-fucose pyrophosphorylase activity of FKP. An inorganic pyrophosphatase cloned from Pasteurella multocida (PmPpA) (Lau et al., 2010) was used to breakdown the pyrophosphate (PPi) generated in FKP reaction to drive the reaction equilibrium towards the direction of GDP-Fuc formation. The GDP-Fuc was used to transfer the fucose moiety to acceptors catalyzed by the Hpα1–3FTΔ66. This three-enzyme system was used in one pot for the formation of the desired Lex and sLex structures from L-fucose, fucosyltransferase acceptors, adenosine 5′-triphosphate, and guanosine 5′-triphosphate without the isolation of intermediates. It avoids the use of high cost sugar nucleotides, allows the access to sugar nucleotide derivatives, simplifies the product purification process, and avoids the product loss during the multiple purification steps required by other approaches. By choosing an appropriate fucosyltransferase, the one-pot three-enzyme process can be applied for synthesizing other fucose-containing structures such as those listed in Figure 1.
Figure 2.
One-pot three-enzyme synthesis of Lewis x trisaccharide Galβ1–4(Fucα1–3)GlcNAcβProN3 or sialyl Lewis x tetrasaccharide Neu5Acα2–3Galβ1–4(Fucα1–3)GlcNAcβProN3 from L-fucose, ATP, GTP, type II disaccharide Galβ1–4GlcNAcβProN3 or trisaccharide Neu5Acα2–3Galβ1–4GlcNAcβProN3. FKP, a recombinant bifunctional L-fucokinase/GDP-fucose pyrophosphorylase (FKP) from Bacteroides fragilis 9343; PmPpA, Pasteurella multocida inorganic pyrophosphatase. Hp1–3FTΔ66, a recombinant Helicobacter pylori α1–3-fucosyltransferase with a C-terminal 66 amino-acid-residue truncation.
The one-pot three-enzyme approach discussed in this protocol is also suitable for the addition of an L-fucose residue, either in its naturally occurring form or with artificial modifications, to suitable glycoconjugates with different fucosidic linkages. Different fucokinases, GDP-fucose pyrophosphorylases, inorganic pyrophosphatases, and fucosyltransferases can be used. The scope of the one-pot three-enzyme fucosylation reaction is dependent on the activities and the substrate specificities of the enzymes used. Nevertheless, some flexibilities in substrate modification have been demonstrated. For example, L-fucose derivatives with unnatural substitutions at the C-6 position of fucose (e.g. C6-CH3 group being replaced by -H, -CH2OH, -CH2N3, -CH2NH2, - CH2CH3, -CH(OH)CH3, -C(O)CH3, -C(O)H, -C≡CH,, -CH2F, -C=CH2) have been demonstrated to be tolerant by FKP or mammalian GDP-fucose salvage pathway, and fucosyltransferases (Rabuka et al., 2006; Sawa et al. 2006; Yi et al., 2009; Wang et al., 2009). The obtained structurally defined synthetic fucosides are important probes to elucidate the biological significance of natural fucosylated structures, their biosynthetic and degradation pathways, and their involvement in the physiological and pathological processes of human and other vertebrates. It can also be used to directly modify the glycan structures on cell surface. This is especially useful for labeling glycoconjugates or cells with biotin, isotopes, chromophores, or fluorophores.
ONE-POT MULTIENZYME SYNTHESIS OF FUCOSIDES, LEWIS X AND SIALYL LEWIS X
Materials
L-Fucose
Galβ1–4GlcNAcβProN3 a
Neu5Acα2–3Galβ1–4GlcNAcβProN 3a
Adenosine 5′-triphosphate (ATP)
Guanosine 5′-triphosphate (GTP)
MgCl2·6H2O
MnCl2·4H2O
Tris base
Hydrochloric acid
Bacteroides fragilis L-fucokinase/GDP-fucose pyrophosphorylase (FKP)b
α1–3-fucosyltransferase from Helicobacter pylori (Hp1–3FTΔ66)b
Pasteurella multocida inorganic pyrophosphatase (PmPpA)b
Thin-layer silica gel plates (silica gel 60 F254)
Ethyl acetate (EtOAc)
Methanol (MeOH)
p-Anisaldehyde
H2SO4
Sodium hydroxide (NaOH)
95% Ethanol
Bio-Gel P-2 Gel, fine
Silica gel 60
Buchi Rotary evaporator
C25KC incubator shaker
Fisher Isotemp economy analog-control water bath Model 105Q
Freeze-dry system
Kontes flexcolumn economy column
Model 2110 fraction collector
Sorvall legend T/RT benchtop centrifuge
Sterile 50 ml centrifuge tubes
aGalβ1–4GlcNAcβProN3 and Neu5Acα2–3Galβ1–4GlcNAcβProN3 were prepared chemoenzymatically as described previously (Lau et al., 2010; Yu et al., 2005; Zhang et al., 2010).
bEnzymes suitable for this protocol can be recombinant proteins or purified proteins obtained in individual laboratories or from commercially available sources. The enzymes used in this protocol including FKP, PmPpA, and Hp1–3FTΔ66 are examples of suitable recombinant enzymes that are expressed and purified in our laboratory. Commercially, FKP from B. fragilis can be obtained from Accendatech (Cat. No.: B-03053, http://www.accendatech.com/index.php/public/page/l_fucokinase_gdp_fucose_pyrophosphorylas e?lang=english). Several inorganic pyrophosphatase are available from Fisher Scientific (inorganic pyrophosphatase from yeast, Cat. No. FEREF0221) and Sigma-Aldrich including inorganic pyrophosphatase from baker’s yeast (S. cerevisiae) (Cat. No. I1643/I1891), inorganic pyrophosphatase from Escherichia coli (Cat. No. I5907), and inorganic pyrophosphatase from Bacillus stearothermophilus (Cat. No. I2891).
Small-scale enzymatic assays
In principle, L-fucose and its derivatives, LacNAc/Neu5Acα2–3LacNAc and their derivatives, different FKPs (or fucokinases and GDP-fucose pyrophosphorylases), PpAs, and fucosyltransferases can be used in this approach. As same enzymes may have different activities on various substrates and different enzymes may have different substrate specificities, it is necessary to carry out small-scale enzymatic assays (Figure 3) before setting up preparative-scale reactions.
- Small scale enzymatic assays are commonly performed in 0.5 ml microcentrifuge tubes.
- Add FKP (10 μg, 0.67 U/mg), PmPpA (10 μg, 1 U/mg) and Hp1–3FTΔ66 (10 μg, 5.8 U/mg) to a Tris-HCl buffer (100 mM, pH = 7.5) containing MgCl2 (15 mM), MnCl2 (10 mM), Galβ1–4GlcNAcβProN (10 mM, for synthesizing Lex) or Neu5Acα2–3Galβ1–4GlcNAcβProN3 (10 mM, for synthesizing sLex), L-fucose (15 mM), ATP (15 mM), and GTP (15 mM). Add water to bring the total volume of the reaction mixture to 15 μl.
- Carry out assays for negative controls at the same time. Control assay I is similar to step 1(i) without adding FKP; Control assay II is similar to step 1(i) without adding Hp1–3FTΔ66.
Incubate the reaction mixtures in the microcentrifuge tubes at 37 °C for 2–6 h in a water bath.
Monitor the reactions using thin layer chromatography (TLC). Run TLC using developing solvent EtOAc:MeOH:H2O = 4:2:1. Dry TLC plates by heating on a hotplate or a hairdryer. Cool the plates down to room temperature and observed under a UV lamp. Stain the plates by dipping the whole plate into p-anisaldehyde sugar stain solution (see REAGENTS AND SOLUTIONS section for stain solution preparation). Wipe the glass side of the plates with a paper towel and dry the plates by heating on a hotplate. Formation of fucosylated product should not be observed in negative controls.
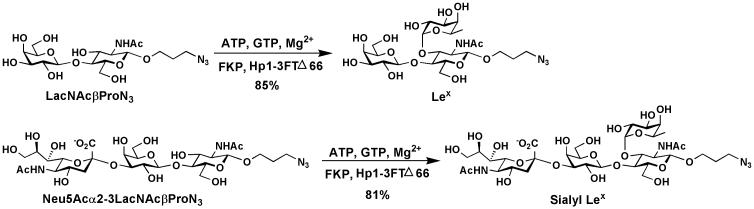
Figure 3.
One-pot three-enzyme synthesis of LexβProN3 and sLexβProN3.
All TLC tests should be performed with care in a fume hood. Cut a TLC plate of desired size. Spot reaction mixtures and solutions of standards (typically 0.5 μl each and the concentration of the solution should be 5–20 mM) on an origin line drawn near (0.5 mm) the bottom of the TLC plate. Dry the TLC plate by carefully heating on a hotplate or using a hairdryer. Cool the plate down to room temperature and place the plate in a closed container containing an appropriate developing solvent/mobile phase in a close to vertical position. Take the plate out when the solvent line almost reaches the top of the plate. Dry the plate completely on a hotplate or using a hairdryer and cool it down to room temperature. Observed under a UV lamp (long wavelength 365 nm) and circle any visible spots. Immerse the plate quickly and completely in p-anisaldehyde sugar stain and remove quickly. Wipe the back of the plate with a paper towel to absorb excess stain. Heat the plate on a hotplate until compound spots can be observed (Yu et al., 2006a). Remove the plate from the hotplate and cool it down to room temperature.
The small-scale assay can be applied to test other fucose analogs or LacNAc/Neu5Acα2–3LacNAc derivatives as potential substrates for the one-pot three-enzyme system. Other enzymes including different FKPs (or fucokinases and GDP-fucose pyrophosphorylases), PpAs, and fucosyltransferases can also be tested. Reaction conditions can be optimized by changing the pH of the reaction mixture (5.0–10.0), the type of buffers, the concentration (0–50 mM) and the type of divalent metals (Mg2+ or Mn2+), reaction temperature (20–40 °C), the concentrations of substrates (1–50 mM) and the amounts of enzymes. For fucosyltransferases that catalyze the formation of different fucosidic linkages (e.g. α1–4-fucosyltransferases or α1–2-fucosyltransferases), different acceptor substrates will be tested, including Neu5Acα2–3Galβ1–3GlcNAcβOR and derivatives (for α1–4-fucosyltransferases), Galβ1–3GalNAcαOR (for α1–4-fucosyltransferases and α1–2-fucosyltransferases), Galβ1–3GalNAcβOR, Galβ1–3GlcNAcβOR, Galβ1–4GlcβOR, and Galβ1–GlcNAcβOR (for α1–2-fucosyltransferases). Reaction conditions can be optimized as described above.
Preparative-scale synthesis of Lex and sLex (Figure 3)
4. Take out the following compounds from -20 °C freezer: Galβ1–4GlcNAcβProN3 (for the synthesis of Lex) or Neu5Acα2–3Galβ1–4GlcNAcβProN3 (for the synthesis of Lex) as the fucosyltransferase acceptor [please see references (Lau et al., 2010; Yu et al., 2005) for experimental details of preparing Galβ1–4GlcNAcβProN3 and Neu5Acα2–3Galβ1–4GlcNAcβProN3 with related NMR spectra and chemical shifts], L-fucose, ATP, and GTP. Allow them to warm up to room temperature. Take out FKP from 4 °C refrigerator and lyophilized Hp1–3FTΔ66 from −20 °C freezer and put them on ice.
5. Weigh out Galβ1–4GlcNAcβProN3 (56 mg, 0.12 mmol) or Neu5Acα2–3Galβ1–4GlcNAcβProN3 (94 mg, 0.12 mmol), L-fucose (30 mg, 0.18 mmol, 1.5 equiv.), ATP (99 mg, 0.18 mmol, 1.5 equiv.), GTP (95 mg, 0.18 mmol, 1.5 equiv.) and add into a 50 ml centrifuge tube.
6. Add 5 ml of deionized H2O to the tube. Cap the tube and vortex to dissolve the compounds completely.
7. Transfer 1 ml of a Tris-HCl buffer stock solution (1 M, pH 7.5) into the tube and swirl the tube to mix.
8. Transfer 0.15 ml of MgCl2 stock solution (1 M) and 0.10 ml of MnCl2 stock solution (1 M) into the tube and swirl the tube to mix.
9. Add FKP (1.5 mg), PpA (0.8 mg), and Hp1–3FTΔ66 (0.6 mg) into the tube. Gently mix the reaction mixture by swirling manually.
10. Add deionized H2O to bring the total volume of the reaction mixture to 10 ml.
11. Cap the tube and incubate the reaction mixture in a C25KC incubator shaker at 37 °C for up to 24 h with rotation at 140 rpm.
12. Monitor the reaction by TLC analysis after 2 h using 1.5 cm × 4.0 cm TLC plate. Use EtOAc:MeOH:H2O = 5:2:1 (by volume) as the mobile phase, and Galβ1–4GlcNAcβProN3 and Neu5Acα2–3Galβ1–4GlcNAcβProN3 as standards. Stain the plate with p-anisaldehyde sugar stain solution (see REAGENTS AND SOLUTIONS section below) by immersing the plate in a jar containing p-anisaldehyde sugar stain for visualizing compounds on the plate as described in Step 3. The Rf values of the substrates and products are: Galβ1–4GlcNAcβProN3, 0.57; Neu5Acα2–3Galβ1–4GlcNAcβProN3, 0.40; LexβProN3, 0.20; Neu5Acα2–3LexβProN3, 0.14.
13. When no further formation of the production is observed, stop the reaction by adding 10 ml of 95% pre-chilled ethanol. Cap the tube and invert back and forth gently to mix the solution. Incubate the mixture at 4 °C for 30 min.
14. Centrifuge the tube for 30 min. at 5,000 × g in a Sorvall legend T/RT benchtop centrifuge. Transfer the clear supernatant to a 100 ml round bottom flask.
15. Rinse the precipitates left in the 50 ml centrifuge tube with 5 ml of deionized water. Centrifuge the tube for 30 min. at 5,000 × g in a Sorvall legend T/RT benchtop centrifuge. Transfer the clear supernatant to the 100 ml round bottom flask containing the previous supernatant of the reaction mixture in Step 14.
16. Concentrate the combined supernatant by removing solvent using a rotary evaporator at 35 °C under reduced pressure.
The round-bottom flask can be sealed using a rubber stopper and stored in a −20 °C freezer for overnight or several days before purification.
Purification and characterization of products
17. Open the outlet of a Bio-Gel P-2 gel filtration column (2.5 × 80 cm) to let the water above the column flow down to the level of the gel bed.
18. Add 1 ml of H2O into the 100 ml round-bottom flask to dissolve the concentrated residue from Step 16. Carefully transfer this solution onto Bio-Gel P-2 gel column and let the sample solution flow down to the top level of the gel bed.
19. Rinse the 100 ml round bottom flask with 0.5 ml of water and carefully transfer the rinsing solution onto the gel bed. Repeat the rinsing and transferring processes for one more time. Let all the solution flows down to the top level of the gel bed before elution.
20. Add 5 ml of water to the column and using deionized water as the mobile phase. Collect the flow through in 6 ml fractions in 10 ml test tubes using a fraction collector.
21. Identify carbohydrate-containing test tubes by drawing 12 squares on a small TLC plate using a lead pencil and spotting sequentially the samples from every third tube within the squares on the TLC plate.
22. Dry the spots on the TLC plate by heating on a hot-plate, stain the TLC plate with p-anisaldehyde solution, wipe the side of the TLC plates that does not contain silica gel with a paper towel, and dry the plate by heating on a hot-plate. Fractions containing carbohydrates (L-fucose, GDP-Fuc, or fucoside product) will appear as green spots on the TLC plate.
23. Using EtOAc:MeOH:H2O = 45:2:1 (by volume) as the mobile phase and Galβ1–4GlcNAcβProN3 and Neu5Acα2–3Galβ1–4GlcNAcβProN3 as standards, carry out TLC assay for all sugar-containing fractions as in Step 12.
24. Combine the fractions containing the fucoside product into a 250 ml round-bottom flask. Remove the solvent of the combined fractions under reduced pressure using a rotary evaporator at 35 °C to yield the product as colorless foam.
The enzyme reaction normally can be scale up to gram scale with typical yields ranging from 60% to 99%.
Further purification of the fucoside products by silica gel flash chromatography
Carry out these steps (steps 25–30) only when necessary. For some times, pure fucoside products can be obtained after one single Bio-Gel P-2 column purification as described above (steps 17–24). In these cases, steps 25–30 are not need. However, in other times, if pure fucoside products were not obtained by a single Bio-Gel P-2 chromatography, additional silica gel chromatography purification procedures are needed.
25. Dissolve the fucoside product obtained from the gel filtration purification in Step 24 in 2 ml of deionized water in a 100 ml flask. Add 1 g of silica gel to the solution and evaporate the water using a rotary evaporator at 30 °C under high vacuum. The fucoside product and impurities are absorbed by the silica gel.
26. Pack a chromatography column (3 cm i.d × 20 cm length with a 250 ml capacity) using 35 g of silica gel.
27. Load the silica gel adsorbed with compounds from Step 25 to the top of the gel bed in the silica gel column using a long-stem funnel. Cover the top with a layer of sand (1 cm thickness).
28. Elute the column in sequence with 250 ml of ethyl acetate (EtOAc) and 500 ml of EtOAc:MeOH:H2O = 7:2:1 mixture (by volume).
29. Collect the flow through in 6 ml fractions (fractions of fucoside may contain tiny amount of silica gel because a high polarity elute system is used. Additional gel permeation chromatography can be used to remove the impurity). Analyze each fraction using TLC.
30. Combine fractions containing the fucoside product. Concentrate the combined solution in a rotary evaporator.
31. Dissolve dried samples in a small amount (0.5–1 ml) of water and transfer to a pre-weighed bottle. Freeze the sample and lyophilize it using a freeze dryer.
32. Weigh the bottle containing the dried compound. Calculate the amount of the pure product obtained and reaction yield.
33. Characterize the fucoside products by 1H and 13C nuclear magnetic resonance (NMR) spectroscopy and high resolution mass spectrometry (HRMS).
REAGENTS AND SOLUTIONS
Preparing p-anisaldehyde sugar stain solution
Cautiously add 50 ml of concentrated H2SO4 drop wisely to 425 ml of cold methanol in a 1000 ml bottle in a water-ice bath at 0 °C with vigorous stirring without splashing. Dissolve 25 ml of p-anisaldehyde to the mixed methanol-acid solution. Store the resulting colorless solution at −20 °C freezer for up to one year.
All reagents are toxic. Handle in the fume hood with care. Wear gloves, safety goggles, and lab coats.
Packing a Bio-Gel P-2 gel filtration column
To pack a column with about 400 ml of hydrated bed volume, slowly add 140 g of dry Bio-Gel P-2 gel to 800 ml of deionized water in a 2000 ml beaker and allow it to incubate without stirring at room temperature for at least 4 h. Decant to remove the fine floating particles fines (such as broken beads and other impurities), transfer the solution to a filter flask and attach to a water aspirator. Degas for 30 min with occasional swirling. Mount a column (2.5 cm i.d ×100 cm length) on a frame to allow it stay perpendicular to the floor and 0.5 foot above floor surface. Close the bottom valve of the column and add water to about 20% volume of the column. Pour the evenly mixed gel slurry into the column in a single, smooth moment, taking care to avoid producing bubbles. Open the valve on the bottom of the column to allow the flow of water until a firm 25 cm bed is formed. Attach the top cap of the column to a tubing line connected to a flask with 2000 ml of degassed deionized water and allow 1000 ml of deionized water to flow through the column by gravity.
The packed column is reusable and can be stored at room temperature for 2 years or longer if maintained at neutral pH in the presence of 0.02% sodium azide.
COMMENTARY
Background Information
Carbohydrate moieties of the cell surface glycolipids or glycoproteins mediate the interactions of various subsets of leukocytes with endothelial cells (Lasky, 1992). Fucosylated glycans have shown to be important as cell adhesion molecules, hematopoietic cell differentiation markers, and tumor antigens. The availability of structural defined synthetic fucosylated glycans and glycoconjugates facilitates the study of their biological significance and helps to explore their biomedical applications. Therefore, robust and practical synthetic methods for fucose-containing glycoconjugates are highly desirable.
Chemical synthetic methods for fucose-containing structures have been developed but the chemical formation of α-fucosidic bond is challenged by its acid lability, multiple tedious protection/deprotection schemes, and the lack of stereo-control. On the other hand, fucosyltransferase-catalyzed enzymatic approaches are highly efficient and selective. They are useful methods for obtaining many biologically important fucosides. A number of bacterial fucosyltransferases have been cloned (Ge et al., 1997; Li et al., 2008a and 2008b; Martin et al., 1997; Pettit et al., 2010; Rabbani et al., 2005; Rasko et al., 2000; Shao et al., 2003; Wang et al., 1999; Zhang et al., 2010) and several have been employed for preparative-scale synthesis (Pettit et al., 2010; Su et al., 2008; Wang et al., 2009; Wang et al., 2008; Zhang et al., 2010). However, a limiting factor of preparative-scale fucosyltransferase-catalyzed synthesis of fucosides is the high cost of sugar nucleotide donor GDP-Fuc required by the fucosyltransferases.
As most of enzymes are active in an overlapping range of reaction conditions, it is possible to carry out reactions containing multiple enzymes which act in sequential in one-pot without the purification of intermediates. The one-pot multiple-enzyme approach does not only simplify the product purification process, but also allows the access of high cost sugar nucleotides or their analogs which are not commercially available from free monosaccharides. Mixing multienzymes which act in sequential in one-pot can also shift the reaction equilibrium towards the formation of the product to improve overall synthetic yields. Our group has recently developed several highly efficient one-pot multienzyme systems for the synthesis of common mammalian glycans including α2–3/6/8-linked sialosides (Yu et al., 2009; 2005, 2006a; 2006b), β1–3-linked galactoside (Yu et al., 2010), β1–4-linked galactosides (Lau et al., 2010), and α1–3-linked fucosides (Sugiarto et al., 2011; Zhang et al., 2010). In this strategy, monosaccharides or derivatives are activated and transferred to simple glycans or glycoconjugates for the formation of more complex glycans or glycoconjugates with the catalysis of two or more enzymes which act sequentially (Figure 4).
Figure 4.
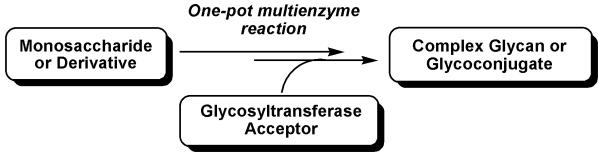
One-pot multienzyme strategy for synthesizing complex glycans or glycoconjugates.
In this protocol, we describe the production of fucosylated glycans LexβProN3 and sLexβProN3 using a one-pot three-enzyme fucosylation system from inexpensive starting material L-fucose instead of expensive GDP-Fuc (Figure 2). The sugar nucleotide GDP-Fuc is generated from L-fucose, ATP, and GTP by a bifunctional enzyme FKP and PmPpA, which is utilized by Hp1–3FTΔ66 for the synthesis of Lex and sLex. A similar one-pot three-enzyme fucosylation strategy can be applied for the synthesis of diverse naturally occurring and non-natural fucosides by choosing an appropriate fucosyltransferase such as α1–2FT, α1–3FT, α1–4FT, or α1–6FT.
The azido tag in the fucosides obtained can be used conveniently for chemoselectively conjugation via Staudinger ligation (Saxon and Bertozzi, 2000) or alkyne-azide 1,3-dipolar cycloaddition (so called "Click Chemistry") (Fazio et al., 2002; Kolb et al., 2001; Tornoe et al., 2002). The azido group can also be reduced to amino group and conjugate to other biomolecules such as proteins through bifunctional crosslinkers (Yu et al., 2007).
Critical Parameters
The success of the one-pot three-enzyme fucosylation approach relies heavily on the activity and substrate promiscuity of both FKP and fucosyltransferse. As the catalytic efficiency of enzymes may vary with the length of the storage period and storage conditions, it is very important to carry out small assays to confirm the activities of FKP and Hp1–3FTΔ66 before setting up preparative-scale reactions. In addition, a positive control with compounds which have been shown to be substrates for the enzymatic system is important. To analyze the crude reaction by thin layer chromatography, it is important to co-spot the reaction mixture with the acceptor substrate, Galβ1–4GlcNAcβProN3 or Neu5Acα2–3Galβ1–4GlcNAcβProN3 and to include product standards, if available, in the same TLC plate. The desired fucosylated product Lex or sLex shows dark green color when stained with p-anisaldehyde sugar stain and heated on a hotplate.
Troubleshooting
If the enzymatic reaction does not work or the yield is low for the positive control, it may due to the loss of the activity of the enzyme during storage. Use another batch of enzymes for testing and synthesis. In order to retain activities, enzymes should be stored in 10% glycerol at 4 °C for short term storage (up to 2 weeks) or in 50% glycerol at −20 °C for long term storage (1–2 years). PmPpA and Hp1–3FTΔ66 used in this study can also be lyophillized from non-glycerolcontaining dialysis buffer (20 mM Tris-HCl, pH 7.5) and stored at -20 °C for 1–2 years without losing activity. Always keep enzymes on ice when setting up reactions. Make sure to use the optimal conditions that have been determined as described in small scale assays in step 3 for carrying out synthesis.
If the pH of the reaction solution drops during the reaction, adjust the pH value by carefully adding NaOH solution (1 M) and test using pH papers.
If the gel filtration chromatography does not separate fucosylated product from the acceptor, the following precautions should be taken: 1) use minimum volume of water (normally 0.5 ml) to dissolve the crude product when load sample to Bio-Gel P-2 column; 2) increase the length of the Bio-gel P-2 column; and 3) further purification using silica gel column chromatography (steps 25–30) is suggested.
If the color of the p-anisaldehyde stain turns into dark red, dispose the stain properly as hazardous waste and prepare a new batch of stain. The stain should be stored at −20 °C in a sealed bottle in freezer. Do not leave the stain solution at room temperature. Wait until the TLC plates are cooled down to room temperature before staining.
Anticipated Results
The described protocol allows preparative-scale synthesis of fucosides with yields ranging from 60–99%. The results for the one-pot three-enzyme preparative synthesis of Lex and sLex using L-fucose as a donor substrate and Galβ1–4GlcNAcβProN3 and Neu5Acα2–3Galβ1–4GlcNAcβProN3 as acceptor substrates are shown in Figure 3. A purity of over 95% of fucosylated product is common after Bio-gel P-2 column purification. The purity can be improved to 99% after further silica gel column purification.
Analytical data
3-Azidopropyl O-β-D-galactopyranosyl-(1→4)-[α-L-fucopyranosyl-(1→3)]-β-D-glucopyranoside (LexβProN3). Yield, 85%; white foam1H NMR (600 MHz, D2O) δ 5.13 (d, 1 H, J = 4.2 Hz), 4.56 (d, 1 H, J = 8.4 Hz), 4.47 (d, 1 H, J = 7.8 Hz), 4.02 (dd, 1 H, J = 10.8 Hz and 3.3 Hz), 3.99–3.59 (m, 17 H), 3.52 (t, 1 H, J = 8.4 Hz), 3.39 (m, 2 H), 2.06 (s, 3 H), 1.86 (m, 2 H, J = 6.0 Hz), 1.19 (d, 3 H, J = 6.6 Hz). 13C NMR (150 MHz, D2O): δ174.42, 102.00, 101.13, 98.78, 75.52, 75.08, 73.56, 72.65, 72.07, 71.23, 69.38, 68.54, 67.89, 67.38, 66.87, 61.66, 59.95, 56.00, 47.95, 28.30, 22.42, 15.48.
3-Azidopropyl O-(5-acetamido-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosylonic acid)-(2→3)-O-β-D-galactopyranosyl-(1→4)-[α-L-fucopyranosyl-(1→3)]-β-D-glucopyranoside (Neu5Acα2–3LexβProN3). Yield, 81%; white foam. 1H NMR (600 MHz, D2O) δ 5.08 (d, 1 H, J = 4.2 Hz), 4.51 (d, 1 H, J = 8.4 Hz), 4.50 (d, 1 H, J = 7.8 Hz), 4.06 (dd, 1 H, J = 9.9 Hz and 3.3 Hz), 4.00–3.81 (m, 11 H), 3.75 (d, 1 H, J = 3.0 Hz), 3.68–3.60 (m, 8 H), 3.58–3.55 (m, 3 H), 3.50 (dd, 1 H, J = 9.6 Hz and 8.4 Hz), 3.37–3.32 (m, 2 H), 2.74 (dd, 1 H, J = 12.3 Hz and 4.2 Hz), 2.02 (s, 3 H), 2.01 (s, 3 H), 1.81 (m, 2 H, J = 6.6 Hz), 1.77 (t, 1 H, J = 12.3 Hz), 1.14 (d, 3 H, J = 6.6 Hz). 13C NMR (100 MHz, D2O) δ 175.19, 174.41, 174.05, 101.78, 101.15, 99.82, 98.76, 75.81, 75.42, 75.07, 74.98, 73.51, 73.07, 72.07, 72.02, 69.42, 69.35, 68.47, 68.28, 67.87, 67.47, 67.36, 66.84, 62.76, 61.64, 59.81, 55.98, 51.86, 47.93, 39.94, 28.27, 22.39, 22.20, 15.43. HRMS (ESI) m/z calcd for C34H57N5O23Na (M+Na) 926.3319, found 926.3342.
Time Considerations
An experienced chemist can anticipate to complete the synthesis, product purification, and characterization in two to three days.
It usually takes 2–12 h to complete small-scale enzymatic assays. For preparative-scale reactions, preparing enzyme reaction mixtures needs 30–60 min and the reaction can take 4–24 h to complete depending on the amounts of the enzymes used. Stop the reaction and remove the solvents using a rotary evaporator will need about 2 h. Purification of the fucosylated product using Bio-gel P-2 column and TLC analysis require 6–8 h. Further purification using silica gel column chromatography usually needs another 6 h. NMR and MS characterization can take 1–3 hr.
Acknowledgments
The authors are grateful for the financial supports from NSF grant CHE1012511, NIH grant R01HD065122, the Camille Dreyfus Teacher-Scholarship, and the UC-Davis Chancellor’s Fellowship.
Literature Cited
- Boehm G, Stahl B. Oligosaccharides from milk. J Nutr. 2007;137:847S–849S. doi: 10.1093/jn/137.3.847S. [DOI] [PubMed] [Google Scholar]
- Chan NW, Stangier K, Sherburne R, Taylor DE, Zhang Y, Dovichi NJ, Palcic MM. The biosynthesis of Lewis X in Helicobacter pylori. Glycobiology. 1995;5:683–688. doi: 10.1093/glycob/5.7.683. [DOI] [PubMed] [Google Scholar]
- Coyne MJ, Reinap B, Lee MM, Comstock LE. Human symbionts use a host-like pathway for surface fucosylation. Science. 2005;307:1778–1781. doi: 10.1126/science.1106469. [DOI] [PubMed] [Google Scholar]
- Danishefsky SJ, Allen JR. From the laboratory to the clinic: A retrospective on fully synthetic carbohydrate-based anticancer vaccines. Angew Chem Int Ed. 2000;39:836–863. doi: 10.1002/(sici)1521-3773(20000303)39:5<836::aid-anie836>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Dube DH, Bertozzi CR. Glycans in cancer and inflammation-potential for therapeutics and diagnostics. Nat Rev. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- Fazio F, Bryan MC, Blixt O, Paulson JC, Wong CH. Synthesis of sugar arrays in microtiter plate. J Am Chem Soc. 2002;124:14397–14402. doi: 10.1021/ja020887u. [DOI] [PubMed] [Google Scholar]
- Ge Z, Chan NW, Palcic MM, Taylor DE. Cloning and heterologous expression of an alpha1,3-fucosyltransferase gene from the gastric pathogen Helicobacter pylori. J Biol Chem. 1997;272:21357–21363. doi: 10.1074/jbc.272.34.21357. [DOI] [PubMed] [Google Scholar]
- Gong E, Hirohashi S, Shimosato Y. Expression of carbohydrate antigen 19-9 and stage-specific embryonic antigen 1 in non-tumorous and tumorous epithelia of the human colon and rectum. J Natl Cancer Inst. 1985;75:447–454. [PubMed] [Google Scholar]
- Green C. The ABO, Lewis and related blood group antigens; a review of structure and biosynthesis. FEMS Microbiol Immunol. 1989;1:321–330. doi: 10.1111/j.1574-6968.1989.tb02417.x. [DOI] [PubMed] [Google Scholar]
- Guo H, Yi W, Shao J, Lu Y, Zhang W, Song J, Wang PG. Molecular analysis of the O-antigen gene cluster of Escherichia coli O86:B7 and characterization of the chain length determinant gene (wzz) Appl Environ Microbiol. 2005;71:7995–8001. doi: 10.1128/AEM.71.12.7995-8001.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. Antigen structure and genetic basis of histo-blood groups A, B and O: their changes associated with human cancer. Biochim Biophys Acta. 1999;1473:247–266. doi: 10.1016/s0304-4165(99)00183-x. [DOI] [PubMed] [Google Scholar]
- Kannagi R. Molecular mechanism for cancer-associated induction of sialyl Lewis X and sialyl Lewis A expression-The Warburg effect revisited. Glycoconj J. 2004;20:353–364. doi: 10.1023/B:GLYC.0000033631.35357.41. [DOI] [PubMed] [Google Scholar]
- Kannagi R. Regulatory roles of carbohydrate ligands for selectins in the homing of lymphocytes. Curr Opin Struct Biol. 2002;12:599–608. doi: 10.1016/s0959-440x(02)00365-2. [DOI] [PubMed] [Google Scholar]
- Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse chemical function from a few good reactions. Angew Chem Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: Structural, functional, and metabolic aspects. Anun Rev Nutr. 2000;20:699–722. doi: 10.1146/annurev.nutr.20.1.699. [DOI] [PubMed] [Google Scholar]
- Lasky LA. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992;258:964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- Lau K, Thon V, Yu H, Ding L, Chen Y, Muthana MM, Wong D, Huang R, Chen X. Highly efficient chemoenzymatic synthesis of beta1-4-linked galactosides with promiscuous bacterial beta1-4-galactosyltransferases. Chem Commun (Camb) 2010;46:6066–6068. doi: 10.1039/c0cc01381a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letts JA, Rose NL, Fang YR, Barry CH, Borisova SN, Seto NO, Palcic MM, Evans SV. Differential recognition of the type I and II H antigen acceptors by the human ABO(H) blood group A and B glycosyltransferases. J Biol Chem. 2006;281:3625–3632. doi: 10.1074/jbc.M507620200. [DOI] [PubMed] [Google Scholar]
- Li M, Liu XW, Shao J, Shen J, Jia Q, Yi W, Song JK, Woodward R, Chow CS, Wang PG. Characterization of a novel alpha1,2-fucosyltransferase of Escherichia coli O128:b12 and functional investigation of its common motif. Biochemistry. 2008a;47:378–387. doi: 10.1021/bi701345v. [DOI] [PubMed] [Google Scholar]
- Li M, Shen J, Liu X, Shao J, Yi W, Chow CS, Wang PG. Identification of a new alpha1,2-fucosyltransferase involved in O-antigen biosynthesis of Escherichia coli O86:B7 and formation of H-type 3 blood group antigen. Biochemistry. 2008b;47:11590–11597. doi: 10.1021/bi801067s. [DOI] [PubMed] [Google Scholar]
- Lowe JB. Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr Opin Cell Biol. 2003;15:531–538. doi: 10.1016/j.ceb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Ma B, Simala-Grant JL, Taylor DE. Fucosylation in prokaryotes and eukaryotes. Glycobiology. 2006;16:158R–184R. doi: 10.1093/glycob/cwl040. [DOI] [PubMed] [Google Scholar]
- Magnani JL. The discovery, biology, and drug development of sialyl Lea and sialyl Lex. Arch Biochem Biophys. 2004;426:122–131. doi: 10.1016/j.abb.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Martin SL, Edbrooke MR, Hodgman TC, van den Eijnden DH, Bird MI. Lewis X biosynthesis in Helicobacter pylori. Molecular cloning of an alpha(1,3)-fucosyltransferase gene. J Biol Chem. 1997;272:21349–21356. doi: 10.1074/jbc.272.34.21349. [DOI] [PubMed] [Google Scholar]
- Milland J, Sandrin MS. ABO blood group and related antigens, natural antibodies and transplantation. Tissue Antigens. 2006;68:459–466. doi: 10.1111/j.1399-0039.2006.00721.x. [DOI] [PubMed] [Google Scholar]
- Miyoshi E, Moriwaki K, Nakagawa T. Biological function of fucosylation in cancer biology. J Biochem. 2008;143:725–729. doi: 10.1093/jb/mvn011. [DOI] [PubMed] [Google Scholar]
- Monteiro MA, Chan KH, Rasko DA, Taylor DE, Zheng PY, Appelmelk BJ, Wirth HP, Yang M, Blaser MJ, Hynes SO, Moran AP, Perry MB. Simultaneous expression of type 1 and type 2 Lewis blood group antigens by Helicobacter pylori lipopolysaccharides. Molecular mimicry between H. pylori lipopolysaccharides and human gastric epithelial cell surface glycoforms. J Biol Chem. 1998;273:11533–11543. doi: 10.1074/jbc.273.19.11533. [DOI] [PubMed] [Google Scholar]
- Moran AP, Prendergast MM, Appelmelk BJ. Molecular mimicry of host structures by bacterial lipopolysaccharides and its contribution to disease. FEMS Immunol Med Microbiol. 1996;16:105–115. doi: 10.1111/j.1574-695X.1996.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Nakamori S, Furukawa H, Hiratsuka M, Iwanaga T, Imaoka S, Ishikawa O,T, K., Sasaki Y, Kameyama M, Ishiguro S, Irimura T. Expression of carbohydrate antigen sialyl Lea: a new functional prognostic factor in gastric cancer. J Clin Oncol. 1997;15:816–825. doi: 10.1200/JCO.1997.15.2.816. [DOI] [PubMed] [Google Scholar]
- Ouerfelli O, Warren JD, Wilson RM, Danishefsky SJ. Synthetic carbohydrate-based antitumor vaccines: challenges and opportunities. Expert Rev Vaccines. 2005;4:677–685. doi: 10.1586/14760584.4.5.677. [DOI] [PubMed] [Google Scholar]
- Pettit N, Styslinger T, Mei Z, Han W, Zhao G, Wang PG. Characterization of WbiQ: An alpha1,2-fucosyltransferase from Escherichia coli O127:K63(B8), and synthesis of H-type 3 blood group antigen. Biochem Biophys Res Commun. 2010;402:190–195. doi: 10.1016/j.bbrc.2010.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudelko M, Bull J, Kunz H. Chemical and chemoenzymatic synthesis of glycopeptide selectin ligands containing sialyl Lewis X structures. Chembiochem. 2010;11:904–930. doi: 10.1002/cbic.201000029. [DOI] [PubMed] [Google Scholar]
- Rabbani S, Miksa V, Wipf B, Ernst B. Molecular cloning and functional expression of a novel Helicobacter pylori alpha-1,4 fucosyltransferase. Glycobiology. 2005;15:1076–1083. doi: 10.1093/glycob/cwj004. [DOI] [PubMed] [Google Scholar]
- Rabuka D, Hubbard SC, Laughlin ST, Argade SP, Bertozzi CR. A chemical reporter strategy to probe glycoprotein fucosylation. J Am Chem Soc. 2006;128:12078–12079. doi: 10.1021/ja064619y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasko DA, Wang G, Palcic MM, Taylor DE. Cloning and characterization of the alpha(1,3/4) fucosyltransferase of Helicobacter pylori. J Biol Chem. 2000;275:4988–4994. doi: 10.1074/jbc.275.7.4988. [DOI] [PubMed] [Google Scholar]
- Rose NL, Palcic MM, Evans SV. Glycosyltranferases A and B: Four critical amino acids determine blood type. J Chem Educ. 2005;82:1846–1852. [Google Scholar]
- Sakamoto S, Watanabe T, Tokumaru T, Takagi H, Nakazato H, Lloyd KO. Expression of Lewisa, Lewisb, Lewisx, Lewisy, siayl-Lewisa, and sialyl-Lewisx blood group antigens in human gastric carcinoma and in normal gastric tissue. Cancer Res. 1989;49:745–752. [PubMed] [Google Scholar]
- Sawa M, Hsu TL, Itoh T, Sugiyama M, Hanson SR, Vogt PK, Wong C-H. Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc Natl Acad Sci U S A. 2006;103:12371–12376. doi: 10.1073/pnas.0605418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon E, Bertozzi CR. Cell surface engineering by a modified Staudinger reaction. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- Seeberger PH, Werz DB. Synthesis and medical applications of oligosaccharides. Nature. 2007;446:1046–1051. doi: 10.1038/nature05819. [DOI] [PubMed] [Google Scholar]
- Shao J, Li M, Jia Q, Lu Y, Wang PG. Sequence of Escherichia coli O128 antigen biosynthesis cluster and functional identification of an alpha-1,2-fucosyltransferase. FEBS Lett. 2003;553:99–103. doi: 10.1016/s0014-5793(03)00980-3. [DOI] [PubMed] [Google Scholar]
- Simanek EE, McGarvey GJ, Jablonowski JA, Wong CH. Selectin-carbohydrate interactions: From natural ligands to designed mimics. Chem Rev. 1998;98:833–862. doi: 10.1021/cr940226i. [DOI] [PubMed] [Google Scholar]
- Su DM, Eguchi H, Yi W, Li L, Wang PG, Xia C. Enzymatic synthesis of tumor-associated carbohydrate antigen Globo-H hexasaccharide. Org Lett. 2008;10:1009–1012. doi: 10.1021/ol703121h. [DOI] [PubMed] [Google Scholar]
- Sugiarto G, Lau K, Yu H, Vuong S, Thon V, Li Y, Huang S, Chen X. Cloning and characterization of a viral alpha2-3-sialyltransferase (vST3Gal-I) for the synthesis of sialyl Lewisx. Glycobiology. 2011;21:387–396. doi: 10.1093/glycob/cwq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada A, Ohmori K, Yoneda T, Tsuyuoka K, Hasegawa A, Kiso M, Kannagi R. Carbohydrate antigens sialyl Lewis A and sialyl Lewis X and adhesion of human cancer cells to vascular endothelium. Cancer Res. 1993;53:354–361. [PubMed] [Google Scholar]
- Tornoe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1,2,3]- triazoles by regiospecific copper(i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. Essentials of Glycobiology. 2nd Ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2008. p. 55. [PubMed] [Google Scholar]
- Wang G, Rasko DA, Sherburne R, Taylor DE. Molecular genetic basis for the variable expression of Lewis Y antigen in Helicobacter pylori: Analysis of the alpha(1,2) fucosyltransferase gene. Mol Microbiol. 1999;31:1265–1274. doi: 10.1046/j.1365-2958.1999.01268.x. [DOI] [PubMed] [Google Scholar]
- Wang W, Hu T, Frantom PA, Zheng T, Gerwe B, Del Amo DS, Garret S, Seidel RD, 3rd, Wu P. Chemoenzymatic synthesis of GDP-L-fucose and the Lewis X glycan derivatives. Proc Natl Acad Sci U S A. 2009;106:16096–16101. doi: 10.1073/pnas.0908248106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gilbert M, Eguchi H, Yu H, Cheng J, Muthana S, Zhou L, Wang PG, Chen X, Huang X. Chemoenzymatic syntheses of tumor-associated carbohydrate antigen Globo-H and stage-specific embryonic antigen 4. Adv Synth Catal. 2008;350:1717–1728. doi: 10.1002/adsc.200800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi W, Liu X, Li Y, Li J, Xia C, Zhou G, Zhang W, Zhao W, Chen X, Wang PG. Remodeling bacterial polysaccharides by metabolic pathway engineering. Proc Natl Acad Sci U S A. 2009;106:4207–4212. doi: 10.1073/pnas.0812432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Cheng J, Ding L, Khedri Z, Chen Y, Chin S, Lau K, Tiwari VK, Chen X. Chemoenzymatic synthesis of GD3 oligosaccharides and other disialyl glycans containing natural and non-natural sialic acids. J Am Chem Soc. 2009;131:18467–18477. doi: 10.1021/ja907750r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Chokhawala H, Karpel R, Wu B, Zhang J, Zhang Y, Jia Q, Chen X. A multifunctional Pasteurella multocida sialyltransferase: a powerful tool for the synthesis of sialoside libraries. J Am Chem Soc. 2005;127:17618–17619. doi: 10.1021/ja0561690. [DOI] [PubMed] [Google Scholar]
- Yu H, Chokhawala HA, Huang S, Chen X. One-pot three-enzyme chemoenzymatic approach to the synthesis of sialosides containing natural and non-natural functionalities. Nat Protoc. 2006a;1:2485–2492. doi: 10.1038/nprot.2006.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Chokhawala HA, Varki A, Chen X. Efficient chemoenzymatic synthesis of biotinylated human serum albumin-sialoglycoside conjugates containing O-acetylated sialic acids. Org Biomol Chem. 2007;5:2458–2463. doi: 10.1039/b706507h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Huang S, Chokhawala H, Sun M, Zheng H, Chen X. Highly efficient chemoenzymatic synthesis of naturally occurring and non-natural alpha-2,6-linked sialosides: a P. damsela alpha-2,6-sialyltransferase with extremely flexible donor-substrate specificity. Angew Chem Int Ed. 2006b;45:3938–3944. doi: 10.1002/anie.200600572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Thon V, Lau K, Cai L, Chen Y, Mu S, Li Y, Wang PG, Chen X. Highly efficient chemoenzymatic synthesis of beta1-3-linked galactosides. Chem Commun (Camb) 2010;46:7507–7509. doi: 10.1039/c0cc02850a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Lau K, Cheng J, Yu H, Li Y, Sugiarto G, Huang S, Ding L, Thon V, Wang PG, Chen X. Helicobacter hepaticus Hh0072 gene encodes a novel alpha1-3-fucosyltransferase belonging to CAZy GT11 family. Glycobiology. 2010;20:1077–1088. doi: 10.1093/glycob/cwq068. [DOI] [PMC free article] [PubMed] [Google Scholar]