Abstract
Heart failure with preserved ejection fraction (HFpEF) is a heterogeneous syndrome, with several underlying etiologic and pathophysiologic factors. While prior heart failure clinical trials have used a “one size fits all” approach, this approach has not proven successful for HFpEF. Furthermore, with the aging population and epidemics of obesity, diabetes, and hypertension, the prevalence of HFpEF will continue to grow over the foreseeable future. Coupled with the high morbidity and mortality of the HFpEF syndrome, there remains a pressing unmet need to improve the clinical care of HFpEF patients and to design better HFpEF clinical trials. Improved classification of the wide HFpEF phenotypic spectrum is therefore essential to advance the HFpEF field and begin to provide targeted treatment for these patients. Here we describe 4 potential classification schemas for HFpEF: (1) pathophysiologic classification; (2) clinical/etiologic classification; (3) classification based on type of clinical presentation; and (4) phenomics (“phenomapping”) of HFpEF. Improved phenotypic categorization of HFpEF using these schemas is now possible given the multitude of tools available to perform “dense phenotyping” of HFpEF patients. Such categorization should lead to clinical care and clinical trials where targeted therapies based on specific mechanisms of disease can be matched to the specific patient subtypes most likely to respond to those therapies. In addition, innovative analytic strategies, such as “phenomapping”, may allow for the use of dense multi-dimensional data to create novel phenotypic signatures, which should help identify HFpEF patients who are particularly responsive to specific treatments.
Keywords: heart failure with preserved ejection fraction, phenotype, phenomics, etiology, pathophysiology, classification, clinical trials
Introduction
Heart failure (HF) is a common clinical syndrome with high morbidity and mortality, and one that is increasing in prevalence with the aging population.1-4 Regardless of underlying ejection fraction (EF), HF is a heterogeneous syndrome.5-7 HF is the result of one or more risk factors that lead to abnormal cardiac structure and function, which ultimately causes reduced cardiac output and/or elevated cardiac filling pressures at rest or with exertion.1 Although HF with reduced EF (HFrEF) can be heterogeneous in etiology, chronic HFrEF in particular has proven to respond to a “one size fits all” approach; several drugs and devices have improved outcomes in randomized clinical trials of patients with HFrEF.1
However, unlike HFrEF, clinical trials of pharmacologic agents in HF with preserved EF (HFpEF, previously termed diastolic HF) have not shown significant benefits, and no treatments have been found to be effective in this group of patients.8,9 In HFpEF, the underlying phenotypic heterogeneity is most likely much greater than HFrEF,5,6 and may be a key reason for the poor track record of HFpEF clinical trials. Therefore, understanding the phenotypic spectrum of HFpEF, which includes the etiologic and pathophysiologic heterogeneity of the syndrome, may allow for more targeted clinical diagnosis and management of HFpEF patients, and more successful clinical trials.
Heterogeneity of HFpEF: Epidemiologic Studies vs. Pathophysiologic Studies
Prior observational registries and epidemiologic studies that have included patients with HFpEF, such as the Acute Decompensated Heart Failure National Registry (ADHERE) and the Rochester Epidemiology Project, have enrolled a broad array of patients with a wide variety of etiologies and pathophysiologies.10,11 However, detailed mechanistic studies of HFpEF often only enroll very specific subsets of HFpEF patients, thereby limiting generalizability to the overall population of HFpEF patients. For example, in a detailed pathophysiologic study of HFpEF,12 Prasad and colleagues began with 1119 patients hospitalized for HF with EF > 50% but ended up with only 23 (2%) patients who were eligible for their study after applying a lengthy list of exclusion criteria, including atrial fibrillation, chronic kidney disease, myocardial infarction, cognitive impairment, and other common HFpEF comorbidities.12
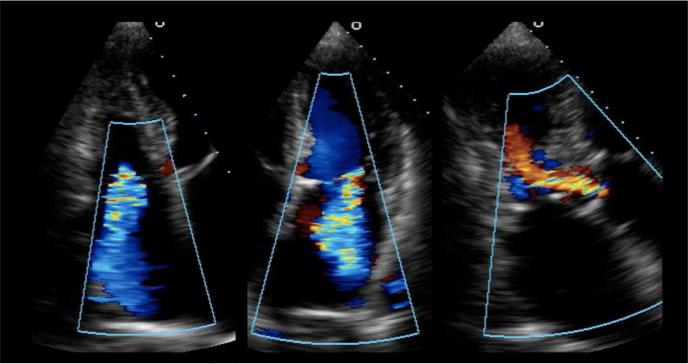
Several studies have now shown that HFpEF is quite heterogeneous from both an etiologic and pathophysiologic standpoint,6,13-15 and previous pathophysiologic studies that have concluded that HFpEF is mainly a disease of diastolic dysfunction have been challenged.16 The complexity and heterogeneity of HFpEF is readily apparent when caring for patients suffering from this syndrome. Consider an 86-year-old woman with systemic hypertension, diabetes, chronic kidney disease, signs and symptoms of HF (New York Heart Association functional class III), a preserved left ventricular (LV) EF of 65%, mild concentric LV hypertrophy with mild superimposed basal septal hypertrophy, severe left atrial enlargement, and moderate (grade II) diastolic dysfunction. This description is typical for a HFpEF patient; however, consider her color Doppler echocardiographic findings (Figure 1), which show moderate aortic regurgitation, moderate mitral regurgitation, and moderate tricuspid regurgitation. Although none of these valvular lesions meets criteria for surgical treatment, they nevertheless combine with the intrinsic myocardial abnormalities to exacerbate the HF syndrome, and treatment of diastolic dysfunction and fluid overload alone may not improve symptoms in this type of patient.
Figure 1. Color Doppler Echocardiography from a Patient with Heart Failure and Preserved Ejection Fraction Showing Multiple Moderate Valvular Lesions.
Caption: Left panel: apical 4-chamber view of the right heart showing moderate tricuspid regurgitation; middle panel: apical 4-chamber view of the left heart showing moderate mitral regurgitation; right panel: apical 3-chamber view showing moderate aortic regurgitation. From Oktay AA, Shah SJ. Heart failure with preserved ejection fraction. In Levine G, ed. Color Atlas of Clinical Cardiology. New Delhi: Jaypee Medical Publishers, 2014; with permission.
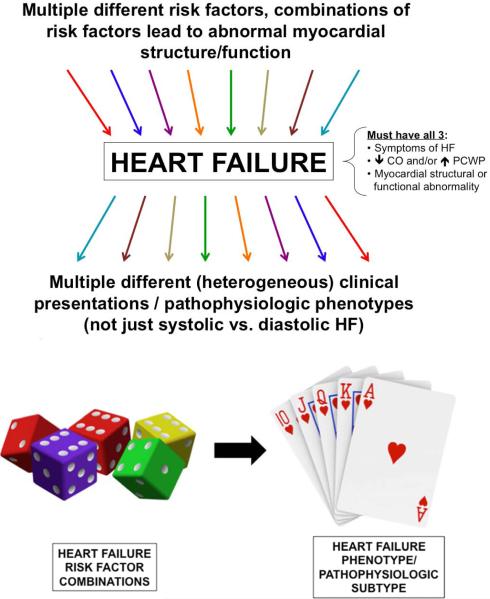
It is now well known that comorbidities are very important in both the development of HFpEF and in driving outcomes in these patients.17-21 Accumulation of comorbidities predisposes to Stage B HF and ultimately leads to Stage C (symptomatic) HF, including HFpEF. However, multiple different combinations of comorbidities can occur, and these combinations of risk factors, along with genetic and environmental factors, lead to different varieties of HF that are more complex than simply “systolic” vs. “diastolic” HF (Figure 2). A critical unmet need is the determination of which combinations of risk factors leads to which specific phenotypes of HFpEF. Such information would allow for targeted screening and diagnostic strategies for the prevention of the spectrum of HFpEF.
Figure 2. Relationship Between Heart Failure Risk Factor Combinations and Heart Failure Phenotypic Heterogeneity.
Caption: Multiple different risk factors can lead to different patterns and types of the HF syndrome. Particular combinations of risk factors (i.e., a roll of dice) may lead to different types of HF phenotypes (i.e., a particular hand of cards). HF—heart failure; CO—cardiac output; PCWP—pulmonary capillary wedge pressure
Phenotypic Classification of HFpEF
An appropriate analogy to help understand the pitfalls of the current diagnosis and treatment strategy in HFpEF is cancer. Imagine a world where: all patients with cancer were viewed similarly; the diagnosis of cancer was based only on symptoms and the presence of a tumor; clinical trials were designed to treat the general disease of cancer; physicians did not further categorize cancer before or after entering patients in clinical trials; and we wonder in amazement that all treatments for cancer have failed.
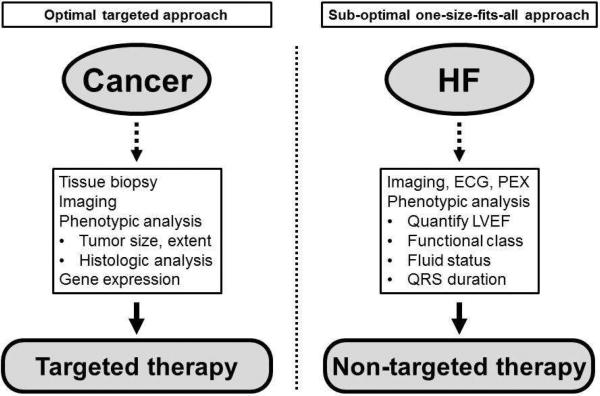
This description of cancer—the concept of cancer as one single disease—sounds foreign and even laughable. However, if we were to replace the word “cancer” with the word “HFpEF” and the word “tumor” with “normal EF” in the preceding paragraph, we would have a description of the current state of affairs for the diagnosis and management of HFpEF. While the field of oncology has benefitted greatly from improved phenotypic classification of cancer (e.g., type of cancer, size of tumor, histologic subtype, extent of growth, presence of metastases, biomarkers levels, and even genetic testing and gene expression of tumor cells), comparably little has been done in HF and even less for HFpEF (Figure 3). Thus, we sorely need new ways to classify and categorize the HFpEF syndrome.
Figure 3. Illustration of a Targeted Diagnostic Approach (e.g., Cancer) versus a One-Size-Fits-All Approach (e.g., Heart Failure).
Caption: Treatment of cancer has benefitted from an increasingly targeted approach whereas current heart failure treatment is more generalized with few exceptions (e.g., cardiac resynchronization therapy). HF—heart failure; ECG—electrocardiography; PEX—physical examination; LVEF—left ventricular ejection fraction
Here we describe the rationale, benefits, and pitfalls of 4 types of classification schemas for HFpEF: (1) pathophysiologic classification; (2) clinical/etiologic classification; (3) classification based on type of clinical presentation; and (4) phenomics (“phenomapping”) of HFpEF.
Pathophysiologic Classification of HFpEF
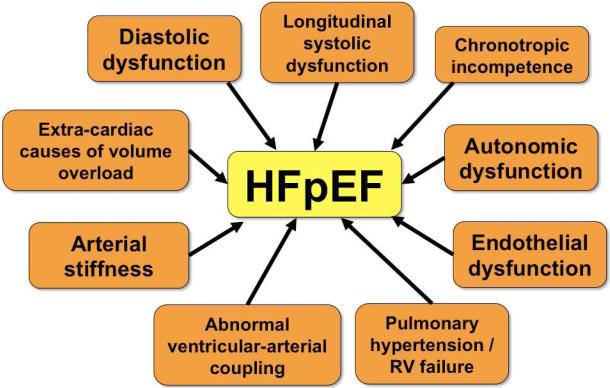
From a pathophysiologic standpoint, the primary abnormality underlying HFpEF was initially thought to be diastolic dysfunction—both impaired LV relaxation and decreased LV compliance, hence the term “diastolic heart failure”.22 While diastolic dysfunction is certainly a prominent part of the HFpEF syndrome, it is now well known that the pathophysiology of HFpEF is heterogeneous (Figure 4).6,8,23 Pathophysiologic abnormalities known to be present in HFpEF include: (1) diastolic dysfunction (impaired relaxation and/or reduced compliance);24 (2) longitudinal systolic dysfunction (e.g., decreased longitudinal systolic tissue velocities, decreased global longitudinal strain;25,26 (3) endothelial dysfunction;20 (4) abnormal ventricular-arterial coupling;27 (5) impaired systemic vasodilator reserve;28 (6) pulmonary hypertension and pulmonary vascular disease with right heart failure in the setting of left heart disease;29,30 (7) chronotropic incompetence; 28,31 and (8) extra-cardiac causes of volume overload in the susceptible heart (examples include obesity, chronic kidney disease, and anemia, each of which can cause diastolic dysfunction along with extracardiac fluid retention, thereby combining to contribute to the HFpEF syndrome).15 Adding to the complexity of HFpEF is the fact that these patients often have more than one pathophysiology of HFpEF that contributes to their clinical syndrome.
Figure 4. Multiple Pathophysiologic Contributors to the Heart Failure with Preserved Ejection Fraction Syndrome.
Caption: HFpEF—heart failure with preserved ejection fraction; RV—right ventricle.
From Oktay AA, Shah SJ. Heart failure with preserved ejection fraction. In Levine G, ed. Color Atlas of Clinical Cardiology. New Delhi: Jaypee Medical Publishers, 2014; with permission.
Clinical/Etiologic Classification of HFpEF
Clinically, several patterns are evident when caring for HFpEF patients, even when those with severe valvular disease, prior history of HFrEF (i.e., “recovered” LVEF), and constrictive pericarditis are excluded. Clinical phenotypes of HFpEF include: (1) “garden variety” HFpEF, which is associated with hypertension, obesity, diabetes/metabolic syndrome, and/or chronic kidney disease; (2) CAD-associated HFpEF25 (these patients typically have multi-vessel CAD, and the CAD seems to be driving the HFpEF syndrome);25 (3) atrial fibrillation-predominant HFpEF (these patients frequently have uncontrolled atrial fibrillation which appears to drive the HFpEF syndrome); (4) right heart failure-predominant HFpEF (these patients have pulmonary venous hypertension [occasionally with super-imposed pulmonary arterial hypertension] and right ventricular dysfunction in the setting of significant diastolic dysfunction; however, the right heart failure drives their clinical course);29,32 (5) hypertrophic cardiomyopathy- induced or hypertrophic-cardiomyopathy-like HFpEF (these patients typically have small LV cavities with thick walls and respond best to negative inotropes); (6) multi-valvular HFpEF (these patients typically have 2 or more moderate valvular lesions that do not meet operative criteria, but nevertheless contribute to HFpEF, usually in the setting of other risk factors and etiologies of HFpEF); and (7) restrictive cardiomyopathies such as cardiac amyloidosis.2
While useful from a clinical standpoint, clinical classification of HFpEF can be problematic because these categories are not mutually exclusive; therefore it is sometimes difficult to place patients into a single category in order to guide treatment. Nevertheless, when diagnosing and managing patients with HFpEF, the clinical/etiologic classification system can be quite helpful in guiding initial treatment (Table 1). However, the clinical/etiologic classification of HFpEF remains empiric and anecdotal for the most part; clinical trials are necessary to prove the utility of the classification and management strategies outlined in Table 1.
Table 1.
Management of Heart Failure with Preserved Ejection Fraction (HFpEF) by Phenotypic Classification
| Phenotypic Classification | Management Strategies |
|---|---|
| Garden-variety HFpEF | • Treat comorbidities |
| • Enroll in HFpEF clinical trial | |
| Coronary artery disease-HFpEF | • Consider revascularization |
| • Aggressive medical management of coronary artery disease | |
| Right heart failure-predominant HFpEF | • Diuresis/ultrafiltration |
| • Digoxin (dose qMWF if elderly and/or if CKD is present) | |
| • Midodrine to support systemic blood pressure if systemically hypotensive | |
| • PDE5 inhibition if superimposed pulmonary arterial hypertension is present (i.e., if PA diastolic pressure – pulmonary capillary wedge pressure > 5 mmHg) | |
| Atrial fibrillation-predominant HFpEF | • Typically require rate/rhythm control more than anti-hypertensive therapy |
| • Trial of cardioversion or ablation, especially if very symptomatic loss of atrial contraction | |
| • Anticoagulation unless contraindicated | |
| Hypertrophic cardiomyopathy-like HFpEF | • Verapamil, diltiazem, long-acting metoprolol; cautious use of diuretics and vasodilators (use only if absolutely necessary) |
| Valvular HFpEF | • Medical treatment of underlying valve disease if possible |
| • Surgical treatment of valvular disease if indicated | |
| High output HFpEF | • Determine underlying cause of high output state (i.e., anemia, liver disease, AV fistula, hyperthyroidism) |
| • Treat underlying cause of high output state | |
| • Diuretics/ultrafiltration typically necessary | |
| Rare causes of HFpEF (“zebras”) | • Determine underlying etiology |
| • Treat underlying cause | |
| • Enroll in clinical trial if possible |
HFpEF—heart failure with preserved ejection fraction; qMWF—every Monday, Wednesday, and Friday; GFR—glomerular filtration rate; PDE5—phosphodiesterase-5 inhibitor; PA—pulmonary artery; LVEDP—LV end-diastolic pressure; AV—arteriovenous.
From Oktay AA, Shah SJ. Diagnosis and Management of Heart Failure with Preserved Ejection Fraction: 10 Key Lessons. Curr Cardiol Rev 2013; with permission.
Classification of HFpEF Based on Clinical Presentation
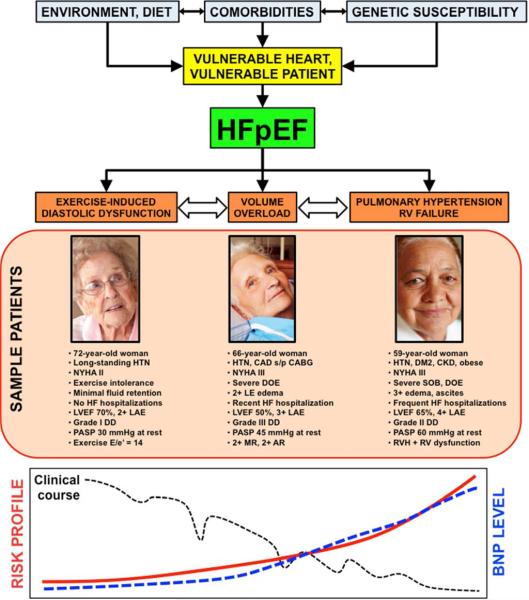
The underlying pathophysiology and etiology of HFpEF is not the only basis for heterogeneity of the HFpEF syndrome. Clinical presentation of HFpEF varies considerably as well. Patients tend to present in 1 of 3 types of categories that correlate with the clinical severity of the HFpEF syndrome,7 as shown in Figure 5.
Figure 5. Theoretical Schema of Heart Failure With Preserved Ejection Fraction Patient Types With Sample Patients, Risk Profiles, and Matched Therapies.
Caption: AR—aortic regurgitation; ARNI—angiotensin receptor/neprilysin inhibitor; BNP—B-type natriuretic peptide; CABG—coronary artery bypass grafting; CAD—coronary artery disease; CKD—chronic kidney disease; DD—diastolic dysfunction; DM2—type 2 diabetes mellitus; DOE—dyspnea on exertion; E/e’—ratio of early mitral inflow to early mitral annular diastolic tissue velocity; HF—heart failure; HTN—hypertension; If—inward “funny” channel; LAE—left atrial enlargement; LVEF—left ventricular ejection fraction; MR—mitral regurgitation; MRA—mineralocorticoid receptor antagonist; NYHA—New York Heart Association; PASP— pulmonary artery systolic pressure; PDE5—phosphodiesterase-5; RV—right ventricular; RVH— right ventricular hypertrophy; SOB—shortness of breath; s/p—status post. From Shah SJ. Matchmaking for the optimization of clinical trials of heart failure with preserved ejection fraction: no laughing matter. J Am Coll Cardiol 2013;62(15):1339-1342; with permission.
The first category, representing the lowest-risk type of patient, but also the most difficult to diagnose, is the patient with exercise-induced increases in LV filling pressures, also known as exercise-induced diastolic dysfunction.33,34 Exertional dyspnea is the predominant symptom in these patients. They typically do not have overt signs of volume overload such as lower extremity edema, and are rarely hospitalized for HF. Therefore, clinical diagnosis rests on a combination of abnormalities in cardiac structure (LV hypertrophy and/or left atrial enlargement) and evidence of exercise-induced elevations in LV filling pressure, either invasively (i.e., pulmonary capillary wedge pressure > 25 mmHg at peak exercise during invasive hemodynamic testing in the cardiac catheterization laboratory)33 or non-invasively (i.e., diastolic stress echocardiography—increased septal E/e’ ratio [> 13] at peak exercise).35-37 Cardiopulmonary exercise testing can also be helpful to exclude poor effort (reduced peak respiratory exchange ratio < 1.0); obesity (normal peak absolute oxygen consumption [VO2] with reduced relative VO2 when indexed to weight); or pulmonary abnormalities as causes of exercise intolerance. HFpEF patients that fit into the category of exercise-induced elevation in LV filling pressure typically have normal or only mildly elevated B-type natriuretic peptide (BNP) levels and the risk of major morbidity or mortality at this stage of the HFpEF syndrome is quite low.33,34,38
The second type of clinical presentation of HFpEF is the common one that clinicians can often easily recognize: overt volume overload. Besides dyspnea, exercise intolerance, and abnormalities in cardiac structure (LV hypertrophy and/or left atrial enlargement), these HFpEF patients have lower extremity edema, elevated jugular venous pressure, and even bibasilar crackles when severe. In addition, these patients typically (but not always) have elevated BNP or NT-proBNP levels and typically have a history of HF hospitalization, after which morbidity and mortality are quite high. These are also the patients that are frequently enrolled in HFpEF clinical trials that require high BNP levels or previous HF hospitalization.39 Although diagnosis is often straightforward in these patients, sometimes the diagnosis is missed, especially in those with concomitant obesity (difficult to visualize JVP and lower BNP levels) or lung disease. In these patients, confirmation of the HFpEF diagnosis with right heart catheterization for evaluation of invasive hemodynamics can be useful.
The third type of HFpEF clinical presentation is that of pulmonary hypertension with right heart failure.29,30,32 This is the highest-risk clinical subset of HFpEF, with high morbidity and mortality. These patients often have the highest BNP levels, but this is not true in all cases. Furthermore, some of these patients have superimposed pulmonary arterial hypertension on top of pulmonary venous hypertension,40 which adds to the propensity for right heart failure. In HFpEF, patients with right ventricular (RV) hypertrophy and RV dysfunction are some of the highest risk, even when controlling for other HFpEF risk factors.23
It is important to note that while some patients can transition from one type of HFpEF clinical presentation to the other, there are patients who first present with one predominant type of HFpEF and do not progress. Whether this phenomenon has to do with duration of the HF syndrome, dietary factors, severity of underlying abnormalities in cardiac structure/function, or other factors is unknown.
Phenomics (“Phenomapping”) of HFpEF
Several sophisticated phenotyping tools ranging from a multitude of biomarkers to comprehensive cardiovascular imaging modalities to environmental characterization using tools such as geocoding are now available in the era of “deep phenotyping”.41 These comprehensive phenotyping tools, along with genomics and systems biology, can improve characterization of heterogeneous syndromes like HFpEF. Combined with “big data” machine learning algorithms to find patterns in dense, multi-dimensional data, novel phenotypic characterization of HFpEF should be possible in the near future. Although these types of analyses are not new, they have been popularized in recent years with the advent of large quantities of genetic data used in research of clinical syndromes and diseases.
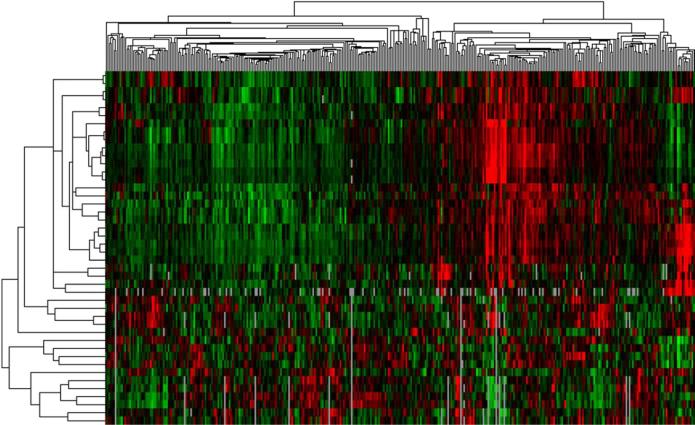
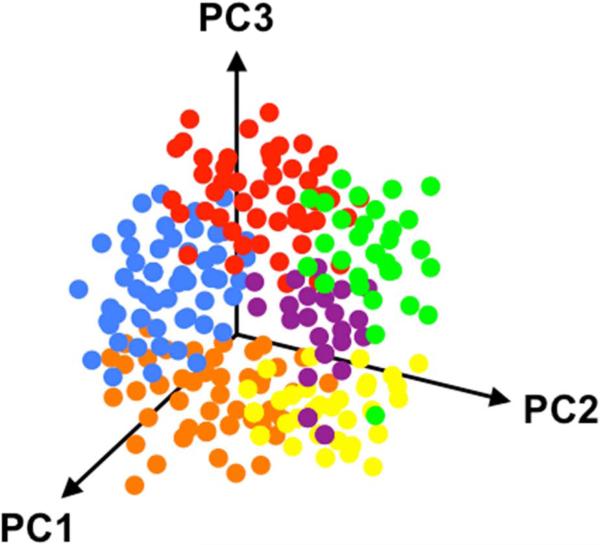
For example, in gene expression analyses, RNA is isolated from a specific tissue, and microarray analyses are used to quantify gene expression with interrogation of several pathways (e.g., inflammation, fibrosis, cell cycle mediators, etc).42 Next, unbiased hierarchical clustering analysis is performed to determine patterns in the differentially expressed genes, and a visual gene expression heatmap is created.43 The resultant gene expression signatures can provide insight into disease pathogenesis and potential therapeutic pathways. A similar type of analysis, unsupervised cluster analysis, can be performed using phenotypic data. In HFpEF, one can take advantage of the large quantity of phenotypic data available, such as a wide variety of quantitative data available from comprehensive echocardiography (including Doppler, tissue Doppler, and speckle-tracking analysis), as shown in Figure 6. One can create phenotypic heat maps that are akin to gene expression heat maps, thereby allowing for novel categorization of patients (Figure 7). In addition, dense, multidimensional phenotypic data from HFpEF clinical trials could be distilled down to a few dimensions using principal components analysis (Figure 8).
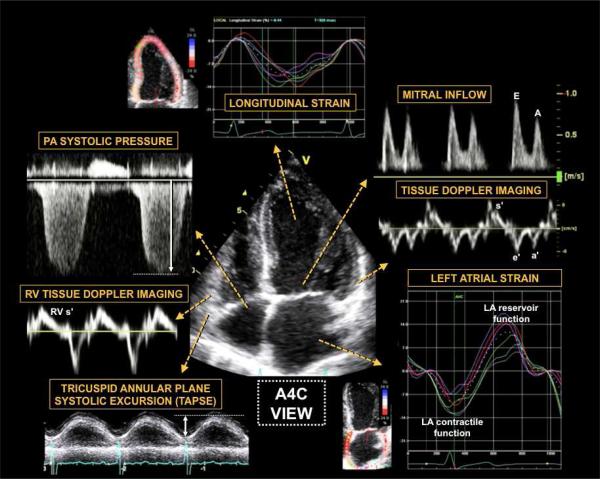
Figure 6. Comprehensive Echocardiographic Phenotypic Analysis of Heart Failure with Preserved Ejection Fraction.
Caption: Comprehensive echocardiography, including two dimensional, Doppler, tissue Doppler, and speckle tracking, allows for detailed phenotypic analysis of cardiac structure, function, and mechanics in patients with heart failure with preserved ejection fraction. The figure shows examples of information that can be obtained from the apical 4-chamber view. Clockwise from the top: speckle-tracking echocardiography for assessment of LV regional and global longitudinal strain (early diastolic strain rate can also be obtained in this view). Mitral inflow and tissue Doppler imaging of the septal and lateral mitral annulus provide information on LV diastolic function grade and estimated LV filling pressure (E/e’ ratio), along with assessment of longitudinal systolic (s’) and atrial (a’) function. Speckle-tracking analysis of LA function provides peak LA contractile function (peak negative longitudinal LA strain) and LA reservoir function (peak positive longitudinal LA strain). Tricuspid annular plane systolic function (TAPSE) and basal RV free wall peak longitudinal tissue Doppler velocity (RV s’) provide information on longitudinal RV function, as does speckle tracking echocardiography of the RV (not shown). Finally, analysis of the tricuspid regurgitant jet Doppler profile, when added to the estimated RA pressure, provides an estimate of the PA systolic pressure. Additional data available from the apical 4-chamber view include assessment of LV volumes and ejection fraction, LA volume, and RV size and global systolic function (e.g., RV fractional area change). LV—left ventricular; LA—left atrial; PA—pulmonary artery; RV—right ventricular; RA—right atrial; A4C—apical 4-chamber From Butler J, Fonarow G, Zile MR, et al. Developing Therapies for Heart Failure with Preserved Ejection Fraction: Current State and Future Directions. JACC Heart Fail 2014 (in press); with permission.
Figure 7. Sample Phenotypic Heat Map (“Pheno-Map”) Developed from Hierarchical Cluster Analysis of Quantitative Echocardiographic Data.
Caption: The rows in the heat map correspond to the various quantitative echocardiographic phenotypes (e.g., septal wall thickness, ejection fraction, early diastolic [e’] tissue velocity, etc.), while the columns represent individual patients. Red = increased values; green = decreased values. The dendrogram across the top of the heat map is a tree diagram that demonstrates the clustering of patients; the dendrogram on the left side of the heat map illustrates clustering of phenotypes.
Figure 8. Three-Dimensional Principal Components Analysis Plot.
Caption: In this theoretical example, patients are grouped based on 3 principal components (PC1, PC2, and PC3) in 3 dimensions. Each color represents a group of patients that correspond to a particular cluster based on phenotypic similarities.
Table 2 summarizes the phenomapping approach to the categorization of clinical syndromes, including HFpEF. These types of analyses have several advantages: (1) they take into consideration immense quantitative phenotypic data; (2) it is possible to visualize the heterogeneity of the clinical syndrome; (3) it provides mutually exclusive classification of a clinical syndrome; and (4) the clustering of patients into categories is “unsupervised” and thus does not rely on knowledge of a specific “outcome”, which is required for more traditional clustering analyses and “supervised” statistical analyses such as classification and regression tree (CART) analysis.44
Table 2.
Analytic Methods for Phenomapping Analyses
| Methodology | Description |
|---|---|
| Preparation of quantitative phenotypic variables | • Collect a large amount of phenotypic data in the patients being studied. |
| • Standardize each quantitative variable to a mean of 0 and standard deviation of 1 (i.e., mean±SD of 0±1). | |
| • Remove any variables with large amounts of missing information, and for all other variables with missing data, perform multiple imputation to fill in missing values. | |
| Explore redundancy among phenotypic variables | • Generate a correlation matrix of phenotypic variables by assigning correlation coefficients to each bivariate comparison. |
| • Visualize correlations among phenotypic variables through the use of hierarchical clustering to create a heatmap of bivariate correlations (with the color intensity of each cell in the matrix corresponding to the strength of the correlation between any two quantitative phenotypes). | |
| • Use the heatmap to highlight similarities among phenotypic variables and visualize and unanticipated correlations across phenotypes. | |
| Principal components analysis | • Perform principal components analysis as an alternate method to find orthogonal axes of phenotypic variation within the dataset. |
| • Interpret top principal components according to combinations of conventional phenotypic features. | |
| • Classify patients according to contribution of individual components. | |
| Model-based clustering with Bayesian information criterion (BIC) analysis | • Use model-based clustering, which achieves parameter fitting and assigns patients to clusters by minimizing a penalized likelihood, to determine phenotypic signatures of patients.46 |
| • Incorporate BIC analysis to penalize increases in model complexity (e.g., greater number of clusters) in order to create the most parsimonious solution (this approach is termed “regularization” in machine learning, and allows increased generalizability to other datasets44). | |
| Multinomial logistic regression with lasso penalty | • Build a generalizable logistic regression model (using multinomial logistic regression with L1 norm [lasso] technique)48 to define membership for each cluster of patients in order to: (1) permit classification of future patients according to a minimal set of quantitative phenotypes; and (2) identify those features most informative for categorization of patients. |
Once performed for HFpEF, phenomapping analyses can be applied to clinical trials to determine whether certain subgroups of patients with a particular phenotype signature are more responsive to the investigational drug or device compared to other types of patients, thereby leading to “theranostics”, a combined diagnostic and therapeutic strategy.45
The Value of Enhanced Classification of HFpEF in Future Clinical Trials
Future clinical trials can harness these advances in phenotypic categorization in two key ways. First, Phase II clinical trials of HFpEF may benefit greatly from advanced phenotyping of potential subjects by matching the mechanism of a particular drug with a specific HFpEF phenotype. For example, a drug that ameliorates ischemia and improves myocyte relaxation would benefit most from enrolling patients with a history of CAD and reduced tissue Doppler e’ velocities on echocardiography. Second, in Phase II and III clinical trials, deep phenotyping of study participants using banked blood and cardiac imaging (such as comprehensive echocardiography), along with other tools (e.g., quality of life, exercise tests, etc) as needed, will allow for the development of “phenomaps”, as described above. Investigators can then use the resultant phenotypic signatures to determine groups of patients most likely to benefit from a particular drug or device.
KEY POINTS.
Patients with HFpEF are united by the presence of (1) increased LV filling pressures and/or reduced cardiac output at rest or with exertion; and (2) a preserved LVEF (typically defined as LVEF > 45-50%); however, the etiology and pathophysiology underlying the HFpEF syndrome—the phenotypic spectrum—varies widely among patients with the syndrome.
The heterogeneity of the HFpEF syndrome may be a key reason why clinical trials have largely failed to show improved outcomes in these patients.
Improved classification of the HFpEF syndrome, whether by etiology, pathophysiology, and/or type of clinical presentation should lead to better matching of appropriate therapies to patients, thereby leading to improved outcomes for these patients.
Phenomapping is a novel technique that uses machine learning to define clusters of patients based on dense phenotypic data, thereby providing an unbiased way to classify hetereogeneous clinical syndromes.
Future clinical trials of HFpEF should account for the heterogeneity of HFpEF when considering inclusion/exclusion criteria, study design, phenotyping tools, and outcome measures, and should a priori consider subgroup analyses to highlight specific HFpEF subgroups that may derive greater benefit from a particular HFpEF drug.
SUMMARY.
HFpEF is a heterogeneous syndrome, a key reason that may explain why: (1) diagnosing and treating HFpEF is so challenging; and (2) clinical trials in HFpEF have failed thus far. Here we have described 4 ways of categorizing HFpEF patients: based on pathophysiology, clinical/etiologic subtype, type of clinical presentation, and quantitative phenomics (phenomapping analysis). Regardless of the classification method used, improved phenotypic characterization of HFpEF patients in both the clinic and in clinical trials, and matching of targeted therapies with specific patient subtypes, will be critical if we are to improve outcomes in this increasingly prevalent patient population.
Acknowledgments
Grant support: National Institutes of Health (NIH) R01 HL107577 and American Heart Association 0835488N to SJS; and NIH K08 HL098361 to RCD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
REFERENCES
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Oktay AA, Shah SJ. Diagnosis and Management of Heart Failure with Preserved Ejection Fraction: 10 Key Lessons. Curr Cardiol Rev. 2013 doi: 10.2174/1573403X09666131117131217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10(4):401–410. doi: 10.1007/s11897-013-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah AM, Pfeffer MA. The many faces of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2012;9(10):555–556. doi: 10.1038/nrcardio.2012.123. [DOI] [PubMed] [Google Scholar]
- 6.Shah AM, Solomon SD. Phenotypic and pathophysiological heterogeneity in heart failure with preserved ejection fraction. Eur Heart J. 2012;33(14):1716–1717. doi: 10.1093/eurheartj/ehs124. [DOI] [PubMed] [Google Scholar]
- 7.Shah SJ. Matchmaking for the optimization of clinical trials of heart failure with preserved ejection fraction: no laughing matter. J Am Coll Cardiol. 2013;62(15):1339–1342. doi: 10.1016/j.jacc.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32(6):670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. 2011;123(18):2006–2013. doi: 10.1161/CIRCULATIONAHA.110.954388. discussion 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47(1):76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 11.Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA : the journal of the American Medical Association. 2006;296(18):2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 12.Prasad A, Hastings JL, Shibata S, et al. Characterization of static and dynamic left ventricular diastolic function in patients with heart failure with a preserved ejection fraction. Circ Heart Fail. 2010;3(5):617–626. doi: 10.1161/CIRCHEARTFAILURE.109.867044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurer MS, King DL, El-Khoury Rumbarger L, Packer M, Burkhoff D. Left heart failure with a normal ejection fraction: identification of different pathophysiologic mechanisms. J Card Fail. 2005;11(3):177–187. doi: 10.1016/j.cardfail.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Kliger C, King DL, Maurer MS. A clinical algorithm to differentiate heart failure with a normal ejection fraction by pathophysiologic mechanism. Am J Geriatr Cardiol. 2006;15(1):50–57. doi: 10.1111/j.1076-7460.2006.05291.x. [DOI] [PubMed] [Google Scholar]
- 15.Bench T, Burkhoff D, O'Connell JB, et al. Heart failure with normal ejection fraction: consideration of mechanisms other than diastolic dysfunction. Curr Heart Fail Rep. 2009;6(1):57–64. doi: 10.1007/s11897-009-0010-z. [DOI] [PubMed] [Google Scholar]
- 16.Burkhoff D, Maurer MS, Packer M. Heart failure with a normal ejection fraction: is it really a disorder of diastolic function? Circulation. 2003;107(5):656–658. doi: 10.1161/01.cir.0000053947.82595.03. [DOI] [PubMed] [Google Scholar]
- 17.Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59(11):998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edelmann F, Stahrenberg R, Gelbrich G, et al. Contribution of comorbidities to functional impairment is higher in heart failure with preserved than with reduced ejection fraction. Clinical research in cardiology : official journal of the German Cardiac Society. 2011;100(9):755–764. doi: 10.1007/s00392-011-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammed SF, Borlaug BA, Roger VL, et al. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: a community-based study. Circ Heart Fail. 2012;5(6):710–719. doi: 10.1161/CIRCHEARTFAILURE.112.968594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 21.Shah SJ, Gheorghiade M. Heart failure with preserved ejection fraction: treat now by treating comorbidities. JAMA : the journal of the American Medical Association. 2008;300(4):431–433. doi: 10.1001/jama.300.4.431. [DOI] [PubMed] [Google Scholar]
- 22.Aurigemma GP, Gaasch WH. Clinical practice. Diastolic heart failure. N Engl J Med. 2004;351(11):1097–1105. doi: 10.1056/NEJMcp022709. [DOI] [PubMed] [Google Scholar]
- 23.Burke MA, Katz DH, Beussink L, et al. Prognostic Importance of Pathophysiologic Markers in Patients with Heart Failure and Preserved Ejection Fraction. Circ Heart Fail. 2013 doi: 10.1161/CIRCHEARTFAILURE.113.000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350(19):1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 25.Shah SJ. Evolving approaches to the management of heart failure with preserved ejection fraction in patients with coronary artery disease. Curr Treat Options Cardiovasc Med. 2010;12(1):58–75. doi: 10.1007/s11936-009-0060-2. [DOI] [PubMed] [Google Scholar]
- 26.Liu YW, Tsai WC, Su CT, Lin CC, Chen JH. Evidence of left ventricular systolic dysfunction detected by automated function imaging in patients with heart failure and preserved left ventricular ejection fraction. J Card Fail. 2009;15(9):782–789. doi: 10.1016/j.cardfail.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107(5):714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 28.Borlaug BA, Melenovsky V, Russell SD, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114(20):2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 29.Thenappan T, Shah SJ, Gomberg-Maitland M, et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2011;4(3):257–265. doi: 10.1161/CIRCHEARTFAILURE.110.958801. [DOI] [PubMed] [Google Scholar]
- 30.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53(13):1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phan TT, Shivu GN, Abozguia K, et al. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3(1):29–34. doi: 10.1161/CIRCHEARTFAILURE.109.877720. [DOI] [PubMed] [Google Scholar]
- 32.Shah SJ. Pulmonary hypertension. JAMA. 2012;308(13):1366–1374. doi: 10.1001/jama.2012.12347. [DOI] [PubMed] [Google Scholar]
- 33.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3(5):588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maeder MT, Thompson BR, Htun N, Kaye DM. Hemodynamic determinants of the abnormal cardiopulmonary exercise response in heart failure with preserved left ventricular ejection fraction. J Card Fail. 2012;18(9):702–710. doi: 10.1016/j.cardfail.2012.06.530. [DOI] [PubMed] [Google Scholar]
- 35.Burgess MI, Jenkins C, Sharman JE, Marwick TH. Diastolic stress echocardiography: hemodynamic validation and clinical significance of estimation of ventricular filling pressure with exercise. J Am Coll Cardiol. 2006;47(9):1891–1900. doi: 10.1016/j.jacc.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 36.Ha JW, Oh JK, Pellikka PA, et al. Diastolic stress echocardiography: a novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise Doppler echocardiography. J Am Soc Echocardiogr. 2005;18(1):63–68. doi: 10.1016/j.echo.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 37.Kane GC, Oh JK. Diastolic stress test for the evaluation of exertional dyspnea. Curr Cardiol Rep. 2012;14(3):359–365. doi: 10.1007/s11886-012-0269-7. [DOI] [PubMed] [Google Scholar]
- 38.Anjan VY, Loftus TM, Burke MA, et al. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic Peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110(6):870–876. doi: 10.1016/j.amjcard.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah SJ, Heitner JF, Sweitzer NK, et al. Baseline Characteristics of Patients in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. 2012 doi: 10.1161/CIRCHEARTFAILURE.112.972794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerges C, Gerges M, Lang MB, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest. 2013;143(3):758–766. doi: 10.1378/chest.12-1653. [DOI] [PubMed] [Google Scholar]
- 41.Tracy RP. ‘Deep phenotyping’: characterizing populations in the era of genomics and systems biology. Curr Opin Lipidol. 2008;19(2):151–157. doi: 10.1097/MOL.0b013e3282f73893. [DOI] [PubMed] [Google Scholar]
- 42.Ballman KV. Genetics and genomics: gene expression microarrays. Circulation. 2008;118(15):1593–1597. doi: 10.1161/CIRCULATIONAHA.107.714600. [DOI] [PubMed] [Google Scholar]
- 43.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome- wide expression patterns. Proc Natl Acad Sci U S A. 1998;95(25):14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med. 2003;26(3):172–181. doi: 10.1207/S15324796ABM2603_02. [DOI] [PubMed] [Google Scholar]
- 45.Shah SJ, Wasserstrom JA. SERCA2a gene therapy for the prevention of sudden cardiac death: a future theranostic for heart failure? Circulation. 2012;126(17):2047–2050. doi: 10.1161/CIRCULATIONAHA.112.138321. [DOI] [PubMed] [Google Scholar]
- 46.Fraley C, Raftery AE. Model-based clustering, discriminant analysis, and density estimation. J Am Stat Assoc. 2002;97:611–631. [Google Scholar]
- 47.Hastie T, Tibshirani R, Friedman J. Unsupervised Learning: Hierarchical Clustering. In: Hastie T, Tibshirani R, Friedman J, editors. The Elements of Statistical Learning. 2nd Ed. Springer; New York: 2009. pp. 520–528. [Google Scholar]
- 48.Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]