Abstract
Depletional induction therapies are routinely used to prevent acute rejection and improve transplant outcome. The effects of depleting agents on T-cell subsets and subsequent T-cell reconstitution are incompletely defined. We used flow cytometry to examine the effects of rabbit antithymocyte globulin (rATG) on the peripheral T-cell repertoire of pediatric and adult renal transplant recipients. We found that while rATG effectively depleted CD45RA+CD27+ naïıve and CD45RO+CD27+ central memory CD4+ T cells, it had little effect on CD45RO+CD27− CD4+ effector memory or CD45RA+CD31−, CD45RO+CD27+ and CD45RO+CD27− CD8+ T cell subsets. When we performed a kinetic analysis of CD31+ recent thymic emigrants and CD45RA+/RO+ T cells, we found evidence for both thymopoiesis and homeostatic proliferation contributing to immune reconstitution.We additionally examined the impact of rATG on peripheral CD4+Foxp3+ T cells.We found that in adults, administration of rATG-induced peripheral expansion and new thymic emigration of T cells with a Treg phenotype, while CD4+Foxp3+ T cells of thymic origin predominated in children, providing the first evidence that rATG induces Treg in vivo. Collectively our data indicate that rATG alters the balance of regulatory to memory effector T cells posttransplant, providing an explanation for how it positively impacts transplant outcome.
Keywords: Antilymphocyte antibodies, kidney allograft, pediatric renal transplant, phenotype, T-cell depletion, T cell
Introduction
New and more potent immunosuppressive strategies have led to significant improvements in allograft survival following solid organ transplantation. As clinical practice has evolved the use of induction therapy has become the pre-dominant practice, increasing from 30% to over 75% of renal allograft recipients over the past decade (1), with depletional therapies being most commonly used. Although the desired effect of lymphocyte depletion, the removal of pathogenic alloreactive T cells capable of damaging the graft, has a clear rationale, the susceptibility of individual T-cell subsets to depletion can vary dependent on the agent used (2). Despite the fact that rATG, specifically Thymoglobulin ®, is the most common form of induction therapy used in the United States, there are currently no long-term data on its effects on T-cell phenotypes in children or adults. The void in the cellular niche created by depletion of lymphocytes triggers the initiation of pathways, thymopoiesis and homeostatic proliferation, aimed at reconstituting the immune system (3). The first, thymopoiesis, is associated with the increased release of näive thymic T cells and is dependent on a functional thymus (3). Because children have larger thymi than adults, thymopoiesis has been postulated to be more likely to contribute to reconstitution in children. Homeostatic proliferation is a predominantly IL-7 driven expansion of the peripheral T cell clones that survived depletion (3). Homeostatic proliferation causes the first wave of T cell repopulation following depletion in adult mice and can induce a change in T-cell function, from näive to memory (4). High frequencies of antidonor memory T cells have been associated with an increased rejection rate in renal transplantation trials, and have been shown to be relatively resistant to immunosuppression (5). Therefore, rATG-induced homeostatic proliferation could have a negatively impact allograft function.
On the other hand, induction agents including rATG may have a protective effect in part through facilitating Treg (6). Initial data suggested that low dose rATG expands Treg in in vitro cultures (7,8). However, more recent data in a small number of patients suggest that rATG may actually cause a reduction in absolute number of regulatory T cells in vivo (9). Treg may modulate the immune response by directly inhibiting alloreactive T cells and homeostatic proliferation (10). To fully understand the impact of rATG it is necessary to define its effects on the kinetics of both effector and regulatory T cells during reconstitution.
To examine the effects of rATG on T-cell phenotypes immune reconstitution, we determined the composition of the peripheral T-cell compartment in adults and children starting at 2 months, after the early posttransplant effects of depletion. We show that thymopoesis is the predominant mechanism of immune reconstitution early posttransplant in both pediatric and adult recipients, whereas homeostatic proliferation predominates later posttransplant. We provide the first in vivo evidence that administration of rATG in adult renal transplant recipients is associated with expansion of T cells of a regulatory phenotype and this expansion occurs initially through the release of FoxP3 T cells from the thymus, followed by the expansion of peripheral FoxP3+ T cells with a memory phenotype.
Materials and Methods
Patients
A total of 100 adult kidney transplant recipients, transplanted between October 2004 and August 2009, 17 pediatric kidney transplant recipients and 6 healthy pediatric controls were prospectively enrolled (Table 1). Approval was obtained from the Internal Review Board of the Mount Sinai School of Medicine. Clinical data were collected and blood was drawn at day 0 and 1, 2, 4 and 6 months posttransplantation.
Table 1.
Patient characteristics
| Adult-rATG | Adult-no rATG | Pediatrics | Healthy Ctrl Peds | |
|---|---|---|---|---|
| Number of patients | 58 | 42 | 17 | 4 |
| Age (year) (SD) | 49.9 ± 13.9 | 55.3 ± 12.6 | 11.9 ± 4.9 | 10 ± 4.2 |
| Gender (%) (M) | 64 | 66 | 59 | 25 |
| Ethnicity (%) | ||||
| Black | 64* | 6* | 29 | 25 |
| Caucasian | 14 | 52 | 41 | 50 |
| Asian | 2 | 9 | 0 | 0 |
| Hispanic | 20 | 33 | 30 | 25 |
| Other | 0 | 0 | 0 | 0 |
| PRA (%) (SD) | 12.4 ± 26.5 | 9.2 ± 18.7 | 1.6 ± 3.8 | n/a |
| Months on dialysis (SD) | 57.5 ± 55.3**,* | 23.3 ± 30.3**,* | 4.8 ± 5.8* | n/a |
| Retransplant (%) | 13.8 | 9.5 | 17.6 | n/a |
| Acute rejection (%) | 13 | 8 | 0 | n/a |
| Rapid steroid withdrawal (%) | 17.2 | 11.2 | 0 | n/a |
| HLA mismatches A, B, DR | 3.6 | 3.4 | 4.2 | n/a |
| Cold ischemia time (h) | 1.3* | 1.8* | 5.6* | n/a |
| Cause of ESRD (%) | ||||
| DM | 10 | 0 | 0 | n/a |
| HTN | 27 | 54 | 0 | |
| DM+HTN | 40 | 26 | 0 | |
| FSGS | 6 | 0 | 47 | |
| IgAN | 6 | 2 | 11 | |
| PKD | 3 | 8 | 11 | |
| Obstructive N | 0 | 0 | 18 | |
| Other | 8 | 10 | 13 | |
| Donor source (%) | ||||
| Deceased | 43 | 32 | 73 | |
| Living | 57 | 68 | 27 | n/a |
p < 0.05 between groups.
p = 0.05 between groups.
Immunosuppression
Pediatric patients, high immunological risk adult recipients (high panel reactive antibodies, African American race, previously transplanted [n = 13]) and patients that received rapid steroid withdrawal (RSW) (n = 9) were given rATG (Thymoglobulin ®) to total of 6 mg/kg on posttransplantion. Patients received either tacrolimus or cyclosporine maintenance immunosuppression in combination with mycophenolate mofetil (Cellcept ®) and steroids tapering to 0.2 mg/kg/day by the end of the first month unless RSW.
Cell isolation
Peripheral blood mononuclear cells (PBMCs) were obtained by Ficoll-Paque density gradient centrifugation as previously described (11). CD4+ and CD8+ T-cell populations were then isolated by positive selection using immunomagnetic microbeads (BD Biosciences, San Jose, CA) and magnetic associated cell sorter (Biosource International, Carlsbad, CA) according to the manufacturer’s instructions.
Flow cytometric analysis
Phenotyping of separated CD4+ and CD8+ T cell was performed using four-color flow cytometry on a FACSCalibur analyzer (Becton Dickin-son, Franklin Lakes, NJ). Subsets included RTE (CD45RA+CD31+), näive (CD45RA+CD27+), central memory (CM) (CD45RO+CD27+), effector memory (EM) (CD45RO+CD27–) and regulatory (FoxP3+) T cells. Antibodies used include CD45 RO APC, CD45RA PerCP-Cy5.5 [Correction made after publication 20 October 2010: PE changed to PerCP], CD27 PE, CD31 APC (BD Biosciences), CD127 PerCP-Cy5.5 and FoxP3 FITC (eBioscience, San Diego, CA, USA). B cells were characterized using CD19-PE-Cy7, CD27-PE, IgM-APC and IgD-FITC (BD Biosciences). Appropriate isotype matched monoclonal antibodies were used as controls.
Statistical analysis
Results were expressed as median and interquartile range (IQR) unless stated otherwise. Comparison of continuous variables between groups was performed by nonparametric tests using the Mann–Whitney test and categorical variables by two-sided chi-square or two-sided Fisher’s exact test, where applicable. p < 0.05 was considered as statistically significant. No correction was made for multiple testing. Statistical analysis was performed using SPSS ® version 14.0 software package (SPSS Inc., Chicago, IL) and GraphpadPrism ® version 4.0b software package (Graphpad Software Inc., San Diego, CA).
Results
Effects of rATG on CD4+ and CD8+ T-cell number
We examined the kinetics of T-cell subset depletion and recovery in adults and children following rATG therapy. In adults, treatment with rATG decreased the total peripheral CD4+ T-cell count by more than 85% at 2 weeks (baseline 501.7 ± 336.7 cells/mm3 to 59.9 ± 92.4 cells/mm3, p = 0.005; Figure 1A [correction made after publication 20 October 2010: cells/cm3 changed to cells/mm3]), followed by a recovery to approximately 35% of the baseline level by 2 months (179 ± 194.8 cells/mm3, p 0.0177 vs. baseline; Figure 1A [correction made after publication = 20 October 2010: Figure 2A changed to 1A and cells/cm3 changed to cells/mm3]), with no further increase at 4 and 6 months. In contrast, administration of rATG decreased the CD8+ T-cell count by only 22% at 2 weeks (338.5 98 cells/mm3 ± publication to 262.2 ± 89 cells/mm3 [correction made after20 October 2010: cells/cm3 changed to cells/mm3]) with full recovery to baseline values by 6 months (Figure 2A). In the absence of rATG, we did not detect changes in peripheral blood CD4+ or CD8+ T-cell counts.
Figure 1. Pattern of CD4+ T cell reconstitution in adult transplant recipients following lymphocyte depletion with rATG.
(A) Absolute CD4+ T cell number over time following rATG [correction made after publication 20 October 2010: CD4+ added after Absolute]. (B) the percentage CD4+ RTE (CD27+CD31+CD45RA+), näive peripheral (CD27+CD31 –CD45RA+), memory effector (CD45RO+CD27–) and central memory (CD45RO+CD27–) T cells in adult CD27 renal transplant recipients that received rATG (n = 40) was determined over the first 6 months post-transplantation = by flow cytometry. Data is expressed as median with IQR. [Correction added after publication 20 October 2010: Figure 2 became Figure 1; Figure 3 became Figure 2 and cm3 was changed to mm3 in Figure 1A]
Figure 2. Pattern of CD8+ T cell reconstitution in adult transplant recipients following lymphocyte depletion with rATG.
(A) The absolute number of CD8+ T cells over time following rATG. (B) the percentage CD8+ RTE (CD27+CD31+CD45RA+), näive peripheral (CD27+CD31 –CD45RA+), (CD45RO+ CD27–) effector memory and central memory (CD45RO+ CD27–) cells in adult renal transplant recipients that received rATG (n = 40) was determined over the first 6 months post-transplantation = by flow cytometry. Data is expressed as median with IQR. [Correction added after publication 20 October 2010: Figure 3 became Figure 2; Figure 1 became Figure 3 and cm3 was changed to mm3 in Figure 2A]
Following the administration of rATG in pediatric patients, we observed that the total lymphocyte count decreased significantly at 1 week (2329 ± 1006 cells/mm3 vs. 221.5±155 cells/mm3, p = 0.0125 × 10−3 [correction made after publication 20 October 2010: cells/cm3 changed to cells/mm3]). We noted significant recovery of the counts by week two (591.8 ± 655.67 cells/mm3, p = 0.0005 vs. baseline [correction made after publication 20 October 2010: cells/cm3 changed to cells/mm3]), with a further increase to 40% of baseline (919.3 ± 111.9 cells/mm3, p = 0.0177 vs. baseline [correction made after publication 20 October 2010: cells/cm3 changed to cells/mm3]) by months 4–6.
Effects of rATG on näive and effector phenotype T cells
To next assess how rATG influences the phenotype of T cell in the peripheral blood and to examine reconstitution following depletion, we quantified näive, effector and memory T cells based on cell surface marker expression at predefined time points following transplantation. Because the relative contribution of thymic emigration following T cell depletion may be different in children as compared to adults, we performed our analyses in both adult and pediatric transplant recipients. We used CD31 expression on näive CD45RA+ cells as a marker of recent thymic emigrants (RTE). Previous work by others revealed that CD31+ strongly correlated with T-cell receptor excision circle (TREC) content (12) and CD31 expression decreased with engagement of the TCR, either by self-MHC in the periphery leading to the expansion of CD31 –CD45RA+ peripheral näive T cells, or by engagement with foreign antigen leading to the development of CD31 –CD45RO+ memory T cells.
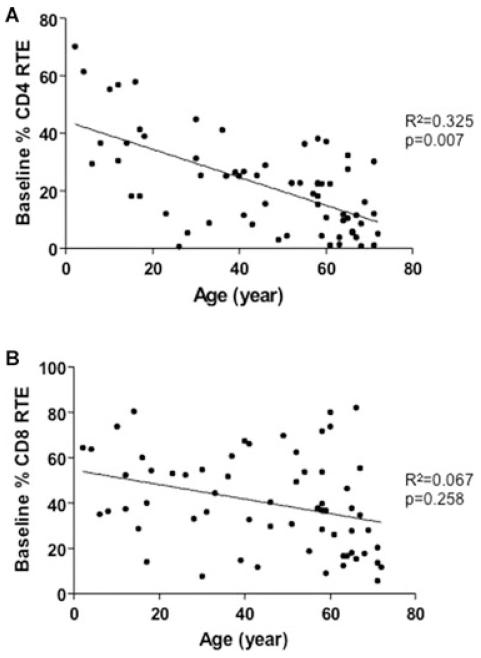
In keeping with previous findings by others, CD45+CD31+CD4+ RTE and CD45+CD31+CD8+RTE individually correlated inversely with age (Figures 3A and B) (12-14). We similarly found an inverse correlation between TREC levels and age (Figure S1).
Figure 3. Percentage recent thymic emigrants at baseline correlates with age.
Percentage (A) CD4+ ((n = 66) and (B) CD8 = 63) CD45RA+CD31+ recent thymic emigrant correlation with age. CD4+ and CD8+ RTE T cells correlated negatively with age. [Correction added after publication 20 October 2010: Figure 1 became Figure 3; Figure 2 became Figure 1]
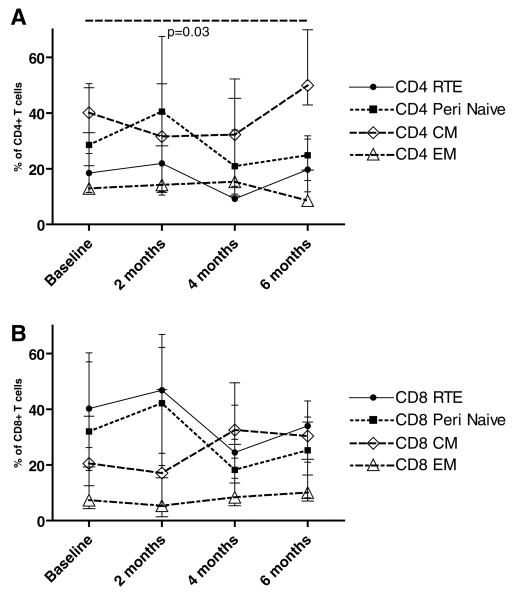
In adults 2 months after transplantation, we noted that rATG induced a significant increase in CD4+ RTE baseline (p = 0.01) over (Figure 1B). Over the same time period we observed a significant decrease in the percentage of CD45RA+ CD31 –CD4+ naïive cells (p = 0.02) (Figure 1B) without a significant effect on the percentage of CD45RO+CD27+ CD4+ CM cells or CD45RO+CD27+CD4+ EM T cells (Figure 1B). Strikingly, we observed a significant twofold expansion in this EM T cell subset by 6 months from 14.2% (IQR 9–20%) at baseline to 29.3% (IQR 9–36%, p = 0.03). We did not detect any significant changes in the memory populations in adults treated without rATG (Figure 4A & 4B) [correction made after publication 20 October 2010: added after (Figure 4A and 4B). Collectively, these data indicate that in adults, rATG administration increases CD4+ RTE, decreases the percentage of näive CD4+ T cells and is associated with a predominant expansion of CD4+ T memory cells by 6 months posttransplant (68.5%, IQR 45–87%, of the total CD4+ T-cell population express a memory phenotype). We found no association between calcineurin inhibitors drug levels and the percentage RTE in the rATG or the no rATG group at any time point.
Figure 4. T-cell phenotypes over time in adult renal transplant recipients that did not receive depletional induction therapy.
(A) The percentage CD4+ and (B) CD8+ RTE, na peripheral, EM and CM T cells in adult näive renal transplant recipients (n = 34) that did not received rATG. Data are expressed as median with IQR.
When we examined effects of rATG on CD8 T cells in adults we similarly noted a significant increase in the percentage of CD31+ RTE at 2 months (p = 0.006; Figure 2B) with a reciprocal but nonsignificant decrease in näive CD8 T cells from 27.8% versus 17% (p = NS; Figure 2B). At 6 months posttransplant, we found that the percentage of RTE had decreased below baseline and peripheral näive CD8+ T cells remained slightly but not significantly lower than pretransplant values (15%, p = NS vs. pretransplant value). With respect to EM and CM CD8+ T cells, we noted that the percentages were unaffected in the early posttransplant period but significantly increased by 6 months posttransplant to 59% (Figure 2B). Interestingly, we observed that a population of CD45RA+/RO+CD8 sitional developed cells in the rATG treated patients, but not the controls, reaching a peak 23.1% (IQR 14–34%) by 4 months. This transitional phenotype has previously been described in associated with inflammatory states, and the presence of these cells suggests that activated näive CD8+ T cells transition to memory T cells (15). The data are consistent with a rATG-induced shift toward memory cells in the CD8+ as well as in the CD4+ T-cell subset (Figures 1B and 2B). Nonetheless, these phenotypic alterations were not associated with a decrease in graft function when compared to the non-rATG treated controls (not shown).
When we examined the T-cell phenotypes in pediatric patients, we found similar results to those observed in adults, with a significant increase in CD4+ and CD8+ RTE at 2 months compared to pretransplant values (p = 0.03. and 0.01, respectively; Figures 5A and B). We observed a significant decrease in peripheral näive and CM CD4+ T cells induced by rATG with recovery of the CD4+ CM, but not the näive CD4+ T cells to baseline values by 6 months posttransplant. We did not detect a significant change in the percentage CD4+ EM T cells over this same time period.
Figure 5. Pattern of immune reconstitution in pediatric transplant recipients following lymphocyte depletion with rATG.
(A) The percentage CD4+ and (B) CD8+ RTE (CD27+CD31+CD45RA+), näive peripheral (CD27+CD31–CD45RA+), EM (CD45RO and (CD45RO+CD27+CD27–) T cells in pediatric renal transplant-recipients (n = 17) that received rATG. Data are expressed as median with IQR.
In contrast to our findings in adults (Figure 2B), we noted a significant decrease in CM CD8+ T cells in the children 1-month after rATG treatment (Figure 5B) (p < 0.001). This decrease recovered to baseline pretransplant values by 6 months. We did not observe significant effects on the EM CD8+ T-cell population. We observed a trend toward a lower percentage of peripheral näive CD8+ T cells at 1 month.
Strikingly, we found large populations of transitional CD4+ and CD8+ CD45RA+/RO+T cells in the children at baseline, comprising 29.9% (IQR 14–34%) and 24.9% (IQR 12–25%) of total CD4+ and CD8+ T respectively. At 6 months, the percentage cells, of transitional T cells remained high at 15.4% (IQR 9–23%) and 34.7% (IQR 19–45%), respectively.
Together these data indicate that the response to rATG is similar in adults and children; memory T cells are relatively resistant to depletion, early repopulation occurs through the release of CD4+ and CD8+ näive emigrants and later repopulation likely occurs thymic via homeostatic proliferation of memory T cells.
rATG is associated with the emergence of regulatory T cells in vivo
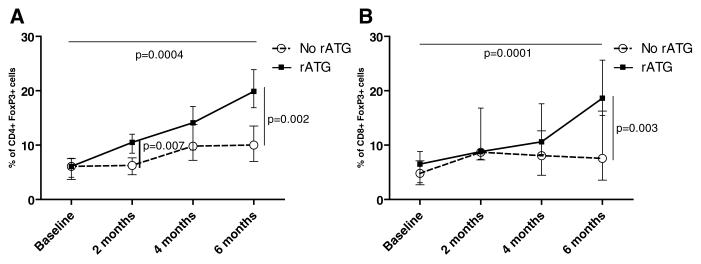
We next examined the impact of rATG on FoxP3+ T cells as a marker for Treg. We found that in adults, treatment with rATG led to the emergence of CD4+ and CD8+ FoxP3+ cells, comprising 19.9% (IQR 16–24%) of total CD4+ T cells (p = 0.004 vs 6.1% pretransplant [Correction made after publication 20 October 2010: p = 0.0028 changed to p = 0.004]) and 18.6% (IQR 15–26%) = of CD8+ T cells (p = 0.0001 vs. 6.5% pretransplant [Correction made after publication 20 October 2010: p = 0.001 changed to p = 0.0001]) by 6 months (Figures 6A and B). We found that the percentage of CD4+ CD45RA-FoxP3hi T cells, recently characterized as the Treg subpopulation with the greatest suppressive abilities (16), was significantly higher in patients that received rATG at all time points posttransplant (Figure 7). We confirmed our findings by using CD127 as a phenotypic marker for Tregs (17). We observed that the percentage of CD25hiCD127lo CD4+ T cells was significantly higher at 6 months versus baseline, specifically in patients treated with rATG (n = 18 rATG, n = 11 non-rATG) (p = 0.03) S2A). Interestingly, patients had a high percentage CD25hiCD127hi FoxP3+ CD4+ T cells at baseline, suggesting CD127 may not be a useful marker for Treg in patients with ESRD (Figure S2B).
Figure 6. CD4+ and CD8+ Treg increase following administration of rATG in vivo.
(A) CD4+ and (B) CD8+ FoxP3 T cells were quantitated by flow cytometry in adult renal transplant patients and compared between patients (n = 28) that did and did not receive=rATG (n = 23). Data are expressed as median with IQR.
Figure 7. CD4+ CD45RA- FoxP3 high Treg are increased post-transplant in patients that received rATG.
PBMCs from patients treated with and without rATG were stained for CD4+, CD45RA+ and FoxP3. Data are expressed as median with IQR.
Because Treg can derive directly from the thymus and can be converted peripherally, and because these distinct types of Tregs may have differential effects on effector cells, we used additional cell surface markers to further phenotype the Foxp3+ cells in each patient. Using CD31 as a marker of thymic emigrants, we observed a significant increase in the percentage of CD4+ and CD8+ FoxP3+ T cells of thymic origin at 2 months posttransplant (p = 0.046 and 0.046 vs. pretransplant values, respectively). In contrast, rATG was associated with a decrease in the percentage of peripheral näive CD4+ and CD8+ CD45RA+ CD31 –FoxP3+ T cells (p = 0.048 and 0.047, respectively), either through deletion of näive FoxP3+ T cells or as a result in the relative increase in other subtypes(Figures 8 and 9). Both CD4+ and CD8+ memory FoxP3+ T cells increased CD45RO between baseline and 6 months (p = 0.002 and 0.002, respectively) (Figures 8 and 9). This pattern of early increase due to thymic release followed by an increase in memory mirrors that of the overall T cell population. Changes in the make up of the phenotype of FoxP3+ T cells were also seen in the absence of rATG, with an expansion of näive FoxP3+ T cells at 2 months (p = 0.0078) and a reciprocal decrease in FoxP3+ T cells with a memory phenotype (p = 0.06) as compared to baseline. However, the total percentage of FoxP3+ T cells remained unchanged.
Figure 8. CD4+ Treg phenotypes over time.
(A) CD45RA+CD31 –FoxP3+ näive thymic emigrant, (B) CD45RA+CD31 –FoxP3+ näive peripheral/, (C) CD45RA– CD45RO+FoxP3 memory Treg were quantitated by flow cytometry in adult transplant recipients that had (n = 25) and had not (n = 20) received rATG over 6 months posttransplant. Data are expressed as median with IQR.
Figure 9. CD8+ Treg phenotypes over time.
(A) CD45RA+CD31 –FoxP3+ näive thymic emigrant, (B) CD45RA+CD31 –FoxP3+ näive peripheral, and (C) CD45RA+CD45RO –FoxP3+ memory Treg were quantified by flow cytometry in adult transplant recipients that had (n = 25) and had not (n = 20) received rATG over 6 months post-transplant. Data are expressed as median with IQR.
We also found a significantly higher percentage of CD4+ FoxP3+ T cells in pediatric transplant recipients after rATG (p = 0.037, compared to healthy controls) (Figure 10A). We did not detect an effect of rATG on CD8+ Foxp3+ T cells (Figure 10B). In contrast to our findings in adults (Figure 5), we found that in children treated with rATG FoxP3+ T cells were largely composed of the subset of CD45RA+ CD31+ T cells of thymic origin at 6 months, with a accompanying large CD45RA+ RO+ transitional population (Figures 10C and D).
Figure 10. Increased regulatory T cells in pediatric transplant recipients compared to healthy pediatric controls.
(A) The percentage CD4+ and (B) CD8+ (FoxP3) regulatory T cells was determined in pediatric renal transplant (Peds Tx) (n = 17) patients at 6 months and compared to healthy pediatric controls (Peds HC) (data presented as median with IQR).
B-cell number and phenotype following rATG
There has been some suggestion that rATG may have an effect on B cells due to binding of antibody by cell surface proteins commonly expressed by both T and B cells. We found that patients treated with rATG (n = 27) had no significant change in B cell number as compared those who did not receive rATG (n = 19) (data not shown). However, patients that received rATG had significantly less memory B cells (CD19+ CD27+ IgM class IgD–) (10.6% vs. 5.27%; p = 0.01) and switch memory B cells (CD19+CD27+IgD–) (15.9% vs. 8.2%; p = 0.02) at 6 months compared to baseline. These data suggest that rATG may impact B cells through its effect on T cells and hence T-cell help.
Discussion
While the clinical benefit of rATG in kidney transplantation is established (18), the mechanisms underlying its therapeutic benefit remain poorly understood. Our results, in which we thoroughly examined the kinetic effects of rATG on peripheral T-cell subsets, provide new information that enhances our understanding of this drug’s complex mechanisms of action. Our data indicate that in both adults and children rATG caused a rapid (over 2 months) decrease in näive CD4+ and CD8+ T cells, but simultaneously enhanced thymic emigration which replaced näive CD4+ and CD8+ cells (Figures 1, 2 and 5) and increased the number of FoxP3+ T cells (Figure 6). This was true despite the fact that thymic involution occurs in adults.
Thymopoiesis contributes new näive cells to the peripheral pool and maintains a diverse T-cell receptor repertoire capable of responding to a broader range of pathogens (19). In addition, thymopoiesis has the potential to replenish naturally occurring Treg within the periphery. Data in humans suggest that over 45 years of age renewal due to thymopoiesis is severely reduced (3). Little is known about the contribution of the thymus to reconstitution following depletion in transplant recipients or its potential effects on the emergence of Tregs. Nickel et al. used CD31 as a marker to examine the influence of conventional immunosuppression on CD4+ RTE (13) posttransplantation and found no significant change in adult transplant recipients. However, only three patients had received depletion therapy in the form of OKT3. Our report is the first to examine the contribution of the thymus to immune reconstitution following lymphocyte depletion in renal transplantation. As expected, we found that thymic emigrants contributed significantly to immune reconstitution in pediatric recipients, with RTE’s accounting for the greatest percentage of both CD4+ and CD8+ T cells at 2 months. Moreover, both CD4+ and CD8+ FoxP3+ T cells were predominantly of thymic origin in children. Surprisingly, we found that RTE also contributed to early repopulation of T cells including T cells with a regulatory phenotype in adults that received rATG. This is contrary to previous data in experimental models in adult mice that suggest that homeostatic proliferation of memory T cells provided the first wave of T cell repopulation following depletion (4,20).
Our data further support the concept that CD4+ and CD8+ memory cells are resistant to depletion by rATG and that these cell subsets in fact expand over the initial 6 months posttransplantation. Previous data examining the effects of depletional agents showed marked decrease in CD4+ and CD8+ T-cell populations with an increase in the relative number of EM T cells at 3 weeks posttransplantation (2). That study was performed in a small number of patients and combined data from patients treated with rATG and Campath-1H. Data from a pediatric population several years out from renal transplant found no difference in the ratio of näive to memory T cells (21). We found that by 6 months memory T cells comprised 68.5% of the total CD4+ T-cell population in adults, predominantly due to the marked expansion of EM cells, while in children this expansion was due CM cells. The development of a dominant memory T-cell phenotype is of concern because memory T cells have a lower threshold for activation and are not confined to the lymphatic system, and thus can circulate to the graft (22). Furthermore, memory cells are less dependent on costimulation for activation (22) and therefore can be activated by nonprofessional antigen presenting cells such as endothelium (23). Thus, a predominance of memory T cells could contribute to early graft injury and loss.
Enhancement of memory by rATG if taken alone is paradoxical given that treatment with rATG is not associated with increased rejection or worse outcomes. The lack of a negative impact suggests additional effects of rATG and may be due to the expansion of Tregs. Several investigators have reported an expansion of Tregs in vitro with low-dose rATG (7,8,24). A more recent report suggested that in vivo rATG decreased the absolute number of Treg, and that lymphocyte recovery was associated with the emergence of a memory Treg phenotype (9). Our data demonstrate for the first time that rATG is associated with the expansion of FoxP3+ T cells in vivo and suggests a shift in the Treg to Teffector ratio. This increase in FoxP3+ T cells resulted from thymic release early posttransplant, suggesting that even in adults the thymus contributes to Treg in the periphery. Over time there is an expansion in peripheral FoxP3+ T cells with a memory phenotype. The functional significance of the predominance of memory versus näive Treg is uncertain since differences in function and trafficking between näive and memory Treg have not been clearly delineated in vivo to date. The functional importance of the increase in Treg in vivo is strongly supported by previous studies in humans. Renal transplant recipients with chronic rejection have been shown to have a lower numbers of CD25hi CD4+ T cells and FoxP3 transcripts in peripheral PBMCs compared to patients with stable renal function and operational tolerance (25). The ratio of memory CD8+ T cells to Treg in the peripheral blood has been identified as a predictor of acute rejection in patients in whom tacrolimus was withdrawn posttransplantation (26). Furthermore, in a recent study a high percentage of intragraft FoxP3 Treg was shown to correlate positively with lower creatinine and higher GFR at 2 years (27).
In conclusion, our data are the first to show that both thymopoiesis and homeostatic proliferation contributed to immune reconstitution after rATG in pediatric and adult renal transplant recipients and that rATG was associated with expansion of Treg in vivo. These data suggest that rATG alters the balance of regulatory to memory T cells posttransplant, in addition to depleting harmful T cells, providing a rationale for its positive impact on allograft outcomes.
Figure S1. Inverse correlation between CD4+ and CD8+ T-cell TREC with age.
Figure S2. Treg phenotypes characterized using CD127.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supplementary Material
Acknowledgment
This work was supported by NIH grant 1U01AI070107.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article:
References
- 1.Merion RM. SRTR report on the state of transplantation. Am J Transplant. 2008;2009;9:867–868. doi: 10.1111/j.1600-6143.2009.02563.x. [DOI] [PubMed] [Google Scholar]
- 2.Pearl JP, Parris J, Hale DA, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 3.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007;19:318–330. doi: 10.1016/j.smim.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sener A, Tang AL, Farber DL. Memory T-cell predominance following T-cell depletional therapy derives from homeostatic expansion of naive T cells. Am J Transplant. 2009;9:2615–2623. doi: 10.1111/j.1600-6143.2009.02820.x. [DOI] [PubMed] [Google Scholar]
- 5.Valujskikh A. Targeting T-cell memory: Where do we stand? Curr Opin Organ Transplant. 2008;13:344–349. doi: 10.1097/MOT.0b013e3283061126. [DOI] [PubMed] [Google Scholar]
- 6.Hale DA. Biological effects of induction immunosuppression. Curr Opin Immunol. 2004;16:565–570. doi: 10.1016/j.coi.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Feng X, Kajigaya S, Solomou EE, et al. Rabbit ATG but not horse ATG promotes expansion of functional CD4+ CD25highFOXP3+ regulatory T cells. Blood. 2008;111:3675–3683. doi: 10.1182/blood-2008-01-130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez M, Clarkson MR, Albin M, et al. A novel mechanism of action for anti-thymocyte globulin: Induction of CD4+ CD25+ Foxp3+ regulatory T cells. J Am Soc Nephrol. 2006;17:2844–2853. doi: 10.1681/ASN.2006050422. [DOI] [PubMed] [Google Scholar]
- 9.Sewgobind VD, Kho MM, Van Der Laan LJ, et al. The effect of rabbit anti-thymocyte globulin induction therapy on regulatory T cells in kidney transplant patients. Nephrol Dial Transplant. 2009;24:16351644. doi: 10.1093/ndt/gfn778. [DOI] [PubMed] [Google Scholar]
- 10.Winstead CJ, Fraser JM, Khoruts A. Regulatory CD4+ CD25+ Foxp3+ T cells selectively inhibit the spontaneous form of lymphopenia-induced proliferation of naive T cells. J Immunol. 2008;180:7305–7317. doi: 10.4049/jimmunol.180.11.7305. [DOI] [PubMed] [Google Scholar]
- 11.Murphy B, Magee CC, Alexander SI, et al. Inhibition of allorecognition by a human class II MHC-derived peptide through the induction of apoptosis. J Clin Invest. 1999;103:859–867. doi: 10.1172/JCI5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimmig S, Przybylski GK, Schmidt CA, et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002;195:789–794. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nickel P, Kreutzer S, Bold G, et al. CD31+ naive Th cells are stable during six months following kidney transplantation: Implications for post-transplant thymic function. Am J Transplant. 2005;5:1764–1771. doi: 10.1111/j.1600-6143.2005.00924.x. [DOI] [PubMed] [Google Scholar]
- 14.Ye P, Kirschner DE. Reevaluation of T cell receptor excision circles as a measure of human recent thymic emigrants. J Immunol. 2002;168:4968–4979. doi: 10.4049/jimmunol.168.10.4968. [DOI] [PubMed] [Google Scholar]
- 15.Hoy MD, O’Donnell JL, Hart DN. Dual CD45RA, CD45RO positive T-lymphocytes within rheumatoid arthritic joints. Pathology. 1993;25:167–173. doi: 10.3109/00313029309084793. [DOI] [PubMed] [Google Scholar]
- 16.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardinger KL, Rhee S, Buchanan P, et al. A prospective, randomized, double-blinded comparison of thymoglobulin versus Atgam for induction immunosuppressive therapy: 10-year results. Transplantation. 2008;86:947–952. doi: 10.1097/TP.0b013e318187bc67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazenberg MD, Verschuren MC, Hamann D, et al. T cell receptor excision circles as markers for recent thymic emigrants: Basic aspects, technical approach, and guidelines for interpretation. J Mol Med (Berlin, Germany) 2001;79:631–640. doi: 10.1007/s001090100271. [DOI] [PubMed] [Google Scholar]
- 20.La Gruta NL, Driel IR, Gleeson PA. Peripheral T cell expansion in lymphopenic mice results in a restricted T cell repertoire. Eur J Immunol. 2000;30:3380–3386. doi: 10.1002/1521-4141(2000012)30:12<3380::AID-IMMU3380>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 21.Klaus G, Mostert K, Reckzeh B, et al. Phenotypic changes in lymphocyte subpopulations in pediatric renal-transplant patients after T-cell depletion. Transplantation. 2003;76:1719–1724. doi: 10.1097/01.TP.0000100396.81490.0C. [DOI] [PubMed] [Google Scholar]
- 22.Valujskikh A. Memory T cells in allograft rejection. Adv Exp Med Biol. 2007;601:247–256. doi: 10.1007/978-0-387-72005-0_26. [DOI] [PubMed] [Google Scholar]
- 23.Al-Lamki RS, Bradley JR, Pober JS. Endothelial cells in allograft rejection. Transplantation. 2008;86:1340–1348. doi: 10.1097/TP.0b013e3181891d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaCorcia G, Swistak M, Lawendowski C, et al. Polyclonal rabbit antithymocyte globulin exhibits consistent immunosuppressive capabilities beyond cell depletion. Transplantation. 2009;87:966–974. doi: 10.1097/TP.0b013e31819c84b8. [DOI] [PubMed] [Google Scholar]
- 25.Louis S, Braudeau C, Giral M, et al. Contrasting CD25hiCD4+ T cells/FOXP3 patterns in chronic rejection and operational drug-free tolerance. Transplantation. 2006;81:398–407. doi: 10.1097/01.tp.0000203166.44968.86. [DOI] [PubMed] [Google Scholar]
- 26.Kreijveld E, Koenen HJ, van Cranenbroek B, et al. Immunological monitoring of renal transplant recipients to predict acute allograft rejection following the discontinuation of tacrolimus. PLoS One. 2008;3:e2711. doi: 10.1371/journal.pone.0002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bestard O, Cruzado JM, Rama I, et al. Presence of FoxP3+ regulatory T Cells predicts outcome of subclinical rejection of renal allografts. J Am Soc Nephrol. 2008;19:2020–2026. doi: 10.1681/ASN.2007111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.