Abstract
Objective
To investigate the characteristics and incidence trends of childhood cancer in Beijing, China, from 2000 to 2009.
Methods
A total of 1,274 cases with childhood cancer in Beijing from 2000 to 2009 were included in the study. All rates were age-standardized using the direct method to the world standard population and expressed per million person-years. Incidence trends were characterized by calculating annual percent change (APC) using Joinpoint Regression Program.
Results
The crude incidence rate was 106.47 per million [age-standardized rate (ASR) 113.34] between 2000 and 2009 in Beijing with the most common diagnoses, leukemia (N=505, 39.64%, ASR 45.20), followed by central nervous system (CNS) tumors (N=228, 17.90%, ASR 19.28) and lymphoma (N=91, 7.14%, ASR 6.97). The incidence for all childhood cancers combined has increased during the study period, with an APC of 5.84% [95% confidence interval (95% CI): 1.0-10.9] after adjusted by world population. The ASR of all combined cancers in boys showed a slight, but no significant increase, with an APC of 5.33% (95% CI: –0.6-11.6); for girls, the trends increased significantly, with an APC of 6.54% (95% CI: 1.5-11.8).
Conclusions
The incidence rate of childhood cancer in Beijing was higher than the average level of China and lower than that of western countries. The incidence trends of childhood cancer, especially leukemia among girls showed a significantly increase from 2000 to 2009. While among boys, no substantially change was seen during the observed time period. Some sex-specific trends by subcategories and trends of major cancers in different age groups by cancer site merit further investigation.
Keywords: Childhood cancer, incidence, epidemiology, Beijing
Introduction
Childhood cancer usually refers to all cancers occurring in children before 15 years of age. Although childhood cancers represent a small percentage of all cancers, only 1% according to the GLOBOCAN 2012, about one in two of these children diagnosed with cancer will die, contributing significantly to the estimated 79,953 childhood cancer deaths in 2012 in the world (1). Thus collectively childhood cancers represent an important global public health problem (2). Describing the epidemiology of childhood cancer, especially over time, will allow for a critical assessment of current protocols for cancer prevention and control. Unfortunately, there is a lack of special reports focusing on childhood cancer in China (3). The aim of our study was to analyze incidence trends and characteristics of childhood cancer in Beijing, the capital of China, among children younger than age 15 years.
Materials and methods
Data source
Data of childhood cancer were derived from Beijing Cancer Registry which has collected population-based cancer incidence data since 1976. Since 1998, the registry surveillance coverage has expanded from 8 to 16 districts, and residents covering changed from 7 to 12 million. Medical records of the newly diagnosed cancer inpatients were monthly required to report to the Beijing Cancer Registry from all the 138 medical hospitals in Beijing. Before 2003, the information of the newly diagnosed cases was reported using a standardized notification card. Since 2003, all designated hospitals have reported cancer data to Beijing Municipal Health Bureau of Statistics Platform using the online Health Information System (HIS). Information is available at the patient level and includes basic demographics, origin site, histology, incidence date, most valid basis of diagnosis, behavior classification, reporting hospital and other relevant variables. Our analysis includes data on all malignant tumors diagnosed in children aged less than 15 years from 2000 to 2009, as well as non-malignant tumors of the central nervous system (CNS). Population data are derived from the Statistic Department of Beijing Municipal Bureau.
Quality control
Childhood cancer data were checked and evaluated by National Central Cancer Registry (NCCR) based on “Guideline for Chinese Cancer Registration” and referring to relevant data quality criterion of “Cancer Incidence in Five Continents Volume IX” by International Agency for Research on Cancer/International Association of Cancer Registries (IARC/IACR) (4). The morphology of newly diagnosed cases was coded by International Classification of Diseases for Oncology, Second Edition (ICD-O-2) and cancer sites were coded by International Statistical Classification of Diseases and Related Health Problems 10th revision (ICD-10) in Beijing since 1998. First, we changed ICD-O-2 and ICD-10 to International Classification of Diseases for Oncology, 3rd edition (ICD-O-3). Then we re-classified childhood cancers according to diagnostic categories of the International Classification of Childhood Cancer, 3rd Edition (ICCC-3), a classification system based on the morphology and topography codes used in ICD-O-3, and used the same ICCC-3 names for cancer categories.
Statistical analysis
All rates were age-standardized using the direct method to the world Segi’s population and expressed per million person-years. Trends were characterized by calculating annual percent change (APC) using Joinpoint 3.4.3 Regression Program (5), developed by the Surveillance, Epidemiology, and End Results (SEER) Program (6). A significance level of 0.05 (two tailed) was used for all analyses. The number of cases was shown by cancer sites, sex and age groups (0 years,1-4 years, 5-9 years, 10-14 years), and the incidence rates of different age-groups were calculated by sex. The age-standardized rates (ASRs) for all children were calculated by cancer sites.
Results
Incidence
Between 2000 and 2009, 1,274 cases with childhood cancers were diagnosed in Beijing (boys =711, girls =563, ratio 1.26:1), and 88.9% of records were based on histological verification (MV%). The crude incidence rate was 106.47 per million (ASR 113.34). The most common diagnoses were leukemia (N=505, 39.64%, ASR 45.20), followed by CNS tumors (N=228, 17.90%, ASR 19.28) and lymphoma (N=91, 7.14%, ASR 6.97) (Tables 1,2). Among leukemia, lymphoid leukemia (57.43%) is more common than acute non-lymphoblastic leukemia (20.59%). In CNS tumors, astrocytoma is the most common tumor (30.26%), followed by primitive neuroectodermal tumor (17.11%,) and ependymoma (8.33%). Among lymphoma, non-Hodgkin lymphoma (NHL) is more common than Hodgkin’s disease (HD) and Burkitt’s lymphoma (67.03%, 14.29% and 13.19%, respectively) (Table 1).
Table 1. Number of childhood cancer cases (0-14 years) by age groups in Beijing, China, 2000-2009.
| ICCC | 0 |
1/4 |
5/9 |
10/14 |
All |
Total | Freq. (%) | Case ratio (M/F) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | ||||||||
| I. Leukemia | 10 | 19 | 104 | 55 | 81 | 53 | 114 | 69 | 309 | 196 | 505 | 39.64 | 1.58 | ||||
| (a) Lymphoid leukemia | 3 | 9 | 81 | 37 | 49 | 27 | 57 | 27 | 190 | 100 | 290 | 57.43 | 1.9 | ||||
| (b) Acute non-lymphocytic leukemia | 2 | 5 | 8 | 7 | 16 | 15 | 30 | 21 | 56 | 48 | 104 | 20.59 | 1.17 | ||||
| (c) Chronic myeloid leukemia | 0 | 0 | 1 | 1 | 5 | 1 | 4 | 2 | 10 | 4 | 14 | 2.77 | 2.5 | ||||
| (e) Unspecified leukemia | 5 | 5 | 14 | 10 | 11 | 10 | 23 | 19 | 53 | 44 | 97 | 19.21 | 1.2 | ||||
| II. Lymphoma and reticuloendothelial neoplasms | 1 | 1 | 11 | 2 | 21 | 6 | 26 | 23 | 59 | 32 | 91 | 7.14 | 1.84 | ||||
| (a) Hodgkin’s disease | 0 | 0 | 1 | 0 | 5 | 0 | 2 | 5 | 8 | 5 | 13 | 14.29 | 1.6 | ||||
| (b) Non-Hodgkin’s lymphoma | 0 | 1 | 5 | 2 | 14 | 6 | 19 | 14 | 38 | 23 | 61 | 67.03 | 1.65 | ||||
| (c) Burkitt’s lymphoma | 0 | 0 | 4 | 0 | 2 | 0 | 5 | 1 | 11 | 1 | 12 | 13.19 | 11 | ||||
| (e) Unspecified lymphoma | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 2 | 3 | 5 | 5.49 | 0.67 | ||||
| III. CNS and miscellaneous intracranial and intraspinal neoplasms | 5 | 5 | 31 | 27 | 29 | 31 | 50 | 50 | 115 | 113 | 228 | 17.9 | 1.02 | ||||
| (a) Ependymoma | 1 | 1 | 5 | 3 | 3 | 4 | 1 | 1 | 10 | 9 | 19 | 8.33 | 1.11 | ||||
| (b) Astrocytoma | 0 | 1 | 8 | 8 | 6 | 11 | 17 | 18 | 31 | 38 | 69 | 30.26 | 0.82 | ||||
| (c) Primitive neuroectodermal tumors | 1 | 1 | 9 | 2 | 7 | 4 | 4 | 11 | 21 | 18 | 39 | 17.11 | 1.17 | ||||
| (d) (e) Other specified or unspecified intracranial and intraspinal neoplasms | 3 | 2 | 9 | 14 | 13 | 12 | 28 | 20 | 53 | 48 | 101 | 44.3 | 1.1 | ||||
| IV. Sympathetic nervous system tumors | 9 | 4 | 22 | 15 | 7 | 3 | 2 | 2 | 40 | 24 | 64 | 5.02 | 1.67 | ||||
| V. Retinoblastoma | 5 | 4 | 9 | 10 | 0 | 1 | 0 | 0 | 14 | 15 | 29 | 2.28 | 0.93 | ||||
| VI. Nephroblastoma etc. | 6 | 3 | 14 | 12 | 2 | 3 | 2 | 3 | 24 | 21 | 45 | 3.53 | 1.14 | ||||
| VII. Hepatoblastoma etc. | 8 | 6 | 5 | 7 | 1 | 1 | 2 | 1 | 16 | 15 | 31 | 2.43 | 1.07 | ||||
| VIII. Malignant bone tumors | 1 | 0 | 1 | 0 | 6 | 14 | 29 | 20 | 37 | 34 | 71 | 5.57 | 1.09 | ||||
| (a) Osteosarcoma | 0 | 0 | 1 | 0 | 3 | 9 | 20 | 13 | 24 | 22 | 46 | 64.79 | 1.09 | ||||
| (c) Ewing’s sarcoma | 0 | 0 | 0 | 0 | 1 | 0 | 4 | 1 | 5 | 1 | 6 | 8.45 | 5 | ||||
| (d) (e) Other specified or unspecified malignant bone tumors | 1 | 0 | 0 | 0 | 2 | 5 | 5 | 6 | 8 | 11 | 19 | 26.76 | 0.73 | ||||
| IX. Soft-tissue sarcoma | 5 | 4 | 7 | 6 | 7 | 7 | 10 | 12 | 29 | 29 | 58 | 4.55 | 1 | ||||
| (a) Rhabdomyosarcoma and embryonal sarcoma | 0 | 0 | 4 | 3 | 3 | 3 | 1 | 3 | 8 | 9 | 17 | 29.31 | 0.89 | ||||
| (b) Fibrosarcoma, neurofibrosarcoma and other fibromatous neoplasms | 2 | 0 | 1 | 2 | 1 | 3 | 4 | 3 | 8 | 8 | 16 | 27.59 | 1 | ||||
| (d) (e) Other specified or unspecified soft-tissue sarcomas | 3 | 4 | 2 | 1 | 3 | 1 | 5 | 6 | 13 | 12 | 25 | 43.1 | 1.08 | ||||
| X. Germ-cell, trophoblastic and other gonadal neoplasms | 1 | 4 | 6 | 6 | 3 | 4 | 14 | 20 | 24 | 34 | 58 | 4.55 | 0.71 | ||||
| (a) Malignant extracranial and extragonadal germ-cell tumors | 0 | 2 | 0 | 4 | 1 | 0 | 1 | 0 | 2 | 6 | 8 | 13.79 | 0.33 | ||||
| (b) Intracranial and intraspinal germ-cell tumors | 0 | 2 | 3 | 1 | 1 | 0 | 13 | 3 | 17 | 6 | 23 | 39.66 | 2.83 | ||||
| (c) Gonadal germ-cell tumors | 1 | 0 | 3 | 1 | 1 | 4 | 17 | 5 | 22 | 27 | 46.55 | 0.23 | |||||
| XI. Other malignant epithelial neoplasms | 0 | 0 | 0 | 1 | 1 | 6 | 11 | 22 | 13 | 28 | 41 | 3.22 | 0.46 | ||||
| XII. Other specified or unspecified malignant neoplasms | 2 | 5 | 13 | 6 | 11 | 3 | 5 | 8 | 31 | 22 | 53 | 4.16 | 1.41 | ||||
| Total | 53 | 55 | 224 | 146 | 169 | 132 | 265 | 230 | 711 | 563 | 1,274 | — | 1.26 | ||||
ICCC, International Classification of Childhood Cancer; M, male; F, female.
Table 2. Age-specific incidence (1/1,000,000) of childhood cancers by sex in Beijing, China, 2000-2009.
| ICCC | 0 |
1/4 |
5/9 |
10/4 |
Total |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | All | M | F | All | M | F | All | M | F | All | M | F | All | ASR* | |||||
| I. Leukemia | 35.94 | 73.44 | 54.01 | 78.49 | 43.88 | 61.66 | 40.73 | 28.21 | 34.65 | 44.46 | 28.52 | 36.72 | 50.19 | 33.74 | 42.2 | 45.2 | ||||
| II. Lymphoma and reticuloendothelial neoplasms | 3.59 | 3.87 | 3.72 | 8.3 | 1.6 | 5.04 | 10.56 | 3.19 | 6.98 | 10.14 | 9.51 | 9.83 | 9.58 | 5.51 | 7.6 | 6.97 | ||||
| III. CNS and miscellaneous intracranial and intraspinal neoplasms | 17.97 | 19.33 | 18.62 | 23.4 | 21.54 | 22.49 | 14.58 | 16.5 | 15.52 | 19.5 | 20.67 | 20.07 | 18.68 | 19.45 | 19.05 | 19.28 | ||||
| IV. Sympathetic nervous system tumors | 32.34 | 15.46 | 24.21 | 16.6 | 11.97 | 14.35 | 3.52 | 1.6 | 2.59 | 0.78 | 0.83 | 0.8 | 6.5 | 4.13 | 5.35 | 7.28 | ||||
| V. Retinoblastoma | 17.97 | 15.46 | 16.76 | 6.79 | 7.98 | 7.37 | 0 | 0.53 | 0.26 | 0 | 0 | 0 | 2.27 | 2.58 | 2.42 | 3.56 | ||||
| VI. Nephroblastoma etc. | 21.56 | 11.6 | 16.76 | 10.57 | 9.57 | 10.08 | 1.01 | 1.6 | 1.29 | 0.78 | 1.24 | 1 | 3.9 | 3.61 | 3.76 | 5.06 | ||||
| VII. Hepatoblastoma etc. | 28.75 | 23.19 | 26.07 | 3.77 | 5.58 | 4.65 | 0.5 | 0.53 | 0.52 | 0.78 | 0.41 | 0.6 | 2.6 | 2.58 | 2.59 | 3.57 | ||||
| VIII. Malignant bone tumors | 3.59 | 0 | 1.86 | 0.75 | 0 | 0.39 | 3.02 | 7.45 | 5.17 | 11.31 | 8.27 | 9.83 | 6.01 | 5.85 | 5.93 | 4.77 | ||||
| IX. Soft-tissue sarcomas | 17.97 | 15.46 | 16.76 | 5.28 | 4.79 | 5.04 | 3.52 | 3.73 | 3.62 | 3.9 | 4.96 | 4.41 | 4.71 | 4.99 | 4.85 | 5.18 | ||||
| X. Germ-cell, trophoblastic and other gonadal neoplasms | 3.59 | 15.46 | 9.31 | 4.53 | 4.79 | 4.65 | 1.51 | 2.13 | 1.81 | 5.46 | 8.27 | 6.82 | 3.9 | 5.85 | 4.85 | 4.68 | ||||
| XI. Other malignant epithelial neoplasms | 0 | 0 | 0 | 0.75 | 0 | 0.39 | 0.5 | 3.19 | 1.81 | 4.29 | 9.09 | 6.62 | 2.11 | 4.82 | 3.43 | 2.63 | ||||
| XII. Other specified or unspecified malignant neoplasms | 7.19 | 19.33 | 13.04 | 9.81 | 4.79 | 7.37 | 5.53 | 1.6 | 3.62 | 1.95 | 3.31 | 2.61 | 5.04 | 3.79 | 4.43 | 5.16 | ||||
| Total | 190.46 | 212.6 | 201.12 | 169.06 | 116.48 | 143.5 | 84.98 | 70.27 | 77.83 | 103.34 | 95.08 | 99.33 | 115.49 | 96.9 | 106.47 | 113.34 | ||||
ICCC, International Classification of Childhood Cancer; M, male; F, female; ASR, age-standardized rate; CNS, central nervous system; *, adjusted by world standard population.
Age-specific incidence rate
The peak age group for crude incidence of childhood cancer was 0 years, equivalent to 201 cases per million per year, followed by 1-4, 10-14 and 5-9 years group, with the crude incidence rates of 143.50, 99.33 and 77.83, respectively (Table 2). The top three incidence rates of childhood cancer were different in different age-groups. Among 0-year cases, the most common cancer is leukemia with crude incidence rate of 54.01 per million per year, followed with hepatoblastoma and sympathetic nervous system tumors, with incidence rates of 26.07 and 24.21 per million per year, respectively. In 1-4 years group, leukemia still shows the highest incidence rate (crude 61.66), followed by CNS tumors (crude 22.49) and sympathetic nervous system tumors (crude 14.35). Among 5-9 years group, leukemia is the most common cancer with the incidence rate of 34.65 per million per year, followed with CNS tumor (crude 15.52) and lymphoma (crude 6.98). Among 10-14 years group, leukemia and CNS tumors show the highest and the second highest incidence (crude 36.72 and 20.07, respectively), while the third one was lymphoma and malignant bone tumor, which had the same incidence rates (crude 9.83) (Table 2).
Incidence trends of childhood cancer
The incidence for all childhood cancers combined has increased during the study period, with an APC of 5.84% [95% confidence interval (95% CI): 1.0-10.9] after adjusted by world population. However, the trends were different among boys and girls. The ASR of all combined cancers in boys during the entire period showed a slight, but non-significant increase, with an APC of 5.33% (95% CI: –0.6-11.6). For girls, the trends for all combined cancers increased significantly, with an APC of 6.54% (95% CI: 1.5-11.8) (Table 3).
Table 3. Incidence rates of childhood cancer by sex in Beijing, China, 2000-2009 (rate: 1/1,000,000).
| Year | Male |
Female |
Total |
|||||
|---|---|---|---|---|---|---|---|---|
| Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | |||
| 2000 | 73.35 (53/722,521) | 67.65 | 72.63 (50/688,444) | 69.61 | 73.00 (103/1,410,965) | 68.64 | ||
| 2001 | 108.49 (75/691,282) | 118.34 | 85.27 (56/656,738) | 94.3 | 97.18 (131/1,348,020) | 106.61 | ||
| 2002 | 95.19 (63/661,837) | 94.57 | 65.48 (41/626,124) | 62.01 | 80.75 (104/1,287,961) | 78.67 | ||
| 2003 | 125.05 (79/631,723) | 152.53 | 73.95 (44/595,016) | 93.17 | 100.27 (123/1,226,739) | 123.71 | ||
| 2004 | 115.06 (69/599,674) | 120.95 | 109.88 (62/564,253) | 128.02 | 112.55 (131/1,163,927) | 124.35 | ||
| 2005 | 146.50 (84/573,388) | 166.29 | 118.60 (64/539,643) | 128.59 | 132.97 (148/1,113,031) | 148.05 | ||
| 2006 | 115.65 (65/562,021) | 121.81 | 113.51 (60/528,609) | 112.7 | 114.61 (125/1,090,630) | 117.46 | ||
| 2007 | 136.91 (77/562,395) | 151.15 | 100.19 (53/528,977) | 100.8 | 119.12 (130/1,091,372) | 126.79 | ||
| 2008 | 136.93 (78/569,615) | 145.66 | 126.98 (68/535,511) | 130.43 | 132.11 (146/1,105,126) | 138.33 | ||
| 2009 | 116.90 (68/581,683) | 119.47 | 118.93 (65/546,548) | 123.7 | 117.88 (133/1,128,231) | 121.48 | ||
| Total | 115.49 (711/6,156,139) | 124.52 | 96.90 (563/5,809,863) | 102.8 | 106.47 (1,274/11,966,002) | 113.34 | ||
| APC | 4.74 | 5.33 | 6.71 | 6.54 | 5.59 | 5.84 | ||
| P | 0.03 | 0.07 | 0.004 | 0.02 | 0.003 | 0.03 | ||
APC, annual percent change.
We calculated the trends of the three most common tumors of childhood cancer by sex. For boys, the incidence trends of all the three tumors, leukemia, CNS tumors and lymphoma, were stable, with the APC of 3.32% (95% CI: –5.0-12.4), 2.87% (95% CI: –7.0-13.7), –0.5% (95% CI: –9.5-9.4), respectively (Figure 1). For girls, the trend for leukemia showed a significant increase (APC =5.33, 95% CI: 0.1-11.1) and showed a non-significant increase for CNS tumors (APC =5.47, 95% CI: –7.7-20.5) (Figure 2). The trend for lymphoma was not calculated because there was no newly diagnosed lymphoma cases of childhood cancer aged less than 15 years in 2009 in Beijing.
Figure 1.
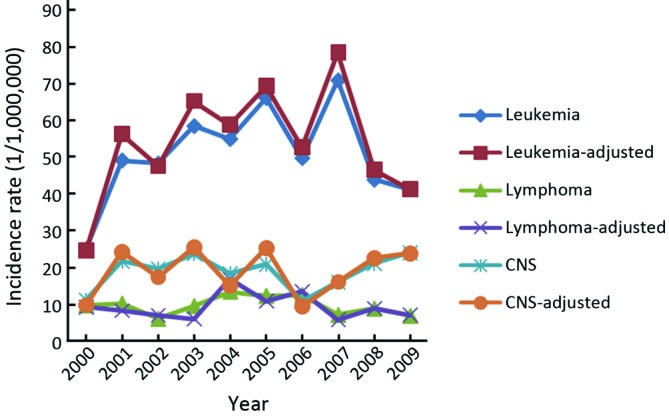
Trends of top 3 childhood cancers (0-14 years) among boys in Beijing, China, 2000-2009.
Figure 2.
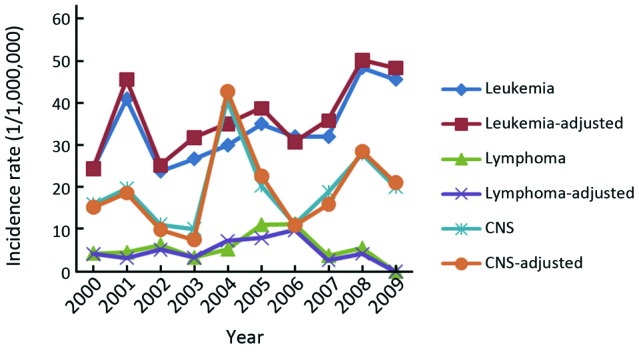
Trends of top 3 childhood cancers (0-14 years) among girls in Beijing, China, 2000-2009.
Discussion
This is the first study in Beijing that surveyed the incidence of childhood cancers by collecting all childhood cancers from the second and tertiary hospitals in the whole city, which covers 12 million residents. According to the GLOBOCAN 2012, the incidence rate of childhood cancer varies in different regions in the world. The Europe and America regions have the higher incidence rates of 126 and 131 per million children, respectively. While the lower incidence rates were seen in Asia and Africa regions with the rates of 75 and 80 per million children, respectively (1). Our analysis found that the ASR of all childhood cancers in Beijing was 113 per million children, which is lower than that of developed countries, but higher than that of developing countries, as well as the national average level in China (1).
The incidence of childhood cancer during 1973 to 2005 in urban Shanghai showed no significant trend (7), while in the United States, the rate has increased slightly at an annual rate of 0.6% per year since 1975 (8). Our findings showed different trends with an average increase of 5.84% (95% CI: 1.0-10.9) annually in Beijing. But the trend among boys showed no significant increase with an APC of 5.33 (95% CI: –0.6-11.6). In terms of the top 3 childhood cancers among boys, the trends also showed no significant increase. Among girls, a significant increase was seen with an APC of 6.54% (95% CI: 1.5-11.8), which is mainly due to the increase of leukemia. The trend of overall incidence in Beijing was the same as that in Australia (9). The reasons for increasing incidence rates in Beijing are largely unknown. Some of the increase may be due to changes in environmental factors or the improved diagnosis. The completeness of cancer registration may also have contributed (10).
Overall cancer in childhood is more common among males than females and the male to female ratio in the most developed countries is around 1.2:1. Our data showed the ratio is 1.26:1, which is the same as other countries (11). However, the male to female ratio varied by diagnostic category. The ratios of Burkitt’s lymphoma and Ewing’s sarcoma are 11:1 and 5:1, respectively. However, some cancers, like germ cell and retinoblastoma, show a slight female preponderance. Like in the childhood cancer elsewhere, leukemia is the most common childhood cancer in Beijing, which accounts for 39.64%, higher than that in other regions in the world. The incidence of leukemia in Beijing was higher than that in England and Wales, Iraq (12,13). Lymphoid leukemia (57.43%) was the commonest among leukemia, and the same results were seen in the United States and Thailand (11). Brain and CNS tumors are the second commonest childhood cancer in Beijing, followed by lymphoma, which was similar to Australia (9). But in Thailand (11), the second one is lymphoma, followed by CNS tumors. Our data showed that the sequence of the top 3 cancers varied in different age groups.
Our knowledge about the etiology of childhood cancer is still quite limited. Environmental causes of childhood cancer have long been suspected by many scientists but have been difficult to pin down, partly because cancer in children is rare and because it is difficult to identify past exposure levels in children, particularly during potentially important periods such as pregnancy, in-utero, or even prior to conception (14). Hence, many of the environmental agents hypothesized for childhood cancer, such as the radiation from nuclear power plants, diagnostic CT, pregnancy and maternal tobacco smoking and exposure to benzene, etc. remain speculative (15-20). Further epidemiological studies to understand on the risk factors on childhood cancer should be conducted in the near future.
This is the first population-based study in Beijing, China that surveyed the incidence of childhood cancer by collecting all childhood cancers from all treating hospitals in Beijing. Our study indicated that incidence rate of childhood cancer in Beijing was higher than the average level of China but lower than western countries. The incidence trends of childhood cancer, especially leukemia among girls, showed a significant increase, while among boys, no substantial change was seen during the observed time period from 2000 to 2009. Some sex-specific trends by subcategories and trends of major cancers in different age groups by cancer sites merit further investigation.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer, 2013. [Google Scholar]
- 2.Rodriguez-Galindo C, Friedrich P, Morrissey L, et al. Global challenges in pediatric oncology. Curr Opin Pediatr 2013;25:3-15 [DOI] [PubMed] [Google Scholar]
- 3.He J, Chen WQ. Chinese cancer registry annual report, 2012. Beijing: Military Medical Science Press, 2012. [Google Scholar]
- 4.Curado MP, Edwards B, Shin HR, et al. Cancer Incidence in Five Continents. Vol. IX. IARC Scientific Publications No.160. Lyon: IARC, 2008:1-187. [Google Scholar]
- 5.Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335-51 [DOI] [PubMed] [Google Scholar]
- 6.National Cancer Institute. Joinpoint Regression Program. Avaiable online: http://surveillance.cancer.gov/joinpoint/
- 7.Bao PP, Zheng Y, Gu K, et al. Trends in childhood cancer incidence and mortality in urban Shanghai, 1973-2005. Pediatr Blood Cancer 2010;54:1009-13 [DOI] [PubMed] [Google Scholar]
- 8.Smith MA, Seibei NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol 2010;28:2625-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baade PD, Youlden DR, Valery PC, et al. Trends in incidence of childhood cancer in Australia, 1983-2006. Br J Cancer 2010;102:620-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arora RS, Eden TO, Kapoor G. Epidemiology of childhood cancer in India. Indian J Cancer 2009;46:264-73 [DOI] [PubMed] [Google Scholar]
- 11.Wiangnon S, Veerakul G, Nuchprayoon I, et al. Childhood cancer incidence and survival 2003-2005, Thailand: study from the Thai Pediatric Oncology Group. Asian Pac J Cancer Prev 2011;12:2215-20 [PubMed] [Google Scholar]
- 12.Kroll ME, Stiller CA, Murphy MF, et al. Childhood leukaemia and socioeconomic status in England and Wales 1976-2005: evidence of higher incidence in relatively affluent communities persists over time. Br J Cancer 2011;105:1783-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alrudainy LA, Hassan JG, Salih HM, et al. Time trends and geographical distribution of childhood leukaemia in basrah, iraq, from 2004 to 2009. Sultan Qaboos Univ Med J 2011;11:215-20 [PMC free article] [PubMed] [Google Scholar]
- 14.Spector LG, Ross JA, Olshan AF. Children’s Oncology Group’s 2013 blueprint for research: epidemiology. Pediatr Blood Cancer 2013;60:1059-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spycher BD, Feller M, Zwahlen M, et al. Childhood cancer and nuclear power plants in Switzerland: a census-based cohort study. Int J Epidemiol 2011;40:1247-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagorio S, Ferrante D, Ranucci A, et al. Exposure to benzene and childhood leukaemia: a pilot case-control study. BMJ Open 2013;3 pii: e002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith MT, Zhang L, McHale CM, et al. Benzene, the exposome and future investigations of leukemia etiology. Chem Biol Interact 2011;192:155-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milne E, Greenop KR, Scott RJ, et al. Parental prenatal smoking and risk of childhood acute lymphoblastic leukemia. Am J Epidemiol 2012;175:43-53 [DOI] [PubMed] [Google Scholar]
- 19.Alibek K, Mussabekova A, Kakpenova A, et al. Childhood cancers: what is a possible role of infectious agents? Infect Agent Cancer 2013;8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milne E, Greenop KR, Fritschi L, et al. Childhood and parental diagnostic radiological procedures and risk of childhood brain tumors. Cancer Causes Control 2014;25:375-83 [DOI] [PubMed] [Google Scholar]


