Abstract
Among the colorectal cancers, the incidence of colon cancer has obviously increased. As a result, the actual incidence of colon cancer has exceeded that of rectal cancer, which dramatically changed the long-existing epidemiological profile. The acute complications of colon cancer include bleeding, obstruction, and perforation, which were among the common acute abdominal surgical conditions. The rapid and accurate diagnosis of these acute complications was very important, and laparoscopic techniques can be applied in abdominal surgery for management of the complications.
Keywords: Colon cancer, bleeding, obstruction, perforation
Introduction
It is estimated that in 2010, there were 102,900 new cases of colon cancer, 39,670 new cases of rectal cancer, and 51,370 deaths related to colon and rectal cancer combined in United States (1). The incidence of colorectal cancer has also witnessed a rapid rise in China, particularly in some socioeconomically developed areas. In Shanghai, the incidence of colorectal cancer had ranked the fourth among maligancies since 1990 (only after the lung cancer and gastric cancer), and rose to the second place in 2002, second only to lung cancer (2,3). Among the colorectal cancers, the incidence of colon cancer has obviously increased (4). As a result, the actual incidence of colon cancer has exceeded that of rectal cancer, which dramatically changed the long-existing epidemiological profile (5). The acute complications of colon cancer include bleeding, obstruction, and perforation, which were among the common acute abdominal surgical conditions (6-10).
Bleeding
Clinical presentation
Anemia
The hemoglobin level, red blood cell count, and hematocrit may not remarkably change at the early stage after acute massive bleeding due to the presence of physiological regulations such as peripheral vasoconstriction and redistribution of red blood cells. Then, after a large number of tissue fluids enter the blood vessels to supplement the plasma deficit, the hemoglobin level and hematocrit decrease due to dilution. This compensation effect usually is completed a few hours or several days after the bleeding, and, on an average, the hemoglobin can be diluted to the maximum extent 32 hours after bleeding. Hemorrhagic will stimulate the hematopoietic system, causing the active proliferation of blood cells and the increase of peripheral blood reticulocytes.
Blood in stool
Blood in stool is a typical clinical manifestation of colon tumor bleeding. Typically, the right-sided colonic bleeding may present with maroon-colored stools, whereas the left-sided colonic bleeding with bright red stools.
Hemorrhagic peripheral circulation failure
The excessive, rapid, and/or persistent bleeding during colon cancer bleeding can cause acute peripheral circulation failure. The symptoms/signs may include: dizziness, fatigue, palpitations, nausea, thirst, cold sweats, amaurosis, or syncope; clammy skin; pale nail beds; poor venous filling and collapse of superficial veins; weak pulse, clammy limbs, rapid heart rate, decreased blood pressure, and even shock; finally, listlessness, irritability, and even unresponsiveness and confusion. The elderly patients are often have low organ functional reserves and chronic diseases; in these patients, even small amount of bleeding can also cause acute multiple organ failure and thus raise the mortality.
Azotemia
Colon cancer bleeding can easily cause renal and pre-renal azotemia. Pre-renal azotemia refers to the retention of nitrogen due to the temporary reduction in renal blood flow and the decrease of glomerular filtration rate and renal excretory function after the hemorrhagic peripheral circulation failure. The blood urea nitrogen can rapidly decline to the normal level after the low blood pressure and shock are corrected. Renal azotemia is clinically manifested as oliguria or anuria, which can be explained by the tubular necrosis (acute renal failure) after severe and persistent shock or the worsened renal damage after bleeding.
Fever
Most patients can experience low-grade fever within 24 hours after bleeding, which can last several days to one week. The fever may be caused by the thermoregulatory center dysfunction due to hypovolemia, anemia, peripheral circulatory failure, and absorption of the blood proteolytic enzymes.
Auxiliary examinations
Suctioning via nasogastric tube to learn whether there is hematocele or active bleeding inside the stomach; also, the gastric tube can be reserved to observe the color of the drainage. Bleeding from lesions in the stomach or duodenum can be excluded if there is no bleeding.
Barium X-ray may help to find bleeding that may be missed under endoscope; however, it is should not be performed immediately after the active bleeding stops since pressing on the abdomen may cause rebleeding; generally, it should be cautiously performed 3-5 days after hemostasis.
Fiber endoscopy has been widely used in the diagnosis of intestinal bleeding. It can directly visualize the scope, property, and severity of lesions; during the examination, it can be used to collect the living tissue for pathological examination; also, hemostatic measures can be taken during the endoscopy.
During the selective arteriography of the superior mesenteric artery, celiac artery, or superior mesenteric artery, a bleeding rate of more than 0.5 mL/min allows the identification of the contrast agents flowing into the intestine via the vascular injury, ensuring a high detection rate of the positive lesions. It has been reported that angiography during the massive bleeding could achieve a positive rate of more than 77%. In addition, vasoconstrictor drugs can also be dripped or embolic agents injected through the tubes for hemostasis. During the angiography, a piece of guidewire can be introduced or methylene blue injected via the tube for marking the bleeding site, which will be helpful for the localizations of the bleeding sites during surgical exploration.
Radionuclide examinations
Technetium-99m (99 mTc) red blood cell scan and abdominal scintigraphy are usually applied. Multiple scans may indicate the areas with high radioactivity concentrations. These techniques can be used for locating the bleeding lesions but cannot make a qualitative diagnosis. The patients can be monitored for 36 hours after rug intake. Thus, they are suitable for detecting chronic minor hemorrhage, particularly intermittent bleeding.
Ultrasound examination
The ultrasound examination of the intestinal space-occupying lesions showed that the intestinal wall becomes thick; presence of hypoechoic mass in the hyperechoic tumor area can be observed, although the margin is unclear.
CT scan
CT can display the extent of tumor spread to the outer region of the intestine and the potential metastasis of the tumor to lymph nodes, liver, and/or peritoneum.
Diagnosis
Identification of bleeding
Some other conditions such as upper gastrointestinal bleeding and oral administration of animal blood, bone charcoal, bismuth agents, and some TCM medicinals can also cause black stools. A small number of patients with acute major bleeding may suffer from peripheral circulatory failure before the occurrence of bloody stool. Thus, for patients presented with symptoms associated with peripheral circulatory failure, the massive lower gastrointestinal hemorrhage due to colon tumors should be carefully considered after other conditions including toxic shock, anaphylactic shock, cardiogenic shock, acute hemorrhagic necrotizing pancreatitis, ruptured ectopic pregnancy, spontaneous or traumatic liver and spleen rupture, aneurysm rupture, and chest bleeding were excluded.
Diagnosis of the arrest of bleeding
Persistent or recurrent bleeding can be considered and timely managed if the following clinical manifestations were found: the patient passes looser or more frequent stools, which are dark red in color, associated with hyperactive bowel sounds; the peripheral circulatory failure is not remarkably improved even after active fluid replacement and blood transfusion; or, the peripheral circulation is improved shortly and then worsens again, and the CVP fluctuates even after rapid fluid replacement and blood transfusion; the red blood cell count, hemoglobin level, and hematocrit continue to decline, whereas the reticulocyte count increase; and high BUN persists or recurs even after sufficient fluid replacement and urination.
Assessing the severity of bleeding
Usually there will be no systemic symptom if the volume of each bleeding episode does not exceed 400 mL; however, if the volume of each bleeding episode exceeds 500 mL and meanwhile the bleed speed is relatively high, the patient can suffer from dizziness, fatigue, tachycardia, and hypotension. In a patient with severe bleeding, blood transfusion is required 1,500 mL within 3 hours to correct shock. The estimation of the volume of the gastrointestinal bleeding is mainly based on the clinical manifestations of peripheral circulatory failure due to hypovolemia, particularly on the dynamic observation results of blood pressure and pulse. The red blood cell count, hemoglobin level, and hematocrit are also helpful for estimating the degree of blood loss.
Treatment
Non-surgical treatment
The general emergency treatment includes: establish adequate intravenous access; rapidly supplement crystalloids and colloids (with a colloid to crystalloid ratio of 3:1) to correct shock; urgently crossmatch and prepare blood; give appropriate blood transfusion based on the estimation of blood loss; and intravenous use of hemostatic agents.
Endoscopic hemostasis has been widely applied for managing gastrointestinal bleeding, with the main measures including: endoscopic spraying of topical hemostatic agents including 5% Monnel solution, 8 mg/dL norepinephrine, thrombin, and medical adhesive; local injection of hemostatic agents: inject 2-3 mL 1/1,000 epinephrine solution around the bleeding site; or, inject the local bleeding site using the mixture of hypertonic saline (3.6% or 7.1%) and 0.005% epinephrine; or, inject the hardener at the bleeding site, mainly using ethanol, 0.2-0.3 mL each time (not exceed 1 mL to avoid ulceration or perforation), around the vessels at the bleeding lesions; use high-frequency electrocoagulation, laser, or microwave to coagulate the tissue proteins, block the blood vessels, and thus stop bleeding.
Angiography and interventional treatment
Selective or super-selective arteriography can not only identify the bleeding site and confirm the diagnosis but also enable effective hemostasis. Also, as a minimally invasive treatment, it is suitable for patients who experience massive bleeding and meanwhile cannot tolerate surgeries. The main interventions include drug infusion and vascular embolization, with the commonly used drugs including vasopressin, epinephrine, norepinephrine, and ephedrine.
Surgical treatment
If the non-surgical treatment fails to achieve satisfactory efficacy in patients who have definite bleeding site and etiologies, emergency surgery or elective surgery may be applied based on the disease conditions. Indications for emergency surgery: patients with stable disease, definite diagnosis, and improved general conditions but have persistent bleeding; persistent bleeding associated with intestinal obstruction, intussusception, intestinal perforation, and/or acute peritonitis; or, bleeding has stopped but a second major bleeding is possible within a short period of time. If hemostasis has been successfully achieved after non-surgical therapy, elective surgery may be arranged after the patient’s general conditions become stable.
Obstruction
Pathophysiology
The obstruction caused by colon cancer is mainly manifested as mechanical obstruction. The main physiological and pathological changes in patients with obstruction due to colon cancer include dilated colon proximal to the obstruction site, fluid and electrolyte loss, and infection (and its related toxemia). The severity of these changes depends on the site of the obstructed site, the duration of obstruction, and presence (or absence) of any blood supply dysfunction on the bowel wall.
Intestinal dilatation
In patients with mechanical intestinal obstruction, the intestinal canal above the obstruction site becomes dilated due to the accumulation of fluid and gas, which is initially manifested as the enhanced intestinal peristalsis, followed by intestinal colic. The upper esophageal sphincter then relaxes, and the patient will literally inhale air into his/her stomach when breathing; thus, 70% of the accumulated gas inside the intestinal canal is the inhaled air, with the majority being nitrogen, which can not be easily absorbed by the stomach or intestine; the remaining 30% of the gas is produced by acid-base neutralization and bacterial fermentation inside the intestine, or are the gases including CO2, H2, and CH4 that are diffused from the blood to the intestine. Every day, a healthy adult’s gastrointestinal tract secrets approximately 8 L of fluids including saliva, gastric juice, bile, pancreatic juice, and intestinal juice. Most of them are absorbed by intestinal mucosa to maintain fluid balance. In a patient with intestinal obstruction, however, a large volume of fluids and gases are accumulated in the proximal intestinal canal, causing intestinal dilatation; they inhibit the absorption function of the intestinal mucosa and meanwhile stimulate its secretion. As a result, an increasing number of fluids are accumulated inside the intestinal canal, and the intestinal dilatation worsens progressively. The intraluminal pressure usually is low (initially below 8 cmH2O) in patients with simple obstruction; however, if the obstruction is prolonged, the pressure can rise to 18 VcmH2O. When the colon becomes obstructed, the intraluminal pressure can be higher than 25 VcmH2O, or even reaches 52 VcmH2O. The raised intraluminal pressure can cause disordered venous return on the bowel wall, resulting in the congestion, edema, and thus increased permeability of the bowel wall; some bacteria can also penetrate into the abdominal cavity, causing peritonitis. If the intraluminal pressure continues to increase, the blood supply on the intestinal wall can be blocked; as a result, the simple obstruction becomes strangulated intestinal obstruction, and ulceration and perforation of the intestinal canal can occur due to ischemia/necrosis. Severe intestinal dilatation can also elevate the diaphragm, affecting the respiratory and circulatory functions.
Fluid and electrolyte loss
During the intestinal obstruction, intestinal dilatation can cause reflex vomiting. In patients with low intestinal obstruction, the decreased absorption function and increased secretions of the intestinal mucosa result in the retention of massive fluid inside the intestinal canal above the obstruction site. These fluids, up to 5-10 L in some patients, contain a large number of sodium bicarbonate. Although not excreted, they are enclosed in the intestine and cannot enter the blood, which equals to fluid loss. Furthermore, excessive intestinal dilatation affects vein reflux, causing intestinal wall edema and plasma extravasation. In patients with strangulated intestinal obstruction and peritonitis, the loss of blood and plasma can be even worse; the patients often suffer from dehydration, oliguria, azotemia, and acidosis. If the dehydration continues, the blood will be further concentrated, resulting in hypotension and hypovolemic shock. Hypokalemia due to potassium loss and/or no feeding can cause intestinal paralysis, thus exacerbating the intestinal obstruction.
Infection (and its related toxemia)
In healthy individuals, due to the effect of gastric acid and because the intestinal contents constantly move forwards and are replaced by new ones under the effect of peristalsis, only a handful of bacteria exist in the small intestine. In patients with simple mechanical intestinal obstruction, the bacteria and toxins, if exist, cannot pass through the mucosal barrier and therefore will not cause notable harm. However, if the obstruction is strangulated, fluids in the affected intestinal canal will contain a large number of bacteria (e.g., Clostridium, Streptococcus, and E. coli), blood, and necrotic tissues, among which the bacterial toxins and necrotic tissue decomposition products are highly toxic. After these fluids enter the abdominal cavity via the damaged or perforated intestinal wall, they can cause severe irritation and infections; when absorbed by the peritoneum, they can cause sepsis. Severe peritonitis and sepsis is the leading cause of death in patients with intestinal obstruction.
In addition to these three major pathophysiological changes, patients with strangulated intestinal obstruction can also be associated with hemorrhage on intestinal wall, in intestinal canal, and/or abdominal cavity. The longer bowel strangulation is associated with larger blood loss, which is also one of the causes of death among patients with intestinal obstruction.
Clinical presentation
Abdominal pain
Most patients with intestinal obstruction have abdominal pain. During the bowel obstruction, if the ileocecal valve is functioning properly, the colonic contents will not flow back to the small intestine; thus, the colonic lumen is gradually enlarged, along with the increased pressure. Therefore, in addition to paroxysmal colic, patients may also suffer from persistent dull pain. Closed-loop obstruction, in which a segment of gut is obstructed at both ends, should also be considered. The persistent dull pain during the intermittent episodes of paroxysmal colic is also the early manifestation of strangulated strangulated ileus. The intestinal ischemic necrosis can be manifested as persistent severe abdominal pain. In the elderly and vulnerable individuals, however, the abdominal pain after intestinal strangulation may be not obvious due to the weak responsiveness.
Vomiting
In patients with colon obstruction, the ileocecal valve can prevents reflux; thus, there will be no vomiting at the early stage. At the later stages, however, the ileocecal valve may not be completely closed due to the over-filling of the intestinal cavity, and therefore the patients may suffer from severe vomiting, with faecal juice inside the stomach contents.
Abdominal distension
Abdominal distension is common in the late stages of colon obstruction. During the closed-loop obstruction, the bowel distension will become obvious, and asymmetric local expansion can often be seen in the abdominal region.
No anal exsufflation and defecation
Anal exsufflation and defecation stop in patients with complete intestinal obstruction. However, Bloody stools and pus can still be discharged in patients with obstruction due to colon cancer.
Systemic symptoms
Dehydration is common in patients with frequent vomiting and severe abdominal bloating. Patients with hypokalemia often have symptoms including weakness, drowsiness, fatigue, and arrhythmia. Patients with strangulated intestinal obstruction have the most remarkable systemic symptoms, which are initially presented as exhausted, soon followed by shock. In patients accompanied by abdominal infection, the abdominal pain can persist and then spread to the whole abdomen, along with infection/toxemia manifestations including chills, fever, and leukocytosis.
Diagnosis
The diagnosis of obstruction was based on: with four main symptoms including abdominal pain, abdominal bloating, vomiting, anal no anal exhaust/defecation; abdominal examination shows visible intestinal peristalsis, abdominal tenderness, and hyperactive or missing bowel sounds; abdominal X-ray fluoroscopy or radiography shows obviously dilated intestinal lumen and multiple fluid levels; and CT identifies both the lumen dilatation and fluid levels and the direct signs of colon cancer (Figure 1).
Figure 1.
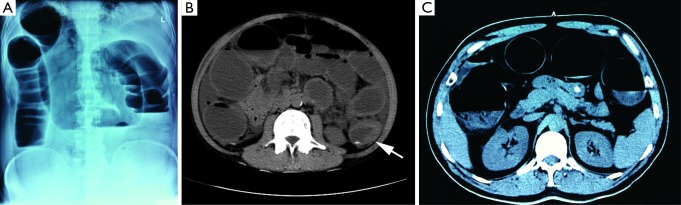
The diagnosis of obstruction: (A) Bowel obstruction on abdominal plain film; (B) CT shows the colon cancer and intestinal obstruction at the sigmoid-descending junction; (C) the colon cancer causes intestinal obstruction; as a result, the colon is remarkably dilated but not the small intestine.
Obstruction caused by colon cancer should also be differentiated from some other conditions. In patients with a history of abdominal surgery, trauma, and/or infection, intestinal adhesion or obstruction due to adhesions should be considered; in patients with lung tuberculosis, intestinal obstruction caused by intestinal tuberculosis or peritoneal tuberculosis should be considered; in patients with rheumatic valvular disease accompanied with atrial fibrillation, atherosclerosis, or arterial occlusive meningitis, which are the common causes of the obstruction of blood flow, mesenteric artery thrombosis should be considered. In young and mid-aged adult patients, the common causes of bowel obstruction are intestinal adhesions, incarcerated external hernia, and volvulus. In the elderly patients, in addition to colon cancer, stool impaction in sigmoid colon or rectum can also play a role; in fact, 90% of the bowel obstruction cases are caused by cancer. In adults, few intestinal obstruction cases are caused by intussusception, which is often secondary to Meckel diverticulum, intestinal polyps, and tumors. Paralytic ileus is often caused by abdominal major surgeries and abdominal infections, but may also be resulted from sepsis, severe pneumonia, drug toxicity, hypokalemia, retroperitoneal bleeding, intestinal bleeding, and ureteric colic.
Treatment
Correcting the dehydration, electrolyte loss, and disorders of acid-base balance
For adult patients with mild symptoms, rehydration of about 1,500 mL is often prescribed; for patients with obvious vomiting, 3,000 mL is recommended; and for patients with peripheral circulatory failure and hypotension, 4,000 mL is needed. If the condition cannot be alleviated within a short period of time, rehydration is also needed to supplement the fluids lost during gastrointestinal decompression and the normal daily requirement (about 2,000 mL). Intravenous infusion of potassium can be prescribed if the urine output is larger than 40 mL/h. In patients with low intestinal obstruction caused by colon cancer, acidosis is common due to the loss of alkaline intestinal fluids. In particular, the loss of plasma and whole blood during the later stages of intestinal obstruction can result in hemoconcentration or hypovolemia. Thus, erythrocytes, plasma, and albumin still need to be supplemented to effectively correct the circulatory disorders.
The treatment plans should be tailored in according with the patient’s vomiting conditions, dehydration, hourly urine output and urine specific gravity, blood sodium, potassium, and chloride concentrations, carbon dioxide combining power, serum creatinine, hematocrit, and CVP. Potassium ions may escape from the cells due to acidosis, and serum potassium determination may not truly reflect the potassium deficiency of cells; thus, ECG should also be performed to facilitate the judgment. The purpose of the fluid and electrolyte supplementation and the correction of acid-base balance disorders is to maintain a relatively stable internal environment, preventing the body from diseases, and thus help the patients to receive a surgical operation under favorable conditions.
Gastrointestinal decompression
Gastrointestinal decompression is a required emergency treatment for intestinal obstruction. A nasogastric tube should be placed in the stomach to aspirate swallowed air and to prevent distention of the fluids, reduce the bacteria and toxins in the intestine, mitigate intestinal dilatation, prevent aspiration pneumonia, and reduce vomiting, improve the circulation and respiratory distress caused by abdominal bloating, thus, to certain extent, improving the intestinal congestion, edema, and blood circulation above the obstruction site.
Controlling infection and its related toxemia
In patients with prolonged or strangulated intestinal obstruction, a variety of intestinal and peritoneal bacterial (such as E. coli, Clostridium, Streptococcus, etc.) infections can occur. Broad-spectrum antibiotic therapies mainly targeting gram-negative bacilli and anaerobic bacteria are particularly important. Animal experiments and clinical studies have proved that antibiotic treatment can significantly reduce intestinal obstruction-related deaths.
Removing the obstruction and restoring the bowel function
Colon cancer often forms closed-loop obstruction, resulting in the excessive dilatation of the intestinal lumen; also, the blood flow on the intestinal wall can easily become disordered, causing necrosis and perforation. If the tumor is near the ileocecal valve, the closed bowel segment tends to be shorter, and the risk of perforation will be higher. Thus, surgery for colon cancer obstruction should be done earlier. Obstruction caused by cecum cancer is often manifested as the signs of low small bowel obstruction. While it has lower risk for necrosis or perforation, the obstruction tends to be complete and cannot be alleviated spontaneously; thus, early surgery is also needed.
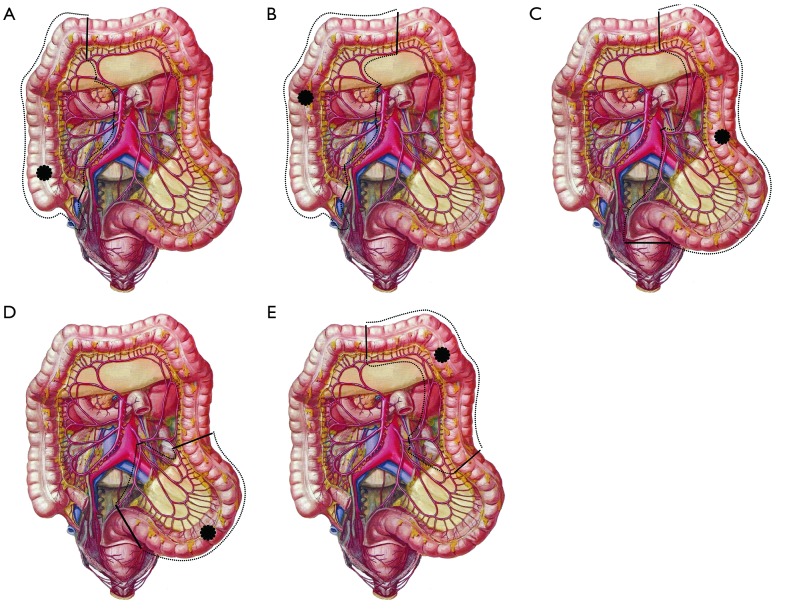
The surgery shall follow the following principles: for patients with right-sided colon cancer accompanied with acute obstruction, right hemicolectomy with primary anastomosis is preferred; for unresectable right-sided colon cancer, a side-to-side anastomosis between the terminal ileum and the transverse colon (the internal bypass) can be applied; the cecal ostomy has been rarely applied due to its poor decompression effectiveness; if condition allows, primary resection of the tumor is preferred for the acute obstruction caused by left-sided colon cancer. Three surgical options have been available: subtotal colectomy, with ileo-sigmoid colon or ileo-rectal anastomosis; left colectomy, with primary anastomosis and terminal ileum ostomy, followed by secondary surgery to close the opening; and left colectomy, with a proximal stoma and distal closure, followed by a secondary surgery for anastomosis; and e) for unresectable left-sided colon cancer, transverse colon or terminal ileum stomy may be performed (Figure 2).
Figure 2.
Resection of tumors in different parts of the colon.
Perforation
Perforation caused by colon cancer is rare in clinical settings (Figure 3). While most perforation cases occur in patients with acute obstruction, ulceration due to the penetration of the tumor mass through the intestinal wall has also been observed in a few patients. Both of them are extremely serious surgical emergencies. Perforation due to acute obstruction is mainly seen in cecum. The extremely high intraluminal pressure can cause the ischemia, necrosis, and even perforation of the local intestinal wall. A large number of fecal intestinal contents then flow into the abdominal cavity, causing diffuse fecal peritonitis and even toxic shock. Infection and toxicity are two major life-threatening factors. Once the perforation due to colon cancer has been confirmed, an emergency surgery should be performed immediately, together with systemic supports and antibiotic treatment. If the systemic conditions allow, primary resection is preferred. For patients with right-sided colon cancer, right hemicolectomy followed by primary anastomosis can be performed; for patients with left-sided colon cancer accompanied with perforation, the tumor should be removed firstly, followed by proximal colostomy. In patients with ruptured tumor that has become unresectable, a proximal double-lumen colostomy should be performed, during which the lesion should be adequately repaired and washed, along with adequate drainage.
Figure 3.
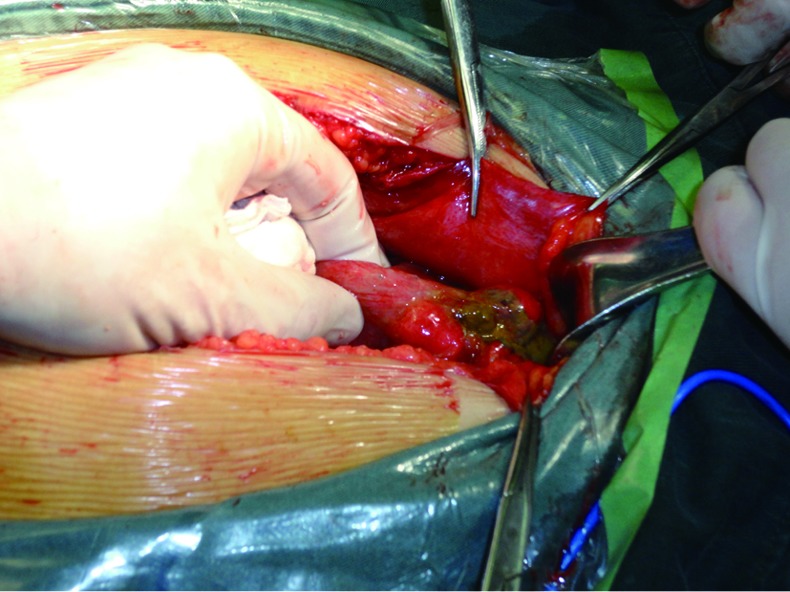
A ruptured and perforated sigmoid colon cancer.
Application of minimally invasive techniques
Laparoscopic surgery
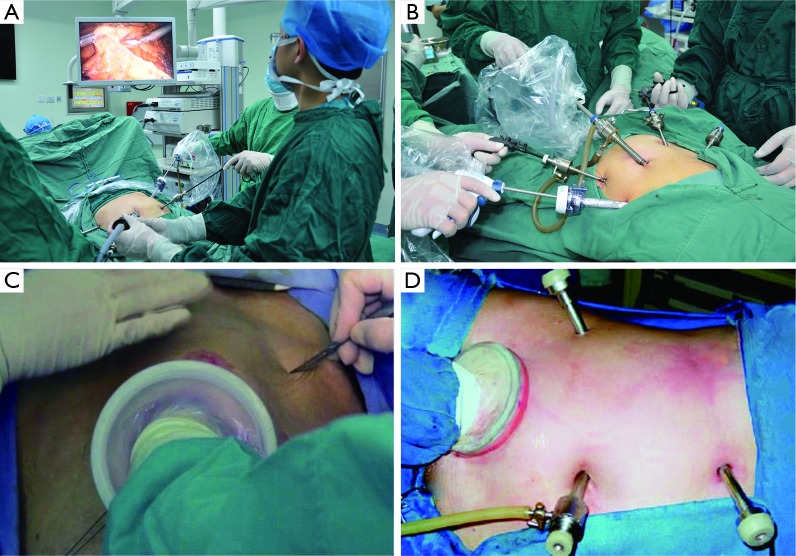
Laparoscopic techniques have been widely applied in abdominal surgery (11,12). During a laparoscopic surgery, the abdominal conditions can be visualized by using the laparoscope and camera, and the surgical operations are performed using long, slim devices (Figure 4). The laparoscopic surgery can avoid larger abdominal incision, along with many advantages including clear surgical field, fine separation, less blood loss, less abdominal disturbances, minimized surgical trauma, and dramatically shortened recovery time. However, the operations are mainly based on the laparoscopic instruments, which do not allow direct touching and are not as skillful as the surgeons’ hands; also, it is difficult to expose the deep structures inside the abdominal cavity, which limits its applications in complicated and highly difficult surgeries. In hand-assisted laparoscopic surgery, the surgeon inserts a hand into the abdomen via a small incision to assist the surgical operations. It was clinically meaningful in simplifying the operation and increasing the safety.
Figure 4.
Laparoscopic techniques in abdominal surgery: (A/B) laparoscopic surgery; (C/D) hand-assisted laparoscopy.
Laparoscopic colectomy has relatively high technical requirements for the surgeon. It has been proven to be a reliable, safe, and minimally invasive surgical approach for the properly selected patients. Laparoscopic colectomy can be divided into two types: laparoscopic assisted colectomy and complete laparoscopic colectomy. During the laparoscopic assisted colectomy, a small incision is made on the abdominal wall after the colon is dissociated under the laparoscope, then the diseased colon segment is lifted outside the body for resection; finally, the colon segment is put back to the abdominal cavity after manual or stapler anastomosis. During the complete laparoscopic colectomy, the cutting and anastomosis of the colon segment is completed inside the abdominal cavity using cutting and stapler equipments under the laparoscope.
In patients with the acute complications of colon cancer, laparoscopic surgery can be used for abdominal exploration to locate the lesion; also, in patients with intestinal obstruction, laparoscopic colostomy can be performed; compared with the open exploration and colostomy, it can remarkably reduce surgical trauma and abdominal adhesions, which facilitates the implementation of the secondary surgery. However, laparoscopic surgeries are contraindicated in patients with massive bleeding, critical conditions, severe heart and lung diseases, and/or peritoneal contamination due to colon cancer perforation. In the emergency setting, laparoscopic colectomy should not be performed without adequate bowel preparation. After the diagnosis is confirmed following laparoscopic exploration, the patients should be converted to open surgery using appropriate procedures.
Robotic surgery
Surgical robot is computerized surgical equipment that can help to locate the diseased sites and carry out surgical operations (13). The laparoscopic techniques have dramatically promoted the applications surgical robots, whereas the introduction of surgical robots also overcomes some shortcomings of laparoscopic techniques (14,15). For example, during the laparoscopic surgeries, the two-dimensional video monitor makes the operator difficult to know the spatial distance, and the operating angles of devices are often limited within the small operating space; also, the relatively poor coordination and flexibility of the laparoscopic surgical instruments limit their applications in fine anatomical dissection.
There were varius surgical robots such as passive robots, active robots, and master-slave robots. Among them the da Vinci Robotic Surgical System represented a true breakthrough and has been widely applied (16). The da Vinci Robotic Surgical System is divided into three main parts: the console operated by the operator; a mobile platform consisting of manipulator arm, camera arm and surgical instruments; and three-dimensional video imaging system. The surgery needs to be performed by two well-trained surgeons: the main operator controls the manipulator arm in the console, and the assistant doctor, standing near the patient, helps to change various laparoscopic surgical instruments that are connected with the manipulator arm. As a video-based system, the da Vinci Robotic Surgical System allows the operator to get connected with the manipulator arm with electronic circuit. The console is equipped with a three-dimensional vision system and a motion scaling system. The motions of the operator’s arms, wrists, and fingers, via the sensors, can be accurately recorded by the computer, and simultaneously translated into the manipulator arm. The ad hoc vibration-eliminating system and action-scaling system enable the manipulator arm to accurately work within the narrow surgical field without any vibration.
Compared with the conventional laparoscopic surgeries, the robotic surgery systems have many advantages. The operator can sit comfortably (relatively far away the patient) when performing the surgery by controlling the console; the three-dimensional imaging system can display the surgical field more vividly; the manipulator arm has seven degrees of freedom, which increases the instrumentation angles, remarkably enhances the operational capabilities of laparoscopic surgeries; more specifically, the large-amplitude motions of the manipulator arm can be proportionally converted fine motions inside the patient’s body. In addition, the robot system can eliminate the vibration of the doctor’s hands through software processing to restore the appropriate eye-hand coordination and the positions that in compliance with human ecology. More than 1,000 surgical robots have been working in hospitals in the United States, performing various surgeries in the departments of head and neck surgery, urology, gastrointestinal surgery, hepatobiliary surgery, thoracic surgery, gynecology, and vascular surgery. The feasibility and advantages of the robotic surgery for colorectal cancer has been demonstrated in more than 1,000 cases. In China, a few large hospitals in Beijing, Chongqing, and Shanghai have introduced robotic surgery for colorectal cancer. Although there have been a small number of reports on the robotic surgical treatment of acute colonic injury or iatrogenic acute suppurative cholangitis in the United States and China, the routine use of robotic systems for surgical emergencies still has a far way to go.
In conclusion, the acute complications of colon cancer include bleeding, obstruction, and perforation, which were among the common acute abdominal surgical conditions. The rapid and accurate diagnosis of these acute complications was very important, and laparoscopic techniques can be applied in abdominal surgery for management of the complications.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zhang S, et al. Report of cancer incidence and mortality in China, 2010. Ann Transl Med. 2014 doi: 10.3978/j.issn.2305-5839.2014.04.05. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen WQ, Zheng RS, Zhang SW, et al. Report of incidence and mortality in china cancer registries, 2008. Chin J Cancer Res 2012;24:171-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hou N, Huo D, Dignam JJ. Prevention of colorectal cancer and dietary management. Chin Clin Oncol 2013;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson VM, Benson AB., 3rd Status of targeted therapies in the adjuvant treatment of colon cancer. J Gastrointest Oncol 2013;4:245-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somasundaram SK, Akritidis G, Alagaratnam S, et al. Extraluminal colonic arteriovenous haemangioma: an unusual cause of chronic lower gastrointestinal bleeding. Ann R Coll Surg Engl 2013;95:e44-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Church DN, Midgley R, Kerr DJ. Stage II colon cancer. Chin Clin Oncol 2013;2:16. [DOI] [PubMed] [Google Scholar]
- 8.Karabulut M, Bas K, Gönenç M, et al. Self-expanding metallic stents in acute mechanical intestinal obstructions resulting from colorectal malignancies. Am Surg 2013;79:1279-82 [PubMed] [Google Scholar]
- 9.Ling TC, Kang JI, Slater JD, et al. Proton therapy for gastrointestinal cancers. Transl Cancer Res 2012;1:150-8 [Google Scholar]
- 10.Kavanagh DO, Nolan B, Judge C, et al. A comparative study of short- and medium-term outcomes comparing emergent surgery and stenting as a bridge to surgery in patients with acute malignant colonic obstruction. Dis Colon Rectum 2013;56:433-40 [DOI] [PubMed] [Google Scholar]
- 11.Tanis PJ, Buskens CJ, Bemelman WA. Laparoscopy for colorectal cancer. Best Pract Res Clin Gastroenterol 2014;28:29-39 [DOI] [PubMed] [Google Scholar]
- 12.Kim BS, Kim HS. Intracorporeal laparoscopic esophagojejunostomy using endoscopic linear staplers: the experiences of 293 cases. Transl Gastrointest Cancer 2013;2:75-8 [Google Scholar]
- 13.Bertani E, Chiappa A, Ubiali P, et al. Robotic colectomy: is it necessary? Minerva Chir 2013;68:445-56 [PubMed] [Google Scholar]
- 14.Park BJ. Robotic lobectomy for non-small cell lung cancer (NSCLC): Multi-center registry study of long-term oncologic results. Ann Cardiothorac Surg 2012;1:24-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rashid OM, Takabe K. Are video-assisted thoracoscopic surgery (VATS) and robotic video-assisted thoracic surgery (RVATS) for pulmonary resection ready for prime time? J Thorac Dis 2012;4:341-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alimoglu O, Atak I, Orhun K, et al. Robot-assisted laparoscopic colorectal surgery. Minerva Chir 2013;68:471-8 [PubMed] [Google Scholar]