Abstract
Background
Erythropoiesis-stimulating agents (ESAs) are widely used in the management of anemia in cancer patients. Despite their apparent effectiveness, recent studies have suggested that ESAs could result in serious adverse events and even higher mortality. The aim of the current study was to evaluate the benefits and risks of ESAs in the management of cancer patients with anemia using a meta-analysis.
Methods
The initial literature search covered Medline, PubMed, Embase, and the Cochrane Center Register of Controlled Trials, and identified 1,569 articles. The final meta-analysis included eight randomized controlled trials (n=2,387) in cancer patients with <11 g/dL hemoglobin (Hb) at the baseline and target Hb (for stopping ESA treatment) at no more than 13 g/dL. The assessment measures included Hb response, blood transfusion rate and adverse events that included venous thromboemblism (VTE), hypertension, and on-study mortality. The results are expressed as pooled odds ratio (OR). Publication bias was assessed using funnel plot analysis.
Results
ESAs significantly increased the Hb concentration [OR 7.85, 95% confidence interval (CI): 5.85 to 10.53, P<0.001] and reduced the red blood cell (RBC) transfusion rate (OR 0.52, 95% CI: 0.42 to 0.65, P<0.001). ESAs did not increase the accumulated adverse events (OR 0.95, P=0.82), or the on-study mortality (OR 1.09, P=0.47).
Conclusions
ESAs are not associated with increased frequency of severe adverse events in anemic cancer patients when the target Hb value is no more than 13 g/dL.
Keywords: Adverse events, anemia, cancer, erythropoiesis-stimulating agents (ESAs)
Introduction
Anemia is a common feature in cancer patients due to the malignant nature of the disease and/or the cytotoxic effects of chemotherapy on hematopoietic cells (1-3). Management of cancer-associated anemia is one of the key issues for improving life quality and reducing morbidity of cancer patients (4). Red blood cell (RBC) transfusion and erythropoiesis-stimulating agents (ESAs) are two major alternatives in the management of anemia. Collective evidence demonstrated that ESAs could increase hemoglobin (Hb) concentration and reduce the frequency of RBC transfusion (5-7). However, severe adverse events such as venous thromboemblism (VTE), cancer progression and even increased mortality, have been associated with the use of ESAs in cancer patients in some studies (8-12). Bohlius and Tonia analyzed all published randomized controlled trials and found that patients receiving ESAs are at higher risk to develop VTE, hypertension, and lower overall survival (13). Increased severe adverse events, however, have not been documented by all relevant studies, particularly those with a relatively low target Hb (14).
At present, there are no well-defined guidelines for the clinicians to follow regarding when to initiate and discontinue the ESA treatment in anemic cancer patients. The 2012 updated National Comprehensive Cancer Network (NCCN) guidelines for cancer- and chemotherapy-induced anemia recommended that cancer patients with Hb <11 g/dL or decreased by more than 2 g/dL from baseline should be treated with ESAs to maintain Hb at a level sufficient to avoid RBC transfusion (6).
In some of the published clinical trials, ESAs were offered when baseline Hb value was higher than 11 g/dL, and discontinued until Hb reached at a value higher than 13 g/dL (15-17). One such study found association between ESAs with reduced survival and increased mortality (17). However, a meta-analysis of randomized controlled trials comparing the use of ESAs versus placebo in cancer patients with chemotherapy-associated anemia (Hb <11 g/dL) did not find a higher mortality rate in the patients treated with ESAs (18). These results suggested that the starting Hb and target Hb are curial factors that determine potential benefits and risks of ESA treatment for cancer patients with anemia.
The current study is a meta-analysis that included only trials in cancer patients with a baseline Hb at <11 g/dL and a target Hb ≤13 g/dL. The results showed that ESA treatment could improve anemia, reduce RBC transfusion frequency, and is not associated with increased on-study mortality, cancer progression and VTE in cancer patients with anemia.
Methods
Selection criteria
All trials in this meta-analysis compared an ESA (epoetin alfa, epoetin beta, epoetin theta, darbepoetin alfa, or recombinant erythropoietin) with a placebo. All trials were conducted in patients with cancer- or chemotherapy-associated anemia. Trials must report one or more of the following measures: Hb response, transfusion rate, quality of life, VTE, accumulated adverse events and on-study mortality (death rate during the study period that may include a period of treatment and a short period after the treatment ended). Non-English papers were excluded from this study.
Based on the 2012 NCCN cancer- and chemotherapy-induced anemia management guidelines (6), the inclusion criteria were set to include the following:
Cancer patients with or without chemotherapy during the study or before the recruitment;
Patients did not receive a treatment four weeks before the study;
Patients with normal liver and renal functions;
The baseline Hb level was less than 11 g/dL;
The targeted Hb level was no more than 13 g/dL.
Patients with any primary hematologic disorders that would cause anemia, uncontrolled severe hypertension and those receiving concomitant radiotherapy were excluded from this study.
Search strategy
The investigators wrote a protocol and carried out a comprehensive search of Medline, PubMed, Embase, and the Cochrane Center Register of Controlled Trials from January 1990 to March 2013. In addition, reference lists of the trials, meta-analysis and relevant review articles were examined for any other eligible trials. A manual search of the reference lists of the initially identified articles and the abstracts presented at relevant scientific meetings was also conducted. During the searching, the terms “erythropoietin”, “recombinant human erythropoietin”, “epoetin alfa (alpha)”, “epoetin beta”, “epoetin delta”, “epoetin theta”, “darbepoetin alfa (alpha)”, “Procrit”, “Aranesp”, “NeoRecormon”, “rHu-EPO”, “an(a)emia”, “an(a)emic”, “quality of life”, “cancer”, “chemotherapy”, “platinum”, “non-platinum” were applied.
Data extraction and quality assessment
We screened abstracts of all retrieved articles and then matched the full texts of all articles selected during screening against the inclusion criteria. Disagreements on which articles met the inclusion criteria were resolved by discussion until a consensus was reached (Figure 1). Data were extracted independently by two authors.
Figure 1.
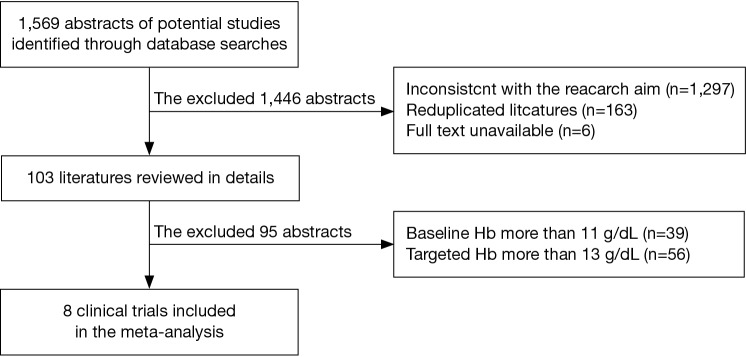
Identification process for eligible studies.
We used the Jadad score (maximum number of points is 5) to assess the quality of the selected studies (19). The score was 5 for three studies, 4 for three studies and 2 for the remaining two studies.
Statistical analysis
The analysis was carried out using Review Manager 5.1. A P value of <0.05 was considered as statistically significant. The data of Hb response, RBC transfusion, on-study mortality, VTE and accumulated adverse events were extracted from each study and combined using the Mantel and Haenszel Method to calculate the pooled odds ratio (OR). The ORs reflected the treatment effects. A pooled OR >1 indicated higher hematological response in the ESA arm, and higher transfusion rate, on-study mortality and serious adverse reactions in the control arm. In order to identify the difference of on-study mortality in different targeted Hb levels, additional subgroup analyses were performed.
Cochran’s Q test (Chi-square χ2 test) and I2 test were used to assess heterogeneity. I2 was calculated from a typical meta-analysis as I2=100%×(Q−df)/Q, where Q is Cochran’s heterogeneity statistic and df is degree of freedom. P>0.01 and I2 <25% were considered as no or low-level heterogeneity (20). I2 >50% was considered as significant heterogeneity; upon such cases, subgroup analysis was carried out by excluding one trial at a time.
Publication bias was assessed visually using a funnel plot (21). It is used primarily as a visual aid to detecting bias or systematic heterogeneity in meta-analysis. A symmetric inverted funnel shape arises from a ‘well-behaved’ data set, in which publication bias is unlikely, while an asymmetric funnel indicates a relationship between treatment effect and study size (Figure 2).
Figure 2.
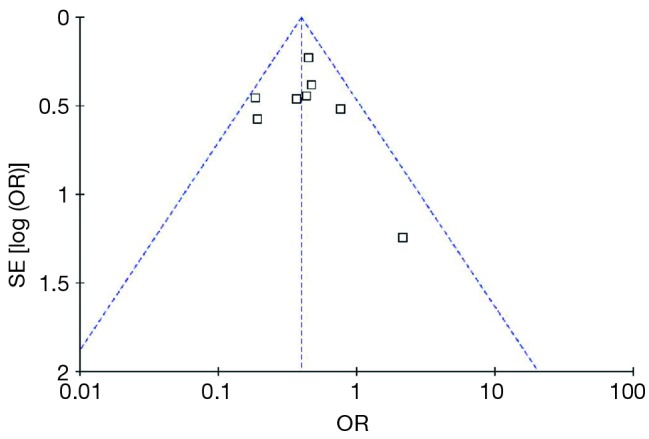
Funnel plot. The funnel plot analysis showed significant asymmetry between the studies.
Results
The initial search identified a total of 1,569 abstracts. Among them, 1,466 trials were excluded from the final analysis for the following reasons: inconsistency with the primary goal of this study (n=1,297), duplicate publication of the same study subjects (n=163), and un-available full text (n=6). In addition, 39 trials with baseline Hb at more than 11 g/L and 56 trials with target Hb at more than 13 g/L were excluded. The final meta-analysis included eight clinical trials (n=2,387) (Figure 1). Among the eight trials (22-29), one trial included two treatment arms (25), one compared recombinant erythropoietin (treatment arm) with placebo (n=100) (22), three compared darbepoetin alfa (treatment arm) with placebo (n=1,588) (23,24,29) and four compared epoetin (treatment arm) with placebo (n=699) (25-28). Three out the eight trials were conducted in patients with solid tumors, one in patients with hematological malignancies, and four in mixed patients with either solid or hematological malignancies. The patients in the trials received chemotherapy either before the recruitment or during the treatment period. Brief information of the eight included studies is summarized in Table 1.
Table 1. Summary of the included studies.
| Study | Patients | Age (Y)* | Mean Hb (g/dL)** | Baseline Hb (g/dL) | Target Hb (g/dL) | Type of malignancy [n] | Treatment agent and duration | Iron-use |
|---|---|---|---|---|---|---|---|---|
| Cascinu et al. [1994] (22) | 100 | NA | 8.6:8.7 | <9 | 12 | Solid [96], hemotologic [4] | rHuEPO 100 U/kg; 9 w | Yes |
| Gordon et al. [2008] (23) | 218 | 70:70 | 10.1:10.2 | <11 | 13 | Solid [157], hematological [44], mixed [17] | Darbepoetin alfa 6.75 μg/kg Q4w; 17 w | Yes |
| Smith et al. [2008] (29) | 985 | 64.3:64.0 | 9.48:9.49 | <11 | 13 | Solid [908], mixed [77] | Darbepoetin alfa 6.75 μg/kg Q4w; 116 w | No |
| Hernandez et al. [2009] (24) | 386 | 63.6:64.5 | 10.0:10.1 | <11 | 13 | Solid [292], hemotological [53], mixed [41] | Darbepoetin alfa 300 mg Q3W; 16 w | Yes |
| Tjulandin et al. [2010] (25) | 223 | 56.0±10.9 | 9.4±1.2 | <11 | 13 | Solid [223] | Epoetin theta, 20,000 IU qw, epoetin beta 150 IU/kg tiw; 12 w | Yes |
| Fujisaka et al. [2011] (26) | 181 | 67:63.5 | NA | 8/10 | 12 | Solid [158], mixed [23] | Epoetin beta 36,000 IU qw; 54 w | Yes |
| Tjulandin et al. [2011] (27) | 186 | 56.3±14.5 | 9.1:9.2 | 11 | 13 | Solid [138], hemotological [48] | Epoetin theta 20,000 IU qw; 12 w | Yes |
| Cabanillas et al. [2012] (28) | 109 | 41:42:00 | 09:08.9 | 10 | 10 | Solid [2], hematological [107] | Epoetin alpha (<18 y): 600 U/kg qw; (>18 y): 40,000 U qw; 5 w | No |
*, age is expressed as the means of ESA treatment group to control group, or means of all recruited patients; **, mean Hb level at the time of recruitment is expressed as the means of ESA treatment to control group, or means of all patients. rHuEPO, recombinant human erythropoietin; NA, not available.
Hemoglobin (Hb) response
Hb response was defined as Hb increase of more than 2 g/dL from the baseline or Hb level reaching to 12 g/dL during the 4-week treatment. The results of the analysis demonstrated significantly higher Hb in the ESA groups than in the placebo controls [OR 7.85, 95% confidence interval (CI): 5.85 to 10.53, I2 =0%, P<0.001] (Figure 3).
Figure 3.
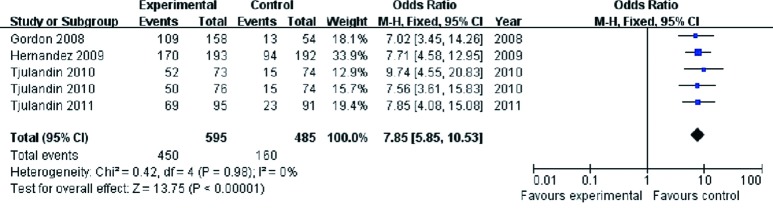
Hb response of cancer patients with anemia treated with ESAs and placebo. Hb, hemoglobin; ESAs, erythropoiesis-stimulating agents.
RBC transfusion
In most of the included trials, Hb at <8.0 or 8.5 g/dL was used as the point to initiate RBC transfusion. The analysis revealed significantly lower frequency of RBC transfusion in the ESA group vs. the placebo control (OR 0.52, 95% CI: 0.42 to 0.65, I2 =50%, P<0.001) (Figure 4). The analysis revealed moderate heterogeneity: one trial (Smith et al. 2008) contributed 34.2% weight to the analysis. Re-analyzing the data after removing this trial (Smith et al. 2008) still showed lower RBC transfusion rate in the ESA than the controls (OR 0.40, 95% CI: 0.31 to 0.53).
Figure 4.
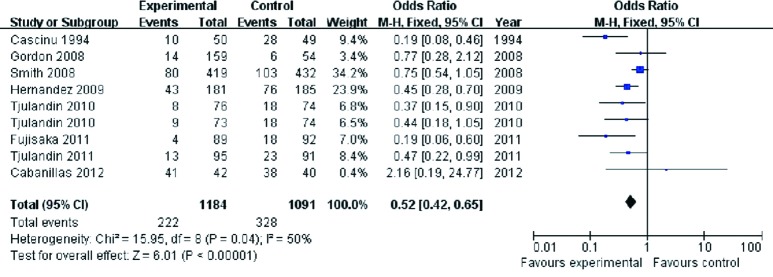
RBC transfusion of cancer patients with anemia treated with ESAs and placebo. RBC, red blood cell; ESAs, erythropoiesis-stimulating agents.
On-study mortality
Our results failed to find significant difference in on-study mortality between the ESA treatment and placebo groups (OR 1.09, 95% CI: 0.87 to 1.38, P=0.45) (Figure 5). There was moderate heterogeneity (the Cochran’s Q test P=0.07; I2 =47%). One trial (29) contributed 52.7% weight to the analysis. Exclusion of this trial also failed to show a statistically significant difference between the two groups (RR 0.72, 95% CI: 0.50 to 1.04; P=0.08).
Figure 5.
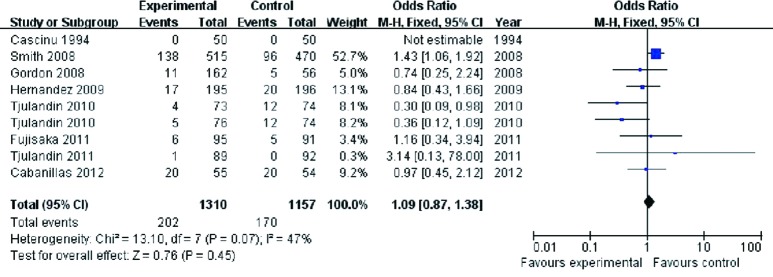
On-study mortality of cancer patients with anemia treated with ESAs and placebo. ESAs, erythropoiesis-stimulating agents.
Three trials were (Cascinu 1994, Fujisaka 2011, Cabanillas 2012) were included in a sub-group analysis of only trials with target Hb at less than 12 g/dL. Such a subgroup analysis revealed practically identical on-study mortality between the two groups (OR 1.02, 95% CI: 0.53 to 1.97, I2 =0%, P=0.95).
Venous thromboembolism (VTE)
Five trials compared the frequency of VTE. A meta-analysis failed to show a statistically significant difference in the rate of VTE (OR 1.58, 95% CI: 0.88 to 2.82, I2 =0%, P=0.13) (Figure 6).
Figure 6.
Venous thromboembolism of cancer patients with anemia treated with ESAs and placebo. ESAs, erythropoiesis-stimulating agents.
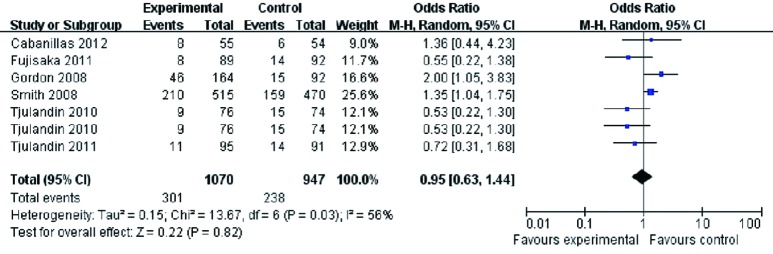
A comparison of the accumulated adverse events did not show a statistically significant difference between the two groups (OR 0.95, 95% CI: 0.63 to 1.44, P=0.82) (Figure 7). The Cochran’s Q test and I2 test indicated a moderate degree of variability (P=0.03 and I2 =56%, respectively).
Figure 7.
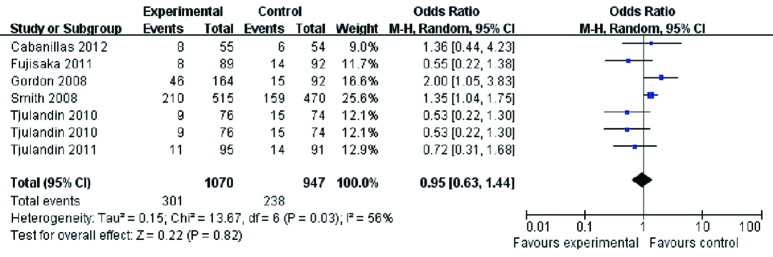
Accumulated adverse events of cancer patients with anemia treated with ESAs and placebo. ESAs, erythropoiesis-stimulating agents.
Quality of life
The quality of life did not differ significantly between the two groups (Table 2).
Table 2. Quality of life scores of the included trials.
| Study | Quality of life score | Mean baseline score |
Mean increase |
Results | |||
|---|---|---|---|---|---|---|---|
| Treatment | Control | Treatment | Control | ||||
| Cascinu et al. [1994] (22) | NA | NA | NA | NA | NA | No significant difference | |
| Gordon et al. [2008] (23) | FACT- F | 30 | 25 | 4.8 | 5 | No significant difference | |
| Smith et al. [2008] (29) | FACT- F | 28.46 | 29.22 | 0.52 | –0.02 | No significant difference | |
| Hernandez et al. [2009] (24) | FACT-F | NA | NA | NA | NA | No significant difference | |
| Tjulandin et al. [2010] (25) | NA | NA | NA | NA | NA | NA | |
| Fujisaka et al. [2011] (26) | FACT-An | NA | NA | 0.3 | –0.99 | No significant difference | |
| Tjulandin et al. [2011] (27) | FACT- F | NA | NA | 2.9±7.9 | 0.6±8.8 | No significant difference | |
| Cabanillas et al. [2012] (28) | FACT-An | NA | NA | NA | NA | No significant difference | |
NA, not available.
Discussion
Anemia is one of the frequent complications in 30-90% of cancer patients (30). Anemia affects the quality life of the patients, and is also associated with increased morbidity and mortality (31). Currently, RBC transfusion and ESAs are routine approaches to treat anemia. However, several clinical trials suggested that ESA may be harmful for the patients if not used properly (17,32).
Major concerns of ESA use include possible cancer progression, increased risk of VTE and increased mortality (14,32). In a meta analysis of 20,102 patients, Tonia et al. concluded that ESA treatment significantly increases mortality during the active study period and decreases overall survival (14). VTE and mortality rate have been reported to increase in patients receiving ESA treatment irrespective of the forms of ESAs as compared to RBC transfusion alone (32). However, a number of studies reached distinct conclusion that ESAs does not significantly affect mortality and cancer progression (33-35).
In the present study, we performed a meta-analysis of eight published trials that compared ESA vs. placebo in cancer patients with baseline Hb at less than 11 g/dL and target Hb at no more than 13 g/dL. The results (Figures 3,4,5,6,7) indicated that ESAs could increase Hb levels and reduce the frequency of RBC transfusion in anemic cancer patients, with no change in on-study mortality, VTE, as well as accumulated adverse events. We believed that such findings could be partly attributed to reduced total ESA dosage and lower target Hb (18,36).
In previous clinical trials showing decreased survival in anemic cancer patients receiving ESAs, patient population was typically not well stratified. For example, the cause of death varies considerably (31,37,38). Also, different forms of erythropoietin might also have distinct biological properties and safety profile (39-41). In line with this notion, one study reported lower two-year survival in darbepoetin alpha treatment group vs. in the controls (35.7% vs. 48.8%) (29). Interestingly, another study concluded that epoetin beta application in anemic cancer patients improved one-year overall survival (42).
Erythropoietin binds to erythropoietin receptor (EPO-R), stimulates proliferation, prevents apoptosis, and induces differentiation of RBC precursors (43). Recent studies of EPO-R mRNA or protein expression in a range of cancer cell lines indicated that ESA could affect cancer cell biology (44,45). Presumably, EPO-R expression may vary among cancer cells of different tissue origins or types. It is conceivable that the benefits vs. risks of ESAs vary among patients with different types of cancers. Therefore, more studies with carefully defined study sample are needed to examine the full-spectrum action of ESAs.
A previous meta-analysis indicated that ESA treatment could improve quality of life in anemic cancer patients (14). In the current study, we did not show a significant improvement in life quality by ESA treatment. The reasons underlying such a major difference remain to be fully investigated. We would like to speculate, as following. ESA treatment clearly could increase Hb, but has been reported to promote cancer progression by some studies (8,10). Expected improvement with increasing Hb by ESA treatment may have been neutralized by expedited cancer progression.
Conclusions
In conclusion, this meta-analysis confirmed that ESA treatment is effective in alleviating anemia in anemic cancer patients. When used in patients with a target Hb at no more than 13 g/dL, ESA treatment is not associated with increased frequency of severe adverse events.
Acknowledgements
This study was partially supported by the Development Projects of Science and Technology of Shandong Province, China (2012GGE27073).
Disclosure: The authors declare no conflict of interest.
References
- 1.Fields SZ, Parshad S, Anne M, et al. Activin receptor antagonists for cancer-related anemia and bone disease. Expert Opin Investig Drugs 2013;22:87-101 [DOI] [PubMed] [Google Scholar]
- 2.Shabaruddin FH, Chen LC, Elliott RA, et al. A systematic review of utility values for chemotherapy-related adverse events. Pharmacoeconomics 2013;31:277-88 [DOI] [PubMed] [Google Scholar]
- 3.Luo W, Nordstrom BL, Fraeman K, et al. Adherence to guidelines for use of erythropoiesis-stimulating agents in patients with chemotherapy-induced anemia: results of a retrospective study of an electronic medical-records database in the United States. Clin Ther 2008;30:2423-35 [DOI] [PubMed] [Google Scholar]
- 4.Ludwig H, Van Belle S, Barrett-Lee P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 2004;40:2293-306 [DOI] [PubMed] [Google Scholar]
- 5.Wauters I, Vansteenkiste J.Darbepoetin alfa in the treatment of anemia in cancer patients undergoing chemotherapy. Expert Rev Anticancer Ther 2012;12:1383-90 [DOI] [PubMed] [Google Scholar]
- 6.Rodgers GM, Becker PS, Blinder M, et al. Cancer- and chemotherapy-induced anemia. J Natl Compr Canc Netw 2012;10:628-53 [DOI] [PubMed] [Google Scholar]
- 7.Aapro M, Spivak JL. Update on erythropoiesis-stimulating agents and clinical trials in oncology. Oncologist 2009;14Suppl 1:6-15 [DOI] [PubMed] [Google Scholar]
- 8.Littlewood TJ, Bajetta E, Nortier JW, et al. Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2001;19:2865-74 [DOI] [PubMed] [Google Scholar]
- 9.Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst 2002;94:1211-20 [DOI] [PubMed] [Google Scholar]
- 10.Hedenus M, Adriansson M, San Miguel J, et al. Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double-blind, placebo-controlled study. Br J Haematol 2003;122:394-403 [DOI] [PubMed] [Google Scholar]
- 11.Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol 2005;23:5960-72 [DOI] [PubMed] [Google Scholar]
- 12.Henke M, Laszig R, Rube C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet 2003;362:1255-60 [DOI] [PubMed] [Google Scholar]
- 13.Bohlius J, Schmidlin K, Brillant C, et al. Erythropoietin or Darbepoetin for patients with cancer--meta-analysis based on individual patient data. Cochrane Database Syst Rev 2009;(3):CD007303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tonia T, Mettler A, Robert N, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev 2012;12:CD003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas G, Ali S, Hoebers FJ, et al. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dL with erythropoietin vs above 10.0 g/dL without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol 2008;108:317-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoskin PJ, Robinson M, Slevin N, et al. Effect of epoetin alfa on survival and cancer treatment-related anemia and fatigue in patients receiving radical radiotherapy with curative intent for head and neck cancer. J Clin Oncol 2009;27:5751-6 [DOI] [PubMed] [Google Scholar]
- 17.Glaspy JA. Erythropoietin in cancer patients. Annu Rev Med 2009;60:181-92 [DOI] [PubMed] [Google Scholar]
- 18.Paladini L, Clark O, Clark L, et al. Erythropoiesis stimulating agents (ESA) for the treatment of chemotherapy induced anemia in patients with hemoglobin levels (Hb)<11 g/dl- a systematic review and meta-analysis. Blood 2008;112:abstr 1305.
- 19.Olivo SA, Macedo LG, Gadotti IC, et al. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther 2008;88:156-75 [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analysis. BMJ 2003;327:557-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salanti G, Amountza G, Ntzani EE, et al. Hardy-weinberg equilibrium in gennetic association studies: An empirical evaluation of reporting, deviations, and power. Eur J Hum Genet 2005;13:840-8 [DOI] [PubMed] [Google Scholar]
- 22.Cascinu S, Fedeli A, Del Ferro E, et al. Recombinant human erythropoietin treatment in cisplatin-associated anemia: a randomized, double-blind trial with placebo. J Clin Oncol 1994;12:1058-62 [DOI] [PubMed] [Google Scholar]
- 23.Gordon D, Nichols G, Ben-Jacob A, et al. Treating anemia of cancer with every-4-week darbepoetin alfa: final efficacy and safety results from a phase II, randomized, double-blind, placebo-controlled study. Oncologist 2008;13:715-24 [DOI] [PubMed] [Google Scholar]
- 24.Hernandez E, Ganly P, Charu V, et al. Randomized, double-blind, placebo-controlled trial of every-3-week darbepoetin alfa 300 micrograms for treatment of chemotherapy-induced anemia. Curr Med Res Opin 2009;25:2109-20 [DOI] [PubMed] [Google Scholar]
- 25.Tjulandin SA, Bias P, Elsasser R, et al. Epoetin Theta in Anaemic Cancer Patients Receiving Platinum-Based Chemotherapy: A Randomised Controlled Trial. Arch Drug Inf 2010;3:45-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujisaka Y, Sugiyama T, Saito H, et al. Randomised, phase III trial of epoetin-beta to treat chemotherapy-induced anaemia according to the EU regulation. Br J Cancer 2011;105:1267-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tjulandin SA, Bias P, Elsasser R, et al. Epoetin Theta with a New Dosing Schedule in Anaemic Cancer Patients Receiving Nonplatinum-Based Chemotherapy: A Randomised Controlled Trial. Arch Drug Inf 2011;4:33-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cabanillas ME, Kantarjian H, Thomas DA, et al. Epoetin alpha decreases the number of erythrocyte transfusions in patients with acute lymphoblastic leukemia, lymphoblastic lymphoma, and Burkitt leukemia/lymphoma: results of a randomized clinical trial. Cancer 2012;118:848-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith RE, Jr, Aapro MS, Ludwig H, et al. Darbepoetin alpha for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: results of a phase III, multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol 2008;26:1040-50 [DOI] [PubMed] [Google Scholar]
- 30.Knight K, Wade S, Balducci L.Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med 2004;116Suppl 7A:11S-26S [DOI] [PubMed] [Google Scholar]
- 31.Caro JJ, Salas M, Ward A, et al. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer 2001;91:2214-21 [PubMed] [Google Scholar]
- 32.Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 2008;299:914-24 [DOI] [PubMed] [Google Scholar]
- 33.Glaspy J, Crawford J, Vansteenkiste J, et al. Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes. Br J Cancer 2010;102:301-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedenus M, Osterborg A, Tomita D, et al. Effects of erythropoiesis-stimulating agents on survival and other outcomes in patients with lymphoproliferative malignancies: a study-level meta-analysis. Leuk Lymphoma 2012;53:2151-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aapro M, Jelkmann W, Constantinescu SN, et al. Effects of erythropoietin receptors and erythropoiesis-stimulating agents on disease progression in cancer. Br J Cancer 2012;106:1249-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang RY, Lee J, Lee YH, et al. Impact of erythropoiesis-stimulating agents on red blood cell transfusion in Korea. Int J Clin Pharm 2012;34:651-7 [DOI] [PubMed] [Google Scholar]
- 37.Vadhan-Raj S, Zhou X, Sizer K, et al. Impact of safety concerns and regulatory changes on the usage of erythropoiesis-stimulating agents and RBC transfusions. Oncologist 2010;15:1359-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boone JD, Fauci JM, Walters CL, et al. The effect of the APPRISE mandate on use of erythropoiesis-stimulating agents and transfusion rates in patients with ovarian cancer receiving chemotherapy. Int J Gynecol Cancer 2013;23:367-71 [DOI] [PubMed] [Google Scholar]
- 39.Halstenson CE, Macres M, Katz SA, et al. Comparative pharmacokinetics and pharmacodynamics of epoetin alfa and epoetin beta. Clin Pharmacol Ther 1991;50:702-12 [DOI] [PubMed] [Google Scholar]
- 40.Storring PL, Tiplady RJ, Gaines Das RE, et al. Epoetin alfa and beta differ in their erythropoietin isoform compositions and biological properties. Br J Haematol 1998;100:79-89 [DOI] [PubMed] [Google Scholar]
- 41.Glaspy J, Henry D, Patel R, et al. Effects of chemotherapy on endogenous erythropoietin levels and the pharmacokinetics and erythropoietic response of darbepoetin alfa: a randomised clinical trial of synchronous versus asynchronous dosing of darbepoetin alfa. Eur J Cancer 2005;41:1140-9 [DOI] [PubMed] [Google Scholar]
- 42.Ohashi Y, Uemura Y, Fujisaka Y, et al. Meta-analysis of epoetin beta and darbepoetin alfa treatment for chemotherapy-induced anemia and mortality: Individual patient data from Japanese randomized, placebo-controlled trials. Cancer Sci 2013;104:481-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osterborg A, Aapro M, Cornes P, et al. Preclinical studies of erythropoietin receptor expression in tumour cells: impact on clinical use of erythropoietic proteins to correct cancer-related anaemia. Eur J Cancer 2007;43:510-9 [DOI] [PubMed] [Google Scholar]
- 44.Sinclair AM, Rogers N, Busse L, et al. Erythropoietin receptor transcription is neither elevated nor predictive of surface expression in human tumour cells. Br J Cancer 2008;98:1059-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paragh G, Kumar SM, Rakosy Z, et al. RNA interference-mediated inhibition of erythropoietin receptor expression suppresses tumor growth and invasiveness in A2780 human ovarian carcinoma cells. Am J Pathol 2009;174:1504-14 [DOI] [PMC free article] [PubMed] [Google Scholar]


