Abstract
Semaphorins were originally identified as axon guidance factors involved in the development of the neuronal system. However, accumulating evidence indicates that several members of semaphorins, so-called ‘immune semaphorins', are crucially involved in various phases of immune responses. These semaphorins regulate both immune cell interactions and immune cell trafficking during physiological and pathological immune responses. Here, we review the following two functional aspects of semaphorins and their receptors in immune responses: their functions in cell–cell interactions and their involvement in immune cell trafficking.
Keywords: semaphorin, immune regulation, immune cell trafficking, tumorigenesis, autoimmune diseases
Introduction
Increasing evidence indicates that the nervous and immune systems have considerable overlap and links.1 For example, some axon guidance molecules, such as slits2, 3, 4 and ephrins,5, 6, 7, 8 have been shown to regulate immune cell migration. In addition, T-cell-antigen-presenting cell contact sites, the so-called ‘immunological synapse', is structurally similar to the ‘neurological synapse' that connects pairs of neurons. These shared molecules and interactions play critical roles in inducing proper immune responses.
Semaphorins were named for their properties that are analogous to the system of flags and lights that is used in rail and maritime communication. They were initially identified as repulsive axon guidance molecules that were required to direct neuronal axons to their appropriate targets.9 More than 20 types of semaphorins have been identified,10 and they have diverse functions in many physiological process,11 including cardiogenesis,12, 13 angiogenesis,14, 15 vasculogenesis,16 tumor metastasis,17, 18, 19 osteoclastogenesis20 and immune regulation.21, 22 In this review, we focus on two functional aspects of semaphorins, their roles in immune cell–cell interactions and immune cell trafficking. In addition, we discuss current perspectives on ‘immune semaphorin' research, including its application for immunological disorders.
Semaphorins and their receptors
Semaphorins are secreted and membrane-associated proteins that are characterized by a conserved extracellular amino-terminal ‘Sema' domain. Based on their C-terminal structures, this diverse group of proteins has been further divided into eight subclasses. Semaphorins in classes I (invertebrate) and IV–VII are membrane-associated, whereas those in classes II (invertebrate), III and VIII (virally encoded) are secreted.10, 23 Two groups of proteins, plexins and neuropilins (NPs), have been identified as the primary semaphorin receptors. Most membrane-bound semaphorins directly bind plexins, whereas class III semaphorins require NPs as obligate coreceptors.24, 25, 26 However, recent reports have suggested that semaphorin receptor usage is more complex than previously thought. For example, Sema3E signals independently of NPs through plexin-D1,16 while Sema7A uses integrins to exert its functions in both the nervous and immune systems.27, 28 In addition, two molecules unrelated to plexins and NPs, CD7229 and T-cell immunoglobulin and mucin domain-containing protein 2 (TIM-2),30 functionally interact with Sema4D and Sema4A, respectively, in the immune system (Figure 1).
Figure 1.
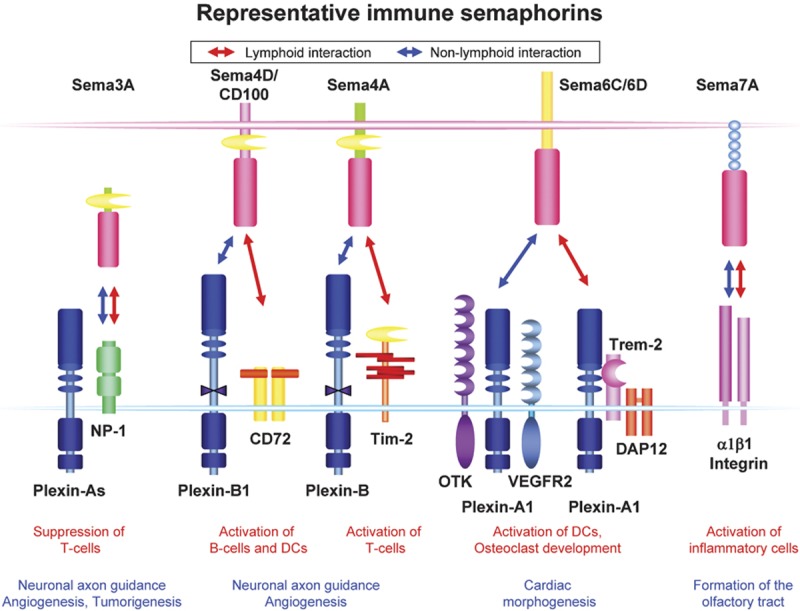
Representative immune semaphorins and their receptors in lymphoid and non-lymphoid cells. Sema3A binds to neuropilin-1 with high affinity to assemble a NP-1/plexin-A1 receptor complex and involves in the axon guidance events. Sema4D binds to plexin-B1 in the brain and transduces chemorepulsive signals. In the immune system, Sema4D uses CD72 as a functional receptor in B cells and DCs and enhances the activation of B cells and DCs. Sema4A binds TIM-2 and is involved in T-cell activation and differentiation in the immune system. In the non-immune system, however, Sema4A recognizes plexin-B proteins and plexin-D1. Sema6D exerts different biological activities through plexin-A1, depending on its coreceptors. During chick embryogenesis, plexin-A1 differentially associates with off-track and VEGFR2, and these receptor complexes have distinct functions in heart development. In the immune system, plexin-A1 forms a receptor complex with TREM-2 and DAP12 and, after Sema6D binds, this complex transduces signals that stimulate DCs and osteoclasts. Sema7A uses β1 integrin as receptors in both the nervous and immune systems. In the immune system, Sema7A expressed on activated T cells stimulates macrophages through α1β1 integrin to promote inflammatory responses. DC, dendritic cell; DAP12, DNAX-activating protein 12; NP-1, neuropilin-1; OTK, off-track kinase; TIM-2, T-cell immunoglobulin and mucin domain-containing protein 2; TREM-2, triggering receptor expressed on myeloid cells 2; VEGFR2, vascular endothelial growth factor receptor 2.
Plexins are canonical semaphorin receptors with a large cytoplasmic region. In the nervous system, semaphorin–plexin signaling has been shown to mediate diverse neural functions by regulating GTPase activities and cytoplasmic/receptor-type protein kinases.11, 31, 32 These plexin-mediated signals are involved in integrin-mediated attachment,15, 33, 34 actomyosin contraction35, 36, 37, 38 and microtubule destabilization.39, 40, 41 In addition, plexins can associate with different coreceptors in distinct tissues to allow semaphorins to exert pleiotropic functions. For instance, plexin-A1 is associated with the tyrosine kinase receptors off-track and vascular endothelial growth factor receptor 2 in heart morphogenesis.42 On the other hand, plexin-A1 forms a receptor complex with triggering receptor expressed on myeloid cell (TREM)-2/DNAX-activating protein 12 (DAP12) in osteoclastogenesis.20 Furthermore, plexin-B1 has been shown to associate with the receptor tyrosine kinases Met and ErbB2, triggering invasive growth of epithelial cells.19, 43
Involvement of ‘immune semaphorins' in cell–cell interactions
Sema4D: a semaphorin involved in B-cell/dendritic cell activation
Sema4D, also known as CD100, is the first semaphorin protein that was determined to have immunoregulatory functions. In the immune system, Sema4D is expressed in T cells, activated B cells and mature dendritic cells (DCs).29, 44, 45 Sema4D promotes the activation of B cells and DCs to induce antibody production and antigen-specific T cells, respectively.29, 46, 47 Plexin-B1 and CD72 were identified as the Sema4D receptors in the nervous and immune systems.11, 48 CD72 negatively regulates B cells by recruiting the tyrosine phosphatase Src homology phosphatase-1 (SHP1) to its immunoreceptor tyrosine-based inhibitory motifs (ITIM).49, 50 Ligation of Sema4D to CD72 causes SHP1 to dissociate from CD72, resulting in B-cell and DC activation.29 Consistent with this function, Sema4D-deficient mice exhibit impaired antibody production and priming of antigen-specific T cells.46, 47 In particular, Sema4D is crucially involved in T-cell-mediated neurological inflammatory diseases. Sema4D-deficient mice are resistant to experimental autoimmune encephalomyelitis (EAE) due to impaired antigen-specific T-cell responses in the draining lymph nodes and attenuated inflammation in the central nervous system.51 In addition, T-cell-derived Sema4D has been implicated in the collapse of process extension of immature oligodendrocytes and the death of immature neural cells in the spinal cords of patients with human T-cell lymphotropic virus type 1-associated myelopathy52 (Table 1).
Table 1. Immune semaphorins, their receptors and diseases.
| Semaphorins/receptors | Expression | Binding partner | Activities | Related diseases |
|---|---|---|---|---|
| Semaphorin | ||||
| Sema3A | T cells | Plexin-A proteins | Inhibition of monocyte migration | Atopic dermatitis |
| Tumor cells | Inhibition of T-cell activation | Cancer | ||
| Endothelial cells | Inhibition of tumor angiogenesis | |||
| Sema4A | Dendritic cells | Plexin-B proteins | T-cell activation | EAE |
| Activated T cells | Plexin-D1 | Promotion of Th1 differentiation | Atopic dermatitis | |
| Th1 cells | TIM-2 | |||
| Sema4D | T cells | Plexin-B1 | B-cell activation | EAE |
| Activated B cells | CD72 | DC activation | HAM | |
| Dendritic cells | Microglial activation | |||
| Injury of oligodendrocytes | ||||
| Sema6D | T cells | Plexin-A1 | DC activation | EAE |
| B cells | Production of type I interferon | Osteopetrosis | ||
| NK cells | Differentiation of osteoclast | Nasu–Halora disease | ||
| Sema7A | Activated T cells | Plexin-C1 | Monocyte/macrophage activation | Contact hypersensitivity |
| Integrin α1β1 | EAE | |||
| Pulmonary fibrosis | ||||
| Receptor | ||||
| Neuropilin-1 | T cells | Class III semaphorins | Inhibition of T-cell activation | Cancer |
| Treg cells | VEGF | Tumor angiogenesis | ||
| Tumor cells | ||||
| Endothelial cells | ||||
| Plexin-A1 | Dendritic cells | Class VI semaphorins | DC activation | EAE |
| Plasmacytoid DCs | Production of type I interferon | Osteopetrosis | ||
| (Osteoclasts) | Differentiation of osteoclast | Nasu–Hakola disease | ||
| Plexin-A4 | T cells | Class VI semaphorins | Inhibition of T-cell activation | EAE |
| Dendritic cells | ||||
| Macrophages | ||||
| Plexin-B1 | Microglia | Class IV semaphorins | Microglial activation | EAE |
| Oligodendrocytes | Injury of oligodendrocytes | HAM | ||
| TIM-2 | Activated T cells | Sema4A | T-cell activation | EAE |
| Th2 cells | Airway atopy | |||
| CD72 | B cells | Sema4D | B-cell activation | |
| (Dendritic cells) | DC activation | |||
| Integrin α1β1 | Monocytes | Sema7A | Monocyte/macrophage activation | EAE |
| Macrophages | Pulmonary fibrosis |
Abbreviations: DC, dendritic cell; EAE, experimental autoimmune encephalomyelitis; HAM, HTLV-1-associated myelopathy; NK, natural killer; Th1, T-helper type 1; Th2, T-helper type 2; TIM-2, T-cell immunoglobulin and mucin domain-containing protein 2; Treg, regulatory T cell; VEGF, vascular endothelial growth factor.
Sema4A: a semaphorin involved in T-cell activation/differentiation
Sema4A, a class IV semaphorin, plays important roles in the immune system. Sema4A is constitutively expressed in DCs and induced in polarized T-helper type 1 (Th1) cells.30, 53 DC-derived Sema4A is crucial for antigen-specific T-cell priming via T cell–DC-cognate cell interactions, while T cell-derived Sema4A is involved in helper T-cell differentiation via T cell–T cell cognate cell interactions. Indeed, Sema4A-deficient mice have impaired Th1 responses to heat-killed Propionibacterium acnes, a Th1-inducing bacteria. Conversely, Sema4A-deficient mice show enhanced T-helper type 2 (Th2) responses against Nippostrongylus brasiliensis, a Th2-inducing intestinal nematode.53 TIM-2, a negative regulator of Th2 cells,54 has been suggested to serve as a functional receptor for Sema4A.30 Consistent with these findings, TIM-2 is preferentially upregulated on Th2 cells.55 Furthermore, Sema4A has been suggested to have several binding partners in addition to TIM-2, and members of plexin-B and plexin-D1 have also been shown to bind to Sema4A.14
Sema4A is also involved in T-cell-mediated autoimmune diseases through mechanisms that are distinct from Sema4D. Indeed, a Sema4A deficiency results in attenuated development of autoimmune myocarditis.56 In addition, Sema4A-deficient mice on a Th2-prone BALB/c background spontaneously develop atopic dermatitis (unpublished data). These results provide further support that Sema4A is physiologically and pathologically involved in the differentiation of helper T cells.
Sema6D and plexin-A1: an interaction involved in the T cell/DC interface
Plexin-A1 is one of the primary semaphorin receptors whose function has been extensively investigated. Class III semaphorins bind to NP-1 and then form a receptor complex with plexin-A1.25 Additionally, plexin-A1 serves as a direct binding receptor for class VI semaphorins, Sema6C and Sema6D.42, 57
In the immune system, plexin-A1 is specifically expressed in DCs, where it mediates the activation of T cells and the production of type I interferon.20, 58, 59 The generation of antigen-specific T cells is impaired in plexin-A1−/− mice.20 Sema6D, which is expressed in T cells, B cell and natural killer cells, was identified as a putative ligand for plexin-A1.20 Indeed, recombinant Sema6D protein binds to and activates DCs and increases type I interferon production. Plexin-A1 forms a receptor complex with the TREM family of proteins and the adaptor molecule DAP12.20, 58 Both DAP12-deficient and plexin-A1-deficient mice not only develop osteopetrosis20, 60, 61 but also are resistant to EAE.62 Interestingly, genetic mutations in human DAP12 or TREM-2 result in a bone-fracture syndrome called Nasu–Hakola disease, further suggesting that plexin-A1 physiologically associates with the TREM/DAP12 complex and that this interaction is relevant to these diseases.
Sema7A: a semaphorin involved in inflammatory responses via T cell–macrophage interactions
Sema7A, also known as CD108, is a membrane-associated glycosylphosphatidylinositol-linked protein.63 In the immune system, Sema7A is induced on activated T cells.27 Sema7A contains an arginine–glycine–aspartate in its Sema domain that is a well-conserved integrin-binding motif.28 Recombinant Sema7A protein stimulates monocytes/macrophages through α1β1 integrin, inducing proinflammatory cytokine production.27 Furthermore, Sema7A receptor usage in the immune system is consistent with that in olfactory nerve outgrowth.28 Sema7A is also involved in pathogenic immune responses. Sema7A-deficient mice are resistant to inflammation, including hapten-induced contact hypersensitivity and EAE.27 In addition, Sema7A plays an important role in the pathogenesis of bleomycin-induced pulmonary fibrosis by regulating transforming growth factor-β signaling.64
NP-1: a class III semaphorin and vascular endothelial growth factor receptor that is necessary to regulate immune responses and tumor angiogenesis
As described above, NP-1 was originally identified as a cell surface glycoprotein that functions as a class III semaphorin receptor.65 In addition, NP-1 is also a receptor for vascular endothelial growth factor (VEGF) in both endothelial cells (ECs) and tumor cells.66 In the immune system, NP-1 is expressed in DCs and T cells,67 where it negatively regulates immune responses. It is also noteworthy that NP-1 plays a key role in tumor angiogenesis through interactions with VEGF.68
NP-1 in CD4+CD25+ regulatory T cells
NP-1 has been shown to help initiate primary immune responses through homophilic interactions at the contact sites between T cells and DCs.67 In addition, NP-1 was identified as a specific marker for CD4+CD25+ regulatory T cells (Tregs).69 Recently, one report suggested that NP-1 in Tregs contributes to prolong contact between Tregs and DCs, resulting in the inhibition of T-cell activation at steady state.70 These findings suggest that NP-1 in Tregs exerts suppressive functions on Tregs, presumably by mediating Treg stop signals on DCs.
NP-1 in effector T cells
Several lines of evidence suggest that Sema3A/NP-1/plexin-A4 functions in the immune system.71, 72 Sema3A is expressed in T cells, while plexin-A4 is expressed in various cells, including T cells, DCs and macrophages. Both NP-1-mutant T cells, in which the Sema3A binding site is specifically disrupted, and plexin-A4-deficient T cells, exhibit enhanced in vitro proliferation after anti-CD3 antibody stimulation.71 Moreover, plexin-A4-deficient mice have enhanced T-cell priming and exacerbated T cell-mediated immune responses such as EAE,71 implying that the Sema3A/NP-1/plexin-A4 interactions are pathologically relevant.
NP-1 in tumor angiogenesis
Tumor progression and dissemination depend not only on the intrinsic properties of cancer cells but also on the tumor microenvironment.73 NP-1 is also expressed by various kinds of human tumor-cell lines and neoplasms.74 Clinical studies suggest that NP-1 plays a role in tumor growth and disease progression due to mediating VEGF signals.75, 76 Recent studies have shown that semaphorins are secreted from tumor cells as well as macrophages and fibroblasts in the tumor microenvironment, thereby influencing cancers and their microenvironments. For instance, Sema3A is secreted from tumors or ECs and suppresses the adhesion and migration of tumor cells and ECs by modulating integrin activities. In addition, Sema3A can inhibit angiogenesis in vivo.77, 78 Similarly, Sema3F inhibits cell spreading and migration in breast carcinoma, melanoma and ECs, resulting in reduced metastatic dissemination.78, 79, 80 Furthermore, since NP-1 is a receptor for both class III semaphorins and VEGF, class III semaphorins may function as antiangiogenic factors by competitively interfering with VEGF receptors.68
Role of semaphorins in immune cell trafficking
In the nervous and cardiovascular systems, semaphorin–plexin signaling regulates cytoskeletal dynamics by activating GTPases, resulting in the modulation of integrin-mediated cell adhesion and actomyosin contractility.32 In this context, it is possible that semaphorins also regulate immune cell trafficking using similar machinery. In addition, it has recently emerged that several semaphorins are involved in immune cell trafficking in both primary and secondary lymphoid organs, although these findings are still preliminary.
Semaphorins in the thymus
The thymus is an organ that supports T-cell differentiation and selection, where interactions with the thymic environment promote the dynamic relocalization of developing lymphocytes.81 The development of thymocytes is regulated by chemokines, sphingosine-1-phosphates, adhesion molecules and cell–cell interactions between thymocytes and thymic epithelial cells or DCs.81 In addition, it has been shown that some chemorepellent molecules, including semaphorins and ephrins, affect thymocyte differentiation during their development.82, 83, 84
Sema3E, which interacts with plexin-D1 in an NP-1-independent manner, was recently reported to participate in thymocyte development.82 Plexin-D1 expression is high in CD4+CD8+ thymocytes (double-positive, DP) but decreased in single-positive cells. Furthermore, its ligand, Sema3E, is preferentially expressed in the medulla rather than in the cortex. Sema3E binds to positively selected CD69+ DP cells and inhibits their CCR9-mediated migration towards corticomedullary junctions. Indeed, fetal liver cell transfer using plexin-D1-deficient embryos showed that CD69+ DP thymocytes are abundantly localized in the cortex and that the boundary of DP and single-positive thymocytes at the corticomedullary junction is disrupted. A similar phenotype was observed in Sema3E-deficient mice, suggesting that the development of thymocytes within the thymus is controlled by Sema3E/plexin-D1 signaling.
Semaphorins in immune cell migration
Class III semaphorins
Sema3A is reported to inhibit immune cell migration. The responsiveness of human monocytes and T cells to chemokine gradients was inhibited by Sema3A.85, 86 Interestingly, it was also shown that the chemokine responsiveness of T cells was enhanced when Sema3A proteins were applied against chemokine gradients.87 Furthermore, this effect could not be abolished by interfering with the expression of collapsing response-mediator protein 2,87 which mediates Sema3A-induced growth-cone guidance. These observations not only indicate that neuron and leukocyte migration is controlled by different molecular mechanisms but also imply that Sema3A-mediated repulsive signals depend on both cell polarity and the site of Sema3A action during immune cell migration.
Other semaphorins
Unlike soluble secreted class III semaphorins, class IV–VII semaphorins are transmembrane proteins that regulate immune cell activities. It was previously reported that these semaphorins are also involved in immune cell migration. For instance, recombinant soluble Sema4D inhibits spontaneous and chemokine (monocyte chemotactic protein-1)-induced human monocyte migration.86, 88 In addition, Sema7A, which can stimulate monocytes/macrophages to produce inflammatory cytokines through α1β1 integrin,27 has been suggested to function as an attractant for human monocytes.89 Furthermore, a viral semaphorin, A39R, which is a ligand for plexin-C1, inhibits DC integrin-mediated adhesion and chemokine (CCL3)-induced migration through actin cytoskeletal rearrangement.90
Possible mechanisms of semaphorin-guided leukocyte migration
Leukocytes must traffic in order to undergo chemokine-/integrin-mediated adhesion and transmigration across ECs through cytoskeletal rearrangement. Recently, it was reported that migrating leukocytes use both integrin-mediated signals and myosin II-mediated actomyosin contraction based on the environmental demands.91, 92 Although the molecular mechanisms that control semaphorin-mediated immune cell trafficking are still elusive, it is plausible that signaling events are differentially used in immune cell movement in the context of different environments and pathological situations.
In recent years, new devices such as time-lapse video imaging and multiphoton microscopy have become powerful tools that can be used to evaluate cell migration and cell–cell interactions. These new technologies will further elucidate how semaphorins and their receptors regulate immune cell trafficking.
Perspectives
Accumulating evidence indicates that semaphorins and their receptors have distinct biological activities in various phases of immune responses, from immune initiation to terminal inflammatory immune responses. These semaphorins form a family of immunoregulatory molecules that are called ‘immune semaphorins'. Consistent with their proposed roles in immunity, they are pathologically involved in several immune disorders, including autoimmune diseases, allergy and congenital bone diseases. Semaphorins and their receptors are crucially responsible for maintaining immunological homeostasis by regulating and coordinating immune cell communication systems. However, several important issues are still unresolved. First, although semaphorins regulate cell motility and morphology through plexins in the nervous system, it has not been fully elucidated how and to what extent they are involved in the dynamics of immune cell movement, particularly ‘in vivo'. Second, semaphorins have been shown to regulate immune cell responses through cell–cell interactions, but it is still unclear how semaphorin-mediated signaling regulates the interface of these cell–cell interactions. Future and ongoing studies using new technologies will not only clarify the complete picture of these unique families but also identify potential therapeutic targets that can be used to treat several immune disorders.
Acknowledgments
This study was supported by research grants from the JSPS Research Fellowships for Young Scientists (H. Takamatsu), the Ministry of Education, Culture, Sports, Science and Technology of Japan, grants-in-aid from the Ministry of Health, Labor, and Welfare, the program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (A. Kumanogoh), the Target Protein Research Program of the Japan Science and Technology Agency (A. Kumanogoh), Uehara Memorial Foundation (A. Kumanogoh) and Takeda Scientific Foundation (A. Kumanogoh). The authors have no conflicting financial interests.
References
- Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5:575–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- Tole S, Mukovozov IM, Huang YW, Magalhaes MA, Yan M, Crow MR, et al. The axonal repellent, Slit2, inhibits directional migration of circulating neutrophils. J Leukoc Biol. 2009;86:1403–1415. doi: 10.1189/jlb.0609391. [DOI] [PubMed] [Google Scholar]
- Prasad A, Qamri Z, Wu J, Ganju RK. Slit-2/Robo-1 modulates the CXCL12/CXCR4-induced chemotaxis of T cells. J Leukoc Biol. 2007;82:465–476. doi: 10.1189/jlb.1106678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Feng L, Park HT, Havlioglu N, Wen L, Tang H, et al. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature. 2001;410:948–952. doi: 10.1038/35073616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Kabuyama Y, Kamataki A, Homma MK, Kobayashi H, Aota S, et al. Enhancement of lymphocyte migration and cytokine production by ephrinB1 system in rheumatoid arthritis. Am J Physiol Cell Physiol. 2008;294:C189–C196. doi: 10.1152/ajpcell.00314.2007. [DOI] [PubMed] [Google Scholar]
- Hjorthaug HS, Aasheim HC. Ephrin-A1 stimulates migration of CD8+CCR7+ T lymphocytes. Eur J Immunol. 2007;37:2326–2336. doi: 10.1002/eji.200737111. [DOI] [PubMed] [Google Scholar]
- Aasheim HC, Delabie J, Finne EF. Ephrin-A1 binding to CD4+ T lymphocytes stimulates migration and induces tyrosine phosphorylation of PYK2. Blood. 2005;105:2869–2876. doi: 10.1182/blood-2004-08-2981. [DOI] [PubMed] [Google Scholar]
- Sharfe N, Freywald A, Toro A, Dadi H, Roifman C. Ephrin stimulation modulates T cell chemotaxis. Eur J Immunol. 2002;32:3745–3755. doi: 10.1002/1521-4141(200212)32:12<3745::AID-IMMU3745>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- Unified nomenclature for the semaphorins/collapsins Semaphorin Nomenclature Committee. Cell. 1999;97:551–552. doi: 10.1016/s0092-8674(00)80766-7. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Gunput RA, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci. 2008;33:161–170. doi: 10.1016/j.tibs.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Yoshida J, Sugimoto T, Yamamoto M, Makino N, Takamatsu H, et al. Repulsive and attractive semaphorins cooperate to direct the navigation of cardiac neural crest cells. Dev Biol. 2008;321:251–262. doi: 10.1016/j.ydbio.2008.06.028. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Kikutani H. Semaphorin signaling during cardiac development. Adv Exp Med Biol. 2007;600:109–117. doi: 10.1007/978-0-387-70956-7_9. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Yabuki M, Kamei J, Kamei M, Makino N, Kumanogoh A, et al. Semaphorin-4A, an activator for T-cell-mediated immunity, suppresses angiogenesis via Plexin-D1. EMBO J. 2007;26:1373–1384. doi: 10.1038/sj.emboj.7601589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, et al. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- Capparuccia L, Tamagnone L. Semaphorin signaling in cancer cells and in cells of the tumor microenvironment – two sides of a coin. J Cell Sci. 2009;122:1723–1736. doi: 10.1242/jcs.030197. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008;8:632–645. doi: 10.1038/nrc2404. [DOI] [PubMed] [Google Scholar]
- Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D, et al. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol. 2002;4:720–724. doi: 10.1038/ncb843. [DOI] [PubMed] [Google Scholar]
- Takegahara N, Takamatsu H, Toyofuku T, Tsujimura T, Okuno T, Yukawa K, et al. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol. 2006;8:615–622. doi: 10.1038/ncb1416. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat Immunol. 2008;9:17–23. doi: 10.1038/ni1553. [DOI] [PubMed] [Google Scholar]
- Kikutani H, Kumanogoh A. Semaphorins in interactions between T cells and antigen-presenting cells. Nat Rev Immunol. 2003;3:159–167. doi: 10.1038/nri1003. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Kolodkin AL. Semaphorin junction: making tracks toward neural connectivity. Curr Opin Neurobiol. 2003;13:79–89. doi: 10.1016/s0959-4388(03)00003-5. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, et al. Plexin–neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- Winberg ML, Noordermeer JN, Tamagnone L, Comoglio PM, Spriggs MK, Tessier-Lavigne M, et al. Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell. 1998;95:903–916. doi: 10.1016/s0092-8674(00)81715-8. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Okuno T, Yamamoto M, Pasterkamp RJ, Takegahara N, Takamatsu H, et al. Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature. 2007;446:680–684. doi: 10.1038/nature05652. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A, Watanabe C, Lee I, Wang X, Shi W, Araki H, et al. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity. 2000;13:621–631. doi: 10.1016/s1074-7613(00)00062-5. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A, Marukawa S, Suzuki K, Takegahara N, Watanabe C, Ch'ng E, et al. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature. 2002;419:629–633. doi: 10.1038/nature01037. [DOI] [PubMed] [Google Scholar]
- Puschel AW. GTPases in semaphorin signaling. Adv Exp Med Biol. 2007;600:12–23. doi: 10.1007/978-0-387-70956-7_2. [DOI] [PubMed] [Google Scholar]
- Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005;6:789–800. doi: 10.1038/nrm1740. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Yoshida J, Sugimoto T, Zhang H, Kumanogoh A, Hori M, et al. FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat Neurosci. 2005;8:1712–1719. doi: 10.1038/nn1596. [DOI] [PubMed] [Google Scholar]
- Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004;305:862–865. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- Barberis D, Casazza A, Sordella R, Corso S, Artigiani S, Settleman J, et al. p190 Rho-GTPase activating protein associates with plexins and it is required for semaphorin signalling. J Cell Sci. 2005;118:4689–4700. doi: 10.1242/jcs.02590. [DOI] [PubMed] [Google Scholar]
- Swiercz JM, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron. 2002;35:51–63. doi: 10.1016/s0896-6273(02)00750-x. [DOI] [PubMed] [Google Scholar]
- Perrot V, Vazquez-Prado J, Gutkind JS. Plexin B regulates Rho through the guanine nucleotide exchange factors leukemia-associated Rho GEF (LARG) and PDZ-RhoGEF. J Biol Chem. 2002;277:43115–43120. doi: 10.1074/jbc.M206005200. [DOI] [PubMed] [Google Scholar]
- Aurandt J, Vikis HG, Gutkind JS, Ahn N, Guan KL. The semaphorin receptor plexin-B1 signals through a direct interaction with the Rho-specific nucleotide exchange factor, LARG. Proc Natl Acad Sci USA. 2002;99:12085–12090. doi: 10.1073/pnas.142433199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Ohshima T, Yamashita N, Ogawara M, Sasaki Y, Nakamura F, et al. Semaphorin3A signaling mediated by Fyn-dependent tyrosine phosphorylation of collapsin response mediator protein 2 at tyrosine 32. J Biol Chem. 2009;284:27393–27401. doi: 10.1074/jbc.M109.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Cheng C, Uchida Y, Nakajima O, Ohshima T, Yagi T, et al. Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron. 2002;35:907–920. doi: 10.1016/s0896-6273(02)00857-7. [DOI] [PubMed] [Google Scholar]
- Mitsui N, Inatome R, Takahashi S, Goshima Y, Yamamura H, Yanagi S. Involvement of Fes/Fps tyrosine kinase in semaphorin3A signaling. EMBO J. 2002;21:3274–3285. doi: 10.1093/emboj/cdf328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, et al. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 2004;18:435–447. doi: 10.1101/gad.1167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiercz JM, Worzfeld T, Offermanns S. ErbB-2 and met reciprocally regulate cellular signaling via plexin-B1. J Biol Chem. 2008;283:1893–1901. doi: 10.1074/jbc.M706822200. [DOI] [PubMed] [Google Scholar]
- Delaire S, Elhabazi A, Bensussan A, Boumsell L. CD100 is a leukocyte semaphorin. Cell Mol Life Sci. 1998;54:1265–1276. doi: 10.1007/s000180050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougeret C, Mansur IG, Dastot H, Schmid M, Mahouy G, Bensussan A, et al. Increased surface expression of a newly identified 150-kDa dimer early after human T lymphocyte activation. J Immunol. 1992;148:318–323. [PubMed] [Google Scholar]
- Kumanogoh A, Suzuki K, Ch'ng E, Watanabe C, Marukawa S, Takegahara N, et al. Requirement for the lymphocyte semaphorin, CD100, in the induction of antigen-specific T cells and the maturation of dendritic cells. J Immunol. 2002;169:1175–1181. doi: 10.4049/jimmunol.169.3.1175. [DOI] [PubMed] [Google Scholar]
- Shi W, Kumanogoh A, Watanabe C, Uchida J, Wang X, Yasui T, et al. The class IV semaphorin CD100 plays nonredundant roles in the immune system: defective B and T cell activation in CD100-deficient mice. Immunity. 2000;13:633–642. doi: 10.1016/s1074-7613(00)00063-7. [DOI] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- Parnes JR, Pan C. CD72, a negative regulator of B-cell responsiveness. Immunol Rev. 2000;176:75–85. doi: 10.1034/j.1600-065x.2000.00608.x. [DOI] [PubMed] [Google Scholar]
- Pan C, Baumgarth N, Parnes JR. CD72-deficient mice reveal nonredundant roles of CD72 in B cell development and activation. Immunity. 1999;11:495–506. doi: 10.1016/s1074-7613(00)80124-7. [DOI] [PubMed] [Google Scholar]
- Okuno T, Nakatsuji Y, Moriya M, Takamatsu H, Nojima S, Takegahara N, et al. Involvement of Sema4D–Plexin-B1 interactions in the central nervous system for pathogenesis of experimental autoimmune encephalomyelitis J Immunol 2009. in press. [DOI] [PubMed]
- Giraudon P, Vincent P, Vuaillat C, Verlaeten O, Cartier L, Marie-Cardine A, et al. Semaphorin CD100 from activated T lymphocytes induces process extension collapse in oligodendrocytes and death of immature neural cells. J Immunol. 2004;172:1246–1255. doi: 10.4049/jimmunol.172.2.1246. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A, Shikina T, Suzuki K, Uematsu S, Yukawa K, Kashiwamura S, et al. Nonredundant roles of Sema4A in the immune system: defective T cell priming and Th1/Th2 regulation in Sema4A-deficient mice. Immunity. 2005;22:305–316. doi: 10.1016/j.immuni.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Kuchroo VK, Umetsu DT, DeKruyff RH, Freeman GJ. The TIM gene family: emerging roles in immunity and disease. Nat Rev Immunol. 2003;3:454–462. doi: 10.1038/nri1111. [DOI] [PubMed] [Google Scholar]
- Chakravarti S, Sabatos CA, Xiao S, Illes Z, Cha EK, Sobel RA, et al. Tim-2 regulates T helper type 2 responses and autoimmunity. J Exp Med. 2005;202:437–444. doi: 10.1084/jem.20050308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino N, Toyofuku T, Takegahara N, Takamatsu H, Okuno T, Nakagawa Y, et al. Involvement of Sema4A in the progression of experimental autoimmune myocarditis. FEBS Lett. 2008;582:3935–3940. doi: 10.1016/j.febslet.2008.10.040. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Han B, Mendelsohn M, Jessell TM. PlexinA1 signaling directs the segregation of proprioceptive sensory axons in the developing spinal cord. Neuron. 2006;52:775–788. doi: 10.1016/j.neuron.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watarai H, Sekine E, Inoue S, Nakagawa R, Kaisho T, Taniguchi M. PDC-TREM, a plasmacytoid dendritic cell-specific receptor, is responsible for augmented production of type I interferon. Proc Natl Acad Sci USA. 2008;105:2993–2998. doi: 10.1073/pnas.0710351105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AW, Brickey WJ, Taxman DJ, van Deventer HW, Reed W, Gao JX, et al. CIITA-regulated plexin-A1 affects T-cell-dendritic cell interactions. Nat Immunol. 2003;4:891–898. doi: 10.1038/ni960. [DOI] [PubMed] [Google Scholar]
- Kaifu T, Nakahara J, Inui M, Mishima K, Momiyama T, Kaji M, et al. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J Clin Invest. 2003;111:323–332. doi: 10.1172/JCI16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker AB, Hoek RM, Cerwenka A, Blom B, Lucian L, McNeil T, et al. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity. 2000;13:345–353. doi: 10.1016/s1074-7613(00)00034-0. [DOI] [PubMed] [Google Scholar]
- Tomasello E, Desmoulins PO, Chemin K, Guia S, Cremer H, Ortaldo J, et al. Combined natural killer cell and dendritic cell functional deficiency in KARAP/DAP12 loss-of-function mutant mice. Immunity. 2000;13:355–364. doi: 10.1016/s1074-7613(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Yamada A, Kubo K, Takeshita T, Harashima N, Kawano K, Mine T, et al. Molecular cloning of a glycosylphosphatidylinositol-anchored molecule CDw108. J Immunol. 1999;162:4094–4100. [PubMed] [Google Scholar]
- Kang HR, Lee CG, Homer RJ, Elias JA. Semaphorin 7A plays a critical role in TGF-beta1-induced pulmonary fibrosis. J Exp Med. 2007;204:1083–1093. doi: 10.1084/jem.20061273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- Tordjman R, Lepelletier Y, Lemarchandel V, Cambot M, Gaulard P, Hermine O, et al. A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat Immunol. 2002;3:477–482. doi: 10.1038/ni789. [DOI] [PubMed] [Google Scholar]
- Serini G, Maione F, Giraudo E, Bussolino F. Semaphorins and tumor angiogenesis. Angiogenesis. 2009;12:187–193. doi: 10.1007/s10456-009-9138-4. [DOI] [PubMed] [Google Scholar]
- Bruder D, Probst-Kepper M, Westendorf AM, Geffers R, Beissert S, Loser K, et al. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol. 2004;34:623–630. doi: 10.1002/eji.200324799. [DOI] [PubMed] [Google Scholar]
- Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008;28:402–413. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Suzuki K, Okuno T, Ogata T, Takegahara N, Takamatsu H, et al. Plexin-A4 negatively regulates T lymphocyte responses. Int Immunol. 2008;20:413–420. doi: 10.1093/intimm/dxn006. [DOI] [PubMed] [Google Scholar]
- Catalano A, Caprari P, Moretti S, Faronato M, Tamagnone L, Procopio A. Semaphorin-3A is expressed by tumor cells and alters T-cell signal transduction and function. Blood. 2006;107:3321–3329. doi: 10.1182/blood-2005-06-2445. [DOI] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellet-Many C, Frankel P, Jia H, Zachary I. Neuropilins: structure, function and role in disease. Biochem J. 2008;411:211–226. doi: 10.1042/BJ20071639. [DOI] [PubMed] [Google Scholar]
- Guttmann-Raviv N, Kessler O, Shraga-Heled N, Lange T, Herzog Y, Neufeld G. The neuropilins and their role in tumorigenesis and tumor progression. Cancer Lett. 2006;231:1–11. doi: 10.1016/j.canlet.2004.12.047. [DOI] [PubMed] [Google Scholar]
- Bielenberg DR, Pettaway CA, Takashima S, Klagsbrun M. Neuropilins in neoplasms: expression, regulation, and function. Exp Cell Res. 2006;312:584–593. doi: 10.1016/j.yexcr.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Acevedo LM, Barillas S, Weis SM, Gothert JR, Cheresh DA. Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood. 2008;111:2674–2680. doi: 10.1182/blood-2007-08-110205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmann-Raviv N, Shraga-Heled N, Varshavsky A, Guimaraes-Sternberg C, Kessler O, Neufeld G. Semaphorin-3A and semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosis. J Biol Chem. 2007;282:26294–26305. doi: 10.1074/jbc.M609711200. [DOI] [PubMed] [Google Scholar]
- Bielenberg DR, Shimizu A, Klagsbrun M. Semaphorin-induced cytoskeletal collapse and repulsion of endothelial cells. Methods Enzymol. 2008;443:299–314. doi: 10.1016/S0076-6879(08)02015-6. [DOI] [PubMed] [Google Scholar]
- Kessler O, Shraga-Heled N, Lange T, Gutmann-Raviv N, Sabo E, Baruch L, et al. Semaphorin-3F is an inhibitor of tumor angiogenesis. Cancer Res. 2004;64:1008–1015. doi: 10.1158/0008-5472.can-03-3090. [DOI] [PubMed] [Google Scholar]
- Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- Choi YI, Duke-Cohan JS, Ahmed WB, Handley MA, Mann F, Epstein JA, et al. PlexinD1 glycoprotein controls migration of positively selected thymocytes into the medulla. Immunity. 2008;29:888–898. doi: 10.1016/j.immuni.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro D, Garcia-Ceca JJ, Cejalvo T, Jimenez E, Jenkinson EJ, Anderson G, et al. EphrinB1–EphB signaling regulates thymocyte–epithelium interactions involved in functional T cell development. Eur J Immunol. 2007;37:2596–2605. doi: 10.1002/eji.200737097. [DOI] [PubMed] [Google Scholar]
- Munoz JJ, Alfaro D, Garcia-Ceca J, Alonso CL, Jimenez E, Zapata A. Thymic alterations in EphA4-deficient mice. J Immunol. 2006;177:804–813. doi: 10.4049/jimmunol.177.2.804. [DOI] [PubMed] [Google Scholar]
- Ji JD, Park-Min KH, Ivashkiv LB. Expression and function of semaphorin 3A and its receptors in human monocyte-derived macrophages. Hum Immunol. 2009;70:211–217. doi: 10.1016/j.humimm.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaire S, Billard C, Tordjman R, Chedotal A, Elhabazi A, Bensussan A, et al. Biological activity of soluble CD100. II. Soluble CD100, similarly to H-SemaIII, inhibits immune cell migration. J Immunol. 2001;166:4348–4354. doi: 10.4049/jimmunol.166.7.4348. [DOI] [PubMed] [Google Scholar]
- Vincent P, Collette Y, Marignier R, Vuaillat C, Rogemond V, Davoust N, et al. A role for the neuronal protein collapsin response mediator protein 2 in T lymphocyte polarization and migration. J Immunol. 2005;175:7650–7660. doi: 10.4049/jimmunol.175.11.7650. [DOI] [PubMed] [Google Scholar]
- Chabbert-de Ponnat I, Marie-Cardine A, Pasterkamp RJ, Schiavon V, Tamagnone L, Thomasset N, et al. Soluble CD100 functions on human monocytes and immature dendritic cells require plexin C1 and plexin B1, respectively. Int Immunol. 2005;17:439–447. doi: 10.1093/intimm/dxh224. [DOI] [PubMed] [Google Scholar]
- Holmes S, Downs AM, Fosberry A, Hayes PD, Michalovich D, Murdoch P, et al. Sema7A is a potent monocyte stimulator. Scand J Immunol. 2002;56:270–275. doi: 10.1046/j.1365-3083.2002.01129.x. [DOI] [PubMed] [Google Scholar]
- Walzer T, Galibert L, Comeau MR, de Smedt T. Plexin C1 engagement on mouse dendritic cells by viral semaphorin A39R induces actin cytoskeleton rearrangement and inhibits integrin-mediated adhesion and chemokine-induced migration. J Immunol. 2005;174:51–59. doi: 10.4049/jimmunol.174.1.51. [DOI] [PubMed] [Google Scholar]
- Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]


