Abstract
Natural killer (NK) cells play critical roles in host immunity against cancer. In response, cancers develop mechanisms to escape NK cell attack or induce defective NK cells. Current NK cell-based cancer immunotherapy aims to overcome NK cell paralysis using several approaches. One approach uses expanded allogeneic NK cells, which are not inhibited by self histocompatibility antigens like autologous NK cells, for adoptive cellular immunotherapy. Another adoptive transfer approach uses stable allogeneic NK cell lines, which is more practical for quality control and large-scale production. A third approach is genetic modification of fresh NK cells or NK cell lines to highly express cytokines, Fc receptors and/or chimeric tumor-antigen receptors. Therapeutic NK cells can be derived from various sources, including peripheral or cord blood cells, stem cells or even induced pluripotent stem cells (iPSCs), and a variety of stimulators can be used for large-scale production in laboratories or good manufacturing practice (GMP) facilities, including soluble growth factors, immobilized molecules or antibodies, and other cellular activators. A list of NK cell therapies to treat several types of cancer in clinical trials is reviewed here. Several different approaches to NK-based immunotherapy, such as tissue-specific NK cells, killer receptor-oriented NK cells and chemically treated NK cells, are discussed. A few new techniques or strategies to monitor NK cell therapy by non-invasive imaging, predetermine the efficiency of NK cell therapy by in vivo experiments and evaluate NK cell therapy approaches in clinical trials are also introduced.
Keywords: cancer, clinical trial, expansion, immunotherapy, natural killer cell
Introduction
Surgery, chemotherapeutic agents and ionizing radiation have been used for decades as primary strategies to eliminate the tumors in patients; however, the development of resistance to drugs or radiation led to a significant incidence of tumor relapse. Therefore, investigating effective strategies to eliminate these resistant tumor cells is urgently needed. The importance of immune system in malignant diseases has been demonstrated by recent major scientific advances.
Both innate and adaptive immune cells actively prevent neoplastic development in a process called ‘cancer immunosurveillance'. Innate immune cells, including monocytes, macrophages, dendritic cells (DCs) and natural killer (NK) cells, mediate immediate, short-lived responses by releasing cytokines that directly lyse tumor cells or capture debris from dead tumor cells. Adaptive immune cells, including T and B cells, mediate long-lived, antigen-specific responses and effective memory.1 Despite these immune responses, malignant cells can develop mechanisms to evade immunosurveillance. Some tumors protect themselves by establishing an immune-privileged environment. For example, they can produce immunosuppressive cytokines IL-10 and transforming growth factor-β (TGF-β) to suppress the adaptive antitumor immune response, or skew the immune response toward a Th2 response with significantly less antitumor capacity.2,3,4 Some tumors alter their expressions of IL-6, IL-10, vascular epithelial growth factor or granulocyte monocyte-colony stimulating factor (GM-CSF), impairing DC functions via inactivation or suppressing maturation.5 In some cases, induced regulatory T cells suppress tumor-specific CD4+ and CD8+ T-cell responses.6 Tumor cells also minimally express or shed tumor-associated antigens, shed the ligands of NK cell-activating receptor such as the NKG2D ligands UL16-binding protein 2, major histocompatibility complex (MHC) class I chain-related molecules A and B molecules (MICA/MICB) or alter MHC-I and costimulatory molecule expression to evade the immune responses.7,8,9 Malignant cells may also actively eliminate immune cells by activation-induced cell death or Fas ligand (FasL) expression.10,11 In addition, primary cancer treatments like chemotherapy and ionizing radiation can compromise antitumor immune responses by their immunosuppressive side effects.
Tumor cells can be eliminated when immune responses are adequate; when they are not, tumor growth and immunourveillance enter into a dynamic balance until tumor cells evade immunosurveillance, at which point neoplasms appear clinically as a consequence. Therapies designed to induce either a potent passive or active antitumor response against malignancies by harnessing the power of the immune system, known as tumor immunotherapy, is an appealing alternative strategy to control tumor growth. Until now, the cancer immunotherapy field has covered a vast array of therapeutic agents, including cytokines, monoclonal antibodies, vaccines, adoptive cell transfers (T, NK and NKT) and Toll-like receptor (TLR) agonists.1,12,13 Adoptive NK cell transfer in particular has held great promise for over three decades. With progress in the NK cell biology field and in understanding NK function, developing NK cells to be a powerful cancer immunotherapy tool has been achieved in recent years. In this article, we will review recent advances in NK cell-based cancer immunotherapy, focusing on potential approaches and large-scale NK cell expansion for clinical practice, as well as on the clinical trials and future perspectives to enhance the efficacy of NK cells.
Conception of NK cells
NK cells were first identified in 1975 as a unique lymphocyte subset that are larger in size than T and B lymphocytes and contain distinctive cytoplasmic granules.14,15 After more than 30 years, our understanding of NK cell biology and function lends important insights into their role in immunosurveillance. It has been known that NK cells develop in bone marrow (BM) from common lymphoid progenitor cells;16 however, NK cell precursors have still not been clearly characterized in humans.17 After development, NK cells distribute widely throughout lymphoid and non-lymphoid tissues, including BM, lymph nodes (LN), spleen, peripheral blood, lung and liver.18
NK cells, defined as CD3−CD56+ lymphocytes, are distinguished as CD56bright and CD56dim subsets. Approximately 90% of peripheral blood and spleen NK cells belong to the CD56dimCD16+ subset with marked cytotoxic function upon interacting with target cells.19,20 In contrast, most NK cells in lymph nodes and tonsils belong to the CD56brightCD16− subset and exhibit predominantly immune regulation properties by producing cytokines such as interferon (IFN)-γ in response to IL-12, IL-15 and IL-18 stimulation.19,21
NK cells rapidly kill certain target cells without prior immunization or MHC restriction, whose activation is dependent on the balance between inhibitory and activating signals from invariant receptors.22,23,24 The activating receptors include the cytotoxicity receptors (NCRs) (NKp46, NKp30 and NKp44), C-type lectin receptors (CD94/NKG2C, NKG2D, NKG2E/H and NKG2F) and killer cell immunoglobulin-like receptors (KIRs) (KIR-2DS and KIR-3DS), while the inhibitory receptors include C-type lectin receptors (CD94/NKG2A/B) and KIRs (KIR-2DL and KIR-3DL). Since some structural families contain both activating and inhibitory receptors, trying to understand how NK cell activity is regulated is often complicated.25 At steady state, the inhibitory receptors (KIRs and CD94/NKG2A/B), which bind to various MHC-I molecules present on almost all cell types, inhibit NK cell activation and prevent NK cell-mediated killing. Under stress conditions, cells downregulate MHC-I expression, causing NK cells to lose inhibitory signaling and be activated in a process called ‘missing-self recognition'. Additionally, the non-MHC self molecules Clr-b (mouse), LLT-1 (human) and CD48 (mouse) recognized by the inhibitory receptors NKR-P1B, NKR-P1A and 2B4, respectively, also perform this function.26,27 In contrast to the self-expressed inhibitory receptor ligands, NK cell-activating receptors can recognize either pathogen-encoded molecules that are not expressed by the host, called ‘non-self recognition', or self-expressed proteins that are upregulated by transformed or infected cells, called ‘stress-induced self recognition'. For example, mouse Ly49H recognizes cytomegalovirus-encoded m157, and NKG2D recognizes the self proteins human UL16-binding proteins and MICA/MICB.28,29 NK cells identify their targets by recognizing a set of receptors on target cells in an NK-target cell zipper formation; this results in the integration of multiple activating and inhibitory signals, the outcome of which depends on the nature of the interacting cells.26 IFNs or DC/macrophage-derived cytokines, such as type I IFN, IL-12, IL-18 and IL-15, enhance the activation or promote the maturation of NK cells, which can also augment NK cell cytolytic activity against tumor cells.30,31,32 Cytotoxic activity of NK cells can increase approximately 20–200 fold after exposure to IFN-α/β or IL-12. Despite these known innate immune cell functions, accumulating evidence in both mice and humans demonstrates that NK cells are educated and selected during development, possess receptors with antigen specificity, undergo clonal expansion during infection and can generate long-lived memory cells.33,34
After over 30 years of researching NK cells, evidence supports that they play critical roles in the early control of viral infection, in hematopoietic stem cell (HSC) transplantation (improved grafting, graft-vs.-host disease and graft-vs.-tumor), in tumor immunosurveillance and in reproduction (uterine spiral artery remodeling). The roles of NK cells in controlling organ transplantation, parasitic and HIV infections, autoimmunity and asthma have also been suggested, but remain to be explored further.26 In particular, therapeutic strategies harnessing the power of NK cells to target multiple malignancies have been designed.
NK cell-mediated antitumor mechanisms
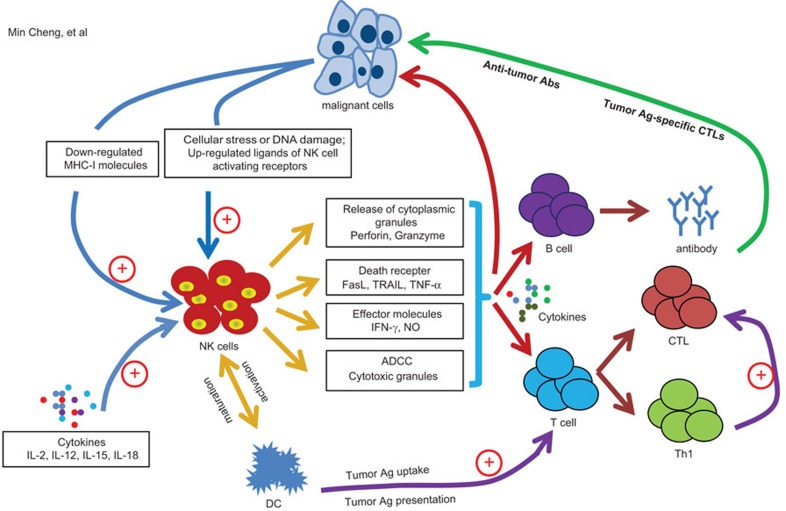
NK cells originally described as large granular lymphocytes, exhibited natural cytotoxicity against certain tumor cells in the absence of preimmunization or stimulation.35,36,37 CD56dim NK cells, which make up the majority of circulating cells, are the most potent cytotoxic NK cells against tumor cells. Evidence gathered from a mouse xenograft tumor model testing functionally deficient NK cells or antibody-mediated NK cell depletion supports that NK cells can eradicate tumor cells.38,39,40,41 An 11-year follow-up study in patients indicated that low NK-like cytotoxicity was associated with increased cancer risk.42 High levels of tumor infiltrating NK cells (TINKs) are associated with a favorable tumor outcome in patients with colorectal carcinoma, gastric carcinoma and squamous cell lung cancer, suggesting that NK-cell infiltration into tumor tissues represents a positive prognostic marker.43,44,45 As described above, NK-cell recognition of tumor cells by inhibitory and activating receptors is complex, and the three recognition models—‘missing-self', ‘non-self' and ‘stress-induced self'—might be used to sense missing- or altered-self cells. Activated NK cells are thus in a position to directly or indirectly exert their antitumor activity to control tumor growth and prevent the rapid dissemination of metastatic tumors by ‘immunosurveillance' mechanisms (Figure 1).
Figure 1.
NK cells in tumor immunosurveillance. The diagram shows the potential roles of NK cells in tumor immunosurveillance. NK cells initially recognize the tumor cells via stress or danger signals. Activated NK cells directly kill target tumor cells through at least four mechanisms: cytoplasmic granule release, death receptor-induced apoptosis, effector molecule production or ADCC. Additionally, NK cells act as regulatory cells when reciprocally interact with DCs to improve their antigen uptake and presentation, facilitating the generation of antigen-specific CTL responses. Also, by producing cytokines such as IFN-γ, activated NK cells induce CD8+ T cells to become CTLs. Activated NK cells can also promote differentiation of CD4+ T cells toward a Th1 response and promote CTL differentiation. Cytokines produced by NK cells might also regulate antitumor Ab production by B cells. Ab, antibody; ADCC, antibody-dependent cellular cytotoxicity; CTL, cytotoxic T lymphocyte; DC, dendritic cell; IFN, interferon; NK, natural killer.
Direct tumor clearance by NK-mediated cytotoxicity
Upon cellular transformation, surface MHC-I expression on tumor cells is often reduced or lost to evade recognition by antitumor T cells. In parallel, cellular stress and DNA damage lead to upregulated expression of ligands on tumor cells for NK cell-activating receptors. Human tumor cells that have lost self MHC-I expression or bear ‘altered-self' stress-inducible proteins are ideal NK cell targets, as NK cells are activated by initially recognizing certain ‘stress' or ‘danger' signals.46 The ‘missing-self' model of tumor cell recognition by NK cells was first demonstrated by observing that MHC-I-deficient syngeneic tumor cells were selectively rejected by NK cells; additionally, NK cell inhibitory receptors were shown to detect this absence of MHC-I expression.47,48,49 NK cells can also kill certain MHC-I-sufficient tumor cells by detecting stress-induced self ligands through their activating receptors. Broad MICA/B expression has been detected on epithelial tumors, melanoma, hepatic carcinoma and some hematopoetic malignancies, representing a counter-measure by the immune system to combat tumor development.31 NK cell-mediated cytotoxicity is also important against tumor initiation and metastasis in vivo.50,51,52
NK cells directly kill target tumor cells through several mechanisms: (i) by releasing cytoplasmic granules containing perforin and granzymes that leads to tumor-cell apoptosis by caspase-dependent and -independent pathways.53,54 Cytotoxic granules reorient towards the tumor cell soon after NK–tumor cell interaction and are released into the intercellular space in a calcium-dependent manner; granzymes are allowed entry into tumor cells by perforin-induced membrane perforations, leading to apoptosis; (ii) by death receptor-mediated apoptosis. Some NK cells express tumor-necrosis factor (TNF) family members, such as FasL or TNF-related apoptosis-inducing ligand (TRAIL), which can induce tumor-cell apoptosis by interacting with their respective receptors, Fas and TRAIL receptor (TRAILR), on tumor cells.55,56,57,58,59 TNF-α produced by activated NK cells can also induce tumor-cell apoptosis;60 (iii) by secreting various effector molecules, such as IFN-γ, that exert antitumor functions in various ways, including restricting tumor angiogenesis and stimulating adaptive immunity.61,62 Cytokine activation or exposure to tumor cells is also associated with nitric oxide (NO) production, where NK cells kill target tumor cells by NO signaling;63,64 (iv) through antibody-dependent cellular cytotoxicity (ADCC) by expressing CD16 to destroy tumor cells.40 The antitumor activity of NK cells can be further enhanced by cytokine stimulation, such as by IL-2, IL-12, IL-18, IL-15 or those that induce IFN production.40,65,66,67,68,69,70
Indirect NK-mediated antitumor immunity
NK cells act as regulatory cells when reciprocally interact with DCs, macrophages, T cells and endothelial cells by producing various cytokines (IFN-γ, TNF-α and IL-10), as well as chemokines and growth factors.26,71 By producing IFN-γ, activated NK cells induce CD8+ T cells to become cytotoxic T lymphocytes (CTLs), and also help to differentiate CD4+ T cells toward a Th1 response to promote CTL differentiation.72,73 NK cell-derived cytokines might also regulate antitumor antibody (Ab) production by B cells.40 In addition, cancer cells killed by NK cells could provide tumor antigens for DCs, inducing them to mature and present antigen.74 By lysing surrounding DCs that have phagocytosed and processed foreign antigens, activated NK cells also could provide additional antigenic cellular debris for other DCs. Thus, activated NK cells promote antitumor immunity by regulating DC activation and maturation,75 as these DCs can facilitate the generation of antigen-specific CTL responses through their ability to cross-present tumor-specific antigens (derived from NK cell-mediated tumor lysis) to CD8+ T cells.76,77
NK cells in tumor immunotherapy
During tumor progression, tumor cells develop several mechanisms to either escape from NK-cell recognition and attack or to induce defective NK cells. These include losing expression of adhesion molecules, costimulatory ligands or ligands for activating receptors, upregulating MHC class I, soluble MIC, FasL or NO expression, secreting immunosuppressive factors such as IL-10, TGF-β and indoleam ine 2,3-d ioxygense (IDO) and resisting Fas- or perforin-mediated apoptosis.31,78,79 In cancer patients, NK cell abnormalities have been observed, including decreased cytotoxicity, defective expression of activating receptors or intracellular signaling molecules, overexpression of inhibitory receptors, defective proliferation, decreased numbers in peripheral blood and in tumor infiltrate, and defective cytokine production.60 Given that NK cells play critical roles in the first-line of defense against malignancies by direct and indirect mechanisms, the therapeutic use of NK cells in human cancer immunotherapy has been proposed and followed in a clinical context (Table 1).
Table 1. NK cells in tumor immunotherapy.
| Administration | Stimulation | Effector | Clinical trial | Limitation | References | |
|---|---|---|---|---|---|---|
| Autologous NK cells | Stimulation with cytokines in vivo;Adoptive transfer after activation/expansion ex vivo | Cytokines: IL-2, IL-12, IL-15, IL-18, IL-21, type I IFNAntibody: KIR Ab | Upregulated adhesion molecules NKp44, perforin, granzymes, FasL, and TRAIL;Enhanced proliferation ability and cytokine production. | Limited activity;metastatic RCC, malignant glioma and breast cancer | Toxicity of systemic cytokine administration;cytokine-activated NK cell apoptosis;suppressed by recognition of self-MHC molecules | [80–100] |
| Allogeneic NK cells | Adoptive transfer after ex vivo activation/expansion;Infusion of unstimulated donor NK cells | IL-15/hydrocortisone;soluble factors, immobilized molecules, cellular activators | Greater tumor killing activity | Safe with minimal toxicity;successful for cancer immunotherapy,including metastatic melanoma, renal cell carcinoma, Hodgkin's disease and poor-prognosis AML; advanced non-small cell lung cancer | Rejection by a patient's immune system | [101–105] |
| NK cells via antibody-dependent cell-mediated cytotoxicity | Systemic administration | Tumor-specific monoclonal antibodies;Altered antibody including class switching, humanization, point mutations;Co-administering cytokines (IL-12, IL-2 and IL-21), TLR agonists (CpG), or agonist antibodies (anti-4-1BB);Antibodies with linked cytokines (Immunocytokines) | Higher cytotoxicity to Ab-coated target cells | CD20-specific mAb (rituximab) in non-Hodgkin's lymphoma patients;HER-2-specific mAb (Trastuzumab/Herceptin) in patients with metastatic breast and gastric carcinomas;Humanized anti-GD2 mAb in melanoma, osteosarcoma, and soft-tissue sarcoma patients | - | [106–124] |
| NK cell lines | Adoptive transfer after ex vivo expansion | Expanded in vitro as necessary | High cytotoxicity to tumor cells; cytokine production | Safe and successful antitumor effectsNK92: advanced malignant melanoma and renal cell carcinoma | Rejection by a patient's immune system | [125–139] |
| Genetic modification of NK cells | Adoptive transfer after genetic modification | Cytokine transgene;Overexpression of activating receptors by genetic modification;Silencing of inhibitory receptor expression by RNA interference;Retargeting NK cells by using a chimeric receptor | Increased tumor-cell killing efficiency;Stronger intracellular signals for activating NK cell cytotoxicity | Successful antitumor effects;IL-2–NK-92; IL-15–NK-92; IL-15–NKL; SCF–NK-92;Anti-HER-2/neu–CD3ξ, anti-CEA–CD3ξ anti-CD33–D3ξ anti-CD19–CD3ξ anti-CD20–CD3ξ | Limited specificity of NK cells via cytokine transgene | [140–150] |
Abbreviations: AML, acute myeloid leukemia; FasL, Fas ligand; IFN, interferon; KIR, killer cell immunoglobulin-like receptors; MHC, major histocompatibility complex; NK, natural killer; RCC, renal cell carcinoma; SCF, stem cell factor; TLR, Toll-like receptor; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
Autologous NK cells
Early studies aimed to improve the antitumor activity of NK cells through activating endogenous NK cells and promoting their proliferation in patients. One major strategy was systemic administration of cytokines such as IL-2, IL-12, IL-15, IL-18, IL-21 and type I IFNs.80,81,82,83,84 Upon cytokine stimulation, NK cells first become lymphokine-activated killer (LAK) cells and exhibit greater cytotoxicity against malignant targets, with upregulated effector molecules, such as adhesion molecules, NKp44, perforin, granzymes, FasL and TRAIL, as well as with enhanced proliferation and cytokine production; however, only limited antitumor activity of LAK cells was observed in cancer patients.85 A poor clinical outcome was also observed, when LAK cells were adoptively transferred into cancer patients in combination with high IL-2 doses, as patients experienced severe life-threatening toxic side effects such as vascular leak syndrome. High IL-2 doses also promote expansion of regulatory T cells that directly inhibit NK-cell functions and induce activation-induced cell death of NK cells.86,87 Another approach using autologous LAK cells in combination with daily administration of low IL-2 doses also resulted in limited clinical success.88 More success was attained when adoptively transferring IL-2-activated LAK cells rather than administering IL-2 systemically.89,90,91 Combining IL-2 and IFN-α with GM-CSF has been shown to be effective, providing a solid basis for using IL-2 to stimulate antitumor activity from endogenous NK cells.60
Other NK cell activators, such as IL-12, IL-15, IL-18 and IL-21, have been successfully tested in preclinical cancer models as part of various vaccination strategies.92,93 In the presence of IL-15 and hydrocortisone (HC), autologous NK cells can be activated and expanded in vitro, and these cells are effective in vivo in a lung metastasis mouse model which accumulated in lung tissue and were retained in the tumor-bearing site for more than 3 days. Extremely high IL-15 doses were necessary to observe any meaningful antitumor effects in vivo. IL-15 was only effective at augmenting NK cell-mediated cytotoxity when presented in trans, as soluble IL-15 at physiological concentrations was not effective. Thus, strategies favoring IL-15 transpresentation to augment NK-mediated immunosurveillance have been proposed.94
There are two important limitations for using cytokines for cancer treatment: toxicity of systemic cytokine administration and cytokine-induced NK-cell apoptosis.95 Adoptively transferring ex vivo expanded and activated autologous NK cells has been evaluated clinically for cancer immunotherapy and was found to greatly improve clinical responses without any obvious adverse side effects in metastatic renal cell carcinoma (RCC), malignant glioma and breast cancer patients.96,97,98 However, these autologous NK cells could not yet exhibit their full cytotoxic capacity in vivo and were not consistently effective in cancer patients;99,100 this may be due to MHC class I expression in cancer patients that suppress autologous NK cells in vivo. Moreover, endogenous NK and LAK cells might be insufficiently cytotoxic to combat advanced tumor cells.40 Therefore, finding ways to overcome autologous NK-cell inhibition by self-HLA molecules is needed to effectively direct autologous NK cells to kill tumor cells. Blocking NK cell expressed inhibitory receptors specific for MHC-I by using anti-KIR Abs can increase NK-cell cytotoxicity against tumor cells, which is currently being tested in a phase I clinical trial in human patients with acute myeloid leukemia (AML).
Allogeneic NK cells
Alloreactive NK cells with KIR mismatch demonstrate greater tumor-killing activity and the ability to better control AML relapse.101,102 Based on the effectiveness of NK-cell alloreactivity in this and other studies, specific criteria for selecting mismatched donors has been established. This convincing clinical evidence also strongly supports a therapeutic role for allogeneic NK cells in controlling human malignancies. Indeed, strategies using adoptively transferred human-mismatched (haploidentical) allogeneic NK cells have been more successful for cancer immunotherapy, including against leukemia and solid cancers, and have been shown to be a safe therapy with minimal toxicity. They can also expand in patients with various malignancies, including metastatic melanoma, renal cell carcinoma, Hodgkin's disease and poor-prognosis AML.103 Adoptive transfer of allogeneic NK cells that were activated and expanded with IL-15/HC in vitro has been demonstrated to be safe and potentially effective in a phase I clinical trial when used in combination with standard chemotherapy in patients with advanced non-small cell lung cancer.104 A disadvantage to this approach is that using KIR mismatched allogeneic NK cells eventually led to immune-mediated rejection due to MHC mismatch.105
ADCC
NK cells express only the activating type IIIA Fc receptor (FcRγIIIa; CD16a) on their surface, which enables NK cells to recognize Ab-coated target cells and trigger NK cell-mediated ADCC, resulting in rapid NK-cell activation and degranulation.1,106 Strong evidence supporting an important role for ADCC comes from anti-CD20 (Rituxumab)-treated non-Hodgkin's lymphoma (NHL) patients as well as from anti-HER2 (Trastuzumab/Herceptin)-treated metastatic breast and gastric carcinoma patients.107,108 Several modifications to alter antibody structure, including class switching, humanization and point mutations to reduce complement activation, have been generated to increase NK-cell ADCC function while reducing antibody-induced toxicity.108,109 Humanized anti-GD2 mAb can stimulate NK cell effectors and simultaneously reduce some toxicity associated with anti-GD2 therapy.110 A CD19-specific mAb with increased FcγRIIIA-binding affinity significantly increased NK cell-mediated ADCC, thus efficiently clearing malignant B cells in cynomolgus macaques in vivo.111,112
The effect of ADCC can be potentiated by coadministering cytokines, TLR agonists or agonist antibodies that activate NK cell receptors. IL-12 increased NK-cell responses to HER2-expressing breast tumor cells when used in combination with Herceptin.113,114 IL-2 also increases ADCC activity against tumor cells for LAK cells.115,116 IL-21 promotes the differentiation of CD56dimCD16+ NK-cell subset, which can potentially direct ADCC.117,118 Combining the TLR agonist CpG with Rituximab increased antitumor NK cell ADCC in a mouse model.119 Activated NK cells by an agonistic antibody to the activating 4-1BB receptor completely regressed subcutaneous murine lymphoma tumors during Rituximab treatment.120 Antibodies containing cytokines linked to their Fc terminus, called immunocytokines (ICs), may wield certain advantages over traditional mAbs.121 ICs enhance synapse formation between the mAb-coated tumor cell and the NK cell by enhancing both Fc and cytokine receptor binding. In several preclinical models, ICs demonstrated much greater antitumor effect than equivalent amounts of naked mAb infused with equivalent cytokine amounts.108,122 Treating with Rituximab combined with an antibody that blocks inhibitory self-recognition or an immunomodulatory agent (Lenalidomide) that upregulates NK-cell activation markers enhanced NK cell-mediated cell lysis.123,124
NK cell lines
Using NK cell lines as the source for therapeutic allogeneic NK cells may be potentially beneficial, as the lack of KIR ligand(s) (recognizing HLA) in the recipient induces NK-cell function.125 Seven established malignant NK cell lines, including NK-92, YT, NKL, HANK-1, KHYG-1, NK-YS and NKG, have been previously reviewed by us.126 Among them, NK-92, KHYG-1, NKL and NKG have been well documented for their antitumor activity, while the other three cell lines YT, NK-YS and HANK-1 are useful for studying the biological characteristics of EBV-associated lymphoma/leukemia.127,128,129,130 NK-92 cells have been demonstrated to be a safe and potentially beneficial therapy with successful antitumor effects, receiving FDA approval for testing in patients with advanced malignant melanoma and renal cell carcinoma.46,131,132,133 NK-92 is currently the only NK cell line that has entered clinical trials and can serve as a platform for studying NK cell-based tumor immunotherapy in the future.
The KHYG-1 cell line is the first human NK leukemia derived from a patient expressing an aberrant p53 gene.130 KHYG-1 cells exhibited greater cytoxicity than NK-92 cells.134 Irradiated KHYG-1 cells with inhibited proliferation does not diminish their enhanced cytolytic activity against tumor targets, suggesting that KHYG-1 cells may be a feasible anticancer immunotherapeutic agent.135 NKL cells showed natural killing ability, ADCC and proliferative responses very similar to CD16−CD56dim NK cells, indicating that they represent a cell line that has likely retained most of the original NK cell characteristics.136 NKL cells have a different antitumor spectrum and are more cytotoxic to some human cancer cells compared with NK-92 cells.137 NKL has potential use in adoptive immunotherapy against tumors.138,139 NKG cells, established in China, was firstly demonstrated to be a promising new human NK cell line candidate for clinical cancer immunotherapy in a xenograft mouse model.127
A distinct advantage of using permanent NK cell lines is that they can easily be maintained in vitro and expanded to large numbers under good manufacturing practice (GMP) conditions for immunotherapy. Most importantly, their antitumor activities can be further enhanced.131 Thus, adoptively transferring established NK cell lines with broad antitumor activity represents an alternative strategy that is more practical for quality control and large-scale production for use in clinical trials.
Genetic modification of NK cells
As summarized in Table 1, expressing cytokine transgenes, overexpressing activating receptors, silencing inhibitory receptors or retargeting NK cells via chimeric receptors might be effective genetic manipulation approaches to modulate and enhance NK–tumor cell interaction.
The cytokine gene transfer approach induces NK cell proliferation and increases survival capacity, further enhancing their activation. By using NK cell lines, modifying genes such as IL-2, IL-12, IL-15 and stem cell factor (SCF) have been demonstrated to restore their cytotoxic capacity as well as increase their proliferative rate, survival ability and in vivo antitumor activity.140,141,142,143,144,145 However, the specificity of NK cells is still limited. The approach focuses on retargeting NK cells to tumor cells by gene transfer of chimeric tumor-antigen specific receptors, such as by fusing a single chain variable fragment receptor (Fv) specific for a certain tumor-associated antigen to intracellular signaling machinery (i.e., a CD3 ζ chain). Indeed, chimeric receptors against HER2/neu, carcinoembryonic antigen (CEA) and CD33 in NK cell lines showed increased specific cytotoxicity both in vitro and in vivo.146,147,148 Additionally, the NK-92 cell line modified to contain a chimeric Ag receptor consisting of a CD20-specific scFv Ab fragment exhibited significantly enhanced cytotoxicity against CD20+ target cells as compared with the control.149 Moreover, NK cells transduced with a chimeric receptor specific for CD19 dramatically enhanced the cytotoxicity against CD19+ malignant B cells.150
Expanding NK cells for clinical practice
For NK cell immunotherapy, obtaining a sufficient number of functional NK cells is critical in clinical protocols. Therefore, the number, purity and state of NK cell proliferation and activation are considered as the key factors.151 In Table 2, the purification/expansion of clinical-grade NK cells developed in recent years is summarized. They can be produced from cord blood, bone marrow, peripheral blood and embryonic stem cells. Overall, the summarized methods suggest that long-term ex vivo expansion of NK cells may present a clinical benefit, but not the short-term activation which is not sufficient for augmenting the functions of NK cells.152
Table 2. Expansion of NK cells in vitro for clinical practice*.
| Starting material | Initial cell number | Medium | Stimulators* Feeder cells | Culture instrument | Culture time/acquired cell number | Fold proliferation | Purity | Cytotoxicity | Phenotype, cytokine production | References** | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cord blood-derived NK cells | CD34+ cell from cord blood (CliniMACS) | (0.89–6.34)×106 | Glycostem Basal Growth Medium +10% HS | SCF, IL-7, IL-15, IL-2, Flt3L, TPO, G-CSF, IL-6, LMWH | Vuelife™ bags, WAVE Bioreactor System 2/10, BIOSTATH CultiBag RM system | 6 weeks(1.6–3.7)×109 | 1435–2657 | >90% | K562 (>40%, 10∶1) | CD56+, CD3−, NKG2D+, NCRs+, CD161+, CD314+, CD244+ | [155] |
| CD34+ cell from cord blood (CliniMACS) | (0.84–2.50)×106 | Glycostem Basal Growth Medium | SCF, TPO, IL-7, Flt3L, IL-15, IL-2, G-CSF, GM-CSF, IL-6, LIF, MIP-1α | 24-well tissue culture plates | 14–35 days(1.9–7.8)×109 | ∼104 (freshly UCB);∼103 (frozen UCB) | >95% | K562, Lama, Kasumi, BLM, nd FM3 (>75%) KG1a (∼30%) (18 h 1∶1) | CD56+, CD3−, NKG2D+, NCRs+, CD107+, 2B4+, CD161+, IFN-γ | [154] | |
| Stem cell/iPSC- derived NK cells | CD34+CD45+ cells (H9 hESC line) | — | RPMI 1640+15% defined fetal bovine serum; DMEM/Ham F12+20% heat-inactivated human serum AB | IL-3, IL-15, IL-7, SCF and Flt3L;Feeder cells: M210-B4; AFT024 | — | 30–35 days | ∼100 | >37.5% | K562, MCF7, PC3 (55%–80%), NTERA2, and U87 (20%–30%) | CD56+, CD45+, CD16+, CD94+, NKG2D+, NKp46+, CD158a+, CD158b+, IFN-γ | [159,162,163] |
| BM CD34+ | — | Dulbecco's medium supplemented with 12.5% fetal calf serum; 12.5% horse serum | IL-2; Feeder cells: stromal cells from irradiated BMMNC | — | — | ∼690 | 75% | K562 (80%, 6.6∶1) | CD3−, CD56+, CD2+, CD7+, CD8+, CD16+ | [157] | |
| PBMCs | CD3−CD56+ cells from PBMCs (CliniMACS) | (0.40±0.16)×108 | CellGro SCGM serum-freeMedium, 5%AB human serum | IL-2, IL-15, anti-CD3 monoclonal antibody(MAb) OKT3 | Baxter LifeCell culture bags | 19 days(85.5±17.2)×108 | 268.3±66.8 | 100% | K562 (>60%, 10∶1) | CD3−, CD56+, NKG2D+, NCRs+, DNAM-1 | [166] |
| CD3−CD56+ cells from PBMCs | 3.0×106 | SCGMMedium and 10% fetal bovine serum | IL-2;Feeder cells: K562-mb15-41BBL | VueLife bag system | 7 days | 90.5 (33–141) | 83.1% (72.9%–85.9%) | K562, HL-60, KG1, and U937 (>40%, 4∶1) | CD3−, CD56+, NKG2D+, NCRs+ | [165] | |
| CD56+ cells from PBMCs | (9.5–85.8)×106 | Alpha-MEM, 20% fetal bovine serum | IL-15, HC | — | 20–23 days | 23 (3.2–131.3) | 97.9% (82.7%–99.6%) | K562, (23.2%, 7.0–54.7%, 1∶1) | CD3−, CD56+, NKG2D+, NCRs+ | [196] | |
| CD56+ cells from PBMCs (CliniMACS) | 2.0×108 | X-VIVO 2010% heat inactivated human AB serum | IL-2;Feeder cells: EBV-TM-LCL cells | Flasks and bags | 21 days3×1010 | 490±260 | 84.3%±7.8% | RCC (27.6±9.3%, 1∶1) | CD3−, CD56+, CD244+, CD48+, NKG2D+sFasL, IFN-γ, GM-CSF, TNF-α, MIP-1α, MIP-1β | [164] | |
| PBMCs | 2×106 NK cells | SCGMMedium and 10% fetal bovine serum | IL-2;Feeder cells: K562-mb15-41BBL | G-Rex100 flasks | 8–10 days | 209 (38–338) | 61% (54%–70%) | K562, U266 and Raji (>40%, 5∶1) | CD3−, CD56+ | [197] | |
| PBMCs | — | Serum-free medium and 10% heat-inactivated human plasma | rhIL-2; OK432;anti-CD16 | Cell-culture bag | 21 days | 637–5712 | 78.9%±11.6% | K562, Raji and Daudi (>20%, 3∶1) | CD3−, CD56+, CD158a+, CD158b1/b2+, CD159a+, CD69+, NKp30+, NKp44+, NKp46+, IFN-γ, TNF-α | [198] | |
| PBMC | 1.5×106 | cRPMI | Il-2;Feeder cells: K562-mbIL15-41BBL cells | T-25 or T-75 culture flasks | 14 days | 165 (4–567) | 45.6% (7.4%–76.4%) | K562, MCF-7, LNCaP, DU145, PC-3 | CD3−, CD56+, NKG2D+, NCRs+ | [199] | |
| PBMCs | (4.6–9.7)×108 | CellGro SCGM serum-free medium5% human serum | IL-2 | Wave Bioreactor System 2/10 | 21 days(9.8×109) | Mean 77-fold | Mean 37.5% | K562 (>25%, 10∶1) | CD3−, CD56+, CD244+, CD11a+, CD69+, NKG2D+, NCR+ | [200] | |
| CD3-depleted PBMCs | 107 CD3−depleted cells | AIMV media 10% hu AB serum | IL-2;Feeder cells: OKT3-loaded autologous PBMC | Cell-culture bags | 21 days(4.70±2.10)×1010 | — | ≥93% | 888 (82±12%, 10∶1) | CD3−, CD56+, CD16+, NKG2D+ | 176 | |
| NK cell lines | NK-92 | (2.5×105/mL)×25 mL/bag | X-Vivo 10 serum-free mediaamino acids and 2.5% human AB plasma | IL-2 (500 IU/ml) | 1 l Vuelife culture bag | 15–17 days>1×109/bag | >200 | ≥80% (viability) | K562 (72%); Raji (58%) (10∶1) | CD3−, CD56+, IL-6, IL-8, IL-10 | [170,171] |
| 1×107/bioreactor | Optimized clinical-grade media | IL-2 (100∼500 IU/ml) | Controlled stirred bioreactor | 11–16 days>1010/bioreactor | >1000 | >95% (viability) | Highly lytic to leukemia, lymphoma, malignant melanoma, prostate cancer, squamous cell carcinoma, breast cancer | Positive: CD56, CD2, CD7, C11a, CD28, CD45, CD54Negative: CD1, CD3, CD4, CD8, CD14, CD16, CD20, CD23, CD34, HLA-DR | [131] | ||
| NKG | (1×105/ml)×200 ml/bag | α-MEM medium10% fetal bovine serum +10% horse serum | IL-2 (100 IU/ml) | WAVE Bioreactor | 12–14 days>1010/bag | >1000 | >90% (viability) | K562 (>50%), Ho-8910 (>60%), Daudi (>70%), LoVo (>35%) (10∶1) | CD56+, CD16−, CD27−, CD3−, αβTCR−, γδTCR−, CD4−, CD8−, CD19−, CD161−, CD45+,CXCR4+, CCR7+, CXCR1−, CX3CR1−;IFN-γ, TNF-α, IL-6, IL-10 | [127] |
Abbreviations: GM-CSF, granulocyte monocyte-colony stimulating factor; hESCs, human embryonic stem cells; IFN, interferon; iPSCs, induced pluripotent stem cells; NCR, natural cytotoxicity receptor; NK, natural killer; PBMC, peripheral blood mononuclear cell; SCF, stem cell factor; UCB, umbilical cord blood.
*Ex vivo expansion of NK cells for clinical immunotherapy under GMP conditions.
**Only references in the most recent 3 years were cited for the ex vivo expansion of NK cells from PBMCs.
Cord blood-derived NK cells
Umbilical cord blood (UCB) is an excellent source of HSCs, which produce a multitude of therapeutic cells, including NK cells. HSCs can be harvested from UCB for clinical applications by using the CliniMACS system to select CD34+ cells.153,154,155 Enriching CD34+ cells from thawed UCB was optimized by using the EloHAES separation method within the CliniMACS system. The CD34+ cell-derived NK cells were generated in static cell culture bags or in an automated bioreactor in order to produce clinical-grade NK cells in a closed environment. Large-scale production of highly active and functional NK cells was obtained by this method for a phase I dose-finding trial in elderly AML patients.155 Another extremely efficient cytokine-based culture system for expanding CD34+ cell-derived NK cells was also reported; this method could be used for both fresh and frozen CD34+ UCB cells. These UCB-derived CD56+ NK cells uniformly expressed high NKG2D levels and natural cytotoxicity receptors, efficiently targeted myeloid leukemia and melanoma cell lines, and lysed primary leukemia cells at low NK/target ratios.154 CD34+ cells expanded ex vivo in optimized serum-free medium provide a promising cell source with significantly higher NK-cell differentiation as well as enhanced IFN-γ secretion and cytotoxic ability compared with the freshly isolated CD34+ cells.153 UCB-derived CD56+ cells separated by an anti-CD56 mAb and immunomagnetic beads could be expanded in an ex vivo culture system in the presence of irradiated autologous lymphocytes and various IL-2 concentrations while maintaining their antileukemic abilities.156 However, prolonged exposure of purified NK cells to cytokines in vitro might induce cell exhaustion, rendering the NK cells unable to effectively kill tumor cells after infusion into the recipient.152
Stem cell/induced pluripotent stem cell (iPSC)-derived NK cells
Human NK cells can be differentiated from BM-derived CD34+ hematopoietic progenitor cells cultured in certain conditions: IL-2 plus an allogeneic feeder cell layer; IL-2 plus other hematopoietic growth factors, such as c-kit ligand; IL-15; or in a marrow stroma-dependent long-term culture system.157,158 CD34+ cells derived from human embryonic stem cells (hESCs) enrich for hematopoietic colony-forming cells, which is similar to CD34+ selection from primary hematopoietic tissues, such as BM and UCB, suggesting that hESCs might be a suitable novel cell source for therapy.159 Studies on hESCs and iPSCs indicate that using hESC- and iPSCs-derived hematopoietic products for diverse clinical therapies is a reasonable expectation for the future.159,160,161 Recently, efficient generation of functional NK cells from hESCs by a two-step culture method has been reported. These NK cells possess the ability to lyse tumor cells by direct cell-mediated cytotoxicity and ADCC, and exhibit a mature NK cell phenotype, including KIR and CD94/NKG2A expression as well as high expression of a variety of effector molecules for natural cytotoxicity, such as FasL, TRAIL, NKp46, NKp44, NKG2D and CD16.162 Furthermore, these hESC-derived NK cells are uniformly CD94+CD117low/− and mediate an effective antitumor response in an in vivo xenogeneic mouse model, which was more effective as compared to UCB-derived NK cells.163 Additionally, hESC- and iPSC-derived NK cells also provide advantages compared with PB-derived NK cells, including effective genetic modification and promoting survival in vivo.161 The most promising future direction for these cells may be to engineer hESCs or iPSCs to express chimeric antigen receptors (CARs) for specific tumor antigens that are capable of directing CTLs to tumor sites; they can also be modified to express cloned T cell receptors for specific tumor antigens, which remains to be tested in vivo.161 Indeed, producing NK cells from CD34+ stem cells has become alluring, since stem cells can be isolated and frozen, and can overcome some obstacles presented by using purified NK cells.152
Peripheral blood mononuclear cells (PBMCs)
NK cells are normally present in low numbers in PBMCs. Thus, many researchers have focused on successfully expanding NK cells ex vivo under GMP conditions for clinical immunotherapy. As summarized in Table 2, Epstein–Barr virus-transformed lymphoblastoid cell lines, genetically modified K562 cells, or irradiated autologous cells were used as feeder cells to promote NK cell expansion from PBMCs.164,165,166,167 The Campana group has developed a master cell bank of K562 feeder cells expressing a membrane-bound form of IL-15 (mbIL15) and 4-1BB ligand (K562-mb15-41BBL) under cGMP guidelines, and demonstrated that large-scale expansion and activation of human NK cells for clinical studies was feasible.165 These NK cells demonstrated cytotoxic activity toward tumor cells even higher than observed in the initial small-scale experiments. Additionally, K562 feeder cells genetically modified to coexpress MICA, 4-1BB ligand and IL-15 (K562-MICA-4-1BBL-IL-15) showed potential for ex vivo NK cell expansion for clinical immunotherapy.167 Compared with mbIL15, K562-based genetically engineered artificial antigen-presenting cells with membrane-bound IL-21 supported human NK cell proliferation with longer telomeres and less senescence, resulting in enhanced expansion and tumor killing.168 Large-scale in vitro-expanded NK cells using irradiated Epstein–Barr virus-transformed lymphoblastoid cell lines feeder cells were also found to be more cytotoxic to tumor cells, with upregulated activating receptors and death receptor ligands as well as altered cytokine secretion profiles.
The safety and antitumor effects of autologous NK cells expanded from PBMCs were investigated in a phase I trial in patients with advanced metastatic tumors and hematological malignancies.164 Large-scale ex vivo alloreactive NK cell expansion suitable for multiple donor lymphocyte infusions in AML was reported. This protocol involved that NK cells purified by CliniMACS were cultured in closed air-permeable culture bags with certified culture medium and other components, including human serum, IL-2, IL-15, anti-CD3 antibody and autologous irradiated feeder cells.166 Furthermore, highly active human NK cells expanded in a large-scale, clinical-grade, feeder-free way were established using an automated bioreactor. Bulk PBMCs were directly cultured without feeder cells or any separation strategies, leading to an NK cell-enriched population that was distinct from either LAK or cytokine-induced killer cells. These expanded NK cells displayed significantly higher cytotoxic capacity and higher NKp44 expression than NK cells expanded in flasks.169 As described earlier in the present review, allogeneic NK cells activated and expanded in vitro with IL-15/HC were safe and potentially effective in a phase I clinical trial when used in combination with chemotherapy in advanced non-small cell lung cancer patients.104
NK cell lines
Compared with autologous or allogeneic NK cells from PBMCs or stem cells, the large-scale expansion of NK cell lines under GMP conditions is easier and more available for clinical adoptive therapy. As shown in Table 2, individual samples of NK-92 cells or NKG cells from a master cell bank can be thawed and expanded in FDA-approved therapeutic-grade media supplemented with the required cytokines and freshly frozen plasma.127,131,170,171 Substantial improvement in the purity and quantity of NK cells could be obtained by using an optimized cell culture medium and a controlled stirred bioreactor for NK-92 cells, or a WAVE bioreactor for NKG cells.
Clinical trials of NK cell-mediated tumor immunotherapy
Results from treating hematological malignancies demonstrated a critical role for NK cells in clinical immunotherapy, as alloreactive NK cells highlighted the graft-vs.-leukemia effect in AML patients.172 The graft-vs.-tumor effect of alloreactive NK cells was also strengthened by mismatched IL-2-activated lymphocytes in patients with solid tumors or hematological malignancies.173 As discussed above, autologous NK cells, allogeneic NK cells, NK cell lines and genetically modified NK cells were investigated for effectiveness as tumor immunotherapies. The clinical study designs evaluating the efficacy of these various NK cell-mediated tumor therapies are summarized in Table 3.
Table 3. Clinical trials of tumor immunotherapy by using NK cells.
| Source of NK cells | Stage | Subjects | Treatment | Styles | Effects | Status | Country | Reference/ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|---|---|---|
| Autologous NK cells (from PBMC) | Phase I | 11 metastatic colorectal cancer; 1 NSCLC | (0.001–0.3)×109 cells/dose; i.v. 1–4 doses/cycle, 1–6 cycle | Used alone | No toxicities | Completed | Germany | [175] |
| Phase I | 7 metastatic melanoma; 1 metastatic renal cell carcinoma | (4.70±2.10)×1010 cells; i.v. | Combined with chemotherapy | No toxicities | Completed | United States | [176] | |
| Phase I | Metastatic nasopharyngeal | i.v. | Used alone | — | Completed | Singapore | NCT00717184 | |
| Phase I | Metastatic cancers; hematological malignancies | i.v. | Used alone | — | Recruiting participants | United States | NCT00720785 | |
| Phase I | Breast cancer, glioma, hepatocellular cancer, squamous cell lung cancer, pancreatic cancer, colon cancer, prostate cancer | i.v. | Used alone | — | Suspended | United States | NCT00909558 | |
| Allogeneic NK cells (from PBMC) | Phase I | 15 advanced NSCLC | (0.2–29)×106 cells/kg/dose; i.v. 2–4 doses | Combined with chemotherapy | No side effects;clinically effective | Completed | Greece | [196] |
| Phase I | Acute myeloid leukemia | i.v. | Combined with chemotherapy | — | Ongoing | United States | NCT00187096 | |
| Phase I | Lymphoma; leukemia; stem cell transplantation | i.v. | Combined with Rituximab | — | Completed | United States | NCT00383994 | |
| Phase I | Lymphoma | 1×107 cells/kg; i.v. | Combined with SCT | No toxicities | Ongoing | United States | NCT00586690; NCT00586703;[178] | |
| Phase I | Non-B lineage hematological malignancies and solid tumors | i.v. | Combined with chemotherapy | — | Recruiting participants | United States | NCT00640796 | |
| Phase I | Lymphoma; myeloma; leukemia | i.v. | Used alone | — | completed | United States | NCT00660166 | |
| Phase I | ALL; CML; JMML; MDS; NHL | i.v. | Combined with chemotherapy and immunotherapy | — | Recruiting participants | United States | NCT00697671 | |
| Phase I | 13 acute myeloid leukemia | (1–5)×106 cells/kg; i.v. | Combined with chemotherapy | No toxicity including GVHD | Unknown | Italy | NCT00799799; [177] | |
| Phase I | Neuroblastoma | i.v. | Combined with chemotherapy | — | Recruiting participants | United States | NCT00877110 | |
| Phase I | Acute lymphoblastic leukemia | i.v | Used alone | — | Recruiting participants | United States | NCT00995137 | |
| Phase I | Malignant lymphomas; solid tumors | Singe-dose infusion:Cohort1: 1×106 cells/kg Cohort2: 1×107 cells/kg;Repeated dose infusion:Cohort3: 1×106 cells/kg; Cohort4: 3×106 cells/kg; Cohort5: 1×107 cells/kg; Cohort6: 3×107 cells/kg; i.v | Used alone | — | Recruiting participants. | Korea | NCT01212341 | |
| Phase I | Leukemia, lymphoma, neuroblastoma, sarcoma, desmoplastic small round cell tumor | 3 dose levels (1×105, 1×106, and 1×107 cells/kg); i.v | Combined with chemotherapy | — | Recruiting participants. | United States | NCT01287104 | |
| Phase I | Acute myeloid leukemia | Four cohorts of escalating doses receiving 0, 1, 10, or 20×106 NK cells/kg; i.v | Combined with chemotherapy and immunotherapy | — | Recruiting participants | United States | NCT01478074 | |
| Phase I | Neuroblastoma | minimum of 0.1×106 cells/kg; maximum of 400×106 CD45+ cells/kg, given once; i.v | Combined with chemotherapy and immunotherapy | — | Recruiting participants | United States | NCT01576692 | |
| Phase I | LeukemiaChronic lymphocytic leukemia | (3–7)×108 cells; however, if the dose of 3×108 cells is not achieved, all available NK cells will be infused; i.v. | Combined with chemotherapy | — | Not yet open for participant recruitment | United States | NCT01619761 | |
| Phase I/II | Acute myelogenous leukemia | (2–3)×107 cells/kg; i.v. | Combined with chemotherapy | — | Ongoing participants | United States | NCT00303667 | |
| Phase I/II | LeukemiaLymphoma | (1.5–8)×107 NK cells/kg; i.v. | Combined with chemotherapy and immunotherapy | — | terminated | United States | NCT00625729 | |
| Phase I/II | Brain and central nervous system tumors; chronic myeloproliferative disorders; leukemia; lymphoma; lymphoproliferative disorder; multiple myeloma and plasma cell neoplasm; myelodysplastic syndromes; myelodysplastic/myeloproliferative neoplasms | i.v. | Combined with SCT | — | Recruiting participants | Korea | NCT00823524 | |
| Phase I/II | Melanoma | i.v. | Combined with chemotherapy | — | Completed | Korea | NCT00846833 | |
| Phase I/II | Multiple myeloma | 3 dose levels (1.5×106 cells/kg, 1.5×107 cells/kg and 1×108 cells/kg), if safe, continuing with maximally 7 doses of 1×108 cells/kg; i.v. | Combined with chemotherapy and SCT | — | Recruiting participants | Switzerland | NCT01040026 | |
| Phase I/II | Acute myeloid leukemia; advanced hematological malignancies; indication for allogeneic stem cell transplantation | 1×107 cells/kg; i.v. | Combined with chemotherapy and radiation therapy and SCT | — | Recruiting participants | Germany | NCT01220544 | |
| Phase I/II | Childhood solid tumor | i.v. | Used alone | — | Recruiting participants | Spain | NCT01337544 | |
| Phase I/II | Acute myeloid leukemia; precursor cell lymphoblastic leukemia-lymphoma; myelodysplastic syndromes; lymphoma; neuroblastoma; rhabdomyosarcoma | ≥1×107 NK cells/kg; i.v. | Combined with HLA-haploidentical HSCT | — | ongoing | Switzerland | NCT01386619 | |
| Phase I/II | Acute myeloid leukemia | 1×106 NK cells/kg or 3×106 cells/kg; i.v. | Used alone | — | Not yet open for participant recruitment | United States | NCT01520558 | |
| Allogeneic NK cells (from PBMC) | Phase II | 14 ovarian cancer; 6 breast cancer | (8.33–39.41)×106 NK cells/kg; i.v. | Combined with chemotherapy | PR (4 patients); SD (12 patients); PD (3 patients) | Completed | United States | [201] |
| Phase II | Acute lymphoblastic leukemia; lymphoma, lymphoblastic | i.v. | Combined with chemotherapy and SCT | — | Recruiting participants | United States | NCT00186875 | |
| Phase II | Metastatic melanoma; metastatic kidney cancer | i.v. | Combined with chemotherapy | — | Completed | United States | NCT00328861 | |
| Phase II | Breast cancer | (1.5–8.0)×107 NK cells/kg; i.v. | Combined with chemotherapy and radiation therapy | — | Terminated | United States | NCT00376805 | |
| Phase II | Leukemia; myelodysplastic syndromes | i.v. | Combined with chemotherapy | — | Recruiting participants. | United States | NCT00526292 | |
| Phase II | Fallopian tube cancer; ovarian cancer; peritoneal cavity cancer | (1.5–8.0)×107 NK cells/kg; i.v. | Combined with chemotherapy and radiation therapy | — | Terminated. | United States | NCT00652899 | |
| Phase II | Neuroblastoma | i.v. | Combined with chemotherapy and SCT | — | Terminated | United States | NCT00698009 | |
| Phase II | Leukemia; pediatric cancer | i.v. | Combined with chemotherapy | — | Completed | United States | NCT00941928 | |
| Phase II | Ovarian cancer; fallopian tube cancer; primary peritoneal cancer; breast cancer | 8.0×107 cells/kg; i.v. | Combined with chemotherapy | — | Recruiting participants | United States | NCT01105650 | |
| Phase II | Acute myelogenous leukemia | ≤8.0×107 nucleated cells//kg; i.v. | combined with chemotherapy | — | Ongoing | United States | NCT01106950 | |
| Phase II | Relapsed lymphoma; B cell non-Hodgkins lymphoma; refractory lymphoma; high risk chronic lymphocytic leukemia | (1.5–8.0)×107 cells/kg; i.v. | Combined with chemotherapy | — | Recruiting participants | United States | NCT01181258 | |
| Phase II | Acute myeloid leukemia; myelodysplastic syndrome | i.v. | Combined with SCT | — | Recruiting participants | United States | NCT01370213 | |
| Phase II | Leukemia; chronic myelogenous leukemia | i.v. | Combined with chemotherapy | — | Recruiting participants | United States | NCT01390402 | |
| Phase II | Myelodysplastic syndrome | i.v. | Combined with chemotherapy | — | Not yet open for participant recruitment | United States | NCT01593670 | |
| Phase II | Acute myelogenous leukemia | i.v. | Combined with chemotherapy | — | Not yet open for participant recruitment | United States | NCT01639456 | |
| NK-92 cells | Phase I | 11 advanced renal cell cancer; 1 melanoma | 1×108 or 3×108 or 1×109 or 3×109 NK-92 cells/m2 body surface; i.v. three doses (3 patients/group) | Used alone | No severe hemodynamic or hematologic toxicities | — | United States | [171] |
| Phase I | Acute myeloid leukemia | 1×109 or 3×109 or 5×109 NK-92 cells/m2 body surface;i.v. two doses | Used alone | Status: suspended | — | United States | NCT00900809 | |
| Phase I | Leukemia; lymphoma; myeloma; Hodgkin's disease | 1×109 or 3×109 or 5×109 NK-92 cells/m2 body surface;i.v. on days 1, 3, and 5 of each cycle; 6 cycles monthly | Used alone | Status: suspended | — | Canada | NCT00990717 | |
| Phase I/II | 4 sarcoma; 2 medulloblastoma; 1 PNET; 1 B-cell ALL | (1–3)×109 NK-92 cells/m2 body surface; i.v. two doses | Used alone | Without any significant side effects;no conclusions as to the efficacy can be drawn | Germany | [131] | ||
| NK cells from UCB | Phase II | Leukemia; myelodysplastic syndromes | i.v. | Combined with chemotherapy and SCT | — | Terminated | United States | NCT00354172 |
Abbreviations: ALL: Acute Lymphoblastic Leukemia; CML: Chronic Myelogenous Leukemia; JMML: Juvenile Myelomonocytic Leukemia; MDS: Myelodysplastic Syndrome; NHL: Non-Hodgkin's Lymphoma; NK, natural killer; PBMC, peripheral blood mononuclear cell; UCB, umbilical cord blood.
Adoptive transfer of autologous NK cells was shown not to have any clinical benefit for treating melanoma, RCC, lymphoma or breast cancer patients in several previously described clinical trials using ex vivo-generated LAK cells.100,174 Based on these studies, a clinical study was initiated in metastatic cancer patients who were adoptively transferred with autologous, in vitro-activated NK cells. While reinfusion of activated autologous NK cells was found to be safe with no negative side effects in metastatic colorectal cancer, non-small cell lung cancer, metastatic melanoma or renal cell carcinoma patients, no significant clinical responses were observed.175,176 Adoptively transferred NK cells persisted in the peripheral circulation for at least 1 week, but they expressed significantly lower levels of the key activating receptors, such as NKG2D, and showed weak ability to kill tumor cells. Additional studies in patients with lower tumor burden, or adoptive NK cell transfer coadministered with an mAb or cytokine, deserve evaluation.175,176 The phase I clinical trials in breast cancer, glioma, squamous cell lung cancer, pancreatic cancer, hepatocellular cancer, colon cancer or prostate cancer patients have since been suspended (www.clincaltrial.gov).
Due to the self-tolerance associated with autologous NK cells, adoptively transferred allogeneic NK cells were explored as an alternative. Highly purified NK cells from haploidentical KIR-ligand mismatched donors were effective in a cohort of elderly patients with high-risk AML (registered at www.clinicaltrial.gov as trial NCT00799799). Further studies are highly warranted to specifically assess the role of NK cell therapy in post-remission management in adult AML patients.177 Adoptive transfer in patients with other malignancies, including lymphoma, leukemia, non-B lineage hematological malignancies and solid tumors, has been proven safe and clinically effective.104,178 Phase II clinical trials for PBMC-derived allogeneic NK cells in patients with hematological malignancies and solid tumors have been completed or are ongoing, as shown in Table 3. Another phase II study of allogeneic NK cell therapy in patients with recurrent ovarian and breast cancer indicated that adoptive NK cell transfer after lympho-depleting chemotherapy was associated with transient donor chimerism. Strategies to augment in vivo NK cell persistence and expansion might be required to reduce this chimeric effect; Treg reconstitution in the recipient, for example, can repress this chimerism.179 Additionally, a phase II clinical trial using UCB-derived allogeneic NK cells has been performed in leukemia and myelodysplastic syndrome patients, but was terminated, and no conclusions can be drawn as to its efficacy.
As allogeneic NK cells, NK-92 cells, as the only NK cell line that has entered clinical trials, were demonstrated to be with no severe hemodynamic toxicities or significant tissue side effects in patients with advanced malignant melanoma and renal cell carcinoma in either Europe or the United States, suggesting that NK-92 cells might be an excellent candidate for adoptive cellular immunotherapy.131,171
Future remarks
Application of tissue-specific NK cells in tumor immunotherapy
Strong evidence supports that NK cells are able to adapt to their microenvironment by expressing divergent phenotypic and functional features that are specific to each organ, such as liver, mucosal tissues, uterus, pancreas, joints, brain and peripheral blood. The various roles NK cells play in different organs are often complex and sometimes even paradoxical.180 Immunotherapeutic approaches targeting NK cells should prove useful in inducing more effective immune responses to improve treatments. In 1985, the idea was put forth that organ-associated NK cell activity was a possible mechanism influencing the therapeutic effects of biological response modifier treatment.67 Liver is the only organ with arterial and venous blood supply from the gut, which is a unique immunological organ with an overwhelming innate immune system. Indeed, the unique features of liver NK cells include higher TRAIL, perforin and granzyme expression, and the lack of Ly-49 inhibitory receptors. Augmented antitumor activity was observed in hepatic NK cells as compared to splenic NK cells; even tumor cells killed by hepatic NK cells are otherwise resistant to killing by splenic NK cells. The important role of NK cells in tumor immunosurveillance within the liver microenvironment makes tissue-specific NK cells an attractive target for immunotherapeutic approaches that aim to control tumor metastasis in the liver.181
NK cell receptor-mediated immunotherapy
NK cell-mediated tumor immunosurveillance was limited in AML patients due to the decreased expression of activating receptors on NK cells and/or the heterogenous expression of ligands on leukemic blasts.182 Since the balance between inhibitory and activating receptors regulates NK cell activation, therapeutic strategies designed to target NK cell receptors may be able to potentiate NK cell activity in treating cancer. For example, reducing inhibitory KIR function by specifically blocking ligand recognition would be particularly effective in treating patients with HLA-expressing tumors that are resistant to NK cell-mediated lysis. A recent report by Binyamin et al.124 demonstrated that antibody-mediated KIR blockade significantly augmented NK cell ADCC responses. However, blocking KIR alone did not significantly increase NK-mediated killing of autologous tumor cells in an in vitro study. A novel human anti-KIR receptor therapeutic antibody that blocks KIR2DL1-3 impressively augments NK-mediated tumor cell killing.183 Study in a preclinical mouse model with all NK cells educated by a single transgenic inhibitory receptor, human KIR2DL3, by engaging its ligand, HLA-Cw3 indicated that anti-KIR mAb treatment induced HLA+ target cell lysis without breaking self-tolerance in vivo, and long-term anti-KIR mAb infusion did not abolish NK cell education or tumor cell recognition.184 Therefore, blocking inhibitory receptors like KIR may be an effective way to enhance NK cell-mediated cytotoxicity toward tumors.185
Drugs affecting NK-cell function
In some instances, immunomodulatory drugs can directly or indirectly activate NK cells. As discussed above, cytokines or growth factors used in combination with NK cells have successfully treated several human cancers (Section on ‘NK cell-mediated antitumor mechanisms'). In addition to these cytokines, broad activators of immune function—some of which also stimulate NK cells—are also implicated in antitumor immunity. In multiple myeloma (MM), thalidomide and immunomodulatory drugs trigger NK cell-mediated tumor-cell lysis by activating their cytotoxicity and ADCC functions. This partially explains the mechanism of action of these drugs and further supports their therapeutic use in MM.186 Immunostimulatory DNA oligonucleotides containing CpG motifs (CpG ODNs) were reported to stimulate immune responses against primary human ALL cells in vivo, reducing systemic leukemia burden, controlling disease, and improving survival. Since NK-cell depletion significantly reduced the CpG ODN-mediated antileukemic activity in vivo, NK-cell activation was partially responsible for the enhanced antitumor activity.187 Additionally, an essential role for NK cells was demonstrated during adjuvant intravesical bacillus Calmette–Guérin immunotherapy when used to treat superficial bladder cancer.188
Monitoring NK cell-based immunotherapy by non-invasive imaging
Evaluating the in vivo accumulation, distribution and quantity of transferred NK cells within tumor regions is clinically necessary to monitor progress of NK cell-based immunotherapies after systemic administration. Using imaging techniques, monitoring NK cells in recipient patients can be performed non-invasively. Since the results can be obtained instantly and in real time, these images may serve as a surrogate readout for tumor response.189,190 Radioisotope imaging techniques, such as positron emission tomography (PET), single photon emission computed tomography (SPECT), magnetic resonance imaging (MRI), optical imaging and fluorescence and bioluminescent imaging, have been investigated as methods to track NK cells in vivo. In addition to these imaging methods currently used in the clinic, new handheld endoscopic and tomographic imaging systems are also being tested.189
Patients with renal cell carcinoma receiving NK cell immunotherapy were monitored by SPECT imaging. NK cells were labeled with a 111In radiotracer during NK cell therapy, and SPECT images provided evidence for the activity, redistribution and tracer accumulation with high sensitivity, indicating an effective immunotherapeutic approach.191 However, many limitations for PET/SPECT still exist, including high cost, low resolution (1–2 mm), radiation exposure, tracer decay and limited ability for follow-up studies (2–3 h for the 18FDG radiotracer or 4–7 days for 111In). The low resolution issue may be improved by integrating the PET/SPECT images with high-resolution CT images (200–400 µm), which is currently being investigated.189 The optical and bioluminescent imaging methods can track transferred NK cells labeled with an exogenous fluorescent dye or those containing a fluorescent protein gene, such as green fluorescent protein or luciferase. OI offers an inexpensive, fast and radiation-free way to monitor NK cells in vivo with high sensitivity and low background noise, but with limited tissue penetration and low resolution (2–3 mm).192,193 The MRI method can track NK cells labeled with iron-oxide nanoparticles that produce a strong negative enhancement on T2-weighted images. This method provides readily available clinical translation, high resolution (100 µm in plane), high soft-tissue contrast, no radiation exposure and longer signal persistence. In addition, several FDA-approved iron-oxide nanoparticles are suitable for clinical use, including ferumoxides, ferumoxytol and ferucarbotran. However, MRI cell-tracking techniques also require high costs, long scan times and limited sensitivity.189 Among these approaches, MRI and PET will likely be at the center stage for whole-body applications for humans in the future; if used in tandem in a combined clinical MRI–PET scanner, these imaging methods can compliment each other by using MRI to localize the tracked cells and PET to measure viability and other functional parameters of the tracked cells.190,194,195 Thus, using imaging techniques to monitor NK cells during immunotherapy can serve as surrogate readouts for NK cell tumor accumulation and tumor response; additionally, monitoring NK cell immunotherapy in real time can help to detect and avoid ineffective treatment in patients who are unknowingly resistant to NK cell therapy.
Conclusion
NK cell-based immunotherapy holds great promise for cancer treatment. However, only modest clinical success has been achieved thus far using NK cell-based therapies in cancer patients. Progress in the field of understanding NK cell biology and function is therefore needed to assist in developing novel approaches to effectively manipulate NK cells for the ultimate benefit of treating cancer patients.
Acknowledgments
This work was supported by the National Mega Project (#2012ZX10002-014), the National Hitech Project (863 project, #2012AA020901) and the project by Department of Health of Chinese Government (#20130211).
The authors have declared that no competing interests exist.
References
- Borghaei H, Smith MR, Campbell KS. Immunotherapy of cancer. Eur J Pharmacol. 2009;625:41–54. doi: 10.1016/j.ejphar.2009.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker Y. Molecular immunological approaches to biotherapy of human cancers—a review, hypothesis and implications. Anticancer Res. 2006;26:1113–1134. [PubMed] [Google Scholar]
- Catchpole B, Gould SM, Kellett-Gregory LM, Dobson JM. Immunosuppressive cytokines in the regional lymph node of a dog suffering from oral malignant melanoma. J Small Anim Pract. 2002;43:464–467. doi: 10.1111/j.1748-5827.2002.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Zagury D, Gallo RC. Anti-cytokine Ab immune therapy: present status and perspectives. Drug Discov Today. 2004;9:72–81. doi: 10.1016/S1359-6446(03)02955-6. [DOI] [PubMed] [Google Scholar]
- Morse MA, Mosca PJ, Clay TM, Lyerly HK. Dendritic cell maturation in active immunotherapy strategies. Expert Opin Biol Ther. 2002;2:35–43. doi: 10.1517/14712598.2.1.35. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Semin Cancer Biol. 2006;16:115–123. doi: 10.1016/j.semcancer.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Salih HR, Goehlsdorf D, Steinle A. Release of MICB molecules by tumor cells: mechanism and soluble MICB in sera of cancer patients. Hum Immunol. 2006;67:188–195. doi: 10.1016/j.humimm.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002;169:4098–4102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- Waldhauer I, Steinle A. Proteolytic release of soluble UL16-binding protein 2 from tumor cells. Cancer Res. 2006;66:2520–2526. doi: 10.1158/0008-5472.CAN-05-2520. [DOI] [PubMed] [Google Scholar]
- Green DR, Bissonnette RP, Glynn JM, Shi Y. Activation-induced apoptosis in lymphoid systems. Semin Immunol. 1992;4:379–388. [PubMed] [Google Scholar]
- Saff RR, Spanjaard ES, Hohlbaum AM, Marshak-Rothstein A. Activation-induced cell death limits effector function of CD4 tumor-specific T cells. J Immunol. 2004;172:6598–6606. doi: 10.4049/jimmunol.172.11.6598. [DOI] [PubMed] [Google Scholar]
- Baxevanis CN, Perez SA, Papamichail M. Cancer immunotherapy. Crit Rev Clin Lab Sci. 2009;46:167–189. doi: 10.1080/10408360902937809. [DOI] [PubMed] [Google Scholar]
- Zhao E, Xu H, Wang L, Kryczek I, Wu K, Hu Y, et al. Bone marrow and the control of immunity. Cell Mol Immunol. 2012;9:11–19. doi: 10.1038/cmi.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975;16:216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Jiang X, Chen Y, Peng H, Tian Z. Single line or parallel lines: NK cell differentiation driven by T-bet and Eomes. Cell Mol Immunol. 2012;9:193–194. doi: 10.1038/cmi.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol. 2006;24:257–286. doi: 10.1146/annurev.immunol.24.021605.090700. [DOI] [PubMed] [Google Scholar]
- Gregoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, et al. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Ferlazzo G, Munz C. NK cell compartments and their activation by dendritic cells. J Immunol. 2004;172:1333–1339. doi: 10.4049/jimmunol.172.3.1333. [DOI] [PubMed] [Google Scholar]
- Roder JC, Pross HF. The biology of the human natural killer cell. J Clin Immunol. 1982;2:249–263. doi: 10.1007/BF00915064. [DOI] [PubMed] [Google Scholar]
- Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1:41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- Miller JS. The biology of natural killer cells in cancer, infection, and pregnancy. Exp Hematol. 2001;29:1157–1168. doi: 10.1016/s0301-472x(01)00696-8. [DOI] [PubMed] [Google Scholar]
- Vitale M, Sivori S, Pende D, Augugliaro R, di Donato C, Amoroso A, et al. Physical and functional independency of p70 and p58 natural killer (NK) cell receptors for HLA class I: their role in the definition of different groups of alloreactive NK cell clones. Proc Natl Acad Sci USA. 1996;93:1453–1457. doi: 10.1073/pnas.93.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- Kumar V, McNerney ME. A new self: MHC-class-I-independent natural-killer-cell self-tolerance. Nat Rev Immunol. 2005;5:363–374. doi: 10.1038/nri1603. [DOI] [PubMed] [Google Scholar]
- Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- Colonna M, Samaridis J. Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-B recognition by human natural killer cells. Science. 1995;268:405–408. doi: 10.1126/science.7716543. [DOI] [PubMed] [Google Scholar]
- Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “l'union fait la force”. Blood. 2005;106:2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- Wu L, Zhang C, Zhang J. HMBOX1 negatively regulates NK cell functions by suppressing the NKG2D/DAP10 signaling pathway. Cell Mol Immunol. 2011;8:433–440. doi: 10.1038/cmi.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paust S, von Andrian UH. Natural killer cell memory. Nat Immunol; 12:500–508. doi: 10.1038/ni.2032. [DOI] [PubMed] [Google Scholar]
- Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8+ T cells. Nat Rev Immunol. 2011;11:645–657. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman RB, Holden HT. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Riccardi C, Santoni A, Barlozzari T, Puccetti P, Herberman RB. In vivo natural reactivity of mice against tumor cells. Int J Cancer. 1980;25:475–486. doi: 10.1002/ijc.2910250409. [DOI] [PubMed] [Google Scholar]
- Barlozzari T, Reynolds CW, Herberman RB. In vivo role of natural killer cells: involvement of large granular lymphocytes in the clearance of tumor cells in anti-asialo GM1-treated rats. J Immunol. 1983;131:1024–1027. [PubMed] [Google Scholar]
- Hayakawa Y, Smyth MJ. Innate immune recognition and suppression of tumors. Adv Cancer Res. 2006;95:293–322. doi: 10.1016/S0065-230X(06)95008-8. [DOI] [PubMed] [Google Scholar]
- Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci USA. 2000;97:2731–2736. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003;90:127–156. doi: 10.1016/s0065-230x(03)90004-2. [DOI] [PubMed] [Google Scholar]
- Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–2328. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577–583. [PubMed] [Google Scholar]
- Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, et al. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23–28. doi: 10.1016/s0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7:329–339. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- Ljunggren HG, Karre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985;162:1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Ljunggren HG, Karre K. In search of the ‘missing self': MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- van den Broek MF, Kagi D, Zinkernagel RM, Hengartner H. Perforin dependence of natural killer cell-mediated tumor control in vivo. Eur J Immunol. 1995;25:3514–3516. doi: 10.1002/eji.1830251246. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Kelly JM, Baxter AG, Korner H, Sedgwick JD. An essential role for tumor necrosis factor in natural killer cell-mediated tumor rejection in the peritoneum. J Exp Med. 1998;188:1611–1619. doi: 10.1084/jem.188.9.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Thia KY, Cretney E, Kelly JM, Snook MB, Forbes CA, et al. Perforin is a major contributor to NK cell control of tumor metastasis. J Immunol. 1999;162:6658–6662. [PubMed] [Google Scholar]
- Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Trapani JA, Davis J, Sutton VR, Smyth MJ. Proapoptotic functions of cytotoxic lymphocyte granule constituents in vitro and in vivo. Curr Opin Immunol. 2000;12:323–329. doi: 10.1016/s0952-7915(00)00094-7. [DOI] [PubMed] [Google Scholar]
- Bradley M, Zeytun A, Rafi-Janajreh A, Nagarkatti PS, Nagarkatti M. Role of spontaneous and interleukin-2-induced natural killer cell activity in the cytotoxicity and rejection of Fas+ and Fas- tumor cells. Blood. 1998;92:4248–4255. [PubMed] [Google Scholar]
- Screpanti V, Wallin RP, Ljunggren HG, Grandien A. A central role for death receptor-mediated apoptosis in the rejection of tumors by NK cells. J Immunol. 2001;167:2068–2073. doi: 10.4049/jimmunol.167.4.2068. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Yamaguchi N, Nakayama M, Takeda K, Akiba H, Tsutsui H, et al. Expression and function of TNF-related apoptosis-inducing ligand on murine activated NK cells. J Immunol. 1999;163:1906–1913. [PubMed] [Google Scholar]
- Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168:1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- Sutlu T, Alici E. Natural killer cell-based immunotherapy in cancer: current insights and future prospects. J Intern Med. 2009;266:154–181. doi: 10.1111/j.1365-2796.2009.02121.x. [DOI] [PubMed] [Google Scholar]
- Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192–197. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, et al. Sequential production of interferon-gamma by NK1.1(+) T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood. 2002;99:1259–1266. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- Cifone MG, D'Alo S, Parroni R, Millimaggi D, Biordi L, Martinotti S, et al. Interleukin-2-activated rat natural killer cells express inducible nitric oxide synthase that contributes to cytotoxic function and interferon-gamma production. Blood. 1999;93:3876–3884. [PubMed] [Google Scholar]
- Furuke K, Burd PR, Horvath-Arcidiacono JA, Hori K, Mostowski H, Bloom ET. Human NK cells express endothelial nitric oxide synthase, and nitric oxide protects them from activation-induced cell death by regulating expression of TNF-alpha. J Immunol. 1999;163:1473–1480. [PubMed] [Google Scholar]
- Talmadge JE, Phillips H, Schindler J, Tribble H, Pennington R. Systematic preclinical study on the therapeutic properties of recombinant human interleukin 2 for the treatment of metastatic disease. Cancer Res. 1987;47:5725–5732. [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- Wiltrout RH, Herberman RB, Zhang SR, Chirigos MA, Ortaldo JR, Green KM, Jr, et al. Role of organ-associated NK cells in decreased formation of experimental metastases in lung and liver. J Immunol. 1985;134:4267–4275. [PubMed] [Google Scholar]
- Kodama T, Takeda K, Shimozato O, Hayakawa Y, Atsuta M, Kobayashi K, et al. Perforin-dependent NK cell cytotoxicity is sufficient for anti-metastatic effect of IL-12. Eur J Immunol. 1999;29:1390–1396. doi: 10.1002/(SICI)1521-4141(199904)29:04<1390::AID-IMMU1390>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Cretney E, Takeda K, Wiltrout RH, Sedger LM, Kayagaki N, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon gamma-dependent natural killer cell protection from tumor metastasis. J Exp Med. 2001;193:661–670. doi: 10.1084/jem.193.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Sgadari C, Furuke K, Bloom ET, Teruya-Feldstein J, Tosato G. Contribution of natural killer cells to inhibition of angiogenesis by interleukin-12. Blood. 1999;93:1612–1621. [PubMed] [Google Scholar]
- Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- Mocikat R, Braumuller H, Gumy A, Egeter O, Ziegler H, Reusch U, et al. Natural killer cells activated by MHC class I(low) targets prime dendritic cells to induce protective CD8 T cell responses. Immunity. 2003;19:561–569. doi: 10.1016/s1074-7613(03)00264-4. [DOI] [PubMed] [Google Scholar]
- Kelly JM, Darcy PK, Markby JL, Godfrey DI, Takeda K, Yagita H, et al. Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat Immunol. 2002;3:83–90. doi: 10.1038/ni746. [DOI] [PubMed] [Google Scholar]
- Nguyen-Pham TN, Yang DH, Nguyen TA, Lim MS, Hong CY, Kim MH, et al. Optimal culture conditions for the generation of natural killer cell-induced dendritic cells for cancer immunotherapy. Cell Mol Immunol. 2012;9:45–53. doi: 10.1038/cmi.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki G, Krystal G, Dougherty G, Takei F, Klingemann HG. Induction of sensitivity to NK-mediated cytotoxicity by TNF-alpha treatment: possible role of ICAM-3 and CD44. Leukemia. 1998;12:1565–1572. doi: 10.1038/sj.leu.2401145. [DOI] [PubMed] [Google Scholar]
- Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, et al. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99:3661–3667. doi: 10.1182/blood.v99.10.3661. [DOI] [PubMed] [Google Scholar]
- Farag SS, Caligiuri MA. Cytokine modulation of the innate immune system in the treatment of leukemia and lymphoma. Adv Pharmacol. 2004;51:295–318. doi: 10.1016/S1054-3589(04)51013-X. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Cretney E, Kershaw MH, Hayakawa Y. Cytokines in cancer immunity and immunotherapy. Immunol Rev. 2004;202:275–293. doi: 10.1111/j.0105-2896.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol. 2005;86:209–239. doi: 10.1016/S0065-2776(04)86006-1. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA. Interleukin-2 and the development of immunotherapy for the treatment of patients with cancer. Cancer J Sci Am. 2000. pp. S2–S7. [PubMed]
- Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA. Immunotherapy of cancer by systemic administration of lymphoid cells plus interleukin-2. J Biol Response Mod. 1984;3:501–511. [PubMed] [Google Scholar]
- Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodella L, Zamai L, Rezzani R, Artico M, Peri G, Falconi M, et al. Interleukin 2 and interleukin 15 differentially predispose natural killer cells to apoptosis mediated by endothelial and tumour cells. Br J Haematol. 2001;115:442–450. doi: 10.1046/j.1365-2141.2001.03055.x. [DOI] [PubMed] [Google Scholar]
- Hayakawa M, Hatano T, Ogawa Y, Gakiya M, Ogura H, Osawa A. Treatment of advanced renal cell carcinoma using regional arterial administration of lymphokine-activated killer cells in combination with low doses of rIL-2. Urol Int. 1994;53:117–124. doi: 10.1159/000282651. [DOI] [PubMed] [Google Scholar]
- Boiardi A, Silvani A, Ruffini PA, Rivoltini L, Parmiani G, Broggi G, et al. Loco-regional immunotherapy with recombinant interleukin-2 and adherent lymphokine-activated killer cells (A-LAK) in recurrent glioblastoma patients. Cancer Immunol Immunother. 1994;39:193–197. doi: 10.1007/BF01533386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes RL, Koslow M, Hiesiger EM, Hymes KB, Hochster HS, Moore EJ, et al. Improved long term survival after intracavitary interleukin-2 and lymphokine-activated killer cells for adults with recurrent malignant glioma. Cancer. 1995;76:840–852. doi: 10.1002/1097-0142(19950901)76:5<840::aid-cncr2820760519>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Keilholz U, Scheibenbogen C, Brado M, Georgi P, Maclachlan D, Brado B, et al. Regional adoptive immunotherapy with interleukin-2 and lymphokine-activated killer (LAK) cells for liver metastases. Eur J Cancer A. 1994;30A:103–105. doi: 10.1016/s0959-8049(05)80028-0. [DOI] [PubMed] [Google Scholar]
- Ma HL, Whitters MJ, Konz RF, Senices M, Young DA, Grusby MJ, et al. IL-21 activates both innate and adaptive immunity to generate potent antitumor responses that require perforin but are independent of IFN-gamma. J Immunol. 2003;171:608–615. doi: 10.4049/jimmunol.171.2.608. [DOI] [PubMed] [Google Scholar]
- Berzofsky JA, Ahlers JD, Belyakov IM. Strategies for designing and optimizing new generation vaccines. Nat Rev Immunol. 2001;1:209–219. doi: 10.1038/35105075. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Dubois S, Sato N, Sabzevari H, Sakai Y, Waldmann TA, et al. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood. 2005;105:721–727. doi: 10.1182/blood-2003-12-4187. [DOI] [PubMed] [Google Scholar]
- Zamai L, Ponti C, Mirandola P, Gobbi G, Papa S, Galeotti L, et al. NK cells and cancer. J Immunol. 2007;178:4011–4016. doi: 10.4049/jimmunol.178.7.4011. [DOI] [PubMed] [Google Scholar]
- Escudier B, Farace F, Angevin E, Charpentier F, Nitenberg G, Triebel F, et al. Immunotherapy with interleukin-2 (IL2) and lymphokine-activated natural killer cells: improvement of clinical responses in metastatic renal cell carcinoma patients previously treated with IL2. Eur J Cancer A. 1994;30A:1078–1083. doi: 10.1016/0959-8049(94)90460-x. [DOI] [PubMed] [Google Scholar]
- Ishikawa E, Tsuboi K, Saijo K, Harada H, Takano S, Nose T, et al. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res. 2004;24:1861–1871. [PubMed] [Google Scholar]
- deMagalhaes-Silverman M, Donnenberg A, Lembersky B, Elder E, Lister J, Rybka W, et al. Posttransplant adoptive immunotherapy with activated natural killer cells in patients with metastatic breast cancer. J Immunother. 2000;23:154–160. doi: 10.1097/00002371-200001000-00018. [DOI] [PubMed] [Google Scholar]
- Miller JS, Tessmer-Tuck J, Pierson BA, Weisdorf D, McGlave P, Blazar BR, et al. Low dose subcutaneous interleukin-2 after autologous transplantation generates sustained in vivo natural killer cell activity. Biol Blood Marrow Transplant. 1997;3:34–44. [PubMed] [Google Scholar]
- Burns LJ, Weisdorf DJ, DeFor TE, Vesole DH, Repka TL, Blazar BR, et al. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transplant. 2003;32:177–186. doi: 10.1038/sj.bmt.1704086. [DOI] [PubMed] [Google Scholar]
- Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- Ruggeri L, Mancusi A, Perruccio K, Burchielli E, Martelli MF, Velardi A. Natural killer cell alloreactivity for leukemia therapy. J Immunother. 2005;28:175–182. doi: 10.1097/01.cji.0000161395.88959.1f. [DOI] [PubMed] [Google Scholar]
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- Iliopoulou EG, Kountourakis P, Karamouzis MV, Doufexis D, Ardavanis A, Baxevanis CN, et al. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother. 2010;59:1781–1789. doi: 10.1007/s00262-010-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist A, McCoy JP, Samsel L, Childs R. Reduction of GVHD and enhanced antitumor effects after adoptive infusion of alloreactive Ly49-mismatched NK cells from MHC-matched donors. Blood. 2007;109:3603–3606. doi: 10.1182/blood-2006-05-024315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sconocchia G, Titus JA, Segal DM. Signaling pathways regulating CD44-dependent cytolysis in natural killer cells. Blood. 1997;90:716–725. [PubMed] [Google Scholar]
- Iannello A, Ahmad A. Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev. 2005;24:487–499. doi: 10.1007/s10555-005-6192-2. [DOI] [PubMed] [Google Scholar]
- Alderson KL, Sondel PM. Clinical cancer therapy by NK cells via antibody-dependent cell-mediated cytotoxicity. J Biomed Biotechnol. 2011;2011:379123. doi: 10.1155/2011/379123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin LS, Otto M, Baldwin WM, 3rd, Vail E, Gillies SD, Handgretinger R, et al. Anti-GD(2) with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain. 2010;149:135–142. doi: 10.1016/j.pain.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navid F, Santana VM, Barfield RC. Anti-GD2 antibody therapy for GD2-expressing tumors. Curr Cancer Drug Targets. 2010;10:200–209. doi: 10.2174/156800910791054167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton HM, Bernett MJ, Pong E, Peipp M, Karki S, Chu SY, et al. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res. 2008;68:8049–8057. doi: 10.1158/0008-5472.CAN-08-2268. [DOI] [PubMed] [Google Scholar]
- Zalevsky J, Leung IW, Karki S, Chu SY, Zhukovsky EA, Desjarlais JR, et al. The impact of Fc engineering on an anti-CD19 antibody: increased Fcgamma receptor affinity enhances B-cell clearing in nonhuman primates. Blood. 2009;113:3735–3743. doi: 10.1182/blood-2008-10-182048. [DOI] [PubMed] [Google Scholar]
- Parihar R, Dierksheide J, Hu Y, Carson WE. IL-12 enhances the natural killer cell cytokine response to Ab-coated tumor cells. J Clin Invest. 2002;110:983–992. doi: 10.1172/JCI15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar R, Nadella P, Lewis A, Jensen R, de Hoff C, Dierksheide JE, et al. A phase I study of interleukin 12 with trastuzumab in patients with human epidermal growth factor receptor-2-overexpressing malignancies: analysis of sustained interferon gamma production in a subset of patients. Clin Cancer Res. 2004;10:5027–5037. doi: 10.1158/1078-0432.CCR-04-0265. [DOI] [PubMed] [Google Scholar]
- Ortaldo JR, Woodhouse C, Morgan AC, Herberman RB, Cheresh DA, Reisfeld R. Analysis of effector cells in human antibody-dependent cellular cytotoxicity with murine monoclonal antibodies. J Immunol. 1987;138:3566–3572. [PubMed] [Google Scholar]
- Schultz KR, Klarnet JP, Peace DJ, Cheever MA, Badger CC, Bernstein ID, et al. Monoclonal antibody therapy of murine lymphoma: enhanced efficacy by concurrent administration of interleukin 2 or lymphokine-activated killer cells. Cancer Res. 1990;50:5421–5425. [PubMed] [Google Scholar]
- Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- Brady J, Hayakawa Y, Smyth MJ, Nutt SL. IL-21 induces the functional maturation of murine NK cells. J Immunol. 2004;172:2048–2058. doi: 10.4049/jimmunol.172.4.2048. [DOI] [PubMed] [Google Scholar]
- Betting DJ, Yamada RE, Kafi K, Said J, van Rooijen N, Timmerman JM. Intratumoral but not systemic delivery of CpG oligodeoxynucleotide augments the efficacy of anti-CD20 monoclonal antibody therapy against B cell lymphoma. J Immunother. 2009;32:622–631. doi: 10.1097/CJI.0b013e3181ab23f1. [DOI] [PubMed] [Google Scholar]
- Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J, et al. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood. 2011;117:2423–2432. doi: 10.1182/blood-2010-08-301945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yamane BH, Hank JA, Albertini MR, Sondel PM. The development of antibody-IL-2 based immunotherapy with hu14.18-IL2 (EMD-273063) in melanoma and neuroblastoma. Expert Opin Investig Drugs. 2009;18:991–1000. doi: 10.1517/13543780903048911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhtoiarov IN, Neal ZC, Gan J, Buhtoiarova TN, Patankar MS, Gubbels JA, et al. Differential internalization of hu14.18-IL2 immunocytokine by NK and tumor cell: impact on conjugation, cytotoxicity, and targeting. J Leukoc Biol. 2011;89:625–638. doi: 10.1189/jlb.0710422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Ilizaliturri FJ, Reddy N, Holkova B, Ottman E, Czuczman MS. Immunomodulatory drug CC-5013 or CC-4047 and rituximab enhance antitumor activity in a severe combined immunodeficient mouse lymphoma model. Clin Cancer Res. 2005;11:5984–5992. doi: 10.1158/1078-0432.CCR-05-0577. [DOI] [PubMed] [Google Scholar]
- Binyamin L, Alpaugh RK, Hughes TL, Lutz CT, Campbell KS, Weiner LM. Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. J Immunol. 2008;180:6392–6401. doi: 10.4049/jimmunol.180.9.6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velardi A, Ruggeri L, Mancusi A, Aversa F, Christiansen FT. Natural killer cell allorecognition of missing self in allogeneic hematopoietic transplantation: a tool for immunotherapy of leukemia. Curr Opin Immunol. 2009;21:525–530. doi: 10.1016/j.coi.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Cheng M, Zhang J, Jiang W, Chen Y, Tian Z. Natural killer cell lines in tumor immunotherapy. Front Med. 2012;6:56–66. doi: 10.1007/s11684-012-0177-7. [DOI] [PubMed] [Google Scholar]
- Cheng M, Ma J, Chen Y, Zhang J, Zhao W, Wei H, et al. Establishment, characterization and successful adaptive therapy against human tumors of NKG cell, a new human NK cell line. Cell Transplant. 2011;20:1731–1746. doi: 10.3727/096368911X580536. [DOI] [PubMed] [Google Scholar]
- Yoneda N, Tatsumi E, Kawano S, Teshigawara K, Oka T, Fukuda M, et al. Detection of Epstein-Barr virus genome in natural-killer-like cell line, YT. Leukemia. 1992;6:136–141. [PubMed] [Google Scholar]
- Tsuchiyama J, Yoshino T, Mori M, Kondoh E, Oka T, Akagi T, et al. Characterization of a novel human natural killer-cell line (NK-YS) established from natural killer cell lymphoma/leukemia associated with Epstein-Barr virus infection. Blood. 1998;92:1374–1383. [PubMed] [Google Scholar]
- Yagita M, Huang CL, Umehara H, Matsuo Y, Tabata R, Miyake M, et al. A novel natural killer cell line (KHYG-1) from a patient with aggressive natural killer cell leukemia carrying a p53 point mutation. Leukemia. 2000;14:922–930. doi: 10.1038/sj.leu.2401769. [DOI] [PubMed] [Google Scholar]
- Tonn T, Becker S, Esser R, Schwabe D, Seifried E. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J Hematother Stem Cell Res. 2001;10:535–544. doi: 10.1089/15258160152509145. [DOI] [PubMed] [Google Scholar]
- Klingemann HG. Natural killer cell-based immunotherapeutic strategies. Cytotherapy. 2005;7:16–22. doi: 10.1080/14653240510018000. [DOI] [PubMed] [Google Scholar]
- Malmberg KJ, Bryceson YT, Carlsten M, Andersson S, Bjorklund A, Bjorkstrom NK, et al. NK cell-mediated targeting of human cancer and possibilities for new means of immunotherapy. Cancer Immunol Immunother. 2008;57:1541–1552. doi: 10.1007/s00262-008-0492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suck G, Branch DR, Smyth MJ, Miller RG, Vergidis J, Fahim S, et al. KHYG-1, a model for the study of enhanced natural killer cell cytotoxicity. Exp Hematol. 2005;33:1160–1171. doi: 10.1016/j.exphem.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Suck G, Branch DR, Keating A. Irradiated KHYG-1 retains cytotoxicity: potential for adoptive immunotherapy with a natural killer cell line. Int J Radiat Biol. 2006;82:355–361. doi: 10.1080/09553000600649653. [DOI] [PubMed] [Google Scholar]
- Robertson MJ, Cochran KJ, Cameron C, Le JM, Tantravahi R, Ritz J. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp Hematol. 1996;24:406–415. [PubMed] [Google Scholar]
- Zhang C, Zhang J, Niu J, Tian Z. Interleukin-15 improves cytotoxicity of natural killer cells via up-regulating NKG2D and cytotoxic effector molecule expression as well as STAT1 and ERK1/2 phosphorylation. Cytokine. 2008;42:128–136. doi: 10.1016/j.cyto.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Garcia-Lora A, Martinez M, Pedrinaci S, Garrido F. Different regulation of PKC isoenzymes and MAPK by PSK and IL-2 in the proliferative and cytotoxic activities of the NKL human natural killer cell line. Cancer Immunol Immunother. 2003;52:59–64. doi: 10.1007/s00262-002-0336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrinaci S, Algarra I, Garrido F. Protein-bound polysaccharide (PSK) induces cytotoxic activity in the NKL human natural killer cell line. Int J Clin Lab Res. 1999;29:135–140. doi: 10.1007/s005990050079. [DOI] [PubMed] [Google Scholar]
- Nagashima S, Mailliard R, Kashii Y, Reichert TE, Herberman RB, Robbins P, et al. Stable transduction of the interleukin-2 gene into human natural killer cell lines and their phenotypic and functional characterization in vitro and in vivo. . Blood. 1998;91:3850–3861. [PubMed] [Google Scholar]
- Zhang J, Sun R, Wei H, Tian Z. Characterization of interleukin-15 gene-modified human natural killer cells: implications for adoptive cellular immunotherapy. Haematologica. 2004;89:338–347. [PubMed] [Google Scholar]
- Jiang W, Zhang J, Tian Z. Functional characterization of interleukin-15 gene transduction into the human natural killer cell line NKL. Cytotherapy. 2008;10:265–274. doi: 10.1080/14653240801965156. [DOI] [PubMed] [Google Scholar]
- Tam YK, Maki G, Miyagawa B, Hennemann B, Tonn T, Klingemann HG. Characterization of genetically altered, interleukin 2-independent natural killer cell lines suitable for adoptive cellular immunotherapy. Hum Gene Ther. 1999;10:1359–1373. doi: 10.1089/10430349950018030. [DOI] [PubMed] [Google Scholar]
- Konstantinidis KV, Alici E, Aints A, Christensson B, Ljunggren HG, Dilber MS. Targeting IL-2 to the endoplasmic reticulum confines autocrine growth stimulation to NK-92 cells. Exp Hematol. 2005;33:159–164. doi: 10.1016/j.exphem.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Zhang J, Sun R, Wei H, Tian Z. Characterization of stem cell factor gene-modified human natural killer cell line, NK-92 cells: implication in NK cell-based adoptive cellular immunotherapy. Oncol Rep. 2004;11:1097–1106. [PubMed] [Google Scholar]
- Schirrmann T, Pecher G. Human natural killer cell line modified with a chimeric immunoglobulin T-cell receptor gene leads to tumor growth inhibition in vivo. . Cancer Gene Ther. 2002;9:390–398. doi: 10.1038/sj.cgt.7700453. [DOI] [PubMed] [Google Scholar]
- Schirrmann T, Pecher G. Specific targeting of CD33+ leukemia cells by a natural killer cell line modified with a chimeric receptor. Leuk Res. 2005;29:301–306. doi: 10.1016/j.leukres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Uherek C, Tonn T, Uherek B, Becker S, Schnierle B, Klingemann HG, et al. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood. 2002;100:1265–1273. [PubMed] [Google Scholar]
- Muller T, Uherek C, Maki G, Chow KU, Schimpf A, Klingemann HG, et al. Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol Immunother. 2008;57:411–423. doi: 10.1007/s00262-007-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S, Klingemann HG. Natural killer cells: can they be useful as adoptive immunotherapy for cancer. Expert Opin Biol Ther. 2005;5:163–172. doi: 10.1517/14712598.5.2.163. [DOI] [PubMed] [Google Scholar]
- Luevano M, Madrigal A, Saudemont A. Generation of natural killer cells from hematopoietic stem cells in vitro for immunotherapy. Cell Mol Immunol. 2012;9:310–320. doi: 10.1038/cmi.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao IT, Yao CL, Kong ZL, Wu ML, Chuang TL, Hwang SM. Generation of natural killer cells from serum-free, expanded human umbilical cord blood CD34+ cells. Stem Cells Dev. 2007;16:1043–1051. doi: 10.1089/scd.2007.0033. [DOI] [PubMed] [Google Scholar]
- Spanholtz J, Tordoir M, Eissens D, Preijers F, van der Meer A, Joosten I, et al. High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy. PLoS One. 2010;5:e9221. doi: 10.1371/journal.pone.0009221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanholtz J, Preijers F, Tordoir M, Trilsbeek C, Paardekooper J, de Witte T, et al. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PLoS One. 2011;6:e20740. doi: 10.1371/journal.pone.0020740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condiotti R, Zakai YB, Barak V, Nagler A. Ex vivo expansion of CD56+ cytotoxic cells from human umbilical cord blood. Exp Hematol. 2001;29:104–113. doi: 10.1016/s0301-472x(00)00617-2. [DOI] [PubMed] [Google Scholar]
- Miller JS, Alley KA, McGlave P. Differentiation of natural killer (NK) cells from human primitive marrow progenitors in a stroma-based long-term culture system: identification of a CD34+7+ NK progenitor. Blood. 1994;83:2594–2601. [PubMed] [Google Scholar]
- Mrozek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87:2632–2640. [PubMed] [Google Scholar]
- Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman DS. Toward clinical therapies using hematopoietic cells derived from human pluripotent stem cells. Blood. 2009;114:3513–3523. doi: 10.1182/blood-2009-03-191304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorr DA, Kaufman DS. Pluripotent stem cell-derived natural killer cells for cancer therapy. Transl Res. 2010;156:147–154. doi: 10.1016/j.trsl.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woll PS, Martin CH, Miller JS, Kaufman DS. Human embryonic stem cell-derived NK cells acquire functional receptors and cytolytic activity. J Immunol. 2005;175:5095–5103. doi: 10.4049/jimmunol.175.8.5095. [DOI] [PubMed] [Google Scholar]
- Woll PS, Grzywacz B, Tian X, Marcus RK, Knorr DA, Verneris MR, et al. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood. 2009;113:6094–6101. doi: 10.1182/blood-2008-06-165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg M, Lundqvist A, McCoy P, Jr, Samsel L, Fan Y, Tawab A, et al. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy. 2009;11:341–355. doi: 10.1080/14653240902807034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler U, Meyer-Monard S, Jorger S, Stern M, Tichelli A, Gratwohl A, et al. Good manufacturing practice-compliant cell sorting and large-scale expansion of single KIR-positive alloreactive human natural killer cells for multiple infusions to leukemia patients. Cytotherapy. 2010;12:750–763. doi: 10.3109/14653241003786155. [DOI] [PubMed] [Google Scholar]
- Gong W, Xiao W, Hu M, Weng X, Qian L, Pan X, et al. Ex vivo expansion of natural killer cells with high cytotoxicity by K562 cells modified to co-express major histocompatibility complex class I chain-related protein A, 4-1BB ligand, and interleukin-15. Tissue Antigens. 2010;76:467–475. doi: 10.1111/j.1399-0039.2010.01535.x. [DOI] [PubMed] [Google Scholar]
- Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One. 2012;7:e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutlu T, Stellan B, Gilljam M, Quezada HC, Nahi H, Gahrton G, et al. Clinical-grade, large-scale, feeder-free expansion of highly active human natural killer cells for adoptive immunotherapy using an automated bioreactor. Cytotherapy. 2010;12:1044–1055. doi: 10.3109/14653249.2010.504770. [DOI] [PubMed] [Google Scholar]
- Tam YK, Martinson JA, Doligosa K, Klingemann HG. Ex vivo expansion of the highly cytotoxic human natural killer-92 cell-line under current good manufacturing practice conditions for clinical adoptive cellular immunotherapy. Cytotherapy. 2003;5:259–272. doi: 10.1080/14653240310001523. [DOI] [PubMed] [Google Scholar]
- Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10:625–632. doi: 10.1080/14653240802301872. [DOI] [PubMed] [Google Scholar]
- Ruggeri L, Mancusi A, Burchielli E, Capanni M, Carotti A, Aloisi T, et al. NK cell alloreactivity and allogeneic hematopoietic stem cell transplantation. Blood Cells Mol Dis. 2008;40:84–90. doi: 10.1016/j.bcmd.2007.06.029. [DOI] [PubMed] [Google Scholar]
- Slavin S, Ackerstein A, Or R, Shapira MY, Gesundheit B, Askenasy N, et al. Immunotherapy in high-risk chemotherapy-resistant patients with metastatic solid tumors and hematological malignancies using intentionally mismatched donor lymphocytes activated with rIL-2: a phase I study. Cancer Immunol Immunother. 2010;59:1511–1519. doi: 10.1007/s00262-010-0878-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- Krause SW, Gastpar R, Andreesen R, Gross C, Ullrich H, Thonigs G, et al. Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: a clinical phase i trial. Clin Cancer Res. 2004;10:3699–3707. doi: 10.1158/1078-0432.CCR-03-0683. [DOI] [PubMed] [Google Scholar]
- Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res. 2011;17:6287–6297. doi: 10.1158/1078-0432.CCR-11-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curti A, Ruggeri L, D'Addio A, Bontadini A, Dan E, Motta MR, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011;118:3273–3279. doi: 10.1182/blood-2011-01-329508. [DOI] [PubMed] [Google Scholar]
- Rizzieri DA, Storms R, Chen DF, Long G, Yang Y, Nikcevich DA, et al. Natural killer cell-enriched donor lymphocyte infusions from A 3-6/6 HLA matched family member following nonmyeloablative allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:1107–1114. doi: 10.1016/j.bbmt.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi FD, Ljunggren HG, la Cava A, van Kaer L. Organ-specific features of natural killer cells. Nat Rev Immunol. 2011;11:658–671. doi: 10.1038/nri3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subleski JJ, Wiltrout RH, Weiss JM. Application of tissue-specific NK and NKT cell activity for tumor immunotherapy. J Autoimmun. 2009;33:275–281. doi: 10.1016/j.jaut.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Correa B, Morgado S, Gayoso I, Bergua JM, Casado JG, Arcos MJ, et al. Human NK cells in acute myeloid leukaemia patients: analysis of NK cell-activating receptors and their ligands. Cancer Immunol Immunother. 2011;60:1195–1205. doi: 10.1007/s00262-011-1050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagne F, Andre P, Spee P, Zahn S, Anfossi N, Gauthier L, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114:2667–2677. doi: 10.1182/blood-2009-02-206532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola C, Andre P, Lemmers C, Fuseri N, Bonnafous C, Blery M, et al. Genetic and antibody-mediated reprogramming of natural killer cell missing-self recognition in vivo. . Proc Natl Acad Sci USA. 2009;106:12879–12884. doi: 10.1073/pnas.0901653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy AK, Campbell KS. Natural killer cells and cancer: regulation by the killer cell Ig-like receptors (KIR) Cancer Biol Ther. 2009;8:2211–2220. doi: 10.4161/cbt.8.23.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Hideshima T, Akiyama M, Podar K, Yasui H, Raje N, et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. Br J Haematol. 2005;128:192–203. doi: 10.1111/j.1365-2141.2004.05286.x. [DOI] [PubMed] [Google Scholar]
- Fujii H, Trudeau JD, Teachey DT, Fish JD, Grupp SA, Schultz KR, et al. In vivo control of acute lymphoblastic leukemia by immunostimulatory CpG oligonucleotides. Blood. 2007;109:2008–2013. doi: 10.1182/blood-2006-02-002055. [DOI] [PubMed] [Google Scholar]
- Brandau S, Riemensberger J, Jacobsen M, Kemp D, Zhao W, Zhao X, et al. NK cells are essential for effective BCG immunotherapy. Int J Cancer. 2001;92:697–702. doi: 10.1002/1097-0215(20010601)92:5<697::aid-ijc1245>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jha P, Golovko D, Bains S, Hostetter D, Meier R, Wendland MF, et al. Monitoring of natural killer cell immunotherapy using noninvasive imaging modalities. Cancer Res. 2010;70:6109–6113. doi: 10.1158/0008-5472.CAN-09-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher MF, Gambhir SS, Grimm J. Noninvasive cell-tracking methods. Nat Rev Clin Oncol. 2011;8:677–688. doi: 10.1038/nrclinonc.2011.141. [DOI] [PubMed] [Google Scholar]
- Meller B, Frohn C, Brand JM, Lauer I, Schelper LF, von Hof K, et al. Monitoring of a new approach of immunotherapy with allogenic (111)In-labelled NK cells in patients with renal cell carcinoma. Eur J Nucl Med Mol Imaging. 2004;31:403–407. doi: 10.1007/s00259-003-1398-4. [DOI] [PubMed] [Google Scholar]
- Tavri S, Jha P, Meier R, Henning TD, Muller T, Hostetter D, et al. Optical imaging of cellular immunotherapy against prostate cancer. Mol Imaging. 2009;8:15–26. [PubMed] [Google Scholar]
- Sutton EJ, Henning TD, Pichler BJ, Bremer C, Daldrup-Link HE. Cell tracking with optical imaging. Eur Radiol. 2008;18:2021–2032. doi: 10.1007/s00330-008-0984-z. [DOI] [PubMed] [Google Scholar]
- Pichler BJ, Kolb A, Nagele T, Schlemmer HP. PET/MRI: paving the way for the next generation of clinical multimodality imaging applications. J Nucl Med. 2010;51:333–336. doi: 10.2967/jnumed.109.061853. [DOI] [PubMed] [Google Scholar]
- Patel D, Kell A, Simard B, Xiang B, Lin HY, Tian G. The cell labeling efficacy, cytotoxicity and relaxivity of copper-activated MRI/PET imaging contrast agents. Biomaterials. 2011;32:1167–1176. doi: 10.1016/j.biomaterials.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Iliopoulou EG, Kountourakis P, Karamouzis MV, Doufexis D, Ardavanis A, Baxevanis CN, et al. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother. 2010;59:1781–1789. doi: 10.1007/s00262-010-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapteva N, Durett AG, Sun J, Rollins LA, Huye LL, Fang J, et al. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy. 2012;14:1131–1143. doi: 10.3109/14653249.2012.700767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Terunuma H, Nieda M, Xiao W, Nicol A. Synergistic cytotoxicity of ex vivo expanded natural killer cells in combination with monoclonal antibody drugs against cancer cells. Int Immunopharmacol. 2012;14:593–605. doi: 10.1016/j.intimp.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Voskens CJ, Watanabe R, Rollins S, Campana D, Hasumi K, Mann DL. Ex-vivo expanded human NK cells express activating receptors that mediate cytotoxicity of allogeneic and autologous cancer cell lines by direct recognition and antibody directed cellular cytotoxicity. J Exp Clin Cancer Res. 2010;29:134. doi: 10.1186/1756-9966-29-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutlu T, Stellan B, Gilljam M, Quezada HC, Nahi H, Gahrton G, et al. Clinical-grade, large-scale, feeder-free expansion of highly active human natural killer cells for adoptive immunotherapy using an automated bioreactor. Cytotherapy. 2010;12:1044–1055. doi: 10.3109/14653249.2010.504770. [DOI] [PubMed] [Google Scholar]
- Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]