Abstract
In clinical therapy, the amount of antigen administered to achieve oral tolerance for allergic diseases is large, and the cost is a major consideration. In this study, we used tobacco plants to develop a large-scale protein production system for allergen-specific immunotherapy, and we investigated the mechanisms of oral tolerance induced by a transgenic plant-derived antigen. We used plants (tobacco leaves) transgenic for the Dermatophagoides pteronyssinus 2 (Der p2) antigen to produce Der p2. Mice received total protein extract from Der p2 orally once per day over 6 days (days 0–2 and days 6–8). Mice were also sensitized and challenged with yeast-derived recombinant Der p2 (rDer p2), after which the mice were examined for airway hyper-responsiveness and airway inflammation. After sensitization and challenge with rDer p2, mice that were fed with total protein extracted from transgenic plants showed decreases in serum Der p2-specific IgE and IgG1 titers, decreased IL-5 and eotaxin levels in bronchial alveolar lavage fluid, and eosinophil infiltration in the airway. In addition, hyper-responsiveness was also decreased in mice that were fed with total protein extracted from transgenic plants, and CD4+CD25+Foxp3+ regulatory T cells were significantly increased in mediastinal and mesenteric lymph nodes. Furthermore, splenocytes isolated from transgenic plant protein-fed mice exhibited decreased proliferation and increased IL-10 secretion after stimulation with rDer p2. The data here suggest that allergen-expressing transgenic plants could be used for therapeutic purposes for allergic diseases.
Keywords: asthma, Der p2, IL-10, oral tolerance, transgenic plant
Introduction
Asthma affects nearly 155 million individuals worldwide, and the incidence is increasing.1 The dysregulation of allergen-specific immune responses plays a central role in the generation of asthma. Clinical and experimental studies of allergic inflammation and asthma suggest that CD4+ T helper (Th) 2 cells are the major effector cells in airway inflammation, and they are associated with lung dysfunction in their recruitment and activation of eosinophils.2, 3, 4
Oral tolerance, the induction of tolerance by the repeated oral administration of an antigen that induces specific inhibition of cellular and humoral immune responses, has been used in numerous disease therapeutic studies. For example, the administration of an oral antigen suppresses autoimmune diseases in animal models including experimental autoimmune encephalitis, arthritis and non-autoimmune diseases such as asthma, allergy, stroke and Alzheimer's disease.5 Several mechanisms of oral tolerance have been proposed: deletion of antigen-specific T cells (clonal deletion),6 immune deviation,7 induction of anergy8 and suppression of antigen-reactive cells by regulatory T cells (Tregs).9
In clinical settings, oral tolerance has been used to treat allergic diseases. For example, specific immunotherapy has been practiced on patients in an attempt to achieve tolerance by the administration of low-dose allergen over long periods of time. This administration has been tested via different routes including subcutaneous, sublingual, oral, local bronchial and local nasal delivery.10 The traditional subcutaneous route has a risk of severe adverse events; therefore, safer routes of administration, such as the sublingual route, have been tested in numerous controlled trials and have shown show long-lasting efficacy in asthma and rhinitis in adults and children.11, 12, 13, 14 Sublingual immunotherapy is now accepted by the World Health Organization as a valid alternative to the subcutaneous route of administration in asthmatic children.15, 16
However, sublingual immunotherapy has limitations because the duration of allergen administration is long and its costs are considerable.11 Development of a convenient and cost-effective delivery method is still required. Allergen-transgenic plants can serve this purpose because the cost of large-scale production is very low, and extraction procedures may not be needed if edible plants are used. In addition, the exceptional stability of transgenic proteins allows for ease of storage and transportation.17 Additionally, using plants to express recombinant proteins prevents production of unwanted byproducts such as endotoxin production, which can be produced from Escherichia coli expression systems.
Here we have shown that feeding transgenic plant-derived allergen protein in a murine model of asthma can suppress airway inflammation and hyper-responsiveness. This study further indicates that oral delivery of allergen proteins extracted from transgenic plants may be a feasible approach for the treatment of allergic diseases.
Materials and methods
Generation of Dermatophagoides pteronyssinus 2 (Der p2)-transgenic plants and recombinant Der p2 (rDer p2)
Transfection was performed by coculturing 100 µl of Der p2-pCambia2300 (CAMBIA, Canberra, Australia) plasmid-transformed Agrobacterium-containing MSG medium (MS salts, Gamborg's B5 vitamins, 3% sucrose, pH 5.8) and 0.5 ml 200 µM acetosyringone with 3-week-old tobacco (Nicotiana tabacum L. cv. W38 strain) leaf slices (1 cm2) on a BM agar plate (MS salts, Gamborg's B5 vitamins, 0.4 µg/ml BAP, 3% sucrose, 0.8% agar, pH 5.7) for 2 days in the dark at 27 °C.
Infected leaf slices were transferred to BM agar plates containing 200 µg/ml kanamycin and 300 µg/ml cefotaxime–HCl. Plates were incubated in 16 h light/8 h dark cycle at 27 °C. The leaves germinated within 3–4 weeks. Transfected Agrobacterium clones were confirmed by PCR. To measure the Der p2 expression level of each clone, we performed western blotting using the anti-Der p2 monoclonal antibody kindly provided by Dr KT Lee's lab at the Institution of Agriculture Chemistry, National Taiwan University (Taipei, Taiwan).
Plant cell suspension cultures and protein extraction was performed by Dr KT Lee's lab at the Institution of Agriculture Chemistry, National Taiwan University. Briefly, callus from a selected positive clone (the clone was named +11; this clone expressed Der p2 in the cytosol at a concentration of 0.5% of total protein) was added to a suspension culture without light. Total protein was extracted and concentrated by ultrafiltration, which excluded low molecular weight molecules such as nicotine and tobacco tar. The total protein was frozen in liquid nitrogen and stored at −20 °C. Each vial was thawed only once and stored at 4 °C after being centrifuged at 12,000 rpm to remove precipitate. Der p2 protein concentrations were quantified by Western blot using rDer p2 as a standard.
rDer p2 was generated by pichia (His+ Muts), which was kindly provided by Dr KY Chua (Department of Pediatrics, National University of Singapore). For 1 l of BMGY medium culture (o.d.600=6), induction was performed in 200 ml BMMY medium for 48 h at 30 °C. Culture supernatant was collected and dialyzed against 20 mM CHCOONa, pH 5.0 (binding buffer). Then the sample was applied to HighTrap SP Sepharose (GE Healthcare (Niskayuna, NY, USA)) for ion-exchange purification. Protein samples, binding buffer, and wash buffer containing 10 mM NaCl and elution buffer containing 150 mM NaCl were all passed through a 0.22 µm filter before use.
Establishment of murine model of asthma
Female 6–8-week-old BALB/c mice were obtained from the Animal Center of the College of Medicine, National Taiwan University. Animal care and handling protocols were approved by the Animal Committee of National Taiwan University. The BALB/c mice were immunized with intraperitoneal injections of 30 µg of rDer p2 mixed with 4 mg alum (Pierce Biotechnology, Rockford, IL, USA) and 200 ng pertussis toxin (List Biological Lab, Cambell, CA, USA) on days 1, 14 and 28. On days 1–3 and 6–8, mice were fed once per day with 651 µg protein extracted from transgenic plants (containing 100 µg rDer p2), 651 µg protein extracted from wild-type (WT) plants, 100 µg rDer p2 extracted from yeast, or buffer. All proteins were dissolved in 3% Na2CO3 buffer. On day 21, all mice were bled to measure the Der p2-specific IgG and IgE titers by sandwich ELISA. On days 34 and 36, all mice were challenged by intratracheal injections of 5 µg of rDer p2 antigen to induce airway inflammation. Mice that were not immunized with Der p2 were used as the negative control group.
Bronchoalveolar lavage and lung histology
Bronchoalveolar lavages were performed using 1 ml HBSS instilled bilaterally with a syringe. The bronchoalveolar lavage fluid (BALF) was harvested three times by gentle aspiration and then centrifuged. Differential cell counts were assessed using cytological preparations. Slides of cells were prepared with a cytospin and stained with Liu staining. A total of 300 cells were counted using microscopy. BALF supernatants were then assayed by ELISA.
The lungs were fixed with 10% neutral phosphate-buffered formalin. Sections were prepared and stained with hematoxylin/eosin to quantitate the number of infiltrating inflammatory cells by microscopy.
Determination of cytokine expression
IL-4, IL-5, IL-10, transgenicF-β, eotaxin, IL-13 and IFN-γ levels were measured by ELISA according to the manufacturer's instructions (R&D, Minneapolis, MN, USA).
Splenocyte proliferation assay
Cells were cultured in AIM-V medium supplemented with 2% TCM (mouse serum replacement; Celox Corp., Oakdale, MN, USA) in the presence or absence of rDer p2 (5, 10 and 20 µg/ml) or anti-CD3 plus anti-CD28 (1 µg/ml each). After incubation for 2 days, 1 µCi/well of [H3]TdR was added and incubated for 17 h. Counts per minute (c.p.m) were read with a β-counter (Packard Instrument Co., Meriden, CT, USA). The stimulation index was defined as the counts per minute of stimulated cultures divided by the counts per minute of cultures in media only.
Whole body plethysmography
Airway responsiveness was measured in unrestrained animals by barometric whole body plethysmography (Buxco, Troy, NY, USA).18 Briefly, mice were placed in the main chamber, and baseline readings were taken and averaged for 3 min. Aerosolized phosphate-buffered saline (PBS) or methacholine (MCh) was nebulized in increasing concentrations (6.25–50 mg/ml) through an inlet of the main chamber for 3 min, and readings were taken and averaged for 3 min after each nebulization. Recordings of every 10 breaths were analyzed to define the respiratory rate in breaths per minute. Airway reactivity was expressed as an enhanced pause (Penh), and data were expressed as the ratio of PenhMCh values compared to PenhPBS from three independent experiments.
Measurement of airway resistance in anesthetized mice
Airway resistance was assessed as an increase in pulmonary resistance after challenge with aerosolized MCh in anesthetized mice using a modification of the techniques described by Glaab et al.19 Mice were anesthetized with 70–90 mg/kg pentobarbital sodium (Sigma-Aldrich, St Louis, MO, USA), tracheostomized and mechanically ventilated at a rate of 150 breaths/min, a tidal volume of 0.3 ml and a positive end-expiratory pressure of 3–4 cmH2O with a computer-controlled small animal ventilator (Harvard Rodent Ventilator, model 683; Harvard Apparatus, South Natick, MA, USA). PE-50 tubing was inserted into the esophagus to the level of the thorax and coupled with a pressure transducer (LDS GOULD, Valley View, OH, USA). Flow was measured by electronic differentiation of volume signal. Pressure, flow and volume changes were recorded. Pulmonary resistance was calculated by a software program (Model PNM-PCT100W, LDS PONEMAH Physiology Platform; LDS GOULD). MCh of 25 mg/ml aerosol was generated with an inline nebulizer and administrated directly through the ventilator. The resistance of the orotracheal tube (0.48 cmH2O s/ml) was subtracted from all airway resistance measurements.
Der p2-specific antibody assay
Total sera and anti-Der p2 IgE and IgG1 antibody titers were determined by ELISA. Briefly, 96-well microtiter plates were coated with 10 µg/well Der p2 (Pharmingen, San Diego, CA, USA) in NaHCO3 buffer (pH 9.6). After overnight incubation at 4 °C, the plates were washed and blocked with 3% bovine serum albumin in PBS for 2 h at room temperature. Serum samples were diluted and added to each well overnight at 4 °C. Plates were then washed. Either biotin-conjugated anti-mouse IgE or IgG1 (0.5 mg/ml; Pharmingen) diluted in 3% bovine serum albumin–PBS buffer (1∶500) was added for 45 min at room temperature. Avidin-conjugated horseradish peroxidase (1∶5000; Pierce Biotechnology) was then added for another 30 min at room temperature. The reaction was developed with addition of the peroxidase substrate 3,3′,5,5′-tetramethylbenzidine (KPL, Gaithersburg, MD, USA) and then stopped by addition of 2 N H2SO4. Absorbance was determined at 450 nm in a microplate reader. Antibody levels were compared to standard serum, and IgG1 and IgE concentrations in standard serum were arbitrarily assigned 1 ELISA unit.
Lymph node preparation
Mediastinal and mesenteric lymph nodes were harvested and pooled from each group at the time of killing. Single-cell suspensions were obtained by mechanical disruption. Cells were stimulated in vitro with 5 µg/ml Der p2 for 72 h. The cell medium was collected for cytokine analysis.
Flow cytometry analysis
Freshly isolated lymph nodes were incubated with FITC- and allophycocyanin-labeled mAbs to mouse CD4 and CD25 (eBioscience, San Diego, CA, USA). For analysis of intracellular Foxp3, stained cells were fixed and permeabilized with Cytofix/Cytoperm solution (eBioscience) according to the manufacturer's suggested protocol and then incubated with PE-conjugated anti- Foxp3 (eBioscience) at 4 °C for 30 min in the dark. Cells were then washed and analyzed by flow cytometry (FACSCalibur; BD Bioscience).
Statistical analysis
All values refer to mean±s.e.m. using one-way ANOVA followed by Newman–Keuls post hoc comparisons. The criterion significance was set at P<0.05.
Results
Generation of transgenic plants
We used the pCambia2300 vector containing transferred DNA (T-DNA) for the expression of Der p2. This vector was transferred into tobacco leaf slices by Agrobacterium-mediated transformation.
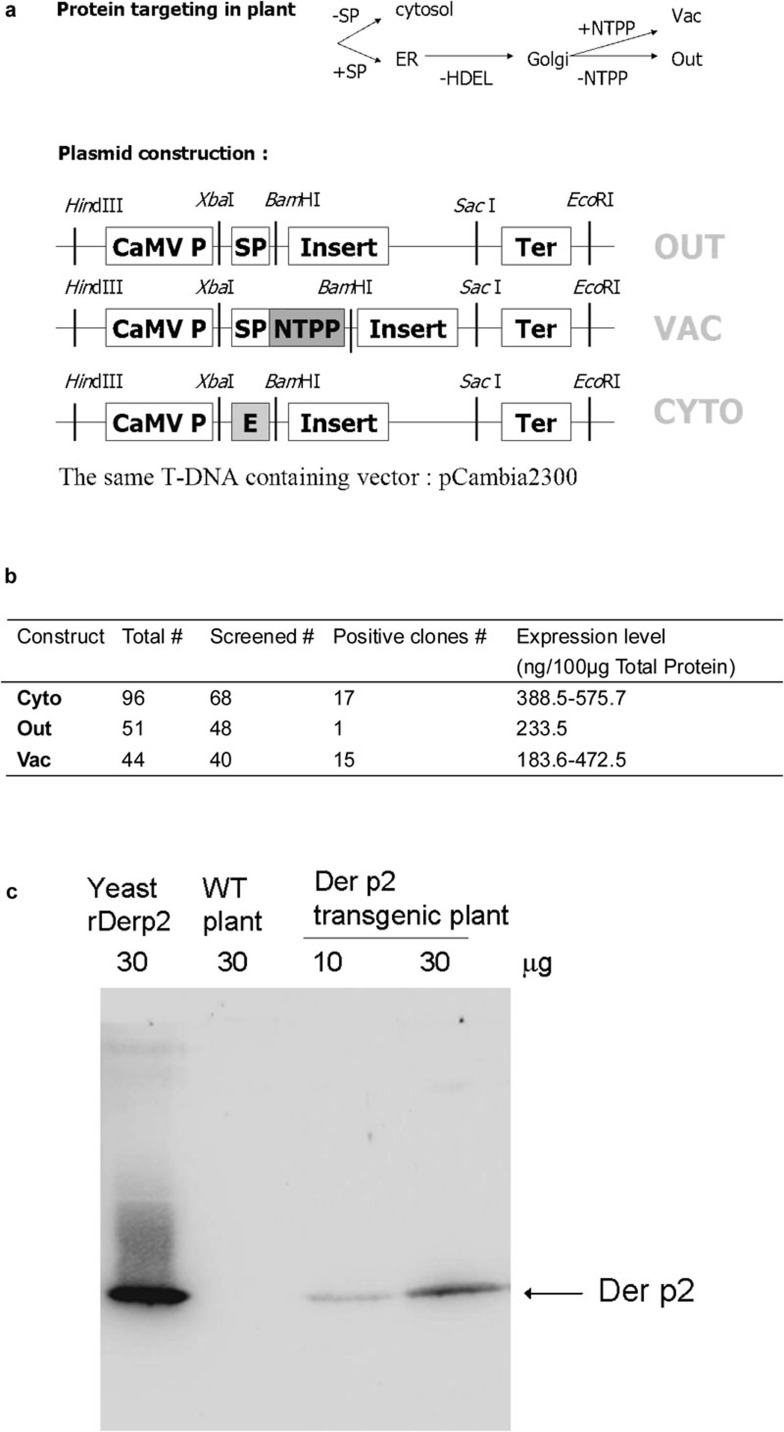
To obtain an optimal expression level in different cellular compartments, three different plasmid constructs that target protein secretion to different organelles were constructed and are summarized in Figure 1a. One construct, derived from Jobling and Gehrke in 1987,20 contains an enhancer sequence for translation in the N′-terminus of the target gene and expresses the protein product in the cytosol. The other two constructs contain signal peptides that direct the protein product to a secretory pathway and then direct excision of the signal peptide after localization. One of these plasmids lacks a stop codon and allows transport of the protein product out of the cell membrane. The other plasmid has a vacuolar targeting signal that transports the protein product to the vacuole,21 the largest organelle in plant cells. Soramin is a protein that accumulates in large quantities in vacuoles, and hence, it is thought that larger spaces might be important for enhancing protein secretion. Thus, we wanted to target our protein to either the cellular matrix or the vacuole, both of which are larger than the cytosol.
Figure 1.
T-DNA regions of plasmids constructs used to create the transgenic plant. (a) T-DNA regions of plasmids constructs used to create the transgenic plant. (b) The structure of T-DNA regions and screening of the three transgene constructs in the resulting plant clones. (c) Der p2 protein levels extracted from transgenic plants were measured by western–eastern blotting analysis. CaMV P, CaMV35S promoter; Der p2, Dermatophagoides pteronyssinus 2; ER, endoplasmic reticulum; HDEL, ER retention signal; NTPP, vacuole-targeting signal; SP, signal peptide; T-DNA, transferred DNA; WT, wild type.
The highest expression level of Der p2 protein obtained in the cytosol-targeting group was 0.5% of total protein (Figure 1b), while the group with the highest frequency of Der p2 positive clones, as determined by western blotting, was in the vacuole-targeting group. However, the overall expression levels were similar between the cytosol-targeting group and vacuole-targeting group (Figure 1b). The ratio of target protein to total protein in our system was higher than that obtained from a previous study.17 In this study, we chose the clone with the highest Der p2 expression level (Der p2 expressed in the cytosol) for the protein extraction. The amount of Der p2 from a total protein extraction of 35 g dry weight callus can be up to 0.185 g. The Der p2 expression in transgenic tobacco plant cells is shown in Figure 1c. After extraction and concentration, the Der p2 expression level was elevated to about 15% of total protein.
Total protein extracted from Der p2-transgenic plants suppresses Der p2-specific IgE and IgG1 titers in sensitized mice
The in vivo effect of Der p2 feeding was analyzed by feeding BALB/c mice with Der p2 from different sources. First, we investigated whether oral feeding of total protein extracted from Der p2-transgenic plants (transgenic plants) could decrease serum Der p2-specific IgE and IgG1 titers.
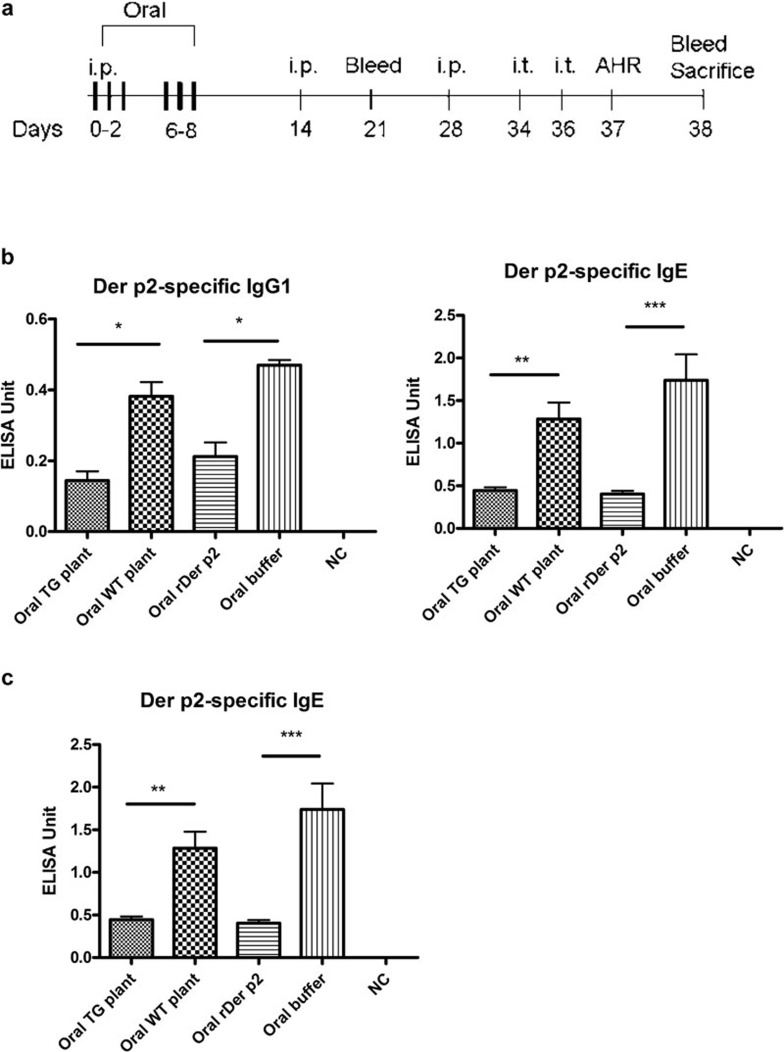
Starting on the day of immunization, mice were fed for 3 days (days 0–2) in the first week and another 3 days in the following week (days 6–8) with 0.1 mg/day yeast-derived rDer p2 or total protein extracts from transgenic plants containing 0.1 mg Der p2. We fed the mice orally at time points when the immune response to Der p2 was not established (Figure 2a). Seven days after the boost, serum levels of Der p2-specific antibodies were detected in these mice. Both Der p2-specific IgE and IgG1 titers decreased after feeding (Figure 2b). In addition, the inhibition of Der p2-specific IgE titer was sustained after Der p2 challenge (Figure 2c).
Figure 2.
Detection of specific antibodies after sensitization of mice fed with Der p2 protein. (a) A time course of Der p2 oral feeding and experimental set-up of asthma murine model is shown. (b) Mice fed with either recombinant Der p2 or Der p2-TG plant extract expressed diminished Der p2-specific IgE and IgG1 titers when mice were bled a week after the first boost. (c) Der p2-specific IgE titers are shown from mice that were bled 1 day after final challenge. NC represents a negative control group where the mice were not immunized with Der p2. Data are expressed as mean±s.e.m. (n≥6). *P<0.05, **P<0.01, ***P<0.001. AHR, airway hyper-responsiveness; Der p2, Dermatophagoides pteronyssinus 2; rDer p2, recombinant Der p2; TG, transgenic; WT, wild type.
Total protein extract from Der p2-transgenic plant suppresses airway inflammation and BALF cytokine release
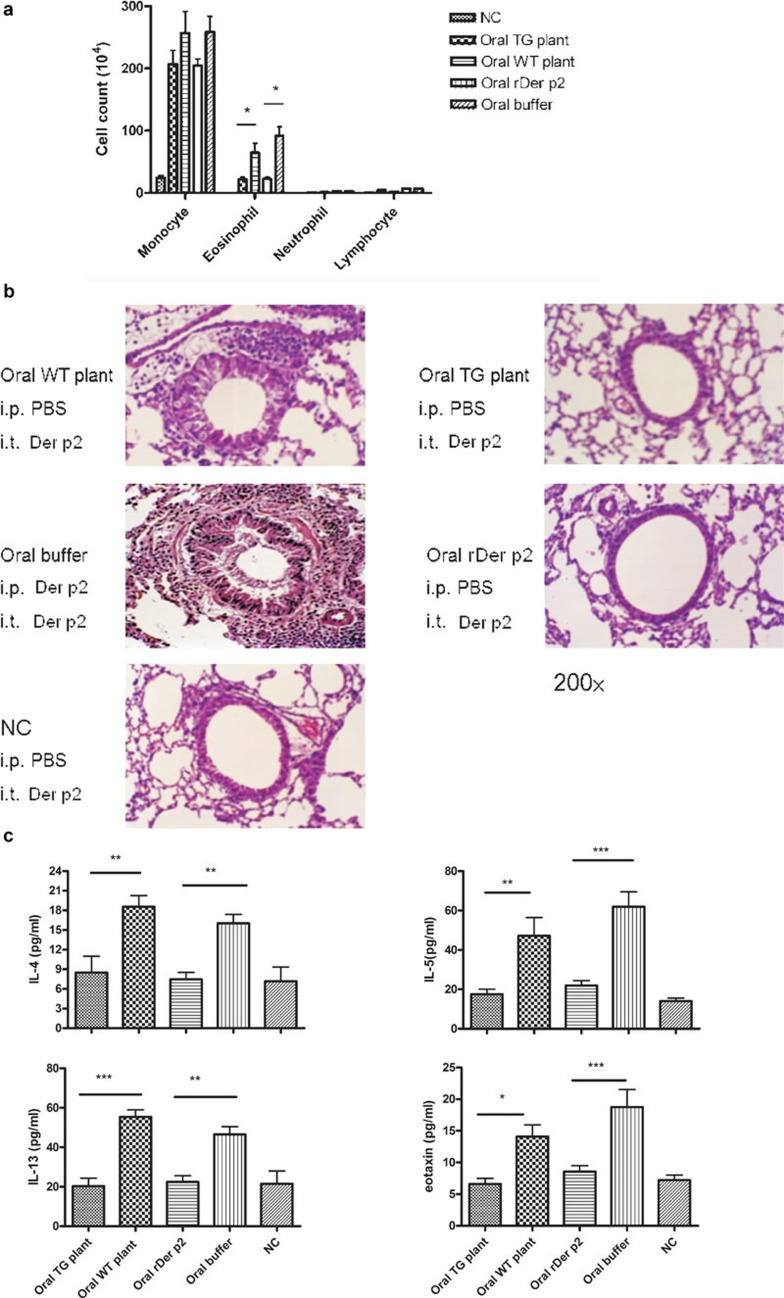
The Der p2-sensitized mice were subsequently challenged via intratracheal (i.t.) administration of rDer p2 (5 µg on days 34 and 36) to induce airway inflammation. Feeding mice with total protein extracted from Der p2-transgenic plants (oral transgenic plant group) or rDer p2 extracted from yeast (oral rDer p2 group) appeared to inhibit the recruitment of eosinophils to the airway in BALF, as compared with mice orally fed with total protein extracted from WT plants (oral WT plant group) or buffer (oral buffer group) (Figure 3a). Histological studies also support this conclusion (Figure 3b). Lung tissue from oral WT plant and oral buffer groups showed streaks of mucus in the lumen and numerous inflammatory cells surrounding the airways. After oral feeding with total protein extracted from transgenic plants or rDer p2 from yeast, Der p2-sensitized and challenged mice showed minimal mucus production and negligible cellular infiltration similar to negative control mice that were not immunized with Der p2.
Figure 3.
Decrease of airway inflammation and BALF cytokines after feeding with total protein extracted from Der p2-TG plants in mice. (a) Inflammatory cell profile in BALF. (b) Histopathological examination of lung tissues. (c) Cytokine levels in BALF. Data are expressed as mean±s.e.m. NC represents the negative control group (n≥6). *P<0.05, **P<0.01, ***P<0.001. BALF, bronchoalveolar lavage fluid; Der p2, Dermatophagoides pteronyssinus 2; PBS, phosphate-buffered saline; rDer p2, recombinant Der p2; TG, transgenic; WT, wild type.
Further investigation of BALF cytokine expression after oral feeding of total protein extracted from transgenic plants or rDer p2 from yeast revealed decreased IL-4, IL-13, IL-5 and eotaxin levels relative to the oral WT plant and oral buffer groups (Figure 3c).
Airway hyper-responsiveness decreases after oral administration of Der p2-transgenic plants
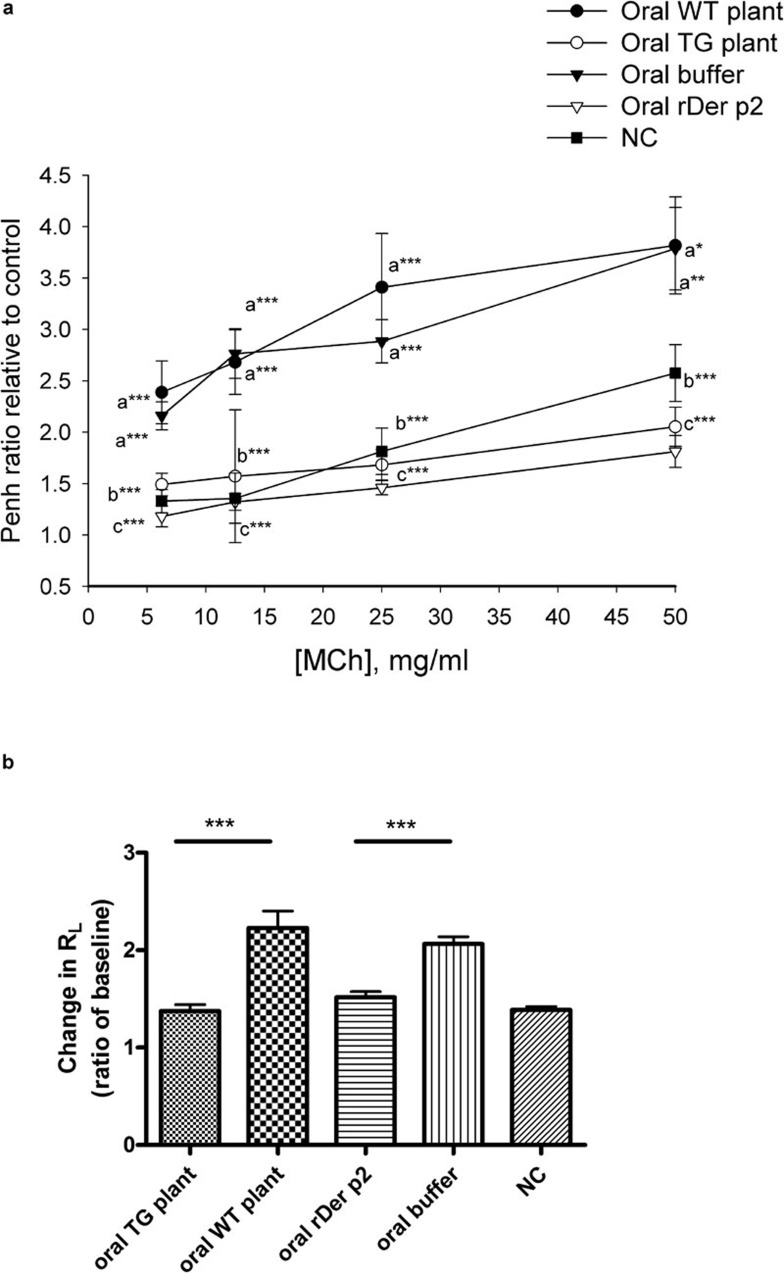
We analyzed whether oral feeding of total protein extracted from transgenic plants would affect the development of airway hyper-responsiveness in a murine model of asthma. One day after the final challenge, airway responsiveness and pulmonary resistance was assessed by non-invasive whole body plethysmography and invasive body plethysmography, respectively. Relative to PBS-sensitized and challenged mice (Figure 4; the oral WT plant and oral buffer groups versus the negative control group), BALB/c mice that had been sensitized and challenged with Der p2 had an increase in the Penh ratio (Figure 4a) and lung resistance (RL) (Figure 4b) after exposure to MCh. Following the oral feeding of total protein extracted from TG plants or rDer p2 from yeast, the levels of Penh ratios and RL were similar to those of the negative control group; therefore, Der p2-sensitization and challenge-induced hyper-responsiveness formation was suppressed in mice fed with transgenic plant Der p2 or rDer p2 relative to the oral buffer or oral WT plant groups.
Figure 4.
Airway hyper-responsiveness is inhibited by oral feeding of total protein extracted from Der p2-TG plants in mice. (a) Airway hyper-responsiveness was measured by non-invasive whole body plethysmography as described in the section on ‘Materials and methods'. Data are expressed as mean±s.e.m. of the Penh value ratio after PBS nebulization from three independent experiments (n≥5). NC represents the negative control group. a*P<0.05, a**P<0.01, a***P<0.001 versus negative control group; b***P<0.001 versus oral WT plant group; c***P<0.001 versus oral buffer group. (b) Airway resistance as measured by invasive body plethysmography. Data are expressed as mean±s.e.m. of the pulmonary resistance (RL) ratio after PBS nebulization from three independent experiments (n≥5). ***P<0.001. NC represents negative control group. Der p2, Dermatophagoides pteronyssinus 2; MCh, methacholine; PBS, phosphate-buffered saline; rDer p2, recombinant Der p2; TG, transgenic; WT, wild type.
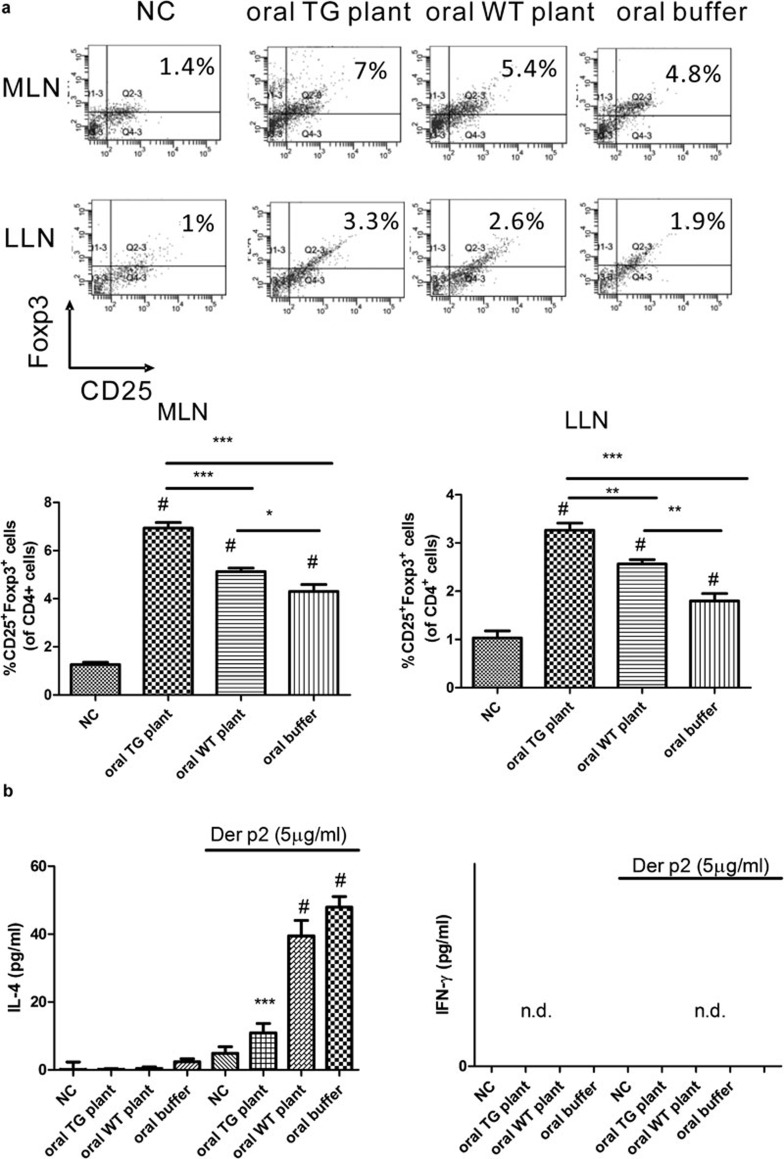
Oral feeding of total protein extracted from Der p2-transgenic plant increases CD4+CD25+Foxp3+ Tregs in mesenteric and mediastinal lymph nodes
We found that oral feeding of total protein extracted from transgenic plants induced mucosal tolerance similar to feeding with rDer p2. Next, we examined the mechanisms of mucosal tolerance induced by oral feeding of total protein extracted from transgenic plants. On day 38, we identified CD4+CD25+Foxp3+ Tregs from mediastinal (LLN) and mesenteric lymph nodes (MLN). After oral feeding of total protein extracted from TG plants, the level of CD4+CD25+Foxp3+ Tregs in MLN and LLN was significantly increased relative to mice that were fed with buffer or total protein extracted from WT plants (Figure 5a). Next, we measured cytokine expression in MLN cells. After stimulation with rDer p2, the level of IL-4 was significantly decreased in mice that were fed with total protein extracted from TG plants relative to mice that were fed with buffer or total protein extracted from WT plants (Figure 5b). We did not detect a difference in IFN-γ expression between different groups.
Figure 5.
Increase in frequency of CD4+CD25+Foxp3+ cells in mesenteric (MLN) and mediastinal lymph node (LLN), and decrease in IL-4 production in mesenteric lymph node cells after oral feeding of total protein extracted from Der p2-TG plants. (a) On day 38, the frequency of CD4+CD25+Foxp3+ cells in MLN and LLN were analyzed by flow cytometry. (b) On day 38, isolated MLN cells were treated with 5 µg/ml rDer p2 for 72 h and supernatants were collected for analysis of cytokine production by ELISA. Data are expressed as mean±s.e.m. (n≥3). NC represents negative control group. #P<0.001 compared with NC group, *P<0.05, **P<0.01, ***P<0.001. Der p2, Dermatophagoides pteronyssinus 2; IFN, interferon; rDer p2, recombinant Der p2; TG, transgenic; WT, wild type.
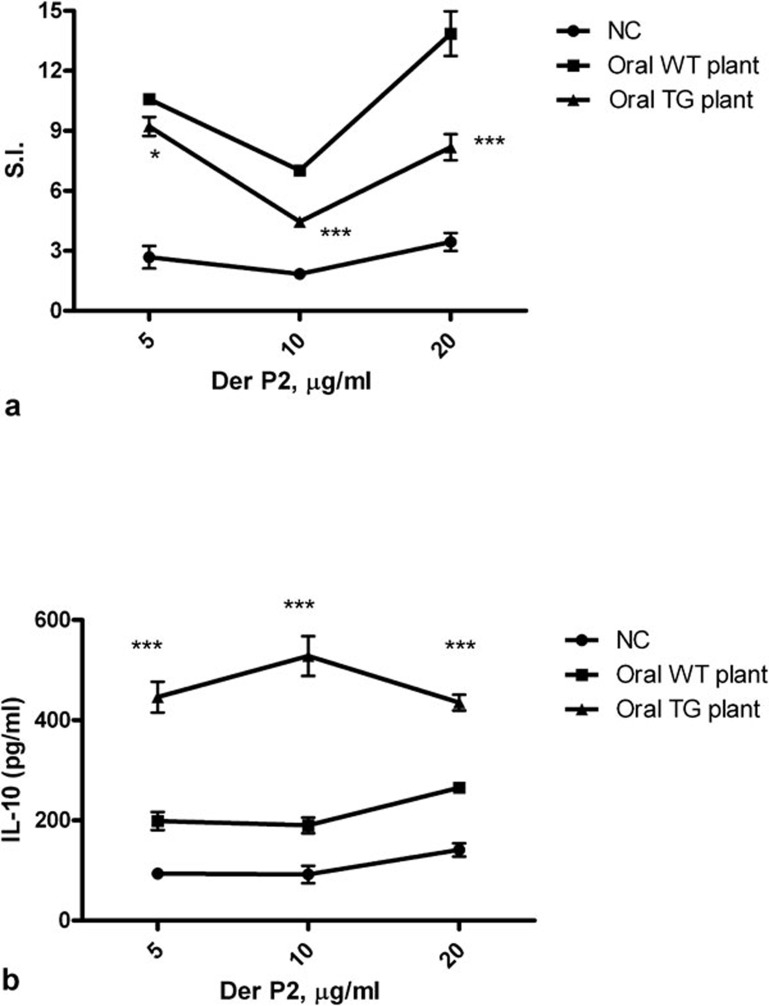
Decreased proliferation response and increased IL-10 secretion in splenocytes from mice fed with transgenic plants upon antigen stimulation
To further investigate the systemic immune response after oral feeding of total protein extracted from TG plants, we cultured splenocytes from both Der p2-sensitized and challenged mice fed with proteins derived from TG or WT plants. After stimulation with rDer p2, the stimulatory index (SI) value of the oral TG-fed group was significantly lower than that of the WT plant group (Figure 6a). IL-10 in the supernatants of the oral TG group was significantly higher than that of the oral WT plant group (Figure 6b).
Figure 6.
Decreased proliferation and increased IL-10 production in splenocytes from mice fed with total protein from Der p2-TG plants. (a) Proliferation of splenocytes upon stimulation with rDer p2. Mice were fed with TG plant extracts containing 100 µg Der p2 or WT plant extracts from the day of immunization. (b) IL-10 secreted in the splenocytes supernatants of splenocyte cultures upon rDp2 stimulation. NC represents the negative control group. Data are expressed as mean±s.e.m. (n≥5). *P<0.05, ***P<0.001 versus the oral WT plant group. Der p2, Dermatophagoides pteronyssinus 2; rDer p2, recombinant Der p2; TG, transgenic; WT, wild type.
Discussion
Der p is a major allergen worldwide. Approximately 80% of asthmatics in Taiwan are sensitized by the house dust mite, Der p. Among those with hypersensitivity to Der p, 87.8% (85.4% of adults and 90.2% of children) of the asthmatic patients in Taiwan were allergic to Der p2.22 However, most of the information about the induction of mucosal tolerance as a treatment for allergic diseases comes from murine models using ovalbumin, derived from eggs, as the model antigen. Ovalbumin is an airway inflammation-inducing allergen used in many murine models because of its availability, but most patients are not allergic to eggs.9 Therefore, in this study, we used Der p2 as an antigen to establish a murine model of asthma. Mice that were orally fed with total protein extracted from TG plants effectively inhibited Der p2-specific IgE and IgG1 titers, BALF IL-4, IL-5, IL-13 and eotaxin production, airway inflammation, airway hyper-responsiveness and displayed suppressed splenocyte proliferation.
Specific immunotherapy with whole allergen could induce unwanted anaphylactic reactions in patients, as indicated by increasing serum antigen-specific IgE levels during the desensitization process;23, 24 however, this phenomenon was not observed in our study, and oral feeding with total protein extracted from TG plants suppressed the Der p2-specific IgE levels in serum. In addition, a previous study found that long-term subcutaneous specific immunotherapy with native allergens Der p1 and Der p2 decreased serum specific IgE and asthma symptoms in patients.25 Therefore, oral feeding with whole allergen is feasible with regard to specific immunotherapy for asthma.
The mechanisms of oral tolerance induced by the feeding of total proteins extracted from TG plants were investigated by performing several experiments. Recently, many researchers have found that orally induced Tregs are functional and inhibit inflammation both locally in the gut and systemically. For example, orally fed ovalbumin-induced adaptive Foxp3+ Tregs inhibited allergic inflammation in mice.26 Therefore, we first looked for the presence of Tregs in LLN and MLN, and we found that Tregs in the LLN and MLN were increased in mice fed with total protein extracted from TG plants. The mechanisms of Treg-induced inhibition of effector T cells could be through expression of inhibitory cytokines such as IL-10 or TGF-β, cytolysis, metabolic disruption, cAMP-mediated inhibition, CD25-mediated apoptosis, CD39- and/or CD73-generated, adenosine receptor 2A-mediated immunosuppression, or inhibition of dendritic cell maturation and function.27 We also investigated levels of suppressive cytokines by measuring IL-10 and TGF-β in BALF and supernatants from splenocyte cultures. These cytokines were not detected in BALF; however, IL-10 levels were increased in supernatants from splenocyte cultures from mice fed with total protein extracted from TG plants. IL-10 is thought to be involved in several regulatory mechanisms including the downregulation of mast cell function, suppression of IgE, decreased eosinophil-sensitive chemokine production and reduced eosinophil survival.28 These results support the notion that suppression of experimental allergy by transgenic plants may be associated with the induction of IL-10. Recently, expression of IL-10 was shown to not be specific for Th2 or Tregs but is a broadly expressed cytokine. IL-10 can be expressed by immune cells of the adaptive immune system including Th1s, Th2s, Tregs, CD8+ T cells and B cells29 and by innate cells such as dendritic cells, macrophages, natural killer cells, mast cells, eosinophils and neutrophils.30 Therefore, any significant contribution of IL-10 in Der p2-transgenic plant-mediated suppression of allergic airway disease would require further investigation.
Second, we measured levels of the Th1 cytokine IFN-γ which is known to exert inhibitory effects on allergic responses31 in the BALF. We found that after Der p2 immunization, BALF, MLN and supernatants from splenocyte cultures from mice fed with the transgenic plants had IFN-γ levels below the detection level (data not shown). We also found that the serum-specific IgG2a levels were also low in mice fed with total protein extracted from the Der p2-transgenic plants, similar to levels found in mice fed with total protein extracted from WT plants (data not shown). Thus, IFN-γ appears not to be involved in the TG-induced oral tolerance in this murine model of asthma.
In addition, we also found decreased lung inflammation in lung tissue sections from mice that were orally fed with total protein extracted from WT plants. We hypothesized that the decrease in inflammation might be associated with increasing numbers of Tregs in MLN; however, the mechanism needs further investigation.
The development of plant-derived pharmaceuticals for disease therapy is an emerging field in vaccine research,32 especially because models using viral and bacterial infections may have unwanted side effects. Current conventional specific immunotherapy is expensive to prepare and requires multiple infections of allergen over a period of years.33 Thus, the availability of proper allergens expressed in transgenic plants in large quantities may facilitate clinical therapy for the establishment of suppressive responses in allergic diseases. According to previous studies,34 one of the limitations in the development of transgenic plants for pharmaceuticals is the low expression level of recombinant foreign protein. In general, levels of recombinant protein produced by transgenic plants range from 0.001% to 1% of total soluble proteins and may not compete with other recombinant protein production systems (E. coli or yeast) at present. However, the advantages of protein expression in plant systems include the potentially high accumulation levels of the protein, appropriate post-translational processing and natural storage stability of proteins produced in plants.35 The cost of downstream processing may determine whether a particular plant production is competitive with traditional fermentation systems. Using partially purified plant proteins or edible plants, which do not need purification, could provide low production costs of recombinant proteins. Thus, an important project currently underway is the generation and examination of the Der p2-transgenic tomato plant.
In this study, the expression levels of Der p2 in the transgenic tobacco plants peaked at 15% of the total extracted protein. This higher protein purity from the extraction system used here can induce oral tolerance in allergic asthma and demonstrated therapeutic potential for other allergic diseases. In conclusion, the remarkable potential therapeutic effects of the Der p2-transgenic plant in our study suggest that Der p2-transgenic plants may be a desirable choice of treatment for allergic patients.
Acknowledgments
We would like to thank Dr Lan Ruth and Dr Ya-Hui Chuang for critical review of the manuscript.
References
- Beasley R. The burden of asthma with specific reference to the United States. J Allergy Clin Immunol. 2002;109:S482–S489. doi: 10.1067/mai.2002.122716. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Mishima H, Renzi PM, Xu LJ, Hamid Q, Martin JG. Transfer of allergic airway responses with antigen-primed CD4+ but not CD8+ T cells in brown Norway rats. J Clin Invest. 1995;96:1303–1310. doi: 10.1172/JCI118165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant Th2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- Corrigan CJ, Kay AB. T cells and eosinophils in the pathogenesis of asthma. Immunol Today. 1992;13:501–507. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- Mcmenamin C, Mckersey M, Kuhnlein P, Hunig T, Holt PG. Gamma delta T cells down-regulate primary IgE responses I rats toinhaled soluble protein antigens. J Immunol. 1995;154:4390–4394. [PubMed] [Google Scholar]
- van Houten N, Blank SF. Direct measurement of anergy of antigen-specific T cells following oral tolerance induction. J Immunol. 1996;157:1337–1341. [PubMed] [Google Scholar]
- Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25+CD4+ regulatory T cells by oral antigen administration. J Immunol. 2001;167:4245–4253. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- Frew AJ. Immunotherapy of allergic disease. J Allergy Clin Immunol. 2003;111:S712–S719. doi: 10.1067/mai.2003.84. [DOI] [PubMed] [Google Scholar]
- Passalacqua G, Baena-Cagnani CE, Berardi M, Canonica GW. Oral and sublingual immunotherapy in paediatric patients. Curr Opin Allergy Clin Immunol. 2003;3:139–145. doi: 10.1097/00130832-200304000-00008. [DOI] [PubMed] [Google Scholar]
- La Rosa M, Ranno C, André C, Carat F, Tosca MA, Canonica GW. Double-blind placebo-controlled evaluation of sublingual-swallow immunotherapy with standardized Parietaria judaica extract in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 1999;104:425–432. doi: 10.1016/s0091-6749(99)70388-x. [DOI] [PubMed] [Google Scholar]
- Giovane AL, Bardare M, Passalacqua G, Ruffoni S, Scordamaglia A, Ghezzi E, et al. A three-year double-blind placebo-controlled study with specific oral immunotherapy to Dermatophagoides: evidence of safety and efficacy in paediatric patients. Clin Exp Allergy. 1994;24:53–59. doi: 10.1111/j.1365-2222.1994.tb00917.x. [DOI] [PubMed] [Google Scholar]
- Di Rienzo V, Marcucci F, Puccinelli P, Parmiani S, Frati F, Sensi L, et al. Long-lasting effect of sublingual immunotherapy in children with asthma due to house dust mite: a 10-year prospective study. Clin Exp Allergy. 2003;33:206–210. doi: 10.1046/j.1365-2222.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- Canonica GW, Passalacqua G. Noninjection routes for immunotherapy. J Allergy Clin Immunol. 2003;111:437–448. doi: 10.1067/mai.2003.129. [DOI] [PubMed] [Google Scholar]
- Peden DB, Bush RK. Advances in environmental and occupational respiratory disease in 2010. J Allergy Clin Immunol. 2011;127:696–700. doi: 10.1016/j.jaci.2011.01.030. [DOI] [PubMed] [Google Scholar]
- Ma SW, Zhao DL, Yin ZQ, Mukherjee R, Singh B, Qin HY, et al. Transgenic plants expressing autoantigens fed to mice to induce oral immune tolerance. Nat Med. 1997;3:793–796. doi: 10.1038/nm0797-793. [DOI] [PubMed] [Google Scholar]
- Hamelmann E, Schwarze J, Takeda A, Oshiba A, Larsen GL, Irvin CG, et al. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care. 1997;156:766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- Glaab T, Mitzner W, Braun A, Ernst H, Hohllfeld JM, Krug N, et al. Repetitive measurements of pulmonary mechanics to inhaled cholinergic challenge in spontaneously breathing mice. J Appl Physiol. 2004;97:1104–1111. doi: 10.1152/japplphysiol.01182.2003. [DOI] [PubMed] [Google Scholar]
- Jobling SA, Gehrke L. Enhanced translation of chimaeric messenger RNAs containing a plant viral untranslated leader sequence. Nature. 1987;325:622–625. doi: 10.1038/325622a0. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Nakamura K. Propeptide of a precursor to a plant vacuolar protein required for vacuolar targeting. Proc Natl Acad Sci USA. 1991;88:834–838. doi: 10.1073/pnas.88.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JJ, Liu YH, Shen HD, Huang SH, Han SH. Prevention of Der p2-induced allergic airway inflammation by Mycobacterium-bacillus Calmette Guerin. J Arch Allergy Immunol. 2002;121:205–210. [PubMed] [Google Scholar]
- Muller U, Akdis CA, Fricker M, Akdis M, Blesken T, Betten F, et al. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J Allergy Clin Immunol. 1998;101:747–754. doi: 10.1016/S0091-6749(98)70402-6. [DOI] [PubMed] [Google Scholar]
- Hoyne GF, Askonas BA, Hetzel C, Tomas WR, Lamb JR. Regulation of house dust mite responses by intranasally administered peptide: transient activation of CD4+ T cells precedes the development of tolerance in vivo. . Int immunol. 1996;8:335–342. doi: 10.1093/intimm/8.3.335. [DOI] [PubMed] [Google Scholar]
- Mastrandrea F, Serio G, Minardi A, Coradduzza G, Rossi N, Scarcia G, et al. IgE responses to Dermatophagoides pteronyssinus native major allergens Der p 1 and Der p 2 during long-term specific immunotherapy. Allergy. 1997;52:1115–1119. doi: 10.1111/j.1398-9995.1997.tb00185.x. [DOI] [PubMed] [Google Scholar]
- Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretolani M. Interleukin-10: an anti-inflammatory cytokine with therapeutic potential. Clin Exp Allergy. 1999;29:1164–1171. doi: 10.1046/j.1365-2222.1999.00456.x. [DOI] [PubMed] [Google Scholar]
- Saraiva M, O'Garra A. The regulation of IL10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin10 and the interleukin10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Mcmenamin C, Holt PG. The natural immune response to inhaled soluble protein antigens major histocompatibility complex (MHC) class I-restricted CD8+ T cell-mediated but class II-restricted CD4+ T cell dependent immune deviation resulting in selective suppression of immunoglobin E production. J Exp Med. 1993;178:889–899. doi: 10.1084/jem.178.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H. SS, Wyeoff K. Medical molecular farming: production of antibodies, biopharmaceuticals, and edible vaccines in plants. Trends Plant Sci. 2001;6:219. doi: 10.1016/S1360-1385(01)01922-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cretico PS. Immunotherapy with allergens. J Am Med Assoc. 1992;268:2834. [PubMed] [Google Scholar]
- Mor TS, Gómez-Lim MA, Palmer KE. Perspective: edible vaccines—a concept coming of age. Trends Microbiol. 1998;6:449–453. doi: 10.1016/s0966-842x(98)01357-2. [DOI] [PubMed] [Google Scholar]
- Kusnadi AR, Nikolov ZL, Howard JA. Transgenic plants expressing autoantigens fed to mice to induce oral immune tolerance. Nat Med. 1997;3:793–796. doi: 10.1038/nm0797-793. [DOI] [PubMed] [Google Scholar]